Minireview: Intensified Low-Temperature Fischer–Tropsch Reactors for Sustainable Fuel Production
Abstract
1. Introduction
2. Fischer–Tropsch Synthesis Reaction
3. Commercial FTS Reactors
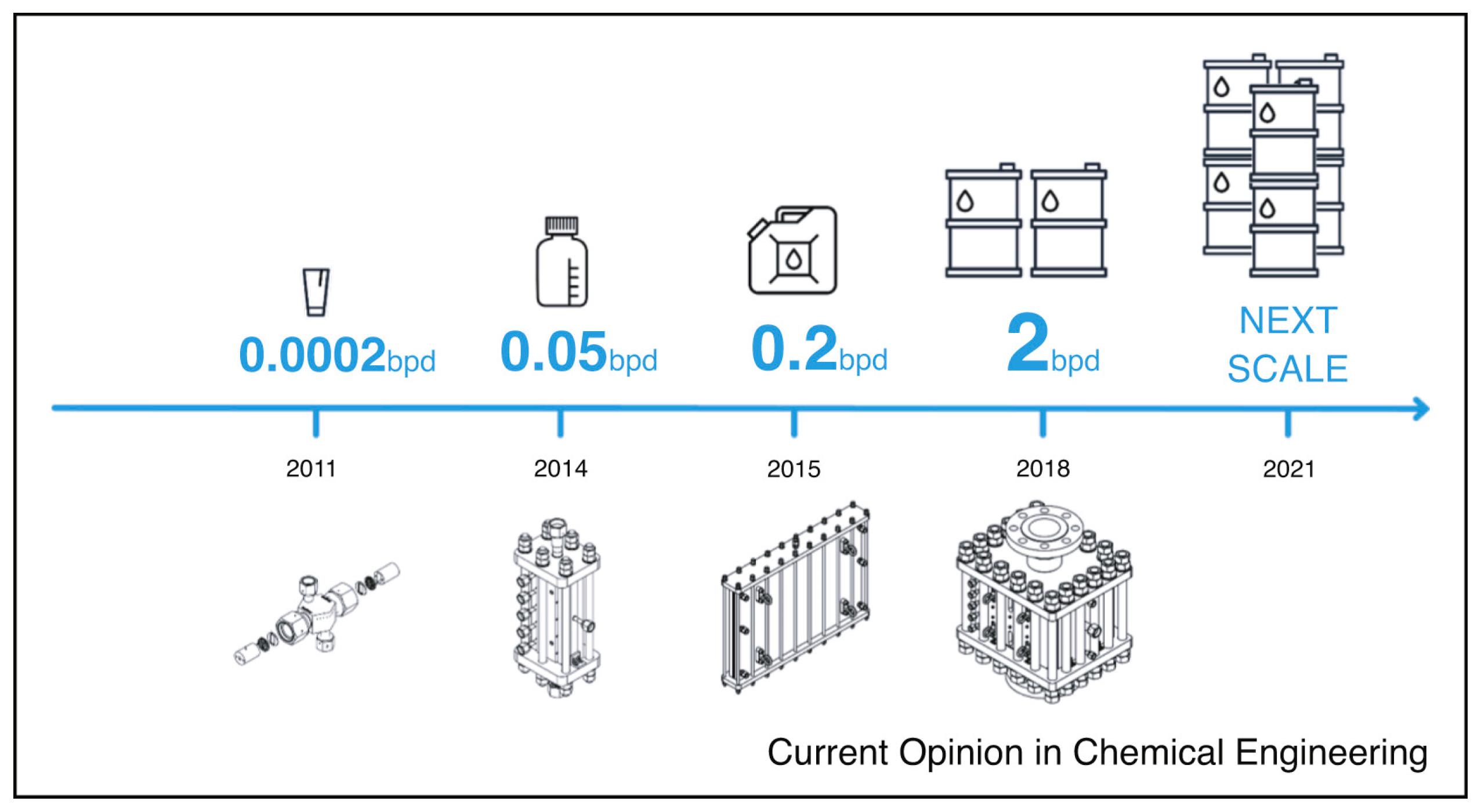
4. Aim of This Minireview
5. Catalyst for Decentralized and Intensified LTFT Reactors
6. Emerging Intensified LTFT Reactors
6.1. Heat Conducting Support
6.2. Microchannel Reactors
6.3. Structured Reactors (Monoliths, Heat-Conducting Inserts, and …)
Random and Ordered Porous Metallic Structures
6.4. Cross Flow Structures
6.5. Heat Adsorbing Materials
6.6. Coupled Reactors
6.7. Fixed-Bed Membrane Reactor
6.8. Catalyst Loading
6.9. Industrialization of Intensified LTFT Technologies
- 2010: 1 BPD SGC Energia, Güssing, Austria; 2011: Petrobras, Fortaleza, Brazil, 6 BPD GTL; and 2012: SGC Energia, Brazil, 50 BPD BTL
7. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LTFT | Low-Temperature Fischer–Tropsch |
| FTS | Fischer–Tropsch Synthesis |
| LCA | Life Cycle Assessment |
| BTL | Biomass-to-Liquid |
| BPD | Barrel per day |
| GTL | Gas-to-Liquid |
| ASF | Anderson–Schulz–Flory |
| WGS | Water Gas Shift |
| rev-WGS | Reverse Water Gas Shift |
| MTFBR | Multi-Tubular Fixed-Bed Reactor |
| SBCR | Slurry Bubble Column Reactor |
| SMDS | Shell Middle Distillate Synthesis |
| PtX | Power-to-X |
| GHSV | Gas Hourly Space Velocity |
| SCR | Self-Catalytic Reactor |
| POCS | Periodic Open Cellular Structure |
| CFR | Cross Flow Reactor |
| PCM | Phase Change Material |
| TRL | Technology Readiness Level |
References
- Schwarz, A.E.; Ligthart, T.N.; Godoi Bizarro, D.; De Wild, P.; Vreugdenhil, B.; van Harmelen, T. Plastic Recycling in a Circular Economy; Determining Environmental Performance through an LCA Matrix Model Approach. Waste Manag. 2021, 121, 331–342. [Google Scholar] [CrossRef]
- Dong, J.; Tang, Y.; Nzihou, A.; Chi, Y.; Weiss-Hortala, E.; Ni, M.; Zhou, Z. Comparison of Waste-to-Energy Technologies of Gasification and Incineration Using Life Cycle Assessment: Case Studies in Finland, France and China. J. Clean. Prod. 2018, 203, 287–300. [Google Scholar] [CrossRef]
- Ouedraogo, A.S.; Frazier, R.S.; Kumar, A. Comparative Life Cycle Assessment of Gasification and Landfilling for Disposal of Municipal Solid Wastes. Energies 2021, 14, 7032. [Google Scholar] [CrossRef]
- Bachmann, M.; Völker, S.; Kleinekorte, J.; Bardow, A. Syngas from What? Comparative Life-Cycle Assessment for Syngas Production from Biomass, CO2, and Steel Mill Off-Gases. ACS Sustain. Chem. Eng. 2023, 11, 5356–5366. [Google Scholar] [CrossRef]
- Weeda, M.; Segers, R. The Dutch Hydrogen Balance, and the Current and Future Representation of Hydrogen in the Energy Statistics; TNO Public Report P10915; TNO: The Hague, The Netherlands, 2020; Available online: https://open.overheid.nl/documenten/ronl-dca8a3f6-1f43-44eb-b8a9-63c496bd2f57/pdf (accessed on 10 March 2024).
- Apolinar-Hernández, J.E.; Bertoli, S.L.; Riella, H.G.; Soares, C.; Padoin, N. An Overview of Low-Temperature Fischer–Tropsch Synthesis: Market Conditions, Raw Materials, Reactors, Scale-Up, Process Intensification, Mechanisms, and Outlook. Energy Fuels 2024, 38, 1–28. [Google Scholar] [CrossRef]
- Wright, M.M.; Brown, R.C.; Boateng, A.A. Distributed Processing of Biomass to Bio-Oil for Subsequent Production of Fischer-Tropsch Liquids. Biofuels Bioprod. Biorefining 2008, 2, 229–238. [Google Scholar] [CrossRef]
- Branco, D.A.C.; Szklo, A.S.; Schaeffer, R. Co2e Emissions Abatement Costs of Reducing Natural Gas Flaring in Brazil by Investing in Offshore GTL Plants Producing Premium Diesel. Energy 2010, 35, 158–167. [Google Scholar]
- Hu, J.; Yu, F.; Lu, Y. Application of Fischer–Tropsch Synthesis in Biomass to Liquid Conversion. Catalysts 2012, 2, 303–326. [Google Scholar] [CrossRef]
- Saeidi, S.; Nikoo, M.K.; Mirvakili, A.; Bahrani, S.; Saidina Amin, N.A.; Rahimpour, M.R. Recent Advances in Reactors for Low-Temperature Fischer-Tropsch Synthesis: Process Intensification Perspective. Rev. Chem. Eng. 2015, 31, 209–238. [Google Scholar] [CrossRef]
- Fratalocchi, L.; Groppi, G.; Visconti, C.G.; Lietti, L.; Tronconi, E. Adoption of 3D Printed Highly Conductive Periodic Open Cellular Structures as an Effective Solution to Enhance the Heat Transfer Performances of Compact Fischer-Tropsch Fixed-Bed Reactors. Chem. Eng. J. 2020, 386, 123988. [Google Scholar] [CrossRef]
- Fratalocchi, L.; Visconti, C.G.; Groppi, G.; Lietti, L.; Tronconi, E. Intensifying Heat Transfer in Fischer-Tropsch Tubular Reactors through the Adoption of Conductive Packed Foams. Chem. Eng. J. 2018, 349, 829–837. [Google Scholar] [CrossRef]
- Cheng, K.; Kang, J.; King, D.L.; Subramanian, V.; Zhou, C.; Zhang, Q.; Wang, Y. Chapter Three—Advances in Catalysis for Syngas Conversion to Hydrocarbons. In Advances in Catalysis; Song, C., Ed.; Academic Press: Cambridge, MA, USA, 2017; Volume 60, pp. 125–208. [Google Scholar] [CrossRef]
- Hondo, E.; Lu, P.; Zhang, P.; Li, J.; Tsubaki, N. Direct Production of Hydrocarbons by Fischer-Tropsch Synthesis Using Newly Designed Catalysts. J. Jpn. Pet. Inst. 2020, 63, 239–247. [Google Scholar] [CrossRef]
- Scholman, E.; van der Schaaf, J.; d’Angelo, M.N.; Aguirre, A.; Filot, I. Open Foam Catalysts for Fischer-Tropsch Synthesis and Reactor Development; Technical University of Eindhoven: Eindhoven, The Netherlands, 2019; Available online: https://pure.tue.nl/ws/portalfiles/portal/195223909/Afstudeerverslag_Esther_Scholman_0910458_pdf (accessed on 1 March 2024).
- de Klerk, A. Fischer–Tropsch Fuels Refinery Design. Energy Environ. Sci. 2011, 4, 1177–1205. [Google Scholar] [CrossRef]
- Li, J.; He, Y.; Tan, L.; Zhang, P.; Peng, X.; Oruganti, A.; Yang, G.; Abe, H.; Wang, Y.; Tsubaki, N. Integrated Tuneable Synthesis of Liquid Fuels via Fischer–Tropsch Technology. Nat. Catal. 2018, 1, 787–793. [Google Scholar] [CrossRef]
- Yamane, N.; Wang, Y.; Li, J.; He, Y.; Zhang, P.; Nguyen, L.; Tan, L.; Ai, P.; Peng, X.; Wang, Y.; et al. Building Premium Secondary Reaction Field with a Miniaturized Capsule Catalyst to Realize Efficient Synthesis of a Liquid Fuel Directly from Syngas. Catal. Sci. Technol. 2017, 7, 1996–2000. [Google Scholar] [CrossRef]
- Li, J.; Yang, G.; Yoneyama, Y.; Vitidsant, T.; Tsubaki, N. Jet Fuel Synthesis via Fischer–Tropsch Synthesis with Varied 1-Olefins as Additives Using Co/ZrO2–SiO2 Bimodal Catalyst. Fuel 2016, 171, 159–166. [Google Scholar] [CrossRef]
- Bao, J.; He, J.; Zhang, Y.; Yoneyama, Y.; Tsubaki, N. A Core/Shell Catalyst Produces a Spatially Confined Effect and Shape Selectivity in a Consecutive Reaction. Angew. Chem. Int. Ed. 2008, 47, 353–356. [Google Scholar] [CrossRef]
- Li, J.; Sun, J.; Fan, R.; Yoneyama, Y.; Yang, G.; Tsubaki, N. Selectively Converting Biomass to Jet Fuel in Large-Scale Apparatus. ChemCatChem 2017, 9, 2668–2674. [Google Scholar] [CrossRef]
- Kang, J.; Cheng, K.; Zhang, L.; Zhang, Q.; Ding, J.; Hua, W.; Lou, Y.; Zhai, Q.; Wang, Y. Mesoporous Zeolite-Supported Ruthenium Nanoparticles as Highly Selective Fischer–Tropsch Catalysts for the Production of C5–C11 Isoparaffins. Angew. Chem. Int. Ed. 2011, 50, 5200–5203. [Google Scholar] [CrossRef]
- Zhang, Q.; Kang, J.; Wang, Y. Development of Novel Catalysts for Fischer–Tropsch Synthesis: Tuning the Product Selectivity. ChemCatChem 2010, 2, 1030–1058. [Google Scholar] [CrossRef]
- Sun, J.; Yang, G.; Peng, X.; Kang, J.; Wu, J.; Liu, G.; Tsubaki, N. Beyond Cars: Fischer-Tropsch Synthesis for Non-Automotive Applications. ChemCatChem 2019, 11, 1412–1424. [Google Scholar] [CrossRef]
- Tsakoumis, N.E.; Rønning, M.; Borg, Ø.; Rytter, E.; Holmen, A. Deactivation of Cobalt Based Fischer–Tropsch Catalysts: A Review. Catal. Today 2010, 154, 162–182. [Google Scholar] [CrossRef]
- Rommens, K.T.; Saeys, M. Molecular Views on Fischer–Tropsch Synthesis. Chem. Rev. 2023, 123, 5798–5858. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, Y.; Gaspard, P.; Kruse, N. The Oscillating Fischer-Tropsch Reaction. Science 2023, 382, 99–103. [Google Scholar] [CrossRef]
- Gholami, Z.; Tišler, Z.; Rubáš, V. Recent Advances in Fischer-Tropsch Synthesis Using Cobalt-Based Catalysts: A Review on Supports, Promoters, and Reactors. Catal. Rev. 2021, 63, 512–595. [Google Scholar] [CrossRef]
- Rytter, E.; Holmen, A. Consorted Vinylene Mechanism for Cobalt Fischer–Tropsch Synthesis Encompassing Water or Hydroxyl Assisted CO-Activation. Top. Catal. 2018, 61, 1024–1034. [Google Scholar] [CrossRef]
- Iglesia, E.; Hibbitts, D. The Fischer-Tropsch Synthesis: A Few Enduring Mechanistic Conundrums Revisited. J. Catal. 2022, 405, 614–625. [Google Scholar] [CrossRef]
- Riedel, T.; Claeys, M.; Schulz, H.; Schaub, G.; Nam, S.-S.; Jun, K.-W.; Choi, M.-J.; Kishan, G.; Lee, K.-W. Comparative Study of Fischer–Tropsch Synthesis with H2/CO and H2/CO2 Syngas Using Fe- and Co-Based Catalysts. Appl. Catal. Gen. 1999, 186, 201–213. [Google Scholar] [CrossRef]
- Yates, I.C.; Satterfield, C.N. Intrinsic Kinetics of the Fischer-Tropsch Synthesis on a Cobalt Catalyst. Energy Fuels 1991, 5, 168–173. [Google Scholar] [CrossRef]
- van der Laan, G.P. Kinetics, Selectivity and Scale Up of the Fischer-Tropsch Synthesis; University of Groningen: Groningen, The Netherlands, 1999; Available online: https://pure.rug.nl/ws/portalfiles/portal/9883465/thesis.pdf (accessed on 1 March 2024).
- Todic, B.; Nowicki, L.; Nikacevic, N.; Bukur, D.B. Fischer–Tropsch Synthesis Product Selectivity over an Industrial Iron-Based Catalyst: Effect of Process Conditions. Catal. Today 2016, 261, 28–39. [Google Scholar] [CrossRef]
- Horáček, J. Fischer–Tropsch Synthesis, the Effect of Promoters, Catalyst Support, and Reaction Conditions Selection. Monatshefte Für Chem.-Chem. Mon. 2020, 151, 649–675. [Google Scholar] [CrossRef]
- Iglesia, E.; Reyes, S.C.; Madon, R.J.; Soled, S.L. Selectivity Control and Catalyst Design in the Fischer-Tropsch Synthesis: Sites, Pellets, and Reactors. In Advances in Catalysis; Eley, D.D., Pines, H., Weisz, P.B., Eds.; Academic Press: Cambridge, MA, USA, 1993; Volume 39, pp. 221–302. [Google Scholar] [CrossRef]
- Gavrilović, L.; Jørgensen, E.A.; Pandey, U.; Putta, K.R.; Rout, K.R.; Rytter, E.; Hillestad, M.; Blekkan, E.A. Fischer-Tropsch Synthesis over an Alumina-Supported Cobalt Catalyst in a Fixed Bed Reactor—Effect of Process Parameters. Catal. Today 2021, 369, 150–157. [Google Scholar] [CrossRef]
- Jahangiri, H.; Bennett, J.; Mahjoubi, P.; Wilson, K.; Gu, S. A Review of Advanced Catalyst Development for Fischer–Tropsch Synthesis of Hydrocarbons from Biomass Derived Syn-Gas. Catal. Sci. Technol. 2014, 4, 2210–2229. [Google Scholar] [CrossRef]
- Fu, T.; Li, Z. Review of Recent Development in Co-Based Catalysts Supported on Carbon Materials for Fischer–Tropsch Synthesis. Chem. Eng. Sci. 2015, 135, 3–20. [Google Scholar] [CrossRef]
- Prieto, G.; De Mello, M.I.S.; Concepción, P.; Murciano, R.; Pergher, S.B.C.; Martínez, A. Cobalt-Catalyzed Fischer–Tropsch Synthesis: Chemical Nature of the Oxide Support as a Performance Descriptor. ACS Catal. 2015, 5, 3323–3335. [Google Scholar] [CrossRef]
- Morales, F.; Weckhuysen, B.M. Promotion Effects in Co-Based Fischer-Tropsch Catalysis. Catalysis 2006, 19, 1–40. [Google Scholar]
- Bahadoran, F.; Moradian, A.; Shirazi, L.; Zamani, Y. Fischer–Tropsch Synthesis: Evaluation of Gd and Ru Promoters Effect on Co/γ-Al2O3 Catalyst at Different Conditions. Chem. Pap. 2018, 72, 309–325. [Google Scholar] [CrossRef]
- Westerterp, K.R.; Fontein, H.J.; van Beckum, F.P. Decoking of Fixed-bed Catalytic Reactors. Chem. Eng. Technol. 1988, 11, 367–375. [Google Scholar]
- Zhu, X.; Lu, X.; Liu, X.; Hildebrandt, D.; Glasser, D. Study of Radial Heat Transfer in a Tubular Fischer−Tropsch Synthesis Reactor. Ind. Eng. Chem. Res. 2010, 49, 10682–10688. [Google Scholar] [CrossRef]
- Koning, G.W.K. Heat and Mass Transport in Tubular Packed Bed Reactors at Reacting and Non-Reacting Conditions. Experiments and Models. Ph.D. Thesis, Twente University Press, Enschede, The Netherlands, 2002. Available online: https://ris.utwente.nl/ws/portalfiles/portal/6073702/t000003e.pdf (accessed on 18 February 2025).
- Eilers, J.; Posthuma, S.A.; Sie, S.T. The Shell Middle Distillate Synthesis Process (SMDS). Catal. Lett. 1990, 7, 253–269. [Google Scholar] [CrossRef]
- Shaikh, A.; Al-Dahhan, M. Scale-up of Bubble Column Reactors: A Review of Current State-of-the-Art. Ind. Eng. Chem. Res. 2013, 52, 8091–8108. [Google Scholar] [CrossRef]
- Vogel, A.P.; Nel, H.G.; Stadler, J.A.; Jordi, R.G.; Breman, B.B. Intensification of the Sasol SPD Reactor—Realizing Potential. Ind. Eng. Chem. Res. 2014, 53, 1768–1774. [Google Scholar] [CrossRef]
- Quinlan, C.W.; Fiato, R.A.; Bienstock, M.G.; Ansell, L.M. Advanced Gas-To-Liquids Technology: AGC-21. In Proceedings of the IPTC, Doha, Qatar, 21–23 November 2005; p. IPTC-10642. [Google Scholar]
- Kim, J.-S.; Lee, S.; Lee, S.-B.; Choi, M.-J.; Lee, K.-W. Performance of Catalytic Reactors for the Hydrogenation of CO2 to Hydrocarbons. Catal. Today 2006, 115, 228–234. [Google Scholar] [CrossRef]
- Pfeifer, P.; Schmidt, S.; Betzner, F.; Kollmann, M.; Loewert, M.; Böltken, T.; Piermartini, P. Scale-up of Microstructured Fischer–Tropsch Reactors—Status and Perspectives. Curr. Opin. Chem. Eng. 2022, 36, 100776. [Google Scholar] [CrossRef]
- Tucker, C.L.; Claeys, M.; Steen, E. van. Decoupling the Deactivation Mechanisms of a Cobalt Fischer–Tropsch Catalyst Operated at High Conversion and ‘Simulated’ High Conversion. Catal. Sci. Technol. 2020, 10, 7056–7066. [Google Scholar] [CrossRef]
- Wolf, M.; Fischer, N.; Claeys, M. Water-Induced Deactivation of Cobalt-Based Fischer–Tropsch Catalysts. Nat. Catal. 2020, 3, 962–965. [Google Scholar] [CrossRef]
- Claeys, M.; Dry, M.E.; van Steen, E.; van Berge, P.J.; Booyens, S.; Crous, R.; van Helden, P.; Labuschagne, J.; Moodley, D.J.; Saib, A.M. Impact of Process Conditions on the Sintering Behavior of an Alumina-Supported Cobalt Fischer–Tropsch Catalyst Studied with an in Situ Magnetometer. ACS Catal. 2015, 5, 841–852. [Google Scholar] [CrossRef]
- Zhou, X.; Price, G.A.; Sunley, G.J.; Copéret, C. Small Cobalt Nanoparticles Favor Reverse Water-Gas Shift Reaction Over Methanation Under CO2 Hydrogenation Conditions. Angew. Chem. Int. Ed. 2023, 62, e202314274. [Google Scholar] [CrossRef]
- Myrstad, R. Scale-up of Microchannel Reactors for Small Scale GTL Processes. In Proceedings of the 2nd Trondheim Gas Technology Conference, Trondheim, Norway, 2–3 November 2011. [Google Scholar]
- Thunman, H.; Seemann, M.; Berdugo Vilches, T.; Maric, J.; Pallares, D.; Ström, H.; Berndes, G.; Knutsson, P.; Larsson, A.; Breitholtz, C.; et al. Advanced Biofuel Production via Gasification—Lessons Learned from 200 Man-Years of Research Activity with Chalmers’ Research Gasifier and the GoBiGas Demonstration Plant. Energy Sci. Eng. 2018, 6, 6–34. [Google Scholar] [CrossRef]
- Borg, Ø.; Hammer, N.; Enger, B.C.; Myrstad, R.; Lindvåg, O.A.; Eri, S.; Skagseth, T.H.; Rytter, E. Effect of Biomass-Derived Synthesis Gas Impurity Elements on Cobalt Fischer–Tropsch Catalyst Performance Including in Situ Sulphur and Nitrogen Addition. J. Catal. 2011, 279, 163–173. [Google Scholar] [CrossRef]
- Gardner, J.L.; He, W.; Li, C.; Wong, J.; Sale, K.L.; Simmons, B.A.; Singh, S.; Tanjore, D. Calorimetric Evaluation Indicates That Lignin Conversion to Advanced Biofuels Is Vital to Improving Energy Yields. RSC Adv. 2015, 5, 51092–51101. [Google Scholar] [CrossRef]
- Myrstad, R.; Eri, S.; Pfeifer, P.; Rytter, E.; Holmen, A. Fischer–Tropsch Synthesis in a Microstructured Reactor. Catal. Today 2009, 147, S301–S304. [Google Scholar] [CrossRef]
- Saeidi, S.; Amiri, M.T.; Amin, N.A.S.; Rahimpour, M.R. Progress in Reactors for High-Temperature Fischer–Tropsch Process: Determination Place of Intensifier Reactor Perspective. Int. J. Chem. React. Eng. 2014, 12, 639–664. [Google Scholar] [CrossRef]
- Delparish, A.; Avci, A.K. Intensified Catalytic Reactors for Fischer-Tropsch Synthesis and for Reforming of Renewable Fuels to Hydrogen and Synthesis Gas. Fuel Process. Technol. 2016, 151, 72–100. [Google Scholar] [CrossRef]
- He, L.; Parra, J.M.S.; Blekkan, E.A.; Chen, D. Towards Efficient Hydrogen Production from Glycerol by Sorption Enhanced Steam Reforming. Energy Environ. Sci. 2010, 3, 1046–1056. [Google Scholar] [CrossRef]
- Dry, M.E. The Fischer–Tropsch Process: 1950–2000. Catal. Today 2002, 71, 227–241. [Google Scholar] [CrossRef]
- Dry, M.E. High Quality Diesel via the Fischer–Tropsch Process—A Review. J. Chem. Technol. Biotechnol. 2002, 77, 43–50. [Google Scholar] [CrossRef]
- Sahir, A.H.; Zhang, Y.; Tan, E.C.D.; Tao, L. Understanding the Role of Fischer–Tropsch Reaction Kinetics in Techno-Economic Analysis for Co-Conversion of Natural Gas and Biomass to Liquid Transportation Fuels. Biofuels Bioprod. Biorefin. 2019, 13, 1306–1320. [Google Scholar] [CrossRef]
- Tripathi, M.; Sahu, J.N.; Ganesan, P. Effect of Process Parameters on Production of Biochar from Biomass Waste through Pyrolysis: A Review. Renew. Sustain. Energy Rev. 2016, 55, 467–481. [Google Scholar] [CrossRef]
- Broer, K.M.; Peterson, C. Gasification. In Thermochemical Processing of Biomass; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2019; pp. 85–123. [Google Scholar] [CrossRef]
- Potgieter, J.H.; Moodley, D.; Botha, T.; Visagie, J.; Manong, T.; Frank, M.; Claeys, M.; van Steen, E.; Böltken, T.; Pfeifer, P. Development of Promoted Cobalt/Alumina Fischer-Tropsch Catalysts for Increased Activity and Selectivity: Micro-Reactor to Piloting Scale. Catal. Today 2024, 432, 114554. [Google Scholar] [CrossRef]
- Cao, C.; Hu, J.; Li, S.; Wilcox, W.; Wang, Y. Intensified Fischer–Tropsch Synthesis Process with Microchannel Catalytic Reactors. Catal. Today 2009, 140, 149–156. [Google Scholar] [CrossRef]
- Jacobs, G.; Davis, B.H. Reactor Approaches for Fischer–Tropsch Synthesis. In Multiphase Catalytic Reactors: Theory, Design, Manufacturing, and Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 269–294. [Google Scholar]
- LeViness, S.; Deshmukh, S.R.; Richard, L.A.; Robota, H.J. Velocys Fischer–Tropsch Synthesis Technology—New Advances on State-of-the-Art. Top. Catal. 2014, 57, 518–525. [Google Scholar] [CrossRef]
- de Deugd, R.M.; Chougule, R.B.; Kreutzer, M.T.; Meeuse, F.M.; Grievink, J.; Kapteijn, F.; Moulijn, J.A. Is a Monolithic Loop Reactor a Viable Option for Fischer–Tropsch Synthesis? Chem. Eng. Sci. 2003, 58, 583–591. [Google Scholar] [CrossRef]
- de Deugd, R.M.; Kapteijn, F.; Moulijn, J.A. Using Monolithic Catalysts for Highly Selective Fischer–Tropsch Synthesis. Catal. Today 2003, 79–80, 495–501. [Google Scholar] [CrossRef]
- Egaña, A.; Sanz, O.; Merino, D.; Moriones, X.; Montes, M. Fischer–Tropsch Synthesis Intensification in Foam Structures. Ind. Eng. Chem. Res. 2018, 57, 10187–10197. [Google Scholar] [CrossRef]
- Park, J.C.; Roh, N.S.; Chun, D.H.; Jung, H.; Yang, J.-I. Cobalt Catalyst Coated Metallic Foam and Heat-Exchanger Type Reactor for Fischer–Tropsch Synthesis. Fuel Process. Technol. 2014, 119, 60–66. [Google Scholar] [CrossRef]
- Almeida, L.C.; González, O.; Sanz, O.; Paul, A.; Centeno, M.A.; Odriozola, J.A.; Montes, M. Fischer-Tropsch Catalyst Deposition on Metallic Structured Supports. In Studies in Surface Science and Catalysis; Bellot Noronha, F., Schmal, M., Falabella Sousa-Aguiar, E., Eds.; Natural Gas Conversion VIII; Elsevier: Berlin/Heidelberg, Germany, 2007; Volume 167, pp. 79–84. [Google Scholar] [CrossRef]
- Nekhamkina, O.; Sheintuch, M. Cross-Flow Reactor Design for Fischer Tropsch Synthesis. Chem. Eng. J. 2019, 372, 277–293. [Google Scholar] [CrossRef]
- Mittal, A.; Roy, S.; Larachi, F. Modeling of Heat Uptake and Release with Embedded Phase-Change Materials in Monolithic Microfluidized Bed Reactors. Ind. Eng. Chem. Res. 2010, 49, 1086–1097. [Google Scholar] [CrossRef]
- Rohde, M.P.; Schaub, G.; Khajavi, S.; Jansen, J.C.; Kapteijn, F. Fischer–Tropsch Synthesis with in Situ H2O Removal—Directions of Membrane Development. Microporous Mesoporous Mater. 2008, 115, 123–136. [Google Scholar] [CrossRef]
- Forghani, A.A.; Elekaei, H.; Rahimpour, M.R. Enhancement of Gasoline Production in a Novel Hydrogen-Permselective Membrane Reactor in Fischer–Tropsch Synthesis of GTL Technology. Int. J. Hydrogen Energy 2009, 34, 3965–3976. [Google Scholar] [CrossRef]
- Bayat, M.; Rahimpour, M.R. Simultaneous Hydrogen Injection and In-Situ H2O Removal in a Novel Thermally Coupled Two-Membrane Reactor Concept for Fischer–Tropsch Synthesis in GTL Technology. J. Nat. Gas Sci. Eng. 2012, 9, 73–85. [Google Scholar] [CrossRef]
- Rahimpour, M.R.; Bahmanpour, A.M. Optimization of Hydrogen Production via Coupling of the Fischer–Tropsch Synthesis Reaction and Dehydrogenation of Cyclohexane in GTL Technology. Appl. Energy 2011, 88, 2027–2036. [Google Scholar] [CrossRef]
- Gavrilović, L.; Kazi, S.S.; Oliveira, A.; Encinas, O.L.I.; Blekkan, E.A. Sorption-Enhanced Fischer-Tropsch Synthesis—Effect of Water Removal. Catal. Today 2024, 432, 114614. [Google Scholar] [CrossRef]
- Wang, D.; Zhong, M.; Xue, J.; Wang, Z.; Li, X.; Hou, B. High Thermal Conductive Al2O3@Al Composites Supported Cobalt Catalysts for Fischer-Tropsch Synthesis. Fuel 2022, 327, 125199. [Google Scholar] [CrossRef]
- Wang, D.; Wang, Z.; Li, G.; Li, X.; Hou, B. SiO2-Modified Al2O3@Al-Supported Cobalt for Fischer–Tropsch Synthesis: Improved Catalytic Performance and Intensified Heat Transfer. Ind. Eng. Chem. Res. 2018, 57, 12756–12765. [Google Scholar] [CrossRef]
- Kulkarni, S.R.; Velisoju, V.K.; Tavares, F.; Dikhtiarenko, A.; Gascon, J.; Castaño, P. Silicon Carbide in Catalysis: From Inert Bed Filler to Catalytic Support and Multifunctional Material. Catal. Rev. 2023, 65, 174–237. [Google Scholar] [CrossRef]
- de la Osa, A.R.; De Lucas, A.; Romero, A.; Valverde, J.L.; Sánchez, P. Influence of the Catalytic Support on the Industrial Fischer–Tropsch Synthetic Diesel Production. Catal. Today 2011, 176, 298–302. [Google Scholar] [CrossRef]
- Fratalocchi, L.; Groppi, G.; Visconti, C.G.; Lietti, L.; Tronconi, E. The Pivotal Role of an Interconnected Cellular Conductive Structure to Manage Heat Removal in Compact Fischer–Tropsch Fixed-Bed Reactors. React. Chem. Eng. 2019, 4, 1917–1921. [Google Scholar] [CrossRef]
- Jähnisch, K.; Hessel, V.; Löwe, H.; Baerns, M. Chemistry in Microstructured Reactors. Angew. Chem. Int. Ed. 2004, 43, 406–446. [Google Scholar] [CrossRef]
- Yu, L.; Nassar, R.; Fang, J.; Kuila, D.; Varahramyan, K. Investigation of a Novel Microreactor for Enhancing Mixing and Conversion. Chem. Eng. Commun. 2008, 195, 745–757. [Google Scholar] [CrossRef]
- Todić, B.; Ordomsky, V.V.; Nikačević, N.M.; Khodakov, A.Y.; Bukur, D.B. Opportunities for Intensification of Fischer–Tropsch Synthesis through Reduced Formation of Methane over Cobalt Catalysts in Microreactors. Catal. Sci. Technol. 2015, 5, 1400–1411. [Google Scholar] [CrossRef]
- Groppi, G.; Beretta, A.; Tronconi, E. Structured Catalytic Reactors for Selective Oxidations. In Handbook of Advanced Methods and Processes in Oxidation Catalysis; Imperial College Press: London, UK, 2014; pp. 943–997. [Google Scholar] [CrossRef]
- Mesheryakov, V.D.; Kirillov, V.A.; Kuzin, N.A. A Multifunctional Reactor with a Regular Catalyst Packing for Fischer-Tropsch Synthesis. Chem. Eng. Sci. 1999, 54, 1565–1570. [Google Scholar] [CrossRef]
- Twigg, M.V.; Richardson, J.T. Fundamentals and Applications of Structured Ceramic Foam Catalysts. Ind. Eng. Chem. Res. 2007, 46, 4166–4177. [Google Scholar] [CrossRef]
- Jacquot, C.; Vamvakeros, A.; Pavlišič, A.; Price, S.W.T.; Dong, H.; Matras, D.; Protasova, L.; Likozar, B.; Jacques, S.D.M.; Beale, A.M.; et al. A Multi-Scale Study of 3D Printed Co-Al2O3 Catalyst Monoliths versus Spheres. Chem. Eng. J. Adv. 2023, 16, 100538. [Google Scholar] [CrossRef]
- Lacroix, M.; Dreibine, L.; de Tymowski, B.; Vigneron, F.; Edouard, D.; Bégin, D.; Nguyen, P.; Pham, C.; Savin-Poncet, S.; Luck, F.; et al. Silicon Carbide Foam Composite Containing Cobalt as a Highly Selective and Re-Usable Fischer–Tropsch Synthesis Catalyst. Appl. Catal. Gen. 2011, 397, 62–72. [Google Scholar] [CrossRef]
- Iovane, M.; Zennaro, R.; Forzatti, P.; Groppi, G.; Lietti, L.; Tronconi, E.; Visconti, C.G.; Rossini, S.; Mignone, E. Reactor for Exothermic or Endothermic Catalytic Reactions. WO 2010130399A1, 18 November 2010. Available online: https://patents.google.com/patent/WO2010130399A1/en?oq=WO+2010%2f130399+ (accessed on 5 February 2024).
- Paturzo, M.; Favaretto, M.; Piazza, M.; Forzatti, P.; Groppi, G.; Lietti, L.; Tronconi, E.; Visconti, C.G. Multi-Structured Reactor Made of Monolithic Adjacent Thermoconductive Bodies for Chemical Processes with a High Heat Exchange. U.S. Patent 10011776B2, 3 July 2018. Available online: https://patents.google.com/patent/US10011776B2/en (accessed on 5 February 2024).
- Abusrafa, A.E.; Challiwala, M.S.; Wilhite, B.A.; Elbashir, N.O. Thermal Assessment of a Micro Fibrous Fischer Tropsch Fixed Bed Reactor Using Computational Fluid Dynamics. Processes 2020, 8, 1213. [Google Scholar] [CrossRef]
- Sheng, M.; Yang, H.; Cahela, D.R.; Yantz, W.R.; Gonzalez, C.F.; Tatarchuk, B.J. High Conductivity Catalyst Structures for Applications in Exothermic Reactions. Appl. Catal. Gen. 2012, 445–446, 143–152. [Google Scholar] [CrossRef]
- Sheng, M.; Yang, H.; Cahela, D.R.; Tatarchuk, B.J. Novel Catalyst Structures with Enhanced Heat Transfer Characteristics. J. Catal. 2011, 281, 254–262. [Google Scholar]
- Vervloet, D.; Kapteijn, F.; Nijenhuis, J.; van Ommen, J.R. Process Intensification of Tubular Reactors: Considerations on Catalyst Hold-up of Structured Packings. Catal. Today 2013, 216, 111–116. [Google Scholar] [CrossRef]
- Schildhauer, T.J.; Pangarkar, K.; van Ommen, J.R.; Nijenhuis, J.; Moulijn, J.A.; Kapteijn, F. Heat Transport in Structured Packings with Two-Phase Co-Current Downflow. Chem. Eng. J. 2012, 185–186, 250–266. [Google Scholar] [CrossRef]
- Barrera, J.L.; Hartvigsen, J.J.; Hollist, M.; Pike, J.; Yarosh, A.; Fullilove, N.P.; Beck, V.A. Design Optimization of Integrated Cooling Inserts in Modular Fischer-Tropsch Reactors. Chem. Eng. Sci. 2023, 268, 118423. [Google Scholar] [CrossRef]
- Harmel, J.; Peres, L.; Estrader, M.; Berliet, A.; Maury, S.; Fécant, A.; Chaudret, B.; Serp, P.; Soulantica, K. Hcp-Co Nanowires Grown on Metallic Foams as Catalysts for Fischer–Tropsch Synthesis. Angew. Chem. Int. Ed. 2018, 57, 10579–10583. [Google Scholar] [CrossRef]
- Wang, X.; Ren, Y.; Zhang, L. Intensification of High-Flux Fischer-Tropsch Synthesis with Axial Gradient Co-Loading FeCrAl Monolith Catalysts. Chem. Eng. J. 2023, 452, 139188. [Google Scholar] [CrossRef]
- Gryaznov, K.O.; Sineva, L.V.; Asalieva, E.Y.; Mordkovich, V.Z. Comprehensive Comparison of High-Performance Fischer-Tropsch Synthesis Cobalt Catalysts Containing Different Types of Heat-Conducting Frames. Catal. Ind. 2023, 15, 21–35. [Google Scholar] [CrossRef]
- Wei, Q.; Li, H.; Liu, G.; He, Y.; Wang, Y.; Tan, Y.E.; Wang, D.; Peng, X.; Yang, G.; Tsubaki, N. Metal 3D Printing Technology for Functional Integration of Catalytic System. Nat. Commun. 2020, 11, 4098. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Mori, K.; Nakano, T.; Yamashita, H. Advances in Metal 3D Printing Technology for Tailored Self-Catalytic Reactor Design. ChemCatChem 2024, 16, e202301380. [Google Scholar] [CrossRef]
- Almeida, L.C.; Echave, F.J.; Sanz, O.; Centeno, M.A.; Arzamendi, G.; Gandía, L.M.; Sousa-Aguiar, E.F.; Odriozola, J.A.; Montes, M. Fischer–Tropsch Synthesis in Microchannels. Chem. Eng. J. 2011, 167, 536–544. [Google Scholar] [CrossRef]
- Yang, J.-I.; Yang, J.H.; Kim, H.-J.; Jung, H.; Chun, D.H.; Lee, H.-T. Highly Effective Cobalt Catalyst for Wax Production in Fischer–Tropsch Synthesis. Fuel 2010, 89, 237–243. [Google Scholar] [CrossRef]
- Uher, C. Thermal Conductivity of Metals. In Thermal Conductivity: Theory, Properties, and Applications; Tritt, T.M., Ed.; Springer: Boston, MA, USA, 2004; pp. 21–91. [Google Scholar] [CrossRef]
- Al-Alloy AlSi7Mg0,6/EN AC-42200. SLM Solution Group AG. 2024. Available online: https://www.slm-solutions.com/fileadmin/Content/Powder/MDS/MDS_Al-Alloy_AlSi7Mg0_6_0219_EN.pdf (accessed on 18 February 2025).
- Liu, D.-M.; Lin, B.-W. Thermal Conductivity in Hot-Pressed Silicon Carbide. Ceram. Int. 1996, 22, 407–414. [Google Scholar] [CrossRef]
- Graves, R.S.; Kollie, T.G.; McElroy, D.L.; Gilchrist, K.E. The Thermal Conductivity of AISI 304L Stainless Steel. Int. J. Thermophys. 1991, 12, 409–415. [Google Scholar] [CrossRef]
- Engelbrecht, N.; Everson, R.C.; Bessarabov, D.; Kolb, G. Microchannel Reactor Heat-Exchangers: A Review of Design Strategies for the Effective Thermal Coupling of Gas Phase Reactions. Chem. Eng. Process. Process Intensif. 2020, 157, 108164. [Google Scholar] [CrossRef]
- Bracconi, M.; Ambrosetti, M.; Maestri, M.; Groppi, G.; Tronconi, E. A Fundamental Analysis of the Influence of the Geometrical Properties on the Effective Thermal Conductivity of Open-Cell Foams. Chem. Eng. Process. Process Intensif. 2018, 129, 181–189. [Google Scholar] [CrossRef]
- Fratalocchi, L.; Groppi, G.; Visconti, C.G.; Lietti, L.; Tronconi, E. Packed-POCS with Skin: A Novel Concept for the Intensification of Non-Adiabatic Catalytic Processes Demonstrated in the Case of the Fischer-Tropsch Synthesis. Catal. Today 2022, 383, 15–20. [Google Scholar] [CrossRef]
- Visconti, C.G.; Panzeri, M.; Groppi, G.; Tronconi, E. Heat Transfer Intensification in Compact Tubular Reactors with Cellular Internals: A Pilot-Scale Assessment of Highly Conductive Packed-POCS with Skin Applied to the Fischer-Tropsch Synthesis. Chem. Eng. J. 2024, 481, 148469. [Google Scholar] [CrossRef]
- The GLAMOUR Project. Available online: https://www.Glamour-Project.Eu/ (accessed on 10 January 2025).
- Boymans, E.; Ganjkhanlou, Y.; Denneman, M.; Sutens, B.; Lefevere, J.; Grootjes, S. Structured Internals for the Intensified Fischer–Tropsch Synthesis in Fixed-Bed Reactors. React. Chem. Eng. 2025, 10, 686–693. [Google Scholar] [CrossRef]
- Odunsi, A.O.; O’Donovan, T.S.; Reay, D.A. Temperature Stabilisation in Fischer–Tropsch Reactors Using Phase Change Material (PCM). Appl. Therm. Eng. 2016, 93, 1377–1393. [Google Scholar] [CrossRef]
- Rahimpour, M.R.; Mirvakili, A.; Paymooni, K. Simultaneous Hydrogen Production and Utilization via Coupling of Fischer–Tropsch Synthesis and Decalin Dehydrogenation Reactions in GTL Technology. Int. J. Hydrogen Energy 2011, 36, 2992–3006. [Google Scholar] [CrossRef]
- Rahimpour, M.R.; Lotfinejad, M. Co-Current and Countercurrent Configurations for a Membrane Dual Type Methanol Reactor. Chem. Eng. Technol. 2008, 31, 38–57. [Google Scholar] [CrossRef]
- Rahimpour, M.R.; Mirvakili, A.; Paymooni, K.; Moghtaderi, B. A Comparative Study between a Fluidized-Bed and a Fixed-Bed Water Perm-Selective Membrane Reactor with in Situ H2O Removal for Fischer–Tropsch Synthesis of GTL Technology. J. Nat. Gas Sci. Eng. 2011, 3, 484–495. [Google Scholar] [CrossRef]
- Rahimpour, M.R.; Elekaei, H. Optimization of a Novel Combination of Fixed and Fluidized-Bed Hydrogen-Permselective Membrane Reactors for Fischer–Tropsch Synthesis in GTL Technology. Chem. Eng. J. 2009, 152, 543–555. [Google Scholar] [CrossRef]
- Aguirre, A.; Scholman, E.; van der Shaaf, J.; Neira d’Angelo, M.F. Controlling the Selectivity in the Fischer-Tropsch Synthesis Using Foam Catalysts: An Integrated Experimental and Modeling Approach. Chem. Eng. J. 2021, 409, 128139. [Google Scholar] [CrossRef]
- Balzarotti, R.; Beretta, A.; Groppi, G.; Tronconi, E. A Comparison between Washcoated and Packed Copper Foams for the Intensification of Methane Steam Reforming. React. Chem. Eng. 2019, 4, 1387–1392. [Google Scholar] [CrossRef]
- Balzarotti, R.; Ambrosetti, M.; Arnesano, M.; Anglani, A.; Groppi, G.; Tronconi, E. Periodic Open Cellular Structures (POCS) as Enhanced Catalyst Supports: Optimization of the Coating Procedure and Analysis of Mass Transport. Appl. Catal. B Environ. 2021, 283, 119651. [Google Scholar] [CrossRef]
- Guettel, R.; Turek, T. Comparison of Different Reactor Types for Low Temperature Fischer–Tropsch Synthesis: A Simulation Study. Chem. Eng. Sci. 2009, 64, 955–964. [Google Scholar] [CrossRef]
- LeViness, S.; Tonkovich, A.; Jarosch, K.; Fitzgerald, S.; Yang, B.; McDaniel, J. Improved Fischer-Tropsch Economics Enabled by Microchannel Technology. White Pap. Gener. Velocys 2011. Available online: https://tinyurl.com/bdz9p645 (accessed on 1 February 2025).
- Tonkovich, A.L.; Mazanec, T.; Jarosch, K.; Fitzgerald, S.; Yang, B.; Taha, R.; Kilanowski, D.; Lerou, J.; McDaniel, J.; Dritz, T. Improved Fischer-Tropsch Economics Enabled by Microchannel Technology. Velocys 2008. Available online: https://www.adktroutguide.com/files/Microchannel_FT_White_Paper_Sep08.pdf (accessed on 1 February 2025).
- Deshmukh, S.R.; Tonkovich, A.L.Y.; Jarosch, K.T.; Schrader, L.; Fitzgerald, S.P.; Kilanowski, D.R.; Lerou, J.J.; Mazanec, T.J. Scale-Up of Microchannel Reactors For Fischer−Tropsch Synthesis. Ind. Eng. Chem. Res. 2010, 49, 10883–10888. [Google Scholar] [CrossRef]
- Schuetzle, R.; Schuetzle, D. Processes for the Production of Liquid Fuels from Carbon Containing Feedstocks, Related Systems and Catalysts. U.S. Patent 11702599B2, 18 July 2023. Available online: https://patents.google.com/patent/US11702599B2/en (accessed on 31 January 2024).
- Bowe, M.J.; Lee-Tuffnell, C.D.; Baxter, I.K.; Hopper, C. Gas-to-Liquid Plant Using Parallel Units. U.S. Patent 20100186824A1, 29 July 2010. Available online: https://patents.google.com/patent/US20100186824A1/en?oq=US+Patent+12%2f718%2c633 (accessed on 31 January 2024).
- Loewert, M.; Hoffmann, J.; Piermartini, P.; Selinsek, M.; Dittmeyer, R.; Pfeifer, P. Microstructured Fischer-Tropsch Reactor Scale-up and Opportunities for Decentralized Application. Chem. Eng. Technol. 2019, 42, 2202–2214. [Google Scholar] [CrossRef]
- INERATEC Secures State-of-the-Art Catalysts from Sasol for the Production of Sustainable e-Fuels. Available online: https://www.ineratec.de/en/news/ineratec-secures-state-art-catalysts-sasol-production-sustainable-e-fuels (accessed on 5 February 2025).
- Tsubaki Laboratory. Available online: http://www3.u-toyama.ac.jp/tsubaki/eng%202007/topics_en.html (accessed on 12 February 2024).
- Velocys Projects List. Available online: https://velocys.com/projects/ (accessed on 5 February 2025).
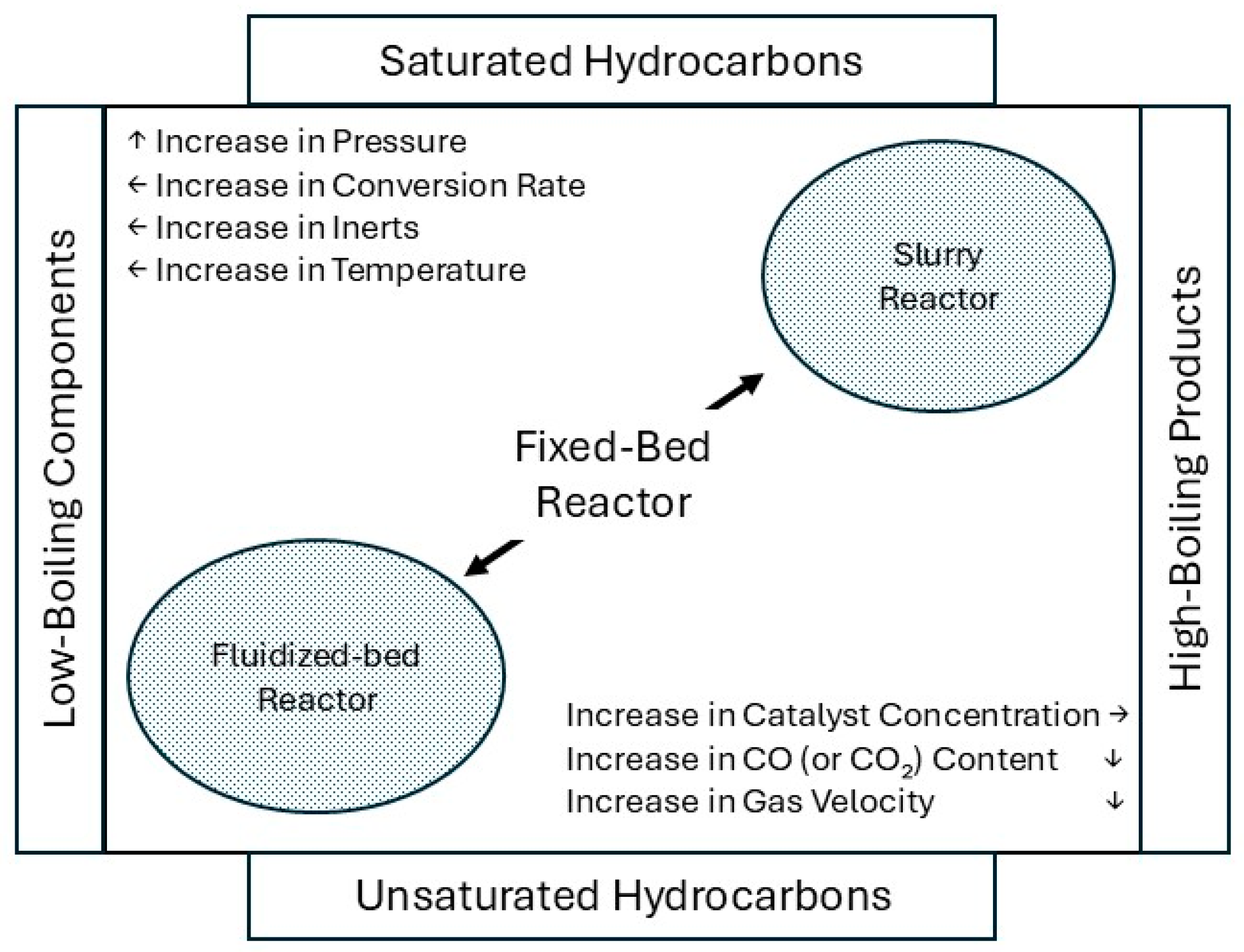

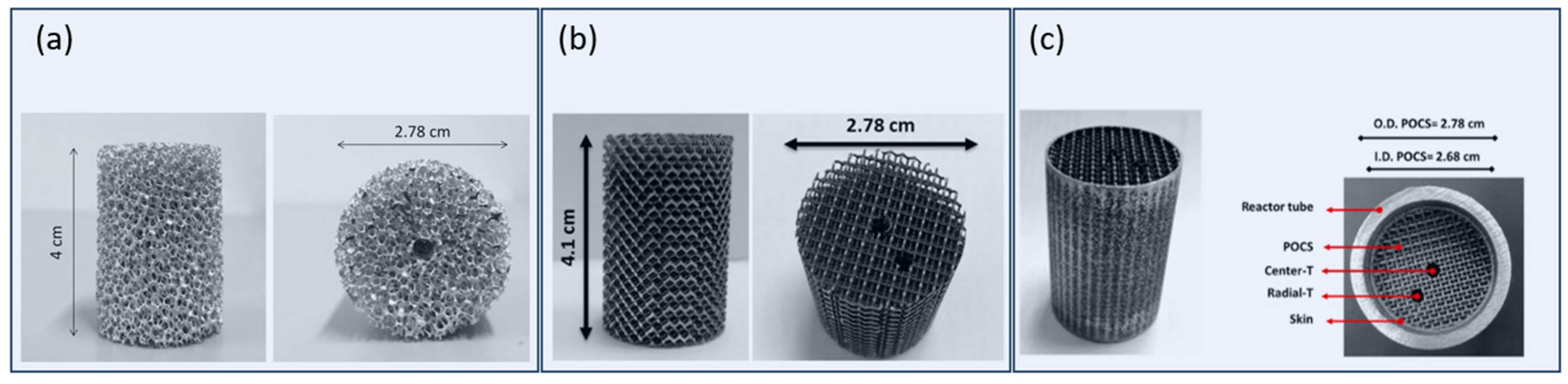
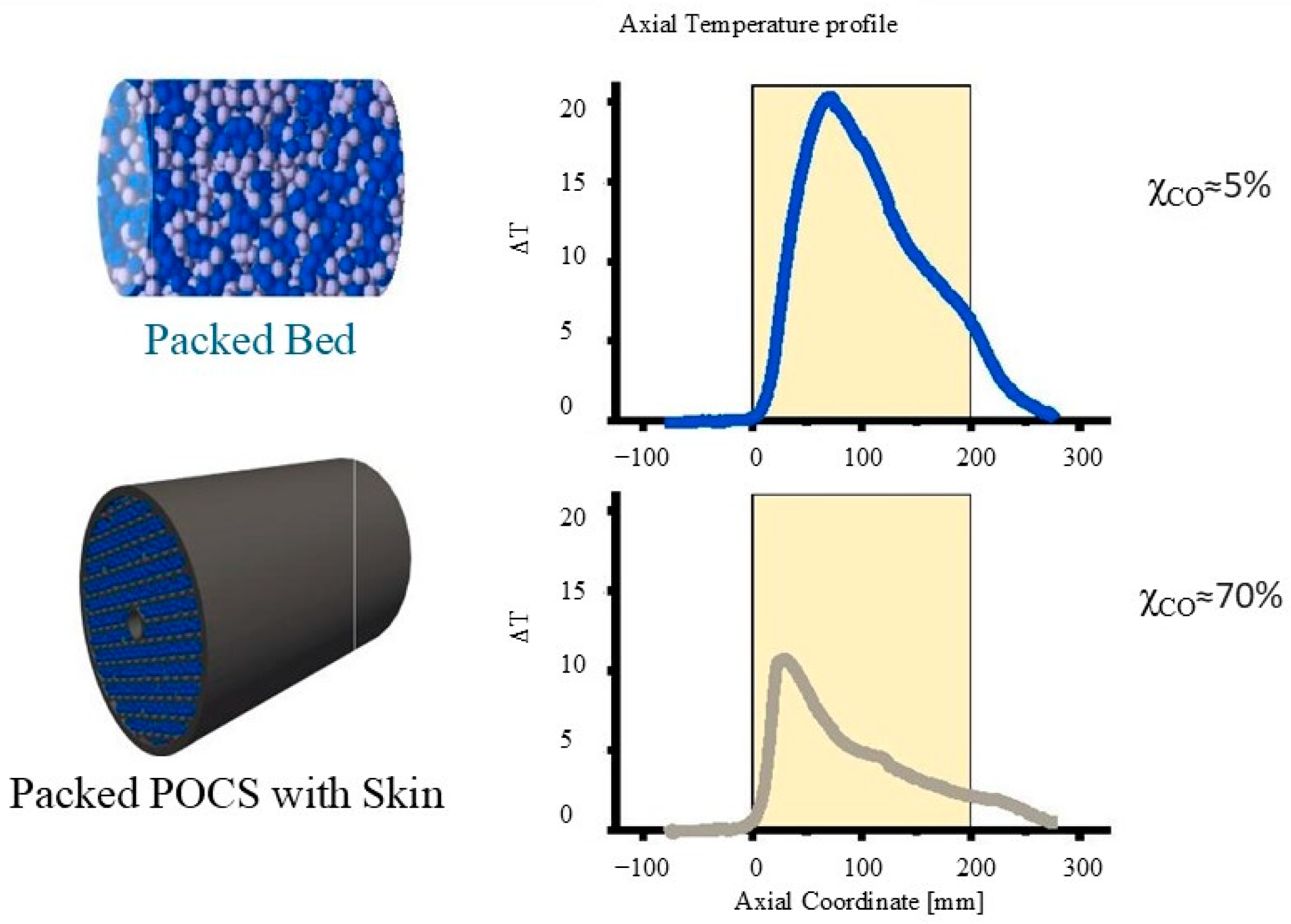
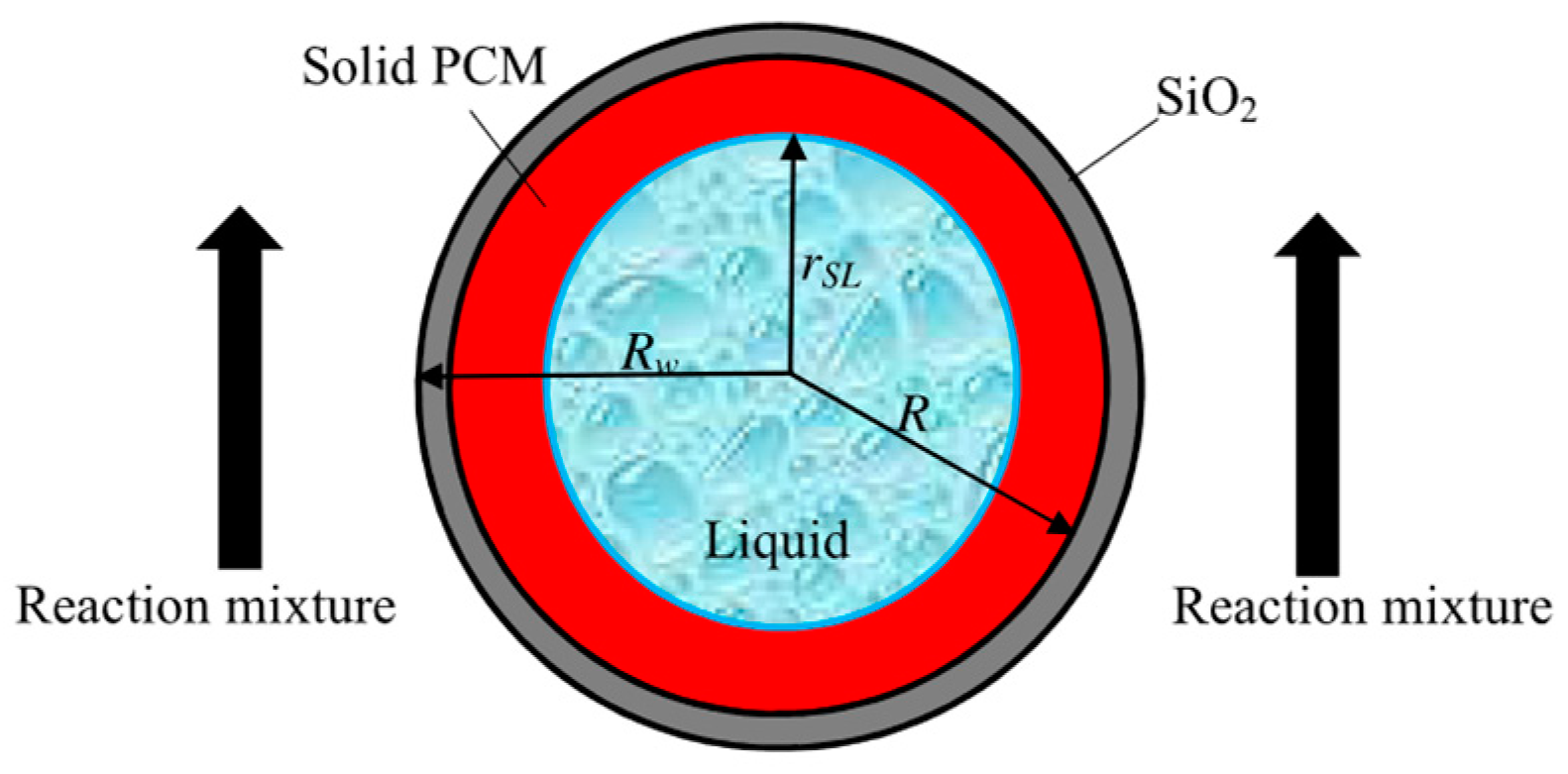

| Parameter | Effect on Product Distribution | Effect on CO Conversion/Activity |
|---|---|---|
| Temperature | Higher temperatures and small α and less C5+ products [13,28]. | Higher temperatures favor higher conversion [13,28,33]. |
| Pressure | Higher pressures and high α and more C5+ products [34]. Gas composition has a superior impact than pressure, especially in the case of the Co catalyst. | Higher pressures favor higher conversion [34,35]. |
| Flow rate of reactants | Basically an effect of residence time. A long residence time (low flow rate) increases α and C5+ products [36]. | High flow rate and short residence time allow conversion [36]. |
| Gas composition | Higher H2/CO favors paraffins and low chain products (small α) [34,37]. | Higher H2/CO increases the conversion but favors methane and undesired product formation [34,37]. |
| Catalyst composition | Co-based catalysts favor paraffins and middle distillate (i.e., diesel, kerosene) yield (high α); Fe-based catalysts favor olefins, oxygenates; Ni-based catalysts are less selective (more CH4 formation) but more active. Fe and Ni are also active for water gas shift (WGS) and reverse WGS in case of low H2/CO ratio or CO2 presence in the feed gas [28,38]. Ru is more active than these metals; however, due to its high price, it only used as a promoter [28]. | These catalysts have different temperature windows (conversion is not comparable) [28,38]. |
| Support material | Affects the dispersion of the metal particles, reducibility, and heat conduction, which can impact the activity of the catalyst [39,40]. | Acid–base character of the support and its porosity affect both conversion and selectivity. TiO2 supports and coating result in outstanding yield and C13+ productivity [40]. |
| Promoter addition | Can improve the activity, reducibility, and selectivity of the catalyst [28,41]. Ru is one of the most common promoters, especially for a Co catalyst, as it enhances the reducibility of Co [42]. | Can affect stability, interaction, and dispersion of NPs and selectivity, as well as conversion and lifetime of the catalyst [41]. |
| Type of Syngas | Production Scale of Fuel |
|---|---|
| Fossil fuels | 15,000–140,000 barrel per day (BPD) [7,56] (≈0.9–8.2 GW, assuming gasoline as fuel, 0.0583 MW/BPD) |
| Biomass BTL unit | ≈21.4–≈342 BPD (1.25 [51]–20 MW [57]) * 500–2000 BPD [56] (≈29–116 MW) |
| Small scale GTL | 1000–2000 BPD [56] (≈58.3–116.6 MW) |
| Material 1 | Heat Conduction Wm−1 K−1 |
|---|---|
| Al | 237 [113] |
| Cu | 402 (at 27 °C) [113] |
| AlSi7Mg0.6/EN AC-42200 | 150–170 [114] |
| SiC | 98.6 [115] |
| Ni | 93 (at 7 °C) [113] |
| Stainless Steel (AISI 304 L) | 16–17 [116] |
| Silica and coerdierite | 1–3 [117] |
| Typical FTS catalyst | 0.2 [70] |
| Type of the Reactor | TRL Level | Example of Heat Duties kW/m3 |
|---|---|---|
| Fixed (packed)-bed reactor | 10 | 79–218 [89,122] 3 |
| Heat-conducting support | 3 | 251 [89] 3 |
| Microchannel reactor | 7 (8) 1 | Similar or higher than POCS 3 |
| Structured reactors—POCS | 5 | 800–2000 [119,120,122] 3 |
| Cross flow structures | 2 | N/A |
| Heat-adsorbing materials (PCMs) | 2 | N/A |
| Coupled reactors | 2 | N/A |
| Fixed-bed membrane reactors | 2 (4) 2 | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ganjkhanlou, Y.; Boymans, E.; Vreugdenhil, B. Minireview: Intensified Low-Temperature Fischer–Tropsch Reactors for Sustainable Fuel Production. Fuels 2025, 6, 24. https://doi.org/10.3390/fuels6020024
Ganjkhanlou Y, Boymans E, Vreugdenhil B. Minireview: Intensified Low-Temperature Fischer–Tropsch Reactors for Sustainable Fuel Production. Fuels. 2025; 6(2):24. https://doi.org/10.3390/fuels6020024
Chicago/Turabian StyleGanjkhanlou, Yadolah, Evert Boymans, and Berend Vreugdenhil. 2025. "Minireview: Intensified Low-Temperature Fischer–Tropsch Reactors for Sustainable Fuel Production" Fuels 6, no. 2: 24. https://doi.org/10.3390/fuels6020024
APA StyleGanjkhanlou, Y., Boymans, E., & Vreugdenhil, B. (2025). Minireview: Intensified Low-Temperature Fischer–Tropsch Reactors for Sustainable Fuel Production. Fuels, 6(2), 24. https://doi.org/10.3390/fuels6020024






