Spray Characteristics of Bioethanol-Blended Fuel under Various Temperature Conditions Using Laser Mie Scattering and Optical Illumination
Abstract
:1. Introduction
- A high-octane number, which induces a strong resistance to knock and, consequently, the ability to optimize the engine (compression ratio and spark-advance, in particular);
- A density similar to gasoline’s;
- The presence of oxygen in the formula, which can decrease unburned or partially burned molecule emissions (HC and CO);
- A high latent heat of vaporization, which enables a “cooling effect” of air and, consequently, can enhance the engine’s volumetric efficiency.
- Conversely, some disadvantages must be considered:
- The oxygen included in the molecule (30%wt) causes an increase in the fuel volumetric consumption;
- The high latent heat of vaporization may have difficulties in cold conditions, especially cold start;
- Ethanol combustion in engines produces aldehyde emissions, which can have a negative impact on health;
- Vapour lock can occur;
- It degrades some metal materials in the fuel system and swells rubber or synthetic resins;
- The phase separation of the fuel becomes easy as a small amount is mixed.
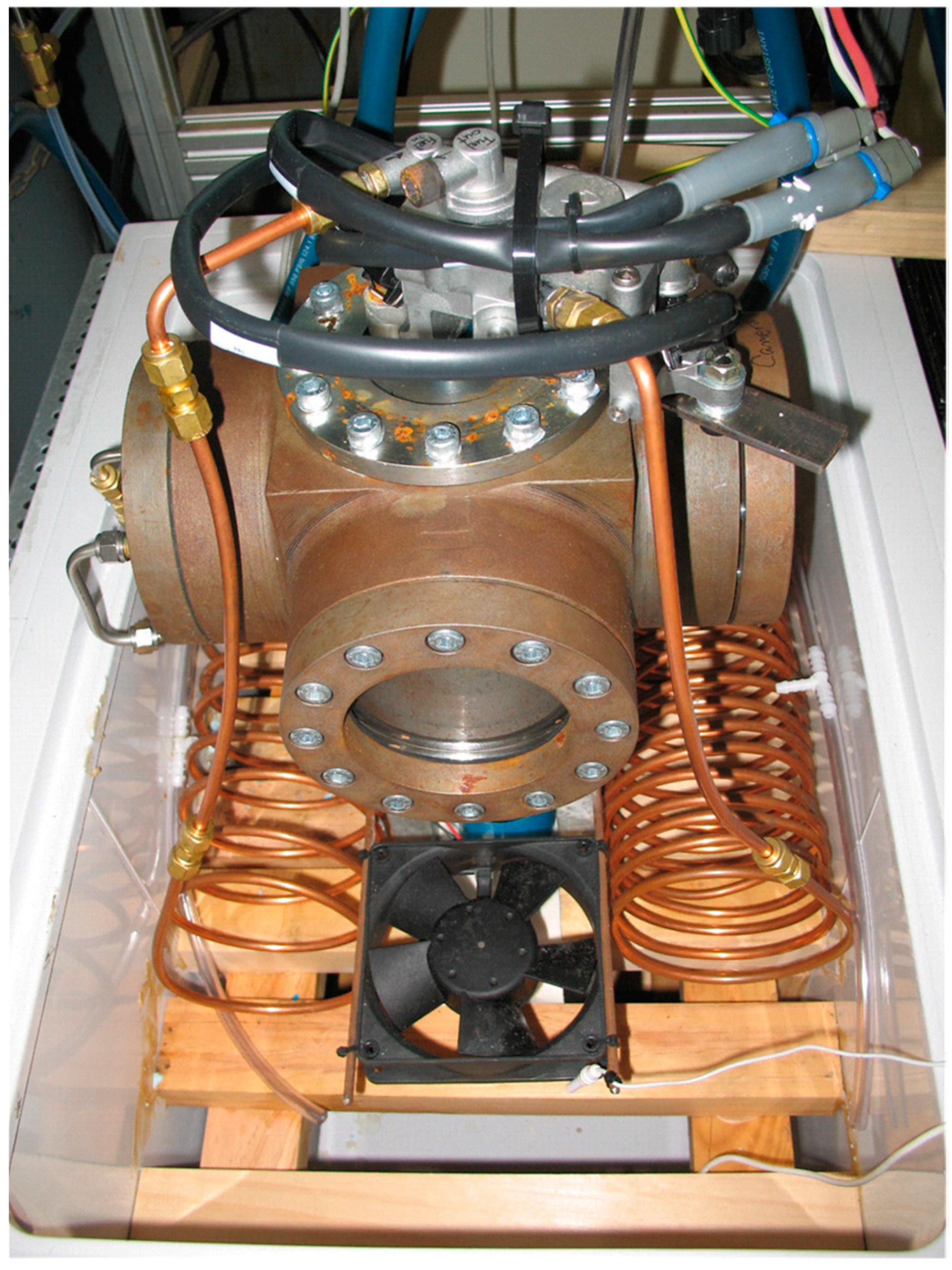
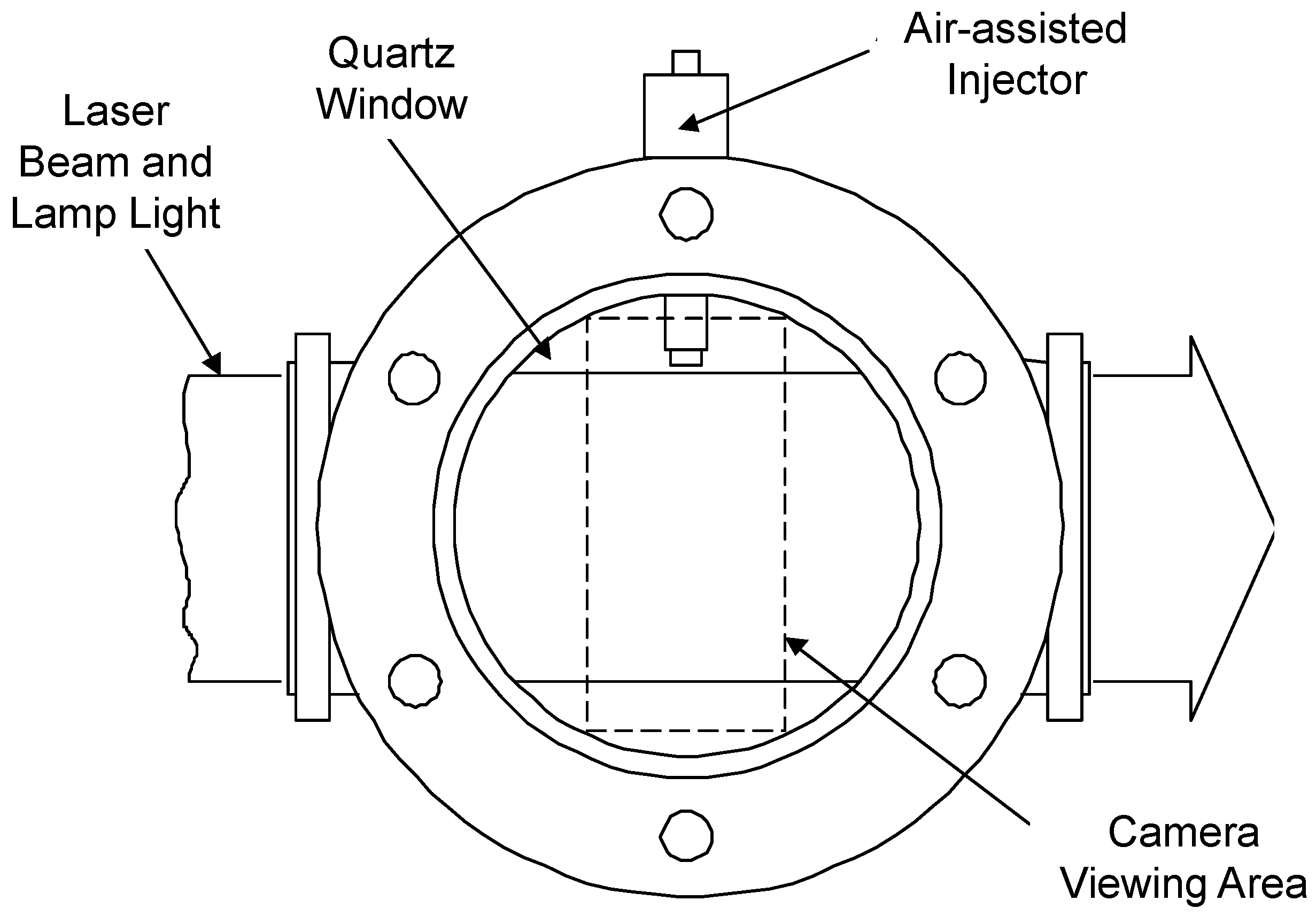
2. Experiment
3. Results
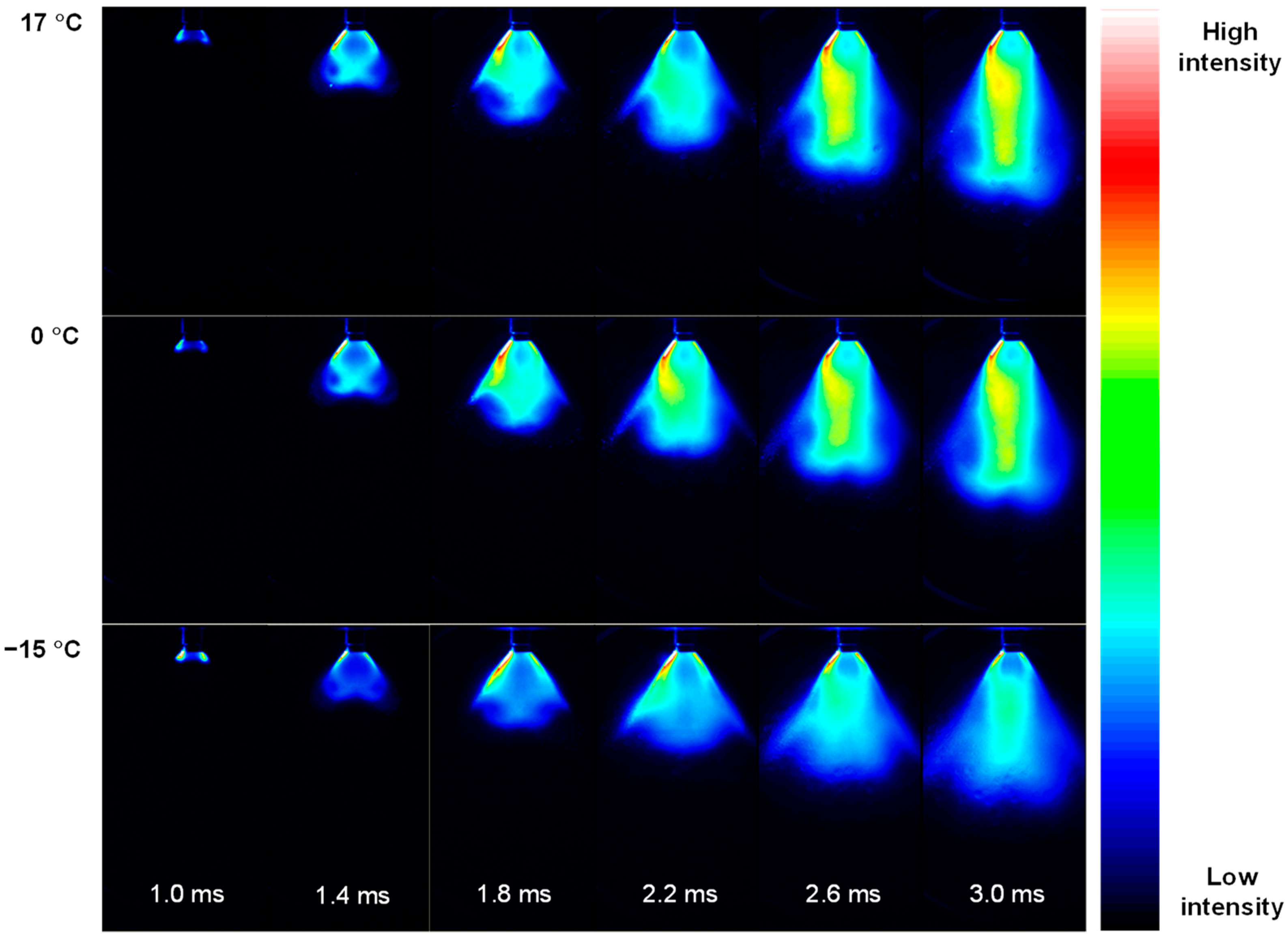
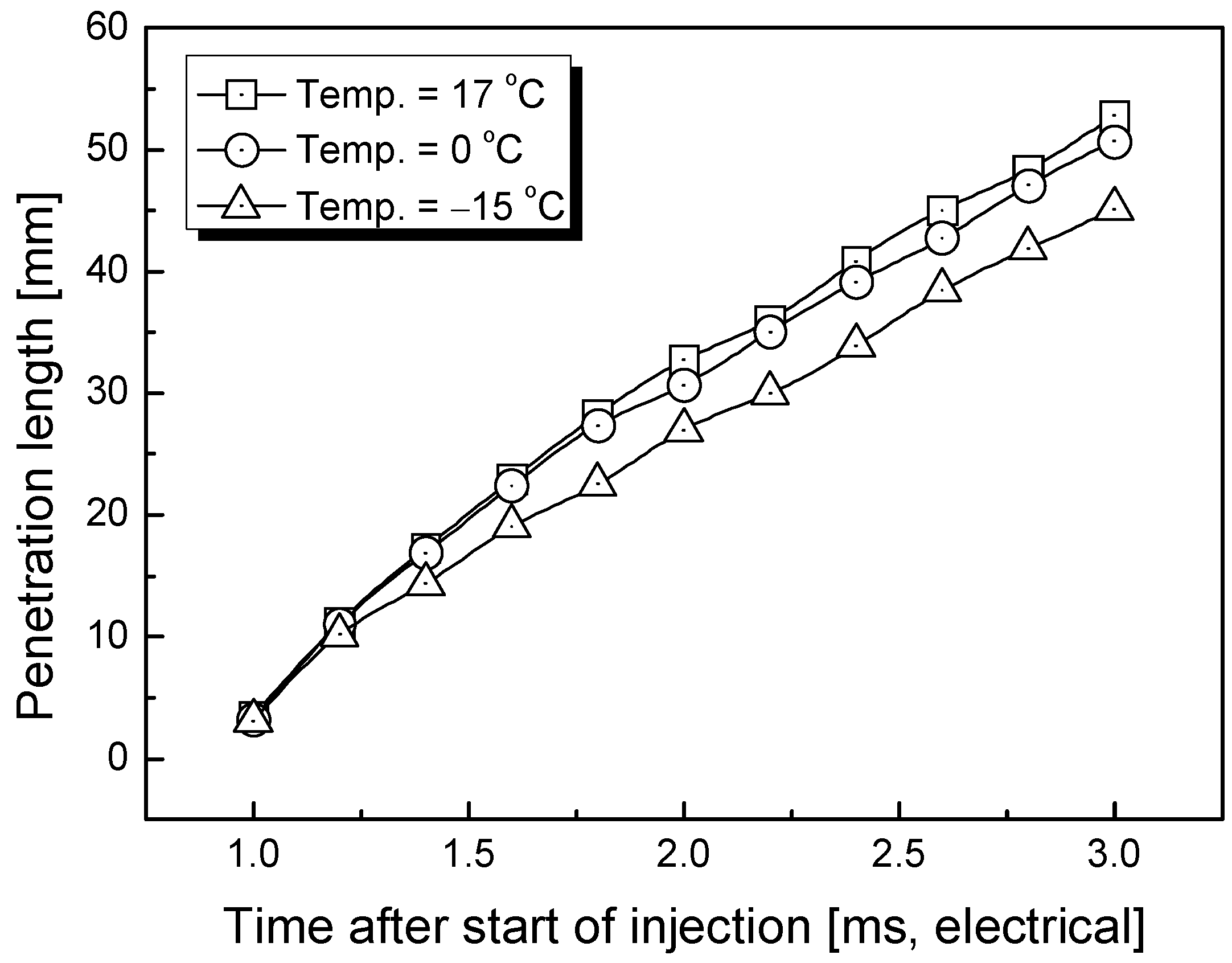
3.1. Penetration Depth Estimation
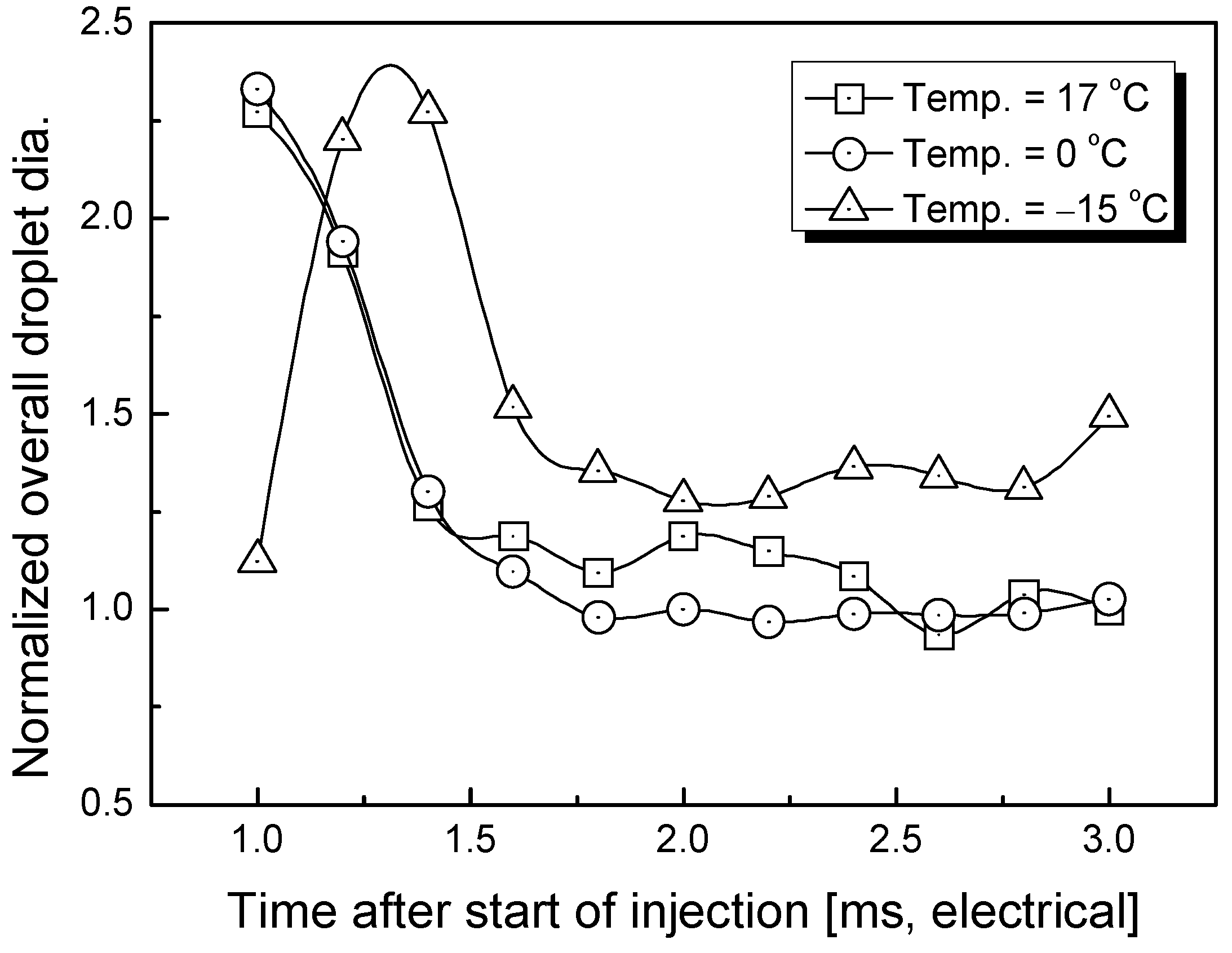
3.2. Estimation of Average Droplet Size
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Union. Directive 2015/1513 of the European Parliament and of the Council of 9 September 2015 Amending Directive 98/70/EC Relating to the Quality of Petrol and Diesel Fuels and Amending Directive 2009/28/EC on the Promotion of the Use of Energy from Renewable Sources, L 239/1 EN. Off. J. Eur. Union 2015, 58, 1–29. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32015L1513 (accessed on 15 February 2022).
- European Union. Directive 2009/28/EC of the European Parliament and of The Council of 23 April 2009 on the Promotion of the Use of Energy from Renewable Sources and Amending and Subsequently Repealing Directives 2001/77/EC and 2003/30/EC, L 140/16 EN. Off. J. Eur. Union 2009, 5, 16–62. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex%3A32009L0028 (accessed on 18 February 2022).
- Topgul, T.; Yucesu, H.S.; Cinar, C.; Koca, A. The effects of ethanol-unleaded gasoline blends and ignition timing on engine performance and exhaust emissions: Technical Note. Renew. Energy 2006, 31, 2534–2542. [Google Scholar] [CrossRef]
- Yuksel, F.; Yuksel, B. The use of ethanol-gasoline blend as a fuel in an SI engine: Technical Note. Renew. Energy 2004, 29, 1181–1191. [Google Scholar] [CrossRef]
- Bata, R.M.; Elond, A.C.; Rice, R.W. Emissions from IC engines fueled with alcohol-gasoline blends: A literature review. Trans. ASME 1989, 111, 421–431. [Google Scholar] [CrossRef]
- Brewster, S.; Railton, D.; Maisey, M.; Frew, M. The effect of E100 water content on high load performance of a spray guide direct injection boosted engine. In SAE Paper 2007-01-2648, Proceedings of the SAE Brasil 2007 Congress and Exhibit, São Paulo, Brazil, 28–30 November 2007; SAE: Warrendale, PA, USA, 2007. [Google Scholar] [CrossRef]
- Balat, M.; Balat, H. Recent trends in global production and utilization of bio-ethanol fuel. Appl. Energy 2009, 86, 2273–2282. [Google Scholar] [CrossRef]
- Demirbas, A. Competitive liquid biofuels from biomass. Appl. Energy 2011, 88, 17–28. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Dragone, G.; Guimarães, P.M.; Silva, J.P.; Carneiro, L.M.; Roberto, I.C. Technological trends, global market, and challenges of bio-ethanol production. Biotechnol. Adv. 2010, 28, 817–830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Singh, N.; Prasad, R. Anhydrous ethanol: A renewable source of energy. Renew. Sustain. Energy Rev. 2010, 14, 1830–1844. [Google Scholar] [CrossRef]
- Niven, R.K. Ethanol in gasoline: Environmental impacts and sustainability review article. Renew. Sustain. Energy Rev. 2005, 9, 535–555. [Google Scholar] [CrossRef]
- Oscar, J.S.; Carlos, A.C. Review: Trends in biotechnological production of fuel ethanol from different feedstocks. Bioresour. Technol. 2008, 99, 5270–5295. [Google Scholar] [CrossRef]
- Limayem, A.; Ricke, S.C. Lignocellulosic biomass for bioethanol production: Current perspectives, potential issues and future prospects. Prog. Energy Combust. Sci. 2012, 38, 449–467. [Google Scholar] [CrossRef]
- Sarkar, N.; Ghosh, S.K.; Bannerjee, S.; Aikat, K. Bioethanol production from agricultural wastes: An overview. Renew Energy 2012, 37, 19–27. [Google Scholar] [CrossRef]
- Cardona, C.A.; Quintero, J.A.; Paz, I.C. Production of bioethanol from sugarcane bagasse: Status and perspectives. Bioresour. Technol. 2010, 101, 4754–4766. [Google Scholar] [CrossRef] [PubMed]
- Kosugi, A.; Kondo, A.; Ueda, M.; Murata, Y.; Vaithanomsat, P.; Thanapase, W. Production of ethanol from cassava pulp via fermentation with a surface engineered yeast strain displaying glucoamylase. Renew. Energy 2009, 34, 1354–1358. [Google Scholar] [CrossRef]
- Rao, K.; Chaudhari, V.; Varanasi, S.; Kim, D.S. Enhanced ethanol fermentation of brewery waste water using the genetically modified strain E. coli KO11. Appl. Microbiol. Biotechnol. 2007, 74, 50–60. [Google Scholar] [CrossRef]
- Linggang, S.; Phang, L.Y.; Wasoh, M.H.; Abd-Aziz, S. Sago pith residue as an alternative cheap substrate for fermentable sugars production. Appl. Biochem. Biotechnol. 2012, 167, 122–131. [Google Scholar] [CrossRef]
- Mood, S.H.; Golfeshan, A.H.; Tabatabaei, M.; Jouzani, G.S.; Najafi, G.H.; Gholami, M. Lignocellulosic biomass to bioethanol, a comprehensive review with a focus on pretreatment. Renew. Sustain. Energy Rev. 2013, 27, 77–93. [Google Scholar] [CrossRef]
- Hendriks, A.T.W.M.; Zeeman, G. Pretreatments to enhance the digestibility of lignocellulosic biomass. Bioresour. Technol. 2009, 100, 10–18. [Google Scholar] [CrossRef]
- Alvira, P.; Tomas, E.; Ballesteros, M.; Negro, M.J. Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: A review. Bioresour. Technol. 2010, 101, 4851–4861. [Google Scholar] [CrossRef]
- Carolina, C.M.; Arturo, J.G.; Mahmoud, E.-H. A comparison of pretreatment methods for bioethanol production from lignocellulosic materials. Process Saf. Environ. 2012, 90, 189–202. [Google Scholar] [CrossRef]
- Utley, T.D.; Brewster, S.; Tilmouth, A.; Jin, S.H.; Brear, M.J. Unassisted Low Temperature Starting of a Pure Ethanol Fuelled Direct Injection Engine. In SAE Paper 2008-36-0270, Proceedings of the Congresso SAE Brasil, São Paulo, Brazil, 7–9 October 2008; SAE: Warrendale, PA, USA, 2008. [Google Scholar] [CrossRef]
- The Engineering Toolbox. Available online: https://www.engineeringtoolbox.com/ethanol-dynamic-kinematic-viscosity-temperature-pressure-d_2071.html (accessed on 21 February 2022).
- Jeuland, N.; Montagne, X.; Gautrot, X. Potentiality of ethanol as a fuel for dedicated engine. Oil Gas Sci. Technol.–Rev. IFP 2004, 59, 559–570. [Google Scholar] [CrossRef] [Green Version]
- Michalopoulou, D.-P.; Komiotou, M.; Zannikou, Y.; Karonis, D. Impact of bio-ethanol, bio-ETBE addition on the volatility of gasoline with oxygen content at the level of E10. Fuels 2021, 2, 29. [Google Scholar] [CrossRef]
- Rodríguez-Antón, L.M.; Hernández-Campos, M.; Sanz-Pérez, F. Experimental determination of some physical properties of gasoline, ethanol and ETBE blends. Fuel 2013, 112, 178–184. [Google Scholar] [CrossRef]
- Weber de Menezes, E.; Cataluña, R.; Samios, D.; Da Silva, R. Addition of an azeotropic ETBE/ethanol mixture in eurosupertype gasolines. Fuel 2006, 85, 2567–2577. [Google Scholar] [CrossRef]
- Karonis, D.; Anastopoulos, G.; Lois, E.; Stournas, S. Impact of simultaneous ETBE and ethanol addition on motor gasoline properties. SAE Int. J. Fuels Lubr. 2009, 1, 1584–1594. [Google Scholar] [CrossRef]
- AlRamadan, A.S.; Sarathy, S.M.; Badra, J. Unraveling the octane response of gasoline/ethanol blends: Paving the way to formulating gasoline surrogates. Fuel 2021, 299, 120882. [Google Scholar] [CrossRef]
- Magnusson, R.; Nilsson, C. The influence of oxygenated fuels on emissions of aldehydes and ketones from a two-stroke spark ignition engine. Fuel 2011, 90, 1145–1154. [Google Scholar] [CrossRef]
- Yücesu, S.H.; Topgül, T.; Çinar, C.; Okur, M. Effect of ethanol–gasoline blends on engine performance and exhaust emissions in different compression ratios. Appl. Therm. Eng. 2006, 26, 2272–2278. [Google Scholar] [CrossRef]
- Jin, S.H.; Brear, M.J.; Watson, H.C.; Brewster, S. An experimental study of the spray from an air-assisted direct fuel injector. Proc. Inst. Mech. Eng. Part D J. Autom. Eng. 2008, 222, 1883–1894. [Google Scholar] [CrossRef]
- Sick, V.; Westbrook, C.K. Diagnostic implications of the reactivity of fluorescence tracers. In Proceedings of the 32nd International Symposium on Combustion, Montreal, QC, Canada, 3–8 August 2008. [Google Scholar] [CrossRef]
- Cabrera, H.; Marcano, A.; Castellanos, Y. Absorption coefficient of nearly transient liquids measured using thermal lens spectrometry. Condens. Matter Phys. 2006, 9, 385–389. [Google Scholar] [CrossRef] [Green Version]
- Pope, R.M.; Fry, E.S. Absorption spectrum (380~700nm) of pure water II: Integrating cavity measurements. Appl. Opt. 1997, 36, 8710–8723. [Google Scholar] [CrossRef] [PubMed]
- LeGal, P.; Farrugia, N.; Greenhalgh, D.A. Laser sheet dropsizing of dense sprays. Opt. Laser Technol. 1999, 31, 75–83. [Google Scholar] [CrossRef]
- Jermy, M.C.; Greenhalgh, D.A. Planar dropsizing by elastic and fluorescence scattering in sprays too dense for phase Doppler measurement. Appl. Phys. B 2000, 71, 703–710. [Google Scholar] [CrossRef]
- Lefebvre, A.H. Atomization and Sprays, 2nd ed.; Taylor & Francis: Boca Raton, FL, USA, 1989; ISBN 9781498736251. [Google Scholar] [CrossRef]


| Property | Gasoline | Ethanol |
|---|---|---|
| molecular formula | ~CH1.85 | C2H5OH |
| C (% mass) | ~86.6 | 52.1 |
| O (% mass) | 0 | 34.7 |
| density at 20 °C (kg/L) | ~0.74 | 0.79 |
| stoichiometric AFR (:1) | ~14.6 | 9.0 |
| lower heating value (MJ/kg) | ~43.5 | 26.8 |
| flash point (°C) | ≤−40 | 14 |
| CO2 (g/MJ) | ~72.9 | 71.3 |
| boiling point (°C) | 25~200 | 78.4 |
| freezing point (°C) | −114 | |
| latent heat of vaporisation (kJ/kg) | ~300 | 855 |
| research octane number | 91~98 | ~110 |
| ignition temperature (°C) | ~300 | 420 |
| viscosity (mPa s) | 1.214 @ 17 °C | |
| 1.720 @ 0 °C | ||
| 2.419 @ −15 °C | ||
| surface tension (N/m) | 0.02239 @ 20 °C |
| Injector | Air-Assisted Injector |
|---|---|
| fuel injection pressure | 800 kPag |
| air injection pressure | 650 kPag |
| fuel injection quantity | 20 mg/injection |
| fuel/air injection delay | 2.5 ms |
| air injection duration | 3.3 ms |
| ambient temperature | 17, 0 and −15 °C |
| chamber pressure | atmospheric |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, S.-H. Spray Characteristics of Bioethanol-Blended Fuel under Various Temperature Conditions Using Laser Mie Scattering and Optical Illumination. Fuels 2022, 3, 207-216. https://doi.org/10.3390/fuels3020013
Jin S-H. Spray Characteristics of Bioethanol-Blended Fuel under Various Temperature Conditions Using Laser Mie Scattering and Optical Illumination. Fuels. 2022; 3(2):207-216. https://doi.org/10.3390/fuels3020013
Chicago/Turabian StyleJin, Seong-Ho. 2022. "Spray Characteristics of Bioethanol-Blended Fuel under Various Temperature Conditions Using Laser Mie Scattering and Optical Illumination" Fuels 3, no. 2: 207-216. https://doi.org/10.3390/fuels3020013
APA StyleJin, S.-H. (2022). Spray Characteristics of Bioethanol-Blended Fuel under Various Temperature Conditions Using Laser Mie Scattering and Optical Illumination. Fuels, 3(2), 207-216. https://doi.org/10.3390/fuels3020013






