Impacts of Light Exposure and Soil Covering on Sweet Potato Storage Roots in a Novel Soilless Culture System
Abstract
:1. Introduction
2. Materials and Methods
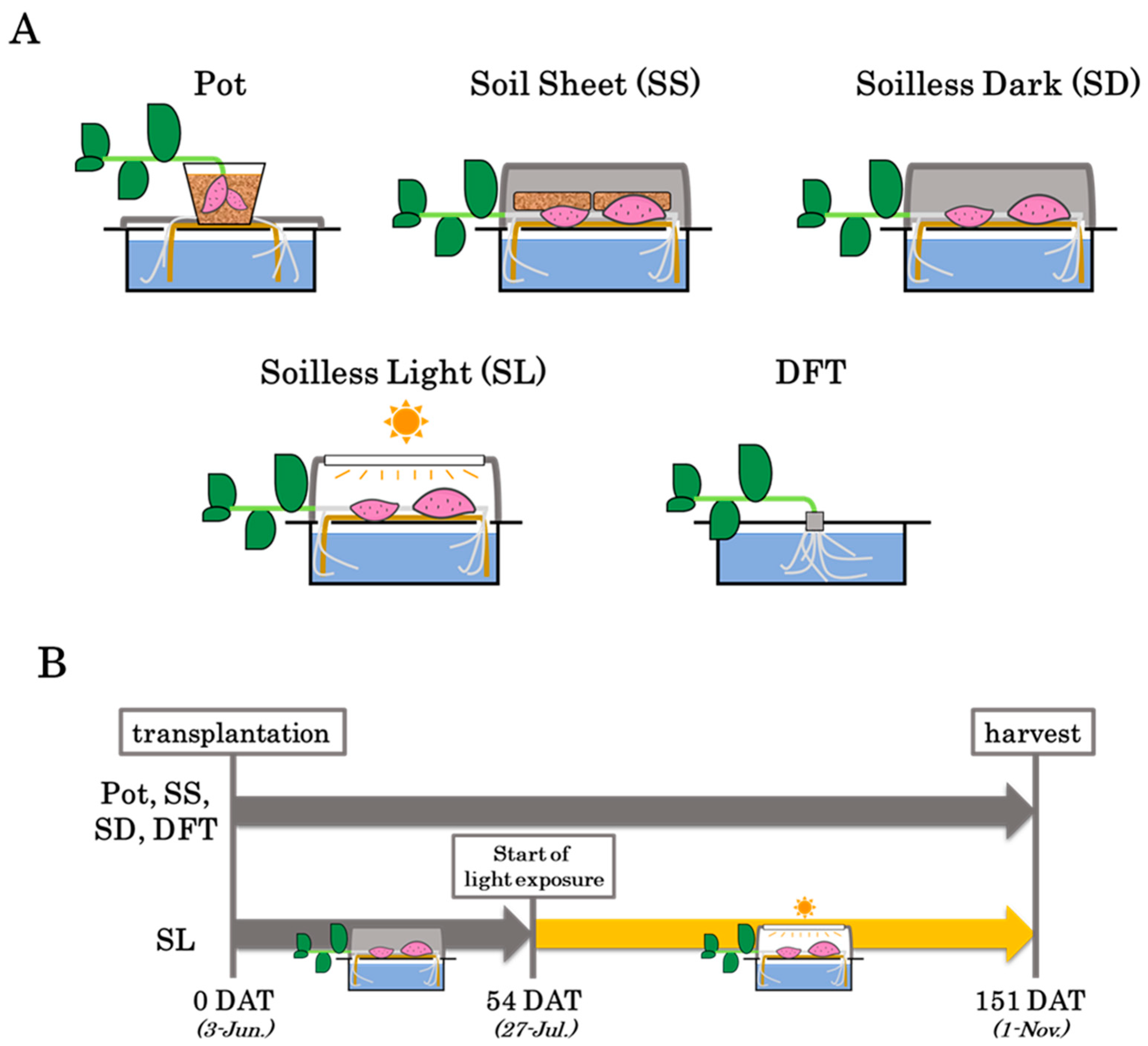
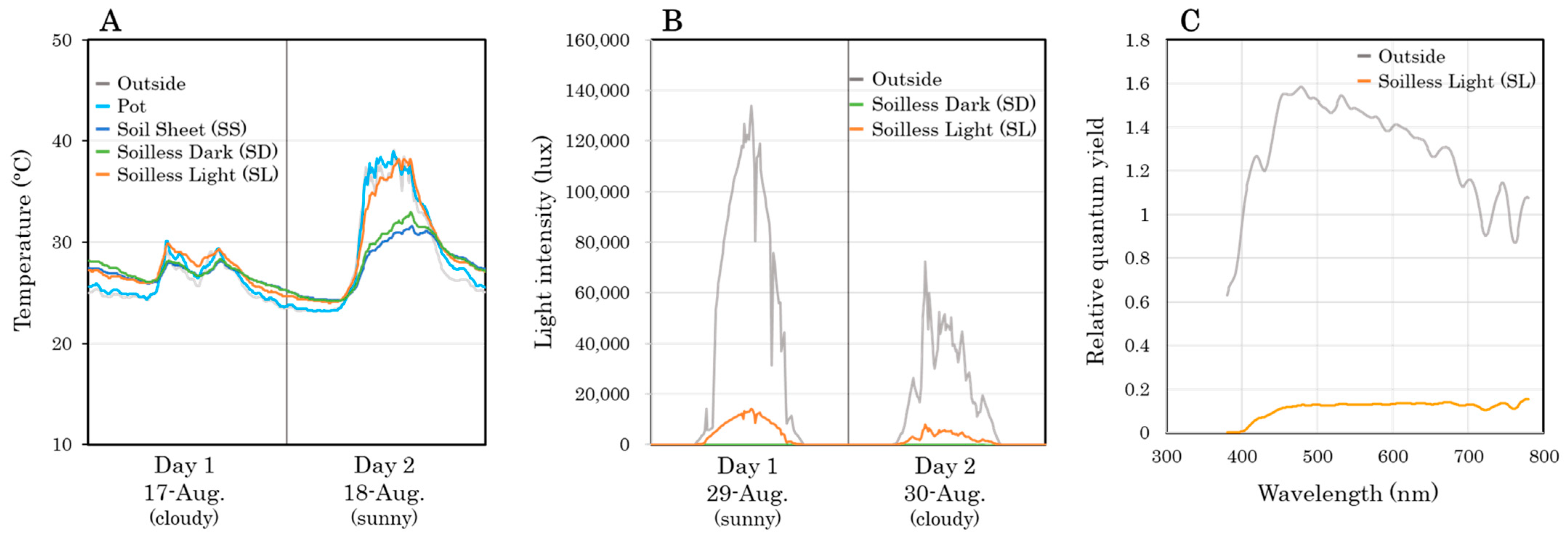
2.1. Experimental Conditions
2.2. Measurement of Plant Growth and Yield
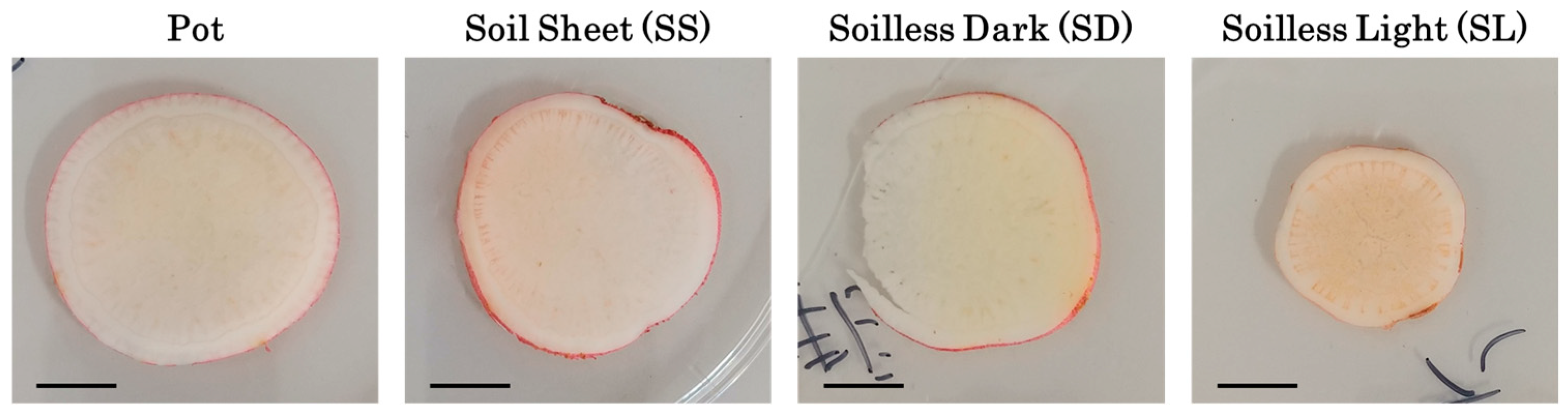
2.3. Measurement of the Color of Storage Roots
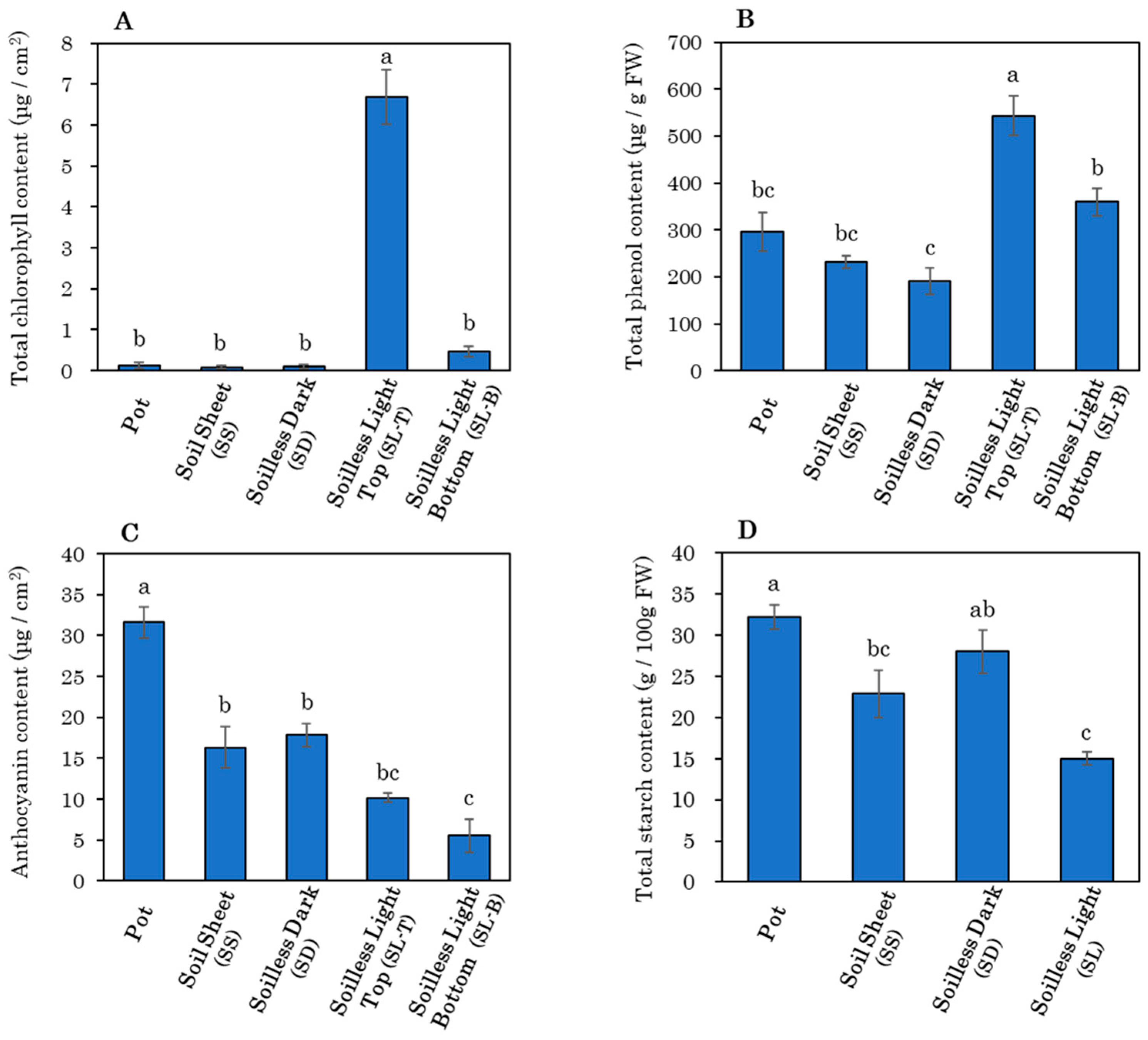
2.4. Measurement of Anthocyanin Content
2.5. Measurement of Total Phenolic Contents
2.6. Measurement of Chlorophyll Content
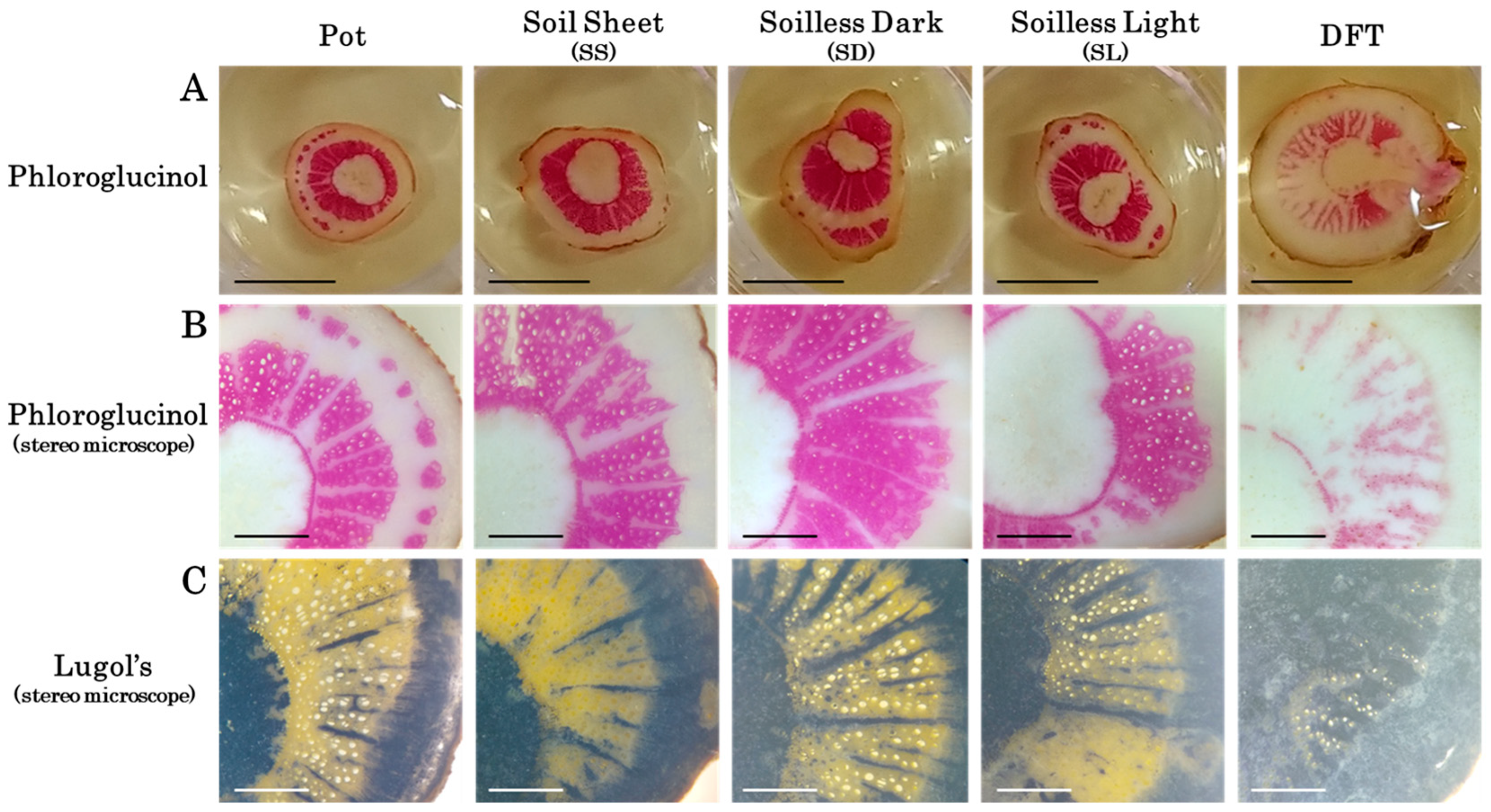
2.7. Measurement of Starch Content
2.8. Starch Staining
2.9. Lignin Staining
2.10. Data Analysis
3. Results
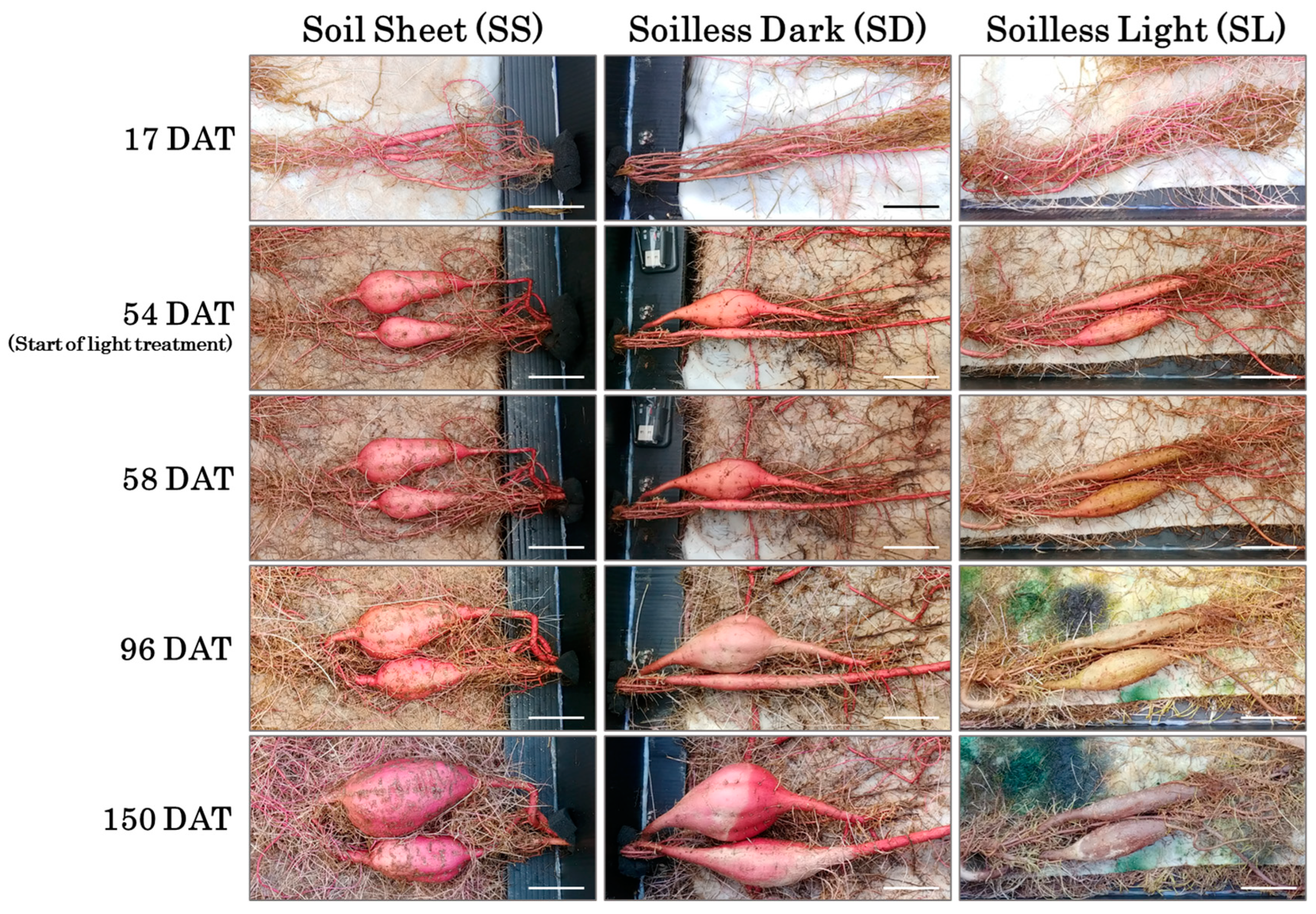
3.1. Time-Course Observations of Storage Root Growth
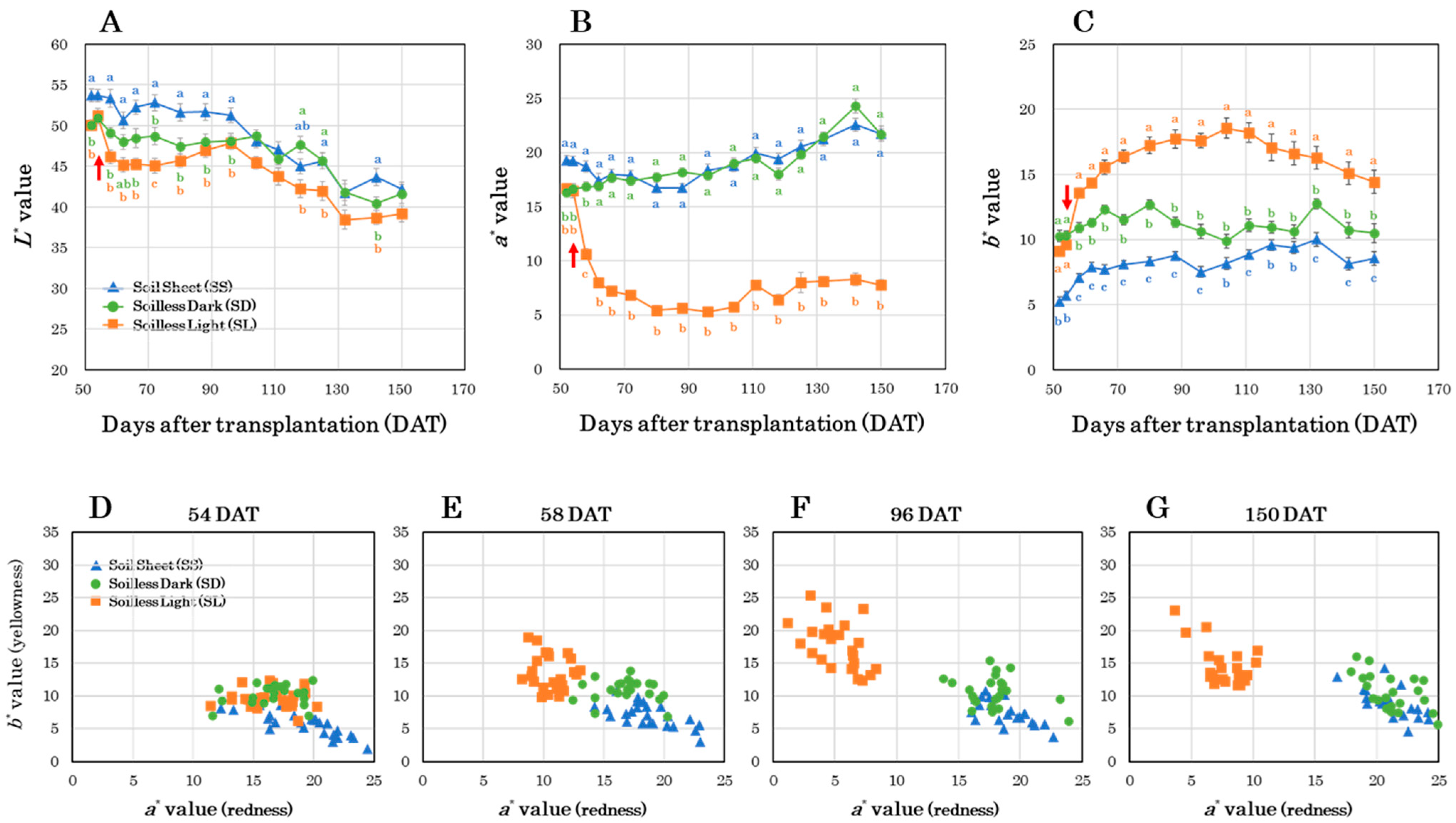
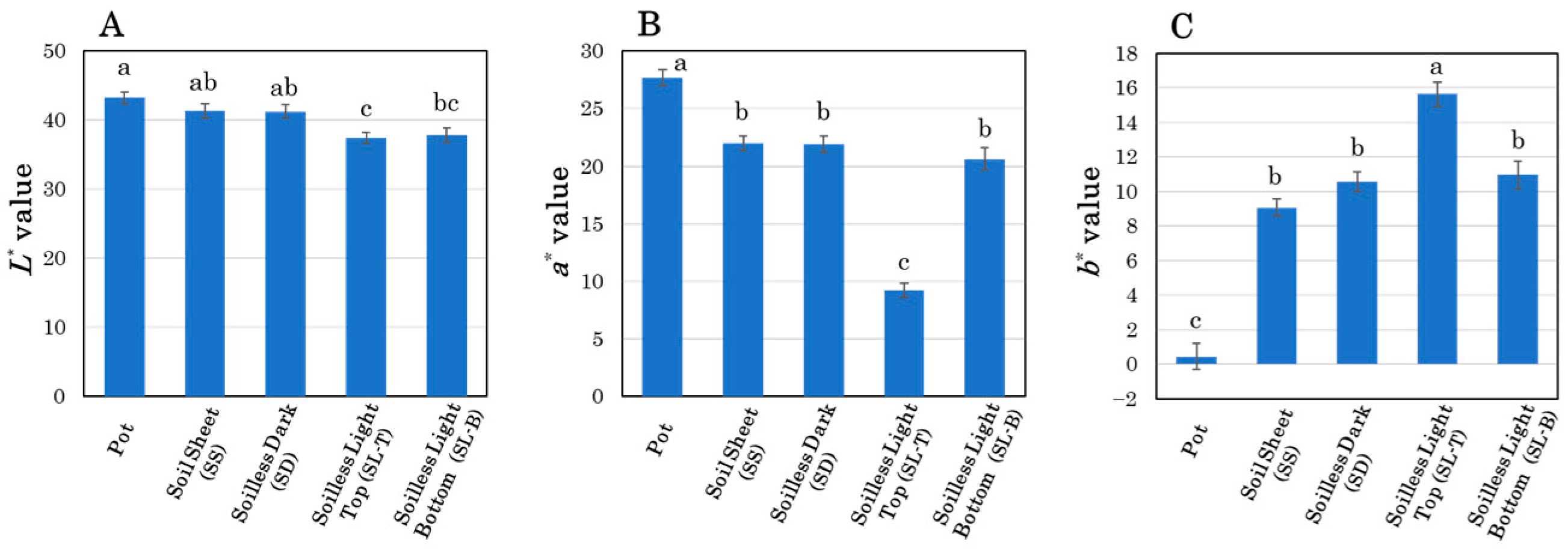
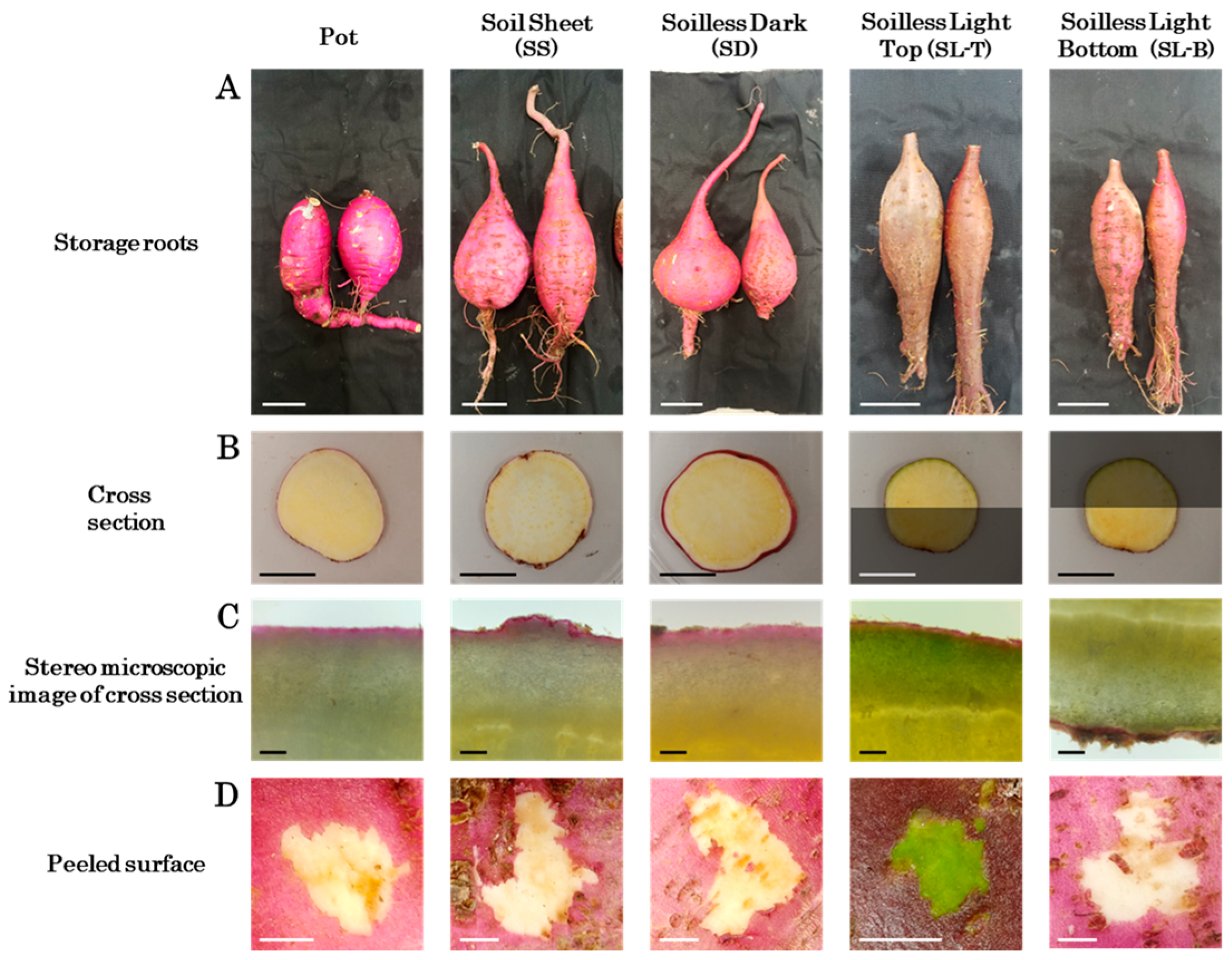
3.2. Time-Course Observations of Storage Root Color
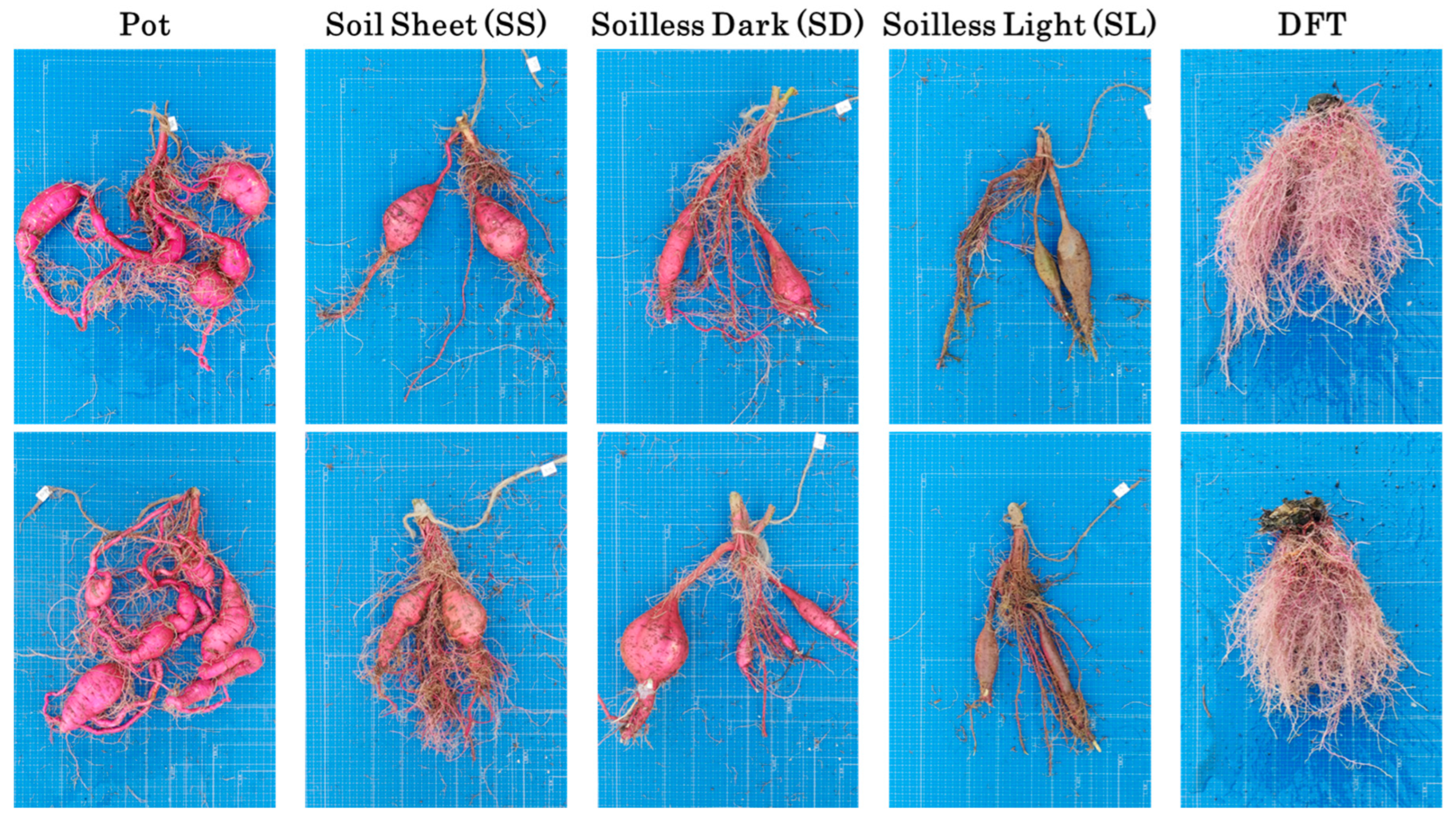
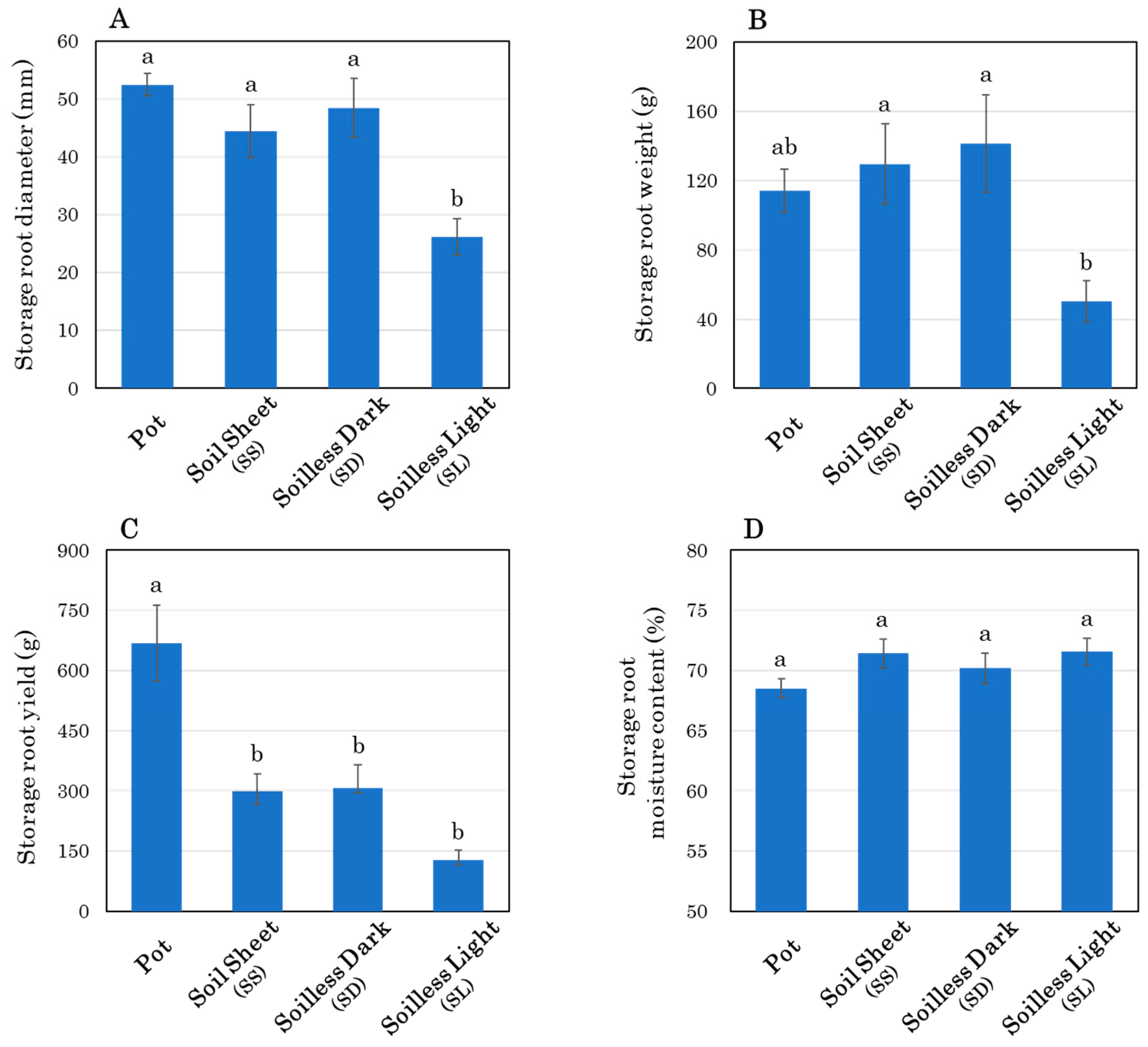
3.3. Storage Root Growth and Morphology at Harvest
3.4. Above-Ground Growth and Morphology at Harvest
4. Discussion
4.1. Comparison of Pot and Novel Soilless Culture System
4.2. Effect of Soil Covering
4.3. Effects of Light Exposure
4.4. DFT Hydroponics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fankhauser, C.; Chory, J. Light Control of Plant Development. Annu. Rev. Cell Dev. Biol. 1997, 13, 203–229. [Google Scholar] [CrossRef] [PubMed]
- Ntagkas, N.; de Vos, R.C.H.; Woltering, E.J.; Nicole, C.C.S.; Labrie, C.; Marcelis, L.F.M. Modulation of the Tomato Fruit Metabolome by LED Light. Metabolites 2020, 10, 266. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Singh, S.K.; Pattanaik, S.; Wang, H.; Yuan, L. Light Regulation of the Biosynthesis of Phenolics, Terpenoids, and Alkaloids in Plants. Commun. Biol. 2023, 6, 1055. [Google Scholar] [CrossRef]
- Zoratti, L.; Karppinen, K.; Luengo Escobar, A.; Häggman, H.; Jaakola, L. Light-Controlled Flavonoid Biosynthesis in Fruits. Front. Plant Sci. 2014, 5, 534. [Google Scholar] [CrossRef]
- Chen, S.; Wang, X.; Cheng, Y.; Gao, H.; Chen, X. Effects of Supplemental Lighting on Flavonoid and Anthocyanin Biosynthesis in Strawberry Flesh Revealed via Metabolome and Transcriptome Co-Analysis. Plants 2024, 13, 1070. [Google Scholar] [CrossRef]
- An, J.-P.; Liu, Y.-J.; Zhang, X.-W.; Bi, S.-Q.; Wang, X.-F.; You, C.-X.; Hao, Y.-J. Dynamic Regulation of Anthocyanin Biosynthesis at Different Light Intensities by the BT2-TCP46-MYB1 Module in Apple. J. Exp. Bot. 2020, 71, 3094–3109. [Google Scholar] [CrossRef]
- Ubi, B.E.; Honda, C.; Bessho, H.; Kondo, S.; Wada, M.; Kobayashi, S.; Moriguchi, T. Expression Analysis of Anthocyanin Biosynthetic Genes in Apple Skin: Effect of UV-B and Temperature. Plant Sci. 2006, 170, 571–578. [Google Scholar] [CrossRef]
- Panjai, L.; Röhlen-Schmittgen, S.; Ellenberger, J.; Noga, G.; Hunsche, M.; Fiebig, A. Effect of Postharvest Irradiation with Red Light on Epidermal Color and Carotenoid Concentration in Different Parts of Tomatoes. Food Meas. 2021, 15, 1737–1746. [Google Scholar] [CrossRef]
- Kim, Y.-H.; Lim, S.; Han, S.-H.; Lee, J.-C.; Song, W.-K.; Bang, J.-W.; Kwon, S.-Y.; Lee, H.-S.; Kwak, S.-S. Differential Expression of 10 Sweetpotato Peroxidases in Response to Sulfur Dioxide, Ozone, and Ultraviolet Radiation. Plant Physiol. Biochem. 2007, 45, 908–914. [Google Scholar] [CrossRef]
- Mo, M.; Yokawa, K.; Wan, Y.; Baluška, F. How and Why Do Root Apices Sense Light under the Soil Surface? Front. Plant Sci. 2015, 6, 775. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, C.; Xu, H.; Shi, X.; Zhen, W.; Hu, Z.; Huang, J.; Zheng, Y.; Huang, P.; Zhang, K.-X.; et al. HY5 Contributes to Light-Regulated Root System Architecture Under a Root-Covered Culture System. Front. Plant Sci. 2019, 10, 1490. [Google Scholar] [CrossRef] [PubMed]
- Kon, S.; Toyofuku, K.; Muto, D.; Kimura, S.; Ogawa, A. Irradiating Roots of Komatsuna (Brassica napus) with Various Light Qualities Affects Growth and Nutrient Content in Leaves, Stems, and Roots. Sci. Hortic. 2024, 331, 113179. [Google Scholar] [CrossRef]
- Agbede, T.M.; Oyewumi, A. Soil Properties, Sweet Potato Growth and Yield under Biochar, Poultry Manure and Their Combination in Two Degraded Alfisols of Humid Tropics. Sci. Hortic. 2022, 304, 111331. [Google Scholar] [CrossRef]
- Hajiaghaei Kamrani, M.; Rahimi Chegeni, A.; Hosseinniya, H. Effects of Different Growing Media on Yield and Growth Parameters of Potato Minitubers (Solanum tuberosum L.). Commun. Soil Sci. Plant Anal. 2019, 50, 1838–1853. [Google Scholar] [CrossRef]
- Tomobe, H.; Tsugawa, S.; Yoshida, Y.; Arita, T.; Tsai, A.Y.-L.; Kubo, M.; Demura, T.; Sawa, S. A Mechanical Theory of Competition between Plant Root Growth and Soil Pressure Reveals a Potential Mechanism of Root Penetration. Sci. Rep. 2023, 13, 7473. [Google Scholar] [CrossRef]
- Johansen, T.J.; Thomsen, M.G.; Løes, A.-K.; Riley, H. Root Development in Potato and Carrot Crops–Influences of Soil Compaction. Acta Agric. Scand. Sect. B Soil Plant Sci. 2015, 65, 182–192. [Google Scholar] [CrossRef]
- Pérez, M.; García, A.; Paredes, A.; Luna, J.; Madriz, P. Soil Mechanical Resistance to Root Penetration and Shape of the Reserve Root of Sweet Potato from the Huaman Descriptor. Agron. Costarric. 2016, 40, 147–159. [Google Scholar] [CrossRef]
- Kudo, K.; Sato, N.; Noborio, K. Tuberous Root Enlargement of Sweet Potato under Low Soil Presser Conditions. JASMAC Proc. 2023, 35, OR1-3. Available online: https://www.jasma.info/jasmac-35/wp-content/uploads/sites/15/2023/09/OR1-3-1.pdf (accessed on 12 September 2024).
- Kozai, T. Sustanable Plant Factory: Closed Plant Production System with Articicial Light for High Resource Use Efficiencies and Quality Produce. Acta Hortic. 2013, 1004, 27–40. [Google Scholar] [CrossRef]
- Sakamoto, M.; Suzuki, T. Effect of Root-Zone Temperature on Growth and Quality of Hydroponically Grown Red Leaf Lettuce (Lactuca sativa L. Cv. Red Wave). Am. J. Plant Sci. 2015, 6, 2350. [Google Scholar] [CrossRef]
- Thapa, U.; Nandi, S.; Rai, R.; Upadhyay, A. Effect of Nitrogen Levels and Harvest Timing on Growth, Yield and Quality of Lettuce under Floating Hydroponic System. J. Plant Nutr. 2022, 45, 2563–2577. [Google Scholar] [CrossRef]
- Lau, V.; Mattson, N. Effects of Hydrogen Peroxide on Organically Fertilized Hydroponic Lettuce (Lactuca sativa L.). Horticulturae 2021, 7, 106. [Google Scholar] [CrossRef]
- Eguchi, T.; Yoshida, S. A Cultivation Method to Ensure Tuberous Root Formation in Sweetpotatoes (Ipomoea batatas (L.) Lam.). Environ. Control. Biol. 2004, 42, 259–266. [Google Scholar] [CrossRef]
- Kusakawa, T.; Inoue, M. Damage to Pot-cultured Carrot Growth due to a Temporarily Raised Groundwater Level and Flooding Period. Hort. Res. 2010, 9, 495–500. [Google Scholar] [CrossRef]
- Siqinbatu; Kitaya, Y.; Hirai, H.; Shibuya, T.; Endo, R. Effects of Soil Water Content on the Growth of Sweet Potato Grown in Sandy Soil. Eco Eng. 2014, 26, 75–80. [Google Scholar] [CrossRef]
- Hayden, A. Aeroponic and Hydroponic Systems for Medicinal Herb, Rhizome, and Root Crops. HortScience 2006, 41, 536–538. [Google Scholar] [CrossRef]
- Tunio, M.H.; Gao, J.; Shaikh, S.A.; Lakhiar, I.A.; Qureshi, W.A.; Solangi, K.A.; Chandio, F.A. Potato Production in Aeroponics: An Emerging Food Growing System in Sustainable Agriculture for Food Security. Chil. J. Agric. Res. 2020, 80, 118–132. [Google Scholar] [CrossRef]
- Selvaraj, M.G.; Montoya-P, M.E.; Atanbori, J.; French, A.P.; Pridmore, T. A Low-Cost Aeroponic Phenotyping System for Storage Root Development: Unravelling the below-Ground Secrets of Cassava (Manihot esculenta). Plant Methods 2019, 15, 131. [Google Scholar] [CrossRef]
- Uewada, T. The solution culture of sweet potatoes. Environ. Control. Biol. 1990, 28, 135–140. [Google Scholar] [CrossRef]
- Sakamoto, M.; Suzuki, T. Effect of Pot Volume on the Growth of Sweetpotato Cultivated in the New Hydroponic System. Sustain. Agric. Res. 2018, 7, 137–145. [Google Scholar] [CrossRef]
- Sakamoto, M.; Komatsu, Y.; Suzuki, T. Nutrient Deficiency Affects the Growth and Nitrate Concentration of Hydroponic Radish. Horticulturae 2021, 7, 525. [Google Scholar] [CrossRef]
- Lee, J.-H.; Goto, E. Ozone Control as a Novel Method to Improve Health-Promoting Bioactive Compounds in Red Leaf Lettuce (Lactuca sativa L.). Front. Plant Sci. 2022, 13, 1045239. [Google Scholar] [CrossRef] [PubMed]
- Japan Meteorological Agency Official Website. Past Weather Data of Wakayama City in 2023. Available online: https://www.data.jma.go.jp/stats/etrn/view/annually_s.php?prec_no=65&block_no=47777&year=2023&month=&day=&view= (accessed on 12 September 2024).
- Sakamoto, M.; Suzuki, T. Synergistic Effects of a Night Temperature Shift and Methyl Jasmonate on the Production of Anthocyanin in Red Leaf Lettuce. Am. J. Plant Sci. 2017, 8, 1534. [Google Scholar] [CrossRef]
- Sakamoto, M.; Suzuki, T. Methyl Jasmonate and Salinity Increase Anthocyanin Accumulation in Radish Sprouts. Horticulturae 2019, 5, 62. [Google Scholar] [CrossRef]
- Chazaux, M.; Schiphorst, C.; Lazzari, G.; Caffarri, S. Precise Estimation of Chlorophyll a, b and Carotenoid Content by Deconvolution of the Absorption Spectrum and New Simultaneous Equations for Chl Determination. Plant J. 2022, 109, 1630–1648. [Google Scholar] [CrossRef]
- Korte, P.; Unzner, A.; Damm, T.; Berger, S.; Krischke, M.; Mueller, M.J. High Triacylglycerol Turnover Is Required for Efficient Opening of Stomata during Heat Stress in Arabidopsis. Plant J. 2023, 115, 81–96. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, B.; Zhao, J.; Guo, J.; Li, Y.; Han, S.; Huang, L.; Du, Y.; Hong, Y.; Tang, D.; et al. Autophagy Contributes to Leaf Starch Degradation. Plant Cell 2013, 25, 1383–1399. [Google Scholar] [CrossRef]
- Valifard, M.; Mohsenzadeh, S.; Kholdebarin, B.; Rowshan, V. Effects of Salt Stress on Volatile Compounds, Total Phenolic Content and Antioxidant Activities of Salvia mirzayanii. S. Afr. J. Bot. 2014, 93, 92–97. [Google Scholar] [CrossRef]
- Gharibi, S.; Tabatabaei, B.E.S.; Saeidi, G.; Goli, S.A.H. Effect of Drought Stress on Total Phenolic, Lipid Peroxidation, and Antioxidant Activity of Achillea Species. Appl. Biochem. Biotechnol. 2016, 178, 796–809. [Google Scholar] [CrossRef]
- Villordon, A.; Gregorie, J.C.; LaBonte, D.; Khan, A.; Selvaraj, M. Variation in ‘Bayou Belle’ and ‘Beauregard’ Sweetpotato Root Length in Response to Experimental Phosphorus Deficiency and Compacted Layer Treatments. HortScience 2018, 53, 1534–1540. [Google Scholar] [CrossRef]
- Liu, H.; Si, C.; Shi, C.; Wang, S.; Sun, Z.; Shi, Y. Switch from Apoplasmic to Symplasmic Phloem Unloading during Storage Roots Formation and Bulking of Sweetpotato. Crop Sci. 2019, 59, 675–683. [Google Scholar] [CrossRef]
- Gajanayake, B.; Reddy, K.R.; Shankle, M.W.; Arancibia, R.A. Early-Season Soil Moisture Deficit Reduces Sweetpotato Storage Root Initiation and Development. HortScience 2013, 48, 1457–1462. [Google Scholar] [CrossRef]
- Sinkovič, L.; Pipan, B.; Meglič, V.; Kunstelj, N.; Nečemer, M.; Zlatić, E.; Žnidarčič, D. Genetic Differentiation of Slovenian Sweet Potato Varieties (Ipomoea batatas) and Effect of Different Growing Media on Their Agronomic and Nutritional Traits. Ital. J. Agron. 2017, 12, 350–356. [Google Scholar] [CrossRef]
- Siose, T.K.; Guinto, D.F. Performance of Improved Sweetpotato (Ipomoea batatas L.) Cultivars under Different Soil Types of Samoa. S. Pac. J. Nat. App. Sci. 2017, 35, 1–9. [Google Scholar] [CrossRef]
- Wang, Z.; Fu, J.; He, M.; Tian, Q.; Cao, H. Effects of Source/Sink Manipulation on Net Photosynthetic Rate and Photosynthate Partitioning during Grain Filling in Winter Wheat. Biol. Plant. 1997, 39, 379–385. [Google Scholar] [CrossRef]
- Bertin, N.; Gautier, H.; Roche, C. Number of Cells in Tomato Fruit Depending on Fruit Position and Source-Sink Balance during Plant Development. Plant Growth Regul. 2002, 36, 105–112. [Google Scholar] [CrossRef]
- Sakamoto, M.; Suzuki, T. Effect of Nutrient Solution Concentration on the Growth of Hydroponic Sweetpotato. Agronomy 2020, 10, 1708. [Google Scholar] [CrossRef]
- Gajanayake, B.; Reddy, K.R.; Shankle, M.W.; Arancibia, R.A.; Villordon, A.O. Quantifying Storage Root Initiation, Growth, and Developmental Responses of Sweetpotato to Early Season Temperature. Agron. J. 2014, 106, 1795–1804. [Google Scholar] [CrossRef]
- Eguchi, T.; Kitano, M.; Eguchi, H. Growth of Tuberous Root as Affected by the Ambient Humidity in Sweetpotato (Ipomoea batatas Lam.). Environ. Control. Biol. 1999, 37, 197–201. [Google Scholar] [CrossRef]
- Noda, T.; Kobayashi, T.; Suda, I. Effect of Soil Temperature on Starch Properties of Sweet Potatoes. Carbohydr. Polym. 2001, 44, 239–246. [Google Scholar] [CrossRef]
- Orman-Ligeza, B.; Morris, E.C.; Parizot, B.; Lavigne, T.; Babé, A.; Ligeza, A.; Klein, S.; Sturrock, C.; Xuan, W.; Novák, O.; et al. The Xerobranching Response Represses Lateral Root Formation When Roots Are Not in Contact with Water. Curr. Biol. 2018, 28, 3165–3173.e5. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Concepcion, M.; Stange, C. Biosynthesis of Carotenoids in Carrot: An Underground Story Comes to Light. Arch. Biochem. Biophys. 2013, 539, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Hozyo, Y.; Kato, S. Thkenig Growth and Re-thickening Growth of Tuberous Roots of Sweet Potato Pants (Ipomoea batatas Poiret). Jpn. J. Crop Sci. 1976, 45, 131–138. [Google Scholar] [CrossRef]
- Fuentes, P.; Pizarro, L.; Moreno, J.C.; Handford, M.; Rodriguez-Concepcion, M.; Stange, C. Light-Dependent Changes in Plastid Differentiation Influence Carotenoid Gene Expression and Accumulation in Carrot Roots. Plant Mol. Biol. 2012, 79, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Noh, S.A.; Lee, H.-S.; Kim, Y.-S.; Paek, K.-H.; Shin, J.S.; Bae, J.M. Down-Regulation of the IbEXP1 Gene Enhanced Storage Root Development in Sweetpotato. J. Exp. Bot. 2013, 64, 129–142. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, J.; Sun, L.; Kong, Y.; Chen, J.; Zhu, M.; Xu, T.; Li, Z.; Dong, T. Progress on Physiological and Molecular Mechanisms of Storage Root Formation and Development in Sweetpotato. Sci. Hortic. 2023, 308, 111588. [Google Scholar] [CrossRef]
- Wahid, A.; Close, T.J. Expression of Dehydrins under Heat Stress and Their Relationship with Water Relations of Sugarcane Leaves. Biol. Plant 2007, 51, 104–109. [Google Scholar] [CrossRef]
- Rodríguez, P.; Torrecillas, A.; Morales, M.A.; Ortuño, M.F.; Sánchez-Blanco, M.J. Effects of NaCl Salinity and Water Stress on Growth and Leaf Water Relations of Asteriscus maritimus Plants. Environ. Exp. Bot. 2005, 53, 113–123. [Google Scholar] [CrossRef]
- Egilla, J.N.; Davies, F.T.; Boutton, T.W. Drought Stress Influences Leaf Water Content, Photosynthesis, and Water-Use Efficiency of Hibiscus Rosa-Sinensis at Three Potassium Concentrations. Photosynthetica 2005, 43, 135–140. [Google Scholar] [CrossRef]
- Ghori, N.-H.; Ghori, T.; Hayat, M.Q.; Imadi, S.R.; Gul, A.; Altay, V.; Ozturk, M. Heavy Metal Stress and Responses in Plants. Int. J. Environ. Sci. Technol. 2019, 16, 1807–1828. [Google Scholar] [CrossRef]
- Yokawa, K.; Fasano, R.; Kagenishi, T.; Baluška, F. Light as Stress Factor to Plant Roots–Case of Root Halotropism. Front. Plant Sci. 2014, 5, 718. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Baba, S.; Obayashi, T.; Sato, M.; Toyooka, K.; Keränen, M.; Aro, E.-M.; Fukaki, H.; Ohta, H.; Sugimoto, K.; et al. Regulation of Root Greening by Light and Auxin/Cytokinin Signaling in Arabidopsis. Plant Cell 2012, 24, 1081–1095. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Ma, X.; Gao, X.; Wu, W.; Zhou, B. Light Induced Regulation Pathway of Anthocyanin Biosynthesis in Plants. Int. J. Mol. Sci. 2021, 22, 11116. [Google Scholar] [CrossRef]
- Ruan, Y.; Chen, K.; Su, Y.; Jiang, S.; Xu, P.; Murray, J.D. A Root Tip-Specific Expressing Anthocyanin Marker for Direct Identification of Transgenic Tissues by the Naked Eye in Symbiotic Studies. Plants 2021, 10, 605. [Google Scholar] [CrossRef]
- Shi, L.; Li, X.; Fu, Y.; Li, C. Environmental Stimuli and Phytohormones in Anthocyanin Biosynthesis: A Comprehensive Review. Int. J. Mol. Sci. 2023, 24, 16415. [Google Scholar] [CrossRef]
- Gao, H.-N.; Jiang, H.; Cui, J.-Y.; You, C.-X.; Li, Y.-Y. Review: The Effects of Hormones and Environmental Factors on Anthocyanin Biosynthesis in Apple. Plant Sci. 2021, 312, 111024. [Google Scholar] [CrossRef]
- Sun, J.; Hui, K.; Guo, Z.; Li, Y.; Fan, X. Cellulose and Lignin Contents Are Negatively Correlated with Starch Accumulation, and Their Correlation Characteristics Vary Across Cassava Varieties. J. Plant Growth Regul. 2023, 42, 658–669. [Google Scholar] [CrossRef]
- Singh, V.; Sergeeva, L.; Ligterink, W.; Aloni, R.; Zemach, H.; Doron-Faigenboim, A.; Yang, J.; Zhang, P.; Shabtai, S.; Firon, N. Gibberellin Promotes Sweetpotato Root Vascular Lignification and Reduces Storage-Root Formation. Front. Plant Sci. 2019, 10, 1320. [Google Scholar] [CrossRef]
- Higuchi, K.; Kanai, M.; Tsuchiya, M.; Ishii, H.; Shibuya, N.; Fujita, N.; Nakamura, Y.; Suzui, N.; Fujimaki, S.; Miwa, E. Common Reed Accumulates Starch in Its Stem by Metabolic Adaptation under Cd Stress Conditions. Front. Plant Sci. 2015, 6, 138. [Google Scholar] [CrossRef]
- Buckseth, T.; Sharma, A.K.; Pandey, K.K.; Singh, B.P.; Muthuraj, R. Methods of Pre-Basic Seed Potato Production with Special Reference to Aeroponics—A Review. Sci. Hortic. 2016, 204, 79–87. [Google Scholar] [CrossRef]
- Ravi, V.; Chakrabarti, S.K.; Makeshkumar, T.; Saravanan, R. Molecular Regulation of Storage Root Formation and Development in Sweet Potato. In Horticultural Reviews: Volume 42; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014; pp. 157–208. ISBN 978-1-118-91682-7. [Google Scholar]
- Michael Turner, G.; Tran, V.; Sun, W.; Federico Rosas, O.; Ravindran, H.; Neculăescu, A.-M.; Kougianos, A.; Keys, S.; Hawkey, M.; Alexandrou, N. Lunar Agriculture: Farming for the Future. In Earth and Space 2021; American Society of Civil Engineers: Reston, VA, USA, 2021; pp. 639–652. [Google Scholar] [CrossRef]
- Wilson, C.D.; Pace, R.D.; Bromfield, E.; Jones, G.; Lu, J.Y. Sweet Potato in a Vegetarian Menu Plan for NASA’s Advanced Life Support Program. Life Support Biosph. Sci. 1998, 5, 347–351. [Google Scholar] [PubMed]
- JAXA (Japan Aerospace Exploration Agency). Release of the Lunar Farm Working Group Review Report. 2019. Available online: https://www.ihub-tansa.jaxa.jp/english/Lunarfarming_en.html (accessed on 12 September 2024).
- Kitaya, Y.; Higashi, K.; Shibuya, T.; Endo, R. Fundamental Study on Plant-Based Regenerative Life-Support Systems with Sweetpotato Culture in Space. Aerosp. Technol. Jpn. 2021, 19, 889–891. [Google Scholar] [CrossRef]
- Miyajima, H. 7. Study of the Lunar Farming System. Study Working Group. 2023, pp. 68–83. Available online: https://jaxa.repo.nii.ac.jp/record/2000110/files/AA2330006000.pdf#page=73 (accessed on 12 September 2024).
- Paul, A.-L.; Elardo, S.M.; Ferl, R. Plants Grown in Apollo Lunar Regolith Present Stress-Associated Transcriptomes That Inform Prospects for Lunar Exploration. Commun. Biol. 2022, 5, 382. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakamoto, M.; Suzuki, T. Impacts of Light Exposure and Soil Covering on Sweet Potato Storage Roots in a Novel Soilless Culture System. AgriEngineering 2024, 6, 3912-3930. https://doi.org/10.3390/agriengineering6040222
Sakamoto M, Suzuki T. Impacts of Light Exposure and Soil Covering on Sweet Potato Storage Roots in a Novel Soilless Culture System. AgriEngineering. 2024; 6(4):3912-3930. https://doi.org/10.3390/agriengineering6040222
Chicago/Turabian StyleSakamoto, Masaru, and Takahiro Suzuki. 2024. "Impacts of Light Exposure and Soil Covering on Sweet Potato Storage Roots in a Novel Soilless Culture System" AgriEngineering 6, no. 4: 3912-3930. https://doi.org/10.3390/agriengineering6040222
APA StyleSakamoto, M., & Suzuki, T. (2024). Impacts of Light Exposure and Soil Covering on Sweet Potato Storage Roots in a Novel Soilless Culture System. AgriEngineering, 6(4), 3912-3930. https://doi.org/10.3390/agriengineering6040222





