Abstract
The push for sustainability in nanomaterials has catalyzed significant advancements in the green synthesis of carbon dots (CDs) from renewable resources. This review uniquely explores recent innovations, including the integration of hybrid techniques, such as micro-wave-assisted and ultrasonic-assisted hydrothermal methods, as well as photocatalytic synthesis. These combined approaches represent a breakthrough, offering rapid production, precise control over CD properties, and enhanced environmental sustainability. In addition, the review emphasizes the growing use of green solvents and bio-based reducing agents, which further reduce the environmental footprint of CD production. This work also addresses key challenges, such as consistently controlling CD properties—size, shape, and surface characteristics—across different synthesis processes. Advanced characterization techniques and process optimizations are highlighted as essential strategies to overcome these hurdles. Furthermore, this review pioneers the integration of circular economy principles into CD production, proposing novel strategies for sustainable material use and waste reduction. By exploring innovative precursor materials, refining doping and surface engineering techniques, and advocating for comprehensive life cycle assessments, this work sets a new direction for future research. The insights provided here represent a significant contribution to the field, paving the way for more sustainable, efficient, and scalable CD production with diverse applications in optoelectronics, sensing, and environmental remediation.
1. Introduction
Carbon dots (CDs) are a novel class of carbon-based nanomaterials, typically smaller than 10 nm, that have recently gained significant attention due to their unique properties, such as high photoluminescent quantum yield, excellent aqueous solubility, robust biocompatibility, resistance to photobleaching, and remarkable electron transferability [1]. These nanostructures are characterized by a quasi-spherical morphology and are predominantly composed of a carbon-based core, often with surface functional groups, including amino, hydroxyl, and carboxyl groups [1,2,3]. The exceptional properties of CDs, such as excellent biocompatibility, low cytotoxicity, and chemical inertness, make them highly attractive for various applications, including biosensing [4], bioimaging [5], and drug discovery [6]. Additionally, CDs exhibit efficient light-harvesting, strong absorption, tunable photoluminescence, and outstanding photoinduced electron-transfer abilities [7], making them desirable for use in optoelectronic devices [8], photocatalysis [9], electrocatalysis [10], and energy conversion and storage applications [2].
However, despite the extensive research efforts, several critical gaps remain in the field. The scalability of CD production, control over their properties, and environmental impact of their synthesis are areas that require further investigation. Traditional synthesis methods for CDs often rely on non-renewable carbon sources, such as graphite and fossil fuel-derived precursors, which are not only unsustainable but also limited by challenges, such as low yields and poor control over CD properties [11,12]. Furthermore, these methods typically involve harsh chemicals and complex setups, which contribute to environmental degradation [11,12,13,14]. This has spurred a paradigm shift towards embracing sustainable materials and renewable resources in CD synthesis [3].
Modern research has begun to explore the immense potential of sustainable materials and renewable resources in revolutionizing the synthesis of CDs. By harnessing the abundance of plant biomass, biowastes from agricultural and industrial processes, and naturally derived compounds, like citric acid and carbohydrates, researchers have unlocked a new realm of eco-friendly and cost-effective CD production [14,15,16]. However, the application of these sustainable practices is still in its infancy, and there is a need for comprehensive studies that compare the efficiency, scalability, and environmental impact of these green synthesis methods against conventional approaches. Additionally, while some studies have explored the use of green solvents and bio-based reducing agents in CD synthesis, the full potential of these techniques remains underexplored [17].
Moreover, the valorization of biowastes through their conversion into value-added CDs presents a compelling opportunity to address waste management challenges while simultaneously generating economic value [14,15,17]. This approach not only reduces the environmental burden but also contributes to the development of a sustainable and circular bioeconomy. Yet, the practical implications of the large-scale implementation of these methods are not well understood, and further research is needed to address the scalability and economic feasibility of biomass-derived CDs.
As the demand for CDs continues to grow across various industries, the development of sustainable and environmentally friendly synthesis methods using renewable resources has become a crucial endeavor [11,12,13,14,17]. However, there is a lack of detailed comparative analyses that evaluate the performance and environmental impact of these emerging green synthesis techniques relative to traditional methods. Such analyses are essential for guiding future research and for the broader adoption of sustainable practices in the industry.
Therefore, the objective of this review is to fill these critical gaps by providing an in-depth exploration of biomass-derived CDs, focusing on their historical evolution, innovative green synthesis methods, and advanced characterization techniques. This review integrates recent trends in CD synthesis, such as the use of green solvents, bio-based reducing agents, and the combination of multiple techniques, with a comprehensive analysis of their properties, applications, and environmental impacts. By synthesizing and consolidating existing literature, it aims to elucidate the potential of sustainable synthesis routes and address the challenges associated with their production and use. What sets this review apart is its detailed comparative analysis of emerging synthesis techniques and its thorough evaluation of the environmental and sustainability considerations, as compared in Table 1 with the latest reviews. Through these unique contributions, this review offers valuable insights and practical recommendations for advancing the field of eco-friendly nanomaterials and guiding future research directions.

Table 1.
The summary of latest reviews and this study’s comparison.
2. History and Synthesis of Carbon Dots
The history of CDs began in 2004, when Xu et al. [19] discovered small, fluorescent nanoparticles while purifying single-walled carbon nanotubes. These nanoparticles were later termed “carbon quantum dots.” In 2006, Sun et al. [20] introduced the term “carbon dots” to describe similar nanoparticles generated via the laser ablation of a carbon target, although the resulting surface-passivated CDs exhibited a quantum yield (QY) of only about 10%.
A significant milestone in the development of CDs occurred in 2013, when Zhu et al. [21] reported a facile, one-step synthesis of polymer-like CDs using citric acid and ethylenediamine as precursors through a hydrothermal method. This method produced CDs with quantum yields reaching up to 80%, offering better control over their size and surface functionalization. This breakthrough greatly enhanced the optical properties of CDs and sparked renewed interest in their potential applications.
Over the years, various synthetic approaches have been developed to produce CDs with tailored structures and functionalities. These approaches can be broadly categorized into top-down and bottom-up methods. Top-down methods include techniques, such as arc discharge [19], laser ablation [20], and electrochemical oxidation [22], which involve breaking down larger carbon structures into nanoscale dots. On the other hand, bottom-up methods, such as pyrolysis/carbonization [23], hydrothermal/solvothermal treatment [21,24,25], and microwave-assisted synthesis [26], involve building CDs from molecular precursors.
These advancements in synthesis methods have allowed for the production of CDs with diverse properties, making them suitable for a wide range of applications, including bioimaging [5], sensing, catalysis [9], energy conversion [2], and optoelectronics [8]. Recent research efforts have focused on improving the quantum yield, tunability of photoluminescence, and stability of CDs, particularly for biomedical applications where biocompatibility and low toxicity are crucial [5,6].
As the field of carbon dots continues to evolve, ongoing studies aim to further refine synthesis techniques, better understand the structure–property relationships, and explore novel applications in emerging areas. The focus of this review is to provide an in-depth exploration of green synthesis methods, highlighting eco-friendly approaches that align with sustainability goals and reduce the environmental impact of CD production.
3. Green Synthesis Methods for Biomass-Derived Carbon Dots
Over the past decade, a multitude of pathways has been proposed for tailoring CDs with specific properties tailored to diverse applications. These well-established synthesis strategies fall within the paradigm of “top-down” and “bottom-up” approaches [3,27,28]. Top-down methodologies encompass “arc-discharge, laser ablation, chemical exfoliation, and electrochemical oxidation” [19,20,22,29], typically necessitating prolonged reaction times, stringent reaction conditions, and the utilization of costly materials and sophisticated equipment [30,31,32]. In contrast, bottom-up techniques involve the synthesis of CDs through the thermal, microwave, or pyrolytic decomposition of chemical precursors [21,23,24,25,26]. Bottom-up methodologies are preferred over top-down approaches due to their economic efficiency, reliability, and environmentally conscious nature in CD synthesis [33], rendering them the preferred choice for many researchers in the field. A comprehensive discussion of the nuances and benefits of employing bottom-up methodologies in CD synthesis is provided in the subsequent sections.
3.1. Microwave-Assisted Synthesis
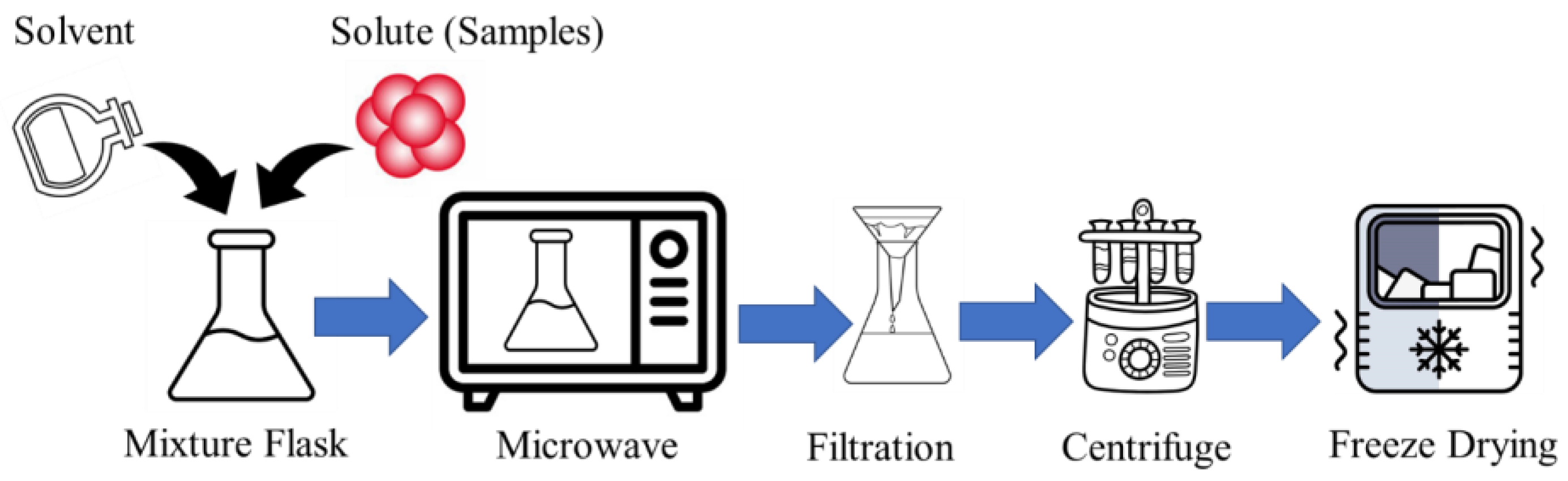
Microwave-assisted chemistry emerges as a rapid and efficient method for synthesizing CDs, leveraging in situ and evanescent heating to bolster both the yield and quality. A common protocol involves dissolving the natural substrate in a solvent, followed by exposure to microwave irradiation (wavelength 1 mm to 1 m range [18]) in a controlled chamber. The resulting CDs can then undergo separation and purification processes [12]. The process flow diagram of the microwave-assisted CD synthesis is shown in Figure 1.
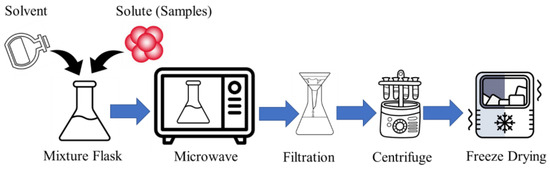
Figure 1.
The process flow diagram of microwave-assisted CD synthesis.
In 2013, a significant milestone was reached as researchers utilized microwave irradiation for the first time to produce fluorescent CDs measuring around 3.7 nm [21]. Building on this breakthrough, further progress was made in 2014 using the same method. The process involved heating a solution containing saccharides and polyethylene glycol in an aqueous medium using a 500 W domestic microwave oven for nearly 3 min [34]. The latest research on CDs synthesis using a microwave-assisted method has been summarized in Table 2.

Table 2.
The summary of the latest studies on microwave-assisted CD synthesis.
The compiled data in Table 2 from various studies on biowaste-derived CD synthesis using microwave irradiation reveal several key takeaways and trends. Firstly, the choice of biowaste feedstocks, ranging from lemon and onion biomasses to sesame seeds, highlights the diverse range of materials that can be utilized in CD synthesis, showcasing the potential of biowastes as sustainable precursors. Secondly, the varying microwave power levels and irradiation times demonstrate the flexibility of microwave-assisted synthesis in achieving efficient and rapid CD production, with higher power and shorter times generally leading to faster synthesis rates. Moreover, the QY and particle sizes of the synthesized CDs varied significantly, underscoring the importance of optimizing synthesis conditions for the desired CD properties. For instance, CDs derived from palm kernel shell exhibited a high QY of 44% with a small particle size of 6–7 nm, showcasing the potential benefits of using specific biowastes for efficient and compact CD synthesis. Overall, the data suggest that microwave-assisted CD synthesis offers a promising approach for harnessing biowastes as renewable precursors, with the potential to produce CDs with desirable properties for various applications. Further research and the optimization of microwave parameters and feedstock selection are warranted to fully realize the benefits and potential of biowaste-derived CDs in sustainable nanomaterial synthesis.
3.2. Ultrasonic-Assisted Synthesis
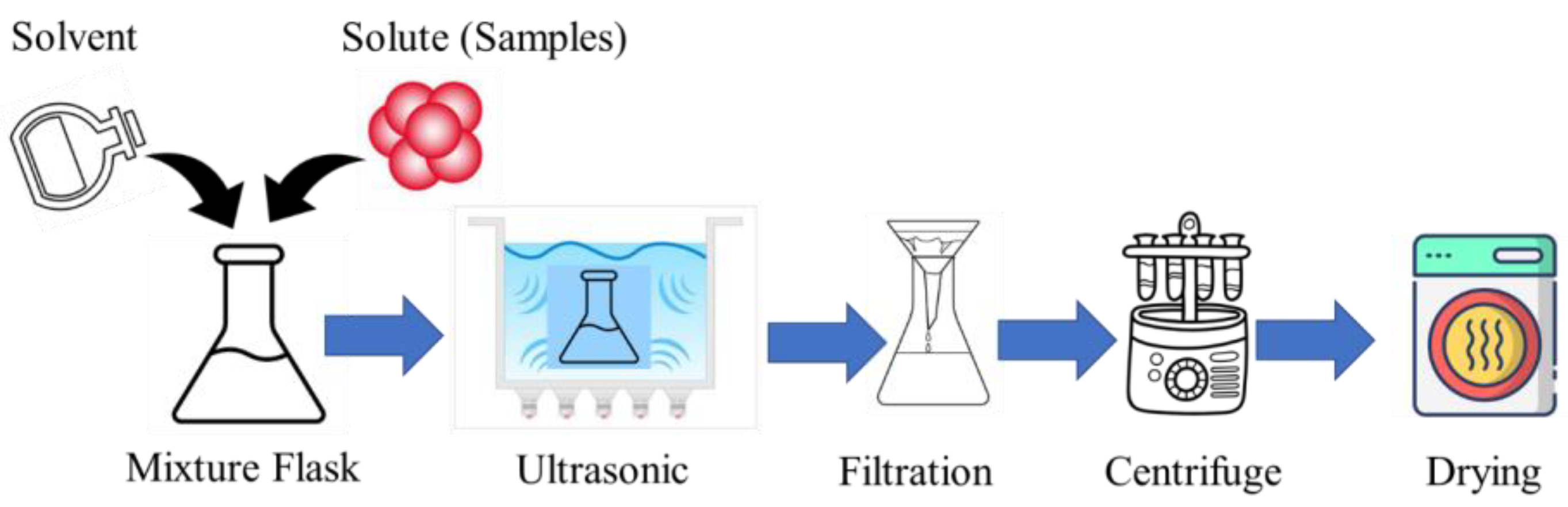
CDs can be synthesized using the ultrasonic method, which is a simple, one-pot, and environmentally friendly approach. The synthesis typically involves dissolving a carbon precursor, like glucose, citric acid, polyethylene glycol, or plant extracts, in a suitable solvent [48,49]. The precursor solution is then subjected to high-intensity ultrasonic irradiation, which induces acoustic cavitation, generating localized high temperatures and pressures that facilitate the carbonization and formation of CDs [48,49,50]. Leveraging the high energy of ultrasonic waves streamlines the synthesis of CDs by eliminating the need for intricate post-treatment procedures, resulting in a straightforward and efficient production route for small-sized CDs [51]. After the ultrasonic treatment, the synthesized CDs are purified using techniques, like centrifugation, filtration, or dialysis, to remove unreacted precursors and byproducts and then isolated through drying methods [48]. The process flow diagram of the ultrasonic-assisted CD synthesis is shown in Figure 2.
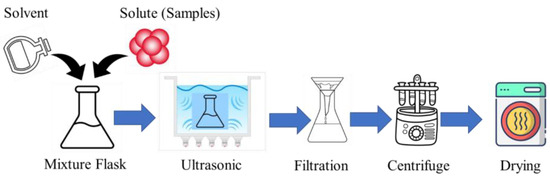
Figure 2.
The process flow diagram of ultrasonic-assisted CD synthesis.
The synthesis of graphene quantum dots using the ultrasonic exfoliation of graphene was first described in 2012 [52]. Subsequently, in 2014, a study introduced a straightforward approach involving ultrasonic treatment for the mass production of water-soluble CDs using carbon sourced from food waste. This method yielded 120 g of CDs from a mixture of 100 kg of food waste [53]. The latest research on CD synthesis using a microwave-assisted method has been summarized in Table 3.

Table 3.
The summary of the latest studies on ultrasonic-assisted CD synthesis.
The data presented in Table 3 offer comprehensive insight into the versatility and effectiveness of ultrasonic-assisted synthesis for producing CDs from a range of renewable feedstocks. Notably, the variation in power levels (ranging from 150 W to 400 W) across different experiments highlights the adaptability of ultrasonic systems to accommodate diverse energy inputs, while the consistent use of a 40 kHz ultrasonic frequency underscores its critical role in ensuring efficient and uniform CD synthesis. The QY values ranging from 2.4% to 60.18% reflect the substantial impact of synthesis conditions on the photoluminescence efficiency of CDs. Higher QY values, particularly those obtained from wild raw neem bark and coconut water, suggest the potential of specific feedstocks in producing CDs with enhanced optical properties. Additionally, the observed range of CDs particle sizes, from 0.233 nm to 10 nm, highlights the tunability of CD size through ultrasonic-assisted synthesis. The trends observed in Table 2 emphasize the effectiveness and versatility of ultrasonic-assisted synthesis in producing high-quality CDs from renewable feedstocks. The findings underscore the importance of optimizing synthesis parameters based on specific feedstock characteristics and desired CD properties, paving the way for further advancements in sustainable nanomaterial synthesis.
3.3. Hydrothermal Synthesis
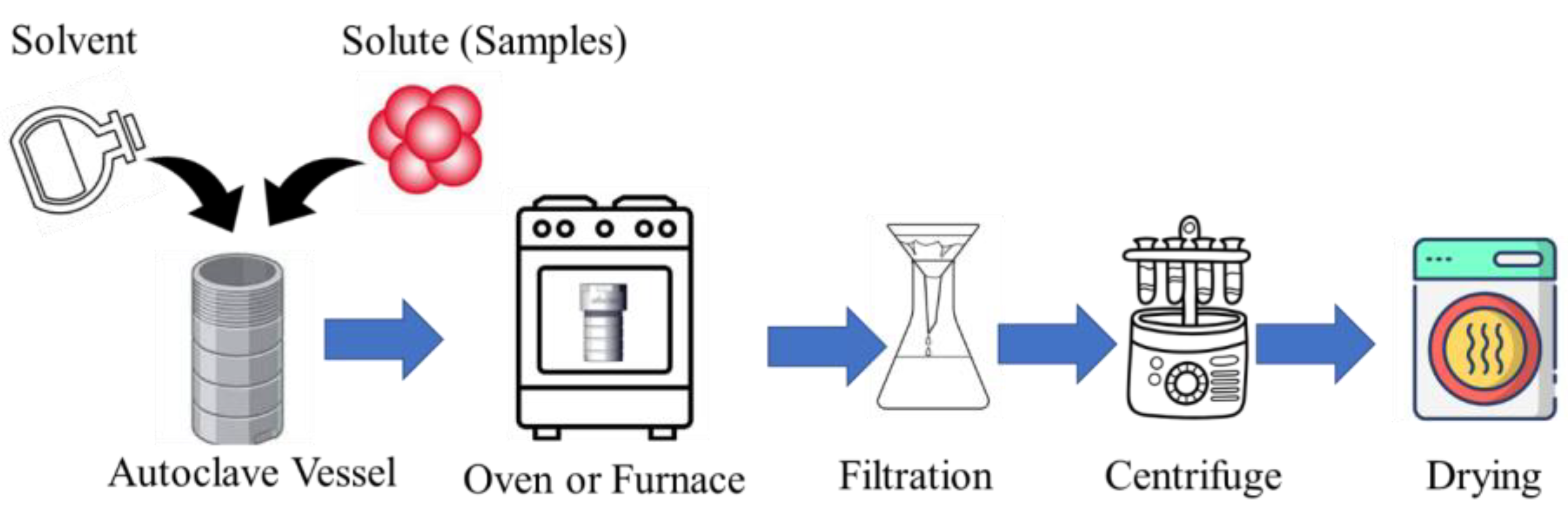
The hydrothermal method is an affordable and environmentally friendly approach to CD production. It offers a straightforward process compared to other synthetic methods. The hydrothermal synthesis of CDs involves several key steps to produce these nanomaterials efficiently. Initially, a precursor solution containing a carbon source, such as citric acid or glucose, along with necessary chemical agents and water (water/biomass ratio may range from 5:1 to 75:1) [62], is prepared. This solution is then loaded into a commonly used Teflon-lined stainless-steel autoclave, which serves as the reaction vessel. The autoclave is sealed tightly to maintain pressure during the hydrothermal reaction. The setup is placed in an air oven capable of maintaining high temperatures, typically ranging from 100 °C to 300 °C [63,64,65,66]. During the hydrothermal reaction, which occurs under high pressure and temperature conditions inside the autoclave, precursor molecules undergo polymerization, carbonization, and other chemical transformations, leading to the formation of CDs [65,66]. After the specified reaction duration, the autoclave is removed from the oven and allowed to cool down naturally. The contents are then retrieved, and the CDs undergo purification (filtration and centrifugation) to remove unreacted precursors or by-products [64,65]. Another technique, known as solvothermal synthesis, operates similarly to the hydrothermal method. The primary distinction lies in the use of a non-aqueous precursor solution in solvothermal synthesis. The process flow diagram of the hydrothermal-assisted CD synthesis is shown in Figure 3.
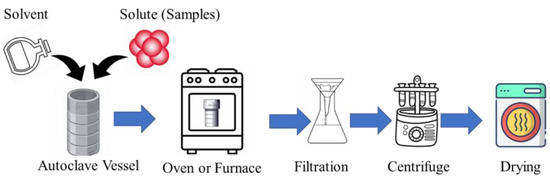
Figure 3.
The process flow diagram of hydrothermal-assisted CD synthesis.
Several feedstocks and diverse biomass materials have been tested for the synthesis of CDs using hydrothermal methods in the literature [67,68,69,70,71,72]. Table 4 below presents a summarized list of the latest research findings regarding the utilization of different feedstocks in hydrothermal synthesis processes for CDs. These studies encompass a wide range of natural and synthetic materials, showcasing the diversity and potential of hydrothermal methods in tailoring CDs for various applications.

Table 4.
The summary of the latest studies on hydrothermal-assisted CD synthesis.
Table 4 presents a comprehensive overview of the hydrothermal synthesis of carbon dots (CDs) utilizing a wide range of renewable feedstocks. The data highlight several key trends and takeaways regarding the synthesis conditions and characteristics of the resulting CDs. One prominent trend is the significant variation in the QY percentages observed across different feedstocks. For instance, CDs synthesized from agarose waste demonstrate a high QY of 62%, indicating efficient photoluminescence properties. On the other hand, waste-tea-derived CDs exhibit a lower QY of 3.26%, suggesting differences in the optical performance based on the feedstock composition and hydrothermal conditions. Another noteworthy observation is the diverse particle sizes of the synthesized CDs. Feedstocks like rose flowers and banana peel result in CDs with particle sizes ranging from 2.5 to 39 nm, demonstrating the tunability of hydrothermal methods in controlling CD dimensions. This variability in particle size is crucial for tailoring CDs for specific applications, such as bioimaging or optoelectronic devices.
Furthermore, the utilization of unconventional feedstocks, such as bacteria (Escherichia coli), and natural materials, like avocado peel and valerian root, for CD synthesis indicates the versatility of hydrothermal techniques in harnessing a broad spectrum of raw materials. This approach not only promotes sustainability by utilizing renewable resources but also opens avenues for exploring novel sources for CD production. Overall, the data in Table 4 underscore the efficacy of hydrothermal synthesis in generating CDs with diverse optical properties from a range of renewable feedstocks. The observed trends emphasize the importance of optimizing synthesis parameters to achieve desired CD characteristics, paving the way for advancements in sustainable nanomaterial synthesis and diverse applications in various fields.
3.4. Carbonization or Pyrolysis Synthesis
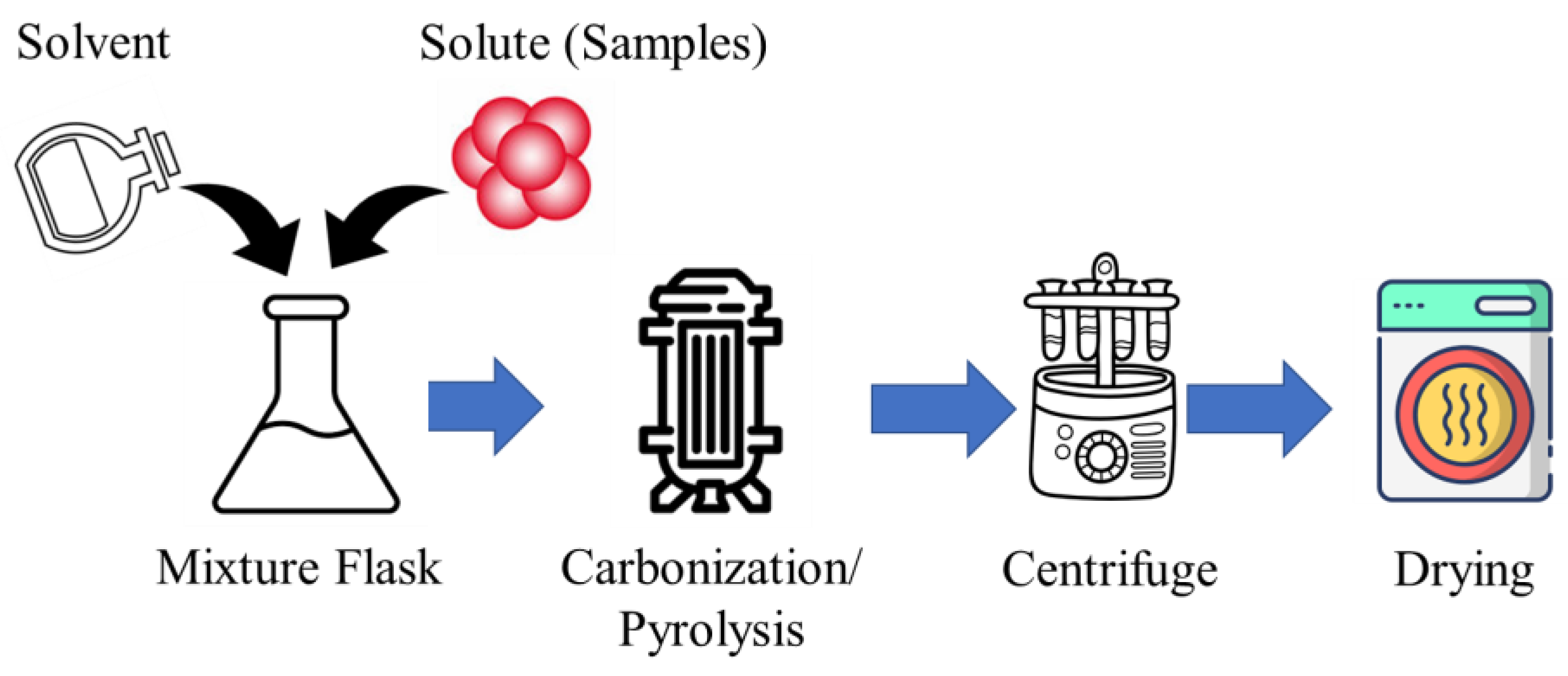
The CD synthesis via carbonization/pyrolysis starts with choosing a carbon source, like citric acid or biomass. Next, a precursor solution is made in a suitable solvent, optionally with stabilizing agents or direct carbonization/pyrolysis without the solution. The solution is then heated at high temperatures (300 °C to 1000 °C) in an inert atmosphere to decompose and form CDs. After controlled cooling, the solution undergoes purification (centrifugation or dialysis) to remove impurities, ensuring the quality and purity of the synthesized CDs. The process flow diagram of the carbonization/pyrolysis-assisted CD synthesis is shown in Figure 4.
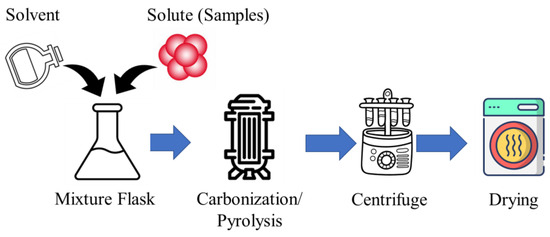
Figure 4.
The process flow diagram of carbonization/pyrolysis-assisted CD synthesis.
Pyrolysis stands out as a robust technique for producing fluorescent CDs, leveraging macroscopic carbon structures as precursors. This method boasts several advantages, such as rapid reaction times, cost-effectiveness, easy operation, solvent-free protocols, and scalability [3,50,88]. The interplay of “heating, dehydration, degradation, and carbonization” processes is pivotal in converting organic-carbon-containing substances into CDs at elevated temperatures [88]. The direct pyrolysis of precursor materials, such as citric acid [89,90], cellulosic biomass [88], and a combination of citric acid and tris-base [90], has been proven to be a facile bottom-up technique for making CDs with a homogenous size distribution. The latest research on CD synthesis using carbonization/pyrolysis methods has been compiled and summarized in Table 5.

Table 5.
The summary of the latest studies on pyrolysis-assisted CD synthesis.
Table 5 summarizes recent studies on CD synthesis using carbonization/pyrolysis methods with diverse renewable feedstocks. Key observations include varying synthesis temperatures, from 1000 °C for “In natura” peat to 200–300 °C for mangosteen peel, demonstrating the flexibility of pyrolysis conditions. Quantum yield percentages and particle sizes also vary across feedstocks, showcasing the influence of biomass sources on CD properties. The utilization of unconventional sources, like chickpea peel and olive solid wastes, highlights the potential of pyrolysis for sustainable CD synthesis.
3.5. Recent Trends in Green Synthesis of Carbon Dots
3.5.1. Combination of Synthesis Methods
Recently, a growing trend in the synthesis of CDs has emerged, emphasizing the utilization of a combination of synthesis methods. This trend stems from the recognition that a single synthesis approach may not fully capture the desired properties or functionalities of CDs. By combining different synthesis techniques, researchers aim to leverage the strengths of each method to tailor the properties of CDs more precisely. Some of the latest research in this area has been discussed below.
Microwave-Assisted Hydrothermal Synthesis
Microwave-assisted hydrothermal synthesis has emerged as a highly promising technique in recent years for the rapid and efficient production of various nanomaterials, including CDs. This method uniquely combines the benefits of microwave irradiation, such as uniform heating and reduced reaction times, with the controlled environment provided by hydrothermal conditions. This synergy enables precise control over the size, morphology, and surface properties of CDs, leading to improved photoluminescence and quantum yield characteristics [33,100].
Several studies have demonstrated the effectiveness of microwave-assisted hydrothermal synthesis in producing high yields and quality of CDs. For instance, the latest research has reported the CDs with high photoluminescence QY, reaching up to 65.8% in the solid state produced through microwave-assisted hydrothermal synthesis [65]. A different study showcased the microwave-assisted hydrothermal synthesis of nitrogen-doped (N-CDs) with exceptional luminescence. These N-CDs exhibited a noteworthy QY of 75.96% and a spectral composition ranging from blue to red (51.48%). The synthesis was conducted under optimized conditions at 180 °C for 8 min, resulting in N-CDs with an average size of 6.06 nm. Characterization also revealed the presence of abundant oxygen- and nitrogen-containing functional groups on the surface of these N-CDs, along with strong ultraviolet-absorption properties [101]. A similar investigation highlighted the synthesis of CDs from starch, achieving a QY of 30% along with blue fluorescence emission. The study revealed the necessity of microwave heating for a specific duration to enhance the yield of these blue-fluorescent CDs [42]. A very recent study introduced an innovative microwave-assisted hydrothermal method for empty fruit bunches, which was subjected to controlled temperatures ranging from 60 to 100 °C using a household microwave for 5 min. The resulting CDs exhibited an average diameter of 4.5 nm, with the highest QY of 15.22 ± 0.84% achieved at the highest temperature of 100 °C [102].
The research on the combination technique of microwave-assisted hydrothermal synthesis is relatively limited; however, the existing studies report its efficiency. This method shows significant improvements in the reaction time, with synthesis times measured in seconds or minutes compared to the conventional heating method, which may take hours [103]. Microwave radiation penetrates samples of varying depths, ensuring uniform heating and avoiding temperature differences due to heat conduction. These advantages contribute to faster response times and the production of more uniform products [99,104,105,106].
Ultrasonic-Assisted Hydrothermal Synthesis
The ultrasonic-assisted hydrothermal synthesis of CDs is a novel approach that combines ultrasonic waves with hydrothermal conditions to produce high-quality CDs. This method has gained attention due to its ability to achieve efficient and rapid synthesis while ensuring excellent control over the size, morphology, and optical properties of the resulting CDs [107]. The ultrasonic waves enhance the dispersion of precursors and promote uniform nucleation and growth during hydrothermal treatment, leading to CDs with enhanced luminescence properties and quantum yields [54,108]. In a recent study utilizing ultrasonic-assisted hydrothermal synthesis, CDs were found to exhibit exceptional optical properties, including a high quantum yield of 40.5%, strong resistance to photobleaching, and superior photostability [107]. A recent study investigated the synthesis of CDs using whole-meal bread and augmented their QY by incorporating natural additives, such as soy flour and lemon juice. Despite employing diverse precursors, the resulting carbon quantum dots (CQDs) exhibited a comparable morphology and showcased enhanced QYs. Notably, the CQDs derived from whole-meal bread combined with soybean flour and lemon juice achieved the highest QY, soaring from 0.8% to 2.31% [108]. Another study showcased the potential of ultrasound-assisted hydrothermal synthesis in producing highly efficient photoluminescent sulfur SCDs, which were then employed to develop a sensitive and selective fluorescent sensor for detecting iron ions (Fe3+) and ascorbic acid (AA) with excellent performance in complex environments [109]. Similar to microwave-assisted hydrothermal synthesis, ultrasonic-assisted hydrothermal synthesis is still in its early stages with limited reported research. However, the available results have demonstrated significant improvements in the reaction efficiency, uniformity of products, and synthesis times, CDs, and properties. Further exploration and research in this area hold promise for advancing the synthesis of carbon dots and other nanomaterials using ultrasonic-assisted hydrothermal methods.
3.5.2. Photocatalytic Synthesis
The photocatalytic synthesis of CDs has emerged as an innovative and environmentally friendly approach for producing these fluorescent nanomaterials. The photocatalytic synthesis of CDs involves the use of semiconductor photocatalysts, like TiO2, ZnO, or g-C3N4. When these photocatalysts are exposed to light, they generate reactive species, such as holes, hydroxyl radicals, and electrons [110,111]. These reactive species initiate the carbonization and nucleation of renewable carbon precursors, leading to the formation of CDs. The photocatalytic synthesis often occurs under mild conditions, such as room temperature or moderate heating, without the need for harsh chemicals or high temperatures [110]. During the photocatalytic synthesis, CDs can be formed directly on the surface of the photocatalyst, leading to the in situ formation of CD/photocatalyst composites or heterostructures. These composites exhibit enhanced photocatalytic performance due to improved charge separation and transfer processes [111,112]. The photocatalytic synthesis also allows for the doping of CDs with heteroatoms, like nitrogen, sulfur, or phosphorus, as well as functionalization with various surface groups [3].
Research studies have demonstrated the successful photocatalytic synthesis of CDs using various photocatalysts and renewable precursors. For example, the synthesis of nitrogen-doped CDs using g-C3N4 and citric acid as precursors has been reported, with the resulting CDs exhibiting excellent photocatalytic activity for hydrogen evolution. Another study utilized TiO2 and biomass-derived precursors to synthesize CDs with tunable optical properties for bioimaging and sensing applications [3,111].
3.5.3. Use of Green Solvents
The use of green solvents in the synthesis of CDs has gained significant attention as a sustainable and environmentally friendly approach, aligning with the principles of green chemistry. Several studies have explored the utilization of various green solvents for CD synthesis, offering several advantages over traditional organic solvents. Water is one of the most commonly used green solvents for the hydrothermal or solvothermal synthesis of CDs [113]. The use of water as a solvent eliminates the need for toxic and hazardous organic solvents, making the synthesis process more environmentally benign. CDs synthesized using water as the solvent often exhibit good biocompatibility and low cytotoxicity, making them suitable for biological applications, such as bioimaging and biosensing.
Researchers have explored the use of organic solvents derived from renewable sources, such as ethanol and acetic acid, for the synthesis of CDs [114,115]. These solvents are considered green alternatives to traditional organic solvents as they are biodegradable, less toxic, and can be obtained from sustainable sources, like biomass or fermentation processes. The use of renewable organic solvents, like ethanol and acetic acid, has been shown to influence the optical properties and surface functionalization of the synthesized CDs [115].
Supercritical Fluids
The use of supercritical fluids (SCFs) has emerged as an innovative and environmentally friendly approach for the synthesis of CDs. Supercritical fluids, such as carbon dioxide (scCO2) and water (scH2O), offer unique properties and advantages in the synthesis of CDs, making them an attractive alternative to conventional methods. SCFs like scCO2 and scH2O are non-toxic, non-flammable, and environmentally benign solvents, aligning with the principles of green chemistry [116,117,118]. This approach involves the direct synthesis of CDs from small organic molecules or carbon precursors in the presence of SCFs. The SCF acts as both the reaction medium and the solvent, facilitating the formation and stabilization of CDs [116]. Several studies have demonstrated the successful synthesis of CDs using SCFs. For example, Pang et al. reported the synthesis of CDs from citric acid and glucose precursors using scCO2 and scH2O, respectively, with tunable optical properties and potential applications in bioimaging and sensing [116]. Additionally, researchers have explored the use of SCFs in combination with ultrasound or continuous flow reactors for efficient and scalable CD synthesis [119].
Ionic Liquids
The use of ionic liquids (ILs), which are salts in a liquid state, offers several advantages over conventional solvents, and they have been explored as green media for CD synthesis. ILs facilitate the rapid and facile synthesis of CDs, often at relatively low temperatures (≤100 °C) and atmospheric pressure [120]. The use of ionic liquids allows for the synthesis of CDs with tunable emission properties, ranging from blue to red [120,121]. Some studies have reported the synthesis of acidophilic CDs using ILs. These CDs exhibit high photoluminescence in acidic environments, making them suitable for many applications in acidic media [122], such as a recent study on CDs synthesized using ILs, which have been reported to exhibit remarkably high photoluminescence QY, reaching up to 98.5% [120]. This exceptional optical property makes these CDs attractive for various applications, such as bioimaging, sensing, and optoelectronic devices.
Deep Eutectic Solvents
Deep Eutectic Solvents (DESs) are considered green and sustainable solvents as they are typically formed by mixing biodegradable and inexpensive components, like choline chloride, urea, glycerol, or organic acids [123,124]. They have low toxicity and low volatility and can be easily prepared from renewable sources, aligning with the principles of green chemistry. The use of DESs facilitates the facile and efficient synthesis of CDs, often requiring lower temperatures (≤100 °C) and shorter reaction times compared to conventional hydrothermal or solvothermal methods [125,126]. DESs can act as both solvents and doping agents, facilitating the incorporation of heteroatoms, like nitrogen, sulfur, or chlorine, into the CD structure [125,127]. DESs can influence the surface functional groups, doping, and optical properties of the synthesized CDs. By varying the DES composition (hydrogen bond donor and acceptor), researchers can tune the fluorescence emission, QY, and surface chemistry of the CDs for specific applications [128,129].
DESs have been used in combination with renewable and sustainable carbon sources, like biomass, cellulose, or plant extracts, for CD synthesis. This approach valorizes abundant and inexpensive resources, promoting sustainability and waste valorization [126,127]. Several studies have demonstrated the successful synthesis of CDs using DESs. For example, Sophora flavescens Aiton was used as a precursor with choline chloride/urea DES to synthesize CDs with strong blue fluorescence and the potential for bioimaging applications [125]. Another study reported the synthesis of nitrogen and sulfur-doped CDs from cellulose using a urea/sulfamic acid DES, exhibiting high sensitivity for detecting copper ions and glutathione [127]. The recent research about CD synthesis using green solvents has been summarized in Table 6.

Table 6.
The summary of CD synthesis using green solvents.
3.5.4. Bio-Based Reducing Agents
For CDs, as a promising class of fluorescent nanomaterials with numerous applications, their synthesis often involves the use of reducing agents, and researchers have been exploring sustainable and eco-friendly alternatives derived from biomass and biowaste. One such approach is the utilization of table sugar (sucrose) derived carbon dots (TSCDs) as reducing agents in the synthesis of metal nanoparticles. TSCDs have proven to be an environmentally benign alternative, facilitating the formation of nanoparticles without the need for harsh chemicals [136]. Another innovative strategy involves the use of carbon dots derived from bioresources as reducing, protecting, and stabilizing agents for the synthesis of silver nanoparticles (AgNPs). These bio-based CDs not only aid in the formation of AgNPs but also contribute to their stability and biocompatibility, making them suitable for applications as antimicrobial agents [137].
Researchers have also explored the synthesis of CDs from various biomass waste sources, such as crab shells, lettuce waste, durian shells, peach leaves, oil palm waste, cashew gum, cellulose-based biowaste, cow manure, green pepper, grapefruit peel, corn straw, and fennel seeds. These sustainable precursors offer an environmentally friendly and cost-effective approach to CD synthesis, while also addressing the issue of waste management [138,139]. Furthermore, lignocellulosic biomass, an abundant and renewable resource, has been utilized as a precursor for the synthesis of CDs. The utilization of lignocellulosic biomass not only reduces the exposure of synthesis processes to chemicals but also facilitates the production of CDs with unique properties and functionalities [140]. The summary of biomass, biowastes, or renewable resources being tested for CD synthesis is provided in Table 2, Table 3, Table 4 and Table 5.
4. Characterization of Synthesized CDs
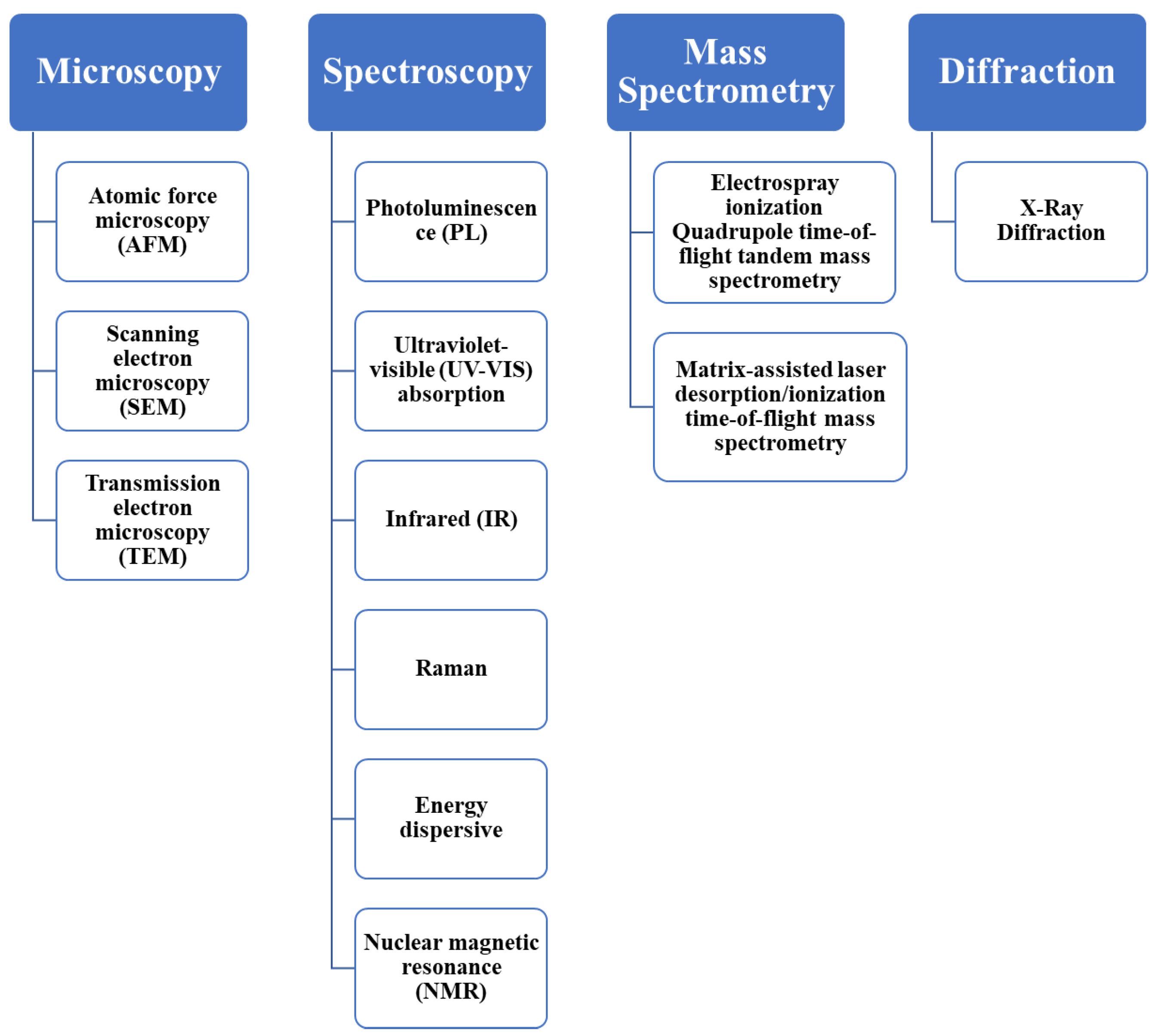
Characterization of CDs is of paramount importance due to the need for understanding their structural, optical, and chemical properties, which directly impact their performance in various applications. Figure 5 presents an overview of several classifications of characterization techniques employed for CDs, which are being discussed in detail below to elucidate their role in unraveling the unique features and functionalities of CDs.
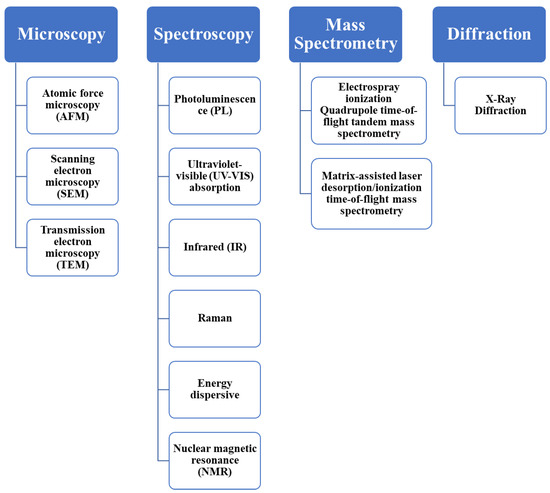
Figure 5.
Characterization techniques for the synthesized CDs.
4.1. Microscopy
Microscopic techniques are commonly employed for the characterization of CDs, facilitating a direct assessment of their morphology at the nanoscale level. These methods allow for the precise measurement of particle size by observing individual nanoparticles. Common microscopic techniques for CDs include “atomic force microscopy (AFM), transmission electron microscopy (TEM), and scanning electron microscopy (SEM)”.
4.1.1. Atomic Force Microscopy (AFM)
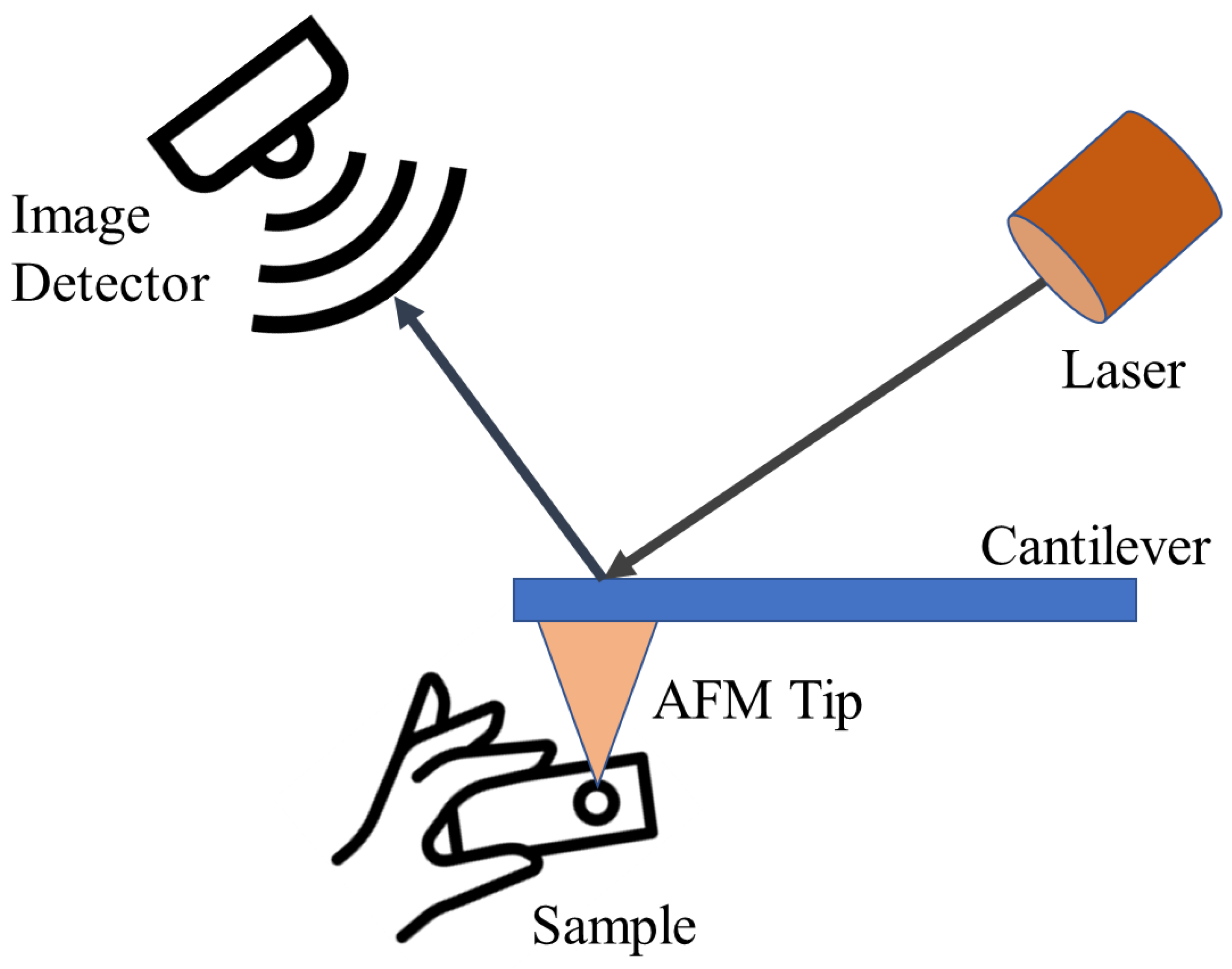
AFM represents a form of scanning probe microscopy renowned for its ability to image surfaces with exceptional resolution, reaching fractions of a nanometer. This resolution surpasses the optical diffraction limit by over 1000 times. AFM operates by employing a sharp tip positioned at the end of a cantilever, enabling it to “sense” and map the surface morphology of a sample through physical contact, in contrast to techniques reliant on light or electrons. The cantilever deflection is measured to generate a high-resolution topographic image of the surface [141]. The general principle of AFM is shown in Figure 6. AFM has a wide range of applications across fields like materials science, polymer engineering, 2D materials, food research, and virology, due to its ability to characterize a diverse set of samples at the nanoscale [141]. In the case of CDs, AFM provides both 2D and 3D information from images of CDs. Particle dimensions are computed by analyzing particle heights in 2D images, while 3D images reveal the CD surface morphology [142,143]. It is a powerful technique that can provide high-resolution, 3D topographic imaging of CDs at the nanoscale, with resolutions lower than 1 nm. AFM imaging can reveal the morphology, size distribution, and even the crystalline structure of CDs [1,144].
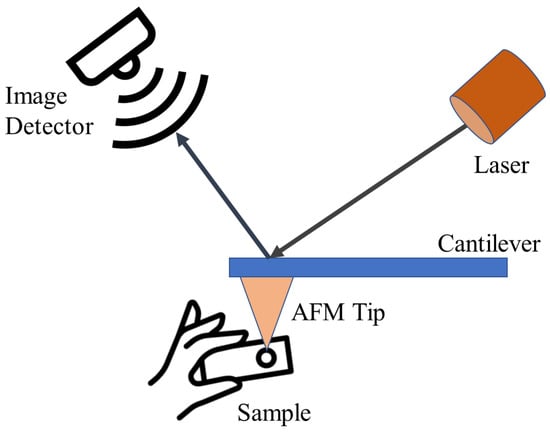
Figure 6.
AFM characterization technique working principle.
4.1.2. Transmission Electron Microscopy (TEM)
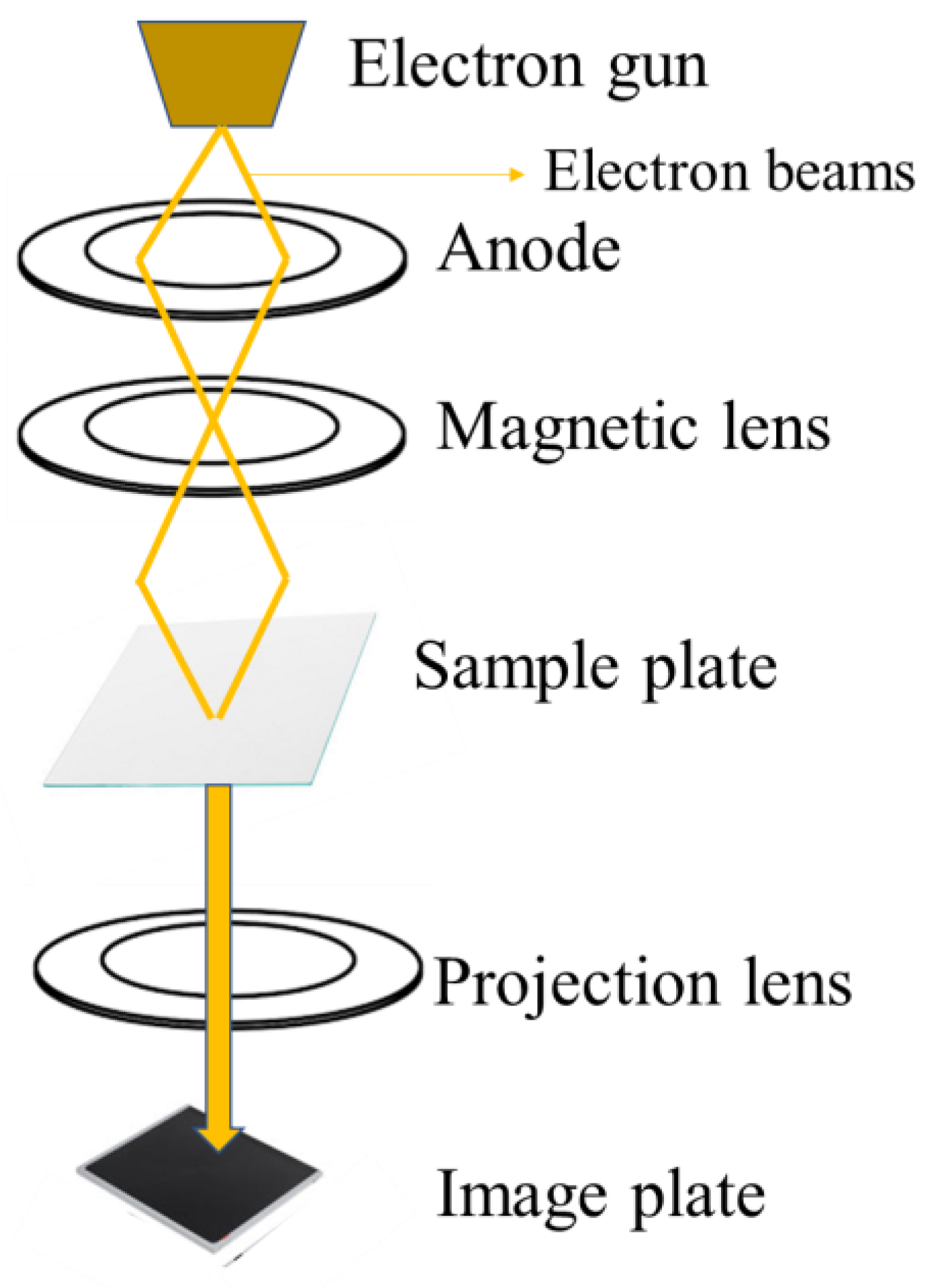
TEM is a powerful microscopy technique that involves passing a beam of high-energy electrons through an ultra-thin specimen. This interaction results in the formation of a high-resolution image, providing detailed insights into the internal structure and composition of the sample at the nanoscale level. The electron beam interacts with the sample, and the transmitted, and scattered electrons are used to generate an image that can resolve details down to the atomic scale, much finer than what is possible with light microscopes [145]. The basic principle of TEM is shown in Figure 7. TEM is an effective method for characterizing CDs at the nanoscale. The produced CDs’ shape and size distribution can be seen via TEM imaging; these images usually display spherical particles with dimensions ranging from 2 to 50 nm [82,146,147,148]. High-resolution TEM (HRTEM) provides even more detailed structural information, allowing researchers to observe the lattice fringes of the CDs and identify their partially crystalline nature [146,147].
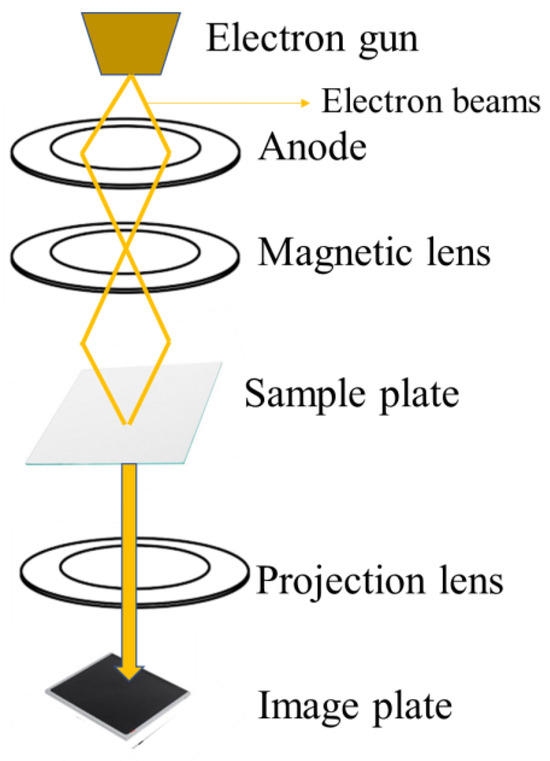
Figure 7.
TEM characterization working principle.
4.1.3. Scanning Electron Microscopy (SEM)
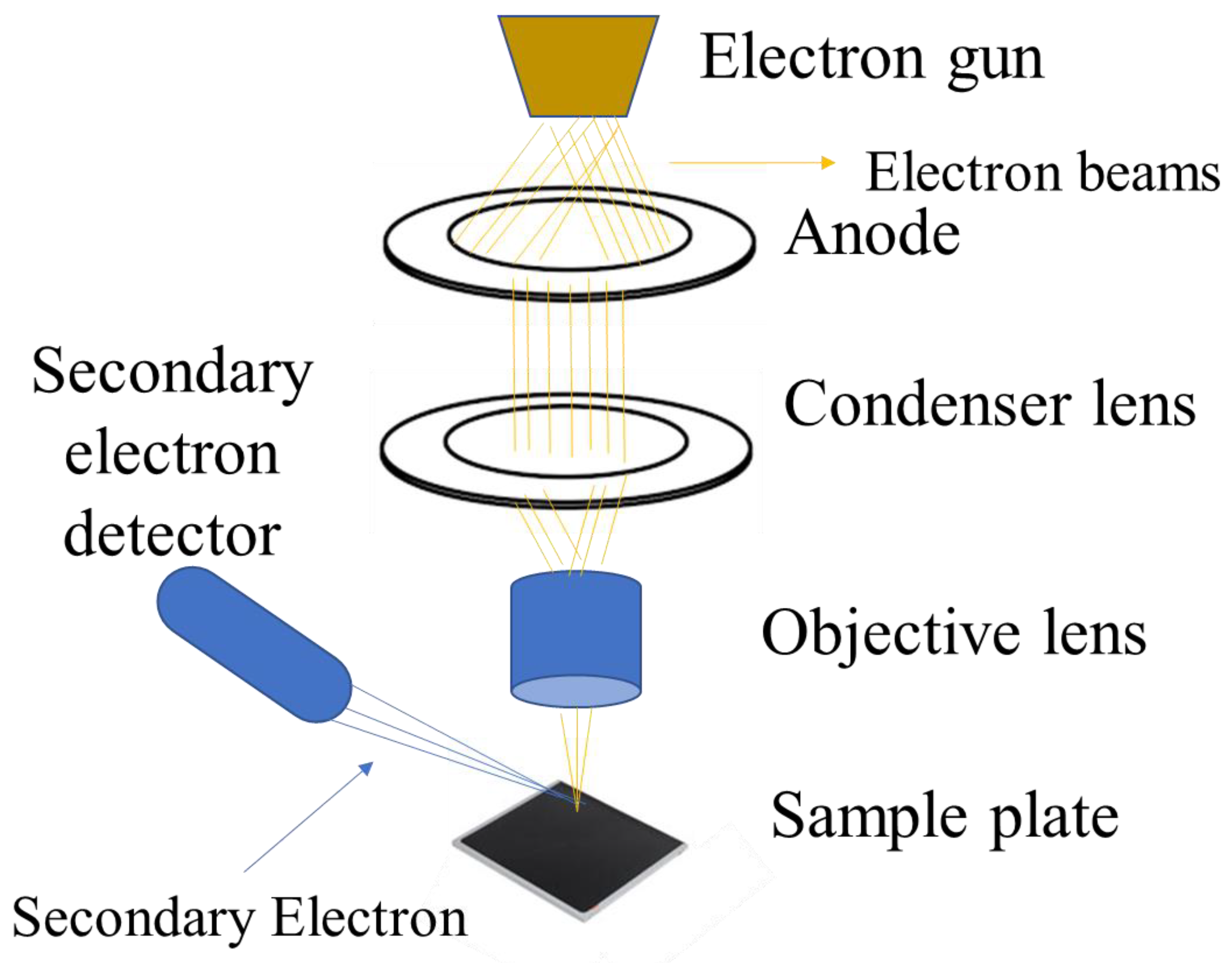
SEM works on the basis of scanning a sample’s surface with a concentrated stream of high-energy electrons. Various signals, such as “secondary electrons, backscattered electrons, and X-rays”, are produced when the electron beam interacts with the sample. These signals provide details on the composition and surface topography of the sample. A 2D image of the sample surface is created by rastering the electron beam across the sample and combining the detected signals with the beam position. The secondary electrons emitted from the sample surface are the primary signal used to generate the topographic image, as they provide information about the surface features and morphology. Backscattered electrons can also be detected to provide information about the compositional contrast in multi-phase samples [149,150,151]. The basic principle of SEM is shown in Figure 8. SEM is a useful tool for determining the morphology, size distribution, and particle size of CDs [1]. It can provide information about the size distribution of CDs, with studies reporting particle sizes ranging from 2–50 nm [146,147]. The high-resolution imaging capabilities of SEM make it a valuable tool for the morphological characterization of carbon dots and other nanomaterials, complementing the structural information that can be obtained from techniques like TEM [1,146,147].
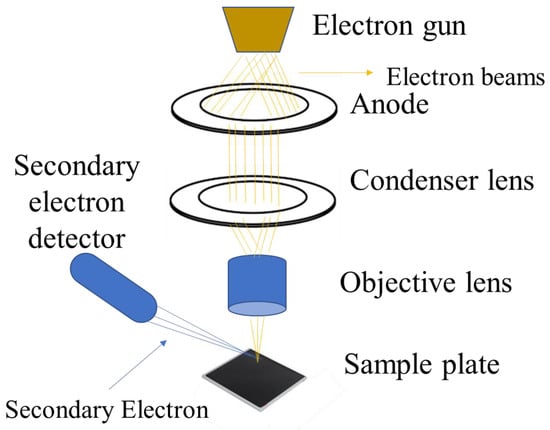
Figure 8.
SEM characterization technique working principle.
4.2. Spectroscopy
CDs are extensively characterized using a range of spectroscopic techniques, encompassing “photoluminescence (PL), ultraviolet-visible (UV-Vis), infrared (IR), Raman spectroscopy (RS), energy dispersive X-ray (EDX), nuclear magnetic resonance (NMR), dynamic light scattering (DLS), and X-ray photoelectron spectroscopy (XPS)”.
4.2.1. Photoluminescence (PL) Spectroscopy
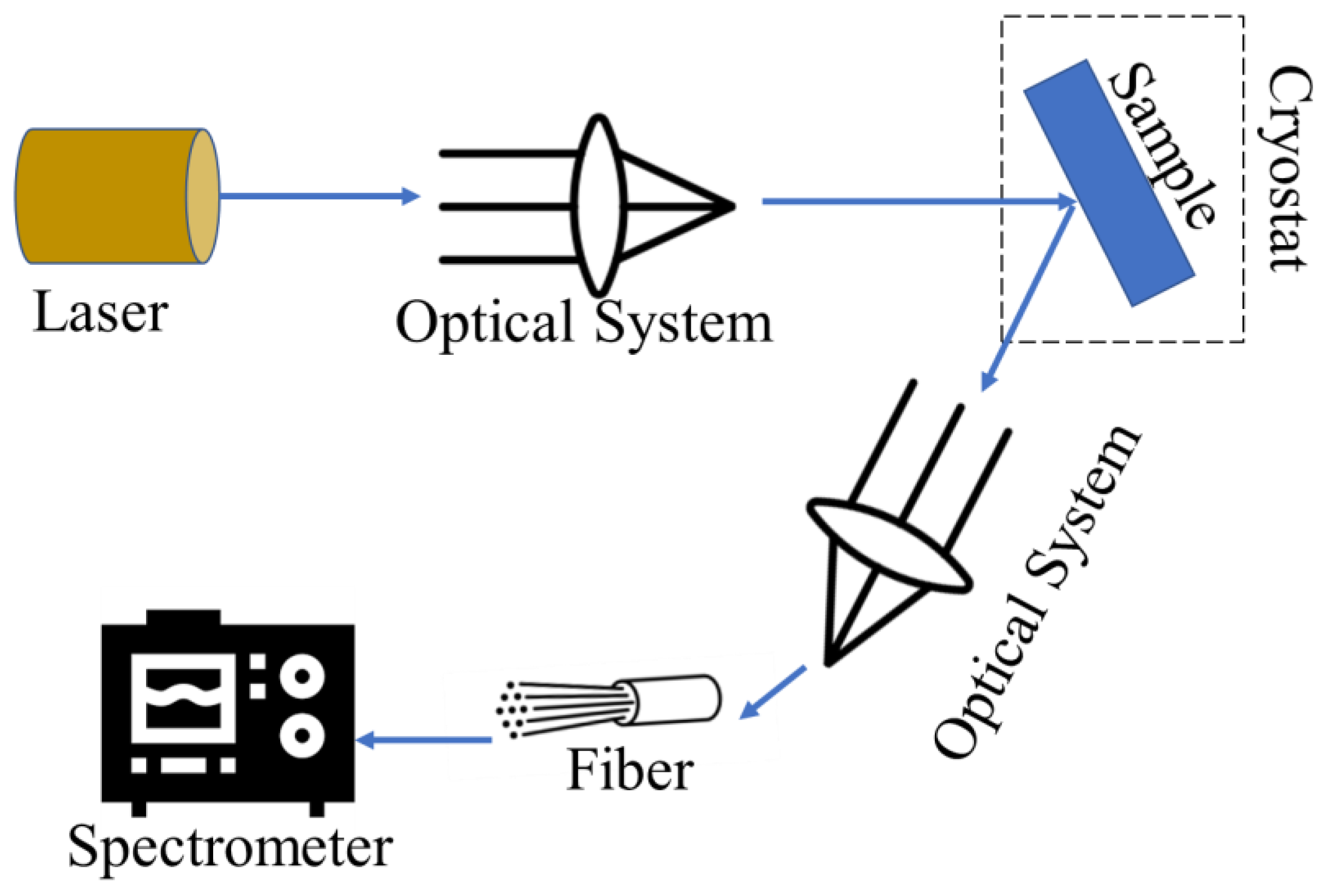
PL spectroscopy is a non-contact, non-destructive method of probing the optical and electronic properties of materials by measuring the light emitted when a sample is excited by light (photons). When a material absorbs light, it can undergo photo-excitation, where electrons are raised to higher energy states. As the electrons relax back down to lower energy states, they can emit photons, a process called photoluminescence. PL spectroscopy has two main types of luminescence: fluorescence, where the emission occurs quickly from a singlet excited state, and phosphorescence, where the emission is delayed due to a triplet excited state [152,153]. The basic principle of PL spectroscopy is shown in Figure 9.
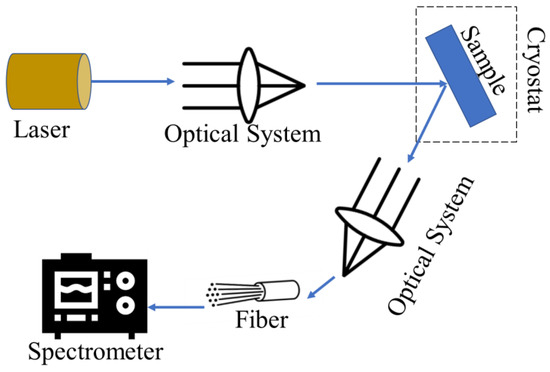
Figure 9.
The working principle of PL spectroscopy technique.
CDs typically exhibit good photoluminescence quantum yields and a characteristic shift in their emission spectrum with the excitation wavelength. PL spectroscopy analysis of CDs has shown a strong emission peak in the blue region, around 460–470 nm, when excited with UV light (325–400 nm) [154,155]. The PL intensity of CDs can be influenced by factors like the purity, surface structure, and presence of electron-withdrawing groups on the CDs and environmental factors like pH, temperature, and the presence of salts, with the CDs generally showing good stability and minimal interference [154].
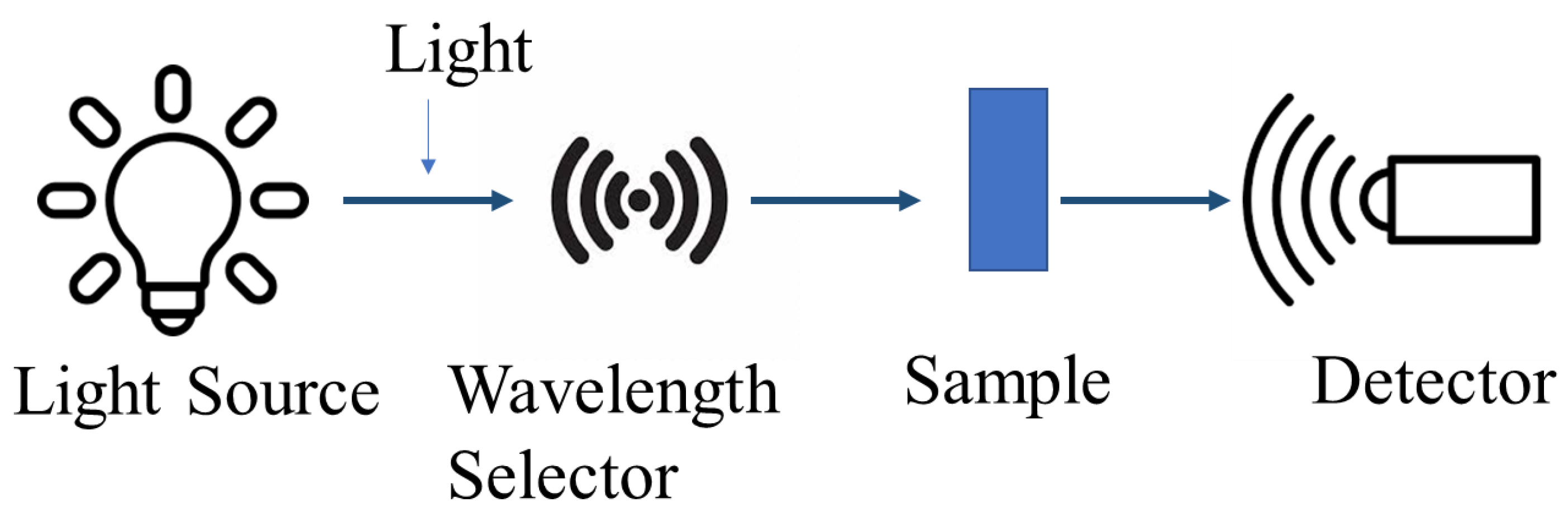
4.2.2. Ultraviolet-Visible (UV-Vis) Spectroscopy
UV-Vis’s spectroscopy, a vital analytical method, measures the absorption or transmission of light within the ultraviolet and visible regions of the electromagnetic spectrum, usually ranging from 200 nm to 800 nm [156,157]. This technique hinges on the Beer–Lambert law, affirming that a solution’s absorbance directly correlates with the concentration of the absorbing species and the path length [157]. A light beam is passed through a sample in order to measure the amount of light that is absorbed or transmitted at various wavelengths. This gives details on the sample’s optical characteristics and electronic transitions [156,157]. The basic principle of UV-Vis spectroscopy is shown in Figure 10.
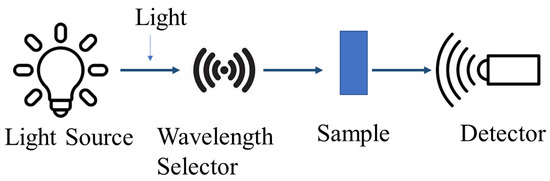
Figure 10.
The working principle of UV-Vis spectroscopy technique.
UV-Vis spectroscopy is a useful technique for analyzing the optical properties of CDs, including their absorption characteristics. CD samples typically exhibit broad absorption spectra extending into the visible region, with characteristic peaks around 255 nm and 272 nm attributed to the π-π transitions of the carbon core [147,158]. The UV-Vis absorption spectra of CDs can provide insights into their chemical composition and structural features, as the peak positions and intensities are influenced by factors like the degree of oxidation and the presence of doping elements [147].
4.2.3. Infrared (IR) Spectroscopy
In IR spectroscopy, molecules absorb infrared light, which boosts their vibrational energy, which is detectable and analyzable. This interaction leads to specific frequency absorption, aligning with the molecular bonds’ vibrational energies within the sample. Consequently, each substance exhibits a unique spectral pattern, unveiling crucial details about its molecular structure and properties [159,160,161]. For a molecule to absorb infrared light, it must have a bond with a dipole moment, where the electrons are not equally shared between the atoms [160]. IR spectroscopy is a widely used technique for characterizing the chemical functionalities and surface properties of CDs [1,162,163,164]. IR serves to assess the presence of surface carbonyl (C=O) and hydroxyl (–OH) groups on CDs, while also enabling the identification of heteroatoms doped within their structure [165,166,167,168]. In addition, IR spectra can provide insights into the chemical composition and structural features of the CDs, as the peak positions and intensities are influenced by factors like the degree of oxidation and the presence of doping elements [162,163].
4.2.4. Raman Spectroscopy (RS)
RS is a vibrational spectroscopic technique that is based on the inelastic scattering of light by molecules. When a molecule is illuminated by monochromatic light, typically from a laser, the light can interact with the molecular bonds and cause them to vibrate, leading to a change in the polarizability of the molecule. This change in polarizability results in the scattering of the incident light at different frequencies, a phenomenon known as the Raman effect [168,169,170]. RS, a non-invasive and non-destructive method, is commonly used to analyze the carbon state in CDs [171,172,173]. The two main bands that are typically seen in the Raman spectra of CDs are the D band, which indicates the vibrations of C-atoms in disordered glassy carbon or graphite, and the G band, which represents the vibrations of sp2 C-atoms in a hexagonal lattice. An understanding of sample purity can be gained from the intensity ratio (D/G) of these bands, which shows the degree of disorder or graphitization [174,175].
4.2.5. Energy Dispersive X-ray (EDX) Spectroscopy
EDX spectroscopy, also known as “Energy Dispersive X-ray Analysis (EDXA) or Energy Dispersive X-ray Microanalysis (EDXMA)”, operates by exposing a sample to an electron beam. This interaction causes the emission of characteristic X-rays, aiding in the identification of the sample’s elemental composition [176,177]. When a high-energy electron beam interacts with a sample, it can eject core electrons from the atoms in the sample. As electrons from higher energy levels fill the vacancies left by the ejected electrons, they emit X-rays with energies characteristic of the specific elements present in the sample. The emitted X-rays are detected and analyzed by the EDX system, which consists of a detector, usually a semiconductor crystal like silicon or germanium, and associated electronics. [176,177,178,179]. EDX spectroscopy, is being used to analyze the elemental composition of CDs, encompassing elements like C, N, O, and potential dopants, such as silicon (Si), while also gauging the purity levels of CDs [180,181,182].
4.2.6. Nuclear Magnetic Resonance (NMR) Spectroscopy
NMR spectroscopy relies on the magnetic properties of atomic nuclei, particularly hydrogen nuclei (protons). When placed in a magnetic field and exposed to radiofrequency pulses, these nuclei absorb and re-emit electromagnetic radiation at characteristic frequencies. By analyzing these signals, NMR can provide detailed information about the chemical environment, structure, and interactions within a molecule [183]. NMR spectroscopy is crucial for obtaining detailed structural insights into CDs, including chemical bonding, the elemental composition, and surface functional groups. It also detects chemical changes during carbonization [1]. However, NMR has drawbacks like lower sensitivity, longer analysis times, and higher costs compared to mass spectrometry [184,185].
4.2.7. Dynamic Light Scattering (DLS)
DLS, also referred to as “Photon Correlation Spectroscopy (PCS) or Quasi-Elastic Light Scattering (QELS)”, is a method for measuring particle size distribution in a solution. It operates by analyzing variations in scattered light intensity due to the Brownian motion of suspended particles [186]. The working principle of DLS involves the measurement of the time-dependent fluctuations in the scattered light intensity. When a laser beam is directed into the sample, particles in the solution scatter light in different directions. The movement of particles due to Brownian motion causes rapid fluctuations in the scattered light intensity. By analyzing these fluctuations over time, DLS can calculate the diffusion coefficient of particles, which is directly related to their size [186,187]. DLS is utilized to determine the hydrodynamic size of CDs by measuring their diffusion rate in liquid media. However, this method may be unreliable and inaccurate, as it provides information solely on the size distribution of CDs based on the generated photon correlation function [188,189,190,191].
4.2.8. X-ray Photoelectron Spectroscopy (XPS)
XPS is an analytical method for assessing the material elemental composition, chemical state, and electronic structure. It relies on the photoelectric effect, where X-rays prompt electrons to exit inner atomic orbitals within the material. The emitted electrons, known as photoelectrons, are then analyzed to provide information about the material’s surface composition and properties [192,193]. XPS is employed for analyzing the electronic state and elemental composition of the CD surface, along with other surface properties [194,195]. However, it cannot discern individual nanoparticles due to limited spatial resolution [143].
4.3. Mass Spectrometry (MS)
Mass spectrometry is a prominent method used for CD characterization, offering insights into their chemical structures [143]. Techniques like “electrospray ionization quadrupole time-of-flight tandem mass spectrometry (ESIQ-TOF-MS/MS) and matrix assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS)” are employed within this method.
4.3.1. Electrospray Ionization Quadrupole Time-of-Flight Tandem Mass Spectrometry (ESIQ-TOF-MS/MS)
ESIQ-TOF-MS/MS operates by first ionizing molecules in a sample using electrospray ionization (ESI). These ions are then subjected to tandem mass spectrometry (MS/MS) analysis. During this process, ions are sorted and isolated according to their mass-to-charge ratio (m/z) by employing a quadrupole mass filter. The filtered ions are then accelerated into a time-of-flight (TOF) analyzer, where their velocities are measured to determine their mass. This method allows for the precise identification and characterization of molecules in a sample based on their mass and fragmentation patterns [143,196,197]. This technique allows for the detailed characterization of the structure and composition of CDs, as it can provide exact molar masses with high mass accuracy in the ppm range [143,198].
4.3.2. Matrix Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry (MALDI-TOF MS)
MALDI-TOF MS operates by first preparing the sample with a matrix that aids in ionization upon laser irradiation. The laser causes the desorption and ionization of molecules, creating ions with a positive charge. These ions are then accelerated into a time-of-flight (TOF) mass analyzer, where their flight times are measured based on their mass-to-charge ratio (m/z). This allows for the determination of the mass of the ions and their subsequent identification and characterization based on their mass spectra. MALDI-TOF MS offers precise mass measurements with an accuracy of up to 0.1 nm [143,199,200]. It serves as a soft ionization method utilized for characterizing CD structures and their fragmentation [197]. While capable of identifying the chemical functionalities of CDs, this method exhibits reduced sensitivity for higher masses and cannot detect larger ions within the higher mass range [143].
4.4. X-ray Diffraction (XRD)
XRD is a technique used to determine the atomic and molecular structure of a crystalline material. It works by directing an X-ray beam at a sample, which causes the X-rays to diffract as they interact with the atoms in the material. By measuring the angles and intensities of the diffracted X-rays, XRD can provide information about the spacing between atoms in the crystal lattice, the crystal structure, and the orientation of crystalline domains within the material [201,202]. The XRD patterns of CDs indicates an amorphous carbon phase. The XRD analysis can provide information about the lattice spacing (d-spacing) of the CDs, which is typically around 0.34 nm, corresponding to the interlayer spacing of graphitic carbon [203,204,205]. XRD, while a powerful technique for crystalline materials, faces limitations in accurately characterizing the structure of amorphous CDs [189,194,206,207]. Table 7 summarizes the characterization techniques and corresponding details regarding CDs.

Table 7.
The summary of CD characterization techniques and corresponding details.
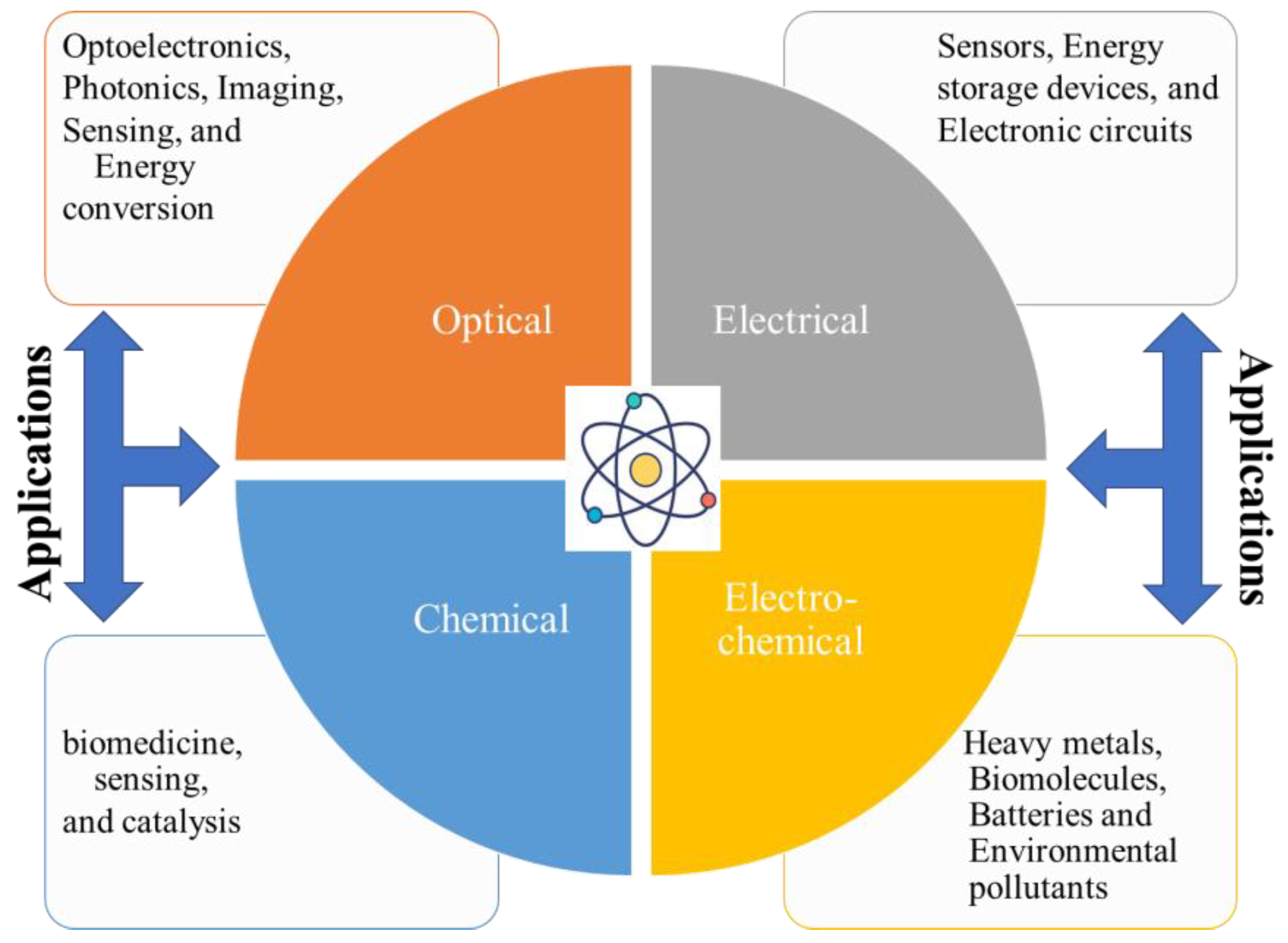
5. Properties and Applications of Carbon Dots
The exploration of renewable CDs spans various properties and applications, making them a subject of extensive research and development. These CDs exhibit a range of optical, electrical, chemical, and biocompatibility attributes that contribute to their versatility and potential in numerous fields. Therefore, an in-depth understanding of the properties of biomass-derived CDs is crucial for developing tailored CDs with specific properties for various applications, as well as for expanding the applicability of CDs through efficient synthesis methods [208]. The graphical presentation of CDs properties and their applications is provided in Figure 11.
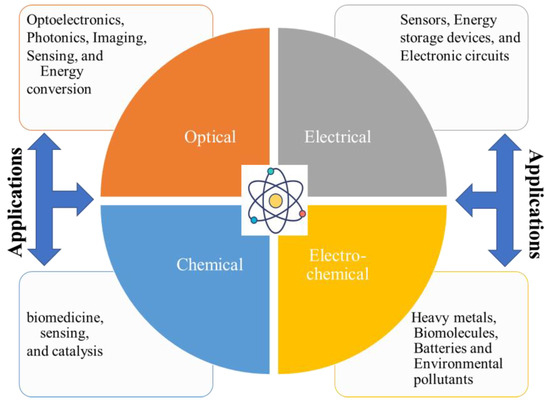
Figure 11.
The CD properties and potential applications.
5.1. Optical Properties
Optical properties refer to the behavior of materials when they interact with light or other electromagnetic radiation. These properties determine how a material absorbs, transmits, reflects, refracts, or scatters light, and they play a crucial role in various applications across different fields [209]. The optical properties of CDs vary widely due to their diverse structures [210,211]. Therefore, understanding optical properties is essential for developing and optimizing materials for various applications, such as optoelectronics, photonics, imaging, sensing, and energy conversion [212].
5.1.1. Fluorescence Property
Fluorescence refers to the emission of light by a substance after absorbing light or electromagnetic radiation. When a material absorbs photons of a particular energy, it can re-emit photons at a longer wavelength, which is characteristic of fluorescence. The fluorescence property of CDs is significant due to several key reasons. Firstly, they exhibit bright and tunable photoluminescence (PL) emission across a wide range, making them versatile for various applications. This emission color can be controlled by adjusting their structure, size, and surface chemistry [212,213,214,215]. Additionally, CDs show excitation-wavelength dependent emission, enabling multi-color emission from a single sample. Their fluorescence can also be attributed to quantum confinement effects, influenced by size and dimensionality [213]. Surface defects and functional groups contribute to their emissive properties. CDs are biocompatible with low toxicity, enhancing their appeal for biological and biomedical uses [214]. Overall, their fluorescence versatility has led to applications in sensing, imaging, optoelectronics, and energy conversion [213,214,215,216].
5.1.2. Multiphoton and Up-Conversion Emission
CDs showcase distinctive nonlinear optical properties like multiphoton and up-conversion emissions. Multiphoton emission involves the simultaneous absorption of multiple photons, causing emission at shorter wavelengths than the excitation. This allows emission in the visible or UV region when excited by longer wavelengths [217]. Up-conversion emission, or anti-Stokes emission, involves converting low-energy photons to higher-energy ones, often via a two-photon absorption process [218,219,220]. These properties, influenced by factors like size and surface chemistry, find use in bioimaging, optical storage, lasing, and nano-sensing, offering benefits like deeper tissue penetration and reduced interference [217,219].
5.1.3. Ultraviolet-Absorption Property
CDs exhibit a remarkable optical property in the form of strong absorption in the ultraviolet (UV) region, particularly in the UVA (320–400 nm) and UVB (280–320 nm) ranges due to conjugated π-domains and surface functional groups [221,222]. This absorption varies based on the preparation methods, surface modifications, and dopants, with peaks typically around 525 nm [222,223]. The strong UV absorption of CDs is an important property that makes them attractive for applications, such as UV-shielding materials, UV-blocking coatings, and UV-protective films for polymers and other materials [221]. These applications are crucial in areas like personal care products, packaging materials, and protective coatings for various surfaces, where shielding from harmful UV radiation is essential.
5.2. Electrical Properties
CDs exhibit unique electrical properties that make them attractive for various electronic and optoelectronic applications. One of the key electrical properties of CDs is their tunable electrical conductivity, which can be modulated through doping or surface functionalization. This tunability allows researchers to tailor the electrical properties of carbon dots to suit specific application requirements [224,225]. A study on poly methyl methacrylate/CDs nanocomposites revealed that the AC conductivity of these materials follows the Jonscher’s power law with double exponents, indicating the presence of two distinct conduction mechanisms. The temperature dependence of the AC conductivity using the Arrhenius representation further confirmed the existence of two mechanisms governing the conductivity in these nanocomposites [226]. This observation suggests that the electrical properties of CDs can be tuned by controlling the synthesis conditions, doping, or incorporation of carbon dots into polymer matrices [224].
The incorporation of CDs into polymer matrices has been shown to enhance the electrical properties of the resulting nanocomposite materials. For example, in azobenzene-clay nanocomposites doped with CDs, the AC conductivity properties could be tuned using UV light, making them promising candidates for photo-switchable optoelectronic devices [227]. The unique electrical properties of CDs are believed to arise from their intrinsic band gap structure. This band gap structure, combined with the ability to tune the electrical properties through doping, surface functionalization, and incorporation into polymer matrices opens up a wide range of potential applications for CDs in electronic and optoelectronic devices [224,225,228].
Furthermore, the electrical properties of CDs can be influenced by their size, shape, and surface chemistry, which can be controlled during the synthesis process. This level of control over the electrical properties is crucial for optimizing the performance of carbon dots in various applications, such as sensors, energy storage devices, and electronic circuits [224,225,228,229,230].
5.3. Chemical Properties
In the realm of nanotechnology, CDs emerge as versatile marvels with distinct chemical attributes. These tiny entities, measuring just a few nanometers, boast surfaces adorned with functional groups, like amines, carboxyls, and hydroxyls, rendering them highly reactive and adaptable [224]. Despite their minute size, CDs exhibit remarkable stability (due to its ability to functionalized with various organic molecules or polymers), enduring harsh conditions while maintaining their reactivity, making them ideal for diverse applications ranging from sensing to biomedical endeavors [223,224,230]. Moreover, their ability to undergo doping with heteroatoms, like nitrogen or sulfur, expands their potential, facilitating tailored properties for specific purposes [224,231]. The interplay of these chemical traits, influenced by synthesis techniques and precursors, unveils a vast spectrum of CD varieties, each with their own unique characteristics, promising boundless possibilities and innovations in the realm of nanotechnology.
In summary, the chemical properties of CDs are characterized by their high reactivity, chemical stability, ease of surface functionalization, presence of various functional groups, and hydrophilicity and the ability to tune their properties through heteroatom doping and different synthesis methods. These chemical properties contribute to the versatility and potential applications of CDs in various fields, including biomedicine, sensing, and catalysis [223,224,231].
5.4. Electrochemical Properties
CDs possess unique electrochemical properties that render them highly promising for various electrochemical applications. Their advantages in electrochemistry include a large surface area that enhances electrical conductivity through electron–hole pair generation, affordability, and low toxicity [1,232,233]. Moreover, CDs boast abundant surface functionalities, such as carboxyl, amino, and hydroxyl groups, facilitating surface modifications that improve particle stability and allow for precise adjustments of their chemical and physical attributes [234]. Additionally, the incorporation of heteroatoms into CDs enhances their electronic properties by promoting intramolecular charge transfer and offering reactive sites crucial for electrocatalysis [235], for various reactions, including “hydrogen evolution reaction, oxygen evolution reaction, and oxygen reduction reaction” [236]. The electrochemical properties of CDs depend on their size, functionalities, and heteroatom doping. Smaller graphene quantum dots (GQDs) exhibit higher specific capacitance, ideal for microelectronics requiring a rapid power response [237]. Additionally, incorporating GQDs into electrodes enhances properties like electrical conductivity and cycle stability, benefiting applications like lithium-ion batteries [238], sodium-ion batteries, and potassium-ion batteries [236]. It has also been reported that the electrochemical properties of CDs make them attractive for developing electrochemical sensors for various analytes, such as heavy metals, biomolecules, and environmental pollutants [239].
6. Environmental Impact and Sustainability
A comprehensive comparison of green synthesis methods for CDs is essential to assess their environmental impact and sustainability. As the demand for CDs continues to rise due to their diverse applications in fields like optoelectronics, bioimaging, and environmental sensing, understanding the eco-friendliness of their production methods becomes paramount. Green synthesis methods are designed to minimize the utilization of hazardous chemicals, lower energy consumption, and minimize waste generation in contrast to conventional techniques. By comparing these green synthesis techniques, this review aims to identify the most sustainable and environmentally friendly options, guiding researchers, industries, and policymakers towards more responsible and sustainable practices in nanomaterial synthesis. Table 8 offers a thorough comparative analysis of various green methods used in CDs synthesis. This includes a detailed examination of methods, materials, reaction conditions, by-products, advantages, and limitations. Such comprehensive comparisons aim to assess the potential and aid in the appropriate selection of these methods for CD synthesis.

Table 8.
The comparison of all green methods for CD synthesis.
Based on the summary provided in the Table 8 detailing the comparison of green synthesis methods for CDs, it becomes evident that each method brings a unique set of advantages and challenges to the forefront of nanomaterial synthesis. Beginning with the utilization of abundant and renewable plant biomass as carbon precursors across all methods, there is a clear commitment to sustainability and eco-friendliness. This foundational choice sets the stage for the entire synthesis process, aligning with the principles of green chemistry and responsible resource utilization.
In this review, microwave-assisted and ultrasonic-assisted syntheses stand out for their rapid and uniform heating techniques. These methods act as catalysts, speeding up reactions to mere minutes and ensuring higher yields with precise control over CD properties. It is akin to witnessing a magical transformation, where plant extracts and biomolecules seamlessly transition into functional nanomaterials. However, the enchantment is not without its challenges, as the need for specialized microwave equipment and vessels underscores the complexity of achieving such rapid and controlled synthesis.
In contrast, hydrothermal synthesis emerges as the methodical artisan, taking its time to craft CDs with meticulous attention to detail. The controlled environment of high temperatures and pressures allows for superior control over CD size, surface properties, and optical characteristics. This method showcases the beauty of slow and steady progress, yet it grapples with the realities of long reaction times and scalability hurdles. The dance between control and complexity is palpable, highlighting the delicate balance in nanomaterial synthesis. Pyrolysis or carbonization adds a fiery dimension to the narrative, where high temperatures transform plant biomass into carbon dots amidst a symphony of gaseous by-products. This method’s strength lies in its ability to control CD surface properties and facilitate doping with heteroatoms like nitrogen. However, the energy-intensive nature of pyrolysis and the challenges in controlling CD size distribution pose formidable obstacles along the synthesis journey.
Amidst these individual narratives, the hybrid heroes—microwave-assisted hydrothermal synthesis and ultrasonic-assisted hydrothermal synthesis—emerge as beacons of innovation. These methods blend the efficiency of rapid heating techniques with the precision of hydrothermal treatment, creating water-soluble, biocompatible CDs with an eye on energy efficiency and cost-effectiveness. Yet, they too encounter hurdles in scaling up and ensuring reproducibility, emphasizing the nuanced nature of green synthesis approaches. In essence, the story of green synthesis methods for carbon dots is a tapestry woven with sustainability, innovation, and scientific exploration. Each method contributes a chapter to the evolving narrative of responsible nanomaterial synthesis, showcasing the ongoing quest for greener, more efficient pathways in materials science.
6.1. Cost and Life Cycle Assessment
In this study, an exploration of the costs and life cycle assessments of different green synthesis methods for CDs reveals intriguing insights. While the existing literature predominantly focuses on the technical aspects of synthesis procedures, reaction conditions, advantages, and limitations, a direct comparison of costs and environmental impacts remains elusive. Nevertheless, drawing on the available information, this study extrapolates certain general inferences regarding potential costs and environmental implications.
6.1.1. Costs
Methods utilizing renewable plant biomass precursors, such as fruit peels, leaves, and agricultural waste, are likely to entail lower raw material costs compared to those relying on pure chemicals or solvents. Synthesis routes employing mild reaction conditions, such as ambient temperature and pressure, as seen in plant extract, biomolecule, microorganism-mediated, and ultrasonic-assisted methods, may result in reduced energy expenditures. Specialized equipment requirements, such as microwave reactors, autoclaves, or ultrasonic reactors, may incur higher initial setup costs but could potentially offset operational expenses through enhanced process intensification. High-temperature methods, like pyrolysis or combustion synthesis, might necessitate increased energy consumption due to elevated temperature demands.
6.1.2. Life Cycle Assessment
Green synthesis methods leveraging renewable biomass precursors and avoiding harsh chemicals or solvents tend to exhibit higher environmental sustainability. Processes generating minimal waste or by-products, exemplified by plant extract, biomolecule, microorganism-mediated, and ultrasonic-assisted methods, typically present a lower environmental impact. Conversely, high-temperature methods, like pyrolysis or combustion synthesis, may contribute to a larger carbon footprint due to heightened energy consumption and potential emissions. The use of specialized equipment, like microwave reactors or autoclaves, can elevate embodied energy and the environmental impact during manufacturing and disposal phases. To conduct a comprehensive assessment of costs and life cycle impacts for each synthesis method, a detailed analysis encompassing factors, such as raw material costs, energy consumption patterns, equipment expenditures, waste treatment strategies, and emissions profiles, would be imperative. Such an in-depth investigation could provide valuable insights into identifying the most cost-effective and environmentally sustainable pathways for carbon dot production, thereby contributing significantly to the advancement of green synthesis methodologies in this emerging field.
7. Challenges and Future Research Directions
The growing demand for sustainable nanomaterials has spurred significant interest in green synthesis methods for CDs derived from renewable sources. While these methods offer promising eco-friendly alternatives to conventional synthesis routes, they also present several challenges that need to be addressed for further advancement.
7.1. Challenges
Control Over CD Properties: Achieving precise control over carbon dot (CD) size, shape, surface properties, and optical characteristics remains a significant challenge. The variability in synthesis conditions and precursor materials can lead to inconsistencies in CD properties, impacting their performance in various applications.
Scale-Up and Reproducibility: Scaling up green synthesis methods for CD production while maintaining reproducibility is another hurdle. Transitioning from laboratory-scale to industrial-scale production requires the optimization of reaction parameters, equipment scalability, and quality control measures to ensure batch-to-batch consistency.
Doping and Functionalization: Incorporating heteroatoms like nitrogen (N) or other functional groups during CD synthesis for enhanced properties poses challenges. Achieving uniform doping levels, maintaining CD stability, and preserving desired optical properties are key considerations in this area.
Energy Efficiency and Process Intensification: While green synthesis methods aim to reduce energy consumption, further improvements in energy efficiency are desirable. Process intensification strategies, such as the optimization of reaction conditions and utilization of alternative energy sources, can contribute to more sustainable CD synthesis processes.
Waste Management and Environmental Impact: Minimizing waste generation and managing by-products from CD synthesis are critical environmental concerns. Developing methods for efficient by-product utilization or conversion into value-added products can enhance the overall sustainability of green synthesis approaches.
7.2. Future Research Directions
Advanced Characterization Techniques: Employing advanced characterization techniques, such as TEM, SEM, XRD, FTIR, and spectroscopic methods, can provide deeper insights into CD structure–property relationships. This can aid in optimizing synthesis methods and understanding the influence of different parameters on CD properties.
Innovative Precursor Materials: Exploring novel renewable precursor materials, such as waste biomass streams, bio-based polymers, and agricultural residues, can diversify the feedstock options for CD synthesis. Tailoring precursor materials for specific CD properties and functionalities can open new avenues for sustainable synthesis routes.
Doping and Surface Engineering: Advancing techniques for precise doping and the surface engineering of CDs can enhance their performance in applications like optoelectronics, sensing, and biomedical imaging. Strategies for controlled doping, surface passivation, and functionalization can be explored to tailor CD properties for targeted applications.
Process Optimization and Automation: Optimizing synthesis processes through automation, microreactor technologies, and AI-driven optimization algorithms can improve reproducibility, scalability, and energy efficiency. Continuous flow systems and the real-time monitoring of reaction parameters can lead to more robust and sustainable CD synthesis methods.
Cost and Life Cycle Assessment: Conduct comprehensive cost and life cycle assessments to evaluate the economic viability and environmental impacts of green synthesis methods for CDs from renewable sources.
Ecotoxicity and Fate of CDs: Investigate the ecotoxicity and environmental fate of CDs to assess their potential effects on ecosystems, human health, and the sustainability of CD production processes.
Circular Economy Approaches: Embracing circular economy principles, such as closed-loop processes, waste valorization, and resource recovery, can address waste management challenges in CD synthesis. Developing eco-friendly strategies for by-product utilization or recycling can reduce environmental impact and enhance overall sustainability.
By addressing these challenges and pursuing innovative research directions, the field of green synthesis methods for carbon dots from renewable sources can advance towards more efficient, sustainable, and scalable approaches with diverse applications in various industries.
8. Implications of This Study
The implications of this study are significant for a diverse audience, including researchers, policymakers, industry professionals, and the broader scientific community.
Guidance for Researchers: This study provides researchers with a comprehensive understanding of CDs, from their historical background to their synthesis methods, properties, and applications. It serves as a valuable resource for guiding future research directions, fostering innovation and promoting collaboration in the field of nanomaterials.
Informed Decision-Making for Policymakers: Policymakers can benefit from the insights presented in this study to make informed decisions regarding regulations, funding priorities, and sustainable development initiatives related to CDs. Understanding the environmental impact and sustainability considerations of CD synthesis methods can guide policy frameworks aimed at promoting responsible nanotechnology practices.
Industry Insights: Industry professionals can gain insights into the diverse applications of CDs, ranging from bioimaging and sensing to optoelectronics and environmental remediation. This knowledge can inspire the development of new products, technologies, and solutions leveraging CDs, while also considering sustainability and environmental impact factors.
Education and Outreach: The study serves as an educational resource for students, educators, and the general public interested in learning about CDs and nanomaterials. It facilitates knowledge dissemination, fosters scientific literacy, and promotes awareness of emerging technologies in the field.
Promotion of Sustainable Practices: By highlighting green synthesis methods, waste management strategies, and considerations for eco-friendly CD applications, this study contributes to the promotion of sustainable practices in nanomaterial synthesis and utilization. It encourages researchers and stakeholders to prioritize environmental sustainability in their work and decision-making processes.
9. Conclusions
This review provides a comprehensive overview of carbon dots (CDs), tracing their historical development, synthesis methods, characterization techniques, properties, and applications. Green synthesis methods, such as microwave-assisted and ultrasonic-assisted hydrothermal approaches, have emerged as significant advancements, offering efficiency, speed, and reduced environmental impact. These techniques provide excellent control over CD properties but come with their own challenges. Microwave-assisted synthesis requires specialized equipment, while ultrasonic-assisted synthesis faces issues with reactant dispersion and scalability. Future research should focus on further developing sustainable synthesis routes and refining characterization techniques to enhance CD properties and applications. Emphasizing circular economy principles and effective waste management will be crucial for minimizing the environmental impact of CD production. Additionally, evaluating the ecotoxicity and environmental fate of CDs is essential for ensuring their safe and sustainable use. In summary, while green synthesis methods have markedly improved the environmental footprint of CD production, ongoing efforts to optimize these techniques and explore novel applications will drive further innovation in the field. Advancing CD technology with a commitment to sustainability will support both scientific progress and environmental stewardship.
Author Contributions
M.U.: conceptualization, methodology, and writing—original draft, S.C.: writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research did not receive any specific grants from funding agencies in the public, commercial, or not-for-profit sectors.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Acknowledgments
The first author would like to acknowledge the Japan Ministry of Education, Culture, Sports, Science and Technology (MEXT) financial support for the MEXT Scholarship for graduate studies. The first author also thanks the Cross Lab at the Tokyo Institute of Technology for supporting and helping to refine his writing skills. In preparing this manuscript, first author used generative AI tools, specifically Quillbot and ChatGPT, to assist in rewriting, rephrasing, and checking the grammar of certain sections. These tools were employed selectively to enhance the clarity and readability of the content, ensuring that the manuscript meets the highest standards of academic communication. All intellectual contributions, research findings, and interpretations remain entirely our own.
Conflicts of Interest
The authors affirm that they do not have any known financial interests or personal relationships that might be perceived as influencing the research presented in this paper.
References
- Mansuriya, B.D.; Altintas, Z. Carbon Dots: Classification, Properties, Synthesis, Characterization, and Applications in Health Care—An Updated Review (2018–2021). Nanomaterials 2021, 11, 2525. [Google Scholar] [CrossRef]
- Liu, J.; Li, R.; Yang, B. Carbon Dots: A New Type of Carbon-Based Nanomaterial with Wide Applications. ACS Cent. Sci. 2020, 6, 2179–2195. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Ren, X.; Sun, M.; Liu, H.; Xia, L. Carbon Dots: Synthesis, Properties and Applications. Nanomaterials 2021, 11, 3419. [Google Scholar] [CrossRef]
- Mansuriya, B.; Altintas, Z. Applications of Graphene Quantum Dots in Biomedical Sensors. Sensors 2020, 20, 1072. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.-R.; Gao, F.; Shu, Z.-Y.; Ren, S.-K.; Zhu, D.; Chao, J. Nucleic Acids-Based Functional Nanomaterials for Bioimaging. J. Anal. Test. 2021, 5, 142–154. [Google Scholar] [CrossRef]
- Bose, R.J.; Ha, K.; McCarthy, J.R. Bio-Inspired Nanomaterials as Novel Options for the Treatment of Cardiovascular Disease. Drug Discov. Today 2021, 26, 1200–1211. [Google Scholar] [CrossRef]
- El-Shafey, A.M. Carbon Dots: Discovery, Structure, Fluorescent Properties, and Applications. Green Process. Synth. 2021, 10, 134–156. [Google Scholar] [CrossRef]
- Otsuka, K.; Fang, N.; Yamashita, D.; Taniguchi, T.; Watanabe, K.; Kato, Y.K. Deterministic Transfer of Optical-Quality Carbon Nanotubes for Atomically Defined Technology. Nat. Commun. 2021, 12, 3138. [Google Scholar] [CrossRef]
- Vitiello, G.; Luciani, G. Photocatalysis: Activity of Nanomaterials. Catalysts 2021, 11, 611. [Google Scholar] [CrossRef]
- Singh, C.; Mukhopadhyay, S.; Hod, I. Metal–Organic Framework Derived Nanomaterials for Electrocatalysis: Recent Developments for CO2 and N2 Reduction. Nano Converg. 2021, 8, 1. [Google Scholar] [CrossRef]
- Chahal, S.; Macairan, J.-R.; Yousefi, N.; Tufenkji, N.; Naccache, R. Green Synthesis of Carbon Dots and Their Applications. RSC Adv. 2021, 11, 25354–25363. [Google Scholar] [CrossRef]
- Sahana, S.; Gautam, A.; Singh, R.; Chandel, S. A Recent Update on Development, Synthesis Methods, Properties and Application of Natural Products Derived Carbon Dots. Nat. Prod. Bioprospecting 2023, 13, 51. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.-H.; Chen, B.-G.; Ngo, L.T.; Huang, W.-T.; Li, C.-H.; Liu, R.-S.; Hsiao, M. Natural Carbon Nanodots: Toxicity Assessment and Theranostic Biological Application. Pharmaceutics 2021, 13, 1874. [Google Scholar] [CrossRef] [PubMed]
- Rabiee, N.; Iravani, S.; Varma, R.S. Biowaste-Derived Carbon Dots: A Perspective on Biomedical Potentials. Molecules 2022, 27, 6186. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.; Huang, Y.; Yang, H.; Yan, X.F.; Chen, Z.P. A Review of Carbon Dots Produced from Biomass Wastes. Nanomaterials 2020, 10, 2316. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.V.; Kavitha, G.; Arulmozhi, R.; Arul, V.; Singaravadivel, S.; Abirami, N. Green Sources Derived Carbon Dots for Multifaceted Applications. J. Fluoresc. 2021, 31, 915–932. [Google Scholar] [CrossRef]
- Ahmed, S.; Ali, W. Green Nanomaterials: Processing, Properties, and Applications; Springer Nature: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Ng, H.K.M.; Lim, G.K.; Leo, C.P. Comparison between Hydrothermal and Microwave-Assisted Synthesis of Carbon Dots from Biowaste and Chemical for Heavy Metal Detection: A Review. Microchem. J. 2021, 165, 106116. [Google Scholar] [CrossRef]
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic Analysis and Purification of Fluorescent Single-Walled Carbon Nanotube Fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737. [Google Scholar] [CrossRef]
- Sun, Y.-P.; Zhou, B.; Lin, Y.; Wang, W.; Fernando, K.a.S.; Pathak, P.; Meziani, M.J.; Harruff, B.A.; Wang, X.; Wang, H.; et al. Quantum-Sized Carbon Dots for Bright and Colorful Photoluminescence. J. Am. Chem. Soc. 2006, 128, 7756–7757. [Google Scholar] [CrossRef]
- Zhu, S.; Meng, Q.; Wang, L.; Zhang, J.; Song, Y.; Jin, H.; Zhang, K.; Sun, H.; Wang, H.; Yang, B. Highly Photoluminescent Carbon Dots for Multicolor Patterning, Sensors, and Bioimaging. Angew. Chem. Int. Ed. 2013, 52, 3953–3957. [Google Scholar] [CrossRef]
- Zhou, J.; Booker, C.; Li, R.; Zhou, X.; Sham, T.-K.; Sun, X.; Ding, Z. An Electrochemical Avenue to Blue Luminescent Nanocrystals from Multiwalled Carbon Nanotubes (MWCNTs). J. Am. Chem. Soc. 2007, 129, 744–745. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.-B.; Zhu, Z.-T.; Wang, H.-X.; Huang, X.; Zhang, X.; Qi, X.; Zhang, H.-L.; Zhu, Y.; Deng, X.; Peng, Y.; et al. A General Solid-State Synthesis of Chemically-Doped Fluorescent Graphene Quantum Dots for Bioimaging and Optoelectronic Applications. Nanoscale 2015, 7, 10162–10169. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Qian, J.; Zhou, Y. Solvothermal Synthesis and Inkjet Printing of Carbon Quantum Dots. ChemistrySelect 2020, 5, 14930–14934. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, X.; Sun, S.; Ma, L.; Zhang, L.; Lin, H. A Facile and High-Efficient Approach to Yellow Emissive Graphene Quantum Dots from Graphene Oxide. Carbon 2017, 124, 342–347. [Google Scholar] [CrossRef]
- Li, L.-L.; Ji, J.; Fei, R.; Wang, C.-Z.; Lu, Q.; Zhang, J.-R.; Jiang, L.-P.; Zhu, J.-J. A Facile Microwave Avenue to Electrochemiluminescent Two-Color Graphene Quantum Dots. Adv. Funct. Mater. 2012, 22, 2971–2979. [Google Scholar] [CrossRef]
- Feng, Z.; Adolfsson, K.H.; Xu, Y.; Fang, H.; Hakkarainen, M.; Wu, M. Carbon Dot/Polymer Nanocomposites: From Green Synthesis to Energy, Environmental and Biomedical Applications. Sustain. Mater. Technol. 2021, 29, e00304. [Google Scholar] [CrossRef]
- Shi, W.; Han, Q.; Wu, J.; Ji, C.; Zhou, Y.; Li, S.; Gao, L.; Leblanc, R.M.; Peng, Z. Synthesis Mechanisms, Structural Models, and Photothermal Therapy Applications of Top-Down Carbon Dots from Carbon Powder, Graphite, Graphene, and Carbon Nanotubes. Int. J. Mol. Sci. 2022, 23, 1456. [Google Scholar] [CrossRef]
- Kuang, T.; Jin, M.; Lu, X.; Liu, T.; Vahabi, H.; Gu, Z.; Gong, X. Functional Carbon Dots Derived from Biomass and Plastic Wastes. Green Chem. 2023, 25, 6581–6602. [Google Scholar] [CrossRef]
- Bacon, M.; Bradley, S.J.; Nann, T. Graphene Quantum Dots. Part. Part. Syst. Charact. 2013, 31, 415–428. [Google Scholar] [CrossRef]
- Hawrylak, P.; Peeters, F.; Ensslin, K. Carbononics—Integrating Electronics, Photonics and Spintronics with Graphene Quantum Dots. Phys. Status Solidi Rapid Res. Lett. 2016, 10, 11–12. [Google Scholar] [CrossRef]
- Hai, X.; Feng, J.; Chen, X.; Wang, J. Tuning the Optical Properties of Graphene Quantum Dots for Biosensing and Bioimaging. J. Mater. Chem. B 2018, 6, 3219–3234. [Google Scholar] [CrossRef]
- Lin, L.; Luo, Y.; Tsai, P.; Wang, J.; Chen, X. Metal Ions Doped Carbon Quantum Dots: Synthesis, Physicochemical Properties, and Their Applications. TrAC Trends Anal. Chem. 2018, 103, 87–101. [Google Scholar] [CrossRef]
- Stefanakis, D.; Philippidis, A.; Sygellou, L.; Filippidis, G.; Ghanotakis, D.; Anglos, D. Synthesis of Fluorescent Carbon Dots by a Microwave Heating Process: Structural Characterization and Cell Imaging Applications. J. Nanoparticle Res. 2014, 16, 2646. [Google Scholar] [CrossRef]
- Monte-Filho, S.S.; Andrade, S.I.E.; Lima, M.B.; Araujo, M.C.U. Synthesis of Highly Fluorescent Carbon Dots from Lemon and Onion Juices for Determination of Riboflavin in Multivitamin/Mineral Supplements. J. Pharm. Anal. 2019, 9, 209–216. [Google Scholar] [CrossRef]
- Ang, W.L.; Mee, C.a.L.B.; Sambudi, N.S.; Mohammad, A.W.; Leo, C.P.; Mahmoudi, E.; Ba-Abbad, M.; Benamor, A. Microwave-Assisted Conversion of Palm Kernel Shell Biomass Waste to Photoluminescent Carbon Dots. Sci. Rep. 2020, 10, 21199. [Google Scholar] [CrossRef] [PubMed]
- Malavika, J.P.; Shobana, C.; Ragupathi, M.; Kumar, P.; Lee, Y.S.; Govarthanan, M.; Selvan, R.K. A Sustainable Green Synthesis of Functionalized Biocompatible Carbon Quantum Dots from Aloe Barbadensis Miller and Its Multifunctional Applications. Environ. Res. 2021, 200, 111414. [Google Scholar] [CrossRef]
- Hu, Y.; Gao, Z. Sewage Sludge in Microwave Oven: A Sustainable Synthetic Approach toward Carbon Dots for Fluorescent Sensing of Para-Nitrophenol. J. Hazard. Mater. 2020, 382, 121048. [Google Scholar] [CrossRef]
- Chatzimitakos, T.; Kasouni, A.; Sygellou, L.; Leonardos, I.; Troganis, A.; Stalikas, C. Human Fingernails as an Intriguing Precursor for the Synthesis of Nitrogen and Sulfur-Doped Carbon Dots with Strong Fluorescent Properties: Analytical and Bioimaging Applications. Sens. Actuators B Chem. 2018, 267, 494–501. [Google Scholar] [CrossRef]
- George, H.S.; Selvaraj, H.; Ilangovan, A.; Chandrasekaran, K.; Kannan, V.R.; Parthipan, P.; Almutairi, B.O.; Balu, R. Green Synthesis of Biomass Derived Carbon Dots via Microwave-Assisted Method for Selective Detection of Fe3+ Ions in an Aqueous Medium. Inorg. Chem. Commun. 2023, 157, 111348. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, C.; Gao, Y.; Yang, L.; Xu, J.; Zhang, X.; Lu, C.; Wang, Y.; Zhu, Y. Biomass-Derived Nitrogen Self-Doped Carbon Dots via a Simple One-Pot Method: Physicochemical, Structural, and Luminescence Properties. Appl. Surf. Sci. 2020, 510, 145437. [Google Scholar] [CrossRef]
- Shibata, H.; Abe, M.; Sato, K.; Uwai, K.; Tokuraku, K.; Iimori, T. Microwave-Assisted Synthesis and Formation Mechanism of Fluorescent Carbon Dots from Starch. Carbohydr. Polym. Technol. Appl. 2022, 3, 100218. [Google Scholar] [CrossRef]
- Venugopalan, P.; Vidya, N. Microwave-Assisted Green Synthesis of Carbon Dots Derived from Wild Lemon (Citrus pennivesiculata) Leaves as a Fluorescent Probe for Tetracycline Sensing in Water. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 286, 122024. [Google Scholar] [CrossRef]
- Yang, P.; Zhu, Z.; Chen, M.; Zhou, X.; Chen, W. Microwave-Assisted Synthesis of Polyamine-Functionalized Carbon Dots from Xylan and Their Use for the Detection of Tannic Acid. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 213, 301–308. [Google Scholar] [CrossRef]
- Zhu, Z.; Yang, P.; Chen, M.; Zhang, T.; Cao, Y.; Zhang, W.; Zhou, X.; Chen, W. Microwave Synthesis of Amphiphilic Carbon Dots from Xylose and Construction of Luminescent Composites with Shape Recovery Performance. J. Lumin. 2019, 213, 474–481. [Google Scholar] [CrossRef]
- Jusuf, B.N.; Sambudi, N.S.; Isnaeni, N.; Samsuri, S. Microwave-Assisted Synthesis of Carbon Dots from Eggshell Membrane Ashes by Using Sodium Hydroxide and Their Usage for Degradation of Methylene Blue. J. Environ. Chem. Eng. 2018, 6, 7426–7433. [Google Scholar] [CrossRef]
- Roshni, V.; Divya, O. One-Step Microwave-Assisted Green Synthesis of Luminescent N-Doped Carbon Dots from Sesame Seeds for Selective Sensing of Fe (III). Curr. Sci. 2017, 112, 385. [Google Scholar] [CrossRef]
- Khayal, A.; Dawane, V.; Amin, M.A.; Tirth, V.; Yadav, V.K.; Algahtani, A.; Khan, S.H.; Islam, S.; Yadav, K.K.; Jeon, B.-H. Advances in the Methods for the Synthesis of Carbon Dots and Their Emerging Applications. Polymers 2021, 13, 3190. [Google Scholar] [CrossRef] [PubMed]
- Zaib, M.; Arshad, A.; Khalid, S.; Shahzadi, T. One Pot Ultrasonic Plant Mediated Green Synthesis of Carbon Dots and Their Application Invisible Light Induced Dye Photocatalytic Studies: A Kinetic Approach. Int. J. Environ. Anal. Chem. 2021, 103, 5063–5081. [Google Scholar] [CrossRef]
- Banger, A.; Gautam, S.; Jadoun, S.; Jangid, N.K.; Srivastava, A.; Pulidindi, I.N.; Dwivedi, J.; Srivastava, M. Synthetic Methods and Applications of Carbon Nanodots. Catalysts 2023, 13, 858. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, V.B.; Gedanken, A. Sonochemical Synthesis of Carbon Dots, Mechanism, Effect of Parameters, and Catalytic, Energy, Biomedical and Tissue Engineering Applications. Ultrason. Sonochem. 2020, 64, 105009. [Google Scholar] [CrossRef]
- Zhuo, S.; Shao, M.; Lee, S.-T. Upconversion and Downconversion Fluorescent Graphene Quantum Dots: Ultrasonic Preparation and Photocatalysis. ACS Nano 2012, 6, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Lee, H.U.; Park, E.S.; Lee, S.C.; Lee, J.-W.; Jeong, S.W.; Kim, C.H.; Lee, Y.-C.; Huh, Y.S.; Lee, J. Photoluminescent Green Carbon Nanodots from Food-Waste-Derived Sources: Large-Scale Synthesis, Properties, and Biomedical Applications. ACS Appl. Mater. Interfaces 2014, 6, 3365–3370. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Cui, K.; Gong, T.; Zhang, J.; Zhai, Z.; Hou, L.; Zaman, F.U.; Yuan, C. Ultrasonic-Assisted Synthesis of N-Doped, Multicolor Carbon Dots toward Fluorescent Inks, Fluorescence Sensors, and Logic Gate Operations. Nanomaterials 2022, 12, 312. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.-B.; Liu, K.-K.; Song, S.-Y.; Zhou, R.; Shan, C.-X. Fluorescent Nano-Biomass Dots: Ultrasonic-Assisted Extraction and Their Application as Nanoprobe for Fe3+ Detection. Nanoscale Res. Lett. 2019, 14, 130. [Google Scholar] [CrossRef]
- Dehvari, K.; Liu, K.Y.; Tseng, P.-J.; Gedda, G.; Girma, W.M.; Chang, J.-Y. Sonochemical-Assisted Green Synthesis of Nitrogen-Doped Carbon Dots from Crab Shell as Targeted Nanoprobes for Cell Imaging. J. Taiwan Inst. Chem. Eng. 2019, 95, 495–503. [Google Scholar] [CrossRef]
- ReddyPrasad, P.; Naidoo, E.B. Ultrasonic Synthesis of High Fluorescent C-Dots and Modified with CuWO4 Nanocomposite for Effective Photocatalytic Activity. J. Mol. Struct. 2015, 1098, 146–152. [Google Scholar] [CrossRef]
- Basha, Z.W.; Muniraj, S.; Kumar, A.S. Neem Biomass Derived Carbon Quantum Dots Synthesized via One Step Ultrasonification Method for Ecofriendly Methylene Blue Dye Removal. Sci. Rep. 2024, 14, 9706. [Google Scholar] [CrossRef]
- Yan, Y.; Manickam, S.; Lester, E.; Wu, T.; Pang, C.H. Synthesis of Graphene Oxide and Graphene Quantum Dots from Miscanthus via Ultrasound-Assisted Mechano-Chemical Cracking Method. Ultrason. Sonochem. 2021, 73, 105519. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, Y.; Liu, K.; Zhou, R.; Shan, C. Multicolor Biomass Based Carbon Nanodots for Bacterial Imaging. Chin. Chem. Lett. 2022, 33, 798–802. [Google Scholar] [CrossRef]
- Manoharan, P.; Dhanabalan, S.C.; Alagan, M.; Muthuvijayan, S.; Ponraj, J.S.; Somasundaram, C.K. Facile Synthesis and Characterisation of Green Luminescent Carbon Nanodots Prepared from Tender Coconut Water Using the Acid-assisted Ultrasonic Route. Micro Nano Lett. 2020, 15, 920–924. [Google Scholar] [CrossRef]
- Abbas, A.; Mariana, L.T.; Phan, A.N. Biomass-Waste Derived Graphene Quantum Dots and Their Applications. Carbon 2018, 140, 77–99. [Google Scholar] [CrossRef]
- Zhu, Z.; Niu, H.; Li, R.; Yang, Z.; Wang, J.; Li, X.; Pan, P.; Liu, J.; Zhou, B. One-Pot Hydrothermal Synthesis of Fluorescent Carbon Quantum Dots with Tunable Emission Color for Application in Electroluminescence Detection of Dopamine. Biosens. Bioelectron. X 2022, 10, 100141. [Google Scholar] [CrossRef]
- Nugroho, D.; Wannakan, K.; Nanan, S.; Benchawattananon, R. The Synthesis of Carbon Dots//Zincoxide (CDs/ZnO-H400) by Using Hydrothermal Methods for Degradation of Ofloxacin Antibiotics and Reactive Red Azo Dye (RR141). Sci. Rep. 2024, 14, 2455. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Wang, Y.; Zhang, F.; Yang, Y.; Liu, X.; Guo, K.; Wang, H.; Xu, B. Microwave-Assisted Hydrothermal Synthesis of Solid-State Carbon Dots with Intensive Emission for White Light-Emitting Devices. J. Mater. Chem. C 2017, 5, 8105–8111. [Google Scholar] [CrossRef]
- Usman, M.; Cheng, S.; Boonyubol, S.; Cross, J.S. From Biomass to Biocrude: Innovations in Hydrothermal Liquefaction and Upgrading. Energy Convers. Manag. 2024, 302, 118093. [Google Scholar] [CrossRef]
- Tajik, S.; Dourandish, Z.; Zhang, K.; Beitollahi, H.; Van Le, Q.; Jang, H.W.; Shokouhimehr, M. Carbon and Graphene Quantum Dots: A Review on Syntheses, Characterization, Biological and Sensing Applications for Neurotransmitter Determination. RSC Adv. 2020, 10, 15406–15429. [Google Scholar] [CrossRef]
- Liu, S.; Cui, J.; Huang, J.; Tian, B.; Jia, F.; Wang, Z. Facile One-Pot Synthesis of Highly Fluorescent Nitrogen-Doped Carbon Dots by Mild Hydrothermal Method and Their Applications in Detection of Cr (VI) Ions. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 206, 65–71. [Google Scholar] [CrossRef]
- Wang, S.; Wu, S.-H.; Fang, W.-L.; Guo, X.-F.; Wang, H. Synthesis of Non-Doped and Non-Modified Carbon Dots with High Quantum Yield and Crystallinity by One-Pot Hydrothermal Method Using a Single Carbon Source and Used for ClO−Detection. Dye. Pigment. 2019, 164, 7–13. [Google Scholar] [CrossRef]
- Xie, N.; Cheng, N.; Liu, N.; Han, N. Green Hydrothermal Synthesis of N-Doped Carbon Dots from Biomass Highland Barley for the Detection of Hg2+. Sensors 2019, 19, 3169. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, M.; Niu, N.; Chen, Z.; Li, S.; Liu, S.; Li, J. Natural-Product-Derived Carbon Dots: From Natural Products to Functional Materials. ChemSusChem 2017, 11, 11–24. [Google Scholar] [CrossRef]
- Wang, G.; Guo, Q.; Chen, D.; Liu, Z.; Zheng, X.; Xu, A.; Yang, S.; Ding, G. Facile and Highly Effective Synthesis of Controllable Lattice Sulfur-Doped Graphene Quantum Dots via Hydrothermal Treatment of Durian. ACS Appl. Mater. Interfaces 2018, 10, 5750–5759. [Google Scholar] [CrossRef] [PubMed]
- Shekarbeygi, Z.; Farhadian, N.; Khani, S.; Moradi, S.; Shahlaei, M. The Effects of Rose Pigments Extracted by Different Methods on the Optical Properties of Carbon Quantum Dots and Its Efficacy in the Determination of Diazinon. Microchem. J. 2020, 158, 105232. [Google Scholar] [CrossRef]
- Atchudan, R.; Edison, T.N.J.I.; Perumal, S.; Muthuchamy, N.; Lee, Y.R. Hydrophilic Nitrogen-Doped Carbon Dots from Biowaste Using Dwarf Banana Peel for Environmental and Biological Applications. Fuel 2020, 275, 117821. [Google Scholar] [CrossRef]
- Chauhan, P.; Saini, J.; Chaudhary, S.; Bhasin, K.K. Sustainable Synthesis of Carbon Dots from Agarose Waste and Prospective Application in Sensing of L-Aspartic Acid. Mater. Res. Bull. 2021, 134, 111113. [Google Scholar] [CrossRef]
- Zhao, Y.; Jing, S.; Peng, X.; Chen, Z.; Hu, Y.; Zhuo, H.; Sun, R.; Zhong, L. Synthesizing Green Carbon Dots with Exceptionally High Yield from Biomass Hydrothermal Carbon. Cellulose 2019, 27, 415–428. [Google Scholar] [CrossRef]
- Sharma, V.D.; Kansay, V.; Bhatia, A.; Bera, M.K. Luminescent Nanocomposite Based on Brassica Juncea-Derived Intentionally Cu-Doped Carbon Quantum Dots Embedded in Bioplastic for UV-Tube down-Conversion Applications. Appl. Phys. A 2023, 129, 632. [Google Scholar] [CrossRef]
- Kasinathan, K.; Samayanan, S.; Marimuthu, K.; Yim, J.-H. Green Synthesis of Multicolour Fluorescence Carbon Quantum Dots from Sugarcane Waste: Investigation of Mercury (II) Ion Sensing, and Bio-Imaging Applications. Appl. Surf. Sci. 2022, 601, 154266. [Google Scholar] [CrossRef]
- Sariga, N.; Kolaprath, M.K.A.; Benny, L.; Varghese, A. A Facile, Green Synthesis of Carbon Quantum Dots from Polyalthia longifolia and Its Application for the Selective Detection of Cadmium. Dye. Pigment. 2023, 210, 111048. [Google Scholar] [CrossRef]
- Meng, W.; Wang, B.; Ai, L.; Song, H.; Lu, S. Engineering White Light-Emitting Diodes with High Color Rendering Index from Biomass Carbonized Polymer Dots. J. Colloid Interface Sci. 2021, 598, 274–282. [Google Scholar] [CrossRef]
- Qing, W.; Chen, K.; Yang, Y.; Wang, Y.; Liu, X. Cu2+-Doped Carbon Dots as Fluorescence Probe for Specific Recognition of Cr(VI) and Its Antimicrobial Activity. Microchem. J. 2020, 152, 104262. [Google Scholar] [CrossRef]
- Wang, C.; Shi, H.; Yang, M.; Yan, Y.; Liu, E.; Ji, Z.; Fan, J. Facile Synthesis of Novel Carbon Quantum Dots from Biomass Waste for Highly Sensitive Detection of Iron Ions. Mater. Res. Bull. 2020, 124, 110730. [Google Scholar] [CrossRef]
- Chen, W.; Li, D.; Tian, L.; Xiang, W.; Wang, T.; Hu, W.; Hu, Y.; Chen, S.; Chen, J.; Dai, Z. Synthesis of Graphene Quantum Dots from Natural Polymer Starch for Cell Imaging. Green Chem. 2018, 20, 4438–4442. [Google Scholar] [CrossRef]
- Jiang, X.; Qin, D.; Mo, G.; Feng, J.; Yu, C.; Mo, W.; Deng, B. Ginkgo Leaf-Based Synthesis of Nitrogen-Doped Carbon Quantum Dots for Highly Sensitive Detection of Salazosulfapyridine in Mouse Plasma. J. Pharm. Biomed. Anal. 2019, 164, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wan, Y.; Zhang, K.; Fu, Q.; Wang, L.; Zeng, J.; Xia, Z.; Gao, D. Green Synthesis of Carbon Dots Using the Flowers of Osmanthus fragrans (Thunb.) Lour. as Precursors: Application in Fe3+ and Ascorbic Acid Determination and Cell Imaging. Anal. Bioanal. Chem. 2019, 411, 2715–2727. [Google Scholar] [CrossRef] [PubMed]
- Sobhani, R.; Rezaei, B.; Shahshahanipour, M.; Ensafi, A.A.; Mohammadnezhad, G. Simple and Green Synthesis of Carbon Dots (CDs) from Valerian Root and Application of Modified Mesoporous Boehmite (AlOOH) with CDs as a Fluorescence Probe for Determination of Imipramine. Anal. Bioanal. Chem. 2019, 411, 3115–3124. [Google Scholar] [CrossRef] [PubMed]
- Qin, K.; Zhang, D.; Ding, Y.; Zheng, X.; Xiang, Y.; Hua, J.; Zhang, Q.; Ji, X.; Li, B.; Wei, Y. Applications of Hydrothermal Synthesis of Escherichia Coli Derived Carbon Dots in in Vitro and in Vivo Imaging and P-Nitrophenol Detection. Analyst 2020, 145, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Marpongahtun, M.; Andriayani, A.; Muis, Y.; Gea, S.; Amaturrahim, S.A.; Attaurrazaq, B.; Daulay, A. Synthesis of Nitrogen-Doped Carbon Dots from Nanocrystalline Cellulose by Pyrolysis Method as Hg2+ Detector. Int. J. Technol. 2023, 14, 219. [Google Scholar] [CrossRef]
- Bagheri, Z.; Ehtesabi, H.; Rahmandoust, M.; Ahadian, M.M.; Hallaji, Z.; Eskandari, F.; Jokar, E. New Insight into the Concept of Carbonization Degree in Synthesis of Carbon Dots to Achieve Facile Smartphone Based Sensing Platform. Sci. Rep. 2017, 7, 11013. [Google Scholar] [CrossRef]
- Zhang, Y.; Fan, X.; Sun, X.; Yang, X.; Li, Z.; Yang, Z.; Dong, C. Synthesis of Oil-Soluble Carbon Dots via Pyrolysis and Their Diverse Applications in Doxycycline Detection, Fluorescent Ink and Film. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2024, 304, 123406. [Google Scholar] [CrossRef]
- Brachi, P. Synthesis of Carbon Dots (CDs) through the Fluidized Bed Thermal Treatment of Residual Biomass Assisted by γ-Alumina. Appl. Catal. B Environ. Energy 2020, 263, 118361. [Google Scholar] [CrossRef]
- Da Costa, R.S.; Da Cunha, W.F.; Pereira, N.S.; Ceschin, A.M. An Alternative Route to Obtain Carbon Quantum Dots from Photoluminescent Materials in Peat. Materials 2018, 11, 1492. [Google Scholar] [CrossRef] [PubMed]
- Aji, M.P.; Susanto, N.; Wiguna, P.A.; Sulhadi, N. Facile Synthesis of Luminescent Carbon Dots from Mangosteen Peel by Pyrolysis Method. Iran. Phys. J. 2017, 11, 119–126. [Google Scholar] [CrossRef]
- Qin, X.; Fu, C.; Zhang, J.; Shao, W.; Qin, X.; Gui, Y.; Wang, L.; Guo, H.; Chen, F.; Jiang, L.; et al. Direct Preparation of Solid Carbon Dots by Pyrolysis of Collagen Waste and Their Applications in Fluorescent Sensing and Imaging. Front. Chem. 2022, 10, 1006389. [Google Scholar] [CrossRef]
- Singh, V.; Chatterjee, S.; Palecha, M.; Sen, P.; Ateeq, B.; Verma, V. Chickpea Peel Waste as Sustainable Precursor for Synthesis of Fluorescent Carbon Nanotubes for Bioimaging Application. Carbon Lett. 2020, 31, 117–123. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Kong, H.; Cheng, J.; Zhang, M.; Sun, Z.; Wang, S.; Liu, J.; Qu, H.; Zhao, Y. Novel Mulberry Silkworm Cocoon-Derived Carbon Dots and Their Anti-Inflammatory Properties. Artif. Cells Nanomed. Biotechnol. 2019, 48, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Dong, Y.; Sun, H.; Chen, N. Highly Fluorescent Carbon Dots from Peanut Shells as Potential Probes for Copper Ion: The Optimization and Analysis of the Synthetic Process. Mater. Today Chem. 2017, 5, 1–10. [Google Scholar] [CrossRef]
- Das, P.; Ganguly, S.; Maity, P.P.; Srivastava, H.K.; Bose, M.; Dhara, S.; Bandyopadhyay, S.; Das, A.K.; Banerjee, S.; Das, N.C. Converting Waste Allium Sativum Peel to Nitrogen and Sulphur Co-Doped Photoluminescence Carbon Dots for Solar Conversion, Cell Labeling, and Photobleaching Diligences: A Path from Discarded Waste to Value-Added Products. J. Photochem. Photobiol. B Biol. 2019, 197, 111545. [Google Scholar] [CrossRef] [PubMed]
- Sawalha, S.; Silvestri, A.; Criado, A.; Bettini, S.; Prato, M.; Valli, L. Tailoring the Sensing Abilities of Carbon Nanodots Obtained from Olive Solid Wastes. Carbon 2020, 167, 696–708. [Google Scholar] [CrossRef]
- Kumar, A.; Kuang, Y.; Liang, Z.; Sun, X. Microwave Chemistry, Recent Advancements, and Eco-Friendly Microwave-Assisted Synthesis of Nanoarchitectures and Their Applications: A Review. Mater. Today Nano 2020, 11, 100076. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, J.; Wang, J.; Yang, Y.; Liu, X. Rapid Microwave-Assisted Synthesis of Highly Luminescent Nitrogen-Doped Carbon Dots for White Light-Emitting Diodes. Opt. Mater. 2017, 73, 319–329. [Google Scholar] [CrossRef]
- Zaman, A.S.; Tan, T.L.; Jamaludin, N.; Sadrolhosseini, A.R.; Rashid, U.; Rashid, S.A. Properties and Molecular Structure of Carbon Quantum Dots Derived from Empty Fruit Bunch Biochar Using a Facile Microwave-Assisted Method for the Detection of Cu2+ Ions. Opt. Mater. 2021, 112, 110801. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, D.; Gao, Y.; Tong, B.; Li, Y.; Zhu, Y. Non-Thermal Effect of Microwave on the Chemical Structure and Luminescence Properties of Biomass-Derived Carbon Dots via Hydrothermal Method. Appl. Surf. Sci. 2021, 552, 149503. [Google Scholar] [CrossRef]
- Ibarra-Prieto, H.D.; Garcia-Garcia, A.; Aguilera-Granja, F.; Navarro-Ibarra, D.C.; Rivero-Espejel, I. One-Pot, Optimized Microwave-Assisted Synthesis of Difunctionalized and B–N Co-Doped Carbon Dots: Structural Characterization. Nanomaterials 2023, 13, 2753. [Google Scholar] [CrossRef]
- Romero, M.P.; Alves, F.; Stringasci, M.D.; Buzzá, H.H.; Ciol, H.; Inada, N.M.; Bagnato, V.S. One-Pot Microwave-Assisted Synthesis of Carbon Dots and in Vivo and in Vitro Antimicrobial Photodynamic Applications. Front. Microbiol. 2021, 12, 662149. [Google Scholar] [CrossRef]
- Abdoon, F.M.; Atawy, H.M. Prospective of Microwave-Assisted and Hydrothermal Synthesis of Carbon Quantum Dots/Silver Nanoparticles for Spectrophotometric Determination of Losartan Potassium in Pure Form and Pharmaceutical Formulations. Mater. Today Proc. 2021, 42, 2141–2149. [Google Scholar] [CrossRef]
- Qi, C.; Wang, H.; Yang, A.; Wang, X.; Xu, J. Facile Fabrication of Highly Fluorescent N-Doped Carbon Quantum Dots Using an Ultrasonic-Assisted Hydrothermal Method: Optical Properties and Cell Imaging. ACS Omega 2021, 6, 32904–32916. [Google Scholar] [CrossRef]
- Anpalagan, K.; Yin, H.; Cole, I.; Zhang, T.; Lai, D.T.H. Quantum Yield Enhancement of Carbon Quantum Dots Using Chemical-Free Precursors for Sensing CR (VI) Ions. Inorganics 2024, 12, 96. [Google Scholar] [CrossRef]
- Fu, W.; Ma, J.; Qiao, Z.; Xu, L.; Wang, L.; Ling, M.; Fu, X.; Li, G.; Han, C.; Zhang, J.; et al. Ultrasound-Assisted Hydrothermal Synthesis of Highly Fluorescent Sulfur Quantum Dots for FE3+ Ion and Ascorbic Acid Detection in Real Samples. Langmuir 2023, 39, 16349–16357. [Google Scholar] [CrossRef] [PubMed]
- Sbacchi, M.; Mamone, M.; Morbiato, L.; Gobbo, P.; Filippini, G.; Prato, M. Shining Light on Carbon Dots: New Opportunities in Photocatalysis. ChemCatChem 2023, 15, e202300667. [Google Scholar] [CrossRef]
- Wang, X.; Cheng, J.; Yu, H.; Yu, J. A Facile Hydrothermal Synthesis of Carbon Dots Modified G-C3N4 for Enhanced Photocatalytic H2-Evolution Performance. Dalton Trans. 2017, 46, 6417–6424. [Google Scholar] [CrossRef]
- Han, M.; Zhu, S.; Lu, S.; Song, Y.; Feng, T.; Tao, S.; Liu, J.; Yang, B. Recent Progress on the Photocatalysis of Carbon Dots: Classification, Mechanism and Applications. Nano Today 2018, 19, 201–218. [Google Scholar] [CrossRef]
- Daulay, A.; Nasution, L.H.; Huda, M.; Amin, M.; Nikmatullah, M.; Supiyani, N.; Yusmiati, N. Green Sources for Carbon Dots Synthesis in Sensing for Food Application—A Review. Biosens. Bioelectron. X 2024, 17, 100460. [Google Scholar] [CrossRef]
- Gu, J.; Li, X.; Hu, D.; Liu, Y.; Zhang, G.; Jia, X.; Huang, W.; Xi, K. Green Synthesis of Amphiphilic Carbon Dots from Organic Solvents: Application in Fluorescent Polymer Composites and Bio-Imaging. RSC Adv. 2018, 8, 12556–12561. [Google Scholar] [CrossRef] [PubMed]
- Huo, X.; Shen, H.; Liu, R.; Shao, J. Solvent Effects on Fluorescence Properties of Carbon Dots: Implications for Multicolor Imaging. ACS Omega 2021, 6, 26499–26508. [Google Scholar] [CrossRef]
- Pang, Y.X.; Li, X.; Zhang, X.; Yeoh, J.X.; Wong, C.; Manickam, S.; Yan, Y.; Wu, T.; Pang, C.H. The Synthesis of Carbon-Based Quantum Dots: A Supercritical Fluid Approach and Perspective. Mater. Today Phys. 2022, 27, 100752. [Google Scholar] [CrossRef]
- Kundu, A.; Maity, B.; Basu, S. Orange Pomace-Derived Fluorescent Carbon Quantum Dots: Detection of Dual Analytes in the Nanomolar Range. ACS Omega 2023, 8, 22178–22189. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Zhou, X.; Fang, R.; Lu, C.; Wang, K.; Gan, Y.; He, X.; Zhang, J.; Huang, H.; Zhang, W.; et al. Supercritical Carbon Dioxide Technology in Synthesis, Modification, and Recycling of Battery Materials. Carbon Neutralization 2023, 2, 169–185. [Google Scholar] [CrossRef]
- Campalani, C.; Rigo, D. Continuous Flow Synthesis and Applications of Carbon Dots: A Mini-Review. Next Sustain. 2023, 1, 100001. [Google Scholar] [CrossRef]
- Cao, M.; Zhao, X.; Gong, X. Ionic Liquid-Assisted Fast Synthesis of Carbon Dots with Strong Fluorescence and Their Tunable Multicolor Emission. Small 2022, 18, 2106683. [Google Scholar] [CrossRef]
- Wan, J.-Y.; Yang, Z.; Liu, Z.-G.; Wang, H.-X. Ionic Liquid-Assisted Thermal Decomposition Synthesis of Carbon Dots and Graphene-like Carbon Sheets for Optoelectronic Application. RSC Adv. 2016, 6, 61292–61300. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, Q.; Xu, P.; Sun, L.; Sun, D.; Zhuo, K. One-Step Synthesis of Acidophilic Highly-Photoluminescent Carbon Dots Modified by Ionic Liquid from Polyethylene Glycol. ACS Omega 2017, 2, 5251–5259. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.; Nakagawa, M.; Cheng, S. Emerging Trends in Green Extraction Techniques for Bioactive Natural Products. Processes 2023, 11, 3444. [Google Scholar] [CrossRef]
- Usman, M.; Cheng, S.; Boonyubol, S.; Cross, J.S. Evaluating Green Solvents for Bio-Oil Extraction: Advancements, Challenges, and Future Perspectives. Energies 2023, 16, 5852. [Google Scholar] [CrossRef]
- Wang, M.; Kang, X.; Deng, L.; Wang, M.; Xia, Z.; Gao, D. Deep Eutectic Solvent Assisted Synthesis of Carbon Dots Using Sophora flavescens Aiton Modified with Polyethyleneimine: Application in Myricetin Sensing and Cell Imaging. Food Chem. 2021, 345, 128817. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, M.; He, B.; Chen, X.; Zeng, J.; Sun, J. Ionothermal Synthesis of Carbon Dots from Cellulose in Deep Eutectic Solvent: A Sensitive Probe for Detecting Cu2+ and Glutathione with “off-on” Pattern. Appl. Surf. Sci. 2022, 599, 153705. [Google Scholar] [CrossRef]
- Wang, N.; Zheng, A.-Q.; Liu, X.; Chen, J.-J.; Yang, T.; Chen, M.-L.; Wang, J.-H. Deep Eutectic Solvent-Assisted Preparation of Nitrogen/Chloride-Doped Carbon Dots for Intracellular Biological Sensing and Live Cell Imaging. ACS Appl. Mater. Interfaces 2018, 10, 7901–7909. [Google Scholar] [CrossRef]
- Tabaraki, R.; Nazari, F. Comparison of Carbon Dots Prepared in Deep Eutectic Solvent and Water/Deep Eutectic Solvent: Study of Fluorescent Detection of Fe3+ and Cetirizine and Their Photocatalytic Antibacterial Activity. J. Fluoresc. 2022, 32, 549–558. [Google Scholar] [CrossRef]
- Tabaraki, R.; Nazari, F. Microwave Synthesis of Carbon Dots in Ten Choline Chloride-Based Deep Eutectic Solvents: Effect of Solvent Molecular Structure on Carbon Dots Fluorescence and Sensing Properties. J. Photochem. Photobiol. A Chem. 2023, 444, 114891. [Google Scholar] [CrossRef]
- Wang, M.; Li, C.; Zhou, M.; Xia, Z.; Huang, Y. Natural Deep Eutectic Solvent Assisted Synthesis and Applications of Chiral Carbon Dots. Green Chem. 2022, 24, 6696–6706. [Google Scholar] [CrossRef]
- Zhao, N.; Song, J.; Zhao, L. Metallic Deep Eutectic Solvents-Assisted Synthesis of Cu, Cl-Doped Carbon Dots as Oxidase-like and Peroxidase-like Nanozyme for Colorimetric Assay of Hydroquinone and H2O2. Colloids Surf. A Physicochem. Eng. Asp. 2022, 648, 129390. [Google Scholar] [CrossRef]
- Yin, Q.; Wang, M.; Fang, D.; Zhu, Y.; Yang, L. Novel N,Cl-Doped Deep Eutectic Solvents-Based Carbon Dots as a Selective Fluorescent Probe for Determination of Morphine in Food. RSC Adv. 2021, 11, 16805–16813. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Cao, S.; Sun, Y.; Liu, D.; Pan, S.; Ma, A.; Kuvarega, A.T.; Mamba, B.B.; Gui, J. Ionic Liquid-Assisted Preparation of N, S-Rich Carbon Dots as Efficient Corrosion Inhibitors. J. Mol. Liq. 2022, 356, 118943. [Google Scholar] [CrossRef]
- Gonçalves, H.M.R.; Pereira, R.F.P.; Lepleux, E.; Pacheco, L.; Valente, A.J.M.; Duarte, A.J.; De Zea Bermudez, V. Non-Newtonian Thermosensitive Nanofluid Based on Carbon Dots Functionalized with Ionic Liquids. Small 2020, 16, 1907661. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, X.; Zhang, Y.; Tang, Y.; Yang, Y.; Wang, B.; Wei, S.; Wang, Z.; Sun, G. Ionic Liquid-Based Carbon Dots as Highly Biocompatible and Sensitive Fluorescent Probe for the Determination of Vitamin P in Fruit Samples. Food Chem. 2023, 406, 134898. [Google Scholar] [CrossRef]
- Ansi, V.A.; Sreelakshmi, P.; Poovathinthodiyil, R.; Renuka, N.K. Table Sugar Derived Carbon Dot—A Promising Green Reducing Agent. Mater. Res. Bull. 2021, 139, 111284. [Google Scholar] [CrossRef]
- Tenkayala, N.K.; Katari, N.K.; Gundla, R.; Jonnalagadda, S.B.; Devaraju, S. Sustainable Approach to Synthesis of Carbon Dot/ Silver Nanoparticles for Biological Evaluation as Antimicrobial Agent. Mater. Res. Express 2023, 11, 015005. [Google Scholar] [CrossRef]
- Abu, N.; Chinnathambi, S.; Kumar, M.; Etezadi, F.; Bakhori, N.M.; Zubir, Z.A.; Salleh, S.N.M.; Shueb, R.H.; Karthikeyan, S.; Thangavel, V.; et al. Development of Biomass Waste-Based Carbon Quantum Dots and Their Potential Application as Non-Toxic Bioimaging Agents. RSC Adv. 2023, 13, 28230–28249. [Google Scholar] [CrossRef]
- Usman, M.; Cheng, S.; Boonyubol, S.; Cross, J.S. The Future of Aviation Soars with HTL-Based SAFs: Exploring Potential and Overcoming Challenges Using Organic Wet Feedstocks. Sustain. Energy Fuels 2023, 7, 4066–4087. [Google Scholar] [CrossRef]
- Gan, J.; Chen, L.; Chen, Z.; Zhang, J.; Yu, W.; Huang, C.; Wu, Y.; Zhang, K. Lignocellulosic Biomass-Based Carbon Dots: Synthesis Processes, Properties, and Applications. Small 2023, 19, 2304066. [Google Scholar] [CrossRef]
- AFM Atomic Force Microscopy—An Overview from Asylum Research. Available online: https://afm.oxinst.com/outreach/atomic-force-microscopy (accessed on 20 August 2024).
- Li, Q.; Zhang, T.; Pan, Y.; Ciacchi, L.C.; Xu, B.; Wei, G. AFM-Based Force Spectroscopy for Bioimaging and Biosensing. RSC Adv. 2016, 6, 12893–12912. [Google Scholar] [CrossRef]
- Hu, Q.; Gong, X.; Liu, L.; Choi, M.M.F. Characterization and Analytical Separation of Fluorescent Carbon Nanodots. J. Nanomater. 2017, 2017, 1804178. [Google Scholar] [CrossRef]
- Muravijova, D.V.; Ermakov, I.A.; Eurov, D.A.; Kirilenko, D.A.; Kurdyukov, D.A.; Rabchinskii, M.K.; Shvidchenko, A.V.; Trofimuk, A.D.; Baidakova, M.V. Atomic Force Microscopy Study of Monodisperse Carbon Nanoparticles. Semiconductors 2018, 52, 2065–2067. [Google Scholar] [CrossRef]
- Kohl, H. Transmission Electron Microscopy; 2016; pp. 4218–4233. [Google Scholar] [CrossRef]
- Oyohwose, U.A.; Omoko, V.I.; Emmanuel, E.; Bello, O.P. Synthesis, Characterization, and Applications of Carbon Dots: A Review. Int. J. Eng. Res. Rev. 2023, 11, 1–13. [Google Scholar] [CrossRef]
- González-González, R.B.; González, L.T.; Madou, M.; Leyva-Porras, C.; Martinez-Chapa, S.O.; Mendoza, A. Synthesis, Purification, and Characterization of Carbon Dots from Non-Activated and Activated Pyrolytic Carbon Black. Nanomaterials 2022, 12, 298. [Google Scholar] [CrossRef]
- Li, S.; Nie, L.; Wen, W.; Wang, Z.; Wang, J.; Wang, S. Hydroxyl Radical Enhanced Carbon Dots Fluorescence Quenching Immunoassays for Simultaneous Detection of Six Kinds of Antibiotics. Food Bioeng. 2022, 1, 212–223. [Google Scholar] [CrossRef]
- Mokobi, F. Scanning Electron Microscope (SEM): Principle, Parts, Uses—Microbe Notes. Available online: https://microbenotes.com/scanning-electron-microscope-sem/ (accessed on 20 August 2024).
- Mahadeshwara, M.R. Scanning Electron Microscope—About Tribology. Available online: https://www.tribonet.org/wiki/scanning-electron-microscope/ (accessed on 20 August 2024).
- Thermofisher Scanning Electron Microscopy | Principles of Scanning Electron Microscopy | Thermo Fisher Scientific—IE. Available online: https://www.thermofisher.com/jp/ja/home/materials-science/learning-center/applications/scanning-electron-microscope-sem-electron-column.html (accessed on 20 August 2024).
- Dubey, V.; Dubey, N.; Domanska, M.M.; Jayasimhadri, M.; Dhoble, S.J. Rare-Earth-Activated Phosphors: Chemistry and Applications; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Horiba What Is Photoluminescence Spectroscopy? Available online: https://www.horiba.com/int/scientific/technologies/photoluminescence-pl/photoluminescence-pl-electroluminescence-el/ (accessed on 20 August 2024).
- Fawaz, W.; Hasian, J.; Alghoraibi, I. Synthesis and Physicochemical Characterization of Carbon Quantum Dots Produced from Folic Acid. Sci. Rep. 2023, 13, 18641. [Google Scholar] [CrossRef]
- Edinburgh Instruments Ltd Carbon Dots | C-Dots | Photoluminescence Spectroscopy. Available online: https://www.edinst.com/photoluminescence-spectroscopy-of-carbon-dots/ (accessed on 20 August 2024).
- Admin UV VIS Spectroscopy—Definition, Theory & Applications with Videos. Available online: https://byjus.com/chemistry/uv-vis-spectroscopy/ (accessed on 20 August 2024).
- Libretexts 4.4: UV-Visible Spectroscopy. Available online: https://chem.libretexts.org/Bookshelves/Analytical_Chemistry/Physical_Methods_in_Chemistry_and_Nano_Science_%28Barron%29/04:_Chemical_Speciation/4.04:_UV-Visible_Spectroscopy (accessed on 20 August 2024).
- Qiu, J.; Zeng, D.; Lin, Y.; Ye, W.; Chen, C.; Xu, Z.; Hu, G.; Liu, Y. Carbon-Polymer Dot-Based UV Absorption and Fluorescence Performances for Heavy Metal Ion Detection. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 285, 121913. [Google Scholar] [CrossRef]
- Excedr What Is Infrared Spectroscopy? Fundamentals & Applications. Available online: https://www.excedr.com/blog/what-is-infrared-spectroscopy (accessed on 20 August 2024).
- The Science of Alcohol. Available online: https://www.open.edu/openlearn/mod/oucontent/view.php?id=83459%C2%A7ion=2.2 (accessed on 20 August 2024).
- Libretexts Infrared Spectroscopy. Available online: https://doi.org/10.1002/9781119373421.ch7 (accessed on 20 August 2024).
- Arroyave, J.M.; Ambrusi, R.E.; Robein, Y.; Pronsato, M.E.; Brizuela, G.; Nezio, D.; Centurión, M.E. Carbon Dots Structural Characterization by Solution-State NMR and UV–Visible Spectroscopy and DFT Modeling. Appl. Surf. Sci. 2021, 564, 150195. [Google Scholar] [CrossRef]
- Rooj, B.; Mandal, U. A Review on Characterization of Carbon Quantum Dots. Vietnam J. Chem. 2023, 61, 693–718. [Google Scholar] [CrossRef]
- Zhu, Q.; Zhou, R.; Liu, J.; Sun, J.; Wang, Q. Recent Progress on the Characterization of Cellulose Nanomaterials by Nanoscale Infrared Spectroscopy. Nanomaterials 2021, 11, 1353. [Google Scholar] [CrossRef]
- Demirci, S.; McNally, A.B.; Ayyala, R.S.; Lawson, L.B.; Sahiner, N. Synthesis and Characterization of Nitrogen-Doped Carbon Dots as Fluorescent Nanoprobes with Antimicrobial Properties and Skin Permeability. J. Drug Deliv. Sci. Technol. 2020, 59, 101889. [Google Scholar] [CrossRef]
- Kazemifard, N.; Ensafi, A.A.; Rezaei, B. Green Synthesized Carbon Dots Embedded in Silica Molecularly Imprinted Polymers, Characterization and Application as a Rapid and Selective Fluorimetric Sensor for Determination of Thiabendazole in Juices. Food Chem. 2020, 310, 125812. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, D.; Varada, O.M.; Venkatanarasimhan, S. Carbon Dots Coated on Amine Functionalized Cellulose Sponge for the Adsorption of the Toxic Herbicide Atrazine. Mater. Today Proc. 2021, 47, 790–799. [Google Scholar] [CrossRef]
- Prochazka, M. Basics of Raman Scattering (RS) Spectroscopy. In Surface-Enhanced Raman Spectroscopy. Biological and Medical Physics, Biomedical Engineering; Springer: Cham, Switzerland, 2015; pp. 7–19. [Google Scholar]
- Libretexts Raman: Application. Available online: https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_%28Physical_and_Theoretical_Chemistry%29/Spectroscopy/Vibrational_Spectroscopy/Raman_Spectroscopy/Raman:_Interpretation (accessed on 20 August 2024).
- Bumbrah, G.S.; Sharma, R.M. Raman Spectroscopy—Basic Principle, Instrumentation and Selected Applications for the Characterization of Drugs of Abuse. Egypt. J. Forensic Sci. 2016, 6, 209–215. [Google Scholar] [CrossRef]
- Saraswat, V.; Yadav, M. Carbon Dots as Green Corrosion Inhibitor for Mild Steel in HCL Solution. ChemistrySelect 2020, 5, 7347–7357. [Google Scholar] [CrossRef]
- Anuar, N.K.K.; Tan, H.L.; Lim, Y.P.; So’aib, M.S.; Bakar, N.F.A. A Review on Multifunctional Carbon-Dots Synthesized from Biomass Waste: Design/Fabrication, Characterization and Applications. Front. Energy Res. 2021, 9, 626549. [Google Scholar] [CrossRef]
- Zaib, M.; Akhtar, A.; Maqsood, F.; Shahzadi, T. Green Synthesis of Carbon Dots and Their Application as Photocatalyst in Dye Degradation Studies. Arab. J. Sci. Eng. 2020, 46, 437–446. [Google Scholar] [CrossRef]
- Carbonaro, N.; Corpino, N.; Salis, N.; Mocci, N.; Thakkar, N.; Olla, N.; Ricci, N. On the Emission Properties of Carbon Dots: Reviewing Data and Discussing Models. C 2019, 5, 60. [Google Scholar] [CrossRef]
- Ma, X.; Li, S.; Hessel, V.; Lin, L.; Meskers, S.; Gallucci, F. Synthesis of Luminescent Carbon Quantum Dots by Microplasma Process. Chem. Eng. Process. Process Intensif. 2019, 140, 29–35. [Google Scholar] [CrossRef]
- Russ, J.C. Fundamentals of Energy Dispersive X-ray Analysis: Butterworths Monographs in MaterialsI; Butterworth-Heinemann: Oxford, UK, 2013. [Google Scholar]
- MST MST|[SEM-EDX] Energy Dispersive X-ray Spectroscopy (SEM). Available online: https://www.mst.or.jp/Portals/0/en/en_sem-edx.htm (accessed on 20 August 2024).
- Raval, N.; Maheshwari, R.; Kalyane, D.; Youngren-Ortiz, S.R.; Chougule, M.B.; Tekade, R.K. Importance of Physicochemical Characterization of Nanoparticles in Pharmaceutical Product Development. In Basic Fundamentals of Drug Delivery; Academic Press: Cambridge, MA, USA, 2019; pp. 369–400. [Google Scholar]
- EDS Principle | West Campus Materials Characterization Core. Available online: https://ywcmatsci.yale.edu/edx-principle (accessed on 20 August 2024).
- Atchudan, R.; Edison, T.N.J.I.; Perumal, S.; Vinodh, R.; Lee, Y.R. In-Situ Green Synthesis of Nitrogen-Doped Carbon Dots for Bioimaging and TiO2 Nanoparticles@nitrogen-Doped Carbon Composite for Photocatalytic Degradation of Organic Pollutants. J. Alloys Compd. 2018, 766, 12–24. [Google Scholar] [CrossRef]
- Chandrasekaran, P.; Arul, V.; Sethuraman, M.G. Ecofriendly Synthesis of Fluorescent Nitrogen-Doped Carbon Dots from Coccinia Grandis and Its Efficient Catalytic Application in the Reduction of Methyl Orange. J. Fluoresc. 2019, 30, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Pudza, M.Y.; Abidin, Z.Z.; Rashid, S.A.; Yasin, F.M.; Noor, A.S.M.; Issa, M.A. Eco-Friendly Sustainable Fluorescent Carbon Dots for the Adsorption of Heavy Metal Ions in Aqueous Environment. Nanomaterials 2020, 10, 315. [Google Scholar] [CrossRef]
- Kraus, R.H.; Espy, M.A.; Magnelind, P.E.; Volegov, P.L. Ultra-Low Field Nuclear Magnetic Resonance: A New MRI Regime; Oxford University Press: Oxford, UK, 2014. [Google Scholar]
- Talib, A.B.; Mohammed, M.H. Preparation, Characterization and Preliminary Cytotoxic Evaluation of 6-Mercaptopurine-Coated Biotinylated Carbon Dots Nanoparticles as a Drug Delivery System. Mater. Today Proc. 2023, 80, 2327–2333. [Google Scholar] [CrossRef]
- Duan, P.; Zhi, B.; Coburn, L.; Haynes, C.L.; Schmidt-Rohr, K. A Molecular Fluorophore in Citric Acid/Ethylenediamine Carbon Dots Identified and Quantified by Multinuclear Solid-state Nuclear Magnetic Resonance. Magn. Reson. Chem. 2020, 58, 1130–1138. [Google Scholar] [CrossRef]
- Stetefeld, J.; McKenna, S.A.; Patel, T.R. Dynamic Light Scattering: A Practical Guide and Applications in Biomedical Sciences. Biophys. Rev. 2016, 8, 409–427. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Danquah, M.K. Research Advances in Dynamic Light Scattering; NOVA: Hauppauge, NY, USA, 2020. [Google Scholar]
- Wu, Y.; Van Der Mei, H.C.; Busscher, H.J.; Ren, Y. Enhanced Bacterial Killing by Vancomycin in Staphylococcal Biofilms Disrupted by Novel, DMMA-Modified Carbon Dots Depends on EPS Production. Colloids Surf. B Biointerfaces 2020, 193, 111114. [Google Scholar] [CrossRef]
- Rojas-Valencia, O.G.; Regules-Carrasco, M.; Hernández-Fuentes, J.; Germán, C.M.R.-S.; Estrada-Flores, M.; Villagarcía-Chávez, E. Synthesis of Blue Emissive Carbon Quantum Dots from Hibiscus sabdariffa Flower: Surface Functionalization Analysis by FT-IR Spectroscopy. Materialia 2021, 19, 101182. [Google Scholar] [CrossRef]
- Kholiya, F.; Jauhari, S.; Meena, R. Seaweed-derived Polymer-based Blue-emitting C-dots: Synthesis, Characterization and Evaluation for Iron Sensing. Polym. Int. 2021, 70, 1309–1315. [Google Scholar] [CrossRef]
- Demir, B.; Moulahoum, H.; Ghorbanizamani, F.; Barlas, F.B.; Yesiltepe, O.; Gumus, Z.P.; Meral, K.; Demirkol, D.O.; Timur, S. Carbon Dots and Curcumin-Loaded CD44-Targeted Liposomes for Imaging and Tracking Cancer Chemotherapy: A Multi-Purpose Tool for Theranostics. J. Drug Deliv. Sci. Technol. 2021, 62, 102363. [Google Scholar] [CrossRef]
- Padhi, S.; Behera, A. Biosynthesis of Silver Nanoparticles: Synthesis, Mechanism, and Characterization. In Agri-Waste and Microbes for Production of Sustainable Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2022; pp. 397–440. [Google Scholar]
- Jafari, S.M. Characterization of Nanoencapsulated Food Ingredients; Academic Press: Cambridge, MA, USA, 2020. [Google Scholar]
- Jlassi, K.; Eid, K.; Sliem, M.H.; Abdullah, A.M.; Chehimi, M.M.; Krupa, I. Rational Synthesis, Characterization, and Application of Environmentally Friendly (Polymer–Carbon Dot) Hybrid Composite Film for Fast and Efficient UV-Assisted Cd2+ Removal from Water. Environ. Sci. Eur. 2020, 32, 12. [Google Scholar] [CrossRef]
- Gao, R.; Wu, Z.; Wang, L.; Liu, J.; Deng, Y.; Xiao, Z.; Fang, J.; Liang, Y. Green Preparation of Fluorescent Nitrogen-Doped Carbon Quantum Dots for Sensitive Detection of Oxytetracycline in Environmental Samples. Nanomaterials 2020, 10, 1561. [Google Scholar] [CrossRef]
- Kailasa, S.K.; Hussain, C.M. Carbon Dots in Analytical Chemistry: Detection and Imaging; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Libretexts 4.10: ESI-QTOF-MS Coupled to HPLC and Its Application for Food Safety. Available online: https://chem.libretexts.org/Bookshelves/Analytical_Chemistry/Physical_Methods_in_Chemistry_and_Nano_Science_%28Barron%29/04:_Chemical_Speciation/4.10:_ESI-QTOF-MS_Coupled_to_HPLC_and_its_Application_for_Food_Safety (accessed on 20 August 2024).
- Ahmad, M.A.; Sumarsih, S.; Chang, J.-Y.; Fahmi, M.Z. Mass Spectrometry-Based Analyses of Carbon Nanodots: Structural Elucidation. ACS Omega 2024, 9, 20720–20727. [Google Scholar] [CrossRef]
- Akimowicz, M.; Bucka-Kolendo, J. MALDI-TOF MS—Application in Food Microbiology. Acta Biochim. Pol. 2020, 67, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Houdová, D.; Soto, J.; Castro, R.; Rodrigues, J.; Pino-González, M.S.; Petković, M.; Bandosz, T.J.; Algarra, M. Chemically Heterogeneous Carbon Dots Enhanced Cholesterol Detection by MALDI TOF Mass Spectrometry. J. Colloid Interface Sci. 2021, 591, 373–383. [Google Scholar] [CrossRef]
- Jaroniec, M.; Sayari, A. Nanoporous Materials III; Elsevier: Amsterdam, The Netherlands, 2002. [Google Scholar]
- Holder, C.F.; Schaak, R.E. Tutorial on Powder X-Ray Diffraction for Characterizing Nanoscale Materials. ACS Nano 2019, 13, 7359–7365. [Google Scholar] [CrossRef]
- Shaikh, A.F.; Tamboli, M.S.; Patil, R.H.; Bhan, A.; Ambekar, J.D.; Kale, B.B. Bioinspired Carbon Quantum Dots: An Antibiofilm Agents. J. Nanosci. Nanotechnol. 2019, 19, 2339–2345. [Google Scholar] [CrossRef]
- Wang, L.; Gu, D.; Su, Y.; Ji, D.; Yang, Y.; Chen, K.; Pan, H.; Pan, W. Easy Synthesis and Characterization of Novel Carbon Dots Using the One-Pot Green Method for Cancer Therapy. Pharmaceutics 2022, 14, 2423. [Google Scholar] [CrossRef]
- Mintz, K.J.; Bartoli, M.; Rovere, M.; Zhou, Y.; Hettiarachchi, S.D.; Paudyal, S.; Chen, J.; Domena, J.B.; Liyanage, P.Y.; Sampson, R.; et al. A Deep Investigation into the Structure of Carbon Dots. Carbon 2021, 173, 433–447. [Google Scholar] [CrossRef]
- Pal, A.; Sk, M.P.; Chattopadhyay, A. Recent Advances in Crystalline Carbon Dots for Superior Application Potential. Mater. Adv. 2020, 1, 525–553. [Google Scholar] [CrossRef]
- Lou, Y.; Hao, X.; Liao, L.; Zhang, K.; Chen, S.; Li, Z.; Ou, J.; Qin, A.; Li, Z. Recent Advances of Biomass Carbon Dots on Syntheses, Characterization, Luminescence Mechanism, and Sensing Applications. Nano Sel. 2021, 2, 1117–1145. [Google Scholar] [CrossRef]
- Fang, M.; Wang, B.; Qu, X.; Li, S.; Huang, J.; Li, J.; Lu, S.; Zhou, N. State-of-the-Art of Biomass-Derived Carbon Dots: Preparation, Properties, and Applications. Chin. Chem. Lett. 2024, 35, 108423. [Google Scholar] [CrossRef]
- Yang, H.-L.; Bai, L.-F.; Geng, Z.-R.; Chen, H.; Xu, L.-T.; Xie, Y.-C.; Wang, D.-J.; Gu, H.-W.; Wang, X.-M. Carbon Quantum Dots: Preparation, Optical Properties, and Biomedical Applications. Mater. Today Adv. 2023, 18, 100376. [Google Scholar] [CrossRef]
- Koutsogiannis, P.; Thomou, E.; Stamatis, H.; Gournis, D.; Rudolf, P. Advances in Fluorescent Carbon Dots for Biomedical Applications. Adv. Phys. X 2020, 5, 1758592. [Google Scholar] [CrossRef]
- Shen, C.-L.; Lou, Q.; Liu, K.-K.; Dong, L.; Shan, C.-X. Chemiluminescent Carbon Dots: Synthesis, Properties, and Applications. Nano Today 2020, 35, 100954. [Google Scholar] [CrossRef]
- Wang, B.; Lu, S. The Light of Carbon Dots: From Mechanism to Applications. Matter 2022, 5, 110–149. [Google Scholar] [CrossRef]
- Yan, F.; Sun, Z.; Zhang, H.; Sun, X.; Jiang, Y.; Bai, Z. The Fluorescence Mechanism of Carbon Dots, and Methods for Tuning Their Emission Color: A Review. Microchim. Acta 2019, 186, 583. [Google Scholar] [CrossRef]
- Vibhute, A.; Patil, T.; Gambhir, R.; Tiwari, A.P. Fluorescent Carbon Quantum Dots: Synthesis Methods, Functionalization and Biomedical Applications. Appl. Surf. Sci. Adv. 2022, 11, 100311. [Google Scholar] [CrossRef]
- Huo, X.; Shen, H.; Xu, Y.; Shao, J.; Liu, R.; Zhang, Z. Fluorescence Properties of Carbon Dots Synthesized by Different Solvents for pH Detector. Opt. Mater. 2022, 123, 111889. [Google Scholar] [CrossRef]
- Liu, M.L.; Chen, B.B.; Li, C.M.; Huang, C.Z. Carbon Dots: Synthesis, Formation Mechanism, Fluorescence Origin and Sensing Applications. Green Chem. 2019, 21, 449–471. [Google Scholar] [CrossRef]
- Deng, Y.; Chen, M.; Chen, G.; Zou, W.; Zhao, Y.; Zhang, H.; Zhao, Q. Visible–Ultraviolet Upconversion Carbon Quantum Dots for Enhancement of the Photocatalytic Activity of Titanium Dioxide. ACS Omega 2021, 6, 4247–4254. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.-B.; Chen, D.-D.; Liu, K.-K.; Wang, Y.; Zhou, R.; Song, S.-Y.; Li, F.-K.; Sui, L.-Z.; Lou, Q.; Hou, L.; et al. Near-Infrared I/II Emission and Absorption Carbon Dots via Constructing Localized Excited/Charge Transfer State for Multiphoton Imaging and Photothermal Therapy. Chem. Eng. J. 2023, 452, 139231. [Google Scholar] [CrossRef]
- Laptinskiy, K.; Khmeleva, M.; Vervald, A.; Burikov, S.; Dolenko, T. Carbon Dots with Up-Conversion Luminescence as pH Nanosensor. Appl. Sci. 2022, 12, 12006. [Google Scholar] [CrossRef]
- Li, D.; Liang, C.; Ushakova, E.V.; Sun, M.; Huang, X.; Zhang, X.; Jing, P.; Yoo, S.J.; Kim, J.; Liu, E.; et al. Thermally Activated Upconversion Near-Infrared Photoluminescence from Carbon Dots Synthesized via Microwave Assisted Exfoliation. Small 2019, 15, 1905050. [Google Scholar] [CrossRef]
- Qiu, J.; Ye, W.; Chen, C.; Xu, Z.; Hu, C.; Zhuang, J.; Dong, H.; Lei, B.; Hu, G.; Liu, Y. Toward Efficient Broad-Spectrum UV Absorption of Carbon Dots: Facile Preparation, Performance Characterization and Its Application as UV Absorbers. J. Ind. Eng. Chem. 2023, 117, 442–449. [Google Scholar] [CrossRef]
- Zhao, Q.; Song, W.; Zhao, B.; Yang, B. Spectroscopic Studies of the Optical Properties of Carbon Dots: Recent Advances and Future Prospects. Mater. Chem. Front. 2020, 4, 472–488. [Google Scholar] [CrossRef]
- Dimos, K. Tuning Carbon Dots’ Optoelectronic Properties with Polymers. Polymers 2018, 10, 1312. [Google Scholar] [CrossRef]
- Ranjan, P.; Khan, R.; Yadav, S.; Sadique, M.A.; Murali, S.; Ban, M.K. Physical and Chemical Properties of Carbon Dots. In Carbon Dots in Agricultural Systems; Elsevier: Amsterdam, The Netherlands, 2022; pp. 117–133. [Google Scholar]
- Ozyurt, D.; Kobaisi, M.A.; Hocking, R.K.; Fox, B. Properties, Synthesis, and Applications of Carbon Dots: A Review. Carbon Trends 2023, 12, 100276. [Google Scholar] [CrossRef]
- Ansary, Z.E.; Bouknaitir, I.; Teixeira, S.S.; Kreit, L.; Panniello, A.; Fini, P.; Striccoli, M.; Hasnaoui, M.E.; Costa, L.C.; Achour, M.E. Electrical Properties in PMMA/Carbon-Dots Nanocomposite Films below the Percolation Threshold. In NATO Science for Peace and Security Series B: Physics and Biophysics; Springer: Berlin/Heidelberg, Germany, 2020; pp. 235–250. [Google Scholar]
- Gogoi, J.; Shishodia, S.; Chowdhury, D. Tunable Electrical Properties of Carbon Dot Doped Photo-Responsive Azobenzene–Clay Nanocomposites. RSC Adv. 2020, 10, 37545–37554. [Google Scholar] [CrossRef]
- Stepanidenko, E.A.; Ushakova, E.V.; Fedorov, A.V.; Rogach, A.L. Applications of Carbon Dots in Optoelectronics. Nanomaterials 2021, 11, 364. [Google Scholar] [CrossRef]
- Jiang, Z.; Guan, L.; Xu, X.; Wang, E.; Wang, C. Applications of Carbon Dots in Electrochemical Energy Storage. ACS Appl. Electron. Mater. 2022, 4, 5144–5164. [Google Scholar] [CrossRef]
- Hasan, A.M.M.; Hasan, M.A.; Reza, A.; Islam, M.M.; Susan, M.A.B.H. Carbon Dots as Nano-Modules for Energy Conversion and Storage. Mater. Today Commun. 2021, 29, 102732. [Google Scholar] [CrossRef]
- Ge, G.; Li, L.; Wang, D.; Chen, M.; Zeng, Z.; Xiong, W.; Wu, X.; Guo, C. Carbon Dots: Synthesis, Properties and Biomedical Applications. J. Mater. Chem. B 2021, 9, 6553–6575. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Feng, Y.; Dong, P.; Huang, J. A Mini Review on Carbon Quantum Dots: Preparation, Properties, and Electrocatalytic Application. Front. Chem. 2019, 7, 671. [Google Scholar] [CrossRef]
- Guin, S.K.; Ambolikar, A.S.; Guin, J.P.; Neogy, S. Exploring the Excellent Photophysical and Electrochemical Properties of Graphene Quantum Dots for Complementary Sensing of Uranium. Sens. Actuators B Chem. 2018, 272, 559–573. [Google Scholar] [CrossRef]
- Cheng, R.; Xiang, Y.; Guo, R.; Li, L.; Zou, G.; Fu, C.; Hou, H.; Ji, X. Structure and Interface Modification of Carbon Dots for Electrochemical Energy Application. Small 2021, 17, 2102091. [Google Scholar] [CrossRef]
- Tian, L.; Li, Z.; Wang, P.; Zhai, X.; Wang, X.; Li, T. Carbon Quantum Dots for Advanced Electrocatalysis. J. Energy Chem. 2021, 55, 279–294. [Google Scholar] [CrossRef]
- Liu, Y.; Roy, S.; Sarkar, S.; Xu, J.; Zhao, Y.; Zhang, J. A Review of Carbon Dots and Their Composite Materials for Electrochemical Energy Technologies. Carbon Energy 2021, 3, 795–826. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, R.; Lang, J.; Yan, X. Insight into the Formation Mechanism of Graphene Quantum Dots and the Size Effect on Their Electrochemical Behaviors. Phys. Chem. Chem. Phys. 2015, 17, 14028–14035. [Google Scholar] [CrossRef]
- Gao, D.; Zhang, Y.; Liu, A.; Zhu, Y.; Chen, S.; Wei, D.; Sun, J.; Guo, Z.; Fan, H. Photoluminescence-Tunable Carbon Dots from Synergy Effect of Sulfur Doping and Water Engineering. Chem. Eng. J. 2020, 388, 124199. [Google Scholar] [CrossRef]
- Hatimuria, M.; Phukan, P.; Bag, S.; Ghosh, J.; Gavvala, K.; Pabbathi, A.; Das, J. Green Carbon Dots: Applications in Development of Electrochemical Sensors, Assessment of Toxicity as Well as Anticancer Properties. Catalysts 2023, 13, 537. [Google Scholar] [CrossRef]
- De Medeiros, T.V.; Manioudakis, J.; Noun, F.; Macairan, J.-R.; Victoria, F.; Naccache, R. Microwave-Assisted Synthesis of Carbon Dots and Their Applications. J. Mater. Chem. C 2019, 7, 7175–7195. [Google Scholar] [CrossRef]
- Lin, X.; Xiong, M.; Zhang, J.; He, C.; Ma, X.; Zhang, H.; Kuang, Y.; Yang, M.; Huang, Q. Carbon Dots Based on Natural Resources: Synthesis and Applications in Sensors. Microchem. J. 2021, 160, 105604. [Google Scholar] [CrossRef]
- Mansi, M.; Bhikhu, M.; Gaurav, S. Synthesis and Applications of Carbon Dots from Waste Biomass. In Carbon Dots in Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2023; pp. 319–328. [Google Scholar]
- Bressi, V.; Balu, A.M.; Iannazzo, D.; Espro, C. Recent Advances in the Synthesis of Carbon Dots from Renewable Biomass by High-Efficient Hydrothermal and Microwave Green Approaches. Curr. Opin. Green Sustain. Chem. 2023, 40, 100742. [Google Scholar] [CrossRef]
- Vishnukumar, P.; Sankaranarayanan, S.; Hariram, M.; Vivekanandhan, S.; Navia, R. Carbon Dots from Renewable Resources: A Review on Precursor Choices and Potential Applications. In Green Nanomaterials: Processing, Properties, and Applications; Springer: Berlin/Heidelberg, Germany, 2020; pp. 159–208. [Google Scholar] [CrossRef]
- Dinç, S.; Kara, M.; Yavuz, E. Synthesis of Carbon Dots from Biomass Resources. In Carbon Dots in Agricultural Systems; Elsevier: Amsterdam, The Netherlands, 2022; pp. 69–116. [Google Scholar]
- Seven, E.S.; Cilingir, E.K.; Bartoli, M.; Zhou, Y.; Sampson, R.; Shi, W.; Peng, Z.; Pandey, R.R.; Chusuei, C.C.; Tagliaferro, A.; et al. Hydrothermal vs Microwave Nanoarchitechtonics of Carbon Dots Significantly Affects the Structure, Physicochemical Properties, and Anti-Cancer Activity against a Specific Neuroblastoma Cell Line. J. Colloid Interface Sci. 2023, 630, 306–321. [Google Scholar] [CrossRef] [PubMed]
- Jorns, M.; Strickland, S.; Mullins, M.; Pappas, D. Improved Citric Acid-Derived Carbon Dots Synthesis through Microwave-Based Heating in a Hydrothermal Pressure Vessel. RSC Adv. 2022, 12, 32401–32414. [Google Scholar] [CrossRef]
- Chauhan, D.S.; Quraishi, M.A.; Verma, C. Carbon Nanodots: Recent Advances in Synthesis and Applications. Carbon Lett. 2022, 32, 1603–1629. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).