1. Introduction
Alumina is a very common support of catalytic systems [
1,
2,
3,
4,
5], including oxidation catalysts for soot combustion. Diesel engines and soot in their exhaust remain critical topics for the environment in large cities today and the catalytic abatement of soot continues to be the most promising solution [
6,
7,
8,
9]. As reported by the European Automobile Manufacturers’ Association (ACEA) [
10], in 2024 the vast majority of registered trucks (95.1%) and vans (84.5%) in the European Union was powered by diesel engines. The share of electrically charged vehicles (ECVs) and hybrid electric vehicles (HEVs) is the highest in the category of buses, but diesel-powered engines are still used in 63.1% of this type of vehicles. The reasons for this trend are the undeniable advantages of diesel engines such as greater durability and lower fuel consumption compared to petrol engines [
11], which is why they continue to be the main type of engine applied in large vehicles used in transportation and why there is a need to cope with the emissions until they are replaced by more environmentally friendly options. Silver is very often studied as the active phase in soot combustion catalysts due to its relatively low price, high activity, and availability of active adsorbed oxygen species when combined with the proper support [
12,
13,
14,
15].
In contrast to alumina, zeolites are crystalline minerals composed of tetrahedral units [
16]. In the center of the tetrahedral units are Si
4+ ions, a part of which are substituted with Al
3+ ions. The Si/Al ratio is one of the main parameters which determine the properties of the final solid. Another important parameter is the arrangement of the tetrahedra into larger units, which then combine to form a 3D network of cages responsible for their high surface area and excellent absorption properties. Synthetic and natural zeolites have been implemented in catalysis for cracking of heavy oil residues on an industrial scale and are currently applied in multiple petrochemical processes, e.g., hydrocracking [
17,
18,
19], ethylbenzene synthesis [
20,
21,
22], and xylene isomerization [
23,
24,
25]. Moreover, zeolites are used in the methanol to propylene (MTP) process [
26,
27,
28] developed by Lurgi and ExxonMobil. The methanol to olefins (MTO) process can be carried out with the use of different types of zeolites as the catalyst [
29,
30,
31]. Fe- and Cu-exchanged ZSM-5 zeolites have also been found active in the catalytic reduction of NO
x [
32]. There are few studies on soot combustion catalysts which employ zeolites as supports. Some notable studies use Ag/HZSM-5 [
33,
34]. A beneficial effect of NO
2 in the gas feed on catalytic activity in low-temperature soot oxidation has been observed on a Cu–zeolite catalyst [
35]. Pt supported on HZSM-5 and USY zeolites has shown exceptional stability in soot combustion despite hydrothermal aging trials and was substantially superior to the alumina-supported catalyst [
36]. In this paper, an A-type and two X-type zeolites were used as supports for silver and compared to catalysts obtained on pure alumina.
The aim of the study was to investigate the impact of the coordination of Al
3+ ions in different types of the supports on their interaction with silver and hence their activity in soot combustion. It is commonly known that the coordination of Al
3+ ions is not the same in low-temperature polymorphs of alumina such as γ-alumina, θ-alumina, etc. and the thermally stable high-temperature form, i.e., α-alumina (corundum) [
37,
38]. In the former, the Al
3+ ion has both tetrahedral and octahedral coordination, whereas in corundum the only coordination is octahedral [
39,
40]. In contrast, in zeolites, Al
3+ ions are tetrahedrally coordinated [
16], though the simultaneous change in composition due to the presence of Si
4+ ions cannot be avoided. The Al/Si ratio varies greatly in zeolites; hence, in order to investigate zeolites as supports it was considered beneficial by the authors to reduce the variables by using three zeolites with the same Al/Si ratio (1.0:1.2). Moreover, the type of zeolites, i.e., LTA and FAU zeolites, selected for this study were chosen to contain the same type of building blocks with four and six alternating Si
4+ and Al
3+ ions [
41]. Because of the difference in the arrangement of the tetrahedra, the pore sizes and other properties of these zeolites are different.
2. Materials and Methods
2.1. Materials
The alumina supports were obtained using thermal decomposition of Al(NO3)3 (pure for analysis, POCh, Gliwice, Poland). Three commercial zeolites were used for the study: 5A zeolite (high purity, Fluka AG, Buchs SG, Switzerland), 10X zeolite (high purity, Fluka AG, Buchs SG, Switzerland), and 13X zeolite (high purity, British Drug Houses Chemicals Ltd., London, United Kingdom). One portion of alumina was calcined at 550 °C and the second one was calcined at 1200 °C, both for 4 h. The zeolites were washed repeatedly with redistilled water before use. Each of the five supports was crushed using an agate mortar, and the 230–500 μm fraction was used for silver deposition. Silver was applied via dry impregnation onto the supports using a solution of silver nitrate (analytical grade, POCh, Gliwice, Poland) to obtain a final silver loading of 14.7 wt.%. This loading was selected because it gave promising results in our preliminary studies. The solvent was evaporated. Next, the prepared catalysts were heated for 1 h at 550 °C in a muffle furnace. In the case of some supports additional samples were made as needed in the light of results of characterization/activity tests with doubled (29.4 wt.%) or half (7.4 wt.%) of the silver loading.
2.2. Characterization Studies
The surface areas and pore size distributions of the samples were determined by nitrogen physisorption in a Micromeritics ASAP 2020 apparatus (Micromeritics Instrument Corp., Norcross, GA, USA). The measurement was performed with approx. 1 g of sample in liquid nitrogen at 77 K after 30 min of degassing at 150 °C under vacuum. The specific surface area was calculated using the Brunauer–Emmett–Teller (BET) equation. Desorption and adsorption isotherms were obtained using the Barrett–Joyner–Halenda (BJH) model in the relative pressure range of 0.1–1.0.
X-ray diffraction studies were performed on all samples in the range of scattering angles from 15 to 140° on a D5000 horizontal goniometer (Bruker AXS GmbH, Karlsruhe, Germany) using Cu Kα radiation (1.5418 Å), with parameters of 40 kV, 40 mA in Bragg–Brentano focusing geometry. A LynxEye detector was used.
The microscopes used for SEM-EDX imaging were Helios 5 and PRISMA E instruments (both from ThermoFisher Scientific, Waltham, MA, USA) with ETD and CBS detectors. To create images, the following parameters were used: working distance of 4 or 10 mm, beam current of 25 pA or spot size 3.5, and beam energy of 3 or 5 kV, respectively. Magnifications of 2000–20,000 times were obtained. EDX data were acquired with the following parameters: 15 kV, spot size 4.0, on PRISMA E.
The aforementioned Helios 5 instrument was also used to carry out the TEM and ToF SIMS measurements. Bright-field TEM images were acquired in the Immersion Mode, using a current beam of 25 pA and 30 kV acceleration voltage. Prior to measurements, a suspension of the samples in 2-propanol was made and four drops were placed onto a copper grid, allowing each to dry before the next one was deposited.
The ToF SIMS detector was used for acquiring data regarding secondary ions emitted from the surface of the studied catalysts. For data quantification the software ToF SIMS Explorer, version 1.12.2.0 (Tofwerk AG, Thun, Switzerland) was used. The UHV chamber had a pressure below 10−7 mbar. The samples were located 4 mm away from the secondary electron gun and 16.5 mm from the ion gun, which was operated at 30 kV with an ion current of 15 nA for spectra collection in both the positive and negative ion modes. A sequence of 10 spectra were obtained with a resolution of 512 × 442 and dwell time of 10 µs from a region of interest of the size 50 by 50 µm and averaged.
2.3. Activity Measurements
The reaction was monitored by thermogravimetric analysis. The mass ratio of catalyst to carbon in the sample for the DTA-TGA measurements (STA 449C apparatus from Netzsch, Selb, Germany) was 5:1. Activated carbon (98% purity, Merck, Darmstadt, Germany) was used as model soot. In order to ensure tight contact between the carbon and the catalyst, 0.02 g of activated carbon and 0.10 g of catalysts were weighed on an analytical balance, mixed, and manually ground for exactly 60 s in an agate mortar. Catalytic combustion of the carbon was carried out under synthetic air with the flow of 90 mL/min (20% oxygen, 80% nitrogen). First, the sample was heated to 30 °C for 30 min, and then measurements were carried out in the temperature range of 30–850 °C with a temperature increase rate of 10 K/min.
Since the soot and the catalyst–soot mixtures were prepared manually, the uncatalyzed soot combustion as well as combustion with selected catalysts were performed thrice to determine the reproducibility of the measurements. The discrepancies were used to determine the margin of error. Appropriate error bars were added in the figures.
3. Results
After the deposition of 14.7 wt.% silver onto each of the supports, the catalysts were imaged using a scanning electron microscope. The secondary electron images of the catalyst at a magnification of 2000 times are shown in
Figure 1. It is clearly visible that the catalysts supported on pure alumina (
Figure 1a and
Figure 1b, Al 550 and Al 1200, respectively) have a different topography than the zeolites. The inserts in the image show the magnification of each of the zeolites. The inserts in the image show images taken at a magnification of 10,000, thanks to which the details of the topography are visible. These supports are composed of small round or cubic particles which form a well-developed, highly porous surface. Indeed, the results of the nitrogen physisorption measurements show that the surface areas of the catalysts greatly varied with the smallest value noted for Al 1200, i.e., below the detection limit (<1 m
2/g), 104 m
2/g for Al 550, and 84 m
2/g for 14.7Ag/5A, and with the two highest values noted for the two X zeolites, namely 381 m
2/g and 326 m
2/g. As expected, the surface area of α-alumina itself, obtained by calcining alumina at 1200 °C, has a very low surface area, i.e., below the detection limit (<1 m
2/g), whereas the zeolites have surface area values of 389 m
2/g for the 5A zeolite, 475 m
2/g for the 10X zeolite, and 442 m
2/g for the 13X zeolite. It is noteworthy that the surface area of the 5A zeolite decreases the most upon silver deposition, i.e., by approx. 300 m
2/g. The comparison of S
BET values before and after silver deposition onto the zeolites is depicted in
Figure S1 and reveals that in the case of the X-type zeolites, the surface area of these catalysts is smaller by approx. 100 m
2/g than that of the zeolites.
The hysteresis loops obtained for the catalysts and the zeolite supports are depicted in
Figure 2. There is a substantial difference in the type and quantity of pores between the catalysts obtained with the two types of alumina supports: 14.7Ag/Al 550 (
Figure 2a) and 14.7Ag/Al 1200 (
Figure 2b). In
Figure 2c–e the top panel shows the hysteresis loop of a zeolite and the bottom panel contains the loop obtained for the corresponding catalyst. The porosity of the 5A zeolite is changed by the silver deposition and results in the open loop hysteresis (
Figure 2c bottom; the experiment was repeated with the same result) which may indicate the presence of open slit-like micropores [
42]. Both X-type zeolites have the H3 hysteresis loop, which shows that (1) the pore type is the same for them both and (2) it is not changed by the deposition of silver.
Both the obtained catalysts and supports were characterized using X-ray diffraction. The diffraction patterns of all of the samples have been obtained and the results are presented in
Figure 3 and
Figure 4. The two pure alumina supports show distinct differences in their crystal structures: the alumina calcined for 4 h at 550 °C, Al 550, is semi-amorphous and exhibits peaks typical for low-temperature forms of alumina [
43,
44,
45] such as θ-alumina (PDF#96-120-0006), whereas that calcined at 1200 °C, Al 1200, has the structure of α-alumina with sharp peaks (PDF#98-006-8591). The deposition of silver nitrate onto Al 550 and subsequent 1 h-long calcination at 550 °C leads to the formation of metallic silver (PDF#00-004-0783) and silver carbonate (PDF#96-100-7036), though the alumina remains in a low-temperature polymorphic form (
Figure 3). The silver carbonate decomposes at temperatures far below the T
max of soot combustion [
46], which means that in both alumina-supported catalysts the active phase is metallic silver.
The diffraction patterns of the three studied zeolites and the catalysts containing 14.7 wt.% obtained with these supports are collected in
Figure 4. The zeolites have diffraction patterns with numerous sharp peaks. For clarity, only six peaks in the diffraction patterns of the supports are marked and labeled. These arise from the (210), (221), (311), (321), (410), and (332) planes of 5A and (331), (511), (440), (533), (642), and (555) planes of the X-type zeolites. The diffraction patterns of the X-type zeolites contain only peaks from the zeolite phase, whereas the diffraction pattern of the A-type zeolite also reveals the presence of a small amount of silica (PDF#00-046-1045). There is a noticeable difference between the position and relative intensity ratio of peaks in the diffraction pattern of zeolite 5A and those of the two X-type zeolites, which shows the pronounced effect of the arrangement of the two types of tetrahedra rings despite the same coordination of the Al
3+ ions. In the case of the catalyst supported on zeolite 5A, there are no peaks arising from the presence of metallic silver (
Figure 4a) whereas the other two do contain them (
Figure 4b,c). It can, however, be stated that for all three zeolites there is a change in the ratio of relative intensities of the peaks.
The values of the relative height ratios, normalized to the most intensive peak, i.e., (321) for 5A and (533) for X-type zeolites, are shown in
Figure S2. In short, the deposition of silver onto both the 5A and 13X zeolites leads to a substantial increase in the intensity of peaks with the higher 2θ values, with the smallest changes noted for the catalyst supported on zeolite 10X. The relative intensities of the peaks with smaller 2θ values were smaller for the X-type zeolites after the deposition of silver. For the 5A-supported catalyst this is true for reflexes from the (221) and (311) planes, though an increase in the relative intensity of (210) was observed. Such substantial changes in the relative intensities of reflexes can signify that during silver deposition Ag-exchanged zeolites are formed. Such a result has been reported by Cametti et al. who noted that the silver–oxygen distances observed in their zeolites were too short to be Ag
0-O, and instead were attributed to Ag
+-O [
47]. They did not detect a reduction of the silver cations to metallic silver upon heating. Location of Ag species within the zeolite cavities was further supported by Rietveld refinement requiring adding substantial occupancies of silver at the cage sites, in comparison to fresh zeolite, to account for changing relative peak intensities and their structure factor. Indeed, for the Ag/10X sample, few Ag sites were located in short proximity of the Al
3+ ion indicating possibility of the exchange. The refinement, however, did not lead with a satisfactory goodness of fit due to a poorly ordered structure of the matter filling the zeolite cages. After deposition of silver, the lattice constant slightly increases for all studied zeolites.
The Circular Backscatter Detector is very useful in the study of supported silver catalysts because the images differentiate between particles that consist of lighter and heavier compounds which show up as darker and brighter spots, respectively. The four images depicted in
Figure 5 show the distribution of metallic silver on alumina (Al 1200,
Figure 5a) and each of the zeolites. It is noteworthy that no silver particles (bright spots) are seen on the 5A zeolite support, which aligns well with the lack of metallic silver in the diffraction pattern of this catalyst (
Figure 4). Similar conclusions can be drawn from TEM images (
Figure S3), which show no metallic silver on the 5A support, though particles of various sizes can be seen on the X-type supports. This issue and the composition of the obtained catalysts was explored further with EDX, which is a technique that allows the detection of element distributions regardless of the phase in which they are present.
The SEM images along with corresponding EDX results, i.e., elemental maps of oxygen, aluminum, silicon, and silver, are collected in
Figure 6. The EDX results corroborate the CBS analysis results revealing no large silver agglomerated on the surface of the catalyst supported on zeolite 5A. They do, however, show the presence of silver, which is also seen in the spectra (
Supplementary Materials, Figure S4). Together with the change in the relative ratio of the zeolite peaks caused by the deposition of silver, it can be deduced that the silver interacts with the zeolite and possibly forms Ag
+ species as mentioned by Ruan et al. [
33]. Such an interaction with the silver would not produce particles of a new phase which could be discerned from the rest of the sample in either SEM or TEM images. The lack of metallic silver particles on the surface of zeolite 5A despite the high silver loading and an even distribution of silver on the surface of the catalyst led to the synthesis of an additional sample with doubled silver loading (29.4 wt.%).
As in the case of zeolite 5A, the same appears to be true with the X-type zeolites, i.e., apart from the visible large silver particles which cover the support (intensive yellow dots on
Figure 6b,c which cause the lower relative intensity of the Al and Si signals in those spots), the whole support has evenly distributed silver throughout the grain. There is no silver in the parts of the images where the grain ends and carbon tape begins. The fact that silver is more abundant across the surface of the 13X zeolite than the 10X zeolite corresponds to the more substantial change in the relative peak height ratio noted in the XRD patterns (
Figure 4 and
Figure S2). Therefore, an additional sample with zeolite 13X with half of the silver loading was synthesized (7.4 wt.%) to check if it is possible to obtain a catalyst without Ag
0.
Figure 7a depicts the CBS image (top left) and elemental maps (bottom left) obtained for 29.4Ag/5A. In contrast to the corresponding catalyst with the lower silver loading, this one has numerous metallic silver agglomerates on the surface, which is in line with the presence of metallic silver peaks in the XRD pattern obtained for this catalyst (
Figure S5a). The oxygen, aluminum, and silicon distribution maps show that the bright particles in the CBS image cover the support, which is indicated by the decrease in intensity of support constituents. Out of these three elements, silicon is different in that there is an increased intensity in the bottom left of its elemental map. This is due to the fact that the sample contains some silica, as already shown in the XRD pattern and the EDX maps obtained for the support and 14.7Ag/5A (
Figure 4 and
Figure 6). The catalyst supported on zeolite 13X with the smaller loading of silver (7.4Ag/13X) contains metallic silver particles on the surface of the zeolite (
Figure 7b) and in the diffraction pattern of this catalyst (
Figure S5b).
The temperature of the maximum rate of soot combustion was used to compare the activity of the supports and catalysts in soot combustion. The results are compiled in
Figure 8. Without a catalyst the maximum rate of soot combustion occurs at approximately 600 °C. The results of all the supports were similar, except for alumina calcined at 1200 °C. It is noteworthy that the alumina calcined at 550 °C is less active despite having a higher surface area and the same composition. This may be due to one of two reasons: different active sites or increased hardness of the alumina, which results in a tighter contact during grinding. Nevertheless, the silver catalyst supported on the high-temperature alumina was much more active in soot combustion than the support itself, indicating that silver is the active component. The activity of the catalysts with 14.7 wt.% falls in the order Ag/Al 1200 > Ag/5A ≈ Ag/13X > Ag/10X ≈ Ag/Al 550. It can be argued that the catalyst obtained with alumina calcined at 550 °C was less active than that obtained at 1200 °C because the latter has a smaller surface area and hence more silver is found on the surface. Therefore, a catalyst with much more silver on Al 550 was synthesized and tested. The XRD pattern of this catalyst is shown in
Figure S5c. The soot combustion results show that an increase in the silver loading does not lead to an increase of activity (
Figure 8), which means that the higher activity of Ag/Al 1200 is most likely due to something else, e.g., interaction of the support with the active phase. This difference is a valuable observation, despite the fact that more active catalysts for soot combustion have been obtained by other research groups, e.g., Shimizu et al. [
48], Sevre et al. [
49], and even in our own, previously reported studies [
46].
It is noteworthy that in the case of zeolite 5A, the activity results show that although 14.7Ag/5A does not exhibit metallic silver reflexes in the XRD pattern, whereas 29.4Ag/5A does, the change does not result in an increase in the activity of the obtained catalytic system. It can be inferred that in these catalysts the most active form of silver is therefore the silver bound to the zeolite, or Ag-exchanged zeolite 5A. In contrast, 14.7 wt.% on both X-structured supports gave metallic silver as well as that which interacted with the supports thus changing the relative intensities of reflexes in the diffraction patterns. Hence, a catalyst with a smaller silver loading was prepared with zeolite 13X to check if the activity of these systems depended on the concentration of metallic silver on the surface. The lower silver loading also led to the presence of metallic silver in the diffraction pattern (
Figure S5b), but the lower concentration resulted in a lower activity of the obtained catalyst in soot combustion (
Figure 8). These differences prompted us to ask the question of what form of silver is the active phase in this reaction. It is quite simple in the case of alumina: the metallic silver is the active phase because it is the only silver-containing phase and the supports are not active in soot combustion. However, the increase in the silver loading beyond a certain point is no longer beneficial. In contrast, the only silver-containing phase noted for zeolite 5A loaded with 14.7 wt.% silver was the silver-exchanged zeolite, and it was found to be the second most active system in these studies. The X-type zeolites, however, contain both a silver-exchanged zeolite and metallic silver. Since the exchange occurred to a lesser degree with the 10X support and the activity of the resulting catalyst was worse than that of the 13X zeolite, it could be postulated that the Ag-exchanged zeolite was a more active phase than the metallic silver supported on it, but the attempt to obtain a solely Ag-exchanged 13X zeolite by decreasing the silver loading by half did not yield a single phase, as metallic silver was seen on the surface. Moreover, it seems that it would not be feasible to synthesize a catalyst with only metallic silver and no Ag-exchanged phase. Hence, a direct comparison of the activity of the two silver-containing phases cannot be made.
The positive ion ToF SIMS spectrum of the 29.4Ag/Al 550 catalyst is shown in
Figure 9a. The most prominent peaks in the spectrum are the two isotopes of silver, present at m/s = 107 and 109, whose abundances are 0.518 and 0.482, respectively. The third most abundant ion in the spectrum is Al
3+ (
m/
z = 27), which comes from the support. In contrast, the positive ion spectrum of zeolites contains more peaks due to the presence of additional components, such as silicon and the counterions. The spectrum and maps of key ions noted for catalyst 29.4Ag/5A are depicted in
Figure 9b. The silicon and aluminum ions are the most abundant ones in the spectrum. The Ca
2+ ion (
m/
z = 40) peak is clearly visible in the spectrum, as well as Na
+ (
m/
z = 23). The two silver peaks are smaller than the peaks which come from the components of the zeolite support.
The analysis of the negative ion spectra results was used to interpret the counter-intuitive results of the activity studies obtained for the alumina-supported catalysts. In the case of pure alumina, the main ions in the spectrum which are associated with the silver catalysts are
m/
z = 16, which is commonly attributed to O
− and
m/
z = 43, which corresponds to AlO
−, as well as those corresponding to the two isotopes of silver (
m/
z = 107 and 109). Two other ions which can be detected from the alumina support are AlO
2− (
m/
z = 59) and AlO
3H
2− (
m/
z = 77). The maps obtained for 14.7Ag/Al 550 for the three most abundant ions in the negative ion spectrum are shown in
Figure 10. It can be seen that the O
− signal is the most abundant one, which is usually the case for metal oxides. The distribution of the aluminum-containing ions is the same as that of the O
− signal. The relative abundances of the ions were normalized to the sum of the intensities of the following four
m/
z signals: 43, 59, 77, and 107, and the values were plotted in
Figure 10b. A comparison of the two catalysts with 14.7 wt.% silver shows that the silver
m/
z = 107 ion is more abundant in the case of the catalyst supported on the alumina calcined at 1200 °C. This can be easily explained by the much smaller surface area of this support. However, in the case of the catalyst supported on alumina calcined at 550 °C, the AlO
− ion is the most abundant aluminum-containing ion, which is not the case for the catalyst with the alumina support calcined at 1200 °C (
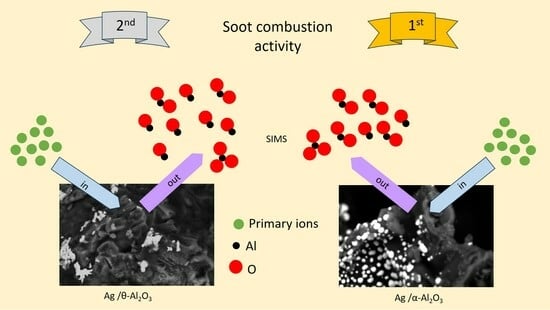
Figure 10b). For this catalyst, the AlO
2− is the most abundant ion. This could be the result of the large difference in the silver concentration on the surface of the catalyst or a difference in the interaction of silver with the two polymorphs of alumina. The pronounced difference in the topography of the silver particles on the alumina calcined at 550 °C and 1200 °C (
Figure 10c) appears to support the latter theory. Moreover, the ToF SIMS results obtained for the 29.4Ag/Al 550 catalytic system (
Figure 10b) also suggest that the latter is the more probable explanation, which could account for the lack of improvement in activity upon the increase in the silver loading. Although in ToF SIMS studies the “matrix effect”, i.e., differences in the relative secondary ion ratios between samples with the same composition due to differences in the chemical environment of the species, is typically considered a drawback of the technique, in these studies it was used to understand the underlying reason for the differences in the silver dispersion on two different alumina supports. These results indicate that the interaction of the silver with each of the alumina supports is different, and hence the dispersion and activity are different. This interesting observation will be further investigated in an upcoming study.