Abstract
Steatotic liver disease is common in the general population and is associated with higher risk for cardiovascular diseases. Early diagnosis and appropriate therapy can prevent the development of irreversible end-stage liver fibrosis and reduce liver-related and cardiovascular mortality. It is important to recognise not only the common causes of metabolic dysfunction-associated steatotic liver disease, such as type 2 diabetes mellitus or morbid obesity, but also rare conditions, because their treatment is different from conventional therapy. Here, we report a female patient with familial partial lipodystrophy, in whom the diagnosis was not confirmed until several years after the initial manifestation, which delayed the start of pathogenetic therapy. After the initiation of leptin replacement therapy, a significant improvement in liver stiffness measurement was achieved within a few months. In addition, we summarise the current treatment options for diabetes and their influence on steatosis hepatis.
1. Introduction
Metabolic dysfunction-associated steatotic liver disease (MASLD) is a heterogeneous group of disorders, characterised by intrahepatic fat accumulation and liver structure and function damage in the absence of alcohol impact.
According to the multi-society Delphi consensus statement, MASLD is diagnosed, if in a patient with steatotic liver disease, identified by liver imaging or biopsy, at least one of five cardiometabolic criteria is met and alcohol intake is excluded. The cardiometabolic criteria are defined as following [1]
- (1)
- BMI > 25 kg/m2 or waist circumference of more than 94 cm in males and more than 80 cm in females;
- (2)
- Fasting serum glucose ≥ 5.6 mmol/L (100 mg/dL) or 2 h post-load glucose levels ≥ 7.8 mmol/L (≥140 mg/dL) or HbA1C ≥ 5.7% (39 mmol/L) or type 2 diabetes or treatment for type 2 diabetes;
- (3)
- Blood pressure > 130/85 mmHg or specific antihypertensive drug treatment;
- (4)
- Plasma triglycerides ≥ 1.70 mmol/L (150 mg/dL) or lipid-lowering treatment;
- (5)
- Plasma HDL-cholesterol ≤ 1.0 mmol/L (40 mg/dL) in males and ≤1.3 mmol/L (50 mg/dL) or lipid-lowering treatment.
The current EASL-EASD-EASO multi-society clinical practice guidelines [2] provide evidence-based recommendations on diagnosis and treatment for MASLD. As advanced liver fibrosis is a predictor of liver-associated mortality, it is recommended to rule out liver fibrosis based on laboratory scores (e.g., FIB-4) and non-invasive imaging techniques.
The most available diagnostic tool for steatosis is a combination of liver sonography with vibration-controlled transient elastography (VCTE) with liver stiffness measurement (LSM) and the assessment of the controlled attenuation parameter (CAP). This diagnostic procedure is comparable with liver biopsy in terms of sensitivity and specificity [3,4] and is widely used in the last decades. Braude et al. [4] demonstrated an association between increased all-cause mortality and liver stiffness on vibration-controlled transient elastography in patients with MASLD. Comparing CAP findings with liver biopsy analyses as the reference standard, Eddowes at al. found that patients with steatosis Grade 1 had a CAP of >302 dB/m, those with Grade 2 had a CAP of >331 dB/m, and those with Grade 3 had a CAP of >337 dB/m, whereas for LSM, the cut-off values for F2, F3, and F4 were 8.2 kPa, 9.7 kPa, and 13.6 kPa, respectively [5]. There are currently no guideline-defined CAP cut-offs, which limits the use of the CAP as a diagnostic criterion for MASLD [6,7,8].
Lipodystrophy syndromes can be a rare cause of MASLD, where the subcutaneous tissue is unable to properly store fat, what leads to fat accumulation in visceral organs, especially in the liver. Patients with generalised or familial partial lipodystrophy usually have low adipocyte hormone leptin levels, measured in serum, and, in most cases, severe metabolic abnormalities such as severe insulin resistance with diabetes mellitus and significant dyslipidaemia (Table 1). Since absolute or relative leptin deficiency is the cause of metabolic derailment in patients with lipodystrophy, human recombinant leptin replacement therapy (Metreleptin) has been tested in several studies in recent decades, with a significant improvement in the metabolic situation achieved and maintained in most patients [9] Metreleptin is available for causal treatment of congenital lipodystrophy, and it can be recommended in addition to diet and standard drug therapy for metabolic complications of FPL, as defined in the Multi-Society Practice Guideline for Diagnosis and Management of Lipodystrophy Syndromes [10].

Table 1.
Diagnostic criteria for familial partial lipodystrophy.
Functional and structural disorders of the liver are the most common organ complication, occurring in the majority of lipodystrophy patients. In LD patients with SLD, steatohepatitis improves due to metreleptin treatment, independent of diet [11]. Also, a reduction in liver volume by 33.8% for generalised lipodystrophy and by 13.4% for partial lipodystrophy after 12 months was observed [12,13]. Akinci demonstrated that 12 months after metreleptin treatment in patients with relative leptin deficiency and partial lipodystrophy liver biopsy, the results confirmed a significant decrease in steatohepatitis. This suggests that leptin deficiency may have regulatory effects in mediating fat deposition and, as consequence, damage the liver [14].
2. Case Report
Here, we report a female patient who presented to our endocrinology outpatient clinic at the age of 30 in the eighth week of pregnancy for treatment of diabetes mellitus (Figure 1).
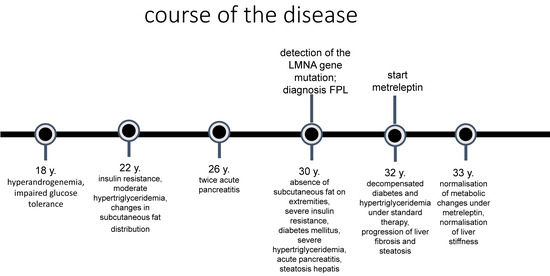
Figure 1.
Course of the disease.
She had hyperandrogenaemia and impaired glucose tolerance with insulin resistance since the age of 18. At that time, her gynaecologist recommended taking oral hormonal contraception until she wanted to have children, metformin therapy (500 mg 3 times a day), and weight reduction, because of a body mass index of 27 kg/m2, to control the hyperandrogenaemia and insulin resistance.
During her first spontaneous pregnancy at the age of 21, she was diagnosed with gestational diabetes. Postpartum, at the age of 22, she was seen in the endocrinology and diabetology department for the first time, where a fat distribution disorder with an accumulation of subcutaneous fat in the neck area with a double chin was first noticed. Furthermore, there was a slight axial and inguinal acanthosis nigricans, and her BMI was 28 kg/m2. Hirsutism and androgenetic alopecia were not present, but the physician noted that there was prominent musculature of the lower and upper extremities, although the patient did not exercise. The laboratory tests revealed very high insulin levels (150.0 µU/mL (6–27)) with normal glucose (80 mg/dL (60–110)) as well as high lipid metabolism parameters (cholesterol: 216 mg/dL (<200); triglycerides: 700 mg/dL (<150)) and hyperandrogenaemia. Ovarian hyperandrogenaemia and hyperinsulinemia, as a sign of insulin resistance, were documented as diagnoses. A low-carbohydrate diet was recommended to reduce her insulin levels, and metformin was prescribed to improve her insulin resistance.
At the age of 26, the patient was admitted to hospital twice at intervals of 3 months due to acute pancreatitis. Very high levels of triglycerides in the serum were conspicuous. After reconvalescence, she presented to the specialised lipid clinic. The lipid disorder was classified as a “diabetogenic lipid constellation” with high LDL, low HDL, and elevated triglyceride levels because of a familial predisposition. Notably, her HbA1c of 5.9% was not significantly increased. Lifestyle modification with an increase in physical activity and a strict change in diet was recommended. Treatment with metformin 1000 mg twice daily was continued. The HbA1c value was between 6.1% and 6.7% in the years prior to presentation at our clinic.
During the first presentation in our clinic, we saw a 30-year-old pregnant patient in the eighth week of pregnancy who was overweight (BMI 30.2 kg/m2) and had slightly elevated blood pressure (140/80 mmHg). Subcutaneous fat accumulation in the neck, throat, and facial area were very conspicuous in contrast to the muscular appearance with prominent musculature, particularly in the lower and upper extremities. There were also prominent veins on the extremities, a protruding navel, acanthosis nigricans located in the axilla and on the inner thighs, and hyperplasia of the labia pudendi. We registered absent or significantly reduced subcutaneous fat on the lower and upper extremities. The skin fold thickness was 4–6 mm on the upper arms, 10 mm on the sides of the navel, 6 mm on the thighs, and 25 mm on the scapulae. Laboratory chemistry, even if blood was taken postprandially, showed derailed lipid metabolism with dramatic hypertriglyceridemia of 8074 mg/dL (<150). The patient was admitted as an inpatient and was given a strict low-fat diet with the use of medium-chain triglyceride (MCT) diet, which led to a rapid improvement in lipid levels and was continued consistently over the course of the disease. HbA1c was only slightly elevated at 6.4%, but as the glucose levels were not within the target range for pregnancy as defined by the national diabetes guideline, insulin therapy was started.
A duplex sonography showed no evidence of relevant peripheral arterial occlusive disease of the upper and lower extremities. There was also no evidence of relevant stenosis of the extracranial arteries. Sonography of the abdomen revealed a liver with a regular contour, smooth surface, and homogeneous echo pattern but significantly enlarged and ubiquitously compacted. The liver stiffness measurement (LSM) registered a median organ density of 5.7 kPa. The assessment of the controlled attenuation parameter (CAP) revealed a high degree of steatosis hepatis (355 dB/m).
A molecular genetic analysis was initiated due to a strong clinical suspicion of familial partial lipodystrophy. A heterozygote mutation, p.(=)+(Arg482Gln), c.(=)+(1445G>A), in Exon 8 of the LMNA gene was detected, which confirmed the diagnosis of familial partial lipodystrophy type 2 (Dunningan type). Her fasting serum leptin levels were at the lower reference limit.
As the pregnancy progressed, the insulin dose had to be steadily increased to keep the glucose levels within the recommended target range. Due to pronounced insulin resistance, which was also aggravated by the pregnancy, the daily insulin doses reached up to almost 1000 IU. This made the patient’s quality of life considerably more difficult, as subcutaneous insulin injections were very painful as she had only a thin layer of subcutaneous fatty tissue. The hypertriglyceridemia could be controlled to some extent by a strict diet with a medium-chain triglyceride (MCT) diet. Nevertheless, moderate pancreatitis occurred once during pregnancy, which was treated with analgesics and volume administration. An insulin deficiency due to pancreatitis could be excluded.
The patient delivered on time by caesarean section. Postpartum, insulin was discontinued, and therapy with metformin 1000 mg twice daily, fenofibrate 250 mg daily, and omega-3 polyunsaturated fatty acids was carried out, under which the metabolic situation remained well controlled for 1.5 years. Treatment with GLP1 receptor analogues or DPP4 inhibitors was deliberately avoided due to concerns about the recurrence of pancreatitis.
After a gastrointestinal infection, metabolic derailment occurred, and insulin therapy was restarted. In the short term, however, there was increasing deterioration in her general condition with blurred vision, a feeling of warmth, malaise, extreme muscle weakness, and hyperphagia. She was unable to exercise due to a lack of muscle strength. Laboratory chemistry revealed hyperglycemic derailment (HbA1c 8.7%) and massive hypertriglyceridemia (2437 mg/dL (<150)). The patient had to be hospitalised, insulin therapy was intensified, and SGLT2 inhibitor therapy was initiated. The transaminases were moderately elevated; sonographically, we registered hepatomegaly with a kissing phenomenon, a rounded contour, and a smooth surface, with a homogeneous but clearly condensed echo pattern. Fibroscan and CAP measurements confirmed moderate to severe liver fibrosis and stage 3 steatosis hepatis.
Thus, 1.5 years postpartum, a metabolically derailed condition of partial familial lipodystrophy was under the consistently implemented standard therapy. The guidelines for the management of lypodystrophy and the criteria for leptin replacement therapy set out in the specialist information were thus fulfilled. This therapy was absolutely necessary in view of the otherwise uncontrollable diabetes and hypertriglyceridemia. The initiation of leptin replacement therapy with metreleptin, starting according to the current recommendations of 3 mg s.c. once daily (https://myaleptainfo.eu, accessed on 10 December 2024), was discussed with the patient. It was also expected that fine-tuning of the insulin therapy in terms of dose reduction would be necessary over the course of the treatment in order to avoid hypoglycaemia. Her subcutaneous FGM3 sensor was attached for continuous glucose level monitoring.
Three months after starting treatment with Metreleptin, the patient reported a significantly improved quality of life: reduced food intake, a reduced abdominal circumference, and a reduction in insulin doses and injections. She felt much stronger, walking was now better, and she no longer had any muscle pain. The distance she could walk had increased significantly since starting the metreleptin injections. The insulin therapy could be stopped. Laboratory tests showed normalisation of the transaminases, triglyceride levels had fallen 100-fold, and her HbA1C was now within the normal range.
Six months after the initiation of leptin replacement therapy, diabetes mellitus was also well controlled (HbA1c 5.4%), and oral diabetes therapy could be de-escalated. Sonographically, we registered a normalisation of liver size, and liver stiffness had decreased significantly.
Nine months after the start of therapy, metreleptin was increased to the recommended standard dose of 5 mg s.c. due to a renewed moderate increase in triglycerides and HbA1c in an otherwise symptom-free patient. Twelve months after the start of therapy, the metabolic parameters were within the target range, and the metreleptin dosage could be maintained (Table 2).

Table 2.
Changes in liver function and structure depending on metabolic parameters and therapy.
3. Conclusions
Metabolic dysfunction-associated steatotic liver disease is a common condition in the general population with the estimated prevalence up to 30% [15,16,17]. Several international clinical practice MASLD guidelines [2,18] emphasise the importance of early detection and evidence-based and complex treatment with regard to liver-related and cardiovascular mortality. The treatment options include lifestyle modifications, pharmacological treatment of diabetes mellitus and obesity (as we summarised in Table 3), specific therapy of fatty liver [6] and therapy of cardiovascular diseases (arterial hypertension, dyslipidaemia).

Table 3.
Treatment options in type 2 diabetes mellitus and fatty liver disease.
In the case of our patient, although the first symptoms of the disease with the phenotype typical of familial lipodystrophy manifested at a young age, the correct diagnosis could only be confirmed 12 years later, and serious and potentially fatal complications, such as acute pancreatitis, had already occurred. The multimodal therapy of the metabolic derailment was not sufficient.
The replacement of the missing hormone leptin (in combination with a diet and glucose- and triglyceride-lowering drugs) with Metreleptin is the only approved causal therapy for leptin deficiency-related consequences in patients with congenital generalised and partial familial lipodystrophy. In the case of our patient, if the situation of uncontrolled diabetes mellitus and hypertriglyceridemia, which cannot be controlled by conventional therapy, was to have persisted, further serious secondary diseases could have developed, such as progression of the existing metabolic dysfunction-associated steatotic liver disease to liver cirrhosis, with the increasing risk of hepatocellular carcinoma, progression of atherosclerosis with cardiovascular secondary diseases such as coronary heart disease to myocardial infarction, cerebrovascular disease to stroke, peripheral arterial occlusive disease, diabetic retinopathy to blindness, diabetic nephropathy to terminal kidney insufficiency requiring dialysis, and diabetes-related peripheral polyneuropathy.
In addition to the known and more common causes of metabolic dysfunction-associated steatotic liver disease, rare disorders such as familial lipodystrophy should be considered in unusual clinical presentations, as specific treatment options are available in these cases.
Author Contributions
Conceptualization E.V.; methodology E.V.; software E.V.; validation, not necessary; formal analysis not necessary; investigation, E.V.; resources, E.V.; data curation, E.V.; writing—original draft preparation, E.V.; writing—review and editing, J.T. and E.S.; visualization, E.S.; supervision, J.T.; project administration, E.V.; funding acquisition, not necessary. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Written informed consent has been obtained from the patient to publish this paper.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology 2023, 78, 1966–1986. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO); European Association for the Study of the Liver (EASL). EASL-EASD-EASO Clinical Practice Guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD). J. Hepatol. 2024, 81, 492–542. [Google Scholar] [CrossRef] [PubMed]
- Mózes, F.E.; Lee, J.A.; Selvaraj, E.A.; Jayaswal, A.N.A.; Trauner, M.; Boursier, J.; Fournier, C.; Staufer, K.; Stauber, R.E.; Bugianesi, E.; et al. Diagnostic accuracy of non-invasive tests for advanced fibrosis in patients with NAFLD: An individual patient data meta-analysis. Gut 2022, 71, 1006–1019. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Braude, M.; Roberts, S.; Majeed, A.; Lubel, J.; Prompen, J.; Dev, A.; Sievert, W.; Bloom, S.; Gow, P.; Kemp, W. Liver stiffness (Fibroscan®) is a predictor of all-cause mortality in people with non-alcoholic fatty liver disease. Liver Int. 2023, 43, 90–99. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Eddowes, P.J.; Sasso, M.; Allison, M.; Tsochatzis, E.; Anstee, Q.M.; Sheridan, D.; Guha, I.N.; Cobbold, J.F.; Deeks, J.J.; Paradis, V.; et al. Accuracy of FibroScan Controlled Attenuation Parameter and Liver Stiffness Measurement in Assessing Steatosis and Fibrosis in Patients with Nonalcoholic Fatty Liver Disease. Gastroenterology 2019, 156, 1717–1730. [Google Scholar] [CrossRef] [PubMed]
- Ciardullo, S.; Vergani, M.; Perseghin, G. Nonalcoholic Fatty Liver Disease in Patients with Type 2 Diabetes: Screening, Diagnosis, and Treatment. J. Clin. Med. 2023, 12, 5597. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ciardullo, S.; Perseghin, G. Trends in prevalence of probable fibrotic non-alcoholic steatohepatitis in the United States, 1999-2016. Liver Int. 2023, 43, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Archer, A.J.; Belfield, K.J.; Orr, J.G.; Gordon, F.H.; Abeysekera, K.W. EASL clinical practice guidelines: Non-invasive liver tests for evaluation of liver disease severity and prognosis. Frontline Gastroenterol. 2022, 13, 436–439. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Oral, E.A.; Simha, V.; Ruiz, E.; Andewelt, A.; Premkumar, A.; Snell, P.; Wagner, A.J.; DePaoli, A.M.; Reitman, M.L.; Taylor, S.I.; et al. Leptin-replacement therapy for lipodystrophy. N. Engl. J. Med. 2002, 346, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.J.; Araujo-Vilar, D.; Cheung, P.T.; Dunger, D.; Garg, A.; Jack, M.; Mungai, L.; Oral, E.A.; Patni, N.; Rother, K.I.; et al. The Diagnosis and Management of Lipodystrophy Syndromes: A Multi-Society Practice Guideline. J. Clin. Endocrinol. Metab. 2016, 101, 4500–4511. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brown, R.J.; Valencia, A.; Startzell, M.; Cochran, E.; Walter, P.J.; Garraffo, H.M.; Cai, H.; Gharib, A.M.; Ouwerkerk, R.; Courville, A.B.; et al. Metreleptin-mediated improvements in insulin sensitivity are independent of food intake in humans with lipodystrophy. J. Clin. Investig. 2018, 128, 3504–3516. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brown, R.J.; Oral, E.A.; Cochran, E.; Araújo-Vilar, D.; Savage, D.B.; Long, A.; Fine, G.; Salinardi, T.; Gorden, P. Long-term effectiveness and safety of metreleptin in the treatment of patients with generalized lipodystrophy. Endocrine 2018, 60, 479–489. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Oral, E.A.; Gorden, P.; Cochran, E.; Araújo-Vilar, D.; Savage, D.B.; Long, A.; Fine, G.; Salinardi, T.; Brown, R.J. Long-term effectiveness and safety of metreleptin in the treatment of patients with partial lipodystrophy. Endocrine 2019, 64, 500–511. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Akinci, B.; Subauste, A.; Ajluni, N.; Esfandiari, N.H.; Meral, R.; Neidert, A.H.; Eraslan, A.; Hench, R.; Rus, D.; McKenna, B.; et al. Metreleptin therapy for nonalcoholic steatohepatitis: Open-label therapy interventions in two different clinical settings. Med 2021, 2, 814–835. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Younossi, Z.M.; Golabi, P.; Paik, J.M.; Henry, A.; Van Dongen, C.; Henry, L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH): A systematic review. Hepatology 2023, 77, 1335–1347. [Google Scholar] [CrossRef] [PubMed]
- Quek, J.; Chan, K.E.; Wong, Z.Y.; Tan, C.; Tan, B.; Lim, W.H.; Tan, D.J.H.; Tang, A.S.P.; Tay, P.; Xiao, J.; et al. Global prevalence of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in the overweight and obese population: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2023, 8, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Le, M.H.; Le, D.M.; Baez, T.C.; Wu, Y.; Ito, T.; Lee, E.Y.; Lee, K.; Stave, C.D.; Henry, L.; Barnett, S.D.; et al. Global incidence of non-alcoholic fatty liver disease: A systematic review and meta-analysis of 63 studies and 1,201,807 persons. J. Hepatol. 2023, 79, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Cusi, K.; Isaacs, S.; Barb, D.; Basu, R.; Caprio, S.; Garvey, W.T.; Kashyap, S.; Mechanick, J.I.; Mouzaki, M.; Nadolsky, K.; et al. American Association of Clinical Endocrinology Clinical Practice Guideline for the Diagnosis and Management of Nonalcoholic Fatty Liver Disease in Primary Care and Endocrinology Clinical Settings: Co-Sponsored by the American Association for the Study of Liver Diseases (AASLD). Endocr. Pract. 2022, 28, 528–562. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).