Nanowire Electrode Structures Enhanced Direct Extracellular Electron Transport via Cell-Surface Multi-Heme Cytochromes in Desulfovibrio ferrophilus IS5
Abstract
1. Introduction
2. Material and Methods
2.1. Fabrication of NW Arrayed Electrodes
2.2. Cultivation of Strain IS5
2.3. SEM
2.4. Water Contact Angle Measurement
2.5. Electrochemical Analysis
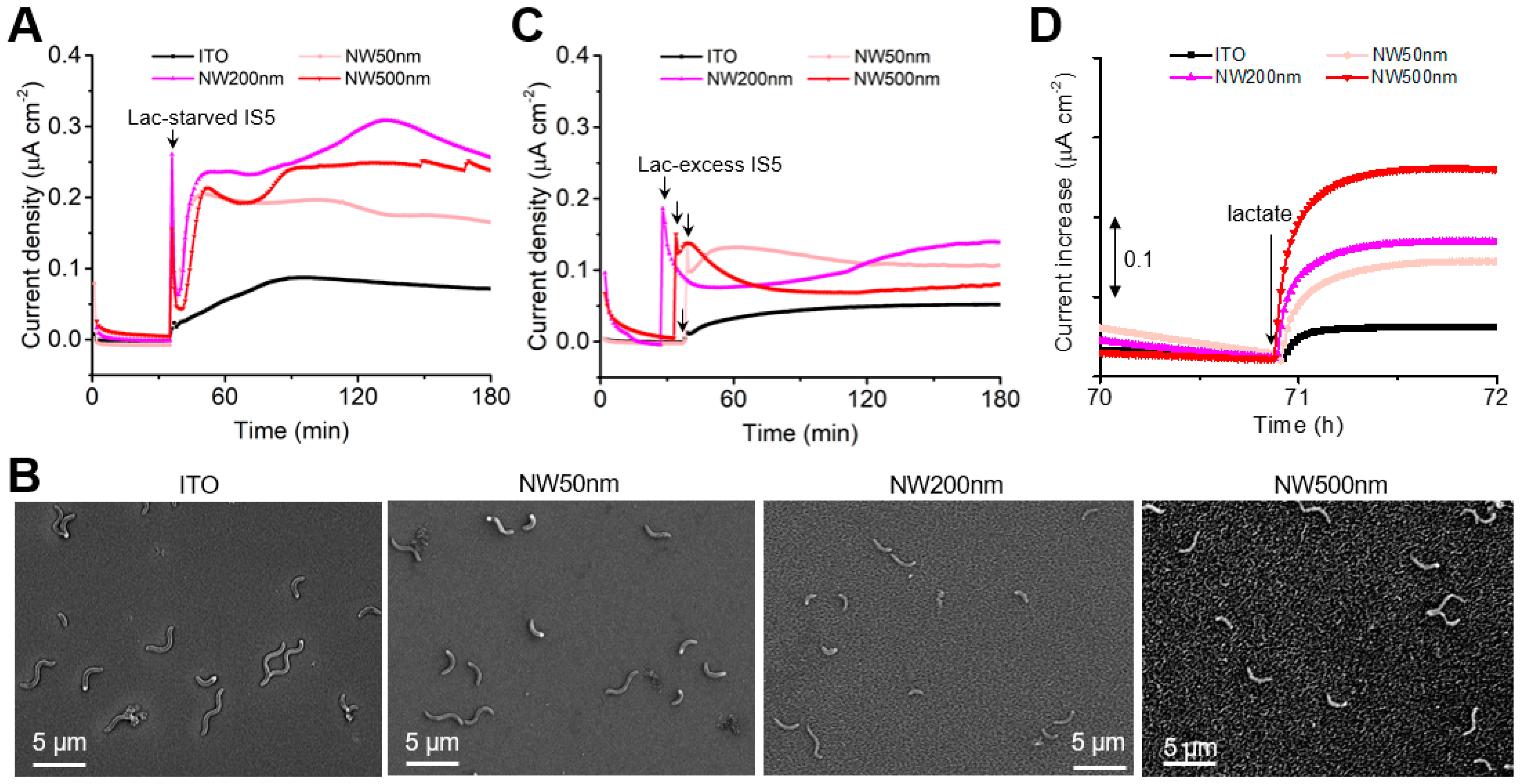
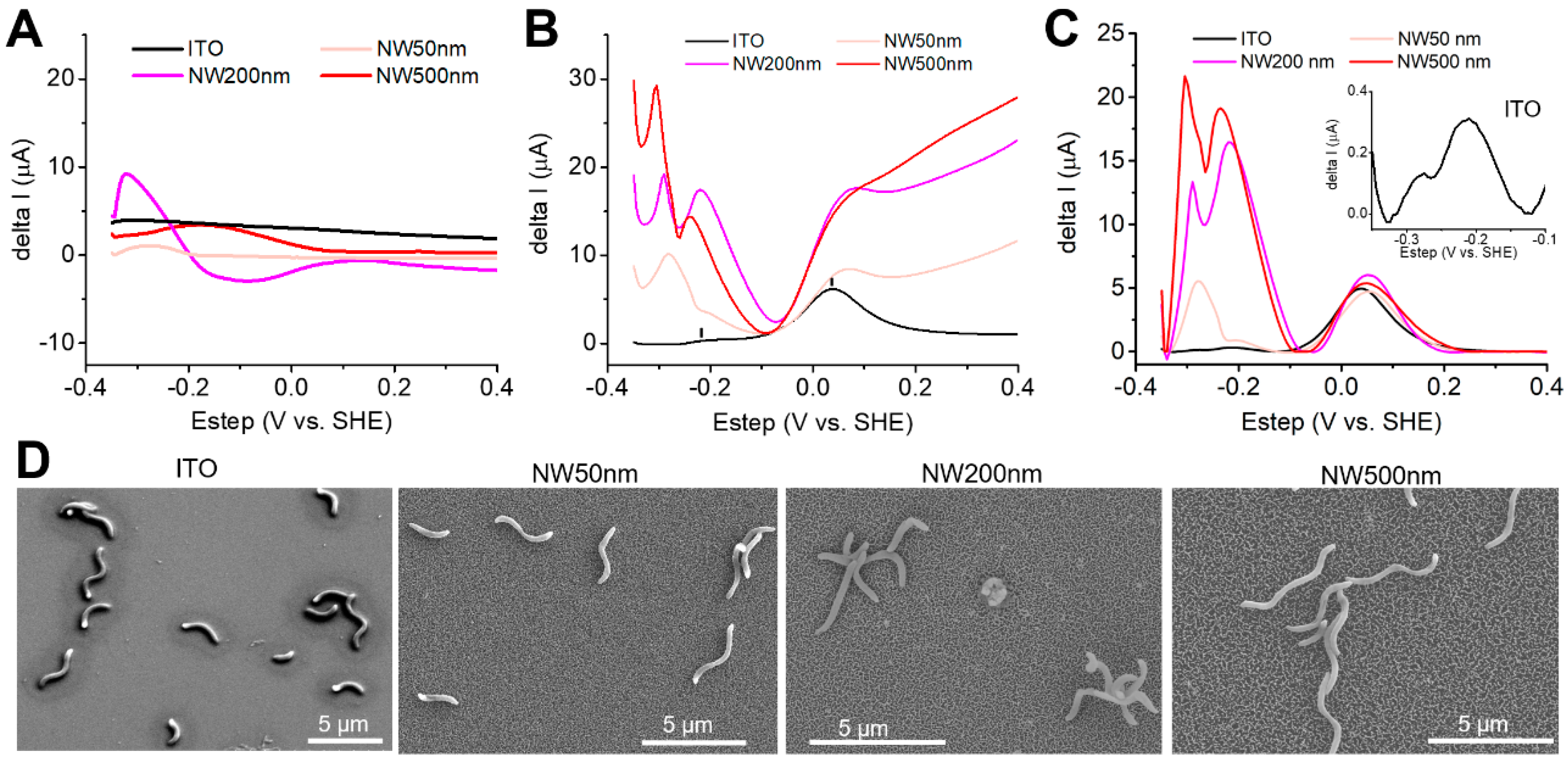
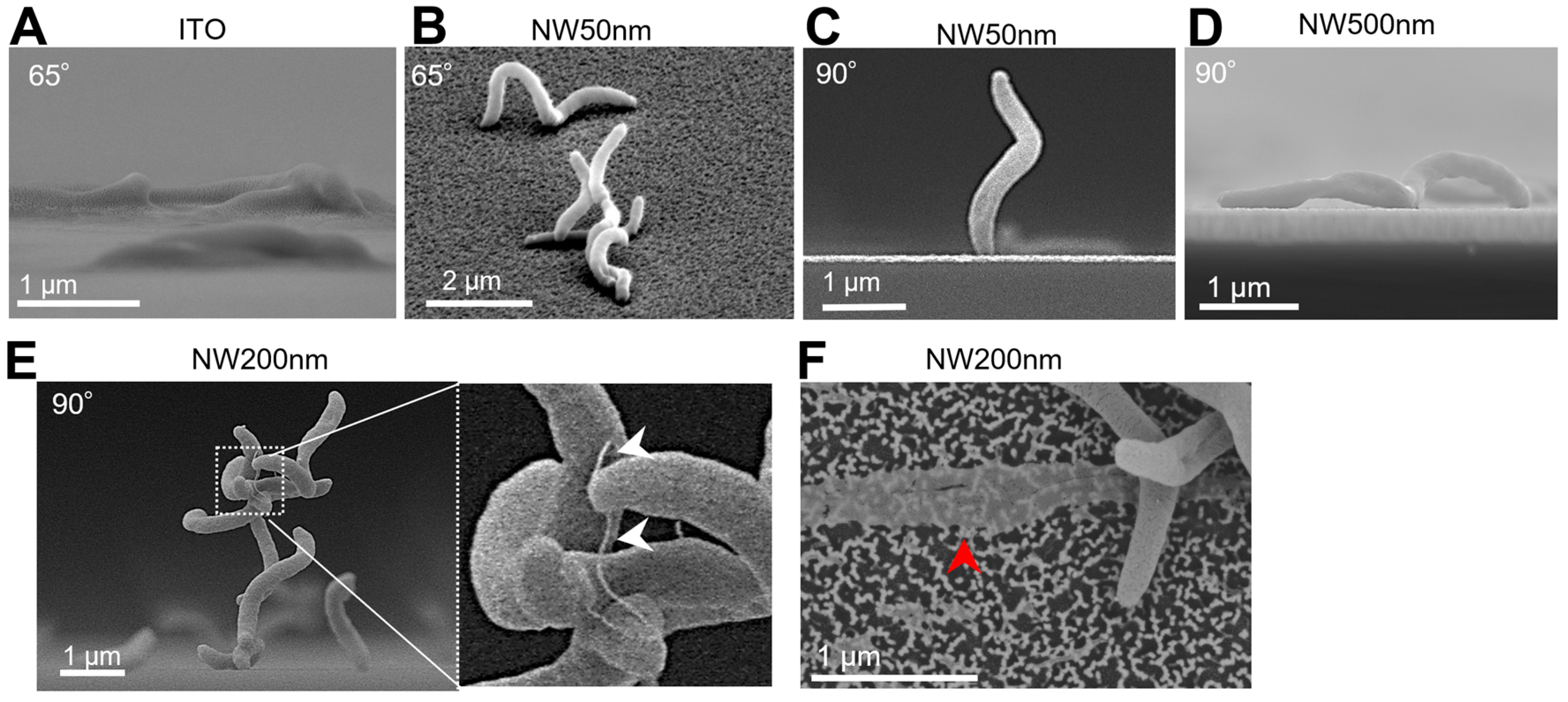
3. Results
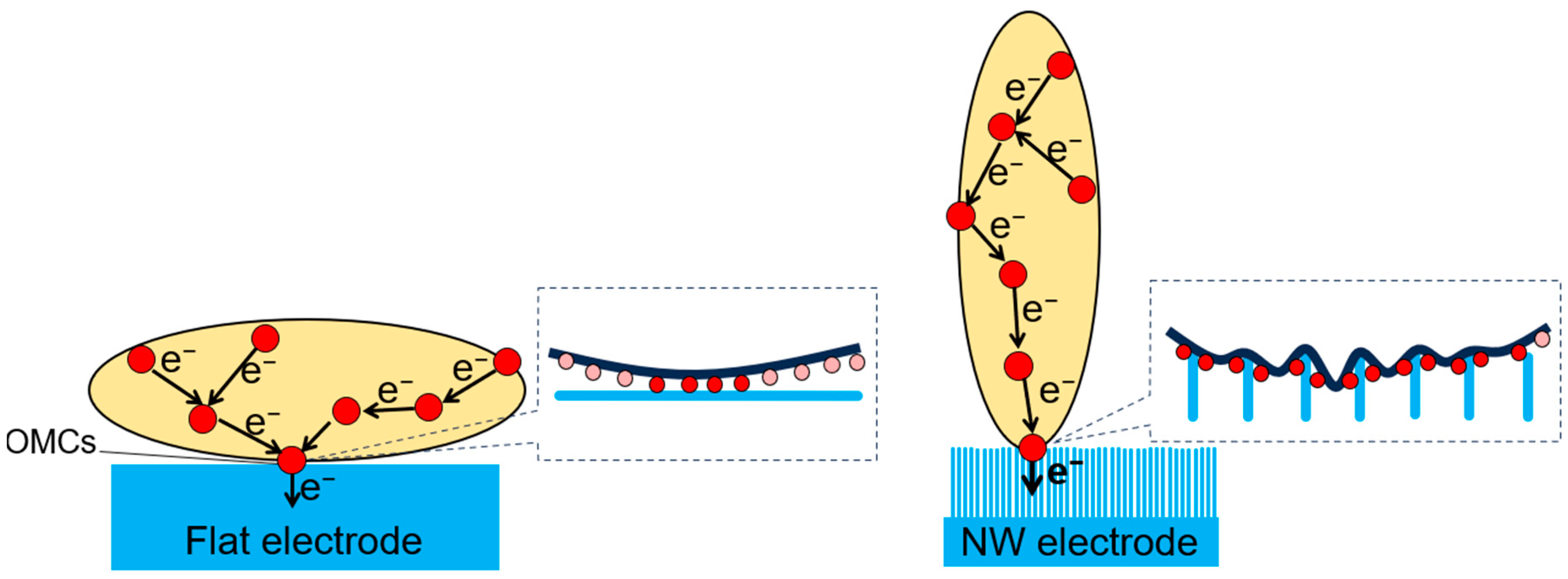
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shi, L.; Dong, H.; Reguera, G.; Beyenal, H.; Lu, A.; Liu, J.; Yu, H.-Q.; Fredrickson, J.K. Extracellular electron transfer mechanisms between microorganisms and minerals. Nat. Rev. Microbiol. 2016, 14, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Myers, C.R.; Nealson, K.H. Bacterial manganese reduction and growth with manganese oxide as the sole electron-acceptor. Science 1988, 240, 1319–1321. [Google Scholar] [CrossRef]
- Lovley, D.R.; Phillips, E.J.P. Novel Mode of Microbial Energy Metabolism: Organic Carbon Oxidation Coupled to Dissimilatory Reduction of Iron or Manganese. Appl. Environ. Microbiol. 1988, 54, 1472–1480. [Google Scholar] [CrossRef] [PubMed]
- Logan, B.E.; Rossi, R.; Ragab, A.; Saikaly, P.E. Electroactive microorganisms in bioelectrochemical systems. Nat. Rev. Microbiol. 2019, 17, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Lovley, D.R. Bug juice: Harvesting electricity with microorganisms. Nat. Rev. Microbiol. 2006, 4, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Coleman, M.L.; Hedrick, D.B.; Lovley, D.R.; White, D.C.; Pye, K. Reduction of Fe(III) in sediments by sulphate-reducing bacteria. Nature 1993, 361, 436–438. [Google Scholar] [CrossRef]
- Barton, L.L.; Fauque, G.D. Biochemistry, physiology and biotechnology of sulfate-reducing bacteria. Adv. Appl. Microbiol. 2009, 68, 41–98. [Google Scholar] [CrossRef] [PubMed]
- Murugan, M.; Miran, W.; Masuda, T.; Lee, D.S.; Okamoto, A. Biosynthesized Iron Sulfide Nanocluster Enhanced Anodic Current Generation by Sulfate Reducing Bacteria in Microbial Fuel Cells. ChemElectroChem 2018, 5, 4015–4020. [Google Scholar] [CrossRef]
- Deng, X.; Okamoto, A. Direct extracellular electron transfer to an indium tin doped oxide electrode via heme redox reactions in Desulfovibrio ferrophilus IS5. Electrochim. Acta 2023, 453, 142293. [Google Scholar] [CrossRef]
- Liang, D.; Liu, X.; Woodard, T.L.; Holmes, D.E.; Smith, J.A.; Nevin, K.P.; Feng, Y.; Lovley, D.R. Extracellular Electron Exchange Capabilities of Desulfovibrio ferrophilus and Desulfopila corrodens. Environ. Sci. Technol. 2021, 55, 16195–16203. [Google Scholar] [CrossRef]
- Wang, R.; Li, H.; Sun, J.; Zhang, L.; Jiao, J.; Wang, Q.; Liu, S. Nanomaterials Facilitating Microbial Extracellular Electron Transfer at Interfaces. Adv. Mater. 2021, 33, 2004051. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.; Chen, X.; Wu, J.; Li, N.; He, W.; Feng, Y. Tailoring Surface Properties of Electrodes for Synchronous Enhanced Extracellular Electron Transfer and Enriched Exoelectrogens in Microbial Fuel Cells. ACS Appl. Mater. Interfaces 2021, 13, 58508–58521. [Google Scholar] [CrossRef]
- Liu, X.; Wu, W.; Gu, Z. Poly (3,4-ethylenedioxythiophene) promotes direct electron transfer at the interface between Shewanella loihica and the anode in a microbial fuel cell. J. Power Sources 2015, 277, 110–115. [Google Scholar] [CrossRef]
- Bian, R.; Jiang, Y.; Wang, Y.; Sun, J.; Hu, J.; Jiang, L.; Liu, H. Highly Boosted Microbial Extracellular Electron Transfer by Semiconductor Nanowire Array with Suitable Energy Level. Adv. Funct. Mater. 2018, 28, 1707408. [Google Scholar] [CrossRef]
- Ding, C.; Liu, H.; Lv, M.; Zhao, T.; Zhu, Y.; Jiang, L. Hybrid bio-organic interfaces with matchable nanoscale topography for durable high extracellular electron transfer activity. Nanoscale 2014, 6, 7866–7871. [Google Scholar] [CrossRef]
- Rehnlund, D.; Lim, G.; Philipp, L.A.; Gescher, J. Nanowired electrodes as outer membrane cytochrome-independent electronic conduit in Shewanella oneidensis. iScience 2022, 25, 103853. [Google Scholar] [CrossRef]
- Patolsky, F.; Zheng, G.; Lieber, C.M. Nanowire Sensors for Medicine and The Life Sciences. Nanomedicine 2006, 1, 51–65. [Google Scholar] [CrossRef]
- Ding, C.-m.; Lv, M.-l.; Zhu, Y.; Jiang, L.; Liu, H. Wettability-Regulated Extracellular Electron Transfer from the Living Organism of Shewanella loihica PV-4. Angew. Chem. Int. Ed. 2015, 54, 1446–1451. [Google Scholar] [CrossRef]
- Jevasuwan, W.; Nakajima, K.; Sugimoto, Y.; Fukata, N. Metal-catalyzed electroless etching and nanoimprinting silicon nanowire-based solar cells: Silicon nanowire defect reduction and efficiency enhancement by two-step H2 annealing. Jpn. J. Appl. Phys. 2016, 55, 065001. [Google Scholar] [CrossRef]
- Jevasuwan, W.; Pradel, K.C.; Subramani, T.; Chen, J.; Takei, T.; Nakajima, K.; Sugimoto, Y.; Fukata, N. Diffused back surface field formation in combination with two-step H2 annealing for improvement of silicon nanowire-based solar cell efficiency. Jpn. J. Appl. Phys. 2017, 56, 04CP01. [Google Scholar] [CrossRef]
- Deng, X.; Dohmae, N.; Nealson, K.H.; Hashimoto, K.; Okamoto, A. Multi-heme cytochromes provide a pathway for survival in energy-limited environments. Sci. Adv. 2018, 4, eaao5682. [Google Scholar] [CrossRef] [PubMed]
- Fourmond, V. QSoas: A Versatile Software for Data Analysis. Anal. Chem. 2016, 88, 5050–5052. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Yiannacou, K.; Karjalainen, M.; Lahtonen, K.; Valden, M.; Sariola, V. Large-scale efficient water harvesting using bioinspired micro-patterned copper oxide nanoneedle surfaces and guided droplet transport. Nanoscale Adv. 2019, 1, 4025–4040. [Google Scholar] [CrossRef] [PubMed]
- Krasowska, A.; Sigler, K. How microorganisms use hydrophobicity and what does this mean for human needs? Front. Cell. Infect. Microbiol. 2014, 4, 112. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Luo, D.; Okamoto, A. Electrode hydrophilicity enhanced the rate of extracellular electron uptake in Desulfovibrio ferrophilus IS5. Electrochim. Acta 2022, 421, 140504. [Google Scholar] [CrossRef]
- Remes, A.; Manea, F.; Motoc, S.; Baciu, A.; Szerb, E.I.; Gascon, J.; Gug, G. Highly Sensitive Non-Enzymatic Detection of Glucose at MWCNT-CuBTC Composite Electrode. Appl. Sci. 2020, 10, 8419. [Google Scholar] [CrossRef]
- Dai, H.; Chen, D.; Li, Y.; Cao, P.; Wang, N.; Lin, M. Voltammetric sensing of dopamine based on a nanoneedle array consisting of NiCo2S4 hollow core-shells on a nickel foam. Mikrochim. Acta 2018, 185, 157. [Google Scholar] [CrossRef] [PubMed]
- Uwimbabazi, E.; Mukasekuru, M.R.; Sun, X. Glucose Biosensor Based on a Glassy Carbon Electrode Modified with Multi-Walled Carbon Nanotubes-Chitosan for the Determination of Beef Freshness. Food Anal. Methods 2017, 10, 2667–2676. [Google Scholar] [CrossRef]
- Jenkins, J.; Mantell, J.; Neal, C.; Gholinia, A.; Verkade, P.; Nobbs, A.H.; Su, B. Antibacterial effects of nanopillar surfaces are mediated by cell impedance, penetration and induction of oxidative stress. Nat. Commun. 2020, 11, 1626. [Google Scholar] [CrossRef]
- Leung, K.M.; Wanger, G.; El-Naggar, M.Y.; Gorby, Y.; Southam, G.; Lau, W.M.; Yang, J. Shewanella oneidensis MR-1 Bacterial Nanowires Exhibit p-Type, Tunable Electronic Behavior. Nano Lett. 2013, 13, 2407–2411. [Google Scholar] [CrossRef]
- Han, T.H.; Parveen, N.; Shim, J.H.; Nguyen, A.T.N.; Mahato, N.; Cho, M.H. Ternary Composite of Polyaniline Graphene and TiO2 as a Bifunctional Catalyst to Enhance the Performance of Both the Bioanode and Cathode of a Microbial Fuel Cell. Ind. Eng. Chem. Res. 2018, 57, 6705–6713. [Google Scholar] [CrossRef]
- Li, J.; Cui, J.; Yang, Q.; Cui, G.; Wei, B.; Wu, Z.; Wang, Y.; Zhou, H. Oxidative Weathering and Microbial Diversity of an Inactive Seafloor Hydrothermal Sulfide Chimney. Front. Microbiol. 2017, 8, 1378. [Google Scholar] [CrossRef] [PubMed]
- Khan, Y.; Durrani, S.K.; Mehmood, M.; Khan, M.R. Mild hydrothermal synthesis of γ-MnO2 nanostructures and their phase transformation to α-MnO2 nanowires. J. Mater. Res. 2011, 26, 2268–2275. [Google Scholar] [CrossRef]
- Zhu, H.; Deng, J.; Chen, J.; Yu, R.; Xing, X. Growth of hematite nanowire arrays during dense pentlandite oxidation. J. Mater. Chem. A 2014, 2, 3008–3014. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, G. Amorphous Goethite as a Catalyst of Chemoselectivity Epoxidation of Alkenes by Hydrogen Peroxide. Russ. J. Gen. Chem. 2022, 92, 1539–1545. [Google Scholar] [CrossRef]
- Okamoto, A.; Hashimoto, K.; Nealson, K.H. Flavin redox bifurcation as a mechanism for controlling the direction of electron flow during extracellular electron transfer. Angew. Chem. Int. Ed. 2014, 53, 10988–10991. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Zhao, Z.; Peng, L.; Shiu, H.-Y.; Ding, M.; Song, F.; Guan, X.; Lee, C.K.; Huang, J.; Zhu, D.; et al. Silver nanoparticles boost charge-extraction efficiency in Shewanella microbial fuel cells. Science 2021, 373, 1336–1340. [Google Scholar] [CrossRef] [PubMed]
- Seong, H.; Higgins, S.G.; Penders, J.; Armstrong, J.P.K.; Crowder, S.W.; Moore, A.C.; Sero, J.E.; Becce, M.; Stevens, M.M. Size-Tunable Nanoneedle Arrays for Influencing Stem Cell Morphology, Gene Expression, and Nuclear Membrane Curvature. ACS Nano 2020, 14, 5371–5381. [Google Scholar] [CrossRef]
- Ivanova, E.P.; Hasan, J.; Webb, H.K.; Gervinskas, G.; Juodkazis, S.; Truong, V.K.; Wu, A.H.F.; Lamb, R.N.; Baulin, V.A.; Watson, G.S.; et al. Bactericidal activity of black silicon. Nat. Commun. 2013, 4, 2838. [Google Scholar] [CrossRef]
- Nakamura, H.; Watano, S. Direct Permeation of Nanoparticles across Cell Membrane: A Review. KONA Powder Part. J. 2018, 35, 49–65. [Google Scholar] [CrossRef]
- Zou, J.; Li, J.; Chen, T.; Li, X. Penetration mechanism of cells by vertical nanostructures. Phys. Rev. E 2020, 102, 052401. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Xu, A.M.; Angle, M.R.; Tayebi, N.; Verma, P.; Melosh, N.A. Mechanical Model of Vertical Nanowire Cell Penetration. Nano Lett. 2013, 13, 6002–6008. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, M.Y.; Wanger, G.; Leung, K.M.; Yuzvinsky, T.D.; Southam, G.; Yang, J.; Lau, W.M.; Nealson, K.H.; Gorby, Y.A. Electrical transport along bacterial nanowires from Shewanella oneidensis MR-1. Proc. Natl. Acad. Sci. USA 2010, 107, 18127–18131. [Google Scholar] [CrossRef]
- Gorby, Y.A.; Yanina, S.; McLean, J.S.; Rosso, K.M.; Moyles, D.; Dohnalkova, A.; Beveridge, T.J.; Chang, I.S.; Kim, B.H.; Kim, K.S.; et al. Electrically conductive bacterial nanowires produced by Shewanella oneidensis strain MR-1 and other microorganisms. Proc. Natl. Acad. Sci. USA 2006, 103, 11358–11363. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, P.; Pirbadian, S.; El-Naggar, M.Y.; Jensen, G.J. Ultrastructure of Shewanella oneidensis MR-1 nanowires revealed by electron cryotomography. Proc. Natl. Acad. Sci. USA 2018, 115, E3246–E3255. [Google Scholar] [CrossRef]
- Filman, D.J.; Marino, S.F.; Ward, J.E.; Yang, L.; Mester, Z.; Bullitt, E.; Lovley, D.R.; Strauss, M. Cryo-EM reveals the structural basis of long-range electron transport in a cytochrome-based bacterial nanowire. Commun. Biol. 2019, 2, 219. [Google Scholar] [CrossRef]
- Tripathi, A.K.; Thakur, P.; Saxena, P.; Rauniyar, S.; Gopalakrishnan, V.; Singh, R.N.; Gadhamshetty, V.; Gnimpieba, E.Z.; Jasthi, B.K.; Sani, R.K. Gene Sets and Mechanisms of Sulfate-Reducing Bacteria Biofilm Formation and Quorum Sensing with Impact on Corrosion. Front. Microbiol. 2021, 12, 754140. [Google Scholar] [CrossRef]
- Heggendorn, F.L.; Fraga, A.G.M.; Ferreira, D.d.C.; Gonçalves, L.S.; Lione, V.d.O.F.; Lutterbach, M.T.S. Sulfate-Reducing Bacteria: Biofilm Formation and Corrosive Activity in Endodontic Files. Int. J. Dent. 2018, 2018, 8303450. [Google Scholar] [CrossRef]
- Wen, J.; Zhao, K.; Gu, T.; Raad, I.I. A green biocide enhancer for the treatment of sulfate-reducing bacteria (SRB) biofilms on carbon steel surfaces using glutaraldehyde. Int. Biodeterior. Biodegrad. 2009, 63, 1102–1106. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, X.; Jevasuwan, W.; Fukata, N.; Okamoto, A. Nanowire Electrode Structures Enhanced Direct Extracellular Electron Transport via Cell-Surface Multi-Heme Cytochromes in Desulfovibrio ferrophilus IS5. Electrochem 2024, 5, 330-340. https://doi.org/10.3390/electrochem5030021
Deng X, Jevasuwan W, Fukata N, Okamoto A. Nanowire Electrode Structures Enhanced Direct Extracellular Electron Transport via Cell-Surface Multi-Heme Cytochromes in Desulfovibrio ferrophilus IS5. Electrochem. 2024; 5(3):330-340. https://doi.org/10.3390/electrochem5030021
Chicago/Turabian StyleDeng, Xiao, Wipakorn Jevasuwan, Naoki Fukata, and Akihiro Okamoto. 2024. "Nanowire Electrode Structures Enhanced Direct Extracellular Electron Transport via Cell-Surface Multi-Heme Cytochromes in Desulfovibrio ferrophilus IS5" Electrochem 5, no. 3: 330-340. https://doi.org/10.3390/electrochem5030021
APA StyleDeng, X., Jevasuwan, W., Fukata, N., & Okamoto, A. (2024). Nanowire Electrode Structures Enhanced Direct Extracellular Electron Transport via Cell-Surface Multi-Heme Cytochromes in Desulfovibrio ferrophilus IS5. Electrochem, 5(3), 330-340. https://doi.org/10.3390/electrochem5030021






