Silicon Treatment on Sorghum Plants Prior to Glyphosate Spraying: Effects on Growth, Nutrition, and Metabolism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Growing Conditions
2.2. Treatments and Experimental Design
2.3. Measurements
2.4. Statistical Analysis
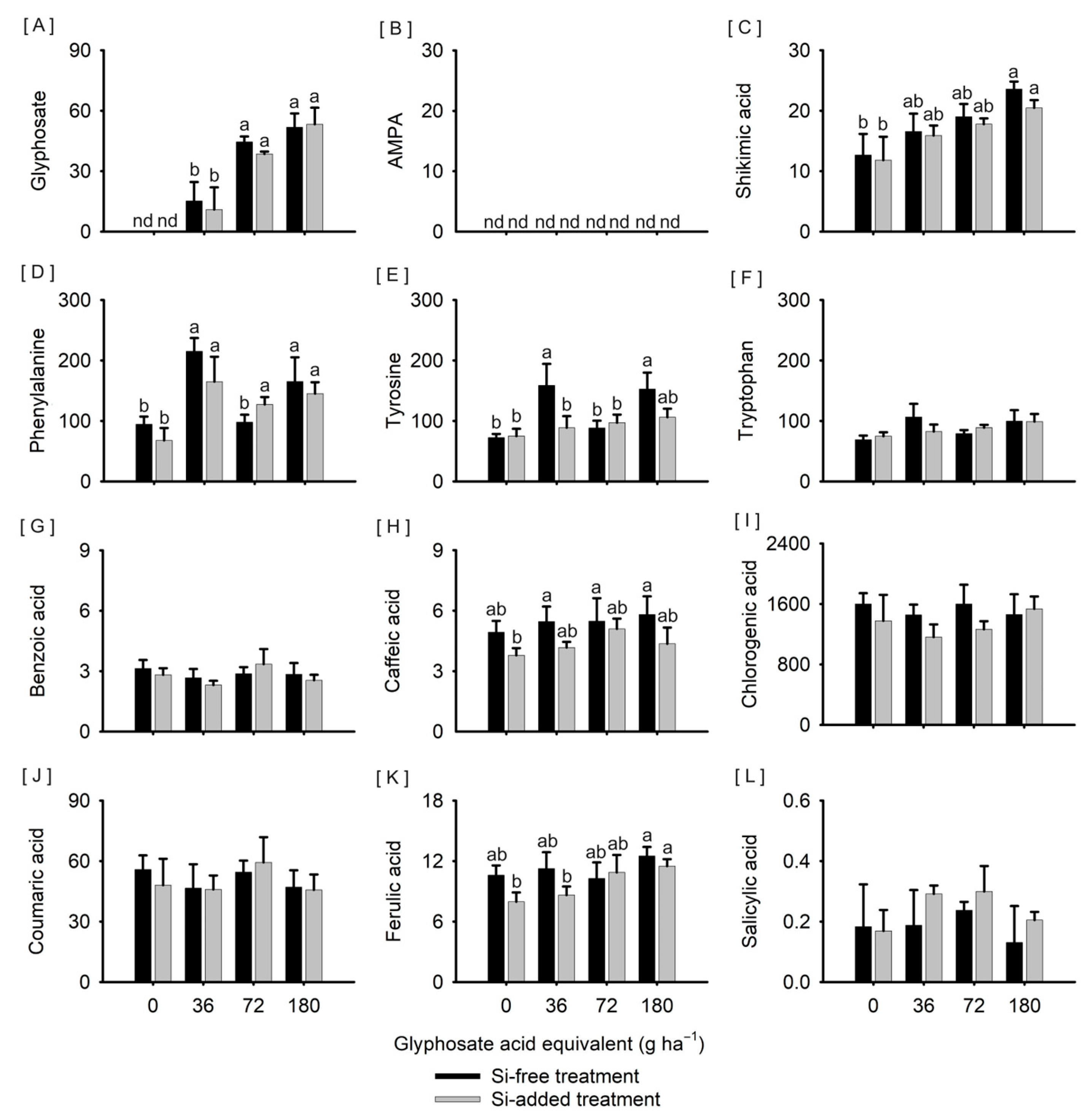
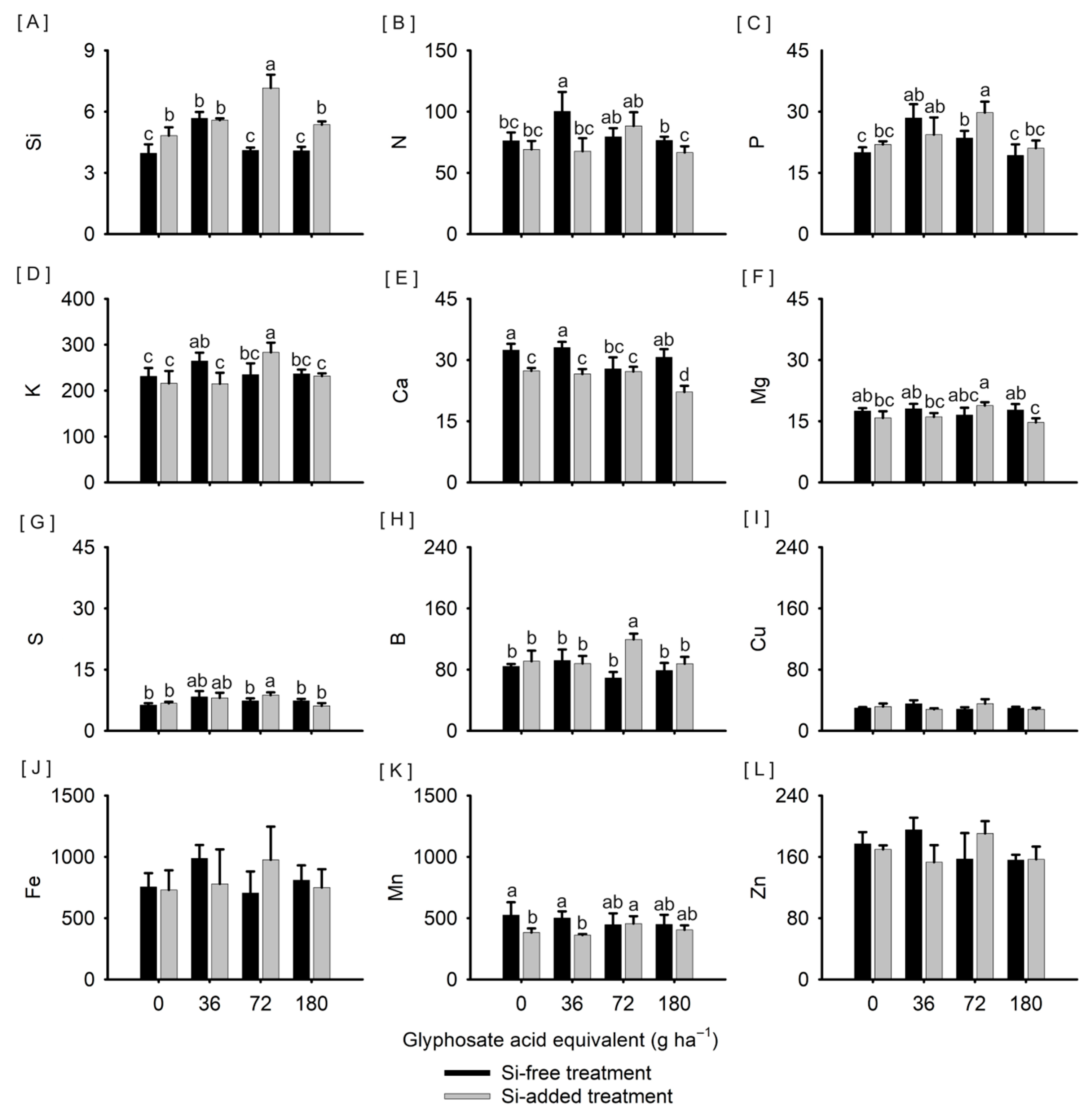
3. Results
4. Discussion
4.1. Si in Sorghum
4.2. Glyphosate and Its Effects on Sorghum
4.3. Combined Effects of Si and Glyphosate on Sorghum
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khoddami, A.; Messina, V.; Komala, V.; Faranhaky, A.; Blanchard, C.L.; Roberts, T.H. Sorghum in foods: Functionality and potential in innovative products. Crit. Rev. Food Sci. Nutr. 2023, 63, 1170–1186. [Google Scholar] [CrossRef] [PubMed]
- Proietti, I.; Frazzoli, C.; Mantovani, A. Exploiting nutritional value of staple foods in the world’s semi-arid areas: Risks, benefits, challenges and opportunities of sorghum. Healthcare 2015, 3, 172–193. [Google Scholar] [CrossRef]
- Kresovich, S.; Barbazuk, B.; Bedell, J.A.; Borrell, A.; Buell, C.R.; Burke, J. Toward sequencing the sorghum genome. A US National Science Foundation-sponsored Workshop Report. J. Plant Physiol. 2005, 138, 1898–1902. [Google Scholar]
- Chadalalavada, K.; Kumari, B.D.R.; Kumar, S. Sorghum mitigates climate variability and change on crop yield and quality. Planta 2021, 253, 113. [Google Scholar] [CrossRef] [PubMed]
- Pandian, B.A.; Sexton-Bowser, S.; Prasad, P.V.V.; Jugulam, M. Current status and prospects of herbicide-resistant grain sorghum (Sorghum bicolor). Pest. Manag. Sci. 2022, 78, 409–415. [Google Scholar] [CrossRef]
- USDA. World Agricultural Production; USDA—United States Department of Agriculture: Washington, DC, USA, 2024. Available online: https://apps.fas.usda.gov/psdonline/circulars/production.pdf (accessed on 13 July 2024).
- Bajwa, A.A.; Nawaz, A.; Farooq, M.; Chauhan, B.S.; Adkins, S. Herbicide program to control Parthenium hysterophorus in grain sorghum in an arid environment. Crops 2023, 3, 292–301. [Google Scholar] [CrossRef]
- Freitas, H.C.; Corrêa, F.R.; Silva, N.F.; Silva, W.S.; Cavalcante, D.; Ribeiro, F.R.E. Efeitos fitotécnicos do manejo de herbicidas aplicados em pré e pós emergência na cultura do sorgo. Braz. J. Sci. 2023, 24, 64–75. [Google Scholar] [CrossRef]
- Tkalich, Y.; Tsyliuryk, O.; Havryushenko, O.; Mytsyk, O.; Kozechko, V.; Rudakov, Y. Weed chemical control in grain sorghum at the steppe zone of Ukraine. Ecol. Quest. 2023, 34, 109–115. [Google Scholar] [CrossRef]
- Werle, R.; Tenhumberg, B.; Linquist, J.L. Modeling shattercane dynamics in herbicide-tolerant grain sorghum cropping systems. Ecol. Model. 2017, 343, 131–141. [Google Scholar] [CrossRef]
- Metlinga, G.V.; Vasilchenko, S.A. Efficacy of Ballerina herbicide on grain sorghum. Grain Econ. Rus. 2021, 73, 68–72. [Google Scholar] [CrossRef]
- ANDEF—Associação Nacional de Defesa Vegetal. Manual de Tecnologia de Aplicação de Produtos Fitossanitários, 1st ed.; Linea Creativa: Campinas, SP, Brazil, 2010; pp. 1–50. [Google Scholar]
- Cunha, J.P.A.R.; Teixeira, M.M.; Coury, J.R.; Ferreira, L.R. Evaluation of strategies to reduce pesticide spray drift. Planta Daninha 2003, 21, 325–332. [Google Scholar] [CrossRef]
- MAPA. Agrofit—Sistema de Agrotóxicos Fitossanitários; MAPA—Ministério da Agricultura, Pecuária e Abastecimento: Brasilia, DF, Brazil, 2003. Available online: https://agrofit.agricultura.gov.br/agrofit_cons/principal_agrofit_cons (accessed on 13 July 2024).
- Reis, L.A.C.; Carvalho, F.P.; França, A.C.; Francino, D.M.T.; Pinto, N.A.V.D.; Freitas, A.F. Leaf morphoanatomy and biochemical variation on coffee cultivars under drift simulation of glyphosate. Planta Daninha 2018, 36, e018143560. [Google Scholar] [CrossRef]
- Andrade, T.C.G.T.; Bacha, A.L.; Camargo, B.M.; Carvalho, L.B. Influence of phosphorus fertilization on the response of pinus genotypes to glyphosate subdoses. New For. 2022, 53, 143–160. [Google Scholar] [CrossRef]
- Nunes, R.T.; Albrecht, A.J.P.; Albrecht, L.P.; Lorenzety, J.B.; Danilussi, M.T.Y.; Silva, R.M.; Silva, A.F.M.; Barroso, A.A.M. Soybean injury caused by the application of subdoses of 2,4-D or dicamba, in simulated drift. J. Environ. Sci. Health Part B 2023, 58, 327–333. [Google Scholar] [CrossRef]
- Zampiroli, R.; Cunha, J.; Alvarenga, C.B. Simulated drift of dicamba and glyphosate on coffee crop. Plants 2023, 12, 3525. [Google Scholar] [CrossRef]
- Cederlund, H. Effects of spray drift of glyphosate on nontarget terrestrial plants: A critical review. Environ. Toxicol. Chem. 2017, 36, 2879–2886. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Zhang, Y.; Lin, M.; Mao, J.; Xing, B.; Li, Y.; Hou, R. Capability of phytoremediation of glyphosate in environment by Vulpia myuros. Ecotoxicol. Environ. Saf. 2023, 265, 115511. [Google Scholar] [CrossRef]
- Duke, S.O. Glyphosate: Uses other than in glyphosate-resistant crops, mode of action, degradation in plants, and effects on non-target plants and agricultural microbes. Rev. Environ. Contam. Toxicol. 2021, 255, 1–65. [Google Scholar]
- Van Eerd, L.L.; Hoagland, R.E.; Zablotowicz, R.M.; Hall, J.C. Pesticide metabolism in plants and microorganisms. Weed Sci. 2003, 51, 472–495. [Google Scholar] [CrossRef]
- Reddy, K.N.; Rimando, A.M.; Duke, S.O. Aminomethylphosphonic acid, a metabolite of glyphosate, causes injury in glyphosate-treated, glyphosate-resistant soybean. J. Agric. Food Chem. 2004, 52, 5139–5143. [Google Scholar] [CrossRef]
- Duke, S.O. Glyphosate degradation in glyphosate-resistant and –susceptible crops and weeds. J. Agric. Food Chem. 2011, 59, 5835–5841. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.P.; Smedbol, A.; Chalifour, L.; Hénault-Ethier, M.; Labrecque, L.L.; Lucotte, M.; Juneau, P. Alteration of plant physiology by glyphosate and its by-product aminomethylphosphonic acid: An overview. J. Exp. Bot. 2014, 65, 4691–4703. [Google Scholar] [CrossRef]
- Guilherme, S.; Santos, M.; Gaivão, I.; Pacheco, M. DNA and chromosomal damage induced in fish (Anguilla anguilla L.) by aminomethylphosphonic acid (AMPA)—The major environmental breakdown product of glyphosate. Environ. Sci. Pollut. Res. 2014, 21, 8730–8739. [Google Scholar] [CrossRef] [PubMed]
- Yanniccari, M.; Tambussi, E.; Istilart, C.; Castro, A.M. Glyphosate effects on gas exchange and chlorophyll fluorescence responses of two Lolium perenne L. biotypes with differential herbicide sensitivity. Plant Physiol. Biochem. 2012, 57, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Zulet-González, A.; Barco-Antoñanzas, M.; Gil-Monreal, M.; Royuela, M.; Zabalza, A. Increased glyphosate-induced gene expression in the shikimate pathway is abolished in the presence of aromatic amino acids and mimicked by shikimate. Front. Plant Sci. 2020, 29, 459. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, K.M.; Weaver, L.M. The shikimate pathway. Ann. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 473–503. [Google Scholar] [CrossRef]
- Zeier, J. New insights into the regulation of plant immunity by amino acid metabolic pathways. Plant Cell Environ. 2013, 36, 2085–2103. [Google Scholar] [CrossRef]
- Häusler, R.E.; Ludewig, F.; Krueger, S. Amino acids: A life between metabolism and signaling. Plant Sci. 2014, 229, 225–237. [Google Scholar] [CrossRef]
- Herrmann, K.M. The Shikimate pathway: Early steps in the biosynthesis of aromatic compounds. Plant Cell. 1995, 7, 907–919. [Google Scholar] [CrossRef]
- Marchiosi, R.; Santos, W.D.; Constantin, R.P.; Lima, R.B.; Soares, A.R.; Finger-Teixeira, A.; Mota, T.R.; Oliveira, D.M.; Foletto-Felipe, M.P.; Abrahão, J.; et al. Biosynthesis and metabolic actions of simple phenolic acids in plants. Phytochem. Rev. 2020, 19, 865–906. [Google Scholar] [CrossRef]
- Saudy, H.S.; Mubarak, M. Mitigating the detrimental impacts of nitrogen deficit and fenoxaprop-p-ethyl herbicide on wheat using silicon. Commun. Soil. Sci. Plant Anal. 2015, 46, 897–907. [Google Scholar] [CrossRef]
- Tripthi, D.K.; Varma, R.K.; Singh, S.; Sachan, M.; Guerriero, G.; Kushwaha, B.K.; Bhardwaj, S.; Ramawat, N.; Sharma, S.; Singh, V.P.; et al. Silicon tackles butachlor toxicity in rice seedlings by regulating anatomical characteristics, ascorbate-glutathione cycle, proline metabolism and levels of nutrients. Sci. Rep. 2020, 10, 14078. [Google Scholar] [CrossRef] [PubMed]
- Soares, C.; Nadais, P.; Sousa, B.; Pinto, E.; Ferreira, I.M.P.L.V.O.; Pereira, R.; Fidalgo, F. Silicon Improves the redox homeostasis to alleviate glyphosate toxicity in tomato plants—Are nanomaterials relevant? Antioxidants 2021, 10, 1320. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, I. Silício como atenuador de estresse causado por glifosato em mudas de laranjeira Valência. Master Thesis, São Paulo State University (UNESP), Jaboticabal, SP, Brazil, 2017. [Google Scholar]
- Prado, R.M. Benefits of Silicon in the Nutrition of Plants, 1st ed.; Springer: Cham, Switzerland, 2023; pp. 1–378. [Google Scholar]
- Olivera-Viciedo, D.; Aguilar, D.S.; Prado, R.M.; Calzada, K.P.; Hurtado, A.C.; Piccolo, M.C.; Soares, M.B.; Toledo, R.L.; Alves, G.R.; Ferreira, D.; et al. Silicon-mediated adjustments in C:N:P ratios for improved beetroot yield under ammonium-induced stress. Agronomy 2024, 14, 1104–1114. [Google Scholar] [CrossRef]
- Olivera-Viciedo, D.; Oliveira, K.S.; de Mello Prado, R.; Habermann, E.; Martínez, C.A.; de Moura Zanine, A. Silicon uptake and utilization on Panicum maximum grass modifies C:N:P stoichiometry under warming and soil water deficit. Soil. Tillage Res. 2024, 235, 105884. [Google Scholar] [CrossRef]
- Cerveira Júnior, W.R.; Costa, Y.K.S.; Carboni, C.A.; Duke, S.O.; Aguiar, P.A.C.; Carvalho, L.B. Growth, morphological, metabolic and photosynthetic responses of clones of eucalyptus to glyphosate. For. Ecol. Manag. 2020, 470–471, 118218. [Google Scholar] [CrossRef]
- Prado, R.M. Introduction to Plant Nutrition. In Mineral Nutrition of Tropical Plants, 1st ed.; Prado, R.M., Ed.; Springer: Cham, Switzerland, 2021; pp. 1–38. [Google Scholar]
- Bortolheiro, F.P.A.P.; Brunelli-Nascentes, M.C.; Carbonari, C.A.; Velini, E.D.; Silva, M.A. Low doses of glyphosate do not damage the secondary metabolism of common bean. J. Environ. Sci. Health Part B 2023, 58, 465–476. [Google Scholar] [CrossRef]
- McWhorter, G.G.; Paul, R.N. The involvement of cork-silica cell pairs in the production of wax filaments in Johnsongrass (Sorghum halepense) leaves. Weed Sci. 1989, 37, 458–470. [Google Scholar] [CrossRef]
- Deshmukh, R.; Sonah, H.; Belanger, R.R. New evidence defining the evolutionary path of aquaporins regulating silicon uptake in land plants. J. Exp. Bot. 2020, 71, 6775–6788. [Google Scholar] [CrossRef]
- Hurtado, A.C.; Chiconato, D.A.; Prado, R.M.; Souza Júnior, G.S.; Gratão, P.L.; Felisberto, G.; Viciedo, D.O.; Santos, D.M.M. Different methods of silicon application attenuate salt stress in sorghum and sunflower by modifying the antioxidative defense mechanism. Ecotoxicol. Environ. Saf. 2020, 203, 110964. [Google Scholar] [CrossRef]
- Thakur, A.; Singh, A.; Tandon, A.; Sharma, V. Insights into the molecular mechanisms of uptake, phytohormone interactions and stress alleviation by silicon: A beneficial but non-essential nutrient for plants. Plant Growth Regul. 2023, 101, 1–13. [Google Scholar] [CrossRef]
- Wani, A.H.; Mir, S.H.; Kumar, S.; Malik, M.A.; Tyub, S.; Rashid, I. Silicon en route-from loam to leaf. Plant Growth Regul. 2023, 99, 465–476. [Google Scholar] [CrossRef]
- Mir, R.A.; Bhat, B.A.; Yousuf, H.; Islam, S.T.; Raza, A.; Rizvi, M.A.; Charagh, S.; Albaqami, M.; Sofi, P.A.; Zargar, S.M. Multidimensional role of silicon to activate resilient plant growth and to mitigate abiotic stress. Front. Plant Sci. 2022, 13, 819658. [Google Scholar] [CrossRef] [PubMed]
- Naidu, S.; Pandey, J.; Mishra, L.C.; Chakraborty, A.; Roy, A.; Singh, I.K.; Singh, A. Silicon nanoparticles: Synthesis, uptake and their role in mitigation of biotic stress. Ecotoxicol. Environ. Saf. 2023, 255, 114783. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Yamaji, N.; Mitani-Ueno, N. Transport of silicon from roots to panicles in plants. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2011, 87, 377–385. [Google Scholar]
- Roohizadeh, G.; Majd, A.; Arbabian, S. The effect of sodium silicate and silica nanoparticles on seed germination and growth in the Vicia faba L. Trop. Plant Res. 2015, 2, 85–89. [Google Scholar]
- Lux, A.; Luxová, M.; Hattori, T.; Inanaga, S.; Sugimoto, Y. Silicification in sorghum (Sorghum bicolor) cultivars with different drought tolerance. Physiol. Plant. 2002, 115, 87–92. [Google Scholar] [CrossRef]
- Hattori, T.; Inanaga, S.; Araki, H.; An, P.; Morita, S.; Luxová, M.; Lux, A. Application of silicon enhanced drought tolerance in Sorghum bicolor. Physiol. Plant. 2005, 123, 459–466. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, V.; Gill, J.P.K.; Datta, S.; Singh, S.; Dhaka, V.; Kapoor, D.; Wani, A.B.; Dhanjal, D.S.; Kumar, M.; et al. Herbicide glyphosate: Toxicity and microbial degradation. Int. J. Environ. Res. Public. Health 2020, 17, 7519. [Google Scholar] [CrossRef]
- Tresnakova, N.; Stara, A.; Velisek, J. Effects of glyphosate and its metabolite AMPA on aquatic organisms. Appl. Sci. 2021, 11, 9004. [Google Scholar] [CrossRef]
- Vasquez-Garcia, J.G.; Palma-Bautista, C.; Rojano-Delgadao, A.M.; de Prado, R.; Menendez, J. The first case of glyphosate-resistance in johnsongrass (Sorghum halepense (L.) in Europe. Plants 2020, 9, 313. [Google Scholar] [CrossRef] [PubMed]
- Freitas-Silva, L.; Araújo, T.O.; Nunes-Nesi, A.; Ribeiro, C.; Costa, A.C.; Silva, L.C. Evaluation of morphological and metabolic responses to glyphosate exposure in two neotropical plant species. Ecol. Indic. 2020, 113, 106246. [Google Scholar] [CrossRef]
- Amrhein, N.; Deus, B.; Gehrke, P.; Steinrucken, H.C. The site of the inhibition of the shikimate pathway by glyphosate. Plant Physiol. 1980, 66, 830–834. [Google Scholar] [CrossRef] [PubMed]
- Cedergreen, N.; Felby, C.; Porter, J.R.; Streibig, J.C. Chemical stress can increase crop yield. Field Crops Res. 2009, 114, 54–57. [Google Scholar] [CrossRef]
- Carvalho, L.B.; Alves, P.L.C.A.; Duke, S. Hormesis with glyphosate depends on coffee growth stage. An. Acad. Bras. Cienc. 2013, 85, 813–822. [Google Scholar] [CrossRef]
- Velini, E.D.; Alves, E.; Godoy, M.C.; Meschede, D.K.; Souza, R.T.; Duke, S.O. Glyphosate applied at low doses can stimulate plant growth. Pest. Manag. Sci. 2008, 64, 489–496. [Google Scholar] [CrossRef]
- Pincelli-Souza, R.P.; Bortolheiro, F.P.A.P.; Carbonari, C.A.; Velini, E.D.; Silva, M.A. Hormetic effect of glyphosate persists during the entire growth period and increases sugarcane yield. Pest. Manag. Sci. 2020, 76, 2388–2394. [Google Scholar] [CrossRef]
- Santos, J.C.C.; Silva, D.M.R.; Amorim, D.J.; Sab, M.P.V.; Almeida Silva, M. Glyphosate hormesis mitigates the effect of water deficit in safflower (Carthamus tinctorius L.). Pest. Manag. Sci. 2021, 77, 2029–2044. [Google Scholar] [CrossRef]
- Brito, P.F.S.; Tropaldi, L.; Carbonari, C.A.; Velini, E.D. Hormetic effects of glyphosate on plants. Pest. Manag. Sci. 2018, 74, 1064–1070. [Google Scholar] [CrossRef]
- Belz, R.G.; Cedergreen, N. Parthenin hormesis in plants depends on growth conditions. Environ. Exp. Bot. 2010, 69, 293–301. [Google Scholar] [CrossRef]
- Carvalho, L.B.; Duke, S.O.; Alves, P.L.C.A. Physiological responses of Eucalyptus x urograndis to glyphosate are dependent on the genotype. Sci. For. 2018, 46, 177–187. [Google Scholar] [CrossRef]
- Bortolheiro, F.P.A.P.; Brunelli-Nascentes, M.C.; Nascentes, R.F.; Silva, M.A. Glyphosate at low doses changes the physiology and increases the productivity of common bean as affected by sowing seasons. J. Environ. Sci. Health Part B 2022, 57, 458–469. [Google Scholar] [CrossRef] [PubMed]
- Orcaray, L.; Igal, M.; Marino, D.; Zabalza, A.; Royuela, M. The possible role of quinate in the mode of action of glyphosate and acetolactate synthase inhibitors. Pest. Manag. Sci. 2010, 66, 262–269. [Google Scholar] [CrossRef]
- Petersen, I.L.; Hansen, H.C.B.; Ravn, H.W.; Sorensen, J.C.; Sorensen, H. Metabolic effects in rapeseed (Brassica napus L.) seedlings after root exposure to glyphosate. Pestic. Biochem. Phys. 2007, 89, 220–229. [Google Scholar] [CrossRef]
- Carbonari, C.A.; Gomes, G.L.G.C.; Velini, E.D.; Machado, R.F.; Simões, P.S.; Macedo, G.C. Glyphosate effects on sugarcane metabolism and growth. Am. J. Plant Sci. 2014, 5, 3585–3593. [Google Scholar] [CrossRef]
- Zabalza, A.; Orcaray, L.; Fernandez-Escalada, M.; Zulet-González, A.; Royuela, M. The Pattern of shikimate pathway and phenylpropanoids after inhibition by glyphosate or quinate feeding in pea roots. Pestic. Biochem. Physiol. 2017, 141, 96–102. [Google Scholar] [CrossRef]
- Ghimire, B.K.; Son, N.Y.; Kim, S.H.; Yu, C.Y.; Chung, I.M. Evaluating water deficit and glyphosate treatment on the accumulation of phenolic compounds and photosynthesis rate in transgenic Codonopsis lanceolata (Siebold & Zucc.) Trautv. over-expressing c-Tocopherol methyltransferase (c-Tmt) gene. Biotech 2017, 7, 167. [Google Scholar]
- Eker, S.; Ozturk, L.; Yazici, A.; Erenoglu, B.; Romheld, V.; Cakmak, I. Foliar-applied glyphosate substantially reduced uptake and transport of iron and manganese in sunflower (Helianthus annus L.) plants. J. Agric. Food Chem. 2006, 54, 10019–10025. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, I.; Yazici, A.; Tutus, Y.; Ozturk, L. Glyphosate reduced seed and leaf concentrations of calcium, manganese, magnesium, and iron in non-glyphosate resistant soybean. Eur. J. Agron. 2009, 31, 114–119. [Google Scholar] [CrossRef]
- Costa, F.R.; Rech, R.; Duke, S.O.; Carvalho, L.B. Lack of effects of glyphosate and glufosinate on growth, mineral content, and yield of glyphosate- and glufosinate-resistant maize. GM Crops Food 2018, 9, 189–198. [Google Scholar] [CrossRef]
- Duke, S.O.; Rimando, A.M.; Reddy, K.N.; Cizdziel, J.V.; Bellalui, N.; Shaw, D.R.; Williams, M.M.; Maul, J.E. Lack of transgene and glyphosate effects on yield and mineral and amino acid content of glyphosate-resistant soybean. Pest. Manag. Sci. 2018, 74, 1166–1173. [Google Scholar] [CrossRef] [PubMed]
- Bortolheiro, F.P.A.P.; Silva, M.A. Low doses of glyphosate can affect the nutrient composition of common beans depending on the sowing season. Sci. Total Environ. 2021, 794, 148733. [Google Scholar] [CrossRef] [PubMed]
- Costa, Y.K.S.; Ribeiro, N.M.; Moura, G.C.P.; Oliveira, A.R.; Bianco, S.; Alcántara, R.; Carvalho, L.B. Effect of glyphosate and P on the growth and nutrition of Coffea arabica cultivars and on weed control. Sci. Rep. 2021, 11, 8095. [Google Scholar] [CrossRef] [PubMed]
- Bidóia, V.S.; Santos Neto, J.C.; Maciel, C.D.G.; Tropaldi, L.; Carbonari, C.A.; Duke, S.O.; Carvalho, L.B. Lack of significant effects of glyphosate on glyphosate-resistant maize in different field locations. Agronomy 2023, 13, 1071. [Google Scholar] [CrossRef]
- Duke, S.O. Glyphosate. In Herbicides: Chemistry, Degradation, and Mode of Action, 1st ed.; Kearney, P.C., Kaufman, D.D., Eds.; Dekker: New York, NY, USA, 1988; Volume 3, pp. 1–70. [Google Scholar]
- Duke, S.O.; Wauchope, R.D.; Hoagland, R.E.; Wills, G.D. Influence of glyphosate on uptake and translocation of calcium ion in soybean seedlings. Weed Res. 1983, 23, 133–139. [Google Scholar] [CrossRef]
- Schoenherr, J.; Schreiber, L. Interactions of calcium ions with weakly acidic active ingredients slow cuticular penetration: A case study with glyphosate. J. Agric. Food Chem. 2004, 52, 6546–6551. [Google Scholar] [CrossRef]
- Mueller, T.C.; Main, C.L.; Thompson, A.; Steckel, L.E. Comparison of glyphosate salts (isopropylamine, diammonium, and potassium) and calcium and magnesium concentrations on the control of various weeds. Weed Technol. 2006, 20, 164–171. [Google Scholar] [CrossRef]
- Kanissery, R.; Gairhe, B.; Kadyampakeni, D.; Batuman, O.; Alferez, F. Glyphosate: Its environmental persistence and impact on crop health and nutrition. Plants 2019, 8, 499–510. [Google Scholar] [CrossRef]
- Duke, S.O.; Lydon, J.; Koskinen, W.C.; Moorman, T.B.; Chaney, R.L.; Hammerschmidt, R. Glyphosate effects on mineral nutrition, crop rhizosphere microbiota, and plant disease in glyphosate-resistant crops. J. Agric. Food Chem. 2012, 60, 10375–10397. [Google Scholar] [CrossRef]
- Ozturk, L.; Yazici, A.; Eker, S.; Gokmen, O.; Romheld, V.; Cakmak, I. Glyphosate inhibition of ferric reductase activity in iron deficient sunflower roots. New Phytol. 2008, 177, 899–906. [Google Scholar] [CrossRef]
- Harris, W.D.; Sammons, R.D.; Grabiak, R.C.; Mehrsheikh, A.; Bleeke, M.S. Computer simulation of the interactions of glyphosate with metal ions in phloem. J. Agric. Food Chem. 2012, 60, 6077–6087. [Google Scholar] [CrossRef] [PubMed]
- Pipke, R.; Schulz, A.; Amrhein, N. Uptake of glyphosate by an Arthrobacter sp. Appl. Environ. Microbiol. 1987, 53, 974–978. [Google Scholar] [CrossRef] [PubMed]
- Hetherington, P.R.; Marshall, G.; Kirkwood, R.C.; Warner, J.M. Absorption and efflux of glyphosate by cell suspensions. J. Exp. Bot. 1998, 29, 527–533. [Google Scholar] [CrossRef]
- Rabello, W.S.; Monnerat, P.H.; Campanharo, M.; Espindula, M.C.; Ribeiro, G. Growth and phosporus absorption by common bean ‘Xodó’ genotype under effect of glyphosate reduced rates. Rev. Bras. Herbic. 2012, 11, 204–212. [Google Scholar]
- Perim, L.; Prando, M.B.; Rosolem, C.A. Cinética de absorção de fósforo em soja transgênica após aplicação de glyphosate. Rev. Bras. Herbic. 2011, 10, 143–150. [Google Scholar] [CrossRef]
- Hetherington, P.R.; Reynolds, T.L.; Marshall, G.; Kirkwood, R.C. The absorption, translocation and distribution of the herbicide glyphosate in maize expressing the CP-4 transgene. J. Exp. Bot. 1999, 50, 1567–1576. [Google Scholar] [CrossRef]
- Reddy, K.N.; Cizdziel, J.V.; Williams, M.M.; Maul, J.E.; Rimando, A.M.; Duke, S.O. Glyphosate resistance technology has minimal effect on maize mineral nutrition and yield. J. Agric. Food Chem. 2018, 66, 10139–10146. [Google Scholar] [CrossRef]
- Ma, J.F. Role of silicon in enhancing the resistance of plants to biotic and abiotic stresses. Soil. Sci. Plant Nutr. 2004, 50, 11–18. [Google Scholar] [CrossRef]
- Ranganathan, S.; Suvarchala, V.; Rajesh, Y.B.R.D.; Prasad, M.S.; Padmakumari, A.P.; Voleti, S.R. Effects of silicon sources on its deposition, chlorophyll content, and disease and pest resistance in rice. Biol. Plant. 2006, 50, 713–716. [Google Scholar] [CrossRef]
- Liang, Y.; Chen, Q.I.N.; Liu, Q.; Zhang, W.; Ding, R. Exogenous silicon (Si) increases antioxidant enzyme activity and reduces lipid peroxidation in roots of salt-stressed barley (Hordeum vulgare L.). J. Plant Physiol. 2003, 160, 1157–1164. [Google Scholar] [CrossRef]
- Gong, H.J.; Chen, K.M.; Chen, G.C.; Wang, S.M.; Zhang, C.L. Silicon alleviates oxidative damage of wheat plants in pots under drought. Plant Sci. 2005, 169, 313–321. [Google Scholar] [CrossRef]
- Liang, Y.; Si, J.; Romheld, V. Silicon uptake and transport is an active process in Cucumis sativus. New Phytol. 2005, 167, 797–804. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simón, L.A.Y.; Viciedo, D.O.; Carbonari, C.A.; Duke, S.O.; de Carvalho, L.B. Silicon Treatment on Sorghum Plants Prior to Glyphosate Spraying: Effects on Growth, Nutrition, and Metabolism. AgriEngineering 2024, 6, 3538-3552. https://doi.org/10.3390/agriengineering6040201
Simón LAY, Viciedo DO, Carbonari CA, Duke SO, de Carvalho LB. Silicon Treatment on Sorghum Plants Prior to Glyphosate Spraying: Effects on Growth, Nutrition, and Metabolism. AgriEngineering. 2024; 6(4):3538-3552. https://doi.org/10.3390/agriengineering6040201
Chicago/Turabian StyleSimón, Lesly Analay Yanes, Dilier Olivera Viciedo, Caio Antonio Carbonari, Stephen Oscar Duke, and Leonardo Bianco de Carvalho. 2024. "Silicon Treatment on Sorghum Plants Prior to Glyphosate Spraying: Effects on Growth, Nutrition, and Metabolism" AgriEngineering 6, no. 4: 3538-3552. https://doi.org/10.3390/agriengineering6040201





