Hexoses Biorefinery: Driving Glucose Dehydration over Sulfonic Polymer and Hybrid Acid Catalysts
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Characterization of Heterogeneous Catalysts
3.2. Catalytic Performance and Kinetics
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LGA | Levoglucosan |
| HMF | 5-hydroxymethylfurfural |
| TEOS | Tetraethyl orthosilicate |
| MPTMS | 3-mercaptopropyltrimethoxysilane |
| FE-SEM | Field Emission Scanning Electron Microscopy |
| SAXRD | Small Angle X-Ray Diffraction |
| HRTEM | High-resolution Transmission Electron Microscopy |
| XRF | X-Ray Fluorescence Spectrometry |
| DMF | N,N-dimethylformamide |
| HPLC | High-Performance Liquid Chromatograph |
| DVB | Divinyl Benzene |
| FA | Formic Acid |
| LGO | Levoglucosenone |
| AG | Anhydroglucoses |
References
- Ashokkumar, V.; Venkatkarthick, R.; Jayashree, S.; Chuetor, S.; Dharmaraj, S.; Kumar, G.; Chen, W.-H.; Ngamcharussrivichai, C. Recent advances in lignocellulosic biomass for biofuels and value-added bioproducts—A critical review. Bioresour. Technol. 2022, 344, 126195. [Google Scholar] [CrossRef]
- Oja, A.O. An Overview of Lignocellulose and Its Biotechnological Importance in High-Value Product Production. Fermentation 2023, 9, 990. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, X.; Yang, Z.; Zhuang, G.; Bai, Z.; Zhang, H.; Guo, Y. Preparation and formation mechanism of levoglucosan from starch using a tubular furnace pyrolysis reactor. J. Anal. Appl. Pyrolysis 2013, 102, 83–88. [Google Scholar] [CrossRef]
- Itabaiana Junior, I.; Nascimento, M.A.; Souza, R.O.M.A.; Dufour, A.; Wojcieszak, R. Levoglucosan: A promising platform molecule? Green Chem. 2020, 22, 5859–5880. [Google Scholar] [CrossRef]
- Lakshmanan, C.M.; Hoelscher, H.E. Production of Levoglucosan by Pyrolysis of Carbohydrates. Pyrolysis in Hot Inert Gas Stream. Ind. Eng. Chem. Prod. Res. Dev. 1970, 9, 57–59. [Google Scholar]
- Kwon, G.-J.; Kim, D.-Y.; Kimura, S.; Kuga, S. Rapid-cooling, continuous-feed pyrolyzer for biomass processing: Preparation of levoglucosan from cellulose and starch. J. Anal. Appl. Pyrolysis 2007, 80, 1–5. [Google Scholar] [CrossRef]
- Maduskar, S.; Maliekkal, V.; Neurock, M.; Dauenhauer, P.J. On the Yield of Levoglucosan from Cellulose Pyrolysis. ACS Sustainable Chem. Eng. 2018, 6, 7017–7025. [Google Scholar] [CrossRef]
- Dobele, G.; Rossinskaja, G.; Telysheva, G.; Meier, D.; Radtke, S.; Faix, O. Levoglucosenone—A Product of Catalytic Fast Pyrolysis of Cellulose. In Progress in Thermochemical Biomass Conversion; Wiley: Hoboken, NJ, USA, 2001; pp. 1500–1508. [Google Scholar]
- Ohara, M.; Takagaki, A.; Nishimura, S.; Ebitani, K. Syntheses of 5-hydroxymethylfurfural and levoglucosan by selective dehydration of glucose using solid acid and base catalysts. Appl. Catal. A Gen. 2010, 383, 149–155. [Google Scholar] [CrossRef]
- Zottola, M.A.; Alonso, R.; Vite, G.D.; Fraser-Reid, B. A practical, efficient large-scale synthesis of 1,6-anhydrohexopyranoses. J. Org. Chem. 1989, 54, 6123–6125. [Google Scholar] [CrossRef]
- Takagaki, A.; Ebitani, K. Glucose to Value-added Chemicals: Anhydroglucose Formation by Selective Dehydration over Solid Acid Catalysts. Chem. Lett. 2009, 38, 650–651. [Google Scholar] [CrossRef]
- Zou, X.; Zhu, C.; Wang, Q.; Yang, G. Catalytic dehydration of hexose sugars to 5-hydroxymethylfural. Biofuels Bioprod. Bioref. 2019, 13, 153–173. [Google Scholar] [CrossRef]
- Crisci, A.J.; Tucker, M.H.; Dumesic, J.A.; Scott, S.L. Bifunctional Solid Catalysts for the Selective Conversion of Fructose to 5-Hydroxymethylfurfural. Top Catal. 2010, 53, 1185–1192. [Google Scholar] [CrossRef]
- Crisci, A.J.; Tucker, M.H.; Lee, M.Y.; Jang, S.G.; Dumesic, J.A.; Scott, S.L. Acid-functionalized SBA-15-type silica catalysts for carbohydrate dehydration. ACS Catal. 2011, 1, 719–728. [Google Scholar] [CrossRef]
- Pagán-torres, Y.J.; Wang, T.; Gallo, J.M.R.; Shanks, B.H.; Dumesic, J.A. Production of 5-Hydroxymethylfurfural from Glucose Using a Combination of Lewis and Brønsted Acid Catalysts in Water in a Biphasic Reactor with an Alkylphenol Solvent. ACS Catal. 2012, 2, 930–934. [Google Scholar] [CrossRef]
- Liu, Y.; Kerton, F.M. Mechanistic studies on the formation of 5-hydroxymethylfurfural from the sugars fructose and glucose. Pure Appl. Chem. 2021, 93, 463–478. [Google Scholar] [CrossRef]
- Zhu, L.; Fu, X.; Hu, Y.; Hu, C. Controlling the Reaction Networks for Efficient Conversion of Glucose to 5-Hydroxymethylfurfural. ChemSusChem 2020, 13, 4812–4832. [Google Scholar] [CrossRef]
- Aida, T.M.; Sato, Y.; Watanabe, M.; Tajima, K.; Nonaka, T.; Hattori, H.; Arai, K. Dehydration of d-glucose in high temperature water at pressures up to 80 MPa. J. Supercrit. Fluids 2007, 40, 381–388. [Google Scholar] [CrossRef]
- Xue, Z.; Ma, M.; Li, Z.; Mu, T. Advances in the conversion of glucose and cellulose to 5-hydroxymethylfurfural over heterogeneous catalysts. RSC Adv. 2016, 6, 98874–98892. [Google Scholar] [CrossRef]
- Wang, J.; Xi, J.; Xia, Q.; Liu, X.; Wang, Y. Recent advances in heterogeneous catalytic conversion of glucose to 5-hydroxymethylfurfural via green routes. Sci. China Chem. 2017, 60, 870–886. [Google Scholar] [CrossRef]
- Agarwala, B.; Kailasamb, K.; Sangwana, R.S.; Elumalai, S. Traversing the history of solid catalysts for heterogeneous synthesis of 5-hydroxymethylfurfural from carbohydrate sugars: A review. Renew. Sustain. Energy. Rev. 2018, 82, 2408–2425. [Google Scholar] [CrossRef]
- Perez, G.P.; Mukherjee, A.; Dumont, M.-J.J. Insights into HMF catalysis. Ind. Eng. Chem. 2019, 70, 1–34. [Google Scholar] [CrossRef]
- Shao, Y.; Ding, Y.; Dai, J.; Long, Y.; Hu, Z.-T. Synthesis of 5-hydroxymethylfurfural from dehydration of biomass-derived glucose and fructose using supported metal catalysts. Green Synth. Catal. 2021, 2, 187–197. [Google Scholar] [CrossRef]
- Tempelman, C.H.L.; Oozeerally, R.; Degirmenci, V. Heterogeneous Catalysts for the Conversion of Glucose into 5-Hydroxymethyl Furfural. Catalysts 2021, 11, 861. [Google Scholar] [CrossRef]
- Park, J.-Y.; Kim, D.-K.; Lee, J.-S. Esterification of free fatty acids using water-tolerable Amberlyst as a heterogeneous catalyst. Bioresource Technol. 2010, 101, S62–S65. [Google Scholar] [CrossRef] [PubMed]
- Hykkerud, A.; Marchetti, J.M. Esterification of oleic acid with ethanol in the presence of Amberlyst 15. Biomass Bioenergy 2016, 95, 340–343. [Google Scholar] [CrossRef]
- Tejero, J.; Cunill, F.; Iborra, M.; Izquierdo, J.F.; Fité, C.J. Dehydration of 1-pentanol to di-n-pentyl ether over ion-exchange resin catalysts. J. Mol. Catal. A Chem. 2002, 182, 541–554. [Google Scholar] [CrossRef]
- Bringué, R.; Iborra, M.; Tejero, J.; Izquierdo, J.F.; Cunill, F.; Fité, C.; Cruz, V.F. Thermally stable ion-exchange resins as catalysts for the liquid-phase dehydration of 1-pentanol to di-n-pentyl ether (DNPE). J. Catal. 2006, 244, 33–42. [Google Scholar] [CrossRef]
- Medina, E.; Bringué, R.; Tejero, J.; Iborra, M.; Fite, C. Conversion of 1-hexanol to di-n-hexyl ether on acidic catalysts. Appl. Catal. A Gen. 2010, 374, 41–47. [Google Scholar] [CrossRef]
- Casas, C.; Bringué, R.; Ramírez, E.; Iborra, M.; Tejero, J. Liquid-phase dehydration of 1-octanol, 1-hexanol and 1-pentanol to linear symmetrical ethers over ion exchange resins. Appl. Catal. A Gen. 2011, 396, 129–139. [Google Scholar] [CrossRef]
- Perez, R.F.; Albuquerque, E.M.; Borges, L.E.P.; Hardacre, C.; Fraga, M.A. Aqueous-phase tandem catalytic conversion of xylose to furfuryl alcohol over [Al]-SBA-15 molecular sieves. Catal. Sci. Technol. 2019, 9, 5350–5358. [Google Scholar] [CrossRef]
- Yang, L.M.; Wang, Y.J.; Luo, G.S.; Dai, Y.Y. Functionalization of SBA-15 mesoporous silica with thiol or sulfonic acid groups under the crystallization conditions. Microporous Mesoporous Mater. 2005, 84, 275–282. [Google Scholar] [CrossRef]
- Melero, J.A.; Stucky, G.D.; Grieken, R.V.; Morales, G. Direct syntheses of ordered SBA-15 mesoporous materials containning arenesulfonic acid groups. J. Mater. Chem. 2002, 12, 1664–1670. [Google Scholar] [CrossRef]
- Amberlysts 15 a Trademark of Rohm and Haas Company and Its Affiliates. A-15 Data Sheet. Available online: https://www.dupont.com/products/amberlyst-15-dry.html (accessed on 6 April 2025).
- Amberlysts 35 is a Trademark of Rohm and Haas Company and Its Affiliates. A-35 Data Sheet. Available online: https://www.dupont.com/products/amberlyst-35-dry.html (accessed on 6 April 2025).
- Amberlyst 36 is a Trademark DuPont. A-36 Data Sheet. Available online: https://www.dupont.com/products/amberlyst-36-wet.html (accessed on 6 April 2025).
- Siril, P.F.; Cross, H.E.; Brown, D.R. New polystyrene sulfonic acid resin catalysts with enhanced acidic and catalytic Properties. J. Mol. Catal. A Chem. 2008, 279, 63–68. [Google Scholar] [CrossRef]
- Zhao, D.; Feng, J.; Huo, Q.; Melosh, N.; Fredrickson, G.H.; Chmelka, B.F.; Stucky, G.D. Triblock Copolymer Syntheses of Mesoporous Silica with Periodic 50 to 300 Angstrom Pores. Science 1998, 279, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Canhaci, S.J.; Perez, R.F.; Borges, L.E.P.; Fraga, M.A. Direct conversion of xylose to furfuryl alcohol on single organic–inorganic hybrid mesoporous silica-supported catalysts. Appl. Catal. B Environ. 2017, 207, 279–285. [Google Scholar] [CrossRef]
- Jeong, G.H.; Kim, E.G.; Kim, S.B.; Park, E.D.; Kim, S.W. Fabrication of sulfonic acid modified mesoporous silica shells and their catalytic performance with dehydration reaction of d-xylose into furfural. Microporous Mesoporous Mater. 2011, 144, 134–139. [Google Scholar] [CrossRef]
- Vlasenko, N.V.; Strizhak, P.E. Hybrid organic-inorganic acid catalysts: The effect of active sites localization on catalytic characteristics in the processes of alcohols’ etherification. A review. J. Appl. Polym. Sci. 2022, 139, 51926. [Google Scholar] [CrossRef]
- Shi, X.; Wu, Y.; Yi, H.; Rui, G.; Li, P.; Yang, M.; Wang, G. Selective Preparation of Furfural from Xylose over Sulfonic Acid Functionalized Mesoporous Sba-15 Materials. Energies 2011, 4, 669–684. [Google Scholar] [CrossRef]
- Sasaki, M.; Takahashi, K.; Haneda, Y.; Satoh, H.; Sasaki, A.; Narumi, A.; Satoh, T.; Kakuchi, T.; Kaga, H. Thermochemical transformation of glucose to 1,6-anhydroglucose in high-temperature steam. Carbohydr. Res. 2008, 343, 848. [Google Scholar] [CrossRef]
- Xu, Z.; Yang, Y.; Yan, P.; Xia, Z.; Liu, X.; Zhang, Z.C. Mechanistic understanding of humin formation in the conversion of glucose and fructose to 5-hydroxymethylfurfural in [BMIM]Cl ionic liquid. RSC Adv. 2020, 10, 34732–34737. [Google Scholar] [CrossRef]
- Vieira, J.L.; Almeida-Trapp, M.; Mithöfer, A.; Plass, W.; Gallo, J.M.R. Rationalizing the conversion of glucose and xylose catalyzed by a combination of Lewis and Brønsted acids. Catal. Today 2020, 344, 92–101. [Google Scholar] [CrossRef]
- Vieira, J.L.; Paul, G.; Iga, G.D.; Cabral, N.M.; Bueno, J.M.C.; Bisio, C.; Gallo, J.M.R. Niobium phosphates as bifunctional catalysts for the conversion of biomass-derived monosaccharides. Appl. Catal. A Gen. 2021, 617, 118099. [Google Scholar] [CrossRef]
- Hu, X.; Wu, L.; Wang, Y.; Mourant, D.; Lievens, C.; Gunawan, R.; Li, C.-Z. Mediating acid-catalyzed conversion of levoglucosan into platform chemicals with various solvents. Green Chem. 2012, 14, 3087–3098. [Google Scholar] [CrossRef]
- Cao, F.; Schwartz, T.J.; McClelland, D.J.; Krishna, S.H.; Dumesic, J.A.; Huber, G.W. Dehydration of cellulose to levoglucosenone using polar aprotic solvents. Energy Environ. Sci. 2015, 8, 1808–1815. [Google Scholar] [CrossRef]
- He, J.; Liu, M.; Huang, K.; Walker, T.W.; Maravelias, C.T.; Dumesic, J.A.; Huber, G.W. Production of levoglucosenone and 5-hydroxymethylfurfural from cellulose in polar aprotic solvent–water mixtures. Green Chem. 2017, 19, 3642–3653. [Google Scholar] [CrossRef]
- Oyola-Rivera, O.; He, J.; Huber, G.W.; Dumesic, J.A.; Cardona-Martínez, N. Catalytic dehydration of levoglucosan to levoglucosenone using Brønsted solid acid catalysts in tetrahydrofuran. Green Chem. 2019, 21, 4988–4999. [Google Scholar] [CrossRef]
- Souza, P.M.; Sousa, L.A.; Noronha, F.B.; Wojcieszak, R. Dehydration of levoglucosan to levoglucosenone over solid acid catalysts. Tuning the product distribution by changing the acid properties of the catalysts. Molecular Catal. 2022, 529, 112564. [Google Scholar] [CrossRef]
- Putten, R.-J.V.; Soetedjo, J.N.M.; Pidko, E.A.; van der Waal, J.C.; Hensen, E.J.M.; Jong, E.; Heeres, H. Dehydration of Different Ketoses and Aldoses to 5-Hydroxymethylfurfural. J. Chem SusChem 2013, 6, 1681–1687. [Google Scholar] [CrossRef]
- Swift, T.D.; Nguyen, H.; Anderko, A.; Nikolakis, V.; Vlachos, D.G. Tandem Lewis/Brønsted homogeneous acid catalysis: Conversion of glucose to 5-hydoxymethylfurfural in an aqueous chromium(iii) chloride and hydrochloric acid solution. Green Chem. 2015, 17, 4725–4735. [Google Scholar] [CrossRef]


| Catalyst | SBET (m2 g−1) | ϕp (Å) | Vp (cm3 g−1) | Acidity (mmol H+ g−1) |
|---|---|---|---|---|
| A-15 | 53 | 300 | 0.40 | 4.7 |
| A-35 | 50 | 300 | 0.35 | 5.2 |
| A-36 | 33 | 240 | 0.20 | 5.4 |
| SBA-15-PSO3H | 579 | 46 | 2.96 | 0.9 |
| Catalyst | k (h−1) | SAG (%) | Carbon Balance (%) |
|---|---|---|---|
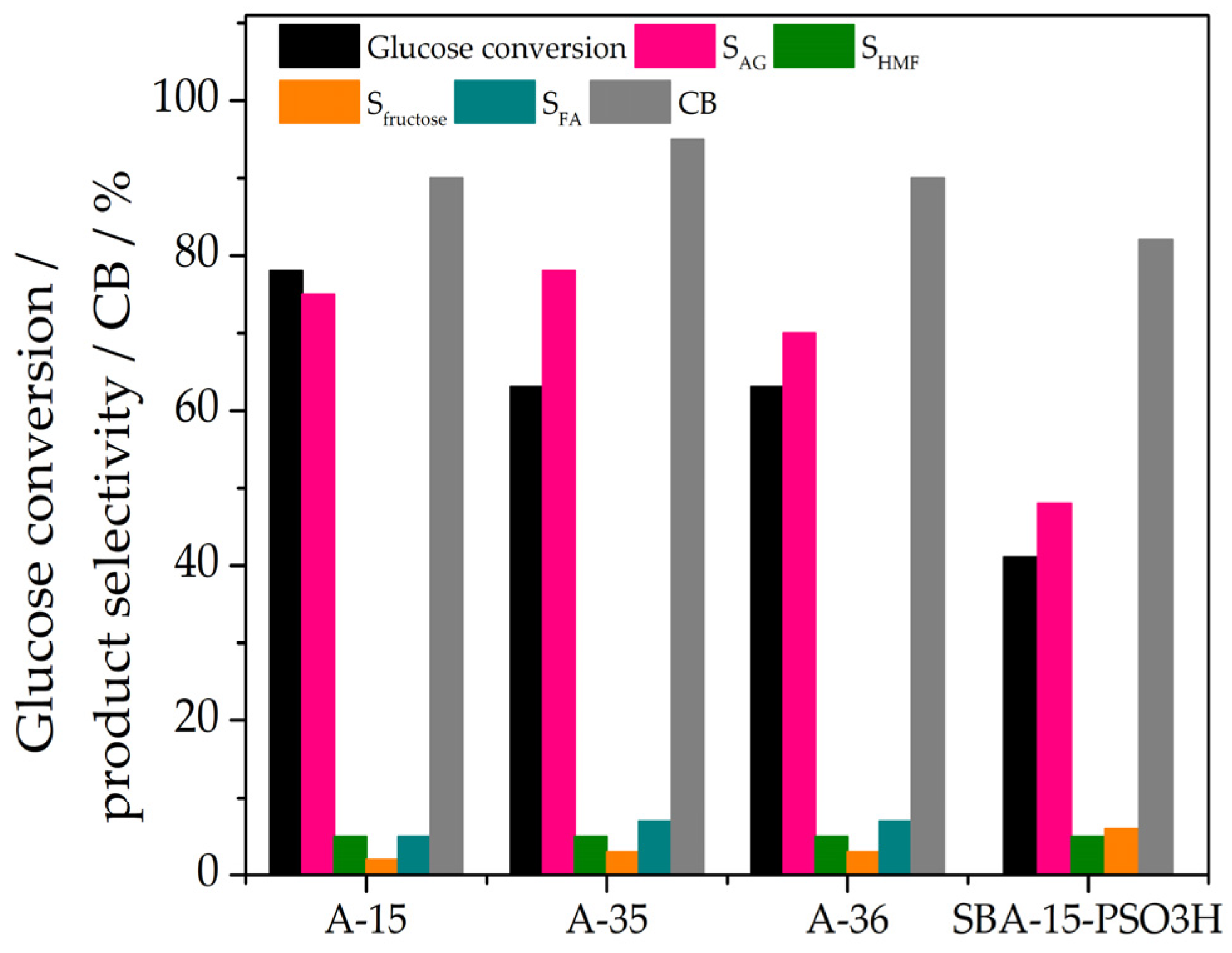
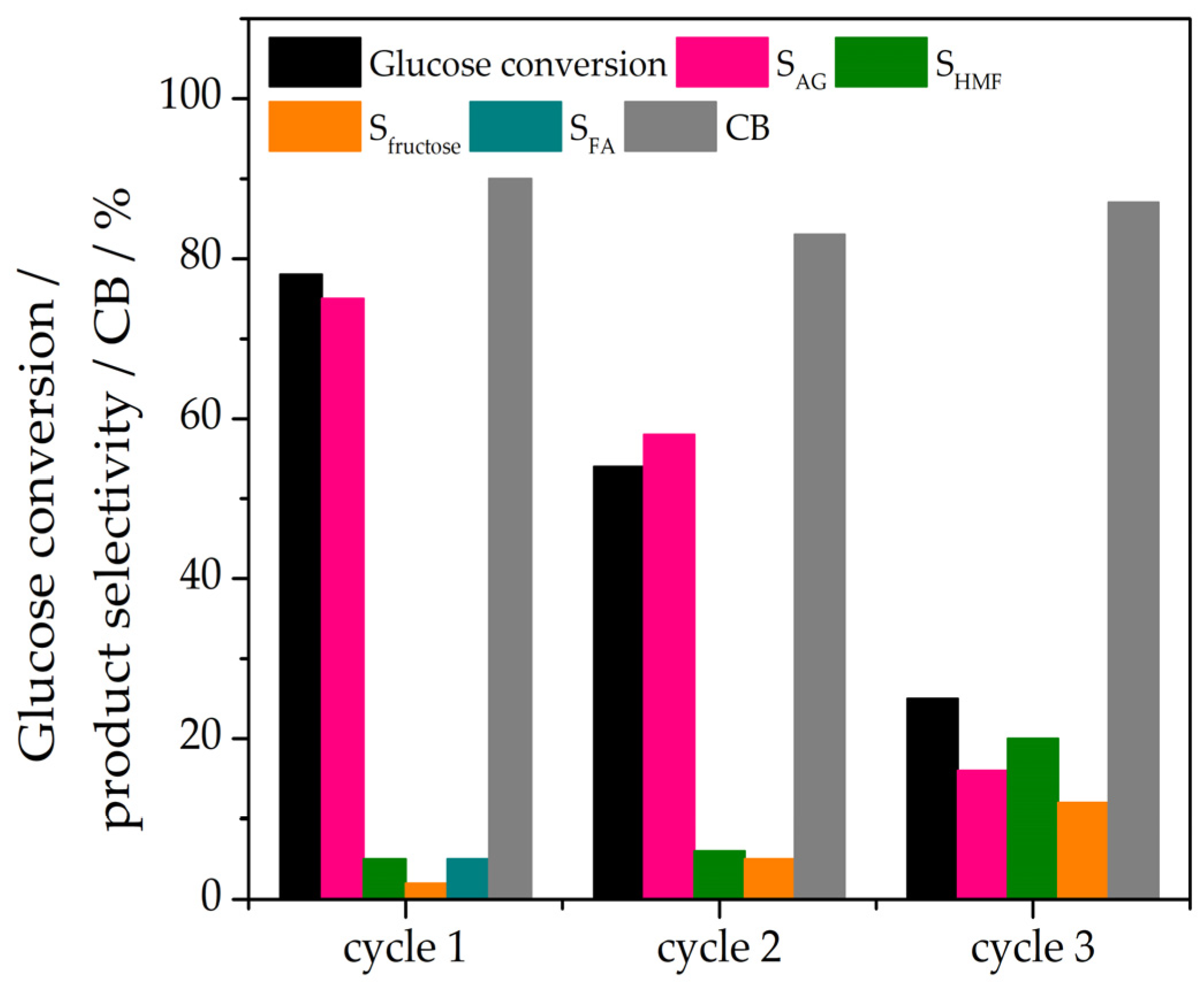
| A-15 | 0.26 | 76 | 90 |
| A-35 | 0.21 | 78 | 95 |
| A-36 | 0.21 | 70 | 90 |
| SBA-15-PSO3H | 0.14 | 48 | 82 |
| Entry | Catalyst | Hexose | Solvent | X (%) | SAG * (%) | Sglucose (%) | Sfructose (%) | Smannose (%) | SHMF (%) | SFA (%) | Carbon Balance (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | No catalyst | Glucose | DMF | 10 | 0 | - | 39 | 0 | 0 | 0 | 94 |
| 2 | A-35 | Glucose | DMF | 85 | 56 | - | 0 | 0 | 6 | 4 | 75 |
| 3 | A-36 | Glucose | DMF | 90 | 58 | - | 0 | 0 | 6 | 6 | 73 |
| 4 | A-36 | Fructose | DMF | 98 | 0 | 0 | - | 0 | 82 | 3 | 86 |
| 5 | No catalyst | Fructose | DMF | 20 | 0 | 13 | - | 0 | 0 | 12 | 85 |
| 6 | A-36 | LGA | DMF | 19 | 19 ** | 0 | 0 | 0 | 0 | 19 | 85 |
| 7 | A-36 | LGA | water | 97 | - | 91 | 0 | 2 | 4 | 3 | 99 |
| 8 | A-36 | Glucose | water | 13 | 33 | - | 0 | 0 | 0 | 0 | 93 |
| 9 | A-36 | Mannose | DMF | 94 | 44 *** | 0 | 0 | - | 4 | 4 | 55 |
| 10 | No catalyst | Mannose | DMF | 13 | 0 | 13 | 0 | - | 0 | 0 | 88 |
| 11 | A-36 | Maltose | DMF | 84 | 33 | 7 | 0 | 0 | 5 | 2 | 55 |
| Entry | Solvent | X (%) | SAG * (%) | Sfructose (%) | SHMF (%) | SFA (%) | Carbon Balance (%) |
|---|---|---|---|---|---|---|---|
| 1 | THF + 20%vol.H2O | 15 | 35 | 0 | 21 | 11 | 95 |
| 2 | DMF | 78 | 75 | 2 | 5 | 5 | 90 |
| 3 | DMF + 2.5%vol.H2O | 58 | 13 | 13 | 3 | 10 | 62 |
| Entry | Catalyst | Mass (g) | Time (h) | X (%) | SAG * (%) | Sfructose (%) | SHMF (%) | SFA (%) | Carbon Balance (%) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | A-15 | 0.16 | 3 | 36 | 63 | 5 | 5 | 0 | 87 |
| 2 | A-15 | 0.80 | 3 | 78 | 75 | 2 | 5 | 5 | 90 |
| 3 | A-35 | 0.14 | 3 | 31 | 66 | 5 | 7 | 0 | 89 |
| 4 | A-35 | 0.80 | 3 | 63 | 78 | 3 | 5 | 7 | 95 |
| 5 | A-36 | 0.14 | 3 | 31 | 66 | 6 | 7 | 0 | 89 |
| 6 | A-36 | 0.80 | 3 | 63 | 70 | 3 | 5 | 7 | 90 |
| 7 | A-15 | 0.80 | 6 | 85 | 72 | 2 | 5 | 4 | 84 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, K.M.A.; Canhaci, S.J.; Perez, R.F.; Fraga, M.A. Hexoses Biorefinery: Driving Glucose Dehydration over Sulfonic Polymer and Hybrid Acid Catalysts. Reactions 2025, 6, 26. https://doi.org/10.3390/reactions6020026
Santos KMA, Canhaci SJ, Perez RF, Fraga MA. Hexoses Biorefinery: Driving Glucose Dehydration over Sulfonic Polymer and Hybrid Acid Catalysts. Reactions. 2025; 6(2):26. https://doi.org/10.3390/reactions6020026
Chicago/Turabian StyleSantos, Kryslaine M. A., Simone J. Canhaci, Rafael F. Perez, and Marco A. Fraga. 2025. "Hexoses Biorefinery: Driving Glucose Dehydration over Sulfonic Polymer and Hybrid Acid Catalysts" Reactions 6, no. 2: 26. https://doi.org/10.3390/reactions6020026
APA StyleSantos, K. M. A., Canhaci, S. J., Perez, R. F., & Fraga, M. A. (2025). Hexoses Biorefinery: Driving Glucose Dehydration over Sulfonic Polymer and Hybrid Acid Catalysts. Reactions, 6(2), 26. https://doi.org/10.3390/reactions6020026






