A Review on the Recent Advancements on Therapeutic Effects of Ions in the Physiological Environments
Abstract
:1. Introduction
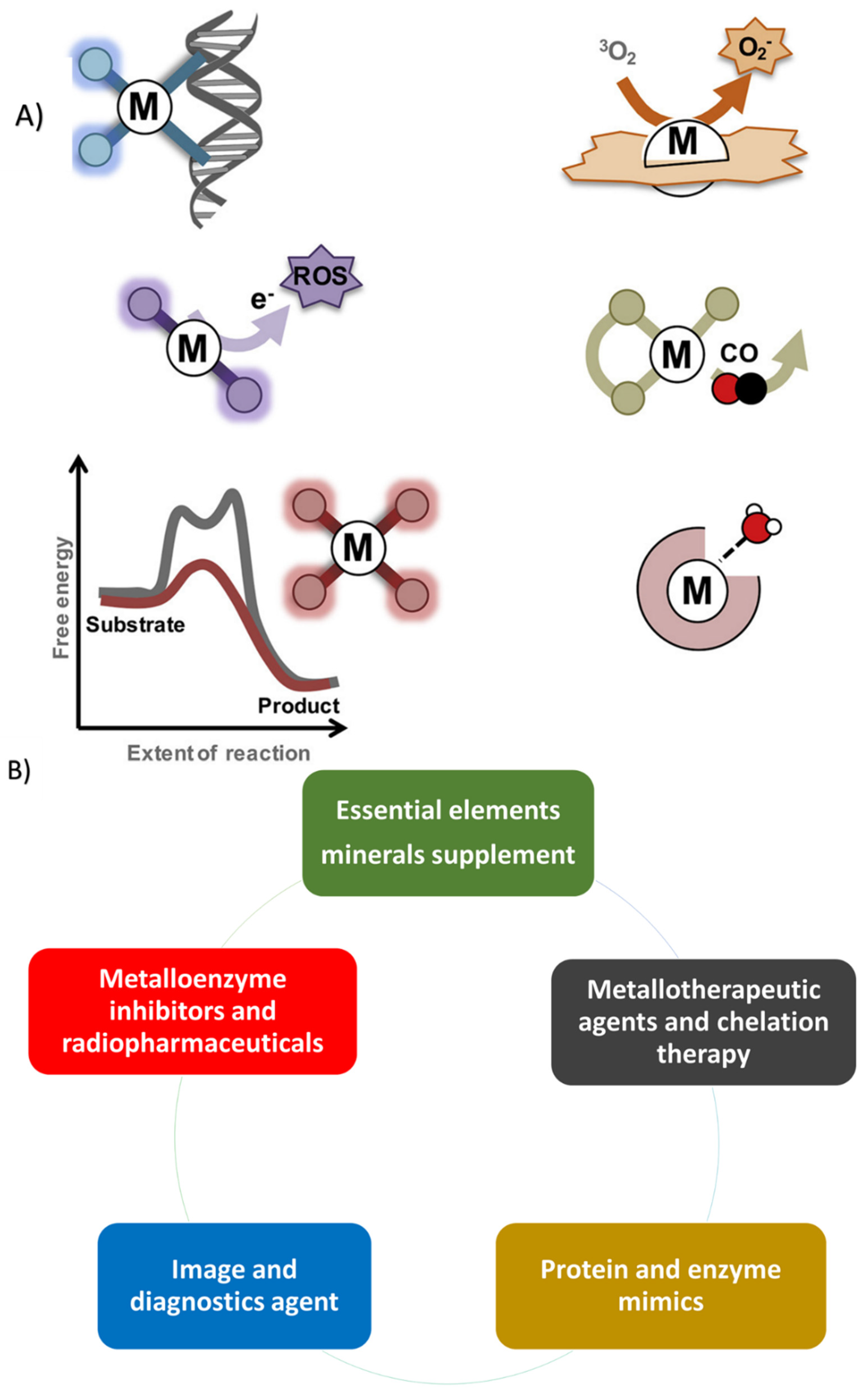
2. Therapeutic Metallic Ions
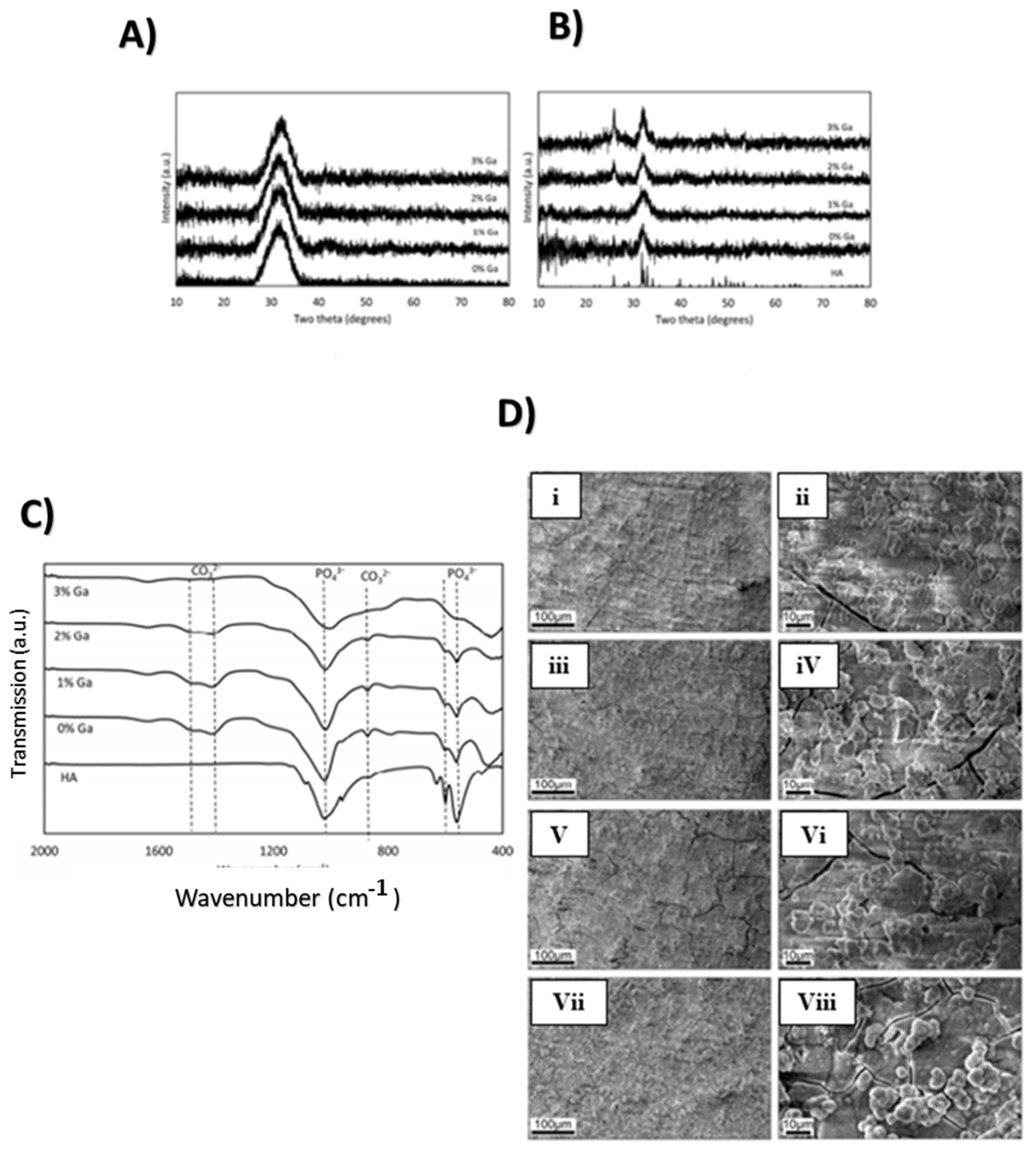
2.1. Gallium
2.1.1. Gallium’s Physical, Chemical, and Biological Properties
2.1.2. Properties and Applications
Anticancer Effects of Gallium
Antimicrobial Activity in Gallium Compounds
Gallium Compounds as Anti-Inflammatory and Immunosuppressive Agents
Effects of Gallium Associated with Hypercalcemia and Bone Metabolism
Antimalarial Agent
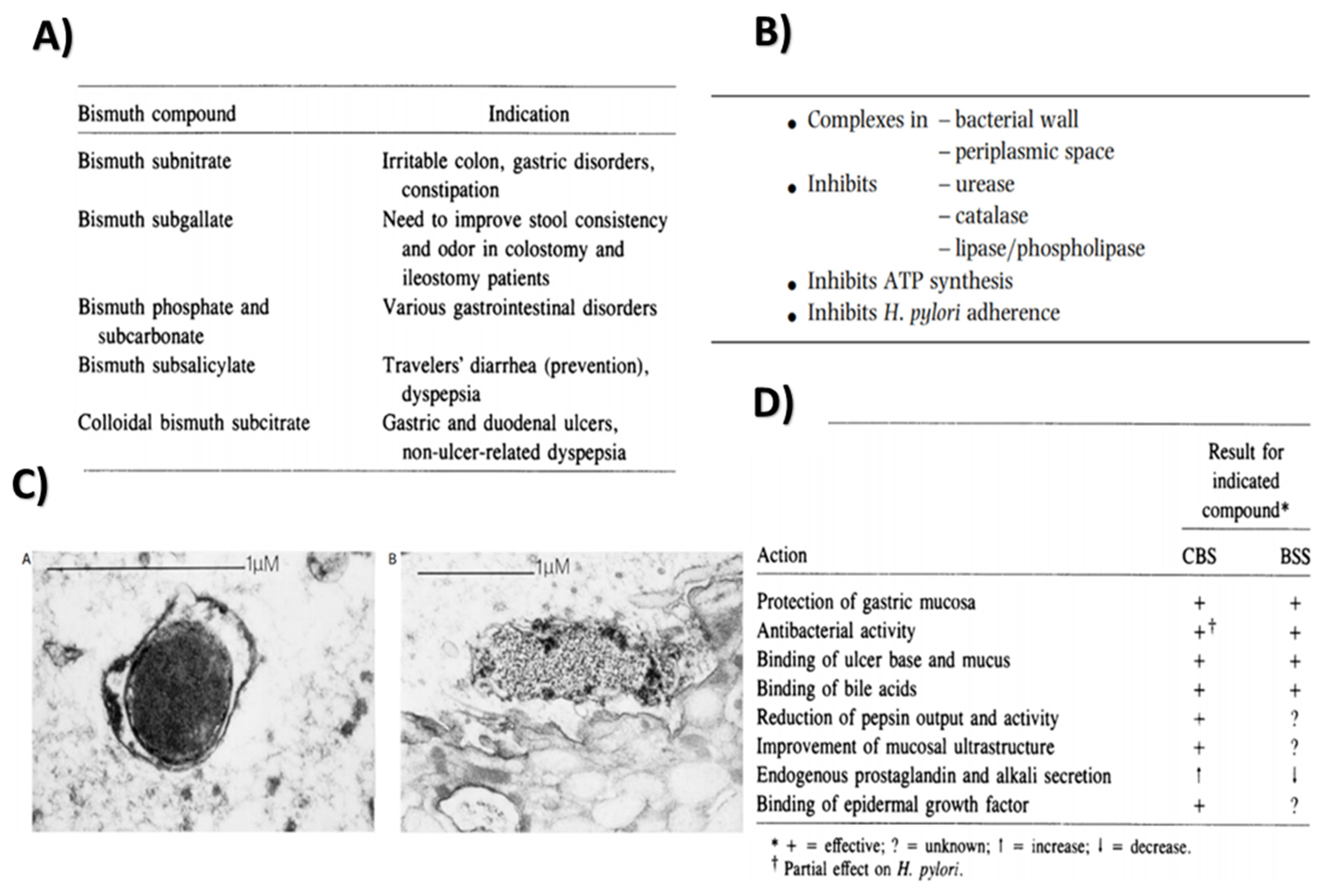
2.2. Bismuth
2.2.1. Properties and Applications
Antibacterial Action
Anti-Leishmaniasis Property
Inhibition of Enzyme Activity
Peptic Ulcer Treatment
2.3. Magnesium
2.3.1. Properties and Applications
Promotion of Osteoblast Cell Proliferation and Differentiation
Mg Ions as a Coagulant
2.4. Calcium
2.4.1. Properties and Applications
Cellular Proliferation and Differentiation
2.5. Germanium
2.5.1. Properties and Applications
Antitumor Activity/Malignant Pathology
Raynaud’s Disease
Antioxidant Effects
As a Protective Agent
As Biocompatible Coatings
2.6. Chromium
2.6.1. Properties and Applications
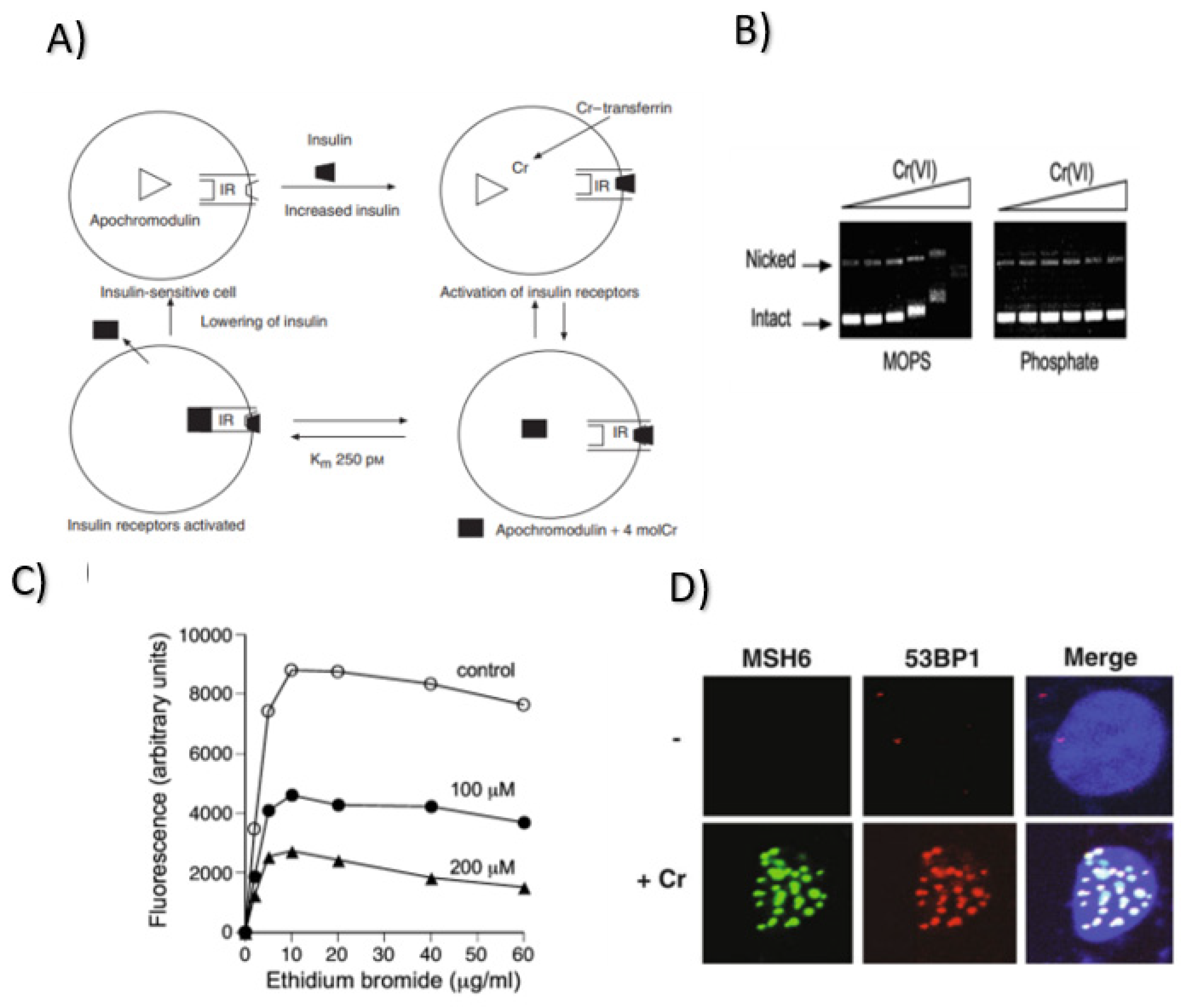
Diabetes Mellitus and Insulin Signaling
Anticarcinogenic Effect
2.7. Lithium
2.7.1. Properties and Applications
Manic Depression Treatment/Bipolar Disorder
Anti-Inflammatory Agent
Wound Healing/Anticoagulating Agent
Schizophrenic Disorders
Major Depressive Disorder
2.8. Potassium
2.8.1. Properties and Applications
Cellular Electrolyte Metabolism
Cell Signaling
Diuretic Agent
Nerve Functioning
Brushes Coated on Artificial Implants
2.9. Strontium
2.9.1. Properties and Applications
Treatment of Cancer
Osteoporosis Treatment
Osteogenic Response
2.10. Boron
2.10.1. Properties and Applications
Bone Mineralization and Proliferation
Anticancer Activity
Antifungal Activity
Antibacterial Activity
Boron in Drug Delivery
Wound Healer
Angiogenic Effect or Stimulate Angiogenesis
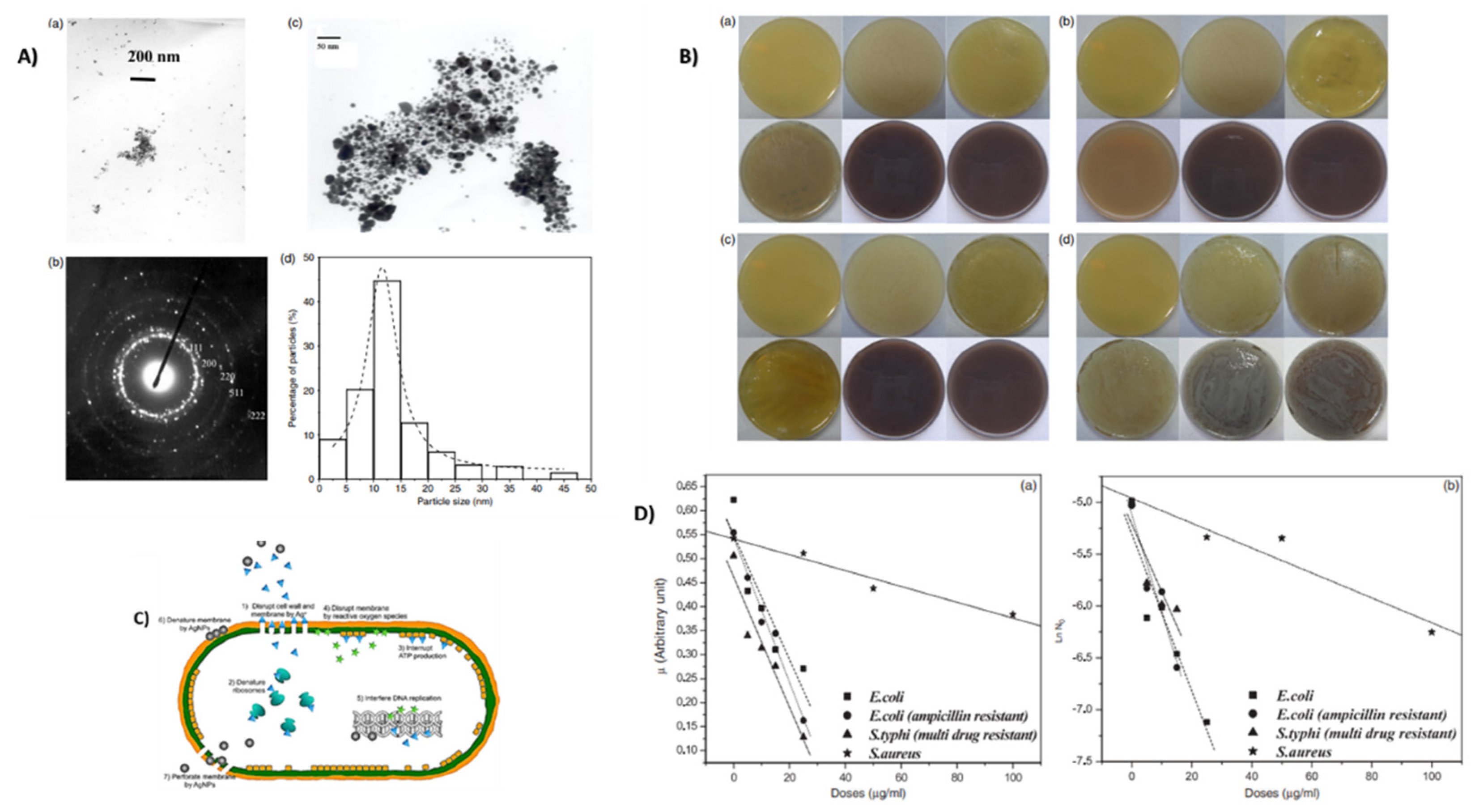
2.11. Silver
2.11.1. Properties and Applications
Antimicrobial Agent
Antibacterial Properties
Wound Healing Activity
Silver Nanoparticles for Bone Healing
Anticancer Activity
2.12. Zinc
2.12.1. Properties and Applications
Total Hip Arthroplasty (THA)
Antibacterial Material
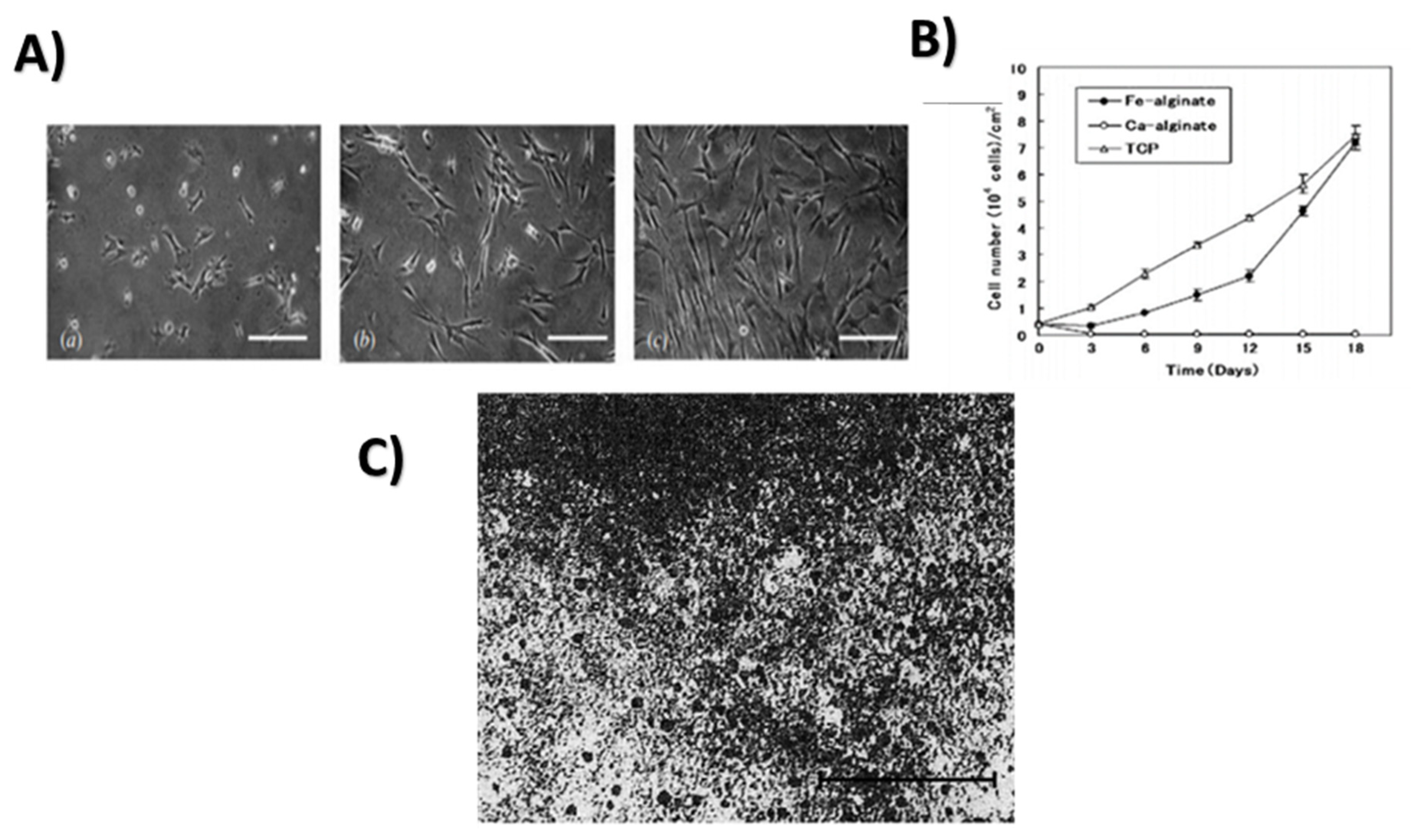
2.13. Iron
2.13.1. Properties and Applications
Antimicrobial Activity
2.14. Cobalt
2.14.1. Properties and Applications
Hypoxia Mimicking Agent
Antibacterial Effect

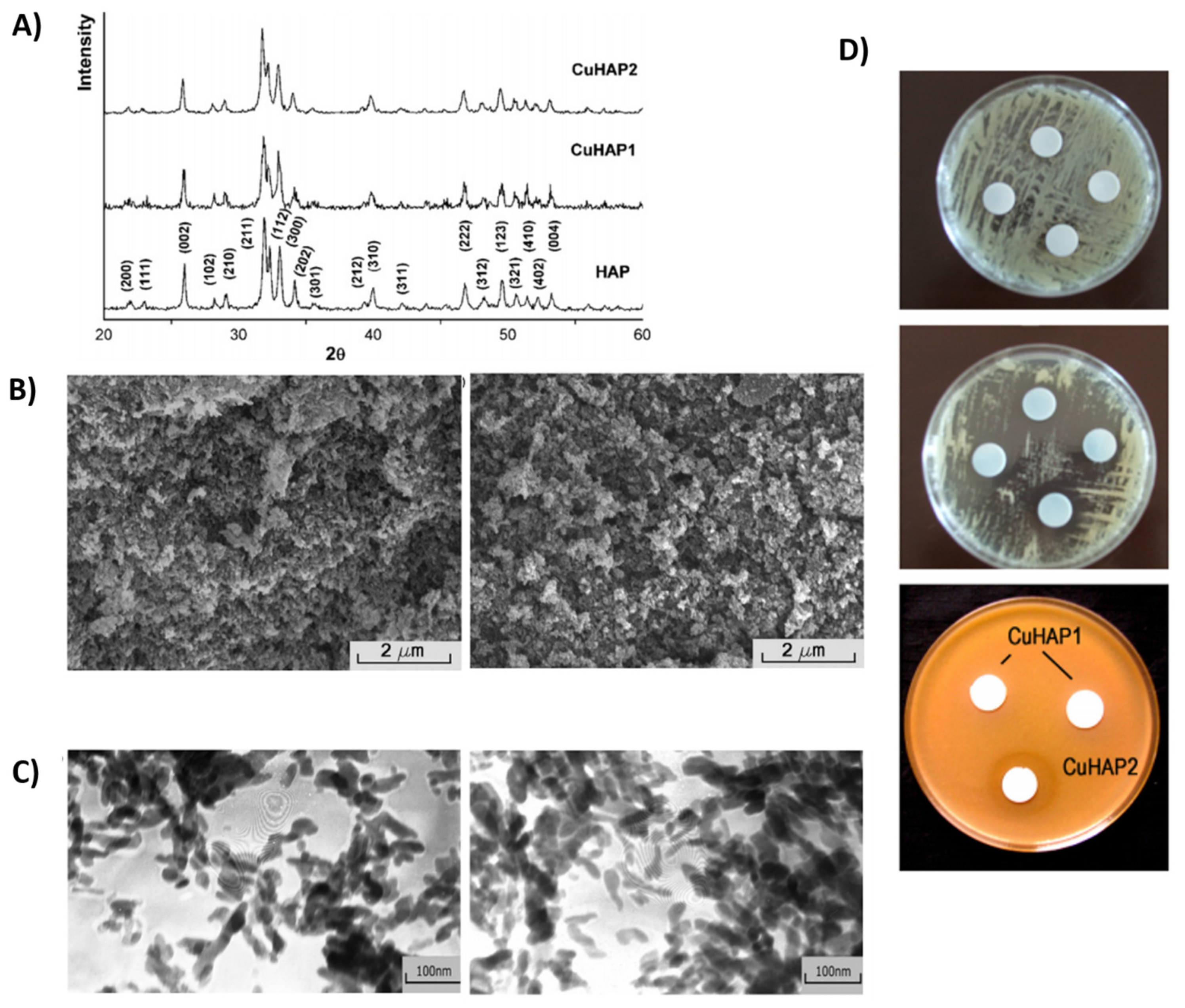
2.15. Copper
2.15.1. Properties and Applications
Inflammation
Cancer
Antimicrobial Potential
2.16. Manganese Ion
2.16.1. Properties and Applications
Antibacterial Property
3. Conclusions
4. Future Prospective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| (ALP) | Alkaline phosphate |
| (BER) | Base excision repair |
| (BGs) | Bioactive glasses |
| (BG) | Bioglass |
| (BHK cells) | Baby hamster kidney fibroblasts |
| (BMSC) | Hypoxia-treated bone marrow stromal cells |
| (BS), | Bismuth subnitrate |
| (BSS) | Bismuth subsalicylate |
| (BSS) | Bismuth subsalicylate |
| (Bi) | Bismuth |
| (Ca) | Calcium |
| (Cr) | Chromium |
| (CBS), | Colloidal bismuth subcitrate |
| (GaDPIX), | Deuteriopropyrin |
| (DABA) | Dual action bone agent |
| (DMEM) | Dulbecco’s Modified Eagle Medium |
| (DNA) | Deoxyribose nucleic acid |
| (DSC) | Differential scanning calorimetry |
| (EDX) | Energy dispersive X-ray analysis |
| (FTIR) | Fourier transform infrared |
| (Ga) | Gallium |
| (GaPPIX) | Gallium protoporphyrin IX |
| (IFN) | Ge-induced interferon |
| (GSH) | Glutathione |
| (GS•) | Glutathione-derived thionyl radical |
| (HAP) | Hydroxy apatite |
| (GaHPIX) | Hematoxylin gallium |
| (Cr(VI)) | Hexavalent chromium |
| (HA) | Hydroxyapatite |
| (HIF) | Hypoxia inducible factor |
| (IR) | Infrared spectroscopy |
| (IR) | Insulin membrane receptor |
| (IRTK) | Insulin receptor tyrosine kinase |
| (Fe) | Iron |
| (LPx) | Lipid peroxidation |
| (LiBBG) | Lithium borate bioglass |
| (LiPBG) | Lithium phosphate bioglass |
| (MDD) | Major depressive disorder |
| (MBG) | Mesoporous bioactive glass |
| (MBGNs) | Mesoporous bioactive glass nanoparticles |
| (m-SXCs) | Mesoporous silica xerogels with variable calcium compositions |
| (MoM) | Metal-on-metal |
| (MMR) | Mismatch repair |
| (NSAIDs) | Nonsteroidal anti-inflammatory drugs |
| (NHDF) | Normal Human Dermal Fibroblasts |
| (NMR) | Nuclear magnetic resonance |
| (NER) | Nucleotide excision repair |
| (OG) | Organic gallium |
| (PSPMAK) | Poly (3-sulfopropyl methacrylate potassium) |
| (RBC) | Ranitidine bismuth citrate |
| (ROS) | Reactive oxygen species |
| (RNA) | Ribonucleic acid |
| (Simulated Body Fluid) | SBF |
| (Scanning Electron Microscopy) | SEM |
| (SLC) | Sodium–lithium countertransport |
| (STAT) | Signal transducer and activator of transcription |
| (TEM) | Transmission electron microscopy |
| (TGA) | Thermogravimetric analysis |
| (TF) | Transferrin |
| (T2D) | Type 2 diabetes mellitus |
| (VEGF). | Vascular endothelial growth factor |
| (XRD) | X-ray powder diffraction |
References
- Bernstein, L.R. Mechanisms of therapeutic activity for gallium. Pharmacol. Rev. 1998, 50, 665–682. [Google Scholar] [PubMed]
- Mukherjee, A.; Sadler, P.J. Metals in Medicine: Therapeutic Agents. In Wiley Encyclopedia of Chemical Biology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; pp. 1–47. [Google Scholar] [CrossRef]
- Ayangbenro, A.S.; Babalola, O.O. A New Strategy for Heavy Metal Polluted Environments: A Review of Microbial Biosorbents. Int. J. Environ. Res. Public Health 2017, 14, 94. [Google Scholar] [CrossRef]
- Brown, T. Design thinking. Harv. Bus. Rev. 2008, 86, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Bundschuh, M.; Filser, J.; Lüderwald, S.; McKee, M.S.; Metreveli, G.; Schaumann, G.E.; Schulz, R.; Wagner, S. Nanoparticles in the Environment: Where Do We Come From, Where Do We Go To? Environ. Sci. Eur. 2018, 30, 6. [Google Scholar] [CrossRef] [Green Version]
- Eliaz, N. Corrosion of Metallic Biomaterials: A Review. Materials 2019, 12, 407. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Zhai, Z.; Sun, D.; Yang, C.; Zhang, X.; Huang, N.; Jiang, X.; Yang, K. Antibacterial durability and biocompatibility of antibacterial-passivated 316L stainless steel in simulated physiological environment. Mater. Sci. Eng. C 2019, 100, 396–410. [Google Scholar] [CrossRef]
- Ge, R.; Lin, M.; Li, X.; Liu, S.; Wang, W.; Li, S.; Zhang, X.; Liu, Y.; Liu, L.; Shi, F.; et al. Cu2+-Loaded Polydopamine Nanoparticles for Magnetic Resonance Imaging-Guided pH- and Near-Infrared-Light-Stimulated Thermochemotherapy. ACS Appl. Mater. Interfaces 2017, 9, 19706–19716. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.C.; Lim, S.; Kim, Y.K. Metal Ion Effects on Aβ and Tau Aggregation. Int. J. Mol. Sci. 2018, 19, 128. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Nguyen, M.; Robert, A.; Meunier, B. Metal Ions in Alzheimer’s Disease: A Key Role or Not? Accounts Chem. Res. 2019, 52, 2026–2035. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, Y.; Chen, X.-H.; Yang, J.-A.; Pan, H.; Chen, D.; Wang, L.; Zhang, J.; Zhu, D.; Wu, S.; et al. Fundamental Theory of Biodegradable Metals—Definition, Criteria, and Design. Adv. Funct. Mater. 2019, 29, 1805402. [Google Scholar] [CrossRef]
- Mohammadinejad, R.; Shavandi, A.; Raie, D.S.; Sangeetha, J.; Soleimani, M.; Hajibehzad, S.S.; Thangadurai, D.; Hospet, R.; Popoola, J.O.; Arzani, A.; et al. Plant molecular farming: Production of metallic nanoparticles and therapeutic proteins using green factories. Green Chem. 2019, 21, 1845–1865. [Google Scholar] [CrossRef] [Green Version]
- Törne, K.; Örnberg, A.; Weissenrieder, J. Influence of strain on the corrosion of magnesium alloys and zinc in physiological environments. Acta Biomater. 2017, 48, 541–550. [Google Scholar] [CrossRef]
- Mohammed, L.; Gomaa, H.G.; Ragab, D.; Zhu, J. Magnetic nanoparticles for environmental and biomedical applications: A review. Particuology 2017, 30, 1–14. [Google Scholar] [CrossRef]
- Zhang, D.; Zheng, A.; Li, J.; Wu, M.; Wu, L.; Wei, Z.; Liao, N.; Zhang, X.; Cai, Z.; Yang, H.; et al. Smart Cu(II)-aptamer complexes based gold nanoplatform for tumor micro-environment triggered programmable intracellular prodrug release, photodynamic treatment and aggregation induced photothermal therapy of hepatocellular carcinoma. Theranostics 2017, 7, 164–179. [Google Scholar] [CrossRef]
- Mouriño, V.; Cattalini, J.P.; Boccaccini, A.R. Metallic ions as therapeutic agents in tissue engineering scaffolds: An overview of their biological applications and strategies for new developments. J. R. Soc. Interface 2012, 9, 401–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, Z.; Sun, S.; Sun, R.; Cui, G.; Hong, L.; Rao, B.; Li, A.; Yu, Z.; Kan, Q.; Mao, Z. A Metal–Polyphenol-Coordinated Nanomedicine for Synergistic Cascade Cancer Chemotherapy and Chemodynamic Therapy. Adv. Mater. 2020, 32, e1906024. [Google Scholar] [CrossRef] [PubMed]
- Rogowska, J.; Olkowska, E.; Ratajczyk, W.; Wolska, L. Gadolinium as a new emerging contaminant of aquatic environments. Environ. Toxicol. Chem. 2018, 37, 1523–1534. [Google Scholar] [CrossRef]
- Talha, M.; Ma, Y.; Kumar, P.; Lin, Y.; Singh, A. Role of protein adsorption in the bio corrosion of metallic implants—A review. Colloids Surf. B Biointerfaces 2019, 176, 494–506. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Yang, H.; Gao, J.; Qin, Y.; Zheng, Y.; Zhu, D. Interfacial Zinc Phosphate is the Key to Controlling Biocompatibility of Metallic Zinc Implants. Adv. Sci. 2019, 6, 1900112. [Google Scholar] [CrossRef]
- Boros, E.; Dyson, P.J.; Gasser, G. Classification of Metal-Based Drugs according to Their Mechanisms of Action. Chem 2020, 6, 41–60. [Google Scholar] [CrossRef] [PubMed]
- Boskey, A.; Ziecheck, W.; Guidon, P.; Doty, S. Gallium nitrate inhibits alkaline phosphatase activity in a differentiating mesenchymal cell culture. Bone Miner. 1993, 20, 179–192. [Google Scholar] [CrossRef]
- Alzoman, H.; Diab, H.M. Effect of gallium aluminium arsenide diode laser therapy onPorphyromonas gingivalisin chronic periodontitis: A randomized controlled trial. Int. J. Dent. Hyg. 2016, 14, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Ehsan, Z. A Poly (Octanediol Citrate)/Gallium-Containing Bioglass Composite for Bone Tissue Regeneration/Ehsan Zeimaran. Ph.D. Thesis, University of Malaya, Kuala Lumpur, Malaysia, 2016. [Google Scholar]
- Rehman, J.C.I.; Knowles, W. Bonfield, Analysis ofin vitro reaction layers formed on Bioglass using thin-film X-ray diffraction and ATR-FTIR microspectroscopy. J. Biomed. Mater. Res. 1998, 41, 162–166. [Google Scholar] [CrossRef]
- Bastos, R.W.; Rossato, L.; Valero, C.; Lagrou, K.; Colombo, A.L.; Goldman, G.H. Potential of Gallium as an Antifungal Agent. Front. Cell. Infect. Microbiol. 2019, 9, 414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adamson, R.H.; Canellos, G.P.; Sieber, S.M. Sieber, Studies on the antitumor activity of gallium nitrate (NSC 15200) and other group IIIa metal salts. Cancer Chemother. Rep. 1975, 59, 599–610. [Google Scholar] [PubMed]
- Kaneko, Y.; Thoendel, M.; Olakanmi, O.; Britigan, B.E.; Singh, P.K. The transition metal gallium disrupts Pseudomonas aeruginosa iron metabolism and has antimicrobial and antibiofilm activity. J. Clin. Investig. 2007, 117, 877–888. [Google Scholar] [CrossRef]
- Kratz, F.; Nuber, B.; Weis, J.; Keppler, B.K. Synthesis and Characterization of Potential Antitumor and Antiviral Gallium(III) Complexes of α-(N)-Heterocyclic Thiosemicarbazones. Synth. React. Inorg. Met.-Org. Chem. 1991, 21, 1601–1615. [Google Scholar] [CrossRef]
- bin Othman, M.F.; Mitry, N.R.; Lewington, V.; Blower, P.; Terry, S.Y. Re-assessing gallium-67 as a therapeutic radionuclide. Nucl. Med. Biol. 2017, 46, 12–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thorson, J.A.; Smith, K.M.; Gomez, F.; Naumann, P.W.; Kemp, J.D. Role of iron in T cell activation: Th1 clones differ from TH2 clones in their sensitivity to inhibition of DNA synthesis caused by IGG mabs against the transferrin receptor and the iron chelator deferoxamine. Cell. Immunol. 1991, 134, 126–137. [Google Scholar] [CrossRef]
- Chitambar, C.R. Medical Applications and Toxicities of Gallium Compounds. Int. J. Environ. Res. Public Health 2010, 7, 2337–2361. [Google Scholar] [CrossRef] [Green Version]
- Goss, C.H.; Kaneko, Y.; Khuu, L.; Anderson, G.D.; Ravishankar, S.; Aitken, M.L.; Lechtzin, N.; Zhou, G.; Czyz, D.M.; McLean, K.; et al. Gallium disrupts bacterial iron metabolism and has therapeutic effects in mice and humans with lung infections. Sci. Transl. Med. 2018, 10, eaat7520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonçalves, R.; Mezencio, J.; Benevides, G.; Matta, S.; Neves, C.; Sarandy, M.; Vilela, E. Effect of gallium-arsenide laser, gallium-aluminum-arsenide laser and healing ointment on cutaneous wound healing in Wistar rats. Braz. J. Med. Biol. Res. 2010, 43, 350–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Z.; Zhao, X.; Chen, X.; Chen, Z.; Xia, Z. Antimicrobial effect of gallium nitrate against bacteria encountered in burn wound infections. RSC Adv. 2017, 7, 52266–52273. [Google Scholar] [CrossRef] [Green Version]
- Chitambar, C.R. The therapeutic potential of iron-targeting gallium compounds in human disease: From basic research to clinical application. Pharmacol. Res. 2017, 115, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Han, B.; Xie, Y.; Wang, H.; Wang, R.; Xia, W.; Li, H.; Sun, H. Combination of gallium(iii) with acetate for combating antibiotic resistant Pseudomonas aeruginosa. Chem. Sci. 2019, 10, 6099–6106. [Google Scholar] [CrossRef] [Green Version]
- Dundar, U.; Evcik, D.; Samli, F.; Pusak, H.; Kavuncu, V. The effect of gallium arsenide aluminum laser therapy in the management of cervical myofascial pain syndrome: A double blind, placebo-controlled study. Clin. Rheumatol. 2006, 26, 930–934. [Google Scholar] [CrossRef]
- Yang, Y.; Ouyang, R.; Xu, L.; Guo, N.; Li, W.; Feng, K.; Ouyang, L.; Yang, Z.; Zhou, S.; Miao, Y. Review: Bismuth complexes: Synthesis and applications in biomedicine. J. Coord. Chem. 2015, 68, 379–397. [Google Scholar] [CrossRef]
- Rana, K.S.; Souza, L.; Isaacs, M.A.; Raja, F.N.S.; Morrell, A.P.; Martin, R.A. Development and Characterization of Gallium-Doped Bioactive Glasses for Potential Bone Cancer Applications. ACS Biomater. Sci. Eng. 2017, 3, 3425–3432. [Google Scholar] [CrossRef] [PubMed]
- Barrioni, B.R.; Oliveira, A.C.; Leite, M.D.F.; Pereira, M. Sol–gel-derived manganese-releasing bioactive glass as a therapeutic approach for bone tissue engineering. J. Mater. Sci. 2017, 52, 8904–8927. [Google Scholar] [CrossRef]
- Lambert, J.R. Pharmacology of Bismuth-Containing Compounds. Clin. Infect. Dis. 1991, 13, S691–S695. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhang, H. Novel Bismuth-Based Nanomaterials Used for Cancer Diagnosis and Therapy. Chem.—A Eur. J. 2018, 24, 17405–17418. [Google Scholar] [CrossRef] [PubMed]
- Cacciotti, I. Bivalent cationic ions doped bioactive glasses: The influence of magnesium, zinc, strontium and copper on the physical and biological properties. J. Mater. Sci. 2017, 52, 8812–8831. [Google Scholar] [CrossRef]
- Cheng, K.; Sano, M.; Jenkins, C.H.; Zhang, G.; Vernekohl, D.; Zhao, W.; Wei, C.; Zhang, Y.; Zhang, Z.; Liu, Y.; et al. Synergistically Enhancing the Therapeutic Effect of Radiation Therapy with Radiation Activatable and Reactive Oxygen Species-Releasing Nanostructures. ACS Nano 2018, 12, 4946–4958. [Google Scholar] [CrossRef] [PubMed]
- Sisin, N.N.T.; Akasaka, H.; Sasaki, R.; Tominaga, T.; Miura, H.; Nishi, M.; Geso, M.; Mat, N.F.C.; Razak, K.A.; Rahman, W.N. Effects of Bismuth Oxide Nanoparticles, Cisplatin and Baicalein-rich Fraction on ROS Generation in Proton Beam irradiated Human Colon Carcinoma Cells. Pol. J. Med Phys. Eng. 2022, 28, 30–36. [Google Scholar] [CrossRef]
- Klauder, J.V. Bismuth in the treatment of syphilis. Arch. Dermatol. Syphilol. 1923, 7, 721–744. [Google Scholar] [CrossRef]
- Ayman, D. Bismuth subnitrate in the treatment of arteriolar (essential) hypertension. J. Am. Med. Assoc. 1932, 98, 545. [Google Scholar] [CrossRef]
- Shavakhi, A.; Tabesh, E.; Yaghoutkar, A.; Hashemi, H.; Tabesh, F.; Khodadoostan, M.; Minakari, M.; Shavakhi, S.; Gholamrezaei, A. The Effects of Multistrain Probiotic Compound on Bismuth-Containing Quadruple Therapy for Helicobacter pylori Infection: A Randomized Placebo-Controlled Triple-Blind Study. Helicobacter 2013, 18, 280–284. [Google Scholar] [CrossRef]
- Alkim, H.; Koksal, A.R.; Boga, S.; Sen, I.; Alkım, C. Role of Bismuth in the Eradication of Helicobacter pylori. Am. J. Ther. 2017, 24, e751–e757. [Google Scholar] [CrossRef]
- Konturek, S.J.; Radecki, T.; Piastucki, I.; Drozdowicz, D. Advances in the Understanding of the Mechanism of Cytoprotective Action by Colloidal Bismuth Subcitrate. Scand. J. Gastroenterol. 1986, 21, 6–10. [Google Scholar] [CrossRef]
- Whitehead, M.W.; Phillips, R.H.; Sieniawska, C.E.; Delves, H.T.; Seed, P.T.; Thompson, R.P.; Powell, J.J. Double-blind comparison of absorbable colloidal bismuth subcitrate and nonabsorbable bismuth subnitrate in the eradication of Helicobacter pylori and the relief of nonulcer dyspepsia. Helicobacter 2001, 5, 169–175. [Google Scholar] [CrossRef]
- Hadrup, N.; Sharma, A.K.; Loeschner, K. Toxicity of silver ions, metallic silver, and silver nanoparticle materials after in vivo dermal and mucosal surface exposure: A review. Regul. Toxicol. Pharmacol. 2018, 98, 257–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, R.; Li, H.; Sun, H. Bismuth: Environmental Pollution and Health Effects. In Encyclopedia of Environmental Health, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 415–423. [Google Scholar] [CrossRef]
- Li, H.; Wang, R.; Sun, H. Systems Approaches for Unveiling the Mechanism of Action of Bismuth Drugs: New Medicinal Applications beyond Helicobacter Pylori Infection. Accounts Chem. Res. 2018, 52, 216–227. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.R.; Midolo, P. The actions of bismuth in the treatment of Helicobacter pylori infection. Aliment. Pharmacol. Ther. 1997, 11, 27–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armstrong, J.A.; Wee, S.H.; Goodwin, C.S.; Wilson, D.H. Response of Campylobacter pyloridis to antibiotics, bismuth and an acid-reducing agent in vitro--an ultrastructural study. J. Med. Microbiol. 1987, 24, 343–350. [Google Scholar] [CrossRef]
- Dore, M.P.; Lu, H.; Graham, D.Y. Role of bismuth in improving Helicobacter pylori eradication with triple therapy. Gut 2016, 65, 870–878. [Google Scholar] [CrossRef]
- Gorbach, S.L. Bismuth therapy in gastrointestinal diseases. Gastroenterology 1990, 99, 863–875. [Google Scholar] [CrossRef]
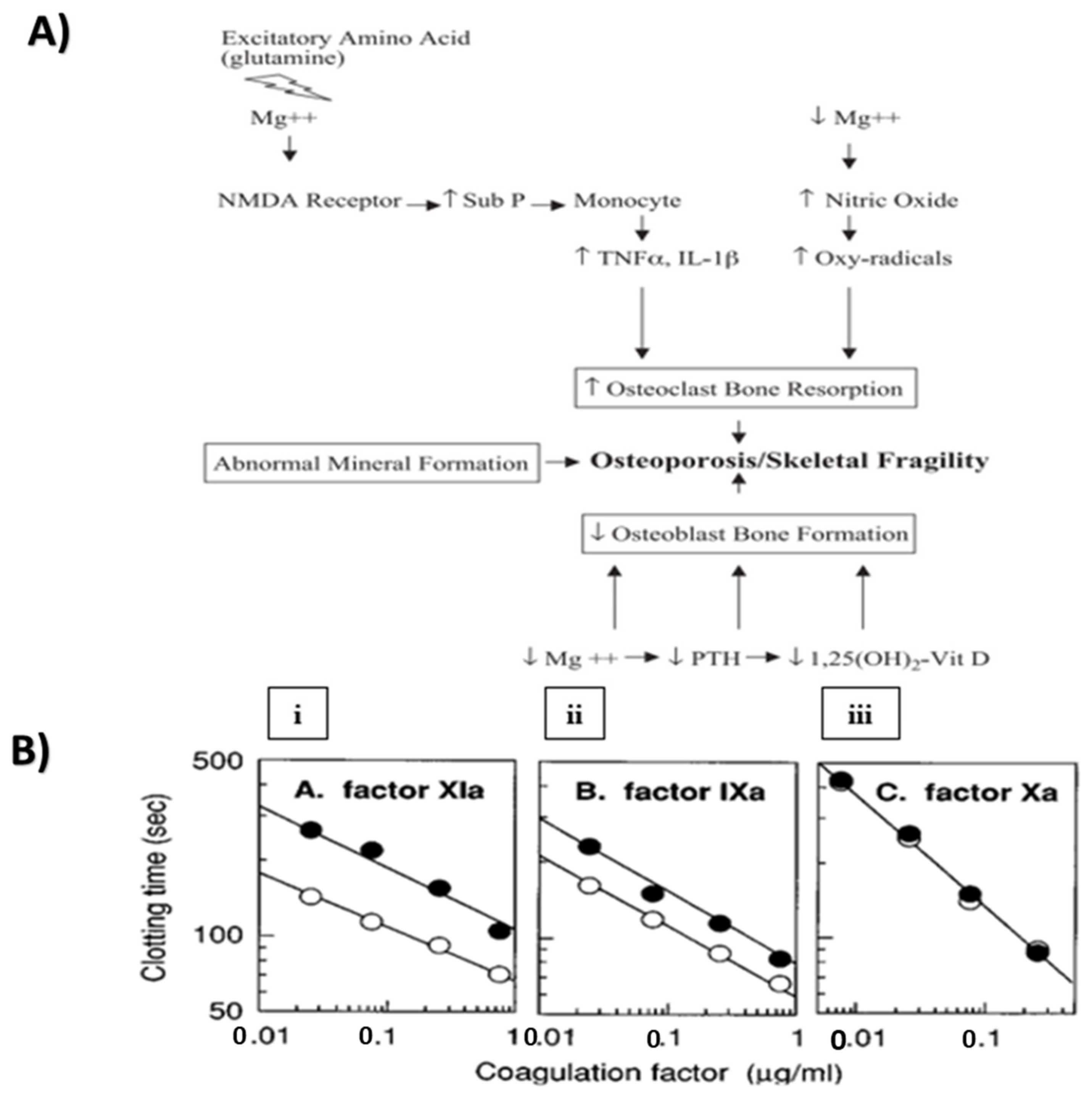
- Rude, R.K.; Gruber, H.E. Magnesium deficiency and osteoporosis: Animal and human observations. J. Nutr. Biochem. 2004, 15, 710–716. [Google Scholar] [CrossRef]
- De Baaij, J.H.F.; Hoenderop, J.G.J.; Bindels, R.J. Magnesium in Man: Implications for Health and Disease. Physiol. Rev. 2015, 95, 1–46. [Google Scholar] [CrossRef]
- Leidi, M.; Dellera, F.; Mariotti, M.; Maier, J.A.M. High magnesium inhibits human osteoblast differentiation in vitro. Magnes. Res. 2011, 24, 1–6. [Google Scholar] [CrossRef]
- Luo, J.; Stewart, R.; Berdeaux, R.; Hu, H. Tonic Inhibition of TRPV3 by Mg2+ in Mouse Epidermal Keratinocytes. J. Investig. Dermatol. 2012, 132, 2158–2165. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Ma, W.; Wei, J.; Mao, Y.; Peng, Z.; Zhang, J.; Kong, X.; Han, Q.; Fan, W.; Yang, Y.; et al. Magnesium promotes root growth and increases aluminum tolerance via modulation of nitric oxide production in Arabidopsis. Plant Soil 2019, 457, 83–95. [Google Scholar] [CrossRef]
- Rude, R.K.; Gruber, H.E.; Norton, H.J.; Wei, L.Y.; Frausto, A.; Mills, B.G. Bone Loss Induced by Dietary Magnesium Reduction to 10% of the Nutrient Requirement in Rats Is Associated with Increased Release of Substance P and Tumor Necrosis Factor-α. J. Nutr. 2004, 134, 79–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Liao, X.; Huang, Z.; You, P.; Chen, C.; Kang, Y.; Yin, G. Synthesis and characterization of novel multiphase bioactive glass-ceramics in the CaO-MgO-SiO2system. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 9999B, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Saboori, A.; Rabiee, M.; Moztarzadeh, F.; Sheikhi, M.H.; Tahriri, M.; Karimi, M. Synthesis, characterization and in vitro bioactivity of sol-gel-derived SiO2–CaO–P2O5–MgO bioglass. Mater. Sci. Eng. C 2009, 29, 335–340. [Google Scholar] [CrossRef]
- Sekiya, F.; Yoshida, M.; Yamashita, T.; Morita, T. Magnesium(II) Is a Crucial Constituent of the Blood Coagulation Cascade. J. Biol. Chem. 1996, 271, 8541–8544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, H.M.; Zhao, Y.; Leung, F.K.L.; Xi, T.; Zhang, Z.; Zheng, Y.; Wu, S.; Luk, K.D.K.; Cheung, K.; Chu, P.K.; et al. Functionalized Polymeric Membrane with Enhanced Mechanical and Biological Properties to Control the Degradation of Magnesium Alloy. Adv. Healthc. Mater. 2017, 6, 1601269. [Google Scholar] [CrossRef]
- Sgambato, A.; Wolf, F.I.; Faraglia, B.; Cittadini, A. Magnesium depletion causes growth inhibition, reduced expression of cyclin D1, and increased expression of p27(KIP1) in normal but not in transformed mammary epithelial cells. J. Cell. Physiol. 1999, 180, 245–254. [Google Scholar] [CrossRef]
- Tommasino, F.; Scifoni, E.; Durante, M. New Ions for Therapy. Int. J. Part. Ther. 2015, 2, 428–438. [Google Scholar] [CrossRef] [Green Version]
- Lankford, K.L.; Letourneau, P.C. Evidence that calcium may control neurite outgrowth by regulating the stability of actin filaments. J. Cell Biol. 1989, 109, 1229–1243. [Google Scholar] [CrossRef]
- Maeno, S.; Niki, Y.; Matsumoto, H.; Morioka, H.; Yatabe, T.; Funayama, A.; Toyama, Y.; Taguchi, T.; Tanaka, J. The effect of calcium ion concentration on osteoblast viability, proliferation and differentiation in monolayer and 3D culture. Biomaterials 2005, 26, 4847–4855. [Google Scholar] [CrossRef]
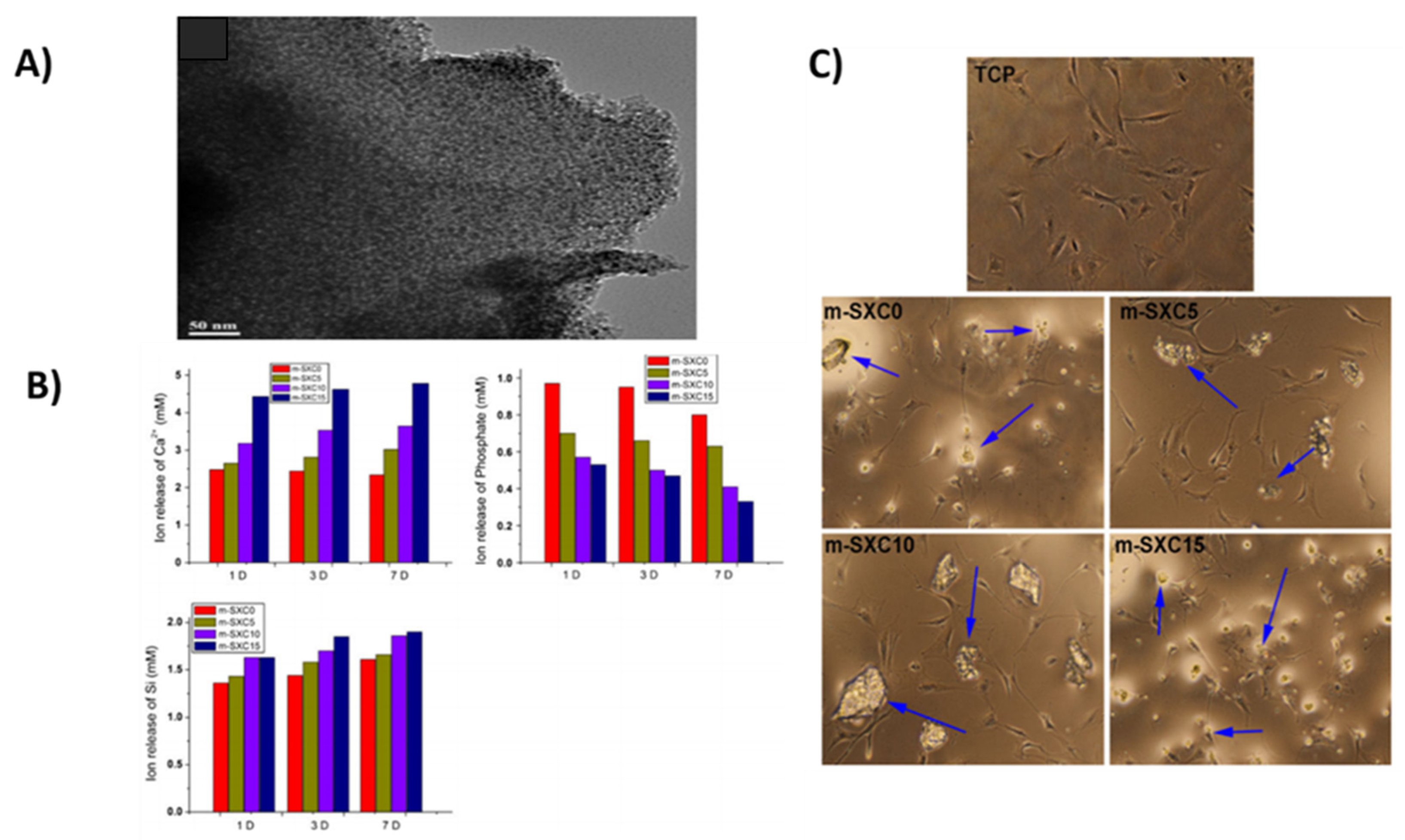
- Zhou, H.; Wei, J.; Wu, X.; Shi, J.; Liu, C.; Jia, J.; Dai, C.; Gan, Q. The bio-functional role of calcium in mesoporous silica xerogels on the responses of osteoblasts in vitro. J. Mater. Sci. Mater. Med. 2010, 21, 2175–2185. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.P. Roles of metals in human health. MOJ Bioorganic Org. Chem. 2018, 2, 1. [Google Scholar] [CrossRef] [Green Version]
- Moriyama, M.; Moriyama, H.; Uda, J.; Kubo, H.; Nakajima, Y.; Goto, A.; Akaki, J.; Yoshida, I.; Matsuoka, N.; Hayakawa, T. Beneficial Effects of the Genus Aloe on Wound Healing, Cell Proliferation, and Differentiation of Epidermal Keratinocytes. PLoS ONE 2016, 11, e0164799. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.Y.; Nie, Y.; Ma, Y.X.; Chen, Y.; Cheng, R.; Yinrg, W.Y.; Hu, Y.; Xu, W.M.; Xu, L.Z. Irisin promotes osteoblast proliferation and differentiation via activating the MAP kinase signaling pathways. Sci. Rep. 2016, 6, 18732. [Google Scholar] [CrossRef] [Green Version]
- Haimi, S.; Gorianc, G.; Moimas, L.; Lindroos, B.; Huhtala, H.; Räty, S.; Kuokkanen, H.; Sándor, G.K.; Schmid, C.; Miettinen, S. Characterization of zinc-releasing three-dimensional bioactive glass scaffolds and their effect on human adipose stem cell proliferation and osteogenic differentiation. Acta Biomater. 2009, 5, 3122–3131. [Google Scholar] [CrossRef]
- Dimeloe, S.; Burgener, A.-V.; Grählert, J.; Hess, C. T-cell metabolism governing activation, proliferation and differentiation; a modular view. Immunology 2017, 150, 35–44. [Google Scholar] [CrossRef] [Green Version]
- Bunting, S.; Di Silvio, L.; Deb, S.; Hall, S. Bioresorbable glass fibres facilitate peripheral nerve regeneration. J. Hand Surg. 2005, 30, 242–247. [Google Scholar] [CrossRef]
- Dhivya, S.; Narayan, A.K.; Kumar, R.L.; Chandran, S.V.; Vairamani, M.; Selvamurugan, N. Proliferation and differentiation of mesenchymal stem cells on scaffolds containing chitosan, calcium polyphosphate and pigeonite for bone tissue engineering. Cell Prolif. 2018, 51, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Fan, J.; Wei, Q.; Liao, J.; Zou, Y.; Song, D.; Xiong, D.; Ma, C.; Hu, X.; Qu, X.; Chen, L.; et al. Noncanonical Wnt signaling plays an important role in modulating canonical Wnt-regulated stemness, proliferation and terminal differentiation of hepatic progenitors. Oncotarget 2017, 8, 27105–27119. [Google Scholar] [CrossRef] [Green Version]
- Huang, Q.; Elkhooly, T.A.; Liu, X.; Zhang, R.; Yang, X.; Shen, Z.; Feng, Q. Effects of hierarchical micro/nano-topographies on the morphology, proliferation and differentiation of osteoblast-like cells. Colloids Surf. B Biointerfaces 2016, 145, 37–45. [Google Scholar] [CrossRef]
- Li, L.; Peng, X.; Qin, Y.; Wang, R.; Tang, J.; Cui, X.; Wang, T.; Liu, W.; Pan, H.; Li, B. Acceleration of bone regeneration by activating Wnt/β-catenin signalling pathway via lithium released from lithium chloride/calcium phosphate cement in osteoporosis. Sci. Rep. 2017, 7, 45204. [Google Scholar] [CrossRef] [PubMed]
- Nandi, S.K.; Mahato, A.; Kundu, B.; Mukherjee, P. Doped Bioactive Glass Materials in Bone Regeneration. In Advanced Techniques in Bone Regeneration; Zorzi, A.R., de Miranda, J.B., Eds.; IntechOpen: London, UK, 2016; pp. 275–328. [Google Scholar]
- Goodman, S. Therapeutic effects of organic Germanium. Med. Hypotheses 1988, 26, 207–215. [Google Scholar] [CrossRef]
- Abrantes, A.M.; Pires, A.S.; Monteiro, L.; Teixo, R.; Neves, A.R.; Tavares, N.T.; Marques, I.A.; Botelho, M.F. Tumour functional imaging by PET. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2020, 1866, 165717. [Google Scholar] [CrossRef] [PubMed]
- Fukazawa, H.; Ohashi, Y.; Sekiyama, S.; Hoshi, H.; Abe, M.; Takahashi, M.; Sato, T. Multidisciplinary treatment of head and neck cancer using BCG, OK-432, and GE-132 as biologic response modifiers. Head Neck 1994, 16, 30–38. [Google Scholar] [CrossRef]
- Jumina, J.; Harizal, H. Dermatologic Toxicities and Biological Activities of Chromium. In Trace Metals in the Environment-New Approaches and Recent Advances; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef] [Green Version]
- Qi, S.S.; Zheng, H.X.; Jiang, H.; Yuan, L.P.; Dong, L.C. Protective Effects of Chromium Picolinate Against Diabetic-Induced Renal Dysfunction and Renal Fibrosis in Streptozotocin-Induced Diabetic Rats. Biomolecules 2020, 10, 398. [Google Scholar] [CrossRef] [Green Version]
- Bozkurt, M.F.; Virgolini, I.; Balogova, S.; Beheshti, M.; Rubello, D.; Decristoforo, C.; Ambrosini, V.; Kjaer, A.; Delgado-Bolton, R.; Kunikowska, J.; et al. Guideline for PET/CT imaging of neuroendocrine neoplasms with 68Ga-DOTA-conjugated somatostatin receptor targeting peptides and 18F-DOPA. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1588–1601, Erratum in 2017, 44, 2150–2151. [Google Scholar] [CrossRef]
- Wang, C.; Chen, Z.; Pan, Y.; Gao, X.; Chen, H. Anti-diabetic effects of Inonotus obliquus polysaccharides-chromium (III) complex in type 2 diabetic mice and its sub-acute toxicity evaluation in normal mice. Food Chem. Toxicol. 2017, 108, 498–509. [Google Scholar] [CrossRef]
- Ali, M.M.; Noaman, E.; Kamal, S.; Soliman, S.; Ismail, D.A. Role of germanium L-cysteine α-tocopherol complex as stimulator of some antioxidant defense systems in gamma-irradiated rats. Acta Pharm. 2007, 57, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Ngala, R.A.; Awe, M.A.; Nsiah, P. The effects of plasma chromium on lipid profile, glucose metabolism and cardiovascular risk in type 2 diabetes mellitus. A case—Control study. PLoS ONE 2018, 13, e0197977. [Google Scholar] [CrossRef]
- Sala, A.; Anderson, D.J.; Brennan, P.M.; Butler, H.J.; Cameron, J.M.; Jenkinson, M.D.; Rinaldi, C.; Theakstone, A.G.; Baker, M.J. Biofluid diagnostics by FTIR spectroscopy: A platform technology for cancer detection. Cancer Lett. 2020, 477, 122–130. [Google Scholar] [CrossRef]
- Prasad, A. Role of chromium compounds in diabetes. Indian J. Pharm. Pharmacol. 2016, 3, 17. [Google Scholar] [CrossRef]
- Léonard, A.; Hantson, P.; Gerber, G. Mutagenicity, carcinogenicity and teratogenicity of lithium compounds. Mutat. Res. Genet. Toxicol. 1995, 339, 131–137. [Google Scholar] [CrossRef]
- Anderson, R.A. Chromium and insulin resistance. Nutr. Res. Rev. 2003, 16, 267–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jain, S.K.; Kannan, K. Chromium Chloride Inhibits Oxidative Stress and TNF-α Secretion Caused by Exposure to High Glucose in Cultured U937 Monocytes. Biochem. Biophys. Res. Commun. 2001, 289, 687–691. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Liu, P.; Pattar, G.R.; Tackett, L.; Bhonagiri, P.; Strawbridge, A.B.; Elmendorf, J.S. Chromium Activates Glucose Transporter 4 Trafficking and Enhances Insulin-Stimulated Glucose Transport in 3T3-L1 Adipocytes via a Cholesterol-Dependent Mechanism. Mol. Endocrinol. 2006, 20, 857–870. [Google Scholar] [CrossRef] [Green Version]
- Thompson, C.M.; Wolf, J.C.; McCoy, A.; Suh, M.; Proctor, D.M.; Kirman, C.R.; Haws, L.C.; Harris, M.A. Comparison of Toxicity and Recovery in the Duodenum of B6C3F1 Mice Following Treatment with Intestinal Carcinogens Captan, Folpet, and Hexavalent Chromium. Toxicol. Pathol. 2017, 45, 1091–1101. [Google Scholar] [CrossRef] [Green Version]
- Deger, Y.; DeDe, S.; Belge, A.; Mert, N.; Kahraman, T.; Alkan, M. Effects of X-Ray Radiation on Lipid Peroxidation and Antioxidant Systems in Rabbits Treated with Antioxidant Compounds. Biol. Trace Elem. Res. 2003, 94, 149–156. [Google Scholar] [CrossRef]
- Yao, H.; Guo, L.; Jiang, B.-H.; Luo, J.; Shi, X. Oxidative Stress and Chromium(VI) Carcinogenesis. J. Environ. Pathol. Toxicol. Oncol. 2008, 27, 77–88. [Google Scholar] [CrossRef]
- Moradi, F.; Maleki, V.; Saleh-Ghadimi, S.; Kooshki, F.; Gargari, B.P. Potential roles of chromium on inflammatory biomarkers in diabetes: A Systematic. Clin. Exp. Pharmacol. Physiol. 2019, 46, 975–983. [Google Scholar] [CrossRef] [Green Version]
- Zhitkovich, A.; Song, Y.; Quievryn, G.; Voitkun, V. Non-Oxidative Mechanisms Are Responsible for the Induction of Mutagenesis by Reduction of Cr(VI) with Cysteine: Role of Ternary DNA Adducts in Cr(III)-Dependent Mutagenesis. Biochemistry 2001, 40, 549–560. [Google Scholar] [CrossRef]
- Jing, H.; Wang, F.; Gao, X. Lithium intoxication induced pyroptosis via ROS / NF-κB / NLRP3 inflammasome regulatory networks in kidney of mice. Environ. Toxicol. 2022, 37, 825–835. [Google Scholar] [CrossRef] [PubMed]
- Sakrajda, K.; Szczepankiewicz, A. Inflammation-Related Changes in Mood Disorders and the Immunomodulatory Role of Lithium. Int. J. Mol. Sci. 2021, 22, 1532. [Google Scholar] [CrossRef] [PubMed]
- Pessin, J.E.; Saltiel, A. Signaling pathways in insulin action: Molecular targets of insulin resistance. J. Clin. Investig. 2000, 106, 165–169. [Google Scholar] [CrossRef] [Green Version]
- Undurraga, J.; Sim, K.; Tondo, L.; Gorodischer, A.; Azua, E.; Tay, K.H.; Tan, D.; Baldessarini, R.J. Lithium treatment for unipolar major depressive disorder: Systematic review. J. Psychopharmacol. 2019, 33, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Aydin, M.; Ilhan, B.C.; Calisir, S.; Yildirim, S.; Eren, I. Continuing clozapine treatment with lithium in schizophrenic patients with neutropenia or leukopenia: Brief review of literature with case reports. Ther. Adv. Psychopharmacol. 2016, 6, 33–38. [Google Scholar] [CrossRef] [Green Version]
- Li, N.; Zhang, X.; Dong, H.; Zhang, S.; Sun, J.; Qian, Y. Lithium Ameliorates LPS-Induced Astrocytes Activation Partly via Inhibition of Toll-Like Receptor 4 Expression. Cell. Physiol. Biochem. 2016, 38, 714–725. [Google Scholar] [CrossRef]
- Abramovic, L.; Boks, M.P.; Vreeker, A.; Bouter, D.C.; Kruiper, C.; Verkooijen, S.; van Bergen, A.H.; Ophoff, R.A.; Kahn, R.S.; van Haren, N.E. The association of antipsychotic medication and lithium with brain measures in patients with bipolar disorder. Eur. Neuropsychopharmacol. 2016, 26, 1741–1751. [Google Scholar] [CrossRef]
- Szklarska, D.; Rzymski, P. Is Lithium a Micronutrient? From Biological Activity and Epidemiological Observation to Food Fortification. Biol. Trace Element Res. 2019, 189, 18–27. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Peti-Peterdi, J.; Brandes, A.U.; Riquier-Brison, A.; Carlson, N.G.; Müller, C.E.; Ecelbarger, C.M.; Kishore, B.K. Prasugrel suppresses development of lithium-induced nephrogenic diabetes insipidus in mice. Purinergic Signal. 2017, 13, 239–248. [Google Scholar] [CrossRef] [Green Version]
- Ge, W.; Jakobsson, E. Systems Biology Understanding of the Effects of Lithium on Affective and Neurodegenerative Disorders. Front. Neurosci. 2018, 12, 933. [Google Scholar] [CrossRef]
- Zhang, K.; Alaohali, A.; Sawangboon, N.; Sharpe, P.T.; Brauer, D.S.; Gentleman, E. A comparison of lithium-substituted phosphate and borate bioactive glasses for mineralised tissue repair. Dent. Mater. 2019, 35, 919–927. [Google Scholar] [CrossRef] [PubMed]
- Duvall, A.E.; Gallicchio, V.S. Lithium Treatment in Clinical Medicine: History, Current Status and Future Use. J. Cell Sci. Ther. 2017, 08, 1–9. [Google Scholar] [CrossRef]
- Murphy, N.; Redahan, L.; Lally, J. Management of lithium intoxication. BJPsych Adv. 2022, 1–10. [Google Scholar] [CrossRef]
- Youssef, F.S.; Menze, E.T.; Ashour, M.L. A Potent Lignan from Prunes Alleviates Inflammation and Oxidative Stress in Lithium/Pilocarpine-Induced Epileptic Seizures in Rats. Antioxidants 2020, 9, 575. [Google Scholar] [CrossRef]
- Agrawal, S.; Gollapudi, S.; Gupta, S.; Agrawal, A. Dendritic cells from the elderly display an intrinsic defect in the production of IL-10 in response to Lithium Chloride. Exp. Gerontol. 2013, 48, 1285–1292. [Google Scholar] [CrossRef] [Green Version]
- Gaddam, A.; Allu, A.R.; Ganisetti, S.; Fernandes, H.R.; Stan, G.E.; Negrila, C.C.; Jamale, A.P.; Mear, F.; Montagne, L.; Ferreira, J.M.F. Effect of Vanadium Oxide on the Structure and Li-Ion Conductivity of Lithium Silicate Glasses. J. Phys. Chem. C 2021, 125, 16843–16857. [Google Scholar] [CrossRef]
- Ahmed, T.A.; Suso, H.-P.; Maqbool, A.; Hincke, M.T. Processed eggshell membrane powder: Bioinspiration for an innovative wound healing product. Mater. Sci. Eng. C 2019, 95, 192–203. [Google Scholar] [CrossRef]
- Young, W. Review of Lithium Effects on Brain and Blood. Cell Transplant. 2009, 18, 951–975. [Google Scholar] [CrossRef]
- Gitlin, M. Lithium side effects and toxicity: Prevalence and management strategies. Int. J. Bipolar Disord. 2016, 4, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Nassar, A.; Azab, A.N. Effects of Lithium on Inflammation. ACS Chem. Neurosci. 2014, 5, 451–458. [Google Scholar] [CrossRef] [Green Version]
- Luo, D.-Z.; Chang, C.-Y.; Huang, T.-R.; Studer, V.; Wang, T.-W.; Lai, W.-S. Lithium for schizophrenia: Supporting evidence from a 12-year, nationwide health insurance database and from Akt1-deficient mouse and cellular models. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maçon, A.L.B.; Jacquemin, M.; Page, S.J.; Li, S.; Bertazzo, S.; Stevens, M.M.; Hanna, J.V.; Jones, J.R. Lithium-silicate sol–gel bioactive glass and the effect of lithium precursor on structure–property relationships. J. Sol-Gel Sci. Technol. 2016, 81, 84–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stone, M.S.; Martyn, L.; Weaver, C.M. Potassium Intake, Bioavailability, Hypertension, and Glucose Control. Nutrients 2016, 8, 444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Granados, A.M.; Vaquero, M.P. Silicon, aluminium, arsenic and lithium: Essentiality and human health implications. J. Nutr. Health Aging 2002, 6, 154–162. [Google Scholar]
- Villette, J.; Cuéllar, T.; Verdeil, J.-L.; Delrot, S.; Gaillard, I. Grapevine Potassium Nutrition and Fruit Quality in the Context of Climate Change. Front. Plant Sci. 2020, 11, 123. [Google Scholar] [CrossRef] [Green Version]
- Mierlo, L.A.J.V.; Arends, L.R.; Streppel, M.T.; A Zeegers, M.P.; Kok, F.J.; E Grobbee, D.; Geleijnse, J.M. Blood pressure response to calcium supplementation: A meta-analysis of randomized controlled trials. J. Hum. Hypertens. 2006, 20, 571–580. [Google Scholar] [CrossRef] [Green Version]
- Marie, P.J. Strontium as therapy for osteoporosis. Curr. Opin. Pharmacol. 2005, 5, 633–636. [Google Scholar] [CrossRef]
- Hackl, B.; Todt, H.; Kubista, H.; Hilber, K.; Koenig, X. Psilocybin Therapy of Psychiatric Disorders Is Not Hampered by hERG Potassium Channel–Mediated Cardiotoxicity. Int. J. Neuropsychopharmacol. 2022, 25, 280–282. [Google Scholar] [CrossRef]
- Grynpas, M.; Hamilton, E.; Cheung, R.; Tsouderos, Y.; Deloffre, P.; Hott, M.; Marie, P. Strontium increases vertebral bone volume in rats at a low dose that does not induce detectable mineralization defect. Bone 1996, 18, 253–259. [Google Scholar] [CrossRef]
- Cabrera, W.E.; Schrooten, I.; De Broe, M.E.; D’Haese, P.C. Strontium and Bone. J. Bone Miner. Res. 1999, 14, 661–668. [Google Scholar] [CrossRef]
- Schrooten, I.; Cabrera, W.; Goodman, W.G.; Dauwe, S.; Lamberts, L.V.; Marynissen, R.; Dorriné, W.; De Broe, M.E.; D’Haese, P.C. Strontium causes osteomalacia in chronic renal failure rats. Kidney Int. 1998, 54, 448–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brandi, M.L. New treatment strategies: Ipriflavone, strontium, vitamin D metabolites and analogs. Am. J. Med. 1993, 95, S69–S74. [Google Scholar] [CrossRef]
- Weaver, C. White Vegetables: A Forgotten Source of Nutrients Potassium and Health 1–3. Am. Soc. Nutr. 2013, 4, 3685–3775. [Google Scholar] [CrossRef]
- Lanham-New, S.A.; Lambert, H.; Frassetto, L. Potassium. Adv. Nutr. Int. Rev. J. 2012, 3, 820–821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandakhbayar, N.; El-Fiqi, A.; Lee, J.-H.; Kim, H.-W. Evaluation of Strontium-Doped Nanobioactive Glass Cement for Dentin–Pulp Complex Regeneration Therapy. ACS Biomater. Sci. Eng. 2019, 5, 6117–6126. [Google Scholar] [CrossRef] [PubMed]
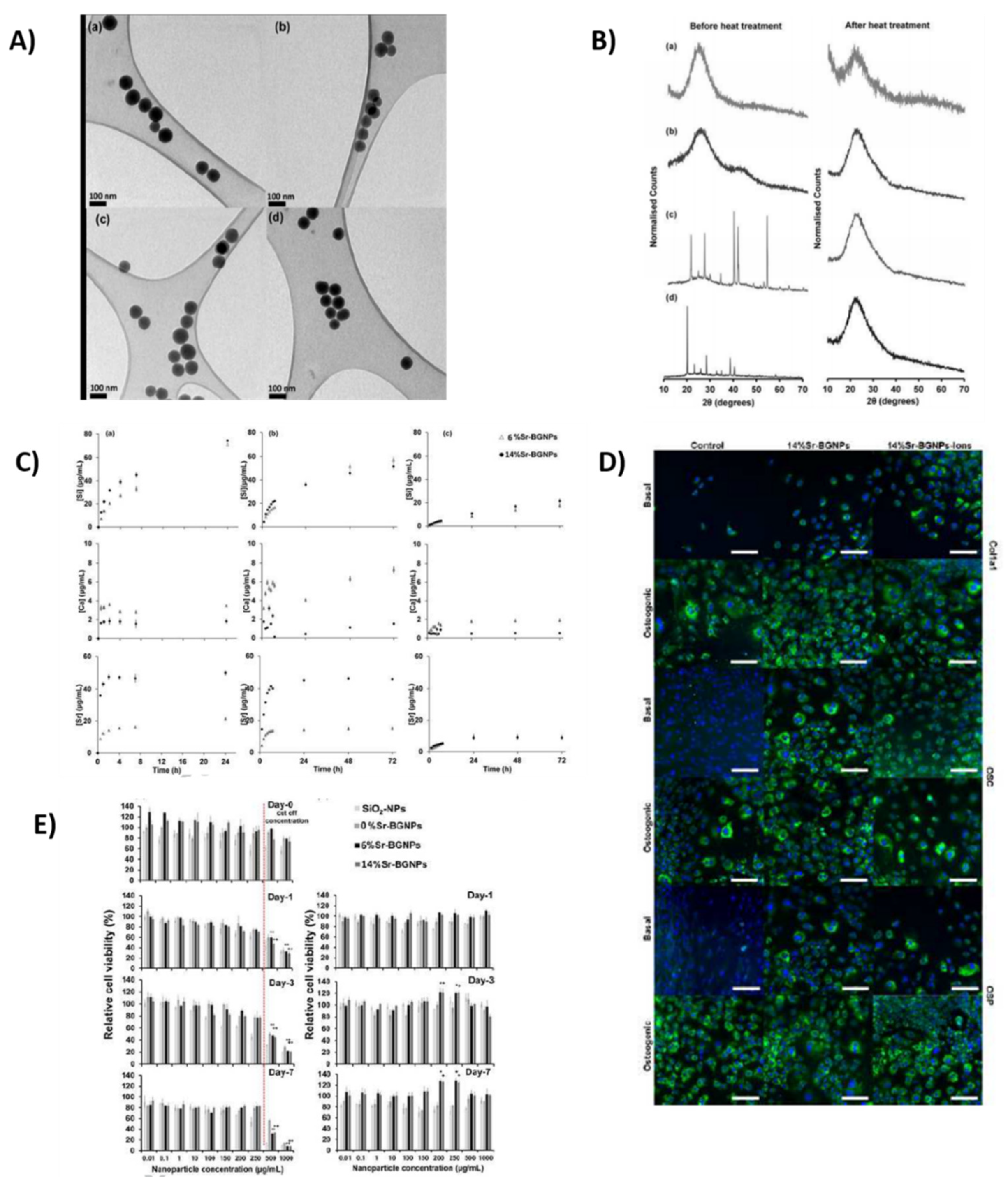
- Naruphontjirakul, P.; Porter, A.E.; Jones, J.R. In vitro osteogenesis by intracellular uptake of strontium containing bioactive glass nanoparticles. Acta Biomater. 2018, 66, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Aqib, R.; Kiani, S.; Bano, S.; Wadood, A.; Rehman, M.A.U. Ag–Sr doped mesoporous bioactive glass nanoparticles loaded chitosan/gelatin coating for orthopedic implants. Int. J. Appl. Ceram. Technol. 2021, 18, 544–562. [Google Scholar] [CrossRef]
- Saleem, O.; Wahaj, M.; Akhtar, M.A.; Rehman, M.A.U. Fabrication and Characterization of Ag–Sr-Substituted Hydroxyapatite/Chitosan Coatings Deposited via Electrophoretic Deposition: A Design of Experiment Study. ACS Omega 2020, 5, 22984–22992. [Google Scholar] [CrossRef]
- Hoppe, A.; Sarker, B.; Detsch, R.; Hild, N.; Mohn, D.; Stark, W.; Boccaccini, A. In vitro reactivity of Sr-containing bioactive glass (type 1393) nanoparticles. J. Non-Crystalline Solids 2014, 387, 41–46. [Google Scholar] [CrossRef]
- Bano, S.; Akhtar, M.; Yasir, M.; Maqbool, M.S.; Niaz, A.; Wadood, A.; Rehman, M.U. Synthesis and Characterization of Silver–Strontium (Ag-Sr)-Doped Mesoporous Bioactive Glass Nanoparticles. Gels 2021, 7, 34. [Google Scholar] [CrossRef]
- Khaliq, H.; Juming, Z.; Ke-Mei, P. The Physiological Role of Boron on Health. Biol. Trace Elem. Res. 2018, 186, 31–51. [Google Scholar] [CrossRef] [PubMed]
- Kargozar, S.; Montazerian, M.; Fiume, E.; Baino, F. Multiple and Promising Applications of Strontium (Sr)-Containing Bioactive Glasses in Bone Tissue Engineering. Front. Bioeng. Biotechnol. 2019, 7, 161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kot, F.S. Boron sources, speciation and its potential impact on health. Rev. Environ. Sci. Bio/Technol. 2009, 8, 3–28. [Google Scholar] [CrossRef]
- Growth, B.E. Variables, Studies of the Interaction Between Boron and Calcium, and its Modification by Magnesium and Potassium. Rats 1992, 35, 225–237. [Google Scholar]
- Naruphontjirakul, P.; Tsigkou, O.; Li, S.; Porter, A.E.; Jones, J.R. Human mesenchymal stem cells differentiate into an osteogenic lineage in presence of strontium containing bioactive glass nanoparticles. Acta Biomater. 2019, 90, 373–392. [Google Scholar] [CrossRef] [PubMed]
- Ghanizadeh, G.; Babaei, M.; Naghii, M.R.; Mofid, M.; Torkaman, G.; Hedayati, M. The effect of supplementation of calcium, vitamin D, boron, and increased fluoride intake on bone mechanical properties and metabolic hormones in rat. Toxicol. Ind. Health 2012, 30, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Devirian, T.A.; Volpe, S.L. The Physiological Effects of Dietary Boron. Crit. Rev. Food Sci. Nutr. 2003, 43, 219–231. [Google Scholar] [CrossRef]
- Barranco, W.T.; Eckhert, C.D. Boric acid inhibits human prostate cancer cell proliferation. Cancer Lett. 2004, 216, 21–29. [Google Scholar] [CrossRef]
- Uysal, T.; Ustdal, A.; Sonmez, M.F.; Ozturk, F. Stimulation of bone formation by dietary boron in an orthoped cally expanded suture in rabbits. Angle Orthod. 2009, 79, 984–990. [Google Scholar] [CrossRef] [Green Version]
- Gümüşderelioğlu, M.; Tunçay, E.; Kaynak, G.; Demirtaş, T.T.; Aydın, S.T.; Hakkı, S.S. Encapsulated boron as an osteoinductive agent for bone scaffolds. J. Trace Elem. Med. Biol. 2015, 31, 120–128. [Google Scholar] [CrossRef]
- Hakki, S.S.; Bozkurt, B.S.; Hakki, E.E. Boron regulates mineralized tissue-associated proteins in osteoblasts (MC3T3-E1). J. Trace Elem. Med. Biol. 2010, 24, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Omri, N.; Bu, Y. Azomethine ylide addition impact on functionalized Fullerene and Boron-Nitride: Anticancer Doxorubicin and Boronic Chalcone drugs binding characteristics with mono- and bis-nanocarriers. Colloids Surf. B Biointerfaces 2020, 196, 111277. [Google Scholar] [CrossRef] [PubMed]
- Duverger, E.; Balme, S.; Bechelany, M.; Miele, P.; Picaud, F. Natural payload delivery of the doxorubicin anticancer drug from boron nitride oxide nanosheets. Appl. Surf. Sci. 2019, 475, 666–675. [Google Scholar] [CrossRef]
- Vatanparast, M.; Shariatinia, Z. Hexagonal boron nitride nanosheet as novel drug delivery system for anticancer drugs: Insights from DFT calculations and molecular dynamics simulations. J. Mol. Graph. Model. 2019, 89, 50–59. [Google Scholar] [CrossRef]
- Sharker, S. Hexagonal Boron Nitrides (White Graphene): A Promising Method for Cancer Drug Delivery. Int. J. Nanomed. 2019, 14, 9983–9993. [Google Scholar] [CrossRef] [Green Version]
- Iyigundogdu, Z.U.; Demir, O.; Asutay, A.B.; Sahin, F. Developing Novel Antimicrobial and Antiviral Textile Products. Appl. Biochem. Biotechnol. 2017, 181, 1155–1166. [Google Scholar] [CrossRef] [PubMed]
- Erper, I.; Yıldırım, E.; Türkkan, M. Antifungal Effect of Boron Compounds Against Three Rhizoctonia solani AG-4 Subgroups Causing Root and Crown Rot. Gesunde Pflanz. 2019, 71, 61–71. [Google Scholar] [CrossRef]
- Alnoman, R.B.; Parveen, S.; Hagar, M.; Ahmed, H.A.; Knight, J.G. A new chiral boron-dipyrromethene (BODIPY)-based fluorescent probe: Molecular docking, DFT, antibacterial and antioxidant approaches. J. Biomol. Struct. Dyn. 2020, 38, 5429–5442. [Google Scholar] [CrossRef]
- Frontistis, Z.; Antonopoulou, M.; Venieri, D.; Konstantinou, I.; Mantzavinos, D. Boron-doped diamond oxidation of amoxicillin pharmaceutical formulation: Statistical evaluation of operating parameters, reaction pathways and antibacterial activity. J. Environ. Manag. 2017, 195, 100–109. [Google Scholar] [CrossRef]
- Sopchenski, L.; Cogo, S.; Dias-Ntipanyj, M.; Elifio-Espósito, S.; Popat, K.; Soares, P. Bioactive and antibacterial boron doped TiO2 coating obtained by PEO. Appl. Surf. Sci. 2018, 458, 49–58. [Google Scholar] [CrossRef]
- Leśnikowski, Z.J. Recent developments with boron as a platform for novel drug design. Expert Opin. Drug Discov. 2016, 11, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Benderdour, M.; Hess, K.; Dzondo-Gadet, M.; Nabet, P.; Belleville, F.; Dousset, B. Boron Modulates Extracellular Matrix and TNFα Synthesis in Human Fibroblasts. Biochem. Biophys. Res. Commun. 1998, 246, 746–751. [Google Scholar] [CrossRef] [PubMed]
- Benderdour, M.; Van Bui, T.; Hess, K.; Dicko, A.; Belleville, F.; Dousset, B. Effects of boron derivatives on extracellular matrix formation. J. Trace Elements Med. Biol. 2000, 14, 168–173. [Google Scholar] [CrossRef]
- Nzietchueng, R.M.; Dousset, B.; Franck, P.; Benderdour, M.; Nabet, P.; Hess, K. Mechanisms implicated in the effects of boron on wound healing. J. Trace Elements Med. Biol. 2002, 16, 239–244. [Google Scholar] [CrossRef]
- Wang, G.; Ye, J.; Wang, M.; Qi, Y.; Zhang, S.; Shi, L.; Fang, Y.; Tian, Y.; Ning, G. Copper boron–imidazolate framework incorporated chitosan membranes for bacterial-infected wound healing dressing. Carbohydr. Polym. 2022, 291. [Google Scholar] [CrossRef] [PubMed]
- Arango-Ospina, M.; Hupa, L.; Boccaccini, A.R. Bioactivity and dissolution behavior of boron-containing bioactive glasses under static and dynamic conditions in different media. Biomed. Glas. 2019, 5, 124–139. [Google Scholar] [CrossRef]
- Khodke, P.B.; Popat, R.R.; Burakale, P.V.; Chinchole, P.P.; Shrikhande, V.N. Silver nanoparticles-A review. Res. J. Pharm. Technol. 2017, 10, 1820–1833. Available online: https://indianjournals.com/ijor.aspx?target=ijor:rjpt&volume=10&issue=6&article=044 (accessed on 7 April 2022). [CrossRef]
- Hou, J.; Tamura, Y.; Lu, H.-Y.; Takahashi, Y.; Kasugai, S.; Nakata, H.; Kuroda, S. An In Vitro Evaluation of Selenium Nanoparticles on Osteoblastic Differentiation and Antimicrobial Properties against Porphyromonas gingivalis. Nanomaterials 2022, 12, 1850. [Google Scholar] [CrossRef]
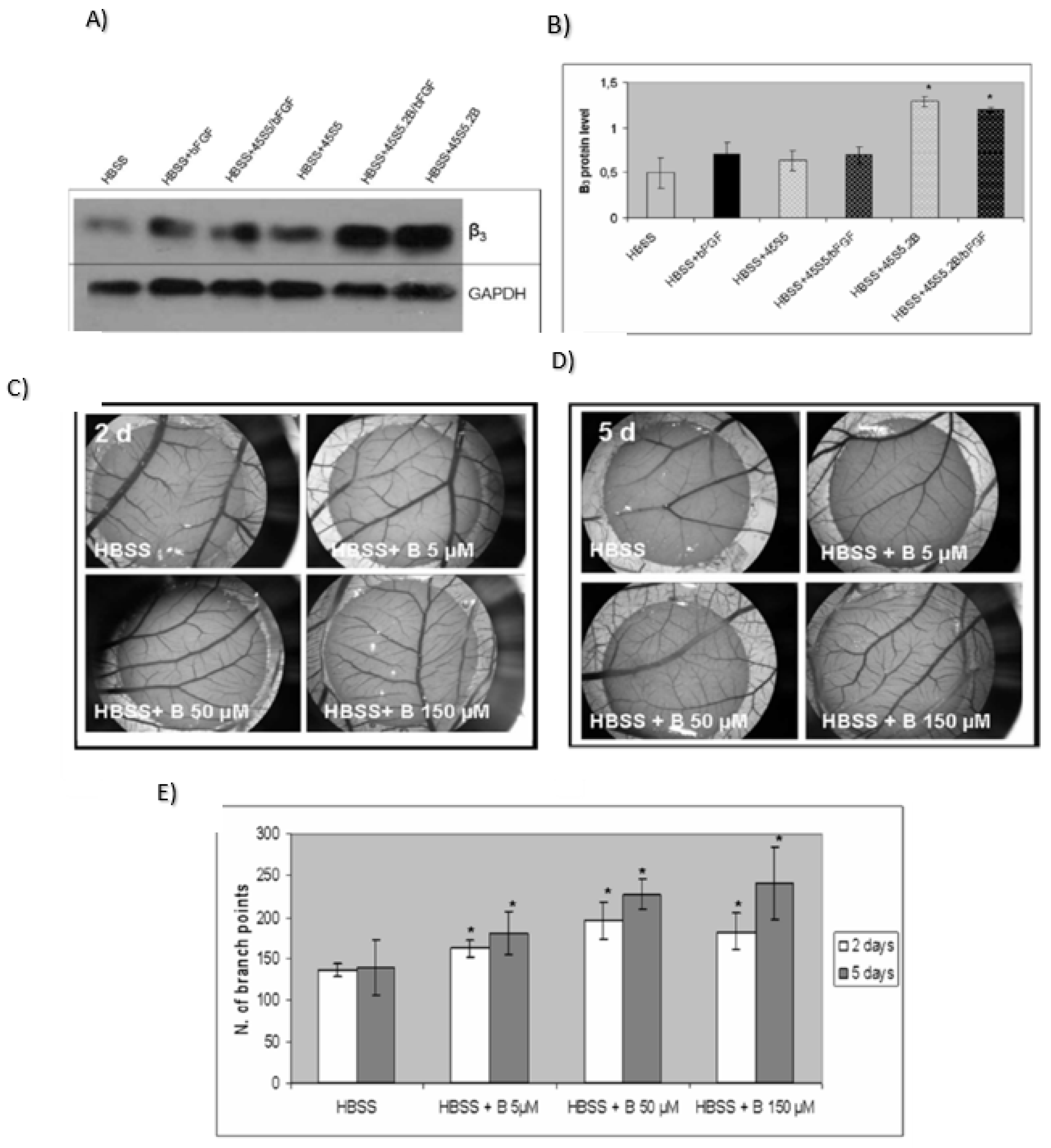
- Haro Durand, L.A.; Vargas, G.E.; Romero, N.M.; Vera-Mesones, R.; Porto-López, J.M.; Boccaccini, A.R.; Zago, M.P.; Baldi, A.; Gorustovich, A. Angiogenic effects of ionic dissolution products released from a boron-doped 45S5 bioactive glass. J. Mater. Chem. B 2015, 3, 1142–1148. [Google Scholar] [CrossRef] [Green Version]
- Sondi, I.; Salopek-Sondi, B. Silver nanoparticles as antimicrobial agent: A case study on E. coli as a model for Gram-negative bacteria. J. Colloid Interface Sci. 2004, 275, 177–182. [Google Scholar] [CrossRef]
- Panacek, A.; Kvitek, L.; Prucek, R.; Kolar, M.; Vecerova, R.; Pizurova, N.; Sharma, V.K.; Nevecna, T.; Zboril, R. Silver colloid nanoparticles: Synthesis, characterization, and their antibacterial activity. J. Phys. Chem. B 2006, 110, 16248–16253. [Google Scholar] [CrossRef] [PubMed]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramírez, J.T.; Yacaman, M.J. The bactericidal effect of silver nanoparticles. Nanotechnology 2005, 16, 2346–2353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baker, C.; Pradhan, A.; Pakstis, L.; Pochan, D.; Shah, S.I. Synthesis and Antibacterial Properties of Silver Nanoparticles. J. Nanosci. Nanotechnol. 2005, 5, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Pandian, S.R.K.; Deepak, V.; Kalishwaralal, K.; Viswanathan, P.; Gurunathan, S. Mechanism of bactericidal activity of Silver Nitrate - a concentration dependent bi-functional molecule. Braz. J. Microbiol. 2010, 41, 805–809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoppe, A.; Güldal, N.S.; Boccaccini, A.R. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials 2011, 32, 2757–2774. [Google Scholar] [CrossRef]
- Qing, T.; Mahmood, M.; Zheng, Y.; Biris, A.S.; Shi, L.; Casciano, D.A. A genomic characterization of the influence of silver nanoparticles on bone differentiation in MC3T3-E1 cells. J. Appl. Toxicol. 2017, 38, 172–179. [Google Scholar] [CrossRef]
- Shrivastava, S.; Bera, T.; Roy, A.; Singh, G.; Ramachandrarao, P.; Dash, D. Characterization of enhanced antibacterial effects of novel silver nanoparticles. Nanotechnology 2007, 18, 225103. [Google Scholar] [CrossRef]
- Shimabukuro, M.; Hayashi, K.; Kishida, R.; Tsuchiya, A.; Ishikawa, K. No-Observed-Effect Level of Silver Phosphate in Carbonate Apatite Artificial Bone on Initial Bone Regeneration. ACS Infect. Dis. 2021, 8, 159–169. [Google Scholar] [CrossRef]
- Burdușel, A.-C.; Gherasim, O.; Grumezescu, A.M.; Mogoantă, L.; Ficai, A.; Andronescu, E. Biomedical Applications of Silver Nanoparticles: An Up-to-Date Overview. Nanomaterials 2018, 8, 681. [Google Scholar] [CrossRef] [Green Version]
- Rehman, M.A.U.; Ferraris, S.; Goldmann, W.H.; Perero, S.; Bastan, F.E.; Nawaz, Q.; di Confiengo, G.G.; Ferraris, M.; Boccaccini, A.R. Antibacterial and Bioactive Coatings Based on Radio Frequency Co-Sputtering of Silver Nanocluster-Silica Coatings on PEEK/Bioactive Glass Layers Obtained by Electrophoretic Deposition. ACS Appl. Mater. Interfaces 2017, 9, 32489–32497. [Google Scholar] [CrossRef]
- Nawaz, Q.; Fastner, S.; Rehman, M.A.U.; Ferraris, S.; Perero, S.; di Confiengo, G.G.; Yavuz, E.; Ferraris, M.; Boccaccini, A.R. Multifunctional stratified composite coatings by electrophoretic deposition and RF co-sputtering for orthopaedic implants. J. Mater. Sci. 2021, 56, 7920–7935. [Google Scholar] [CrossRef]
- Orlowski, P.; Zmigrodzka, M.; Tomaszewska, E.; Soliwoda, K.; Czupryn, M.; Antos-Bielska, M.; Szemraj, J.; Celichowski, G.; Grobelny, J.; Krzyzowska, M. Tannic acid-modified silver nanoparticles for wound healing: The importance of size. Int. J. Nanomed. 2018, 13, 991–1007. [Google Scholar] [CrossRef] [Green Version]
- Paladini, F.; Pollini, M. Antimicrobial Silver Nanoparticles for Wound Healing Application: Progress and Future Trends. Materials 2019, 12, 2540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akther, T.; Mathipi, V.; Kumar, N.S.; Davoodbasha, M.; Srinivasan, H. Fungal-mediated synthesis of pharmaceutically active silver nanoparticles and anticancer property against A549 cells through apoptosis. Environ. Sci. Pollut. Res. 2019, 26, 13649–13657. [Google Scholar] [CrossRef] [PubMed]
- Khorshidi, H.; Haddadi, P.; Raoofi, S.; Badiee, P.; Nazhvani, A.D. Does Adding Silver Nanoparticles to Leukocyte- and Platelet-Rich Fibrin Improve Its Properties? BioMed Res. Int. 2018, 2018, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Jadhav, K.; Deore, S.; Dhamecha, D.; R, R.H.; Jagwani, S.; Jalalpure, S.; Bohara, R. Phytosynthesis of Silver Nanoparticles: Characterization, Biocompatibility Studies, and Anticancer Activity. ACS Biomater. Sci. Eng. 2018, 4, 892–899. [Google Scholar] [CrossRef]
- Abdel-Fattah, W.I.; Ali, G.W. On the anti-cancer activities of silver nanoparticles. J. Appl. Biotechnol. Bioeng. 2018, 5, 1. [Google Scholar] [CrossRef] [Green Version]
- Lansdown, A.B.G.; Mirastschijski, U.; Stubbs, N.; Scanlon, E.; Ågren, M.S. Zinc in wound healing: Theoretical, experimental, and clinical aspects. Wound Repair Regen. 2007, 15, 2–16. [Google Scholar] [CrossRef]
- Chasapis, C.T.; Loutsidou, A.C.; Spiliopoulou, C.A.; Stefanidou, M.E. Zinc and human health: An update. Arch. Toxicol. 2012, 86, 521–534. [Google Scholar] [CrossRef]
- Prasad, A.S. Discovery of Human Zinc Deficiency: Its Impact on Human Health and Disease. Adv. Nutr. 2013, 4, 176–190. [Google Scholar] [CrossRef]
- Bashandy, S.A.E.-M.; Omara, E.A.A.; Ebaid, H.; Amin, M.M.; Soliman, M.S. Role of zinc as an antioxidant and anti-inflammatory to relieve cadmium oxidative stress induced testicular damage in rats. Asian Pac. J. Trop. Biomed. 2016, 6, 1056–1064. [Google Scholar] [CrossRef] [Green Version]
- Kwun, I.-S.; Cho, Y.-E.; Lomeda, R.-A.R.; Shin, H.-I.; Choi, J.-Y.; Kang, Y.-H.; Beattie, J.H. Zinc deficiency suppresses matrix mineralization and retards osteogenesis transiently with catch-up possibly through Runx 2 modulation. Bone 2010, 46, 732–741. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, J.P.; Kanjilal, D.; Teitelbaum, M.; Lin, S.S.; Cottrell, J.A. Zinc as a Therapeutic Agent in Bone Regeneration. Materials 2020, 13, 2211. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Cockerill, I.; Wang, Y.; Qin, Y.-X.; Chang, L.; Zheng, Y.; Zhu, D. Zinc-Based Biomaterials for Regeneration and Therapy. Trends Biotechnol. 2019, 37, 428–441. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Xia, D.; Zheng, Y.; Zhu, Y.; Liu, Y.; Zhou, Y. A pure zinc membrane with degradability and osteogenesis promotion for guided bone regeneration: In vitro and in vivo studies. Acta Biomater. 2020, 106, 396–409. [Google Scholar] [CrossRef]
- Stanić, V.; Dimitrijević, S.; Antić-Stanković, J.; Mitrić, M.; Jokić, B.; Plećaš, I.B.; Raičević, S. Synthesis, characterization and antimicrobial activity of copper and zinc-doped hydroxyapatite nanopowders. Appl. Surf. Sci. 2010, 256, 6083–6089. [Google Scholar] [CrossRef]
- Su, Y.; Wang, K.; Gao, J.; Yang, Y.; Qin, Y.-X.; Zheng, Y.; Zhu, D. Enhanced cytocompatibility and antibacterial property of zinc phosphate coating on biodegradable zinc materials. Acta Biomater. 2019, 98, 174–185. [Google Scholar] [CrossRef]
- Boyd, D.; Li, H.; Tanner, D.A.; Towler, M.R.; Wall, G. The antibacterial effects of zinc ion migration from zinc-based glass polyalkenoate cements. J. Mater. Sci. Mater. Electron. 2006, 17, 489–494. [Google Scholar] [CrossRef] [Green Version]
- Basnet, S.; Mathisen, M.; Strand, T.A. Oral zinc and common childhood infections—An update. J. Trace Elem. Med. Biol. 2015, 31, 163–166. [Google Scholar] [CrossRef]
- Qamar, M.A.; Javed, M.; Shahid, S. Designing and investigation of enhanced photocatalytic and antibacterial properties of 3d (Fe, Co, Ni, Mn and Cr) metal-doped zinc oxide nanoparticles. Opt. Mater. 2022, 126, 112211. [Google Scholar] [CrossRef]
- Darai, R.; Dhakal, K.H.; Pandey, M.P. Effect of Genotype by Environment Interaction (GEI), Correlation, and GGE Biplot analysis for high concentration of grain Iron and Zinc biofortified lentils and their agronomic traits in multi-environment domains of Nepal. Fields Interests 2020, 24. [Google Scholar] [CrossRef]
- Read, S.A.; Obeid, S.; Ahlenstiel, C.; Ahlenstiel, G. The Role of Zinc in Antiviral Immunity. Adv. Nutr. Int. Rev. J. 2019, 10, 696–710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suara, R.O.; Crowe, J.E. Effect of Zinc Salts on Respiratory Syncytial Virus Replication. Antimicrob. Agents Chemother. 2004, 48, 783–790. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Zhang, L.; Wen, D.; Ding, Y. Role of physical and chemical interactions in the antibacterial behavior of ZnO nanoparticles against E. coli. Mater. Sci. Eng. C 2016, 69, 1361–1366. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Yan, Y.-L.; Liu, C.; Bo, L.; Li, G.; Wang, H.; Xu, Y.-J. Therapeutic Effect of Deferoxamine on Iron Overload-Induced Inhibition of Osteogenesis in a Zebrafish Model. Calcif. Tissue Res. 2014, 94, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Ciceri, P.; Falleni, M.; Tosi, D.; Martinelli, C.; Cannizzo, S.; Marchetti, G.; Monforte, A.D.; Bulfamante, G.; A Block, G.; Messa, P.; et al. Therapeutic Effect of Iron Citrate in Blocking Calcium Deposition in High Pi-Calcified VSMC: Role of Autophagy and Apoptosis. Int. J. Mol. Sci. 2019, 20, 5925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coban, E.; Ozdogan, M.; Timuragaoglu, A. Effect of Iron Deficiency Anemia on the Levels of Hemoglobin A1c in Nondiabetic Patients. Acta Haematol. 2004, 112, 126–128. [Google Scholar] [CrossRef]
- Li, Y.; He, H.; Yang, L.; Luo, S. Therapeutic effect of Colla corii asini on improving anemia and hemoglobin compositions in pregnant women with thalassemia. Int. J. Hematol. 2016, 104, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Masaldan, S.; Bush, A.I.; Devos, D.; Rolland, A.S.; Moreau, C. Striking while the iron is hot: Iron metabolism and ferroptosis in neurodegeneration. Free. Radic. Biol. Med. 2019, 133, 221–233. [Google Scholar] [CrossRef]
- Theurl, I.; Aigner, E.; Theurl, M.; Nairz, M.; Seifert, M.; Schroll, A.; Sonnweber, T.; Eberwein, L.; Witcher, D.R.; Murphy, A.T.; et al. Regulation of iron homeostasis in anemia of chronic disease and iron deficiency anemia: Diagnostic and therapeutic implications. Blood 2009, 113, 5277–5286. [Google Scholar] [CrossRef] [Green Version]
- Cohen-Solal, A.; Leclercq, C.; Deray, G.; Lasocki, S.; Zambrowski, J.-J.; Mebazaa, A.; de Groote, P.; Damy, T.; Galinier, M. Iron deficiency: An emerging therapeutic target in heart failure. Heart 2014, 100, 1414–1420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Yu, L.; Ding, J.; Chen, Y. Iron Metabolism in Cancer. Int. J. Mol. Sci. 2019, 20, 95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, B.R.; Bogdan, A.R.; Miyazawa, M.; Hashimoto, K.; Tsuji, Y. Siderophores in Iron Metabolism: From Mechanism to Therapy Potential. Trends Mol. Med. 2016, 22, 1077–1090. [Google Scholar] [CrossRef] [Green Version]
- Crielaard, B.J.; Lammers, T.; Rivella, S. Targeting iron metabolism in drug discovery and delivery. Nat. Rev. Drug Discov. 2017, 16, 400–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Zhabyeyev, P.; Wang, S.; Oudit, G.Y. Role of iron metabolism in heart failure: From iron deficiency to iron overload. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2018, 1865, 1925–1937. [Google Scholar] [CrossRef]
- Ganz, T. Iron in innate immunity: Starve the invaders. Curr. Opin. Immunol. 2009, 21, 63–67. [Google Scholar] [CrossRef] [Green Version]
- Machida-Sano, I.; Matsuda, Y.; Namiki, H. In vitroadhesion of human dermal fibroblasts on iron cross-linked alginate films. Biomed. Mater. 2009, 4, 025008. [Google Scholar] [CrossRef]
- Sipos, P. Formation of spherical iron(III) oxyhydroxide nanoparticles sterically stabilized by chitosan in aqueous solutions. J. Inorg. Biochem. 2003, 95, 55–63. [Google Scholar] [CrossRef]
- Haase, V.H. HIF-prolyl hydroxylases as therapeutic targets in erythropoiesis and iron metabolism. Hemodial. Int. 2017, 21 (Suppl. 1), S110–S124. [Google Scholar] [CrossRef] [Green Version]
- Heath, J.L.; Weiss, J.M.; Lavau, C.P.; Wechsler, D.S. Iron Deprivation in Cancer—Potential Therapeutic Implications. Nutrients 2013, 5, 2836–2859. [Google Scholar] [CrossRef] [Green Version]
- Manyapu, M.; Warraich, G.J.; Kugathasan, S.; Syed, S. Two-Year-Old With a Limp and Suspected Nonaccidental Injury. J. Pediatr. Gastroenterol. Nutr. 2018, 67, e11. [Google Scholar] [CrossRef] [PubMed]
- Cárdenas-Triviño, G.; Elgueta, C.; Vergara, L.; Ojeda, J.; Valenzuela, A.; Cruzat, C. Chitosan doped with nanoparticles of copper, nickel and cobalt. Int. J. Biol. Macromol. 2017, 104, 498–507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carter, K.; Lee, H.J.; Na, K.-S.; Fernandes-Cunha, G.M.; Blanco, I.J.; Djalilian, A.; Myung, D. Characterizing the impact of 2D and 3D culture conditions on the therapeutic effects of human mesenchymal stem cell secretome on corneal wound healing in vitro and ex vivo. Acta Biomater. 2019, 99, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Jiang, T.; She, Y.; Xu, J.; Li, C.; Zhou, S.; Shen, H.; Shi, H.; Liu, S. Effects of Cobalt Chloride, a Hypoxia-Mimetic Agent, on Autophagy and Atrophy in Skeletal C2C12 Myotubes. BioMed Res. Int. 2017, 2017, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Sun, P.; Zhao, X.; Yi, R.; Qian, J.; Shi, Y.; Wang, R. Gardenia jasminoides has therapeutic effects on L-NNA-induced hypertension in vivo. Mol. Med. Rep. 2017, 15, 4360–4373. [Google Scholar] [CrossRef] [Green Version]
- de Laia, A.G.S.; Barrioni, B.R.; Valverde, T.M.; de Goes, A.M.; de Sá, M.A.; Pereira, M.D.M. Therapeutic cobalt ion incorporated in poly(vinyl alcohol)/bioactive glass scaffolds for tissue engineering. J. Mater. Sci. 2020, 55, 8710–8727. [Google Scholar] [CrossRef]
- Erukainure, O.L.; Ijomone, O.M.; Oyebode, O.; Chukwuma, C.I.; Aschner, M.; Islam, S. Hyperglycemia-induced oxidative brain injury: Therapeutic effects of Cola nitida infusion against redox imbalance, cerebellar neuronal insults, and upregulated Nrf2 expression in type 2 diabetic rats. Food Chem. Toxicol. 2019, 127, 206–217. [Google Scholar] [CrossRef]
- Fucich, E.A.; Paredes, D.; A Morilak, D. Therapeutic Effects of Extinction Learning as a Model of Exposure Therapy in Rats. Neuropsychopharmacology 2016, 41, 3092–3102. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Yang, Y.; Deng, Y. Dual therapeutic cobalt-incorporated bioceramics accelerate bone tissue regeneration. Mater. Sci. Eng. C 2019, 99, 770–782. [Google Scholar] [CrossRef]
- Iqbal, S.; Fakhar-E-Alam, M.; Atif, M.; Amin, N.; Alimgeer, K.; Ali, A.; Ahmad, A.U.; Hanif, A.; Farooq, W.A. Structural, morphological, antimicrobial, and in vitro photodynamic therapeutic assessments of novel Zn+2-substituted cobalt ferrite nanoparticles. Results Phys. 2019, 15, 102529. [Google Scholar] [CrossRef]
- Kwak, J.; Choi, S.J.; Oh, W.; Yang, Y.S.; Jeon, H.B.; Jeon, E.S. Cobalt Chloride Enhances the Anti-Inflammatory Potency of Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells through the ERK-HIF-1α-MicroRNA-146a-Mediated Signaling Pathway. Stem Cells Int. 2018, 2018, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Li, Z.; Chen, L.; Hu, Y.; Hu, S.; Miao, Z.; Sun, Y.; Besenbacher, F.; Yu, M. Polyethylene glycol-modified cobalt sulfide nanosheets for high-performance photothermal conversion and photoacoustic/magnetic resonance imaging. Nano Res. 2018, 11, 2436–2449. [Google Scholar] [CrossRef]
- Silva, L.H.A.; Cruz, F.F.; Morales, M.M.; Weiss, D.J.; Rocco, P.R.M. Magnetic targeting as a strategy to enhance therapeutic effects of mesenchymal stromal cells. Stem Cell Res. Ther. 2017, 8, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, J.; Liu, Y.; Sun, J.; Zhu, H.; Chen, Y.; Dong, T.; Sang, R.; Gao, X.; Yang, W.; Deng, Y. Magnetic targeting cobalt nanowire-based multifunctional therapeutic system for anticancer treatment and angiogenesis. Colloids Surf. B Biointerfaces 2020, 194, 111217. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, E.C.; Patra, P.H.; Whalley, B.J. Therapeutic effects of cannabinoids in animal models of seizures, epilepsy, epileptogenesis, and epilepsy-related neuroprotection. Epilepsy Behav. 2017, 70, 319–327. [Google Scholar] [CrossRef] [Green Version]
- Tende, J.A.; Ezekiel, I.; Dikko, A.A.U.; Goji, A.D.T. Effect of ethanolic eleaves extract of moringa oOleifera on blood glucose levels of streptozocin-induced diabetics and normoglycemic wistar rats. Br. J. Pharmacol. Toxicol. 2011, 2, 1–4. Available online: https://www.airitilibrary.com/Publication/alDetailedMesh?docid=20442467-201102-201507070011-201507070011-1-4 (accessed on 7 April 2022).
- Chang, E.L.; Simmers, C.; Knight, D.A. Cobalt Complexes as Antiviral and Antibacterial Agents. Pharmaceuticals 2010, 3, 1711–1728. [Google Scholar] [CrossRef] [Green Version]
- Arun, T.; Verma, S.K.; Panda, P.K.; Joseyphus, J.; Jha, E.; Akbari-Fakhrabadi, A.; Sengupta, P.; Ray, D.; Benitha, V.; Jeyasubramanyan, K.; et al. Facile synthesized novel hybrid graphene oxide/cobalt ferrite magnetic nanoparticles based surface coating material inhibit bacterial secretion pathway for antibacterial effect. Mater. Sci. Eng. C 2019, 104, 109932. [Google Scholar] [CrossRef]
- Afrin, S.; Giampieri, F.; Gasparrini, M.; Forbes-Hernandez, T.Y.; Varela-López, A.; Quiles, J.L.; Mezzetti, B.; Battino, M. Chemopreventive and Therapeutic Effects of Edible Berries: A Focus on Colon Cancer Prevention and Treatment. Molecules 2016, 21, 169. [Google Scholar] [CrossRef] [Green Version]
- Baye, E.; Ukropcova, B.; Ukropec, J.; Hipkiss, A.; Aldini, G.; De Courten, B. Physiological and therapeutic effects of carnosine on cardiometabolic risk and disease. Amino Acids 2016, 48, 1131–1149. [Google Scholar] [CrossRef]
- Chakrawarti, L.; Agrawal, R.; Dang, S.; Gupta, S.; Gabrani, R. Therapeutic effects of EGCG: A patent review. Expert Opin. Ther. Pat. 2016, 26, 907–916. [Google Scholar] [CrossRef]
- Dalecki, A.G.; Crawford, C.L.; Wolschendorf, F. Copper and Antibiotics. Adv. Microb. Physiol. 2017, 70, 193–260. [Google Scholar] [CrossRef] [PubMed]
- Giampietro, R.; Spinelli, F.; Contino, M.; Colabufo, N.A. The Pivotal Role of Copper in Neurodegeneration: A New Strategy for the Therapy of Neurodegenerative Disorders. Mol. Pharm. 2018, 15, 808–820. [Google Scholar] [CrossRef] [PubMed]
- Islam, W.; Fang, J.; Imamura, T.; Etrych, T.; Subr, V.; Ulbrich, K.; Maeda, H. Augmentation of the Enhanced Permeability and Retention Effect with Nitric Oxide–Generating Agents Improves the Therapeutic Effects of Nanomedicines. Mol. Cancer Ther. 2018, 17, 2643–2653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Lin, Z.; Zhang, B.; Guo, L.; Liu, S.; Li, H.; Zhang, J.; Ye, Q. β-elemene sensitizes hepatocellular carcinoma cells to oxaliplatin by preventing oxaliplatin-induced degradation of copper transporter 1. Sci. Rep. 2016, 6, 21010. [Google Scholar] [CrossRef]
- Liu, C.; Wang, D.; Zhang, S.; Cheng, Y.; Yang, F.; Xing, Y.; Xu, T.; Dong, H.; Zhang, X. Biodegradable Biomimic Copper/Manganese Silicate Nanospheres for Chemodynamic/Photodynamic Synergistic Therapy with Simultaneous Glutathione Depletion and Hypoxia Relief. ACS Nano 2019, 13, 4267–4277. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, Z.; Li, F.; Xiao, Y.; Zhang, Y.; Bu, T.; Jia, P.; Zhe, T.; Wang, L. Multifunctional Magnetic Copper Ferrite Nanoparticles as Fenton-like Reaction and Near-Infrared Photothermal Agents for Synergetic Antibacterial Therapy. ACS Appl. Mater. Interfaces 2019, 11, 31649–31660. [Google Scholar] [CrossRef]
- Tao, W.; Zeng, X.; Wu, J.; Zhu, X.; Yu, X.; Zhang, X.; Zhang, J.; Liu, G.; Mei, L. Polydopamine-Based Surface Modification of Novel Nanoparticle-Aptamer Bioconjugates for In Vivo Breast Cancer Targeting and Enhanced Therapeutic Effects. Theranostics 2016, 6, 470–484. [Google Scholar] [CrossRef]
- Lu, X.; Gao, S.; Lin, H.; Yu, L.; Han, Y.; Zhu, P.; Bao, W.; Yao, H.; Chen, Y.; Shi, J. Bioinspired Copper Single-Atom Catalysts for Tumor Parallel Catalytic Therapy. Adv. Mater. 2020. [Google Scholar] [CrossRef]
- Oliver, S.; Vittorio, O.; Cirillo, G.; Boyer, C. Enhancing the therapeutic effects of polyphenols with macromolecules. Polym. Chem. 2016, 7, 1529–1544. [Google Scholar] [CrossRef]
- Rao, P.V.; Krishnan, K.T.; Salleh, N.; Gan, S.H. Biological and therapeutic effects of honey produced by honey bees and stingless bees: A comparative review. Rev. Bras. de Farm. 2016, 26, 657–664. [Google Scholar] [CrossRef] [Green Version]
- Yu, C.; Qian, L.; Ge, J.; Fu, J.; Yuan, P.; Yao, S.C.L.; Yao, S.Q. Cell-Penetrating Poly(disulfide) Assisted Intracellular Delivery of Mesoporous Silica Nanoparticles for Inhibition of miR-21 Function and Detection of Subsequent Therapeutic Effects. Angew. Chem. Int. Ed. 2016, 55, 9272–9276. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Clavijo, A.; Hurt, A.P.; Kotha, A.K.; Coleman, N.J. Effect of Calcium Precursor on the Bioactivity and Biocompatibility of Sol-Gel-Derived Glasses. J. Funct. Biomater. 2019, 10, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tokuda, E.; Furukawa, Y. Copper Homeostasis as a Therapeutic Target in Amyotrophic Lateral Sclerosis with SOD1 Mutations. Int. J. Mol. Sci. 2016, 17, 636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xi, J.; Wei, G.; An, L.; Xu, Z.; Xu, Z.; Fan, L.; Gao, L. Copper/Carbon Hybrid Nanozyme: Tuning Catalytic Activity by the Copper State for Antibacterial Therapy. Nano Lett. 2019, 19, 7645–7654. [Google Scholar] [CrossRef]
- Lun, X.; Wells, J.C.; Grinshtein, N.; King, J.C.; Hao, X.; Dang, N.-H.; Wang, X.; Aman, A.; Uehling, D.; Datti, A.; et al. Disulfiram when Combined with Copper Enhances the Therapeutic Effects of Temozolomide for the Treatment of Glioblastoma. Clin. Cancer Res. 2016, 22, 3860–3875. [Google Scholar] [CrossRef] [Green Version]
- Barrioni, B.R.; Naruphontjirakul, P.; Norris, E.; Li, S.; Kelly, N.L.; Hanna, J.V.; Stevens, M.M.; Jones, J.R.; Pereira, M.D.M. Effects of manganese incorporation on the morphology, structure and cytotoxicity of spherical bioactive glass nanoparticles. J. Colloid Interface Sci. 2019, 547, 382–392. [Google Scholar] [CrossRef]
- Shin, S.-W.; Choi, C.; Lee, G.-H.; Son, A.; Kim, S.-H.; Park, H.C.; Batinic-Haberle, I.; Park, W. Mechanism of the Antitumor and Radiosensitizing Effects of a Manganese Porphyrin, MnHex-2-PyP. Antioxidants Redox Signal. 2017, 27, 1067–1082. [Google Scholar] [CrossRef]
- Sheng, Y.; Li, H.; Liu, M.; Xie, B.; Wei, W.; Wu, J.; Meng, F.; Wang, H.Y.; Chen, S. A Manganese-Superoxide Dismutase From Thermus thermophilus HB27 Suppresses Inflammatory Responses and Alleviates Experimentally Induced Colitis. Inflamm. Bowel Dis. 2019, 25, 1644–1655. [Google Scholar] [CrossRef]
- Xiang, J.; Zhang, J.; Cai, X.; Yang, F.; Zhu, W.; Zhang, W.; Cai, M.; Yu, Z.; Li, X.; Wu, T.; et al. Bilobalide protects astrocytes from oxygen and glucose deprivation-induced oxidative injury by upregulating manganese superoxide dismutase. Phytother. Res. 2019, 33, 2329–2336. [Google Scholar] [CrossRef]
- Zhou, B.; Zhao, J.; Qiao, Y.; Wei, Q.; He, J.; Li, W.; Zhong, D.; Ma, F.; Li, Y.; Zhou, M. Simultaneous multimodal imaging and photothermal therapy via renal-clearable manganese-doped copper sulfide nanodots. Appl. Mater. Today 2018, 13, 285–297. [Google Scholar] [CrossRef]
- Barrioni, B.R.; Norris, E.; Li, S.; Naruphontjirakul, P.; Jones, J.R.; Pereira, M.D.M. Osteogenic potential of sol–gel bioactive glasses containing manganese. J. Mater. Sci. Mater. Med. 2019, 30, 1–15. [Google Scholar] [CrossRef]
- Cho, M.H.; Choi, E.-S.; Kim, S.; Goh, S.-H.; Choi, Y. Redox-Responsive Manganese Dioxide Nanoparticles for Enhanced MR Imaging and Radiotherapy of Lung Cancer. Front. Chem. 2017, 5, 109. [Google Scholar] [CrossRef] [PubMed]
- da Silva, L.H.A.; Antunes, M.A.; Dos Santos, C.C.; Weiss, D.J.; Cruz, F.F.; Rocco, P.R.M. Strategies to improve the therapeutic effects of mesenchymal stromal cells in respiratory diseases. Stem Cell Res. Ther. 2018, 9, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Ding, X.; Liu, J.; Li, J.; Wang, F.; Wang, Y.; Song, S.; Zhang, H. Polydopamine coated manganese oxide nanoparticles with ultrahigh relaxivity as nanotheranostic agents for magnetic resonance imaging guided synergetic chemo-/photothermal therapy. Chem. Sci. 2016, 7, 6695–6700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ollivier, A.; Foresti, R.; El Ali, Z.; Martens, T.; Kitagishi, H.; Motterlini, R.; Rivard, M. Design and Biological Evaluation of Manganese- and Ruthenium-Based Hybrid CO-RMs (HYCOs). ChemMedChem 2019, 14, 1684–1691. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Liang, C.; Zhu, J.; Yang, N.; Jiao, A.; Wang, W.; Song, X.; Dong, X. Manganese-Based Nanoplatform As Metal Ion-Enhanced ROS Generator for Combined Chemodynamic/Photodynamic Therapy. ACS Appl. Mater. Interfaces 2019, 11, 41140–41147. [Google Scholar] [CrossRef]
- Feng, Y.; Ding, D.; Sun, W.; Qiu, Y.; Luo, L.; Shi, T.; Meng, S.; Chen, X.; Chen, H. Magnetic Manganese Oxide Sweetgum-Ball Nanospheres with Large Mesopores Regulate Tumor Microenvironments for Enhanced Tumor Nanotheranostics. ACS Appl. Mater. Interfaces 2019, 11, 37461–37470. [Google Scholar] [CrossRef]
- Fu, L.-H.; Hu, Y.-R.; Qi, C.; He, T.; Jiang, S.; Jiang, C.; He, J.; Qu, J.; Lin, J.; Huang, P. Biodegradable Manganese-Doped Calcium Phosphate Nanotheranostics for Traceable Cascade Reaction-Enhanced Anti-Tumor Therapy. ACS Nano 2019, 13, 13985–13994. [Google Scholar] [CrossRef]
- Kim, J.; Kim, H.Y.; Song, S.Y.; Go, S.-H.; Sohn, H.S.; Baik, S.; Soh, M.; Kim, K.; Kim, D.; Kim, H.-C.; et al. Synergistic Oxygen Generation and Reactive Oxygen Species Scavenging by Manganese Ferrite/Ceria Co-decorated Nanoparticles for Rheumatoid Arthritis Treatment. ACS Nano 2019, 13, 3206–3217. [Google Scholar] [CrossRef]
- He, T.; Qin, X.; Jiang, C.; Jiang, D.; Lei, S.; Lin, J.; Zhu, W.-G.; Qu, J.; Huang, P. Tumor pH-responsive metastable-phase manganese sulfide nanotheranostics for traceable hydrogen sulfide gas therapy primed chemodynamic therapy. Theranostics 2020, 10, 2453–2462. [Google Scholar] [CrossRef]
- Nawaz, Q.; Rehman, M.A.U.; Burkovski, A.; Schmidt, J.; Beltrán, A.M.; Shahid, A.; Alber, N.K.; Peukert, W.; Boccaccini, A.R. Synthesis and characterization of manganese containing mesoporous bioactive glass nanoparticles for biomedical applications. J. Mater. Sci. Mater. Med. 2018, 29, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Tian, C.; Yan, Y.; Zhang, L.; Zhang, H.; Zhang, Z. Manganese-Based Nanoactivator Optimizes Cancer Immunotherapy via Enhancing Innate Immunity. ACS Nano 2020, 14, 3927–3940. [Google Scholar] [CrossRef] [PubMed]
- Lüthen, F.; Bulnheim, U.; Müller, P.D.; Rychly, J.; Jesswein, H.; Nebe, J.B. Influence of manganese ions on cellular behavior of human osteoblasts in vitro. Biomol. Eng. 2007, 24, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Fujitani, W.; Hamada, Y.; Kawaguchi, N.; Mori, S.; Daito, K.; Uchinaka, A.; Matsumoto, T.; Kojima, Y.; Daito, M.; Nakano, T.; et al. Synthesis of hydroxyapatite contining manganese and its evaluation of biocompatibility. Nano Biomed. 2010, 2, 37–46. [Google Scholar] [CrossRef]





| Group 1 | Group 2 | Transition Metals | Group 13 | Group 15 | Metalloids |
|---|---|---|---|---|---|
| Li, K | Mg, Ca, Sr | Cr, Mn, Fe, Co, Cu, Zn, Ag | Ga | Bi | B, Ge |
| Ion | Ion Function in Biological System | Ref |
|---|---|---|
| Ga |
| [1,2] [270,271] [3] [11,27,29,274] |
| Bi |
| [6] [7] [89,90] [9] [10] [11] |
| Mg |
| [17] [18] [19] [20] [21] [22] |
| Ca |
| [23] [24] [19] [25] [25] [26] [27] [28,29] |
| Ge |
| [30] [31,66] [32] [92,93] [30,66] |
| Cr |
| [35] [36] [36] |
| Li |
| [37] [38] [39] [40] [41] [42,43] [44,45] |
| K |
| [46] [47] [48] [49] [50] |
| Sr |
| [51] [52] [53] |
| B |
| [54] [55] [56] [57] [10,11] [56,60] [61] |
| Ag |
| [62,63] [64] [65,66] |
| Zn |
| [67], [68] [69] [187] [70,71] [74] |
| Fe |
| [204,211] [77] [78] [79] [75,80] [80] |
| Co |
| [81,222] [84,222,223] [86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180,181,182,183,184,185,186,187,188,189,190,191,192,193,194,195,196,197,198,199,200,201,202,203,204,205,206,207,208,209,210,211,212,213,214,215,216,217,218,219,220,221,222,223,224,225,226,227] [219] [231] |
| Cu |
| [89] [90] [91,92] [92] [93] [235,236] [245,246,247] [248] [248] |
| Mn |
| [102] [103] [104] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Awais, M.; Aizaz, A.; Nazneen, A.; Bhatti, Q.u.A.; Akhtar, M.; Wadood, A.; Atiq Ur Rehman, M. A Review on the Recent Advancements on Therapeutic Effects of Ions in the Physiological Environments. Prosthesis 2022, 4, 263-316. https://doi.org/10.3390/prosthesis4020026
Awais M, Aizaz A, Nazneen A, Bhatti QuA, Akhtar M, Wadood A, Atiq Ur Rehman M. A Review on the Recent Advancements on Therapeutic Effects of Ions in the Physiological Environments. Prosthesis. 2022; 4(2):263-316. https://doi.org/10.3390/prosthesis4020026
Chicago/Turabian StyleAwais, Muhammad, Aqsa Aizaz, Arooba Nazneen, Qurat ul Ain Bhatti, Memoona Akhtar, Abdul Wadood, and Muhammad Atiq Ur Rehman. 2022. "A Review on the Recent Advancements on Therapeutic Effects of Ions in the Physiological Environments" Prosthesis 4, no. 2: 263-316. https://doi.org/10.3390/prosthesis4020026
APA StyleAwais, M., Aizaz, A., Nazneen, A., Bhatti, Q. u. A., Akhtar, M., Wadood, A., & Atiq Ur Rehman, M. (2022). A Review on the Recent Advancements on Therapeutic Effects of Ions in the Physiological Environments. Prosthesis, 4(2), 263-316. https://doi.org/10.3390/prosthesis4020026






