Abstract
By employing the HSAB principle and the “assisted self-assembly” approach and using 2-pyridylaldoximate (pao−) as the primary ligand and pivalate (piv−) as the ancillary co-ligand, tetranuclear [CoIII2LnIII2(NO3)4(pao)4(piv)4] complex polynuclear compounds were isolated (Ln = Dy, Gd, Tb, Pr, Y). The structure of the Dy(III) complex was determined via single-crystal X-ray crystallography, revealing a metal topology of two {CoIIIDyIII2} triangles that shared a common DyIII…DyIII edge. Microanalytical, PXRD (for the two first members)d and spectroscopic (IR, EDX) data for all complexes provided strong evidence that the complexes were isostructural. The nuclearity and metal topology of the crystallographically characterized [CoIII2LnIII2(NO3)4(pao)4(piv)4] are new in the previously investigated CoIII/LnIII/pao− chemistry emphasizing utility of the “assisted self-assembly” approach.
1. Introduction
Heterometallic first-series transition metal (3d)/rare earth metals (Ln) complexes were first introduced a century ago [1], but it is in the past two decades that they have been explored more extensively due to their exciting properties (magnetic, optical, catalytic, etc.) [2,3,4,5,6], which have been and will be used in diverse applications. The synthesis of 3d/4f-metal complexes has been widely investigated [7,8,9,10,11,12] but in many cases requires a proper design. In particular, the synthesis of trivalent cobalt compared to divalent cobalt compounds is a non-routine event in the field of 3d/4f coordination chemistry. There are in many cases fewer heterobimetallic complexes of trivalent cobalt compared to divalent cobalt. In addition, in many cases one-pot routes involving the reaction of 3d- and 4f-metal starting materials with a polydentate organic ligand often results in the preparation of pure 3d- and 4f-metal complexes. In 1963, Pearson introduced the “Hard and Soft Acids and Bases” (HSAB) model [13], which afterward was established as a useful synthetic tool for the synthesis of coordination complexes. Based on this model, the thermodynamically strongest possible bonding could be promoted by pairing different Lewis acids and bases, leading to the synthesis of 3d/4f-metal complexes. It is therefore anticipated that the LnIII ions that behave as hard acids and 3d metals (divalent or sometimes trivalent) that are less hard (borderline) acids tend to bind to oxygen (hard bases) and nitrogen (borderline) donor atoms, respectively. It is thus clear that the choice of the ligands is of paramount importance for a successful synthetic work.
One route and one strategy (or “designed assembly”) have been popular for the synthesis of 3d/4f-metal compounds [4]. The route is a “one-pot” procedure. This requires a mixture of simple 3d- and 4f-metal “salts” and a designed polydentate organic ligand that possesses parts (coordination “components” or “pockets”) for selective capture of the 4f-metal ion. The strategy, often called the “metal complex as ligand” or “metalloligand” approach, uses isolated mononuclear or dinuclear 3d-metal ion complexes with uncoordinated (free) O-sites, which can further react with the oxophilic (hard acid) LnIII ion; alternatively, the metalloligands can be mononuclear or dinuclear 4f-metal ion complexes with uncoordinated (free) N-sites, which can further react with less oxophilic (borderline acid) 3d-metal ions [4,14,15,16,17,18,19]. There is also a third approach named “assisted self-assembly” in which the introduction of an appropriate co-ligand (e.g., a simple carboxylate or β-dikeonate ion) provides significant assistance to the self-assembly procedure, often leading to high-nuclearity coordination polynuclear compounds or coordination polymers [20].
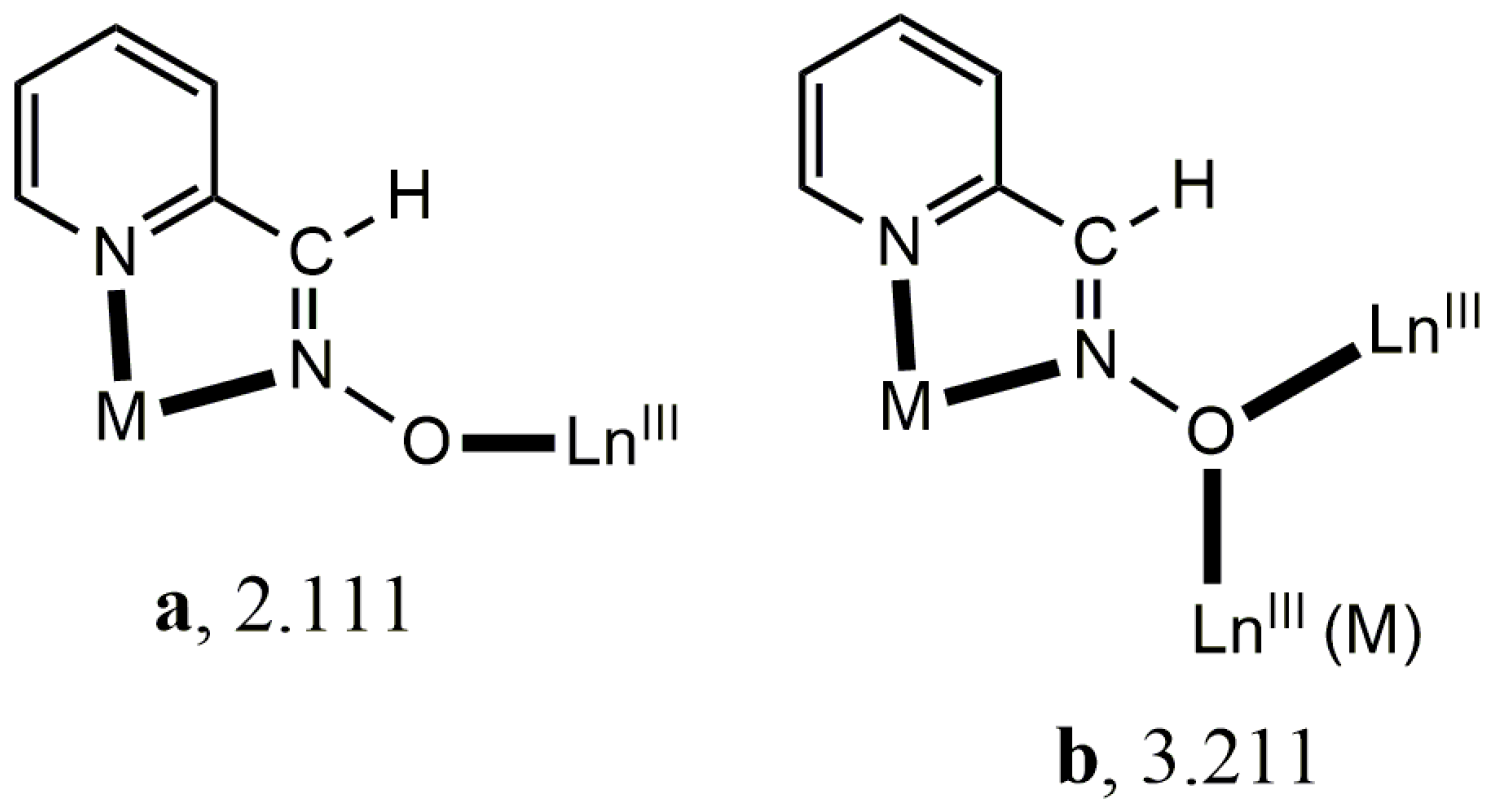
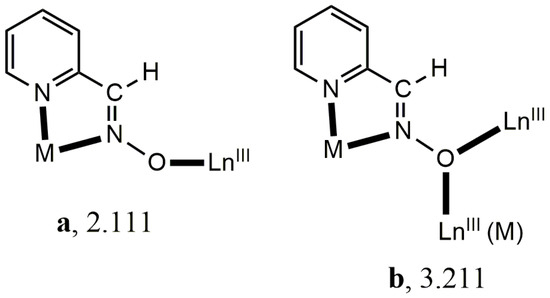
Anionic 2-pyridyl oximes are ideal candidates for the construction of 3d/4f-metal complexes [4]. They possess two N atoms that can form a stable 5-membered chelating ring with the 3d-metal ion and a deprotonated O atom that can bind to one or two LnIII ions (Scheme 1). Restricting further discussion to the simplest 2-pyridyl oxime (2-pyridyl aldoxime, paoH) and cobalt, the “one-pot” route [21] and the “metalloligand” approach [22] have led to dinuclear {CoIIILnIII} and trinuclear {CoIII2LnIII} complexes. We suspected that higher nuclearity CoIII/LnIII/pao− complexes may arise by combining the paoH as the primary ligand with the pivalate (piv−) as the ancillary co-ligand (these are known for their effective bridging of 3d/4f metal ions) following an “assisted self-assembly” approach. We were pleased to see that the outcome was a family of {CoIII2LnIII2} complex polynuclear compounds. Preliminary synthetic, structural, and characterization efforts for this family are described in this paper.
Scheme 1.
Crystallographically established coordination modes of the ligand pao− in 3d/4f complex polynuclear compound chemistry. Harris notation is used to describe these modes. M = a transition metal ion; LnIII = a trivalent rare earth metal. Coordination bonds are drawn with bold lines.
2. Materials and Methods
2.1. Materials and Spectroscopic–Physical Measurements
All synthetic procedures were performed under aerobic conditions. Distilled water was received from the in-house facility. Solvents and reagents were purchased from Sigma-Aldrich (Tanfrichen, Germany) and Alfa Aesar (Karlsruhe, Germany) and used as received without further purification. The cobalt(II) carbonate hydrate (CAS: 57454-67-8) and pivalic acid (CAS: 75-98-9) used for the preparation of the starting material [CoII2(OH2)(piv)4(pivH)4]/Ln(NO3)3·xH2O were of analytical grade. The 2-pyridylaldoxime (CAS: 1193-96-0) and the sodium methoxide (CAS: 124-41-4) were of reagent grade with a purity >99.9% and >95%, respectively. The purity of the products was checked via carbon, hydrogen, and nitrogen microanalyses performed by the Instrumental Analysis Center of the University of Patras. KBr pellets of the complexes were prepared under pressure, and the FT-IR spectra were recorded using a Perkin-Elmer spectrometer (16PC, Perkin-Elmer, Waltham, MA, USA). The elemental analyses of the samples were also performed with a Zeiss ZUPRA 35 VP-FEG instrument (Zeiss, Oberkochen, Germany) operating at 5−20 keV and equipped with EDS (Bruker GmbH, Quanta 200) and BSE (K E Developments, Ltd., London, UK). The XRD patterns of the samples were recorded with a PANalytical X’Pert Pro Materials Powder Diffractometer (MPD) system equipped with a Cu Kα source. For the XRD measurements, the crystals of the samples were isolated and analyzed directly after their removal from the mother liquor.
2.2. Preparation of the Complexes
The dimer starting material [Co2(OH2)(piv)4(pivH)2] was synthesized based on a method found in the literature [23].
[CoIII2DyIII2(NO3)4(pao)4(piv)4].2MeCN (1·2MeCN): A solution of [Co2(OH2)(piv)4(pivH)4] (0.190 g, 0.20 mmol) and Dy(NO3)3.5H2O (0.439 g, 0.60 mmol) in MeCN (15 mL) was added to a solution of paoH (0.073 g, 0.60 mmol) and NaOMe (0.032 g, 0.60 mmol) in MeCN (15 mL). The resulting brownish suspension was stirred for 10 min and filtered, and the filtrate was allowed to slowly evaporate at room temperature. X-ray-quality red crystals of the product were obtained within 7 d. The crystals were collected via filtration, washed with cold MeCN (2 × 0.5 mL) and Et2O (2 × 2 mL), and dried in vacuo over anhydrous CaCl2 (yield: 52%). The sample was satisfactorily analyzed as lattice-MeCN-free. Anal. Calcd. (%) for C44H56Co2Dy2N12O24: C, 33.45; H, 3.58; N, 10.64. Found (%): C, 33.54; H, 3.54; N, 10.78. IR (KBr, cm−1): 3058 (w), 2958 (w), 2852 (s), 2494 (w), 2250 (w), 1608 (m), 1582 (s), 1562 (m), 1480 (s), 1412 (m), 1372 (w), 1358 (w), 1308 (m), 1228 (m), 1150 (m), 1134 (w), 1112 (w), 1032 (w), 904 (w), 856 (w), 816(w), 776 (m), 746 (w), 680 (m), 660 (w), 568 (w), 516 (w), 468 (m), 418 (w).
[CoIII2LnIII2(NO3)4(pao)4(piv)4]·2MeCN (Ln = Gd, 2; Ln = Tb, 3; Ln = Pr, 4; Ln = Y, 5: These complexes, which were in the form of microcrystalline solids, were obtained in an identical manner to that described above for 1·2MeCN (yields: 44% for 2, 51% for 3, 43% for 4, and 41% for 5). The complexes were analyzed satisfactorily as lattice-MeCN-free. Anal. Calcd. (%) for C44H56Co2Gd2N12O24 (2): C, 33.68; H, 3.60; N, 10.71. Found (%): C, 33.31; H, 3.75; N, 10.83. Anal. Calcd. (%) for C44H56Co2Tb2N12O24 (3): C, 33.60; H, 3.59; N, 10.69. Found (%): C, 33.72; H, 3.46; N, 10.80. Anal. Calcd. (%) for C44H56Co2Pr2N12O24 (4): C, 34.39; H, 3.67; N, 10.94. Found (%): C, 34.52; H, 3.55; N, 10.73. Anal. Calcd. (%) for C44H56Co2Y2N12O24 (5): C, 36.89; H, 3.94; N, 11.73. Found (%): C, 36.76; H, 3.74; N, 11.96. The IR spectra of 2–5 were identical to that of the Dy(III) analogue (1).
2.3. Single-Crystal X-ray Crystallography
Red crystals of 1·2MeCN (0.06 × 0.42 × 0.43 mm) were taken from the mother liquor and immediately cooled to −113 °C. Diffraction measurements were made on a Rigaku R-AXIS SPIDER Image Plate diffractometer using graphite-monochromated Cu Kα radiation. Data collection (ω-scans) and processing (cell refinement, data reduction, and numerical absorption correction) were performed using the CrystalClear program package [24]. Important crystallographic data are listed in Table 1. The structure was solved via direct methods using SHELΧS ver. 2013/1 and refined via full-matrix least-squares techniques on F2 with SHELXL ver. 2014/6 [25,26]. Further experimental crystallographic details for 1·2MeCN: 2θmax = 130°; reflections collected/unique/used = 19,083/5063 [Rint = 0.1326]/5063; 427 parameters refined; (Δ/σ)max = 0.001; (Δρ)max/(Δρ)min = 2.033/−2.012 e/Å3; R1/wR2 (for all data) = 0.1245/0.2715. All hydrogen atoms were introduced at calculated positions as riding on their corresponding bonded atoms. All non-H atoms were refined anisotropically. The methyl groups of one of the pivalate ligands were found to be disordered and were refined over two positions with occupation factors 0.54 and 0.46, respectively. Plots of the structure were drawn using the Diamond 3 program package [27].

Table 1.
Crystallographic data for 1·2MeCN.
Crystallographic data were deposited with the Cambridge Crystallographic Data Center (No. 2246567). Copies of the data can be obtained free of charge upon application to CCDC, 12 Union Road, Cambridge, CB2 1EZ, UK; tel.: +(44)-1223-762910; fax: +(44)-1223-336033; e-mail: deposit@ccdc.cam.ac.uk; or via http://www.ccdc.cam.ac.uk/conts/retrieving.html (accessed on 23 March 2023).
3. Results and Discussion
3.1. Synthetic Comments
As described in the Introduction, we were interested in using the “assisted self-assembly approach” in CoII/LnIII/pao− chemistry with the hope to isolate CoIII/LnIII complexes other than the {CoIIILnIII} and {CoIII2LnIII} previously obtained using the “one-pot” [21] and “metalloligand” [22] approaches, respectively. After preliminary experimentation, it was clear that the ancillary pivalate (piv−) ion could assist the self-assembly process and result in higher-nuclearity products. A reaction mixture comprising [CoII2(OH2)(piv)4(pivH)4]/Ln(NO3)3.xH2O/paoH/NaOMe (1:3:3:3) in MeCN resulted in a red solution from which red cubic crystals or microcrystalline powders of [CoIII2Ln2(NO3)4(piv)4(pao)4] (Ln = Dy, 1·2MeCN; Ln = Gd, 2; Ln = Tb, 3; and Ln = Pr, 4) were subsequently isolated in moderate to good yields (40–50%) (Equation (1)). The oxidation of the Co(II) to Co(III) was observed. This was achieved by performing the reaction under aerobic and alkaline conditions, while the oxime group probably further promoted the oxidation of divalent Co(II). Red crystals of 1·2MeCN suitable for single-crystal X-ray analysis were obtained from closed vials. Compounds 1 and 2 were characterized via powder X-ray diffraction measurements and compared with the theoretical PXRD of 1·2MeCN (Figure S1).
The {CoIII2Y2} complex 5 was also isolated. The addition of the NaOMe base was important for the isolation of the products since its absence did not result in the complexes. It was clear that CoII was oxidized to CoIII during the reaction, an effect that is common in Co/2-pyridyloximate chemistry [4,5]. After the isolation and the determination of the structure of 1·2 MeCN through single-crystal X-ray crystallography, we also attempted to isolate the products at a stoichiometric ratio of 1:2:4:2, which successfully led to the same complexes (microanalytical and IR evidence). However, when we attempted to isolate the products with a different solvent or mixture of solvents (i.e., MeOH, CH2Cl2, EtOH, etc.), our efforts were unsuccessful; we did not observe precipitation of the product or any other product. This indicated that the co-crystallized MeCN molecules were crucial in the crystallization of the complexes.
3.2. Characterization of the Products
3.2.1. Vibrational Spectroscopy
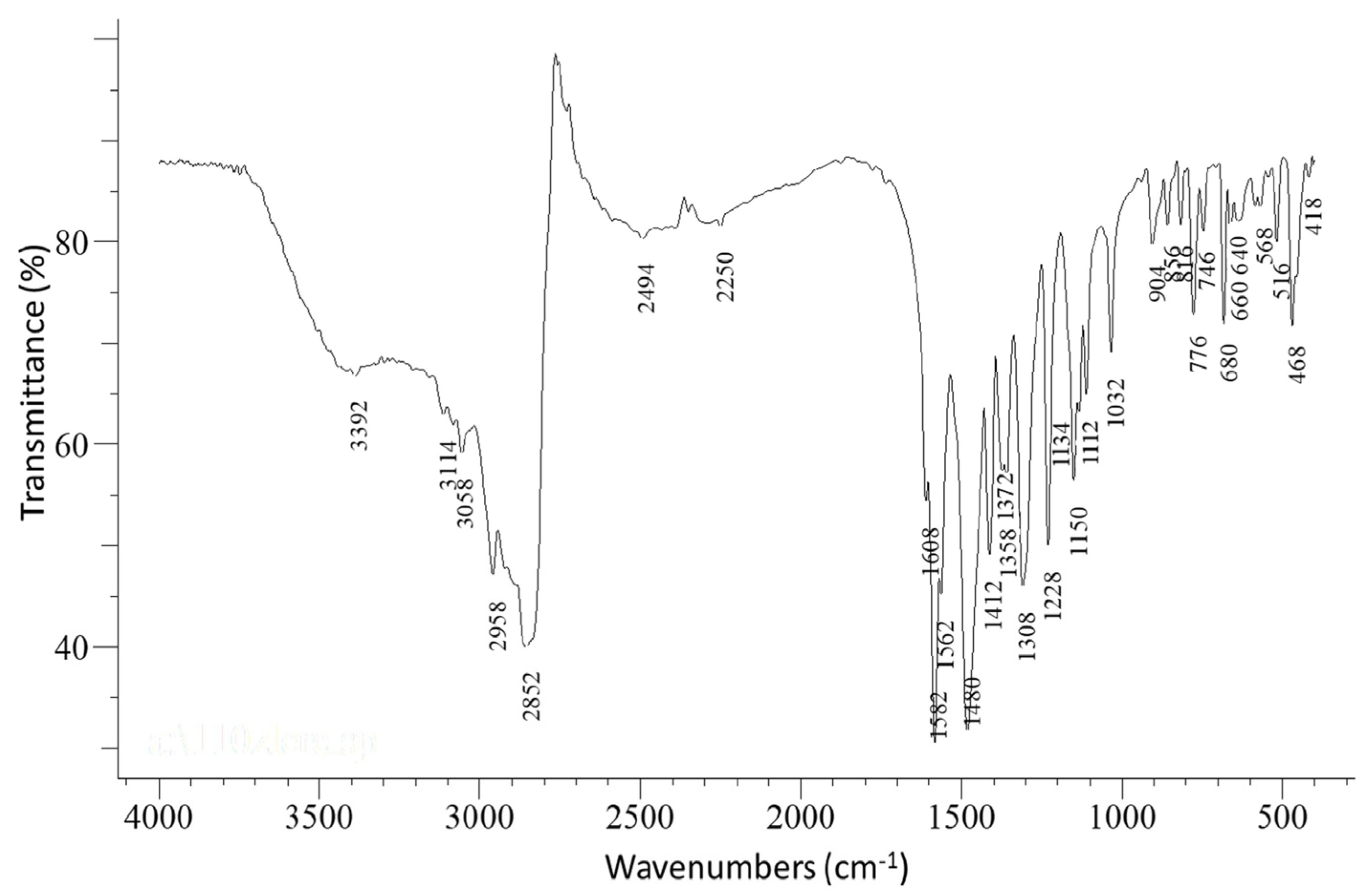
The IR spectrum of the complex [CoIII2Dy2(NO3)4(pao)4 (piv)4] (1) is presented in Figure 1, while in Figures S2–S6 the IR spectra of complexes 2–5 and the free paoH are shown. The in-plane (δ(py)) and out-of-plane (γ(py)) deformation vibrations of the 2-pyridyl ring of free paoH appeared at 627 and 404 cm−1, respectively. These peaks were shifted upwards at 640 and 468 cm−1, respectively, in 1 suggesting coordination of the ring-N atom [21]. The ν(C=N) and ν(N-O) vibrations of the oximate group in the IR spectrum appeared at 1582 cm−1, and 1150 cm−1, respectively. The contribution of the nitrato ligands was also apparent in the IR spectrum of the complex. The bands at 1308 cm−1 and 1480 cm−1 were assigned [21] to the νas(NO2) and ν(N=O) vibrations, respectively; the latter overlapped with the antisymmetric carboxylate sketch. The stretching vibration ν3(E’)[νd(NO)] was not present in the spectrum, which was in accordance with the absence of ionic nitrates (D3h) in the structure of the complex. The considerable separation of the ν(Ν=O) and νas(ΝO2) vibrations (172 cm−1) implied the bidentate ligation of the nitrato ligands [28]. On the other hand, the presence of the carboxylate ligands was indicated by the bands at 1372 and 1480 cm−1 assigned to νs(CO2) and νas(CO2), respectively, while Δ = νas(CO2)-νs(CO2) = 108 cm−1 (which was lower than the corresponding Δ = 220 cm−1 value of the pivalate sodium salt) also indicated the bridging ligation of the carboxylate anions [29].
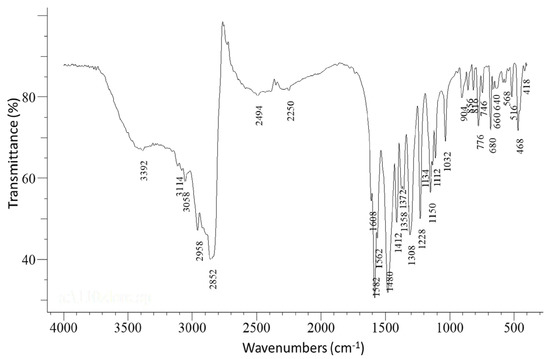
Figure 1.
The FT-IR spectrum of a well-dried (i.e., without lattice MeCN) sample of [CoIII2Dy2(NO3)4(pao)4(piv)4] (1). The weak broad band at 3392 cm−1 was due to the humidity in the sample.
3.2.2. Energy-Dispersive X-ray (EDX) and Powder X-ray Diffraction (PXRD) Analysis of the Complexes
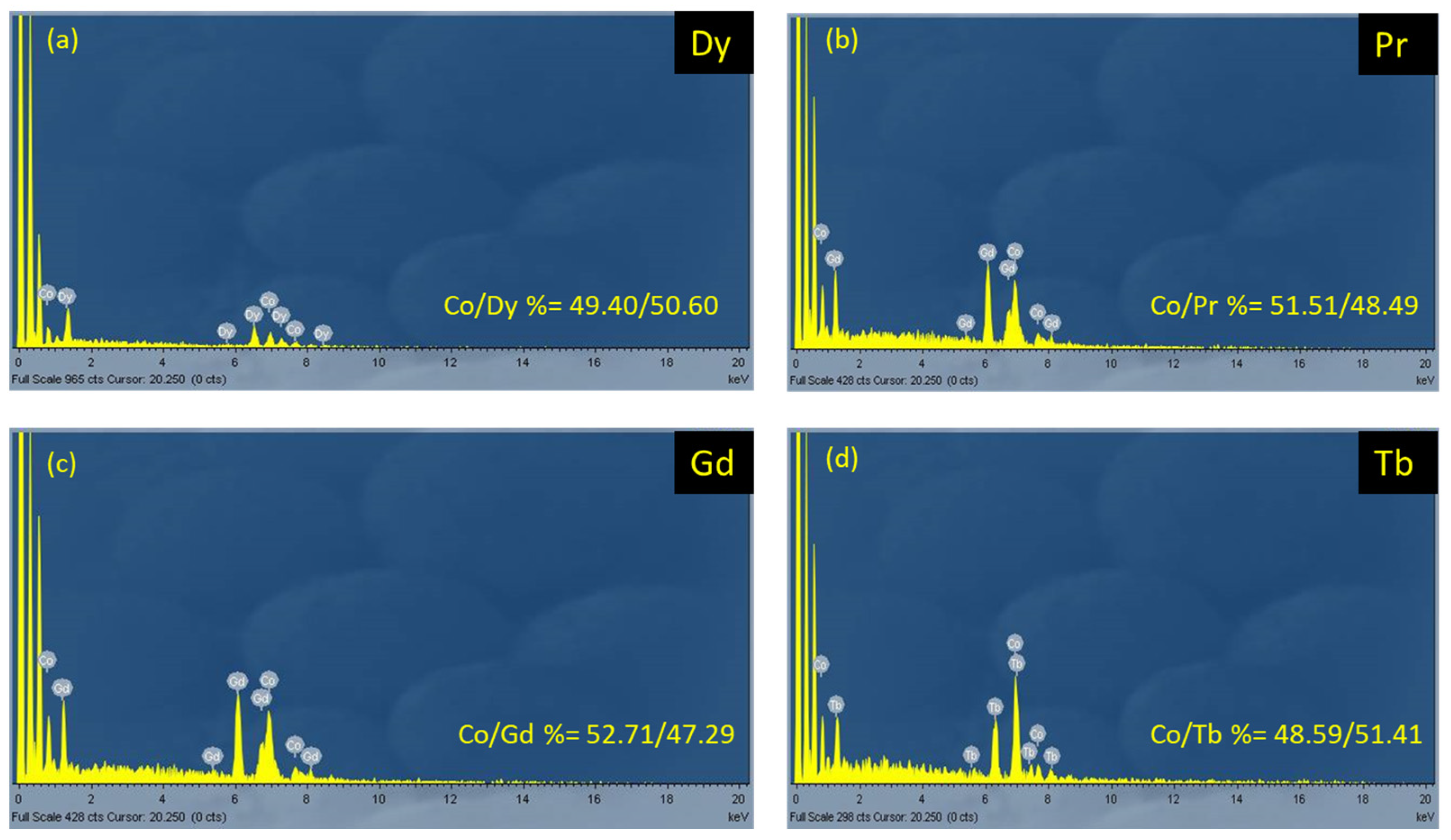
The structure of 1·2MeCN was solved by using single-crystal X-ray crystallography. Complexes 2–5 were proposed to be analogous with 1·2MeCN based on elemental analyses, IR spectra (Section 3.2.1, Figures S3–S6), powder XRD patterns (Figure S1), and EDX measurements (Figure 2). In the EDX spectra of complexes 1–4, the Co/Ln ratio was calculated to be 1:1 (based on the atomic ratio percentage), which was in accordance with the crystal structure of 1, indicating analogous composition for the other compounds. Based on the PXRD, we could observe the similarity of complexes 1 and 2; the XRD diagrams of 1 and 2 were similar with each other and similar to the theoretical one from X-ray diffraction. It is important to note that the IR spectra of all complexes were also similar, thereby indicating the similar chemical structures, and the EDX analysis of complexes 1–4 indicated the similar chemical composition of these complexes.
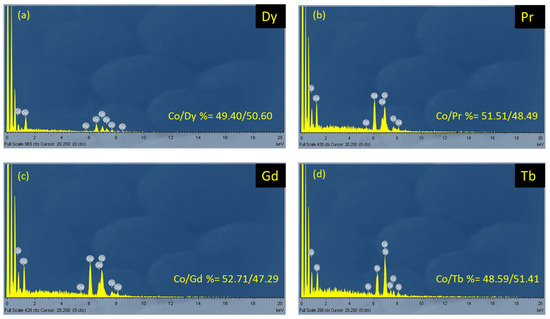
Figure 2.
The EDX spectra of the samples of [CoIII2Ln2(NO3)4(pao)4(piv)4] (Ln = Dy, 1·2MeCN (a); Ln = Pr, 4 (b); Ln = Gd, 2 (c); Ln = Tb, 3 (d)).
3.3. Description of the Structure
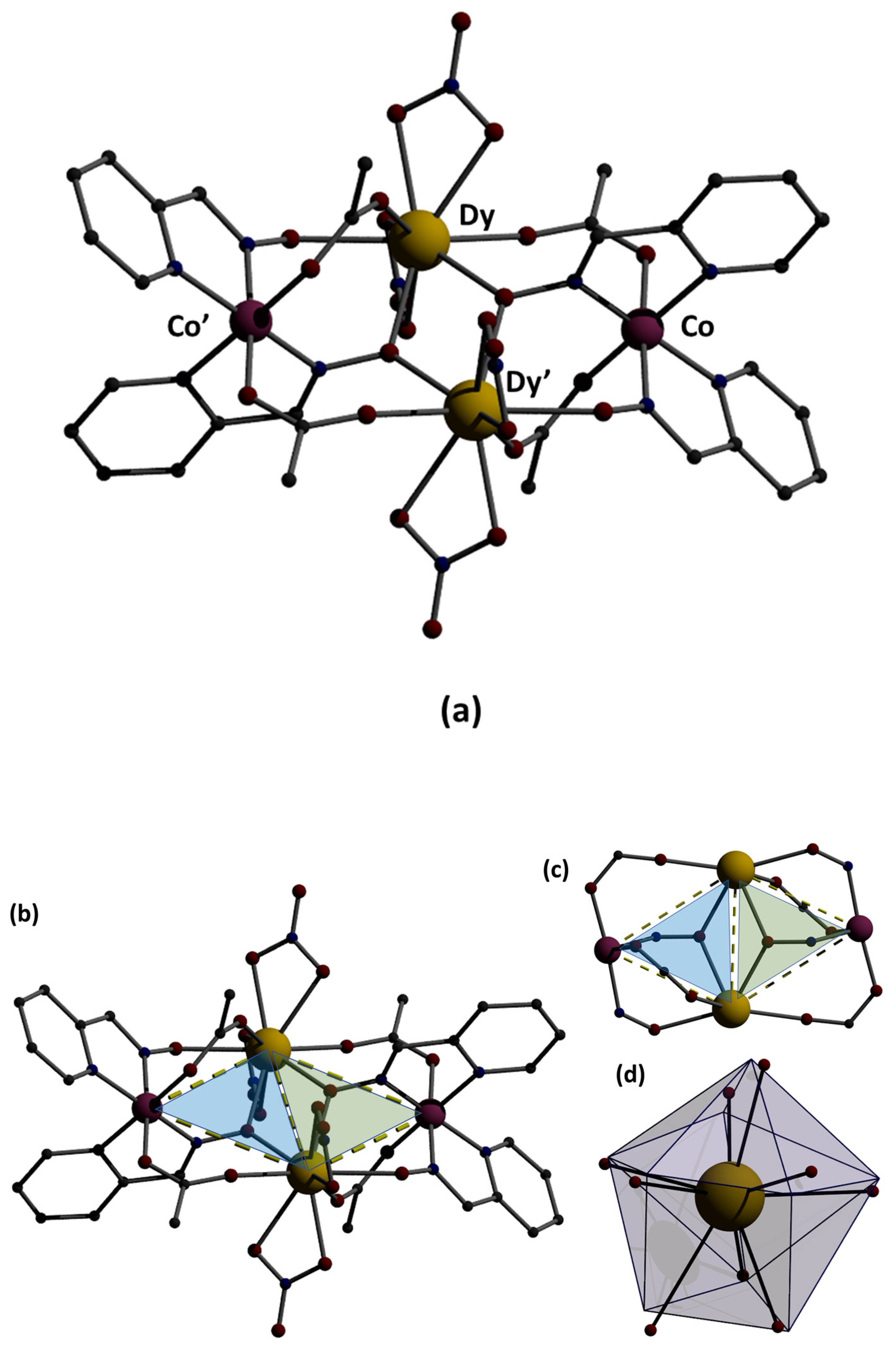
Structural aspects of the molecule [CoIII2Dy2(NO3)4(pao)4(piv)4] are illustrated in Figure 3. Selected interatomic distances are listed in Table 2. This was a centrosymmetric molecule with a topology that could be described as two triangles sharing a common edge, i.e., Dy…Dy’ (Figure 3b). The four metal ions were held together through bridging piv− and pao−ligands adopting three different coordination modes. The CoIII…DyIII vectors of the two triangles were each linked through a 2.11 (Harris notation) pivalate group, while a 3.211 pao− ligand (Figure 3c) bridged the three metal ions of each triangle, and a 2.111 pao− ligand (Figure 3a) bridged the CoIII ion and one DyIII ion of each triangle. Two bidentate chelating nitrato groups completed 9-coordination at each DyIII center. The octahedral coordination sphere of CoIII ions was of the type {CoIIIN4O2}, the bond lengths had a low-spin configuration, and the 9-coordinated DyIII centers were found in a muffin-type geometry (MFF-9) (with CShM = 2.08680 calculated by using the SHAPE program [30]. This butterfly-type topology was new in the 3d/4f/pao−. The oxidation state assignment for the cobalt ions were confirmed by using charge considerations, bond valence sum (BVS) calculations, and interatomic distances (Co-N and Co-O; see Table 2). Interatomic distances (Co-O and Co-N) lay in the ranges 1.865–1.944 Å and 1.899 Å, respectively, which were characteristic for Co(III). Based on the bond valence sum (BVS) calculations using bond length data from the literature [31] and the interatomic distances, the BVS value for complex 1·2MeCN was 3.52, typical for Co(III).
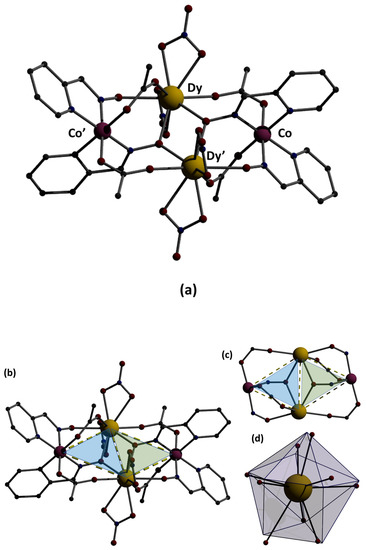
Figure 3.
(a) Representation of the structure of the molecule [CoIII2Dy2(NO3)4(pao)4(piv)4]. The primes denote atoms generated by symmetry operation 1-x, 2-y, -z. The C atoms of the three Me groups of each pivalate are not shown for clarity reasons. (b) The two triangles (illustrated in blue and green) formed in the metal ion bridging topology; (c) the butterfly-type topology through the linkage of the metal ions; (d) the muffin-type polyhedron of the DyIII center.

Table 2.
Selected interatomic distances in 1·2MeCN.
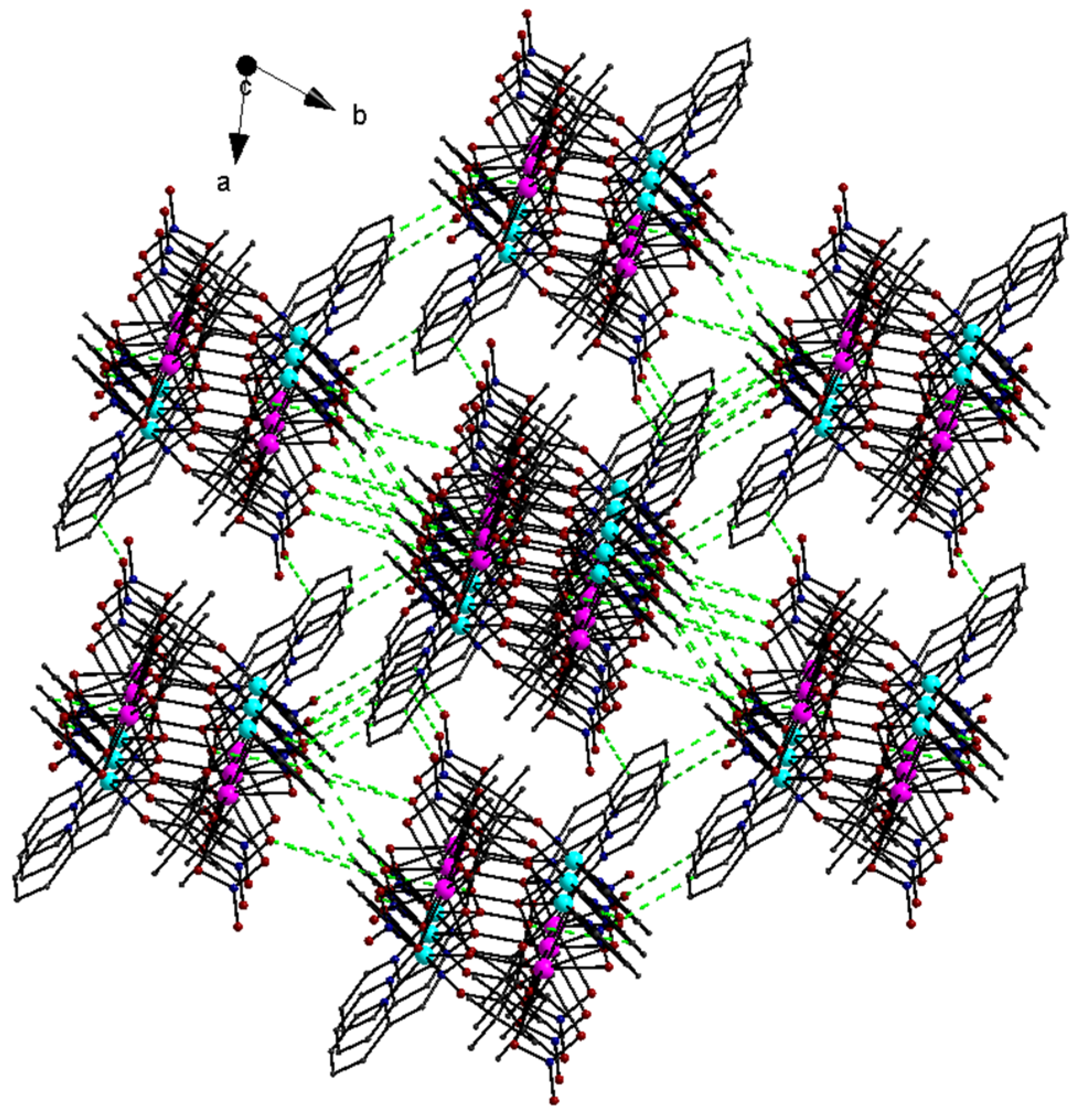
The lattice structure of 1 was constructed by using intermolecular interactions of the C-H…O type (Table S1) that were developed between the C-H moieties of the pao− ligands and the oxygen atoms of the NO3− groups from neighboring molecules. There were also intermolecular interactions involving the MeCN solvent molecules and the coordinated NO3− ions as well as intramolecular C-H…O/N interactions. The intermolecular C-H…O interactions resulted in the formation of a 3D supramolecular network (Figure 4).
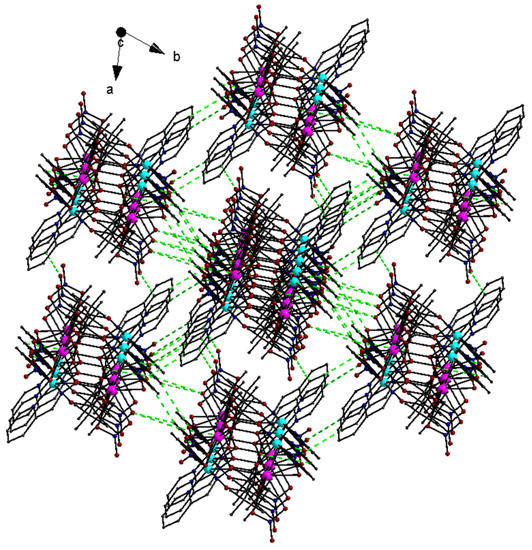
Figure 4.
The 3D supramolecular network in the lattice structure of 1·2MeCN due to the intermolecular C-H…O interactions (dashed green lines).
4. Concluding Comments
The missing {CoIII2LnIII2} nuclearity in the Co/LnIII/pao− chemistry; i.e., the butterfly-type topology, was discovered by using the “assisted self-assembly” process; the ancillary ligand used was the piv− bridging ligand. This work also demonstrated the flexibility of the pao− ligand, which adopted the 2.111 and 3.211 coordination modes, both of which conformed with the HSAB principle. Research efforts are in progress to: (i) prepare other members of the described CoIII/LnIII/pao− family (e.g., with Ln = Nd, Sm, Eu, Ho, Er, and Yb) and study their magnetic and optical properties; and (ii) to use the “assisted self-assembly” approach in systems containing other 2-pyridylaldoximate and carboxylate ligands. The results described herein presage new families of 3d/4f-metal complexes with several primary (i.e., pyridine-2,6-dimethanol) and ancillary (i.e., β-diketonate) 3d/4f-metal complexes.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/chemistry5020068/s1, Figure S1: Comparison of the theoretical PXRD diagram with that of 1·2MeCN for the experimental compounds 1 and 2; Figures S2–S6: IR spectra; Table S1: Intra- and intermolecular interactions in the crystal structure of 1·2MeCN.
Author Contributions
Z.G.L.: writing—review and editing, supervision, project administration; E.K.: resources, investigation, data curation; C.D.P.: investigation, data curation; C.P.R. and V.P.: methodology, data curation, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- James, C.; Willand, P.S. The rare earth cobalticyanide. J. Am. Chem. Soc. 1916, 38, 1497–1500. [Google Scholar] [CrossRef]
- Wang, H.-S.; Zhang, K.; Song, Y.; Pan, Z.-Q. Recent advances in 3d-4f magnetic complexes with several types of non-carboxylate organic ligands. Inorg. Chim. Acta 2021, 521, 120318. [Google Scholar] [CrossRef]
- Sharples, J.W.; Collison, D. The coordination chemistry and magnetism of some 3d–4f and 4f amino-polyalcohol compounds. Coord. Chem. Rev. 2014, 260, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Lada, Z.G.; Polyzou, C.D.; Nika, V.; Stamatatos, T.C.; Konidaris, K.F.; Perlepes, S.P. Adventures in the coordination chemistry of 2-pyridyl oximes: On the way to 3d/4f-metal coordination clusters. Inorg. Chim. Acta 2022, 539, 120954. [Google Scholar] [CrossRef]
- Zheng, Y.-Z.; Evangelisti, M.; Winpenny, R.E.P. Co-Gd phosphonate complexes as magnetic refrigerants. Chem. Sci. 2011, 2, 99–102. [Google Scholar] [CrossRef]
- Boulkedid, A.-L.; Long, J.; Beghidja, C.; Guari, Y.; Beghidja, A.; Larionova, J. A luminescent Schiff-base heterotrinuclear Zn2Dy single-molecule magnet with an axial crystal field. Dalton Trans. 2018, 47, 1402–1406. [Google Scholar] [CrossRef]
- Nikolaevskii, S.A.; Petrov, P.A.; Sukhikh, T.S.; Yambulatov, D.S.; Kiskin, M.A.; Sokolov, M.N.; Eremenko, I.L. Simple synthetic protocol to obtain 3d-4f-heterometallic carboxylate complexes of N-heterocyclic carbenes. Inorg. Chim. Acta 2020, 508, 119643. [Google Scholar] [CrossRef]
- Nikolaevskii, S.A.; Yambulatov, D.S.; Voronina, J.K.; Melnikov, S.N.; Babeshkin, K.A.; Efimov, N.N.; Goloveshkin, A.S.; Kiskin, M.A.; Sidorov, A.A.; Eremenko, I.L. The First Example of 3 d-4 f-Heterometallic Carboxylate Complex Containing Phosphine Ligand. Chem. Sel. 2020, 5, 12829–12834. [Google Scholar] [CrossRef]
- Astaf’eva, T.V.; Yambulatov, D.S.; Nikolaevskii, S.A.; Shmelev, M.A.; Babeshkin, K.A.; Efimov, N.N.; Poddel’sky, A.I.; Eremenko, I.L.; Kiskin, M.A. The First Tetranuclear Iron(II)-Gadolinium(III) Carboxylate Complex [Fe2Gd2(piv)10(bpy)2]: Synthesis, Structure Elucidation and Magnetic Properties. Chem. Sel. 2022, 7, e202203612. [Google Scholar]
- Chorazy, S.; Zychowicz, M.; Ohkoshi, S.-I.; Sieklucka, B. Wide-Range UV-to-Visible Excitation of Near-Infrared Emission and Slow Magnetic Relaxation in LnIII(4,4′-Azopyridine-1,1′-dioxide)[CoIII(CN)6]3– Layered Frameworks. Inorg. Chem. 2019, 58, 165–179. [Google Scholar] [CrossRef]
- Andruh, M.; Costes, J.-P.; Diaz, C.; Gao, S. 3d−4f Combined Chemistry: Synthetic Strategies and Magnetic Properties. Inorg. Chem. 2009, 48, 3342–3359. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zakrzewski, J.J.; Heczko, M.; Zychowicz, M.; Nakagawa, K.; Nakabayashi, K.; Sieklucka, B.; Chorazy, S.; Ohkoshi, S.-I. Proton Conductive Luminescent Thermometer Based on Near-Infrared Emissive {YbCo2} Molecular Nanomagnets. J. Am. Chem. Soc. 2020, 142, 3970–3979. [Google Scholar] [CrossRef]
- Pearson, R.G. Hard and Soft Acids and Bases. J. Am. Chem. Soc. 1963, 85, 3533–3539. [Google Scholar] [CrossRef]
- Alexandru, M.-G.; Visinescu, D.; Shova, S.; Oliveira, W.X.C.; Lloret, F.; Julve, M. Design of 3d–4f molecular squares through the [Fe{(HB(pz)3)}(CN)3]− metalloligand. Dalton Trans. 2018, 47, 6005–6017. [Google Scholar] [CrossRef]
- Sessoli, R.; Powell, A.K. Strategies towards single-molecule magnets based on lanthanide ions. Coord. Chem. Rev. 2009, 253, 2328–2341. [Google Scholar] [CrossRef]
- Li, Y.; Li, D.; Qin, T.; Shi, Z.; Fu, P.; Xiong, D.; Dong, X. A comparative study of proton conduction between two new Cd(II) and Co(II) complexes and in vitro antibacterial study of the Cd(II) complex. Appl. Organomet. Chem. 2023, 37, e6920. [Google Scholar] [CrossRef]
- Qin, T.; Shi, Z.; Zhang, W.; Dong, X.; An, N.; Sakiyama, H.; Muddassir, M.; Srivastava, D.; Kumar, A. 2D isostructural Ln(III)-based coordination polymer derived from Imidazole carboxylic acid: Synthesis, structure and magnetic behavior. J. Mol. Struct. 2023, 1282, 135220. [Google Scholar] [CrossRef]
- Dong, X.; Shi, Z.; Li, D.; Li, Y.; An, N.; Shang, Y.; Sakiyama, H.; Muddassir, M.; Si, C. The regulation research of topology and magnetic exchange models of CPs through Co(II) concentration adjustment. J. Solid State Chem. 2023, 318, 123713. [Google Scholar] [CrossRef]
- Zhang, Y.; Pan, X.; Xu, M.; Xiong, C.; Hong, D.; Fang, H.; Cui, P. Dinitrogen Complexes of Cobalt(−I) Supported by Rare-Earth Metal-Based Metalloligands. Inorg. Chem. 2023, 62, 3836–3846. [Google Scholar] [CrossRef]
- Liu, K.; Shi, W.; Cheng, P. Toward heterometallic single-molecule magnets: Synthetic strategy, structures and properties of 3d–4f discrete complexes. Coord. Chem. Rev. 2015, 289, 74–122. [Google Scholar] [CrossRef]
- Polyzou, C.D.; Koumousi, E.S.; Lada, Z.G.; Raptopoulou, C.P.; Psycharis, V.; Rouzières, M.; Tsipis, A.C.; Mathonière, C.; Clérac, R.; Perlepes, S.P. “Switching on” the single-molecule magnet properties within a series of dinuclear cobalt(iii)–dysprosium(iii) 2-pyridyloximate complexes. Dalton Trans. 2017, 46, 14812–14825. [Google Scholar] [CrossRef]
- Anastasiadis, N.C.; Lada, Z.G.; Polyzou, C.D.; Raptopoulou, C.P.; Psycharis, V.; Konidaris, K.F.; Perlepes, S.P. Synthetic strategies to {CoIII2LnIII} complexes based on 2-pyridyl oximes (Ln = lanthanide). Inorg. Chem. Commun. 2019, 108, 107478. [Google Scholar] [CrossRef]
- Aromí, G.; Batsanov, A.S.; Christian, P.; Helliwell, M.; Parkin, A.; Parsons, S.; Smith, A.A.; Timco, G.A.; Winpenny, R.E.P. Synthetic and Structural Studies of Cobalt–Pivalate Complexes. Chem. A Eur. J. 2003, 9, 5142–5161. [Google Scholar] [CrossRef] [PubMed]
- Rigaku MSC CrystalClear; Rigaku MSC Inc.: The Woodlands, TX, USA, 2005.
- Sheldrick, G. A short history of SHELX. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A 2015, 71, 3–8. [Google Scholar] [CrossRef]
- DIAMOND—Crystal and Molecular Structure Visualization, version 3.1; Crystal Impact: Bonn, Germany, 2014.
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, 4th ed.; Wiley: New York, NY, USA, 1986; pp. 254–257. [Google Scholar]
- Deacon, G.B.; Phillips, R.J. Relationships between the carbon-oxygen stretching frequencies of carboxylato complexes and the type of carboxyalte coordination. Coord. Chem. Rev. 1980, 33, 227–250. [Google Scholar] [CrossRef]
- SHAPE, version 20; Universitat de Barcelona: Barcelona, Spain, 2010.
- Palenik, G.J. Bond Valence Sums in Coordination Chemistry Using Oxidation State Independent R0 Values. Inorg. Chem. 1997, 36, 122. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).