Optimization of Solid-State Fermentation of Switchgrass Using White-Rot Fungi for Biofuel Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Statistical Experimental Design
2.2. Fungal Strains and Feedstock Preparation
2.3. Fungal Pretreatment
2.4. Compositional Analysis
2.5. Enzymatic Hydrolysis
2.6. Pelletization, Raw Materials and Pellet Characterization
3. Results and Discussion
3.1. Characterization of the Raw Material
3.2. Fungal Growth
3.3. Fungal Pretreatment
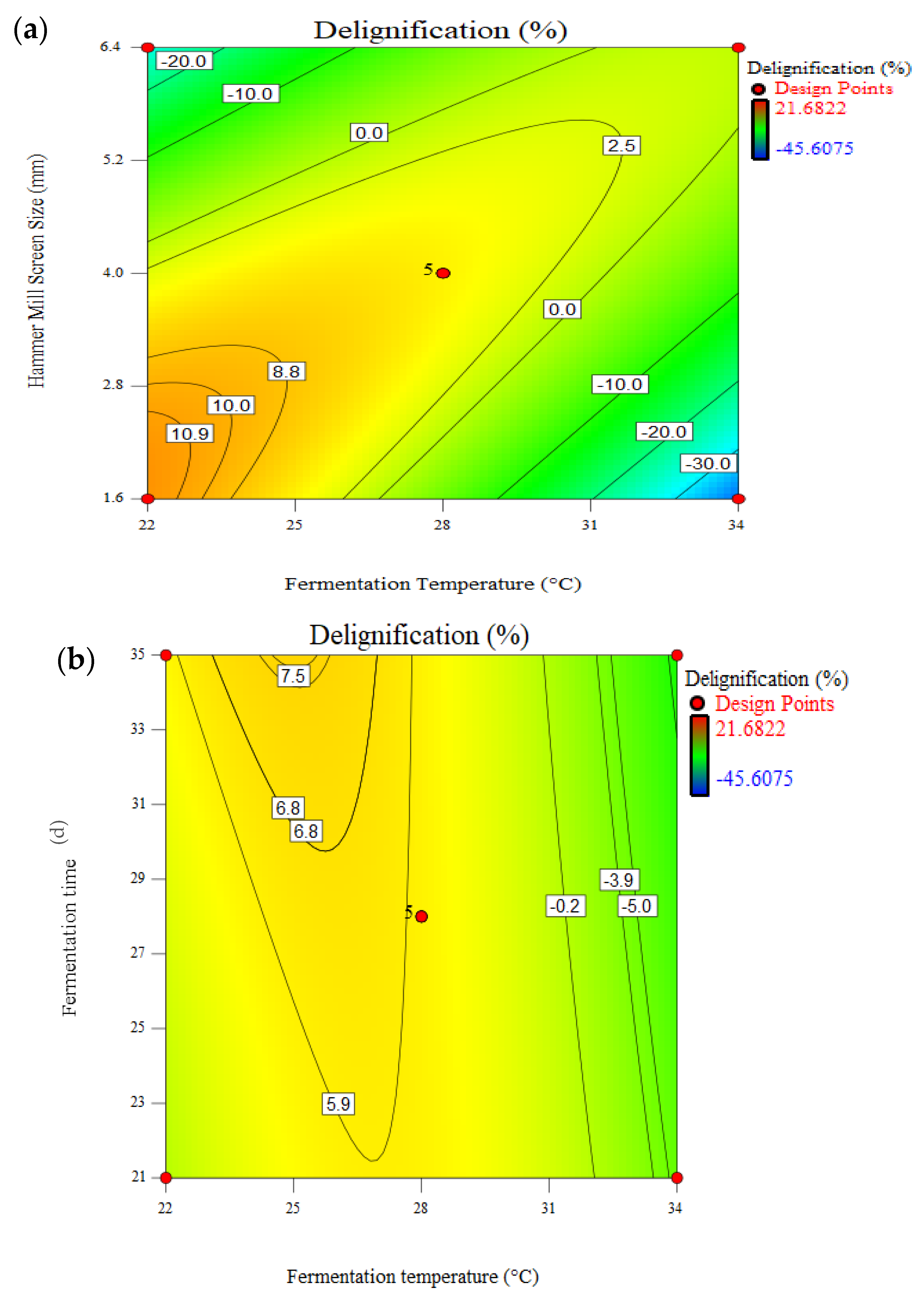
3.4. Effect of Pretreatment on Delignification
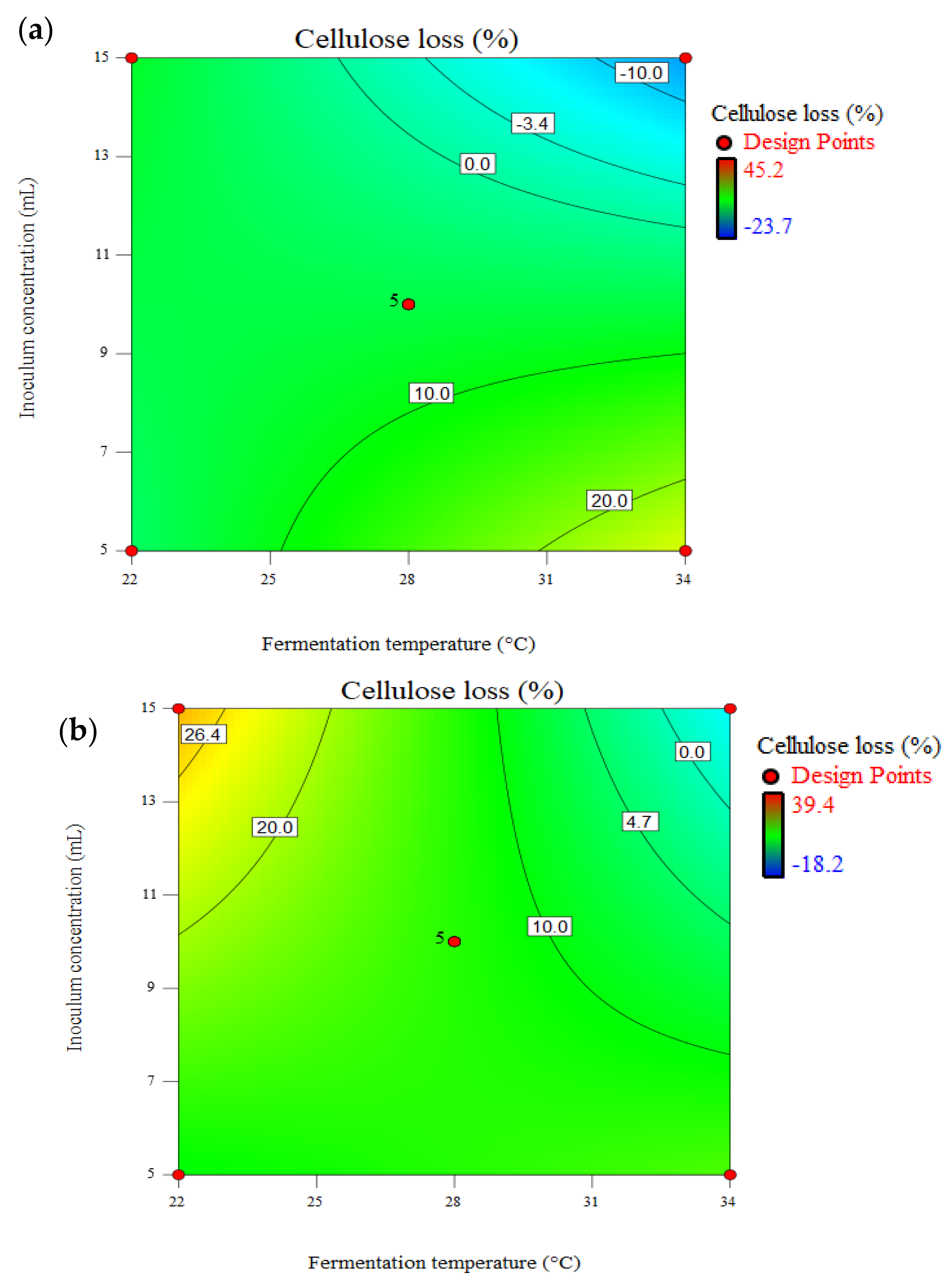
3.5. Effect of Pretreatment on Total Available Carbohydrate (TAC) and Cellulose Loss
3.6. Optimization and Enzymatic Hydrolysis
3.7. Characterization of the Fungal Treated Switchgrass and Its Pellets
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kou, L.; Song, Y.; Zhang, X.; Tan, T. Comparison of Four Types of Energy Grasses as Lignocellulosic Feedstock for the Production of Bio-Ethanol. Bioresour. Technol. 2017, 241, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Wright, L.L. Historical Perspective on How and Why Switchgrass Was Selected as a “Model” High-Potential Energy Crop; Oak Ridge National Laboratory: Oak Ridge, TN, USA, 2007. [Google Scholar]
- Sanderson, M.A.; Adler, P.R.; Boateng, A.A.; Casler, M.D.; Sarath, G. Switchgrass as a Biofuels Feedstock in the USA. Can. J. Plant Sci. 2006, 86, 1315–1325. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Knoll, J.E. Conventional and Molecular Breeding for Improvement of Biofuel Crops: Past, Present, and Future. In Handbook of Bioenergy Crop Plants; Kole, C., Josh, C.P., Shonnard, D.R., Eds.; CRC Press: Boca Raton, FL, USA, 2011; pp. 3–21. [Google Scholar]
- Alizadeh, H.; Teymouri, F.; Gilbert, T.I.; Dale, B.E. Pretreatment of Switchgrass by Ammonia Fiber Explosion (AFEX). Appl. Biochem. Biotechnol. 2005, 121–124, 1133–1141. [Google Scholar] [CrossRef] [PubMed]
- Tae, H.K.; Lee, Y.Y.; Sunwoo, C.; Jun, S.K. Pretreatment of Corn Stover by Low-Liquid Ammonia Recycle Percolation Process. Appl. Biochem. Biotechnol. 2006, 133, 41–57. [Google Scholar] [CrossRef]
- Swain, M.R.; Singh, A.; Sharma, A.K.; Tuli, D.K. Bioethanol Production from Rice- and Wheat Straw: An Overview. In Bioethanol Production from Food Crops; Ramesh, C., Ray, S., Ramachandran, Eds.; Academic Press: San Diego, CA, USA, 2019; pp. 213–231. [Google Scholar]
- Taherzadeh, M.J.; Karimi, K. Pretreatment of Lignocellulosic Wastes to Improve Ethanol and Biogas Production: A Review. Int. J. Mol. Sci. 2008, 9, 1621–1651. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Liu, J.; Chang, X.; Chen, D.; Xue, Y.; Liu, P.; Lin, H.; Han, S. A Review on the Pretreatment of Lignocellulose for High-Value Chemicals. Fuel Process. Technol. 2017, 160, 196–206. [Google Scholar] [CrossRef]
- Sindhu, R.; Binod, P.; Pandey, A. Biological Pretreatment of Lignocellulosic Biomass—An Overview. Bioresour. Technol. 2016, 199, 76–82. [Google Scholar] [CrossRef]
- Pramanik, K.; Sahu, S. Biological Treatment of Lignocellulosic Biomass to Bioethanol. Adv. Biotechnol. Microbiol. 2017, 5, 555674. [Google Scholar] [CrossRef]
- Itoh, H.; Wada, M.; Honda, Y.; Kuwahara, M.; Watanabe, T. Bioorganosolve Pretreatments for Simultaneous Saccharification and Fermentation of Beech Wood by Ethanolysis and White Rot Fungi. J. Biotechnol. 2003, 103, 273–280. [Google Scholar] [CrossRef]
- Kumar, A.G.; Sekaran, G.; Krishnamoorthy, S. Solid State Fermentation of Achras Zapota Lignocellulose by Phanerochaete Chrysosporium. Bioresour. Technol. 2006, 97, 1521–1528. [Google Scholar] [CrossRef]
- Nazarpour, F.; Kuang Abdullah, D.; Abdullah, N.; Zamiri, R.; My, N.A. Evaluation of Biological Pretreatment of Rubberwood with White Rot Fungi for Enzymatic Hydrolysis. Materials 2013, 6, 2059–2073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, C.; Li, Y. Effectiveness of Microbial Pretreatment by Ceriporiopsis subvermispora on Different Biomass Feedstocks. Bioresour. Technol. 2011, 102, 7507–7512. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Chinn, M.S.; Sharma-Shivappa, R.R. Microbial Pretreatment of Cotton Stalks by Solid State Cultivation of Phanerochaete Chrysosporium. Bioresour. Technol. 2008, 99, 6556–6564. [Google Scholar] [CrossRef] [PubMed]
- Asgher, M.; Asad, M.J.; Legge, R.L. Enhanced Lignin Peroxidase Synthesis by Phanerochaete Chrysosporium in Solid State Bioprocessing of a Lignocellulosic Substrate. World J. Microbiol. Biotechnol. 2006, 22, 449–453. [Google Scholar] [CrossRef]
- Knežević, A.; Đokić, I.; Tosti, T.; Popović, S.; Vukojević, J. Biological Pretreatment of Wheat Straw: Effect of Fungal Culturing on Enzymatic Hydrolysis of Carbohydrate. Polymers 2021. preprint. [Google Scholar] [CrossRef]
- Zhou, S.; Herpoël-Gimbert, I.; Grisel, S.; Sigoillot, J.C.; Sergent, M.; Raouche, S. Biological Wheat Straw Valorization: Multicriteria Optimization of Polyporus Brumalis Pretreatment in Packed Bed Bioreactor. Microbiologyopen 2018, 7, e00530. [Google Scholar] [CrossRef] [Green Version]
- Du, W.; Yu, H.; Song, L.; Zhang, J.; Weng, C.; Ma, F.; Zhang, X. The Promoting Effect of Byproducts from Irpex Lacteus on Subsequent Enzymatic Hydrolysis of Bio-Pretreated Cornstalks. Biotechnol. Biofuels 2011, 4, 37–45. [Google Scholar] [CrossRef] [Green Version]
- Mienda, B.S.; Idi, A.; Umar, A. Microbiological Features of Solid State Fermentation and Its Applications—An Overview. Res. Biotechnol. 2011, 2, 21–26. [Google Scholar]
- Sahuand, S.; Pramanik, K. Evaluating Fungal Mixed Culture for Pretreatment of Cotton Gin Waste to Bioethanol by Enzymatic Hydrolysis and Fermentation Using Co-Culture. Pol. J. Environ. Stud. 2017, 26, 1215–1223. [Google Scholar] [CrossRef]
- Martău, G.A.; Unger, P.; Schneider, R.; Venus, J.; Vodnar, D.C.; López-Gómez, J.P. Integration of Solid State and Submerged Fermentations for the Valorization of Organic Municipal Solid Waste. J. Fungi. 2021, 7, 766. [Google Scholar] [CrossRef]
- Vasco-Correa, J.; Shah, A. Techno-Economic Bottlenecks of the Fungal Pretreatment of Lignocellulosic Biomass. Fermentation 2019, 5, 30. [Google Scholar] [CrossRef]
- Hasanly, A.; Talkhoncheh, M.K.; Alavijeh, M.K. Techno-Economic Assessment of Bioethanol Production from Wheat Straw: A Case Study of Iran. Clean Technol. Environ. Policy 2018, 20, 357–377. [Google Scholar] [CrossRef]
- Harun, N.Y.; Parvez, A.; Afzal, M. Process and Energy Analysis of Pelleting Agricultural and Woody Biomass Blends. Sustainability 2018, 10, 1770. [Google Scholar] [CrossRef] [Green Version]
- Gilvari, H.; de Jong, W.; Schott, D.L. Quality Parameters Relevant for Densification of Bio-Materials: Measuring Methods and Affecting Factors—A Review. Biomass. Bioenergy 2019, 120, 117–134. [Google Scholar] [CrossRef]
- Adapa, P.K.; Tabil, L.G.; Schoenau, G.J. Factors Affecting the Quality of Biomass Pellet for Biofuel and Energy Analysis of Pelleting Proc. Int. J. Agric. Biol. Eng. 2013, 6, 1–12. [Google Scholar] [CrossRef]
- Bak, J.S.; Ko, J.K.; Choi, I.-G.; Park, Y.-C.; Seo, J.-H.; Kim, K.H. Fungal Pretreatment of Lignocellulose by Phanerochaete Chrysosporium to Produce Ethanol from Rice Straw. Biotechnol. Bioeng. 2009, 104, 471–482. [Google Scholar] [CrossRef]
- Gao, W.; Tabil, L.G.; Dumonceaux, T.; Ríos, S.E.; Zhao, R. Optimization of Biological Pretreatment to Enhance the Quality of Wheat Straw Pellets. Biomass Bioenergy 2017, 97, 77–89. [Google Scholar] [CrossRef]
- Olughu, O.O.; Tabil, L.G.; Dumonceaux, T.; Mupondwa, E.; Cree, D. Comparative Study on Quality of Fuel Pellets from Switchgrass Treated with Different White-rot Fungi. Energy 2021, 14, 7670. [Google Scholar] [CrossRef]
- Gao, W.; Tabil, L.G.; Liu, Q.; Zhao, R.; Zhang, M. Experimental Study on the Effect of Solid-State Fermentation on Pellet Density and Strength of Corn Stover. J. Biobased Mater. Bioenergy 2019, 13, 840–847. [Google Scholar] [CrossRef]
- Shi, J.; Sharma-Shivappa, R.R.; Chinn, M.; Howell, N. Effect of Microbial Pretreatment on Enzymatic Hydrolysis and Fermentation of Cotton Stalks for Ethanol Production. Biomass Bioenergy 2009, 33, 88–96. [Google Scholar] [CrossRef]
- Dumonceaux, T.; Bartholomew, K.; Valeanu, L.; Charles, T.; Archibald, F. Cellobiose Dehydrogenase Is Essential for Wood Invasion and Nonessential for Kraft Pulp Delignification by Trametes Versicolor. Enzym. Microb. Technol. 2001, 29, 478–489. [Google Scholar] [CrossRef]
- Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, A.; Sluiter, J.; Templeton, D. Preparation of Samples for Compositional Analysis. Laboratory Analytical Procedure (LAP). Tech. Rep. NREL/TP-510-42620. Natl. Renew. Energy Lab. 2008, 1–9. [Google Scholar]
- Sluiter, A.; Hames, B.; Ruiz, R.O.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D.; Energy, D. of Determination of Structural Carbohydrates and Lignin in Biomass. National Renewable Energy Laboratory (NREL) Laboratory Analytical Procedures (LAP) for Standard Biomass Analysis. Biomass Anal. Technol. Team Lab. Anal. Proced. 2007, 2011, 1–14. [Google Scholar]
- Resch, M.G.; Baker, J.O.; Decker, S.R. Low Solids Enzymatic Saccharification of Lignocellulosic Biomass NREL/TP-510-42629; Technical Report NREL/TP-5100-63351; National Renewable Energy Laboratory: Golden, CO, USA, 2015; pp. 1–9. [Google Scholar]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 2002, 31, 426–428. [Google Scholar] [CrossRef]
- Mæhre, H.K.; Dalheim, L.; Edvinsen, G.K.; Elvevoll, E.O.; Jensen, I.J. Protein Determination—Method Matters. Foods 2018, 7, 5. [Google Scholar] [CrossRef] [Green Version]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Ash in Biomass; Technical Report NREL/TP-510-42622; National Renewable Energy Laboratory: Golden, CO, USA, 2005. [Google Scholar]
- Hoover, A.; Emerson, R.; Williams, C.L.; Ramirez-Corredores, M.M.; Ray, A.; Schaller, K.; Hernandez, S.; Li, C.; Walton, M. Grading Herbaceous Biomass for Biorefineries: A Case Study Based on Chemical Composition and Biochemical Conversion. Bioenergy Res. 2019, 12, 977–991. [Google Scholar] [CrossRef] [Green Version]
- Hu, Z.; Sykes, R.; Davis, M.F.; Brummer, E.C.; Ragauskas, A.J. Chemical Profiles of Switchgrass. Bioresour. Technol. 2010, 101, 3253–3257. [Google Scholar] [CrossRef]
- Hu, Z.; Wen, Z. Enhancing Enzymatic Digestibility of Switchgrass by Microwave-Assisted Alkali Pretreatment. Biochem. Eng. J. 2008, 38, 369–378. [Google Scholar] [CrossRef]
- Karunanithy, C.; Muthukumarappan, K. Optimization of Switchgrass and Extruder Parameters for Enzymatic Hydrolysis Using Response Surface Methodology. Ind. Crops Prod. 2011, 33, 188–199. [Google Scholar] [CrossRef]
- Canam, T.; Town, J.R.; Tsang, A.; McAllister, T.A.; Dumonceaux, T.J. Biological Pretreatment with a Cellobiose Dehydrogenase-Deficient Strain of Trametes Versicolor Enhances the Biofuel Potential of Canola Straw. Bioresour. Technol. 2011, 102, 10020–10027. [Google Scholar] [CrossRef]
- Kalinoski, R.M.; Flores, H.D.; Thapa, S.; Tuegel, E.R.; Bilek, M.A.; Reyes-Mendez, E.Y.; West, M.J.; Dumonceaux, T.J.; Canam, T. Pretreatment of Hardwood and Miscanthus with Trametes Versicolor for Bioenergy Conversion and Densification Strategies. Appl. Biochem. Biotechnol. 2017, 183, 1401–1413. [Google Scholar] [CrossRef] [PubMed]
- Kamcharoen, A.; Champreda, V.; Eurwilaichitr, L.; Boonsawang, P. Screening and Optimization of Parameters Affecting Fungal Pretreatment of Oil Palm Empty Fruit Bunch (EFB) by Experimental Design. Int. J. Energy Environ. Eng. 2014, 5, 303–312. [Google Scholar] [CrossRef] [Green Version]
- Shirkavand, E.; Baroutian, S.; Gapes, D.J.; Young, B.R. Pretreatment of Radiata Pine Using Two White Rot Fungal Strains Stereum Hirsutum and Trametes Versicolor. Energy Convers. Manag. 2017, 142, 13–19. [Google Scholar] [CrossRef]
- Yadav, D.; Wati, L. Microbial Delignification and Hydrolysis of Paddy Straw for Ethanol Production. Agric. Res. J. 2016, 53, 528–531. [Google Scholar] [CrossRef]
- Zadrazil, F.; Puniya, A.K. Studies on the Effect of Particle Size on Solid-State Fermentation of Sugarcane Bagasse into Animal Feed Using White-Rot Fungi. Bioresour. Technol. 1995, 54, 85–87. [Google Scholar] [CrossRef]
- Membrillo, I.; Sánchez, C.; Meneses, M.; Favela, E.; Loera, O. Effect of Substrate Particle Size and Additional Nitrogen Source on Production of Lignocellulolytic Enzymes by Pleurotus Ostreatus Strains. Bioresour. Technol. 2008, 99, 7842–7847. [Google Scholar] [CrossRef]
- Reid, I.D. Solid-State Fermentations for Biological Delignification. Enzym. Microb. Technol. 1989, 11, 786–803. [Google Scholar] [CrossRef]
- Wan, C.; Li, Y. Fungal Pretreatment of Lignocellulosic Biomass. Biotechnol. Adv. 2012, 30, 1447–1457. [Google Scholar] [CrossRef]
- Velmurugan, R.; Muthukumar, K. Ultrasound-Assisted Alkaline Pretreatment of Sugarcane Bagasse for Fermentable Sugar Production: Optimization through Response Surface Methodology. Bioresour. Technol. 2012, 112, 293–299. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, J.; He, J.; Liu, Z.; Yu, Z. Combinations of Mild Physical or Chemical Pretreatment with Biological Pretreatment for Enzymatic Hydrolysis of Rice Hull. Bioresour. Technol. 2009, 100, 903–908. [Google Scholar] [CrossRef]
- Mupondwa, E.; Li, X.; Tabil, L. Large-Scale Commercial Production of Cellulosic Ethanol from Agricultural Residues: A Case Study of Wheat Straw in the Canadian Prairies. Biofuels Bioprod. Biorefining 2017, 11, 955–970. [Google Scholar] [CrossRef]
- Liu, J.; Wang, M.L.; Tonnis, B.; Habteselassie, M.; Liao, X.; Huang, Q. Fungal Pretreatment of Switchgrass for Improved Saccharification and Simultaneous Enzyme Production. Bioresour. Technol. 2013, 135, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Ma, F.; Zhang, X.; Chen, S. Biological Pretreatment of Corn Stover by Irpex Lacteus for Enzymatic Hydrolysis. J. Agric. Food Chem. 2010, 58, 10893–10898. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ren, X.; Chen, W.; Bao, J. Biological Pretreatment of Corn Stover by Solid State Fermentation of Phanerochaete Chrysosporium. Front. Chem. Sci. Eng. 2012, 6, 146–151. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, X.; Song, L.; Ke, J.; Xu, C.; Du, W.; Zhang, J. Evaluation of White-Rot Fungi-Assisted Alkaline/Oxidative Pretreatment of Corn Straw Undergoing Enzymatic Hydrolysis by Cellulase. J. Biosci. Bioeng. 2010, 110, 660–664. [Google Scholar] [CrossRef]
- Liu, J.; Sidhu, S.S.; Wang, M.L.; Tonnis, B.; Habteselassie, M.; Mao, J.; Huang, Q. Evaluation of Various Fungal Pretreatment of Switchgrass for Enhanced Saccharification and Simultaneous Enzyme Production. J. Clean. Prod. 2015, 104, 480–488. [Google Scholar] [CrossRef]
- Olughu, O.O.; Tabil, L.G.; Dumonceaux, T.; Mupondwa, E.; Cree, D. Physicochemical and Ultrastructural Changes in Fungal Treated Switchgrass and Their Impact on Enzymatic Digestibility. Bioresour. Technol. Rep. 2022, 20, 101211. [Google Scholar] [CrossRef]
- Mani, S.; Tabil, L.G.; Sokhansanj, S. Grinding Performance and Physical Properties of Wheat and Barley Straws, Corn Stover and Switchgrass. Biomass Bioenergy 2004, 27, 339–352. [Google Scholar] [CrossRef]
- Xu, J.; Cheng, J.J.; Sharma-Shivappa, R.R.; Burns, J.C. Sodium Hydroxide Pretreatment of Switchgrass for Ethanol Production. Energy Fuels 2010, 24, 2113–2119. [Google Scholar] [CrossRef]
- Capolupo, L.; Faraco, V. Green Methods of Lignocellulose Pretreatment for Biorefinery Development. Appl. Microbiol. Biotechnol. 2016, 100, 9451–9467. [Google Scholar] [CrossRef] [Green Version]
- García-Torreiro, M.; López-Abelairas, M.; Lu-Chau, T.A.; Lema, J.M. Fungal Pretreatment of Agricultural Residues for Bioethanol Production. Ind. Crops Prod. 2016, 89, 486–492. [Google Scholar] [CrossRef]
- Tiwari, R.; Rana, S.; Singh, S.; Arora, A.; Kaushik, R.; Agrawal, V.V.; Saxena, A.K.; Nain, L. Biological Delignification of Paddy Straw and Parthenium Sp. Using a Novel Micromycete Myrothecium Roridum LG7 for Enhanced Saccharification. Bioresour. Technol. 2013, 135, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.; Li, Y. Microbial Delignification of Corn Stover by Ceriporiopsis Subvermispora for Improving Cellulose Digestibility. Enzym. Microb. Technol. 2010, 47, 31–36. [Google Scholar] [CrossRef]
- Zhou, S.; Raouche, S.; Grisel, S.; Sigoillot, J.-C.; Herpoël-Gimbert, I. Efficient Biomass Pretreatment Using the White-Rot Fungus Polyporus Brumalis. Fungal Genom. Biol. 2017, 7, 1–6. [Google Scholar] [CrossRef]
- Gao, W.; Lei, Z.; Tabil, L.G.; Zhao, R. Biological Pretreatment by Solid-State Fermentation of Oat Straw to Enhance Physical Quality of Pellets. J. Chem. 2020, 2020, 3060475. [Google Scholar] [CrossRef]
- Wiberg, C. Understanding Ash Fusibility. Biomass Magazine, 26 May 2020. Available online: http://biomassmagazine.com/articles/17068/understanding-ash(accessed on 1 October 2022).
- Wang, Y.; Shao, Y.; Matovic, M.D.; Whalen, J.K. Exploring Switchgrass and Hardwood Combustion on Excess Air and Ash Fouling/Slagging Potential: Laboratory Combustion Test and Thermogravimetric Kinetic Analysis. Energy Convers. Manag. 2015, 97, 409–419. [Google Scholar] [CrossRef]


| Code | Actual Value | |||
|---|---|---|---|---|
| Fermentation Temperature, (°C) | Fermentation Temperature, (d) | Inoculum Concentration, (mL) | Hammer-Mill Screen Size, (mm) | |
| 1 | 34 | 35 | 15 | 6.4 |
| 0 | 28 | 28 | 10 | 3.2 |
| −1 | 22 | 21 | 5 | 1.6 |
| Fungal Strain | Analysis of Variance (ANOVA) | |||||
|---|---|---|---|---|---|---|
| Sum of | Mean | F-Value | p-Value | |||
| Source | Squares | df | Square | |||
| Model | 5492.82 | 7 | 784.69 | 6.30 | 0.0005 | |
| 824.01 | 1 | 824.01 | 6.61 | 0.0178 | ||
| 573.95 | 1 | 573.95 | 4.61 | 0.0437 | ||
| 568.79 | 1 | 568.79 | 4.56 | 0.0446 | ||
| P. chrysosporium | 896.13 | 1 | 896.13 | 7.19 | 0.0140 | |
| 1810.57 | 1 | 1810.57 | 14.53 | 0.0010 | ||
| 1052.01 | 1 | 1052.01 | 8.44 | 0.0085 | ||
| 1661.83 | 1 | 1661.83 | 13.33 | 0.0015 | ||
| Lack of Fit | 2388.60 | 17 | 140.51 | 2.46 | 0.1989 | |
| Model | 3761.84 | 5 | 752.37 | 8.93 | <0.0001 | |
| 373.14 | 1 | 373.14 | 4.43 | 0.0465 | ||
| 1540.75 | 1 | 1540.75 | 18.29 | 0.0003 | ||
| T. versicolor 52J | 734.56 | 1 | 734.56 | 8.72 | 0.0071 | |
| 470.95 | 1 | 470.95 | 5.59 | 0.0269 | ||
| 788.78 | 1 | 788.78 | 9.36 | 0.0056 | ||
| Lack of Fit | 1383.35 | 19 | 72.81 | 0.53 | 0.8487 | |
| Model | 930.30 | 3 | 310.10 | 6.60 | 0.0019 | |
| 543.35 | 1 | 543.35 | 11.57 | 0.0023 | ||
| T. versicolor m4D | 176.19 | 1 | 176.19 | 3.75 | 0.0641 | |
| 210.76 | 1 | 210.76 | 4.49 | 0.0442 | ||
| Lack of Fit | 1126.15 | 21 | 53.63 | 4.50 | 0.0769 | |
| Fungal Strain | Total Available Carbohydrate | Cellulose Loss | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sum of | Mean | F-Value | p-Value | Sum of | Mean | F-Value | p-Value | ||||
| Source | Squares | df | Square | Squares | df | Square | |||||
| PC | Model | 515.20 | 4 | 128.80 | 4.33 | 0.0089 | 1390.65 | 3 | 463.55 | 4.30 | 0.0142 |
| 192.80 | 1 | 192.80 | 6.48 | 0.0178 | 615.62 | 1 | 615.62 | 5.70 | 0.0248 | ||
| 113.42 | 1 | 113.42 | 3.81 | 0.0627 | 361.31 | 1 | 361.31 | 3.35 | 0.0792 | ||
| 92.81 | 1 | 92.81 | 3.12 | 0.0902 | 413.72 | 1 | 413.72 | 3.83 | 0.0615 | ||
| 143.96 | 1 | 143.96 | 4.84 | 0.0378 | 2451.76 | 21 | 116.75 | 1.90 | 0.2833 | ||
| Lack of Fit | 680.58 | 20 | 34.03 | 4.01 | 0.0933 | ||||||
| Model | 361.64 | 3 | 120.55 | 5.19 | 0.0063 | 1753.37 | 3 | 584.46 | 7.26 | 0.0012 | |
| 197.64 | 1 | 197.64 | 8.51 | 0.0074 | 937.40 | 1 | 937.40 | 11.65 | 0.0022 | ||
| Tv52J | 94.09 | 1 | 94.09 | 4.05 | 0.0550 | 461.72 | 1 | 461.72 | 5.74 | 0.0244 | |
| 69.91 | 1 | 69.91 | 3.01 | 0.0951 | 354.25 | 1 | 354.25 | 4.40 | 0.0462 | ||
| Lack of Fit | 526.85 | 21 | 25.09 | 1.87 | 0.2890 | 1870.86 | 21 | 89.09 | 2.53 | 0.1905 | |
| Model | 611.05 | 3 | 203.68 | 6.06 | 0.0030 | 2813.24 | 3 | 937.75 | 5.35 | 0.0055 | |
| 141.11 | 1 | 141.11 | 4.20 | 0.0511 | 2813.24 | 3 | 937.75 | 5.35 | 0.0055 | ||
| Tvm4D | 382.17 | 1 | 382.17 | 11.37 | 0.0024 | 1672.41 | 1 | 1672.41 | 9.54 | 0.0049 | |
| 143.12 | 1 | 143.12 | 4.26 | 0.0496 | 727.02 | 1 | 727.02 | 4.15 | 0.0524 | ||
| Lack of Fit | 674.34 | 21 | 32.11 | 0.78 | 0.6942 | 3562.31 | 21 | 169.63 | 0.83 | 0.6624 | |
| Fungal Strain | Solution Number | X1 (°C) | X2 (d) | X3 (mL) | X4 (mm) | TAC (%) | Delignification (%) | CL (%) |
|---|---|---|---|---|---|---|---|---|
| Untreated | _ | _ | 0 | 3.2 | 51.4 | 0.0 | 0.0 | |
| Tvm4D | 1 | 34.0 | 35.0 | 15.0 | 1.9 | 63.0 | 22.5 | −14.4 |
| 2 | 22.0 | 31.0 | 5.0 | 6.4 | 61.4 | 13.2 | −11.0 | |
| TV52J | 1 | 34.0 | 35.0 | 15.0 | 6.4 | 66.2 | 14.1 | −20.3 |
| 2 | 22.0 | 21.0 | 5.0 | 3.2 | 58.1 | 21.5 | −2.6 | |
| PC | 1 | 34.0 | 21.0 | 15.0 | 1.6 | 66.2 | 42.5 | −11.77 |
| 2 | 22.0 | 21.0 | 5.0 | 6.4 | 58.2 | 39.9 | 2.6 |
| Sample | Unit Density (kg m−3) | Relaxed Density (kg m−3) | Tensile Strength (MPa) | Porosity (%) |
|---|---|---|---|---|
| Untreated | 1075.01 ± 81.25 | 984.36 ± 113.54 | 1.03 ± 0.28 | 15.7 |
| Pe-T2 | 987.19 ± 66.58 | 947.24 ± 62.03 | 0.65 ± 0.28 | 28.3 |
| Pe-P2 | 898.45 ± 99.94 | 852.34 ± 52.56 | 0.87 ± 0.19 | 16.7 |
| Sample | Nitrogen (%) | Carbon (%) | Hydrogen (%) | Sulfur (%) | Ash Content (%) | Oxygen (%) |
|---|---|---|---|---|---|---|
| Untreated | 0.25 ± 0.01 | 44.98± 0.49 | 6.18 ± 0.08 | 0.05 ± 0.03 | 3.25 ± 0.43 | 45.29 |
| Tv 52J-2 | 0.33 ± 0.02 | 44.42 ± 0.15 | 6.10 ± 0.01 | 0.07 ± 0.00 | 2.68 ± 0.70 | 46.40 |
| PC-2 | 0.22 ± 0.06 | 44.47 ± 0.13 | 6.09 ± 0.00 | 0.16 ± 0.00 | 3.78 ± 0.10 | 45.28 |
| Elements | Untreated | Tv 52J-2 | PC-2 |
|---|---|---|---|
| Basic Elements | |||
| K | 3.78 | 2.42 | 3.92 |
| Ca | 4.26 | 4.30 | 5.11 |
| Mg | 1.47 | 1.36 | 1.74 |
| Na | 0.07 | 0.09 | 0.10 |
| P | 1.38 | 1.36 | 2.04 |
| S | 1.13 | 1.01 | 1.38 |
| Al | 0.07 | 0.09 | 0.11 |
| Subtotal | 12.16 | 10.63 | 14.40 |
| Heavy Metals | |||
| Fe | 0.119 | 0.099 | 0.147 |
| Cu | 0.008 | 0.005 | 0.007 |
| Zn | 0.025 | 0.022 | 0.045 |
| Mn | 0.025 | 0.029 | 0.036 |
| Pb | 0.000 | 0.001 | 0.001 |
| Cr | 0.001 | 0.001 | 0.001 |
| Co | 0.003 | 0.001 | 0.001 |
| Mo | 0.004 | 0.004 | 0.003 |
| Ni | 0.005 | 0.005 | 0.005 |
| Subtotal | 0.19 | 0.17 | 0.25 |
| Total | 12.35 | 10.79 | 14.65 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Onu Olughu, O.; Tabil, L.G.; Dumonceaux, T.; Mupondwa, E.; Cree, D. Optimization of Solid-State Fermentation of Switchgrass Using White-Rot Fungi for Biofuel Production. Fuels 2022, 3, 730-752. https://doi.org/10.3390/fuels3040043
Onu Olughu O, Tabil LG, Dumonceaux T, Mupondwa E, Cree D. Optimization of Solid-State Fermentation of Switchgrass Using White-Rot Fungi for Biofuel Production. Fuels. 2022; 3(4):730-752. https://doi.org/10.3390/fuels3040043
Chicago/Turabian StyleOnu Olughu, Onu, Lope G. Tabil, Tim Dumonceaux, Edmund Mupondwa, and Duncan Cree. 2022. "Optimization of Solid-State Fermentation of Switchgrass Using White-Rot Fungi for Biofuel Production" Fuels 3, no. 4: 730-752. https://doi.org/10.3390/fuels3040043
APA StyleOnu Olughu, O., Tabil, L. G., Dumonceaux, T., Mupondwa, E., & Cree, D. (2022). Optimization of Solid-State Fermentation of Switchgrass Using White-Rot Fungi for Biofuel Production. Fuels, 3(4), 730-752. https://doi.org/10.3390/fuels3040043











