Specific Permselectivity and Electrochemical Properties of Homogeneous Bilayer Membranes with a Selective Layer Made of DADMAC and EMA Copolymer
Abstract
1. Introduction
2. Materials and Methods
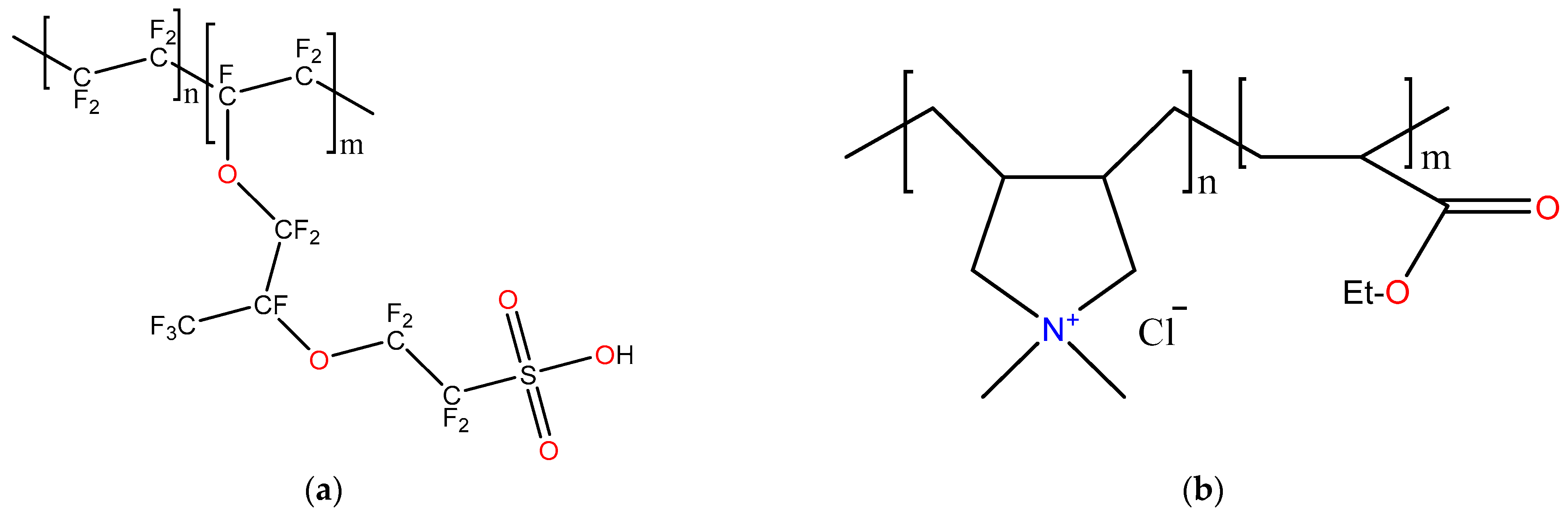
2.1. Materials
2.2. Synthesis of the DADMAC and EMA Copolymer
2.3. Membrane Preparation
2.4. Electrochemical Properties, Sorption, and Permselectivity of the Obtained Ion-Exchange Films
2.5. Separation of Sodium Ions from Simulated Black Sea Water
2.6. SEM Images
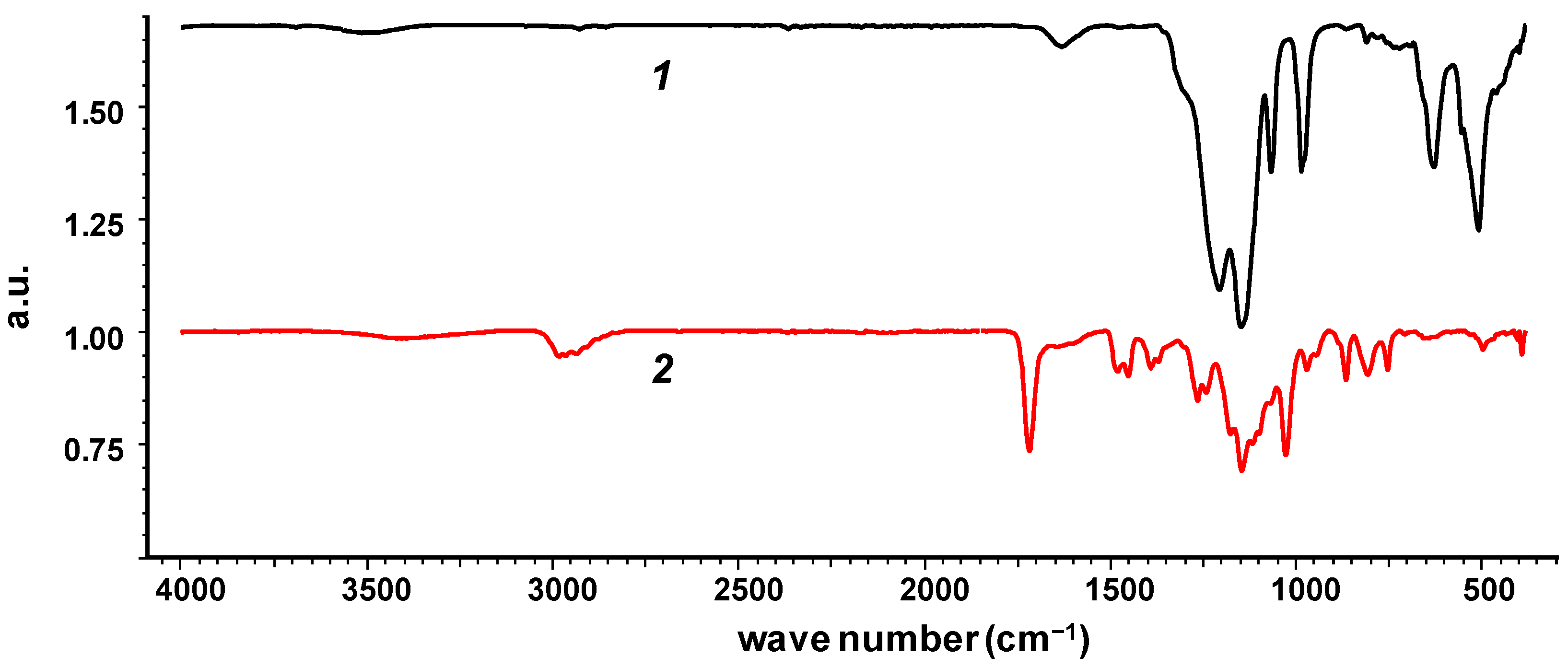
2.7. IR Spectra
3. Results and Discussion
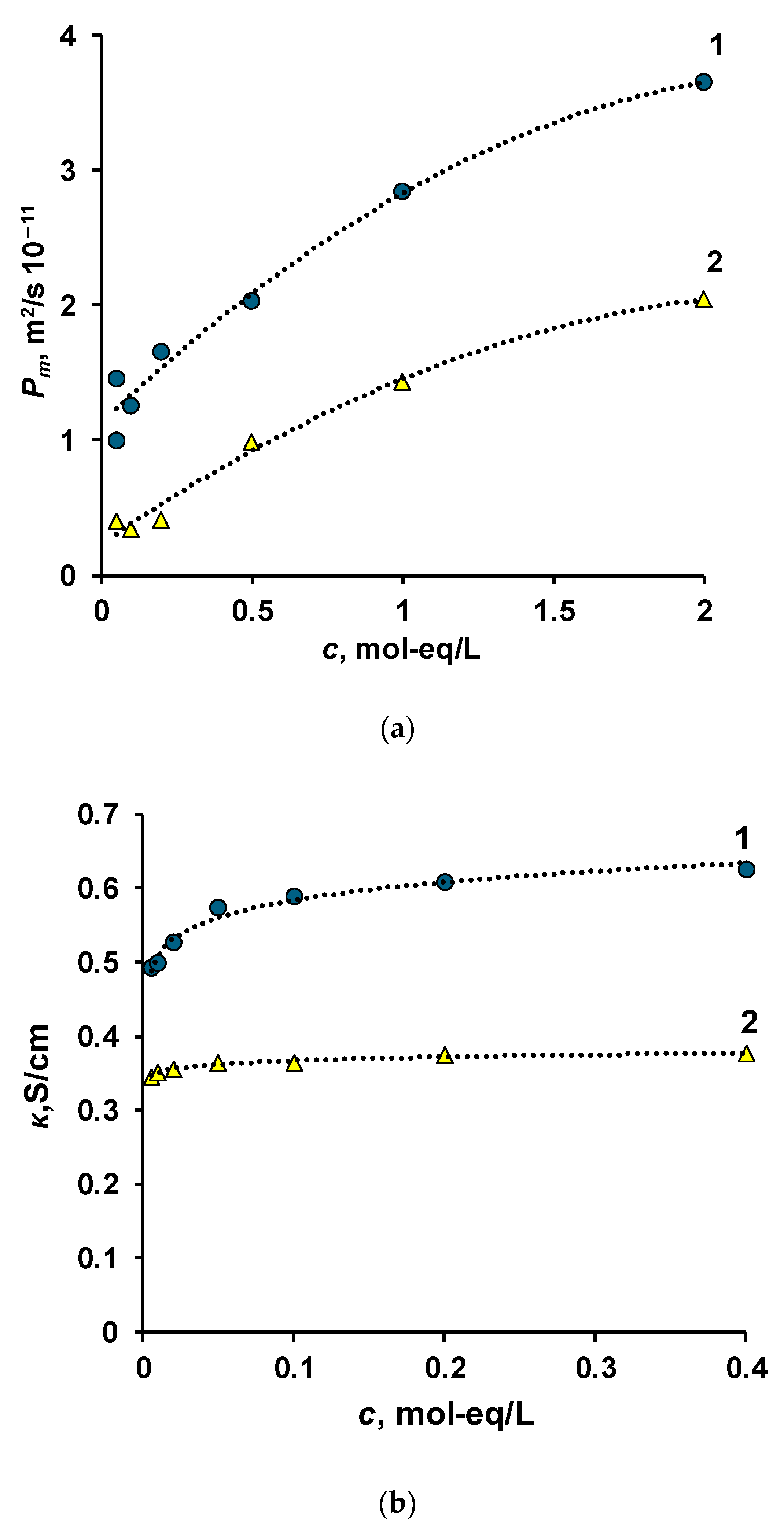
3.1. Equilibrium Conditions
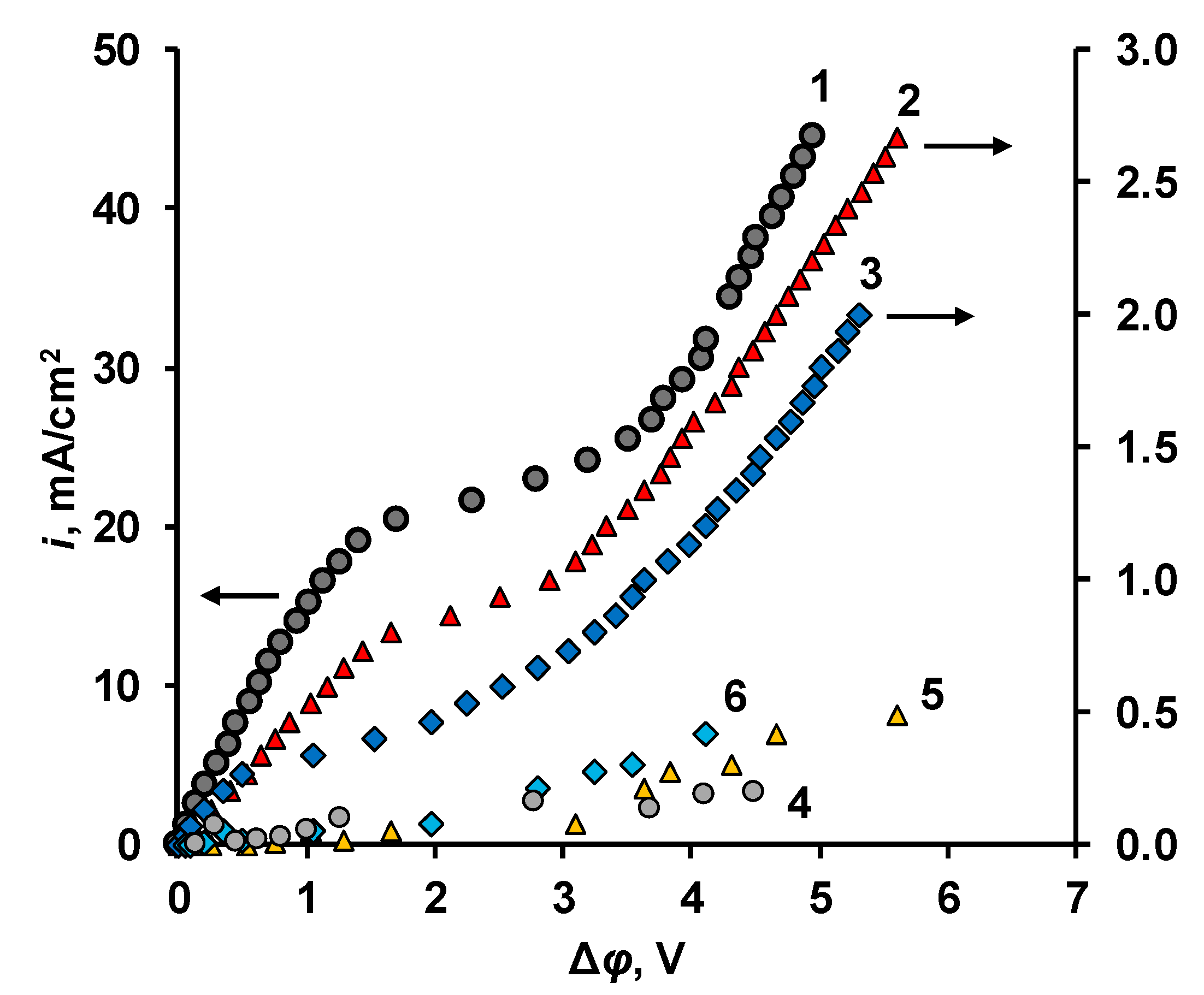
3.2. Current–Voltage Curves
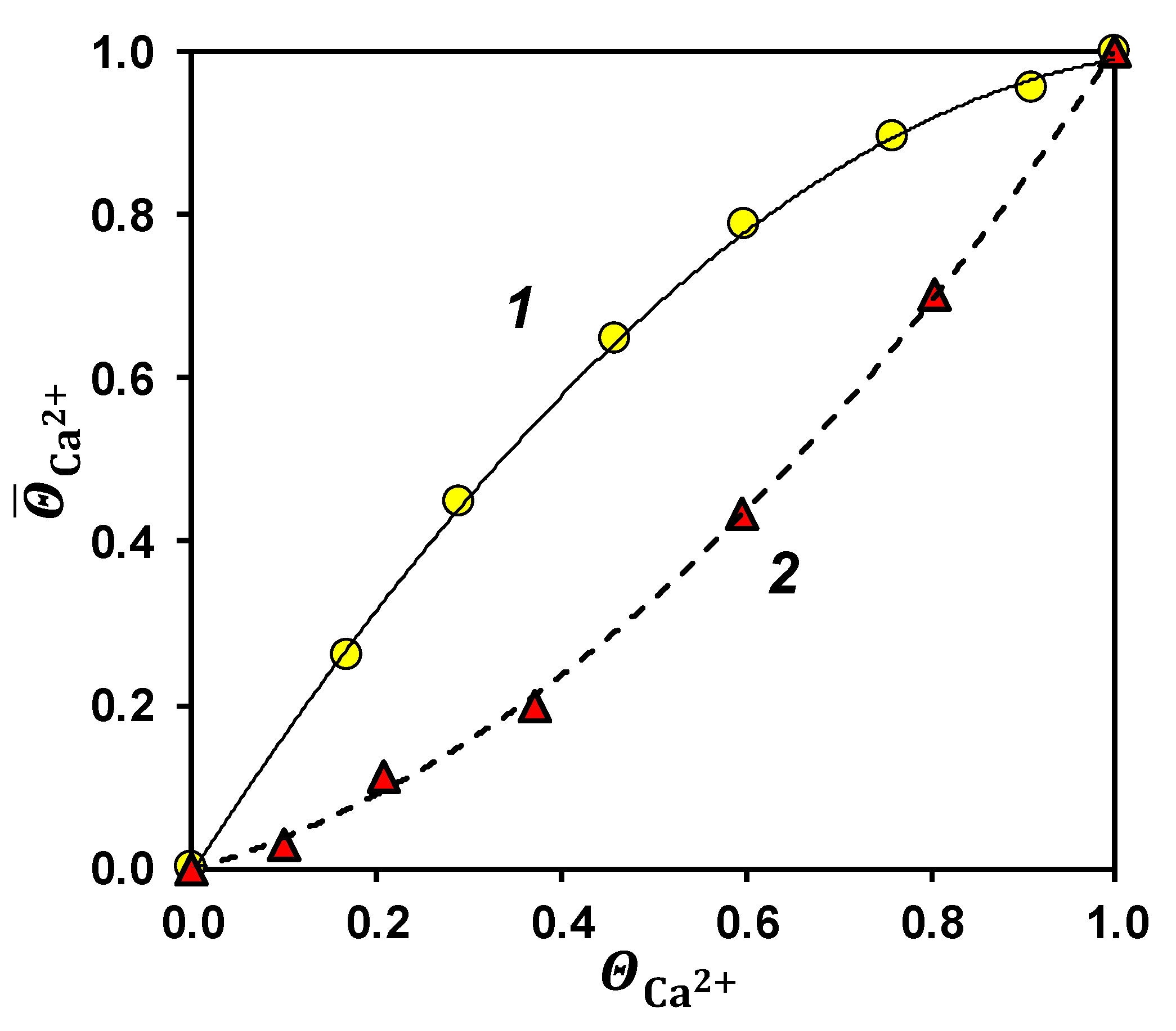
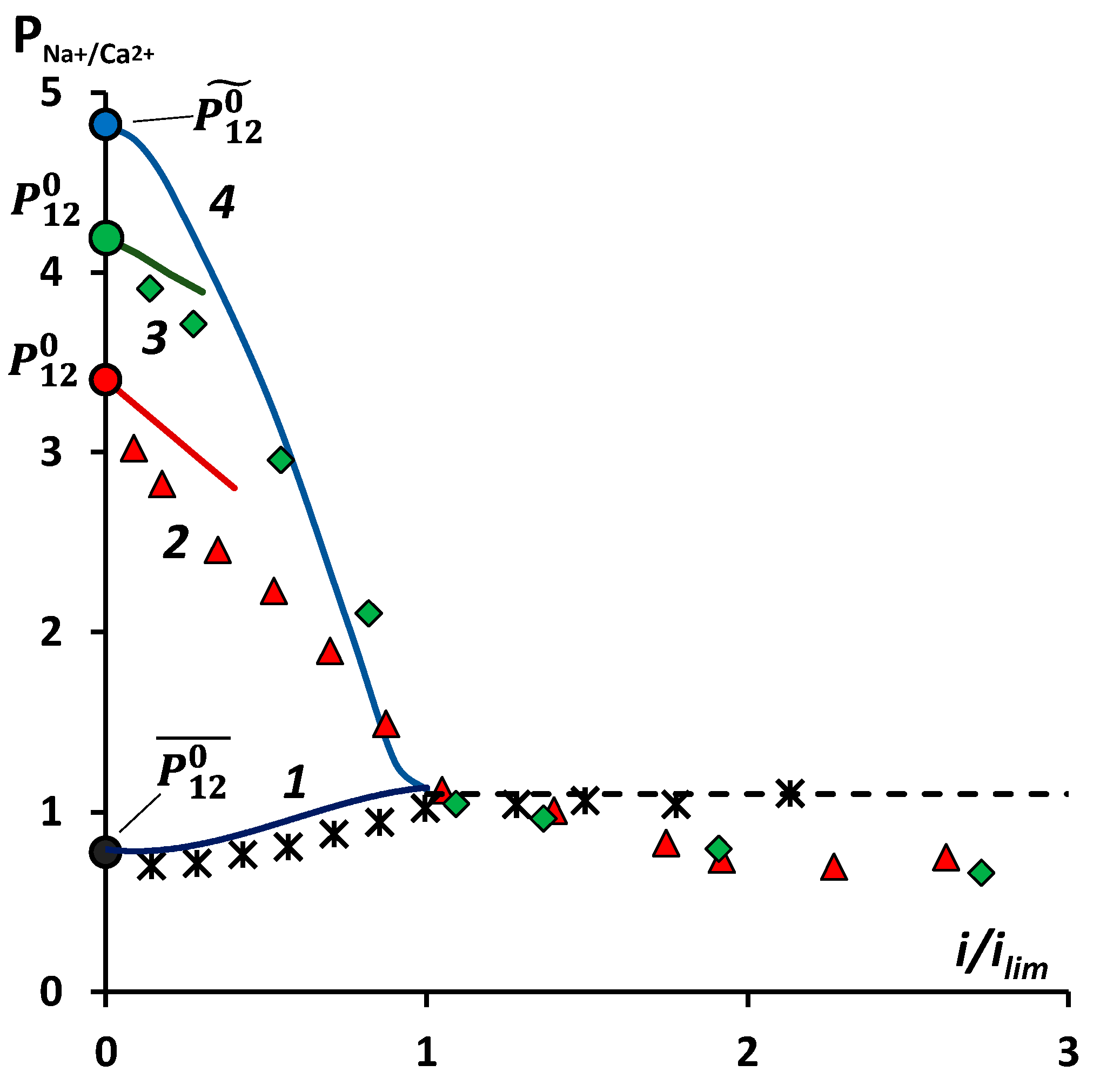
3.3. Specific Permselectivity
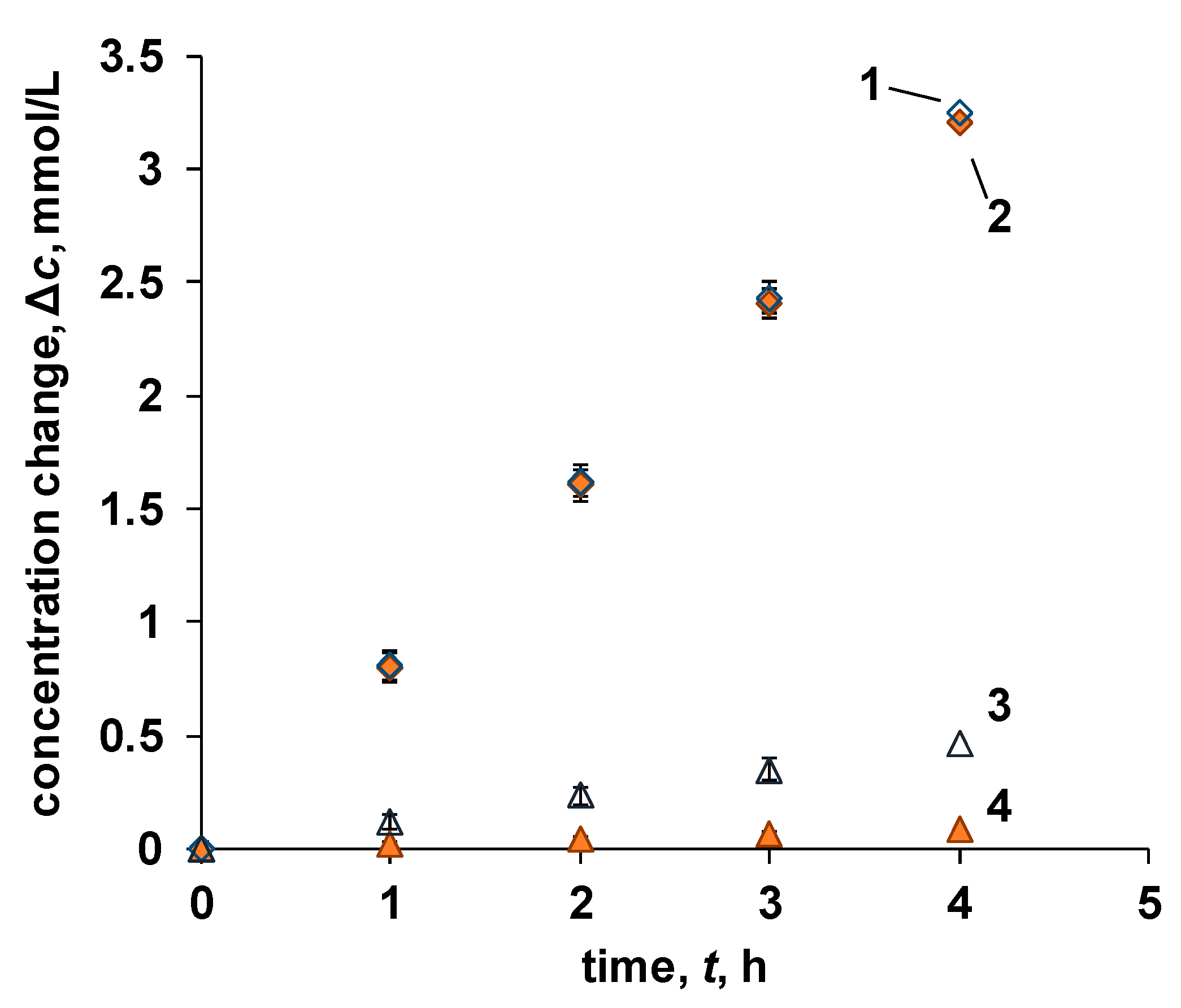
3.4. Concentration of Sodium Chloride from Seawater
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Strathmann, H. Electrodialysis, a mature technology with a multitude of new applications. Desalination 2010, 264, 268–288. [Google Scholar] [CrossRef]
- Kemperman, A.J.B. (Ed.) Handbook Bipolar Membrane Technology; Twente University Press: Enschede, The Netherlands, 2000; ISBN 9036515203. [Google Scholar]
- Vermaas, D.A.; Veerman, J.; Saakes, M.; Nijmeijer, K. Influence of multivalent ions on renewable energy generation in reverse electrodialysis. Energy Environ. Sci. 2014, 7, 1434–1445. [Google Scholar] [CrossRef]
- Güler, E.; van Baak, W.; Saakes, M.; Nijmeijer, K. Monovalent-ion-selective membranes for reverse electrodialysis. J. Memb. Sci. 2014, 455, 254–270. [Google Scholar] [CrossRef]
- Chen, T.; Bi, J.; Sun, M.; Liu, J.; Yuan, J.; Zhao, Y.; Ji, Z. Electrodialysis metathesis for high-value resource conversion and recovery: From sustainable applications to future prospects. Chem. Eng. J. 2023, 473, 145299. [Google Scholar] [CrossRef]
- Wu, Q.; Liang, D.; Lu, S.; Wang, H.; Xiang, Y.; Aurbach, D.; Avraham, E.; Cohen, I. Advances and perspectives in integrated membrane capacitive deionization for water desalination. Desalination 2022, 542, 116043. [Google Scholar] [CrossRef]
- Li, X.; Zhang, H.; Mai, Z.; Zhang, H.; Vankelecom, I. Ion exchange membranes for vanadium redox flow battery (VRB) applications. Energy Environ. Sci. 2011, 4, 1147. [Google Scholar] [CrossRef]
- Qian, Y.; Huang, L.; Pan, Y.; Quan, X.; Lian, H.; Yang, J. Dependency of migration and reduction of mixed Cr2O72−, Cu2+ and Cd2+ on electric field, ion exchange membrane and metal concentration in microbial fuel cells. Sep. Purif. Technol. 2018, 192, 78–87. [Google Scholar] [CrossRef]
- Schmaljohann, D. Thermo- and pH-responsive polymers in drug delivery. Adv. Drug Deliv. Rev. 2006, 58, 1655–1670. [Google Scholar] [CrossRef]
- ter Veen, W.R.; Koene, L. Economic evaluation for an innovative electrochemical closed-loop purification system for industrial process liquids. Met. Finish. 2003, 101, 17–27. [Google Scholar] [CrossRef]
- Boucher, M.; Turcotte, N.; Guillemette, V.; Lantagne, G.; Chapotot, A.; Pourcelly, G.; Sandeaux, R.; Gavach, C. Recovery of spent acid by electrodialysis in the zinc hydrometallurgy industry: Performance study of different cation-exchange membranes. Hydrometallurgy 1997, 45, 137–160. [Google Scholar] [CrossRef]
- Díaz Nieto, C.H.; Palacios, N.A.; Verbeeck, K.; Prévoteau, A.; Rabaey, K.; Flexer, V. Membrane electrolysis for the removal of Mg2+ and Ca2+ from lithium rich brines. Water Res. 2019, 154, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Ran, J.; Wu, L.; He, Y.; Yang, Z.; Wang, Y.; Jiang, C.; Ge, L.; Bakangura, E.; Xu, T. Ion exchange membranes: New developments and applications. J. Memb. Sci. 2017, 522, 267–291. [Google Scholar] [CrossRef]
- Khoiruddin; Ariono, D.; Subagjo; Wenten, I.G. Surface modification of ion-exchange membranes: Methods, characteristics, and performance. J. Appl. Polym. Sci. 2017, 134, 1–13. [Google Scholar] [CrossRef]
- Sata, T. Studies on ion exchange membranes with permselectivity for specific ions in electrodialysis. J. Memb. Sci. 1994, 93, 117–135. [Google Scholar] [CrossRef]
- Abdu, S.; Wessling, M.; Martí-Calatayud, M.-C.; Wong, J.E.; García-Gabaldón, M.; Wessling, M. Layer-by-Layer Modification of Cation Exchange Membranes Controls Ion Selectivity and Water Splitting. ACS Appl. Mater. Interfaces 2014, 6, 1843–1854. [Google Scholar] [CrossRef]
- Luo, T.; Abdu, S.; Wessling, M. Selectivity of ion exchange membranes: A review. J. Memb. Sci. 2018, 555, 429–454. [Google Scholar] [CrossRef]
- Ge, L.; Wu, B.; Yu, D.; Mondal, A.N.; Hou, L.; Afsar, N.U.; Li, Q.; Xu, T.T.; Miao, J.; Xu, T.T. Monovalent cation perm-selective membranes (MCPMs): New developments and perspectives. Chin. J. Chem. Eng. 2017, 25, 1606–1615. [Google Scholar] [CrossRef]
- Melnikov, S.; Bondarev, D.; Nosova, E.; Melnikova, E.; Zabolotskiy, V. Water Splitting and Transport of Ions in Electromembrane System with Bilayer Ion-Exchange Membrane. Membranes 2020, 10, 346. [Google Scholar] [CrossRef] [PubMed]
- White, N.; Misovich, M.; Yaroshchuk, A.; Bruening, M.L. Coating of Nafion membranes with polyelectrolyte multilayers to achieve high monovalent/divalent cation electrodialysis selectivities. ACS Appl. Mater. Interfaces 2015, 7, 6620–6628. [Google Scholar] [CrossRef]
- White, N.; Misovich, M.; Alemayehu, E.; Yaroshchuk, A.; Bruening, M.L. Highly selective separations of multivalent and monovalent cations in electrodialysis through Nafion membranes coated with polyelectrolyte multilayers. Polymer 2015, 103, 478–485. [Google Scholar] [CrossRef]
- Bondarev, D.; Melnikov, S.; Zabolotskiy, V. New homogeneous and bilayer anion-exchange membranes based on N,N-diallyl-N,N-dimethylammonium chloride and ethyl methacrylate copolymer. J. Memb. Sci. 2023, 675, 121510. [Google Scholar] [CrossRef]
- Achoh, A.R.; Zabolotsky, V.I.; Lebedev, K.A.; Sharafan, M.V.; Yaroslavtsev, A.B. Electrochemical Properties and Selectivity of Bilayer Ion-Exchange Membranes in Ternary Solutions of Strong Electrolytes. Membr. Membr. Technol. 2021, 3, 52–71. [Google Scholar] [CrossRef]
- Francis, M.J.; Pashley, R.M.; Rzechowicz, M. The effects of feed water de-gassing on the permeate flux of a small scale SWRO pilot plant. Desalin. Water Treat. 2011, 25, 150–158. [Google Scholar] [CrossRef]
- Gorobchenko, A.; Mareev, S.; Nikonenko, V. Mathematical Modeling of Monovalent Permselectivity of a Bilayer Ion-Exchange Membrane as a Function of Current Density. Int. J. Mol. Sci. 2022, 23, 4711. [Google Scholar] [CrossRef]
- Gorobchenko, A.D.; Gil, V.V.; Nikonenko, V.V.; Sharafan, M.V. Mathematical Modeling of the Selective Transport of Singly Charged Ions Through Multilayer Composite Ion-Exchange Membrane during Electrodialysis. Membr. Membr. Technol. 2022, 4, 423–432. [Google Scholar] [CrossRef]
- Femmer, R.; Mani, A.; Wessling, M. Ion transport through electrolyte/polyelectrolyte multi-layers. Sci. Rep. 2015, 5, 11583. [Google Scholar] [CrossRef]
- Golubenko, D.V.; Yaroslavtsev, A.B. Effect of current density, concentration of ternary electrolyte and type of cations on the monovalent ion selectivity of surface-sulfonated graft anion-exchange membranes: Modelling and experiment. J. Memb. Sci. 2021, 635, 119466. [Google Scholar] [CrossRef]


| Parameter | Layer | |||
|---|---|---|---|---|
| PFSA | MA-1 | |||
| Ca2+ | Na+ | Ca2+ | Na+ | |
| Water uptake (), H2O/gsw, % | 17.4 ± 2 | 15.6 ± 2 | 30.5 ± 2 | 28.9 ± 2 |
| Ion-exchange capacity (), mmol-eq/cm3 | 0.82 ± 0.05 | 0.97 ± 0.05 | ||
| Specific electrical conductivity (), mS/cm | 1.3 ± 0.1 | 1.9 ± 0.1 | 2.0 ± 0.1 | 2.0 ± 0.1 |
| Diffusion coefficients () × , m2/s | 1.4 | 3.9 | 0.2 | 0.7 |
| Counterions equilibrium constant () | 1.6 | – | ||
| Co-ions equilibrium constant () | – | 0.7 | ||
| Diffusion layer thickness, μm | 53.3 ± 1 | |||
| Membrane/solution equilibrium constants | 1.9 | 0.8 | ||
| Layer thickness, μm | 210 | 6 1 or 24 2 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Achoh, A.; Bondarev, D.; Melnikov, S.; Zabolotsky, V. Specific Permselectivity and Electrochemical Properties of Homogeneous Bilayer Membranes with a Selective Layer Made of DADMAC and EMA Copolymer. Electrochem 2024, 5, 393-406. https://doi.org/10.3390/electrochem5040026
Achoh A, Bondarev D, Melnikov S, Zabolotsky V. Specific Permselectivity and Electrochemical Properties of Homogeneous Bilayer Membranes with a Selective Layer Made of DADMAC and EMA Copolymer. Electrochem. 2024; 5(4):393-406. https://doi.org/10.3390/electrochem5040026
Chicago/Turabian StyleAchoh, Aslan, Denis Bondarev, Stanislav Melnikov, and Victor Zabolotsky. 2024. "Specific Permselectivity and Electrochemical Properties of Homogeneous Bilayer Membranes with a Selective Layer Made of DADMAC and EMA Copolymer" Electrochem 5, no. 4: 393-406. https://doi.org/10.3390/electrochem5040026
APA StyleAchoh, A., Bondarev, D., Melnikov, S., & Zabolotsky, V. (2024). Specific Permselectivity and Electrochemical Properties of Homogeneous Bilayer Membranes with a Selective Layer Made of DADMAC and EMA Copolymer. Electrochem, 5(4), 393-406. https://doi.org/10.3390/electrochem5040026







