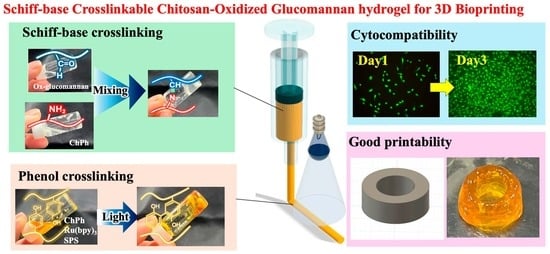
Photo- and Schiff Base-Crosslinkable Chitosan/Oxidized Glucomannan Composite Hydrogel for 3D Bioprinting
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
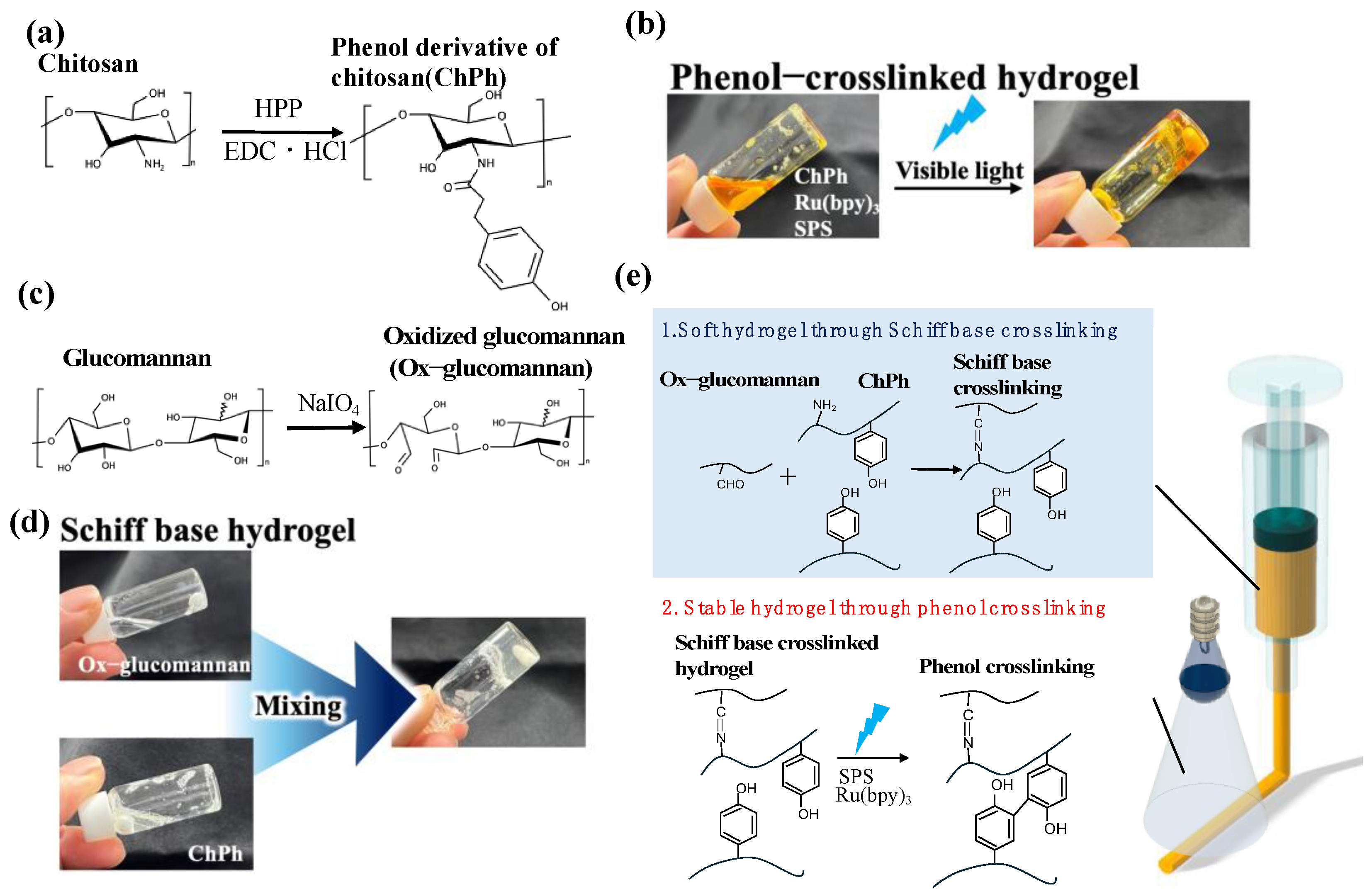
2.2. Synthesis of the Phenol Derivative of Chitosan (ChPh)
2.3. Synthesis of Oxidized Glucomannan (Ox-Glucomannan)
2.4. Fourier-Transform Infrared (FTIR) Spectroscopy
2.5. Preparation of Schiff Base Hydrogels
2.6. Extrudability
2.7. Rheological Properties
2.8. Swelling
2.9. Cell Viabilities
2.10. Antimicrobial Activities
2.11. 3D Printing
2.12. Statistical Analysis
3. Results and Discussion
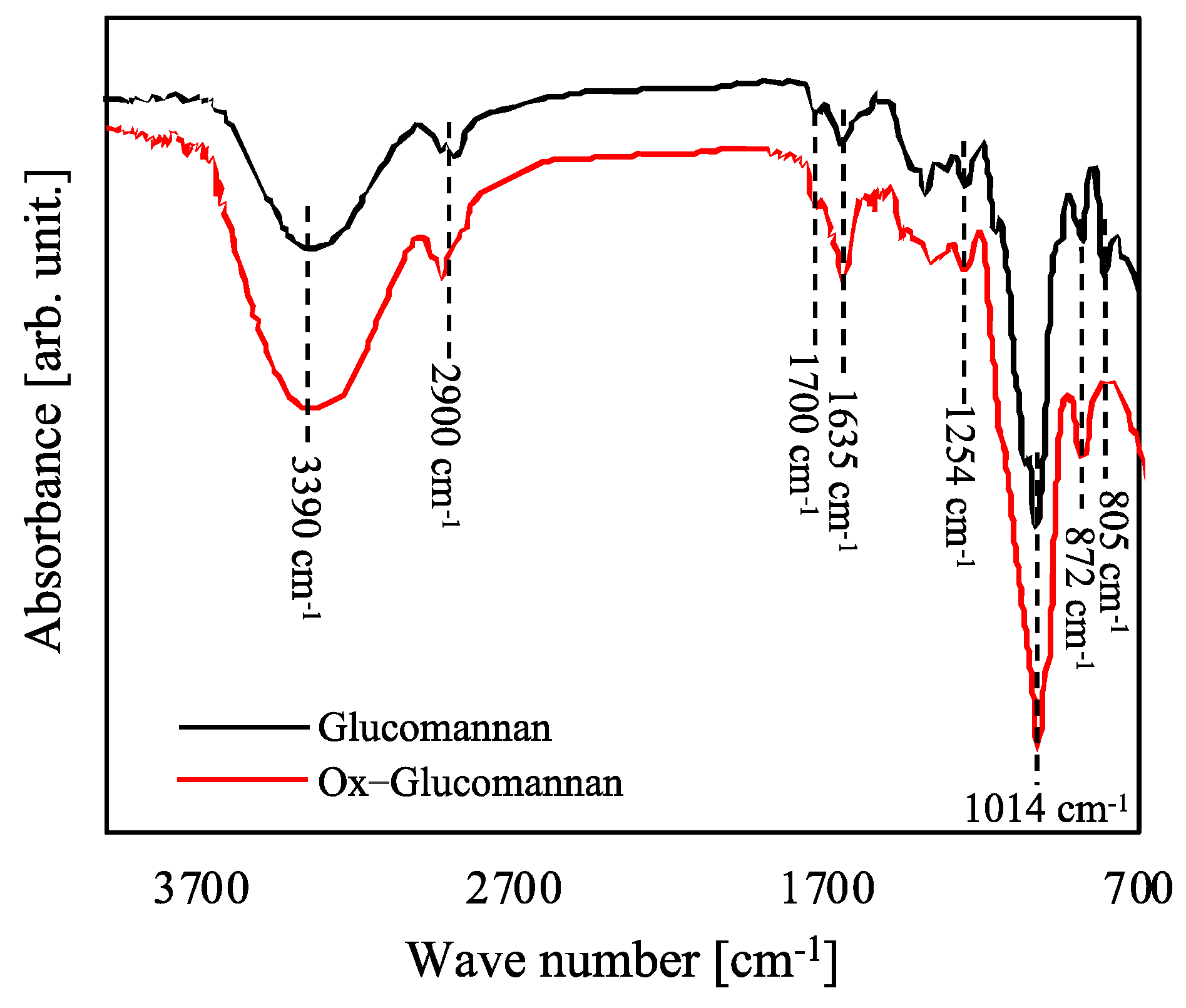
3.1. FTIR Spectra
3.2. Extrudability
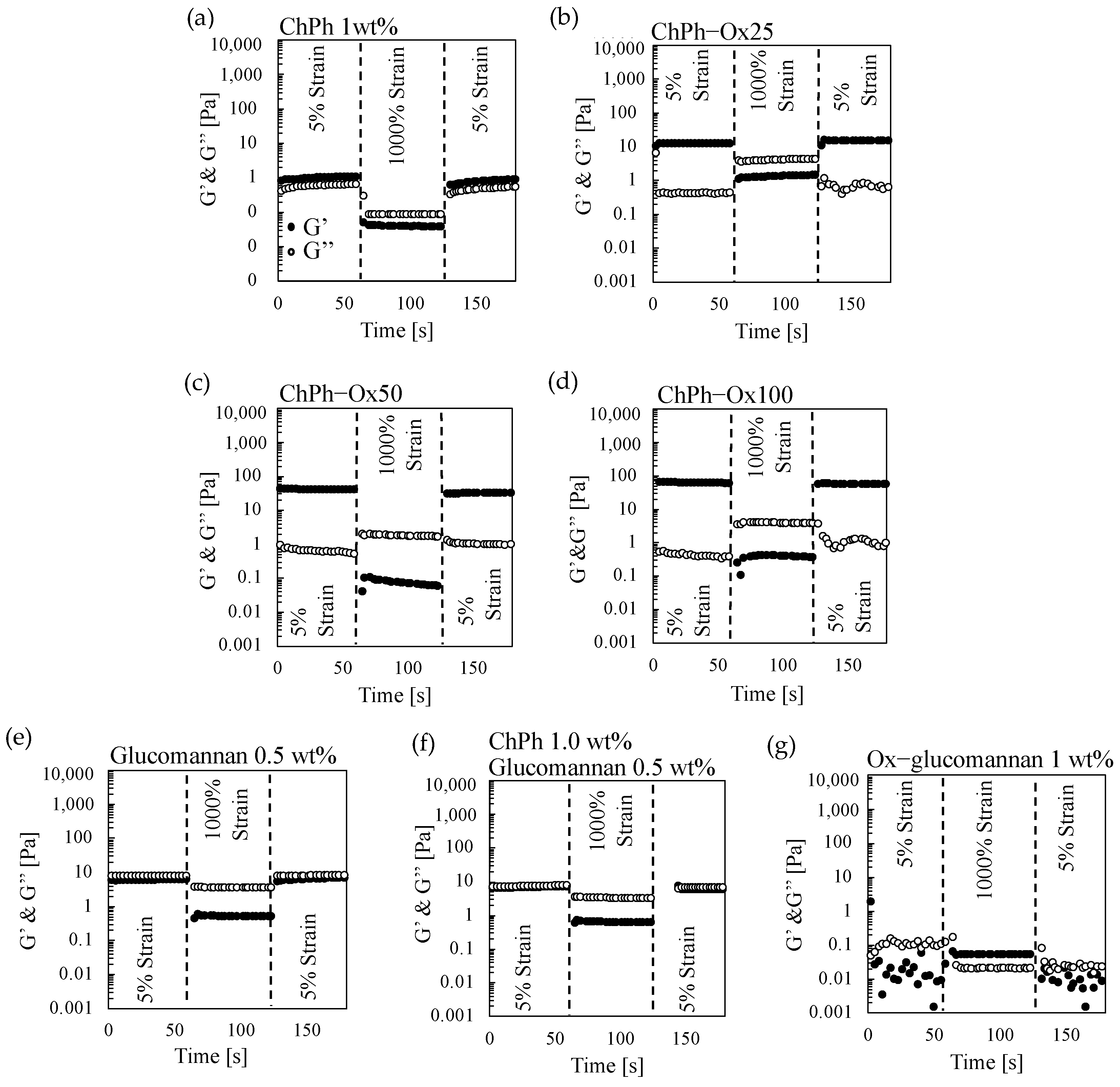
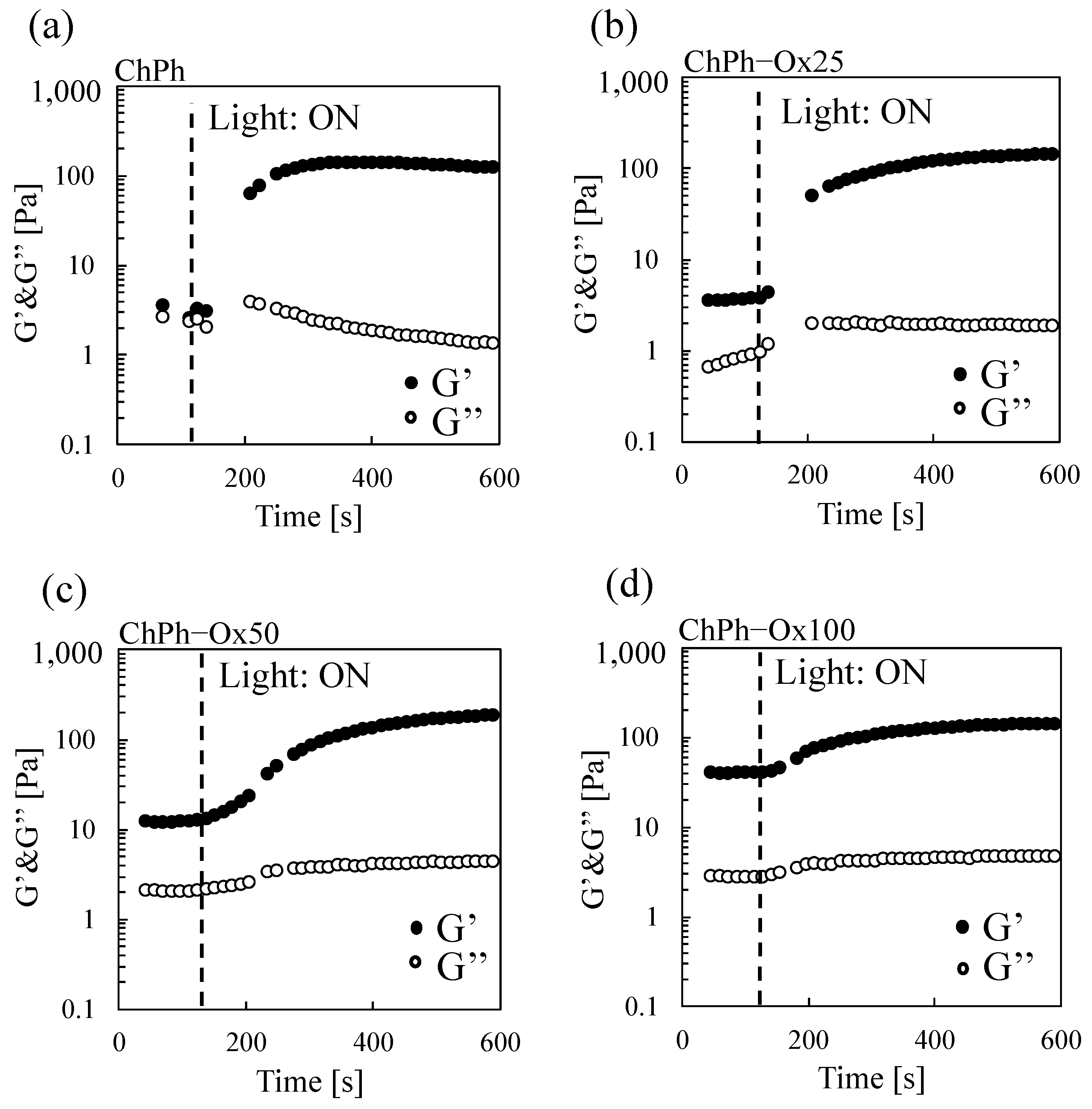
3.3. Rheological Properties of the Hydrogels with Schiff Base and Phenol Crosslinks
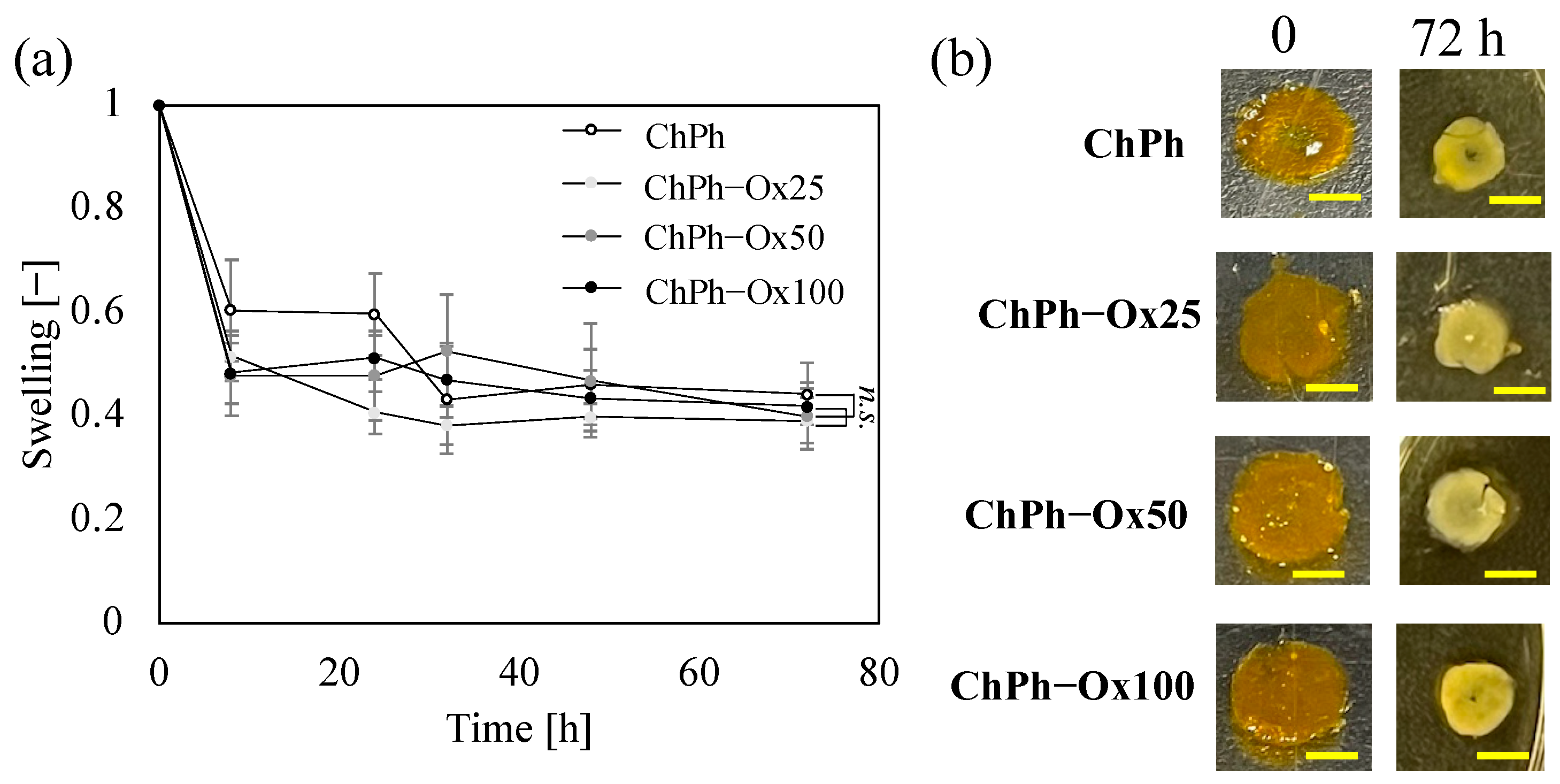
3.4. Swelling
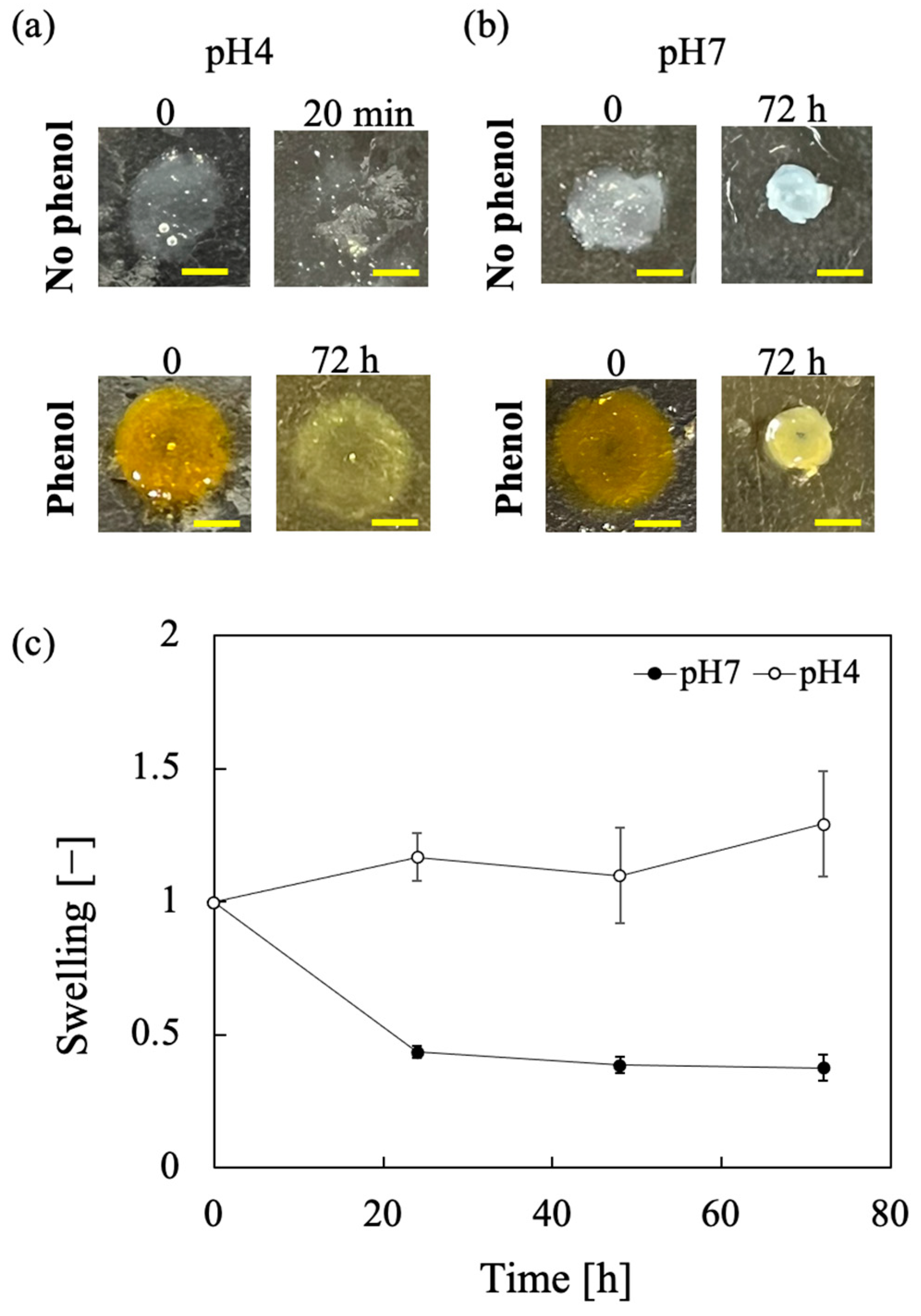
3.5. Effect of pH on Swelling and Stability
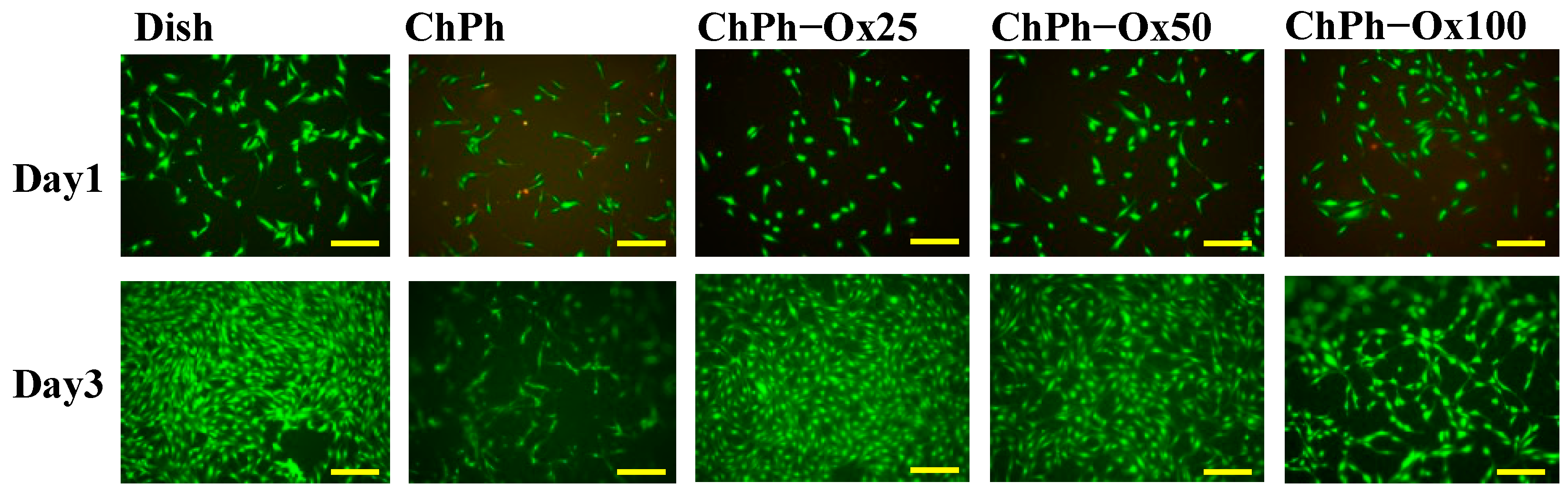
3.6. Cell Viabilities
3.7. Antimicrobial Activities
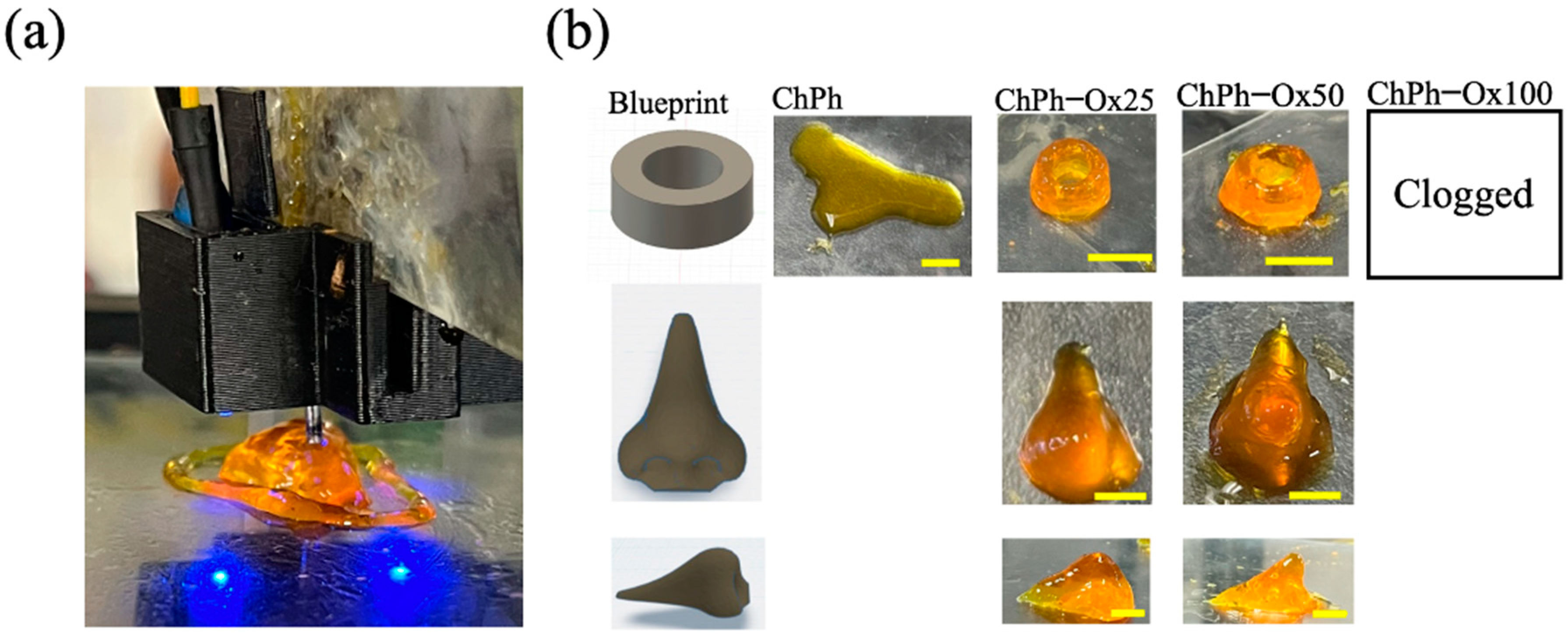
3.8. 3D Printing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gungor-Ozkerim, P.S.; Inci, I.; Zhang, Y.S.; Khademhosseini, A.; Dokmeci, M.R. Bioinks for 3D Bioprinting: An Overview. Biomat. Sci. 2018, 6, 915–946. [Google Scholar] [CrossRef] [PubMed]
- Pedroza-González, S.C.; Rodriguez-Salvador, M.; Pérez-Benítez, B.E.; Moisés Alvarez, M.; Santiago, G.T. De Bioinks for 3D Bioprinting: A Scientometric Analysis of Two Decades of Progress. Int. J. Bioprint. 2021, 7, 68–91. [Google Scholar] [CrossRef]
- Santoni, S.; Gugliandolo, S.G.; Sponchioni, M.; Moscatelli, D.; Colosimo, B.M. 3D Bioprinting: Current Status and Trends—A Guide to the Literature and Industrial Practice. Bio-Des. Manuf. 2022, 5, 14–42. [Google Scholar] [CrossRef]
- Li, X.; Liu, B.; Pei, B.; Chen, J.; Zhou, D.; Peng, J.; Zhang, X.; Jia, W.; Xu, T. Inkjet Bioprinting of Biomaterials. Chem. Rev. 2020, 120, 10793–10833. [Google Scholar] [CrossRef]
- Jessop, Z.M.; Al-Sabah, A.; Gao, N.; Kyle, S.; Thomas, B.; Badiei, N.; Hawkins, K.; Whitaker, I.S. Printability of Pulp Derived Crystal, Fibril and Blend Nanocellulose-Alginate Bioinks for Extrusion 3D Bioprinting. Biofabrication 2019, 11, 045006. [Google Scholar] [CrossRef]
- Liu, F.; Wang, X. Synthetic Polymers for Organ 3D Printing. Polymers 2020, 12, 1765. [Google Scholar] [CrossRef]
- Khoeini, R.; Nosrati, H.; Akbarzadeh, A.; Eftekhari, A.; Kavetskyy, T.; Khalilov, R.; Ahmadian, E.; Nasibova, A.; Datta, P.; Roshangar, L.; et al. Natural and Synthetic Bioinks for 3D Bioprinting. Adv. Nanobiomed. Res. 2021, 1, 2000097. [Google Scholar] [CrossRef]
- Tai, C.; Bouissil, S.; Gantumur, E.; Carranza, M.S.; Yoshii, A.; Sakai, S.; Pierre, G.; Michaud, P.; Delattre, C. Use of Anionic Polysaccharides in the Development of 3D Bioprinting Technology. Appl. Sci. 2019, 9, 2596. [Google Scholar] [CrossRef]
- Yang, Q.; Peng, J.; Xiao, H.; Xu, X.; Qian, Z. Polysaccharide Hydrogels: Functionalization, Construction and Served as Scaffold for Tissue Engineering. Carbohydr. Polym. 2022, 278, 118952. [Google Scholar] [CrossRef]
- Shahidi, F.; Arachchi, J.K.V.; Jeon, Y.J. Food Applications of Chitin and Chitosans. Trends Food Sci. Technol. 1999, 10, 37–51. [Google Scholar] [CrossRef]
- Ramirez Caballero, S.S.; Saiz, E.; Montembault, A.; Tadier, S.; Maire, E.; David, L.; Delair, T.; Grémillard, L. 3-D Printing of Chitosan-Calcium Phosphate Inks: Rheology, Interactions and Characterization. J. Mater. Sci. Mater. Med. 2019, 30, 6. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Liu, Y. Chitosan-Based Biomaterials: From Discovery to Food Application. Polym. Adv. Technol. 2020, 31, 2408–2421. [Google Scholar] [CrossRef]
- Matica, M.A.; Aachmann, F.L.; Tøndervik, A.; Sletta, H.; Ostafe, V. Chitosan as a Wound Dressing Starting Material: Antimicrobial Properties and Mode of Action. Int. J. Mol. Sci. 2019, 20, 5889. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, Z.; Li, Y.; Ding, X.; Li, D.; Shen, C.; Xu, F.J. Dual-Crosslinked Amorphous Polysaccharide Hydrogels Based on Chitosan/Alginate for Wound Healing Applications. Macromol. Rapid Commun. 2018, 39, e1800069. [Google Scholar] [CrossRef]
- El-Araby, A.; Janati, W.; Ullah, R.; Ercisli, S.; Errachidi, F. Chitosan, Chitosan Derivatives, and Chitosan-Based Nanocomposites: Eco-Friendly Materials for Advanced Applications (a Review). Front. Chem. 2023, 11, 1327426. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cheng, F.; Islam, M.R.; Li, H. The Fabrication of the Chitosan-Based Bioink for in Vitro Tissue Repair and Regeneration: A Review. Int. J. Biol. Macromol. 2024, 257, 128504. [Google Scholar] [CrossRef]
- Fatimi, A.; Okoro, O.V.; Podstawczyk, D.; Siminska-Stanny, J.; Shavandi, A. Natural Hydrogel-Based Bio-Inks for 3D Bioprinting in Tissue Engineering: A Review. Gels 2022, 8, 179. [Google Scholar] [CrossRef] [PubMed]
- Fischetti, T.; Celikkin, N.; Contessi Negrini, N.; Farè, S.; Swieszkowski, W. Tripolyphosphate-Crosslinked Chitosan/Gelatin Biocomposite Ink for 3D Printing of Uniaxial Scaffolds. Front. Bioeng. Biotechnol. 2020, 8, 400. [Google Scholar] [CrossRef]
- Zhang, J.; Allardyce, B.J.; Rajkhowa, R.; Zhao, Y.; Dilley, R.J.; Redmond, S.L.; Wang, X.; Liu, X. 3D Printing of Silk Particle-Reinforced Chitosan Hydrogel Structures and Their Properties. ACS Biomater. Sci. Eng. 2018, 4, 3036–3046. [Google Scholar] [CrossRef]
- Hidaka, M.; Kojima, M.; Nakahata, M.; Sakai, S. Visible Light-Curable Chitosan Ink for Extrusion-Based and Vat Polymerization-Based 3d Bioprintings. Polymers 2021, 13, 1382. [Google Scholar] [CrossRef]
- Hong, B.M.; Park, S.A.; Park, W.H. Effect of Photoinitiator on Chain Degradation of Hyaluronic Acid. Biomater. Res. 2019, 23, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Kurisawa, M.; Chung, J.E.; Yang, Y.Y.; Gao, S.J.; Uyama, H. Injectable Biodegradable Hydrogels Composed of Hyaluronic Acid-Tyramine Conjugates for Drug Delivery and Tissue Engineering. Chem. Commun. 2005, 34, 4312–4314. [Google Scholar] [CrossRef] [PubMed]
- Loebel, C.; Rodell, C.B.; Chen, M.H.; Burdick, J.A. Shear-Thinning and Self-Healing Hydrogels as Injectable Therapeutics and for 3D-Printing. Nat. Protoc. 2017, 12, 1521–1541. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jing, X.; Zou, J.; Feng, P.; Wang, G.; Zeng, J.; Lin, L.; Liu, Y.; Mi, H.Y.; Nie, S. Mechanically Robust and Anti-Swelling Anisotropic Conductive Hydrogel with Fluorescence for Multifunctional Sensing. Adv. Funct. Mater. 2024, 34, 2410698. [Google Scholar] [CrossRef]
- Gogoi, D.; Kumar, M.; Singh, J. A Comprehensive Review on Hydrogel-Based Bio-Ink Development for Tissue Engineering Scaffolds Using 3D Printing. Ann. 3D Print. Med. 2024, 15, 100159. [Google Scholar] [CrossRef]
- Saha, D.; Talukdar, D.; Pal, I.; Majumdar, S.; Lepcha, G.; Sadhu, S.; Yatirajula, S.K.; Das, G.; Dey, B. Mechanically Flexible Self-Healing Mg(II)-Metallogel: Approach of Triggering the ROS-Induced Apoptosis in Human Breast Cancer Cells. Langmuir 2024, 40, 19816–19829. [Google Scholar] [CrossRef]
- Heinrich, M.A.; Liu, W.; Jimenez, A.; Yang, J.; Akpek, A.; Liu, X.; Pi, Q.; Mu, X.; Hu, N.; Schiffelers, R.M.; et al. 3D Bioprinting: From Benches to Translational Applications. Small 2019, 15, 1–47. [Google Scholar] [CrossRef]
- Qin, D.; Zhang, A.; Wang, N.; Yao, Y.; Chen, X.; Liu, Y. Hydroxybutyl Chitosan/Oxidized Glucomannan Self-Healing Hydrogels as BMSCs-Derived Exosomes Carriers for Advanced Stretchable Wounds. Appl. Mater. Today 2022, 26, 101342. [Google Scholar] [CrossRef]
- Luo, H.; Xiong, G.; Hu, D.; Ren, K.; Yao, F.; Zhu, Y.; Gao, C.; Wan, Y. Characterization of TEMPO-Oxidized Bacterial Cellulose Scaffolds for Tissue Engineering Applications. Mater. Chem. Phys. 2013, 143, 373–379. [Google Scholar] [CrossRef]
- Heo, D.N.; Alioglu, M.A.; Wu, Y.; Ozbolat, V.; Ayan, B.; Dey, M.; Kang, Y.; Ozbolat, I.T. 3D Bioprinting of Carbohydrazide-Modified Gelatin into Microparticle-Suspended Oxidized Alginate for the Fabrication of Complex-Shaped Tissue Constructs. ACS Appl. Mater. Interfaces 2020, 12, 20295–20306. [Google Scholar] [CrossRef]
- Xu, J.; Liu, Y.; Hsu, S.-H. Hydrogels Based on Schiff Base Linkages for Biomedical Applications. Molecules 2019, 24, 3005. [Google Scholar] [CrossRef] [PubMed]
- Korkiatithaweechai, S.; Umsarika, P.; Praphairaksit, N.; Muangsin, N. Controlled Release of Diclofenac from Matrix Polymer of Chitosan and Oxidized Konjac Glucomannan. Mar. Drugs 2011, 9, 1649–1663. [Google Scholar] [CrossRef]
- Wang, M.; He, W.; Jin, X.; Song, X. Oxidized Konjac Glucomannan as a Paper Strength Agent. BioResources 2015, 10, 8089–8097. [Google Scholar] [CrossRef][Green Version]
- Wang, Y.; Xie, R.; Li, Q.; Dai, F.; Lan, G.; Shang, S.; Lu, F. A Self-Adapting Hydrogel Based on Chitosan/Oxidized Konjac Glucomannan/AgNPs for Repairing Irregular Wounds. Biomater. Sci. 2020, 8, 1910–1922. [Google Scholar] [CrossRef]
- Sakai, S.; Yamada, Y.; Zenke, T.; Kawakami, K. Novel Chitosan Derivative Soluble at Neutral PH and In-Situ Gellable via Peroxidase-Catalyzed Enzymatic Reaction. J. Mater. Chem. 2009, 19, 230–235. [Google Scholar] [CrossRef]
- Wang, H.J.; Chu, Y.Z.; Chen, C.K.; Liao, Y.S.; Yeh, M.Y. Preparation of Conductive Self-Healing Hydrogelsviaan Interpenetrating Polymer Network Method. RSC Adv. 2021, 11, 6620–6627. [Google Scholar] [CrossRef]
- Choi, J.; Yoon, J.; Ahn, K.H.; Choi, S.H.; Char, K. Injectable Hydrogels with Improved Mechanical Property Based on Electrostatic Associations. Colloid Polym. Sci. 2021, 299, 575–584. [Google Scholar] [CrossRef]
- Sakai, S.; Ohi, H.; Hotta, T.; Kamei, H.; Taya, M. Differentiation Potential of Human Adipose Stem Cells Bioprinted with Hyaluronic Acid/Gelatin-Based Bioink through Microextrusion and Visible Light-Initiated Crosslinking. Biopolymers 2018, 109, e23080. [Google Scholar] [CrossRef]
- Wahid, M.H.; Eroglu, E.; LaVars, S.M.; Newton, K.; Gibson, C.T.; Stroeher, U.H.; Chen, X.; Boulos, R.A.; Raston, C.L.; Harmer, S.L. Microencapsulation of Bacterial Strains in Graphene Oxide Nano-Sheets Using Vortex Fluidics. RSC Adv. 2015, 5, 37424–37430. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, L.; Mao, C.; Song, Z.; Li, X.; Liu, C. Preparation and Characterization of Konjac Glucomannan and Gum Arabic Composite Gel. Int. J. Biol. Macromol. 2021, 183, 2121–2130. [Google Scholar] [CrossRef]
- Fan, J.; Wang, K.; Liu, M.; He, Z. In Vitro Evaluations of Konjac Glucomannan and Xanthan Gum Mixture as the Sustained Release Material of Matrix Tablet. Carbohydr. Polym. 2008, 73, 241–247. [Google Scholar] [CrossRef]
- Jiang, Y.; Reddy, C.K.; Huang, K.; Chen, L.; Xu, B. Hydrocolloidal Properties of Flaxseed Gum/Konjac Glucomannan Compound Gel. Int. J. Biol. Macromol. 2019, 133, 1156–1163. [Google Scholar] [CrossRef]
- Chua, M.; Chan, K.; Hocking, T.J.; Williams, P.A.; Perry, C.J.; Baldwin, T.C. Methodologies for the Extraction and Analysis of Konjac Glucomannan from Corms of Amorphophallus Konjac K. Koch. Carbohydr. Polym. 2012, 87, 2202–2210. [Google Scholar] [CrossRef]
- Muhammad, M.; Willems, C.; Rodríguez-fernández, J.; Gallego- Ferrer, G.; Groth, T. Synthesis and Characterization of Oxidized Polysaccharides for in Situ Forming Hydrogels. Biomolecules 2020, 10, 1185. [Google Scholar] [CrossRef]
- Liu, C.; Li, J.; Li, K.; Xie, C.; Liu, J. Oxidized Konjac Glucomannan-Cassava Starch and Sucrose Esters as Novel Excipients for Sustained-Release Matrix Tablets. Int. J. Biol. Macromol. 2020, 156, 1045–1052. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Li, L.; Xing, J.; Cheng, C.; Hu, M.; Luo, Y.; Shi, S.; Liu, Y.; Cui, Z.; Yu, X. Cross-Linking Porcine Peritoneum by Oxidized Konjac Glucomannan: A Novel Method to Improve the Properties of Cardiovascular Substitute Material. Collagen Leather 2023, 5, 5. [Google Scholar] [CrossRef]
- Zhou, Y.; Liao, S.; Chu, Y.; Yuan, B.; Tao, X.; Hu, X.; Wang, Y. An Injectable Bioink with Rapid Prototyping in the Air and In-Situ Mild Polymerization for 3D Bioprinting. Biofabrication 2021, 13, 045026. [Google Scholar] [CrossRef] [PubMed]
- Birman, T.; Seliktar, D. Injectability of Biosynthetic Hydrogels: Consideration for Minimally Invasive Surgical Procedures and 3D Bioprinting. Adv. Funct. Mater. 2021, 31, 29. [Google Scholar] [CrossRef]
- Li, S.; Pei, M.; Wan, T.; Yang, H.; Gu, S.; Tao, Y.; Liu, X.; Zhou, Y.; Xu, W.; Xiao, P. Self-Healing Hyaluronic Acid Hydrogels Based on Dynamic Schiff Base Linkages as Biomaterials. Carbohydr. Polym. 2020, 250, 116922. [Google Scholar] [CrossRef]
- Wu, H.; Bu, N.; Chen, J.; Chen, Y.; Sun, R.; Wu, C.; Pang, J. Construction of Konjac Glucomannan/Oxidized Hyaluronic Acid Hydrogels for Controlled Drug Release. Polymers 2022, 14, 927. [Google Scholar] [CrossRef]
- Chen, H.; Fei, F.; Li, X.; Nie, Z.; Zhou, D.; Liu, L.; Zhang, J.; Zhang, H.; Fei, Z.; Xu, T. A Facile, Versatile Hydrogel Bioink for 3D Bioprinting Benefits Long-Term Subaqueous Fidelity, Cell Viability and Proliferation. Regen. Biomater. 2021, 8, rbab026. [Google Scholar] [CrossRef]
- Sakai, S.; Kamei, H.; Mori, T.; Hotta, T.; Ohi, H.; Nakahata, M.; Taya, M. Visible Light-Induced Hydrogelation of an Alginate Derivative and Application to Stereolithographic Bioprinting Using a Visible Light Projector and Acid Red. Biomacromolecules 2018, 19, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Gaohua, L.; Miao, X.; Dou, L. Crosstalk of Physiological PH and Chemical PKa under the Umbrella of Physiologically Based Pharmacokinetic Modeling of Drug Absorption, Distribution, Metabolism, Excretion, and Toxicity. Expert Opin. Drug Metab. Toxicol. 2021, 17, 1103–1124. [Google Scholar] [CrossRef] [PubMed]
- Szymańska, E.; Winnicka, K. Stability of Chitosan—A Challenge for Pharmaceutical and Biomedical Applications. Mar. Drugs 2015, 13, 1819–1846. [Google Scholar] [CrossRef]
- Filimonova, E.; Bergmann, T.; Zhao, S.; Dyatlov, V.A.; Malfait, W.J.; Wu, T. Effect of Polymer Concentration and Cross-Linking Density on the Microstructure and Properties of Polyimide Aerogels. J. Sol-Gel Sci. Technol. 2024, 110, 747–759. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, Characterization, and Applications: A Review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Rolannd, B.; Agnese, M.; Marco, C. Swelling Behavior of Carboxymethylcellulose Hydrogels in Relation to Cross-Linking, pH, and Charge Density. Macromolucules 2000, 33, 7454–7480. [Google Scholar] [CrossRef]
- Xu, H.; Matysiak, S. Effect of PH on Chitosan Hydrogel Polymer Network Structure. Chem. Commun. 2017, 53, 7373–7376. [Google Scholar] [CrossRef]
- Hidaka, M.; Kojima, M.; Sakai, S. Characterization of Chitosan Hydrogels Obtained through Phenol and Tripolyphosphate Anionic Crosslinking. Polymers 2024, 16, 1274. [Google Scholar] [CrossRef]
- Tang, H.R.; Covington, A.D.; Hancock, R.A. Structure-Activity Relationships in the Hydrophobic Interactions of Polyphenols with Cellulose and Collagen. Biopolymers 2003, 70, 403–413. [Google Scholar] [CrossRef]
- Rumon, M.M.H.; Akib, A.A.; Sultana, F.; Moniruzzaman, M.; Niloy, M.S.; Shakil, M.S.; Roy, C.K. Self-Healing Hydrogels: Development, Biomedical Applications, and Challenges. Polymers 2022, 14, 4539. [Google Scholar] [CrossRef] [PubMed]
- Schmid-Wendtner, M.H.; Korting, H.C. The PH of the Skin Surface and Its Impact on the Barrier Function. Skin. Pharmacol. Physiol. 2006, 19, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Lambers, H.; Piessens, S.; Bloem, A.; Pronk, H.; Finkel, P. Natural Skin Surface PH Is on Average below 5, Which Is Beneficial for Its Resident Flora. Int. J. Cosmet. Sci. 2006, 28, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Mansur, H.S.; Costa, E.d.S.; Mansur, A.A.; Barbosa-Stancioli, E.F. Cytocompatibility Evaluation in Cell-Culture Systems of Chemically Crosslinked Chitosan/PVA Hydrogels. Mater. Sci. Eng. C 2009, 29, 1574–1583. [Google Scholar] [CrossRef]
- Dettin, M.; Zamuner, A.; Roso, M.; Iucci, G.; Samouillan, V.; Danesin, R.; Modesti, M.; Conconi, M.T. Facile and Selective Covalent Grafting of an RGD-Peptide to Electrospun Scaffolds Improves HUVEC Adhesion. J. Pept. Sci. 2015, 21, 786–795. [Google Scholar] [CrossRef]
- Sivaraman, K.; Muthukumar, K.; Shanthi, C. Adhesion and Proliferation Properties of Type I Collagen-Derived Peptide for Possible Use in Skin Tissue Engineering Application. Cell Biol. Int. 2022, 46, 391–402. [Google Scholar] [CrossRef]
- Ji, C.; Khademhosseini, A.; Dehghani, F. Enhancing Cell Penetration and Proliferation in Chitosan Hydrogels for Tissue Engineering Applications. Biomaterials 2011, 32, 9719–9729. [Google Scholar] [CrossRef]
- Ke, C.L.; Deng, F.S.; Chuang, C.Y.; Lin, C.H. Antimicrobial Actions and Applications of Chitosan. Polymers 2021, 13, 904. [Google Scholar] [CrossRef]
- Zheng, K.; Xiao, S.; Li, W.; Wang, W.; Chen, H.; Yang, F.; Qin, C. Chitosan-Acorn Starch-Eugenol Edible Film: Physico-Chemical, Barrier, Antimicrobial, Antioxidant and Structural Properties. Int. J. Biol. Macromol. 2019, 135, 344–352. [Google Scholar] [CrossRef]
- Singh, R.P.; Kumari, P.; Reddy, C.R.K. Antimicrobial Compounds from Seaweeds-Associated Bacteria and Fungi. Appl. Microbiol. Biotechnol. 2015, 99, 1571–1586. [Google Scholar] [CrossRef]
- McCarthy, R.R.; Ullah, M.W.; Pei, E.; Yang, G. Antimicrobial Inks: The Anti-Infective Applications of Bioprinted Bacterial Polysaccharides. Trends Biotechnol. 2019, 37, 1155–1159. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, M.; Bhandari, B.; Wang, Y. 3D Printing: Printing Precision and Application in Food Sector. Trends Food Sci. Technol. 2017, 69, 83–94. [Google Scholar] [CrossRef]
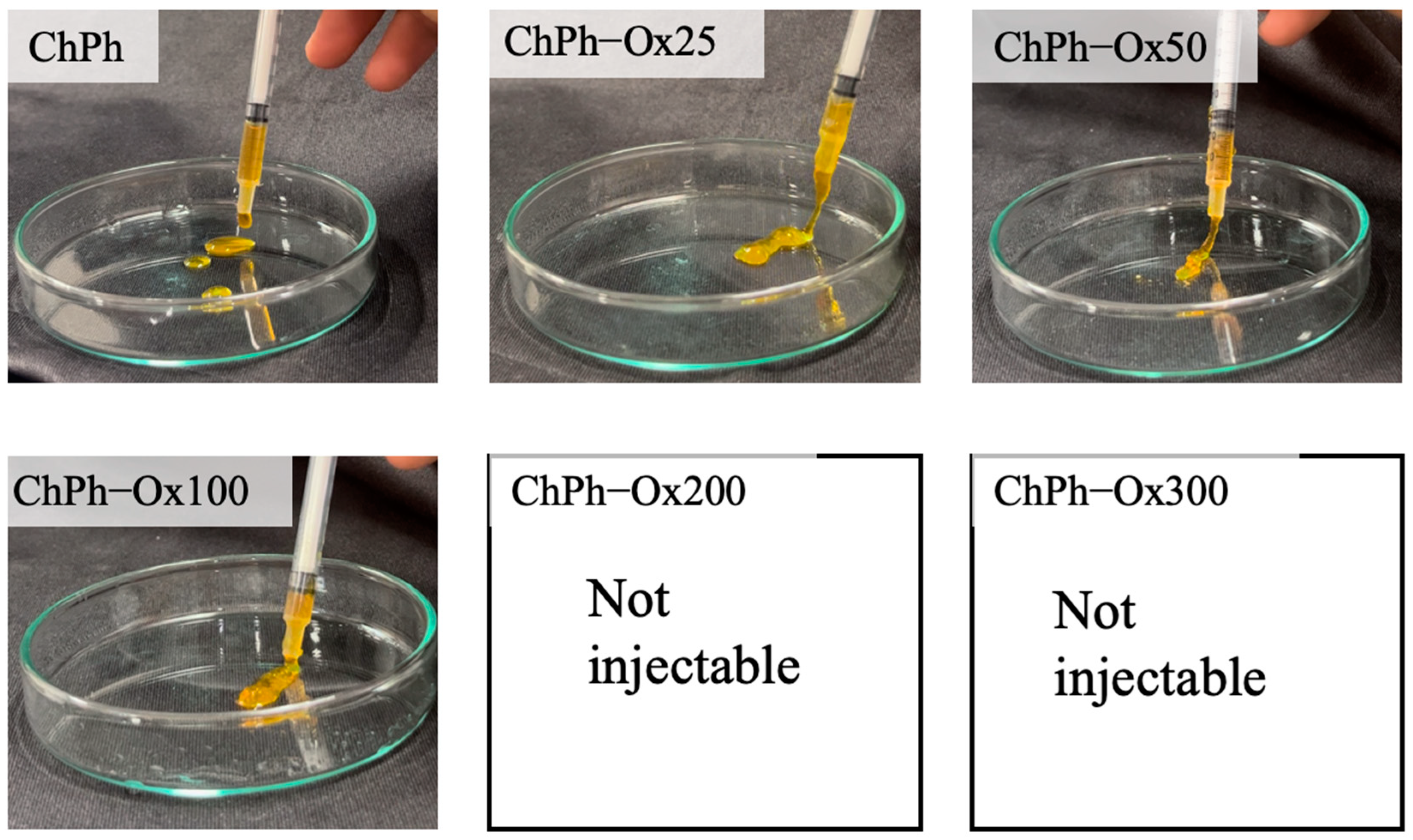

| ChPh [wt%] | Ox-glucomannan [wt%] | The abbreviated symbol |
| 1.0 | - | ChPh |
| 1.0 | 0.25 | ChPh-Ox25 |
| 1.0 | 0.50 | ChPh-Ox50 |
| 1.0 | 1.0 | ChPh-Ox100 |
| 1.0 | 2.0 | ChPh-Ox200 |
| 1.0 | 3.0 | ChPh-Ox300 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hidaka, M.; Sakai, S. Photo- and Schiff Base-Crosslinkable Chitosan/Oxidized Glucomannan Composite Hydrogel for 3D Bioprinting. Polysaccharides 2025, 6, 19. https://doi.org/10.3390/polysaccharides6010019
Hidaka M, Sakai S. Photo- and Schiff Base-Crosslinkable Chitosan/Oxidized Glucomannan Composite Hydrogel for 3D Bioprinting. Polysaccharides. 2025; 6(1):19. https://doi.org/10.3390/polysaccharides6010019
Chicago/Turabian StyleHidaka, Mitsuyuki, and Shinji Sakai. 2025. "Photo- and Schiff Base-Crosslinkable Chitosan/Oxidized Glucomannan Composite Hydrogel for 3D Bioprinting" Polysaccharides 6, no. 1: 19. https://doi.org/10.3390/polysaccharides6010019
APA StyleHidaka, M., & Sakai, S. (2025). Photo- and Schiff Base-Crosslinkable Chitosan/Oxidized Glucomannan Composite Hydrogel for 3D Bioprinting. Polysaccharides, 6(1), 19. https://doi.org/10.3390/polysaccharides6010019