Abstract
Background/Objectives: Hepatitis B virus (HBV) infection is a worldwide health problem responsible for chronic liver disease and hepatocellular carcinoma. Both innate immunity and the adaptive immune response play central roles in the development of chronic hepatitis and liver cancer. We previously performed a comprehensive analysis of gene expression in the livers of HBV-infected chimeric mice and found that several genes associated with cell growth or carcinogenesis via hypoxia and KRAS signaling were upregulated by HBV infection. However, due to the absence of adaptive immunity in uPA/SCID chimeric mice, we were unable to analyze the effect of the host immune response. Methods: In this study, we compared gene expression profiles in the livers obtained from HBV-infected chimeric mice with those of HBV carriers. Results: After HBV infection, the expression of genes associated with inflammation and immune response, especially involving the Th1 and Th2 activation pathways, was altered as HBV-specific intracellular immune responses both in vivo and in clinical samples. Interestingly, the proinflammatory gene IL12A was induced by HBV infection in the chimeric mouse livers but not in the human livers, and associated genes, such as SRDA5A2, AR, and CCR3, showed differential alteration by HBV infection between the chimeric mouse and human livers. Conclusions: These results suggest that hepatocarcinogenesis may be suppressed by host immunity in HBV carriers. This study highlights potential new implications for inhibiting the progression of HBV-related liver diseases, including hepatocarcinogenesis.
1. Introduction
A universal vaccination program for preventing hepatitis B virus (HBV) infection has long been promoted worldwide; nonetheless, an estimated 1.2 million people become newly infected with HBV each year, while 254 million people remain chronically infected with HBV [1]. Chronic HBV infection leads to approximately 1.1 million deaths each year, primarily due to HBV-related liver diseases such as cirrhosis and hepatocellular carcinoma [1]. Once HBV infects human hepatocytes, the viral genome is transported into the nucleus where it forms a covalently closed circular DNA (cccDNA) mini-chromosome, making it difficult to eradicate HBV from hepatocytes through current antiviral therapies using nucleotide/nucleoside analogs (NAs). Therefore, the current guidelines for managing chronic hepatitis B and liver cirrhosis recommend long-term treatment using NAs [2,3,4,5].
HBV-related proteins are continually produced as a result of the continuous transcription of HBV mRNAs from cccDNA, regardless of antiviral therapy. Therefore, HBV infection induces a persistent state of altered gene expression in hepatocytes, and signaling pathways associated with liver inflammation and hepatocarcinogenesis may remain activated. However, these mechanisms have not been fully elucidated, and it is not clear which HBV-associated alterations in gene expression due to infection are critical for inducing hepatic inflammation and hepatocarcinogenesis.
Previously, we succeeded in constructing a human hepatocyte chimeric mouse model that can be continuously infected with HBV [6]. Since the chimeric mice were generated using severe combined immunodeficiency (SCID) mice, they remain in a severely immunodeficient condition, and most mouse hepatocytes are successfully replaced with transplanted human primary hepatocytes with a low rate of rejection [6,7]. This chimeric mouse model can be used to examine the response of human hepatocytes against viral infection without the confounding effects of the host immune response [8,9,10,11]. Using complementary DNA (cDNA) microarrays and next-generation sequencing, we have demonstrated that inflammatory cytokines and chemokines or histone methyltransferases are induced in human hepatocytes after HBV infection [8,9,10,12]. Furthermore, we also reported that genes related to oxidative stress and the Wnt signaling pathway were more highly induced in HBV genotype C-infected hepatocytes than in genotype A-infected hepatocytes, indicating the ability to show the differences in HCC development among HBV genotypes using a humanized mouse model [13]. Although these results reflected clinical features associated with chronic HBV infection, chimeric mouse livers with depleted immune responses to viral infection were used in these analyses, and it is not clear whether similar intracellular responses occur in the human liver during chronic HBV infection. Additionally, in our previous study, we performed comprehensive gene expression analyses using HBV-infected chimeric mice with or without treatment with pegylated interferon (PEG-IFN) plus high-dose entecavir therapy [8]. We found that several genes associated with carcinogenesis signaling were upregulated after HBV infection and were significantly downregulated following antiviral therapy.
Although we examined alterations in gene expression associated with HBV infection, we ignored the effects of host immunity due to the lack of host immunity in the chimeric mice. Consequently, in the present study, we compared gene expression profiles in livers obtained from HBV carriers with those from HBV-infected chimeric mice and analyzed the differences in gene expression between in vivo samples and clinical samples. We found that several genes associated with immune response and inflammation were differentially regulated between the in vivo and clinical samples. In HBV-infected hepatocytes, androgen receptor (AR) activates transcription of interleukin 12A (IL12A), leading to inhibition of hepatocellular carcinoma (HCC) progression. Our results might help to explain the pathogenic mechanism of chronic hepatitis B and provide insight into a potential application for the suppression of hepato-carcinogenesis.
2. Materials and Methods
2.1. Gene Expression Profiles Obtained from Chimeric Mouse Livers
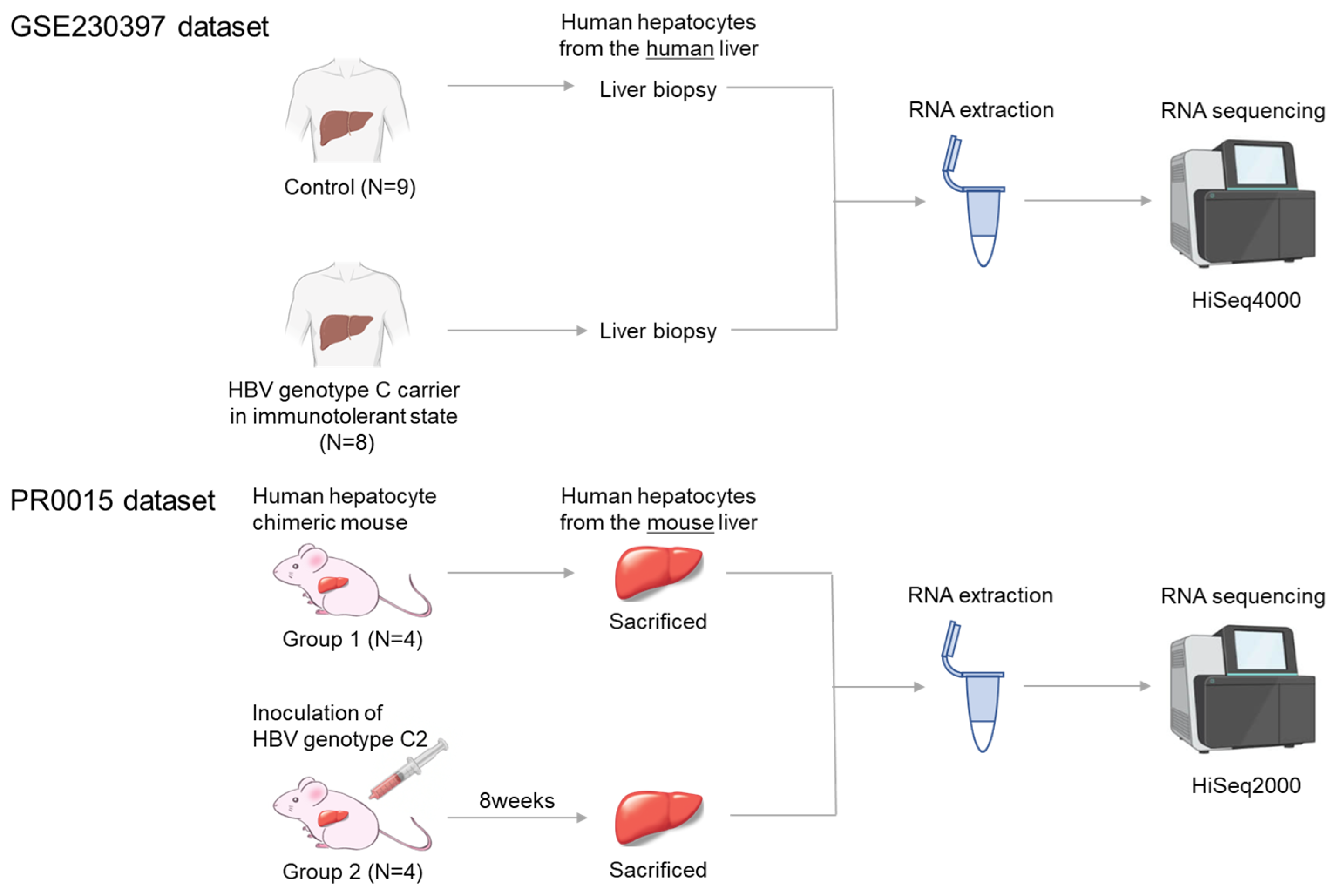
Gene expression profiles obtained from a previous study were used in this study [9]. Briefly, serum samples were obtained with written consent from HBV carriers, and high-viremia HBV genotype C serum (HBV DNA 7.8 log copies/mL) was used to infect mice. The HBV clone sequence was registered in NCBI (Accession: MH891502). The study followed the Declaration of Helsinki and was approved by the Hiroshima University Ethical Committee (Approval ID: E2015-0103). The experimental scheme is shown in Figure 1.
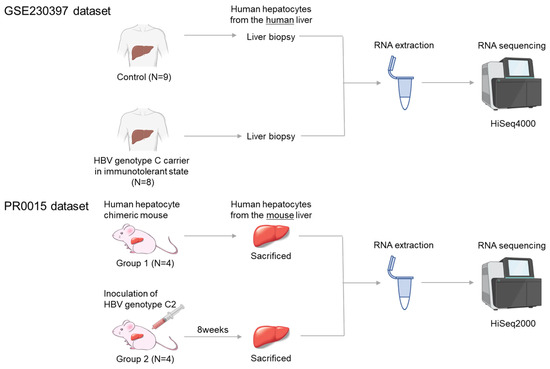
Figure 1.
Experimental scheme. The human GEO GSE230397 dataset consists of 9 healthy samples and 8 HBV carriers. The PR0015 chimeric mouse dataset consists of 4 uninfected control mice and 4 HBV-infected mice. RNA was extracted from liver tissue and sequenced using Illumina HiSeq sequencers.
Chimeric mice were prepared by transplanting frozen human hepatocytes from the same donor into uPA+/+/SCID+/+ mice [8,9,10,11]. Only mice in which over 90% of the liver was repopulated with human hepatocytes were used. Eight chimeric mice were assigned to two groups: four uninfected controls (Group 1) and four mice infected with 1 × 105 copies of HBV via injection into the tail vein (Group 2). Serum was collected at eight weeks post-infection, and HBV DNA was quantified by real-time PCR. All HBV-infected mice developed viremia with high viral load (9–10 Log copies/mL). Serum human albumin was also measured using ELISA, and the levels were maintained at greater than 107 ng/mL, corresponding to a human hepatocyte replacement rate of more than 90%. All 8 mice were sacrificed by anesthesia with diethyl ether.
Human hepatocytes were isolated and stored in RNA later® solution (Applied Biosystems, Foster City, CA, USA), followed by RNA extraction using NucleoSpin RNA II (MACHEREY-NAGEL, Duren, Germany). Quality was verified by absorption analysis, electrophoresis, and Bioanalyzer analysis (Agilent Technologies, Palo Alto, CA, USA). A sequencing library was prepared using SMART-Seq v4 Ultra Low Input RNA Kit (Takara Bio USA, Mountain View, CA, USA), followed by reverse transcription and PCR. Next-generation sequencing (PR0015) was performed on the NovaSeq6000 system (Illumina, Tokyo, Japan) by Takara Bio (Tokyo, Japan), and data analysis was conducted using Expression Miner software (Takara Bio) after excluding mouse-derived sequences.
2.2. Gene Expression Profiles Obtained from Human Livers
Standardized RNA-seq data and clinical information were obtained from the GEO database (https://www.ncbi.nlm.nih.gov/gds accessed on 16 April 2024) using “chronic hepatitis B” as the search criterion. The dataset GSE230397 was deemed suitable for analysis and was downloaded from GEO [14]. The GSE230397 dataset includes data from nine healthy individuals (GSM7221449—GSM7221457) and eight HBV carriers infected with HBV genotype C in the immune-tolerant phase (GSM7221459, GSM7221461, GSM7221462, GSM7221465, GSM7221468, GSM7221469, GSM7221470, and GSM7221471). The dataset authors extracted total RNA using the RNeasy FFPE Kit (Qiagen, Hilden, Germany) and prepared cDNA libraries for each sample using the HiSeq 4000 system (Illumina). Gene expression levels between the two groups were compared using DESeq2.
2.3. Analysis of DEGs
R software (version 4.4.1) was used to read and analyze the gene expression data. Differentially expressed genes (DEGs) were analyzed using the DESeq2 package with the standard comparison model. Adjusted p values for multiple testing were calculated using the embedded Benjamini–Hochberg procedure. DEGs were filtered with |log2(fold change)|≥ 1 and adjusted p ≤ 0.05. Common overlapping DEGs between GSE230397 and PR0015 were selected for further analysis.
2.4. Functional Analysis of DEGs and Data Visualization
We performed functional analysis of the DEGs using the clusterProfiler package in Bioconductor version 3.19. The clusterProfiler package provides gene annotation data for functional profiling of coding and non-coding genes. Gene Ontology (GO) is a comprehensive domain model that classifies genes with respect to three key taxonomies: BP (biological processes), CC (cellular components), and MF (molecular functions). The Kyoto Encyclopedia of Genes and Genomes (KEGG) is a multi-species database of genes and pathways. Molecular functions are represented as networks of interactions in KEGG pathways and modules [15]. Heatmaps were drawn using the base R heatmap package and pheatmap version 1.0.12. DEGs were visualized using volcano plots generated using the R packages ggplot2 version 3.5.1 and ggrepel version 0.9.6.
2.5. Identification of Transcription Factors (TFs) Regulating IL12A
TFs and their downstream genes were searched using the hTF target database (http://bioinfo.life.hust.edu.cn/hTFtarget/).
2.6. Statistical Analysis
All analysis was performed using R software. The embedded Benjamini–Hochberg procedure was used to control the false discovery rate due to multiple testing. An adjusted p value of <0.05 was considered statistically significant.
3. Results
3.1. Identification of DEGs in Two Datasets
To investigate the impact of HBV infection on intrahepatic gene expression in human and mouse livers, comprehensive RNA analysis was performed using total RNA obtained from both HBV-infected and uninfected mouse livers and human livers obtained from healthy volunteers and HBV asymptomatic carriers. In this study, the PR0015 data obtained from four non-HBV-infected and four HBV-infected chimeric mice were analyzed as an in vivo study, and the GSE230397 data obtained from nine healthy volunteers and eight HBV carriers were analyzed as a clinical study (Figure 1).
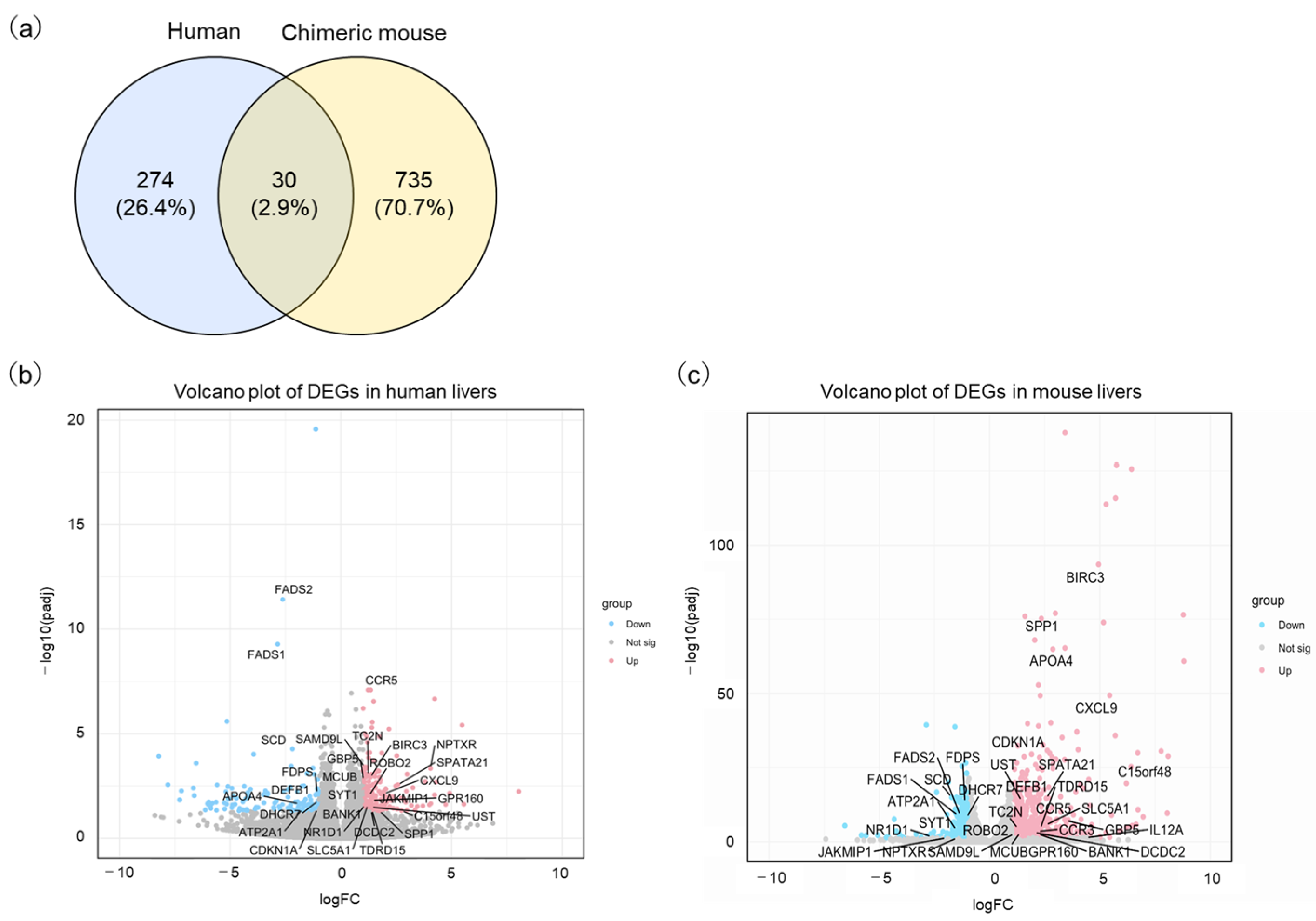
A list of all significantly differentially expressed genes (DEGs) is provided in Supplementary Material Table S1. Specifically, 766 DEGs, including 535 upregulated and 230 downregulated genes, were extracted in the comparison between uninfected and HBV-infected chimeric mouse liver tissues in the PR0015 dataset. In the GSE230397 dataset, 304 DEGs, with 179 genes upregulated and 125 downregulated, were revealed in comparison between HBV asymptomatic carriers and healthy volunteers. Thirty DEGs (ATP2A1, BANK1, BIRC3, C15orf48, CCR5, CDKN1A, CXCL9, DCDC2, DEFB1, DHCR7, FADS1, FADS2, FDPS, GBP5, GPR160, JAKMIP1, MCUB, NPTXR, NR1D1, ROBO2, SAMD9L, SCD, SLC5A1, SPATA2, SPP1, SYT1, TC2N, TDRD15, UST) overlapped in both the in vivo PR0015 and clinical GSE230397 datasets (Figure 2a and Tables S1 and S2). Upregulated and downregulated genes in human and chimeric mouse livers are visualized by a volcano plot in Figure 2b,c.
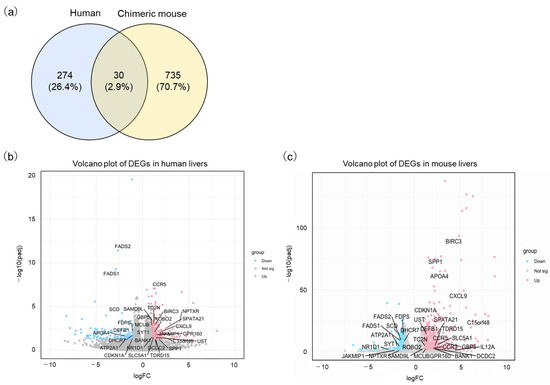
Figure 2.
Identification of differentially expressed genes (DEGs) with HBV infection. (a) Venn diagrams indicate the overlap in DEGs between human and chimeric mice. (b) The volcano plot depicts the distribution of DEGs in the GSE230397 clinical dataset. (c) The volcano plot shows DEGs in the PR0015 mouse dataset. The red color represents upregulated genes, while the blue color represents downregulated genes.
3.2. Functional Enrichment Analysis of HBV Differentially Expressed Genes
To classify DEGs biologically, functional and pathway enrichment analyses were conducted using SRplot [16]. Differential expression analysis was performed on both clinical samples and chimeric mouse samples.
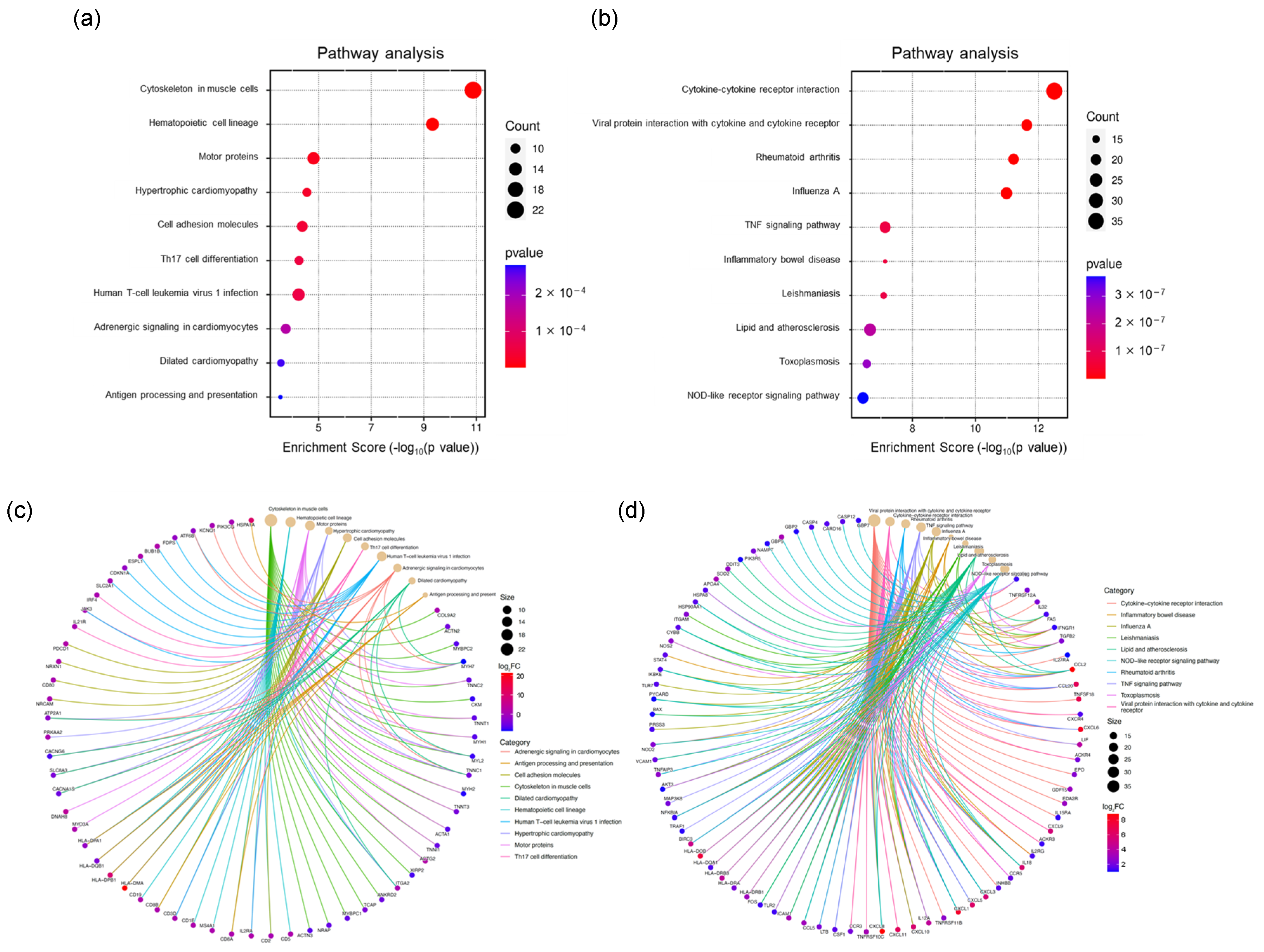
KEGG pathway analysis using the GSE230397 dataset (clinical samples) highlighted pathways involved in hematopoietic cell lineage, cell adhesion molecules, Th17 cell differentiation, Th1 and Th2 cell differentiation, and human T-cell leukemia virus 1 infection, which are primarily regulated by inflammatory responses (Figure 3a and Table S3). In contrast, KEGG pathway analysis using the PR0015 dataset (chimeric mouse livers) emphasized cytokine–cytokine receptor interactions, viral protein interactions with cytokines and cytokine receptors, the TNF signaling pathway, and the IL-17 signaling pathway, which are largely governed by cellular responses (Figure 3b and Table S4). These results suggest similar regulatory mechanisms between human and chimeric mouse data, indicating that inflammation and immune responses play key roles after HBV infection.
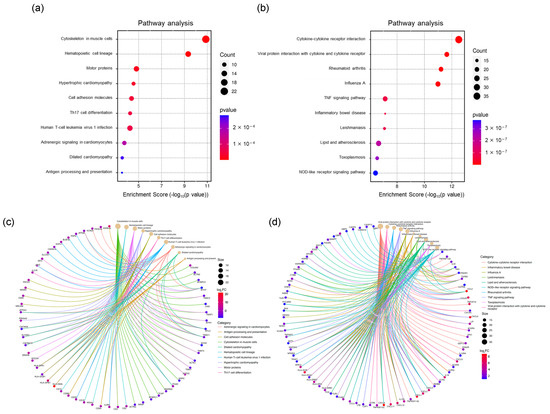
Figure 3.
KEGG analysis of DEGs. (a) Top 10 significantly enriched pathways of DEGs in the GSE230397 dataset. (b) Top 10 significantly enriched pathways of DEGs in PR0015. (c) Significant genes involved in the top 10 pathways in GSE230397. (d) Significant genes involved in the top 10 pathways in PR0015.
A total of 23 overlapping pathways were identified, with several enriched in cell adhesion molecules and Th1/Th2/Th17 cell differentiation, such as the chemokine signaling pathway and the inflammatory bowel disease pathway, highlighting their roles in immune response and inflammation. A complete list of KEGG pathway terms is provided in Supplementary Material Table S2. Key inflammatory genes, including CX3CL1, CCL2, CCL20, and CXCL6, were highly enriched in chimeric mouse samples, whereas HLA-DMA and HLA-DPB1, which are crucial for antigen processing and presentation, were primarily enriched in clinical samples (Figure 3c,d).
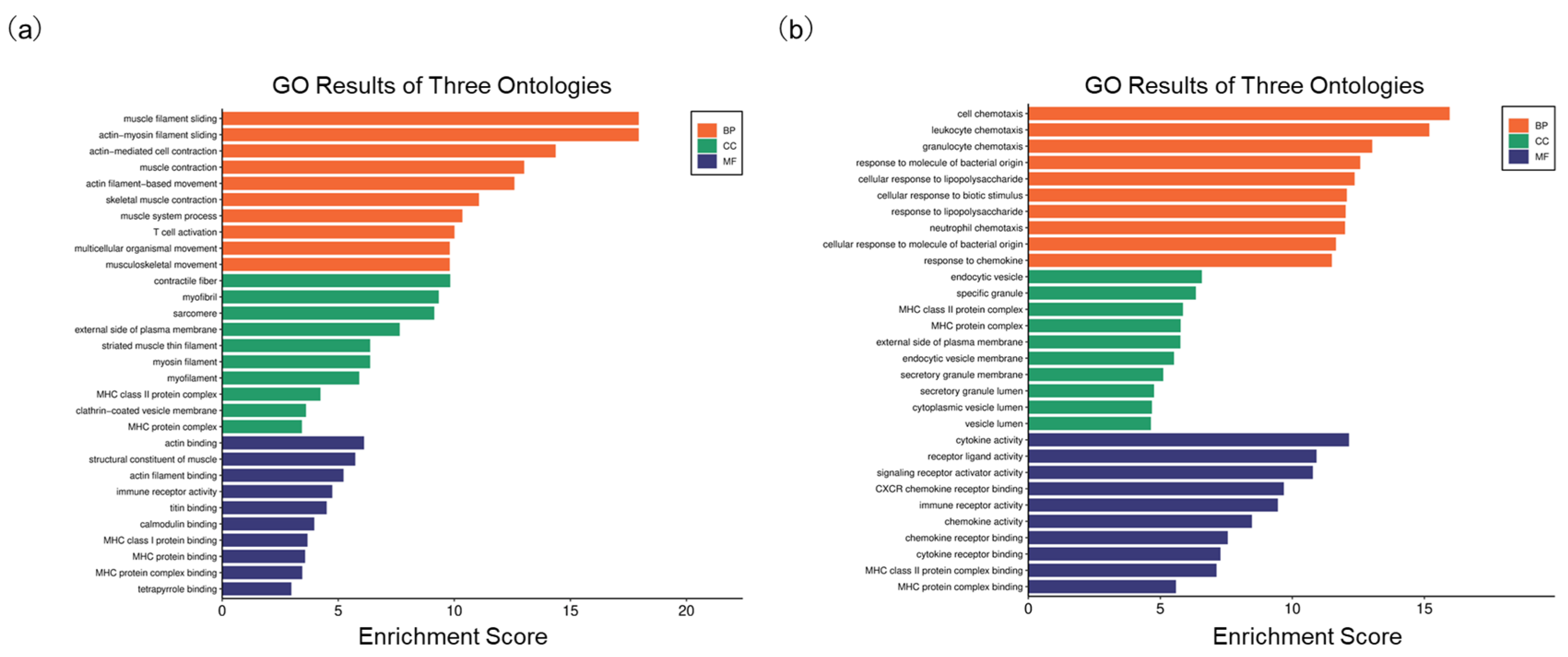
On the other hand, we also compared the alteration of functional signals after HBV infection based on GO ontologies. In the GSE230397 dataset, several biological processes (BP), such as muscle filament sliding, T cell activation, and actin-mediated cell contraction, were highlighted (Figure 4a). In the PR0015 dataset, DEGs were markedly enriched in T cell activation, cell chemotaxis, leukocyte chemotaxis, and response to chemokines (Figure 4b). In the cellular component category (CC), the major histocompatibility complex (MHC) class II protein complex and actin binding classifications were enriched in both clinical and chimeric mouse samples (Tables S5 and S6). In the molecular function (MF) ontology, immune receptor activity was included in both clinical and chimeric mouse samples (Tables S7 and S8). These results indicate that T cell activation, which is closely related to immune recognition and inflammatory response, plays an important role after HBV infection in both clinical and chimeric mouse samples.
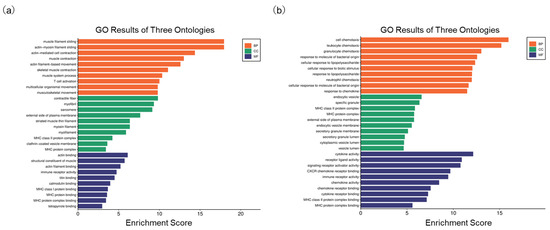
Figure 4.
Go analysis of DEGs. (a) Enriched terms in GO analysis of DEGs in GSE230397; (b) Enriched terms in GO analysis of DEGs in PR0015.
3.3. Th1 and Th2 Responses and Their Effects on Inflammation and Immunity
As the activation of T cells was indicated as an important signal associated with HBV infection, we focused on Th1 and Th2 responses and their effects. A comparison of pathway expression levels revealed a similar regulatory pattern in Th1 and Th2 cell differentiation in both clinical and chimeric mouse samples (Figure 5a,b). However, certain genes exhibited differential expression patterns between the two conditions.
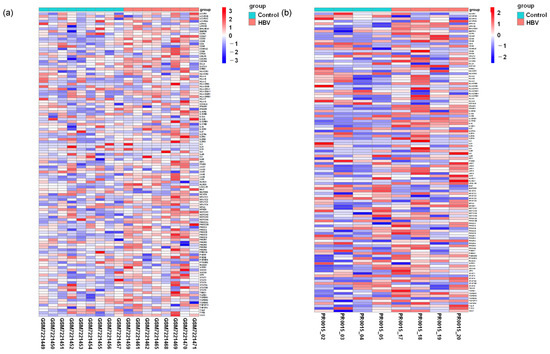
Figure 5.
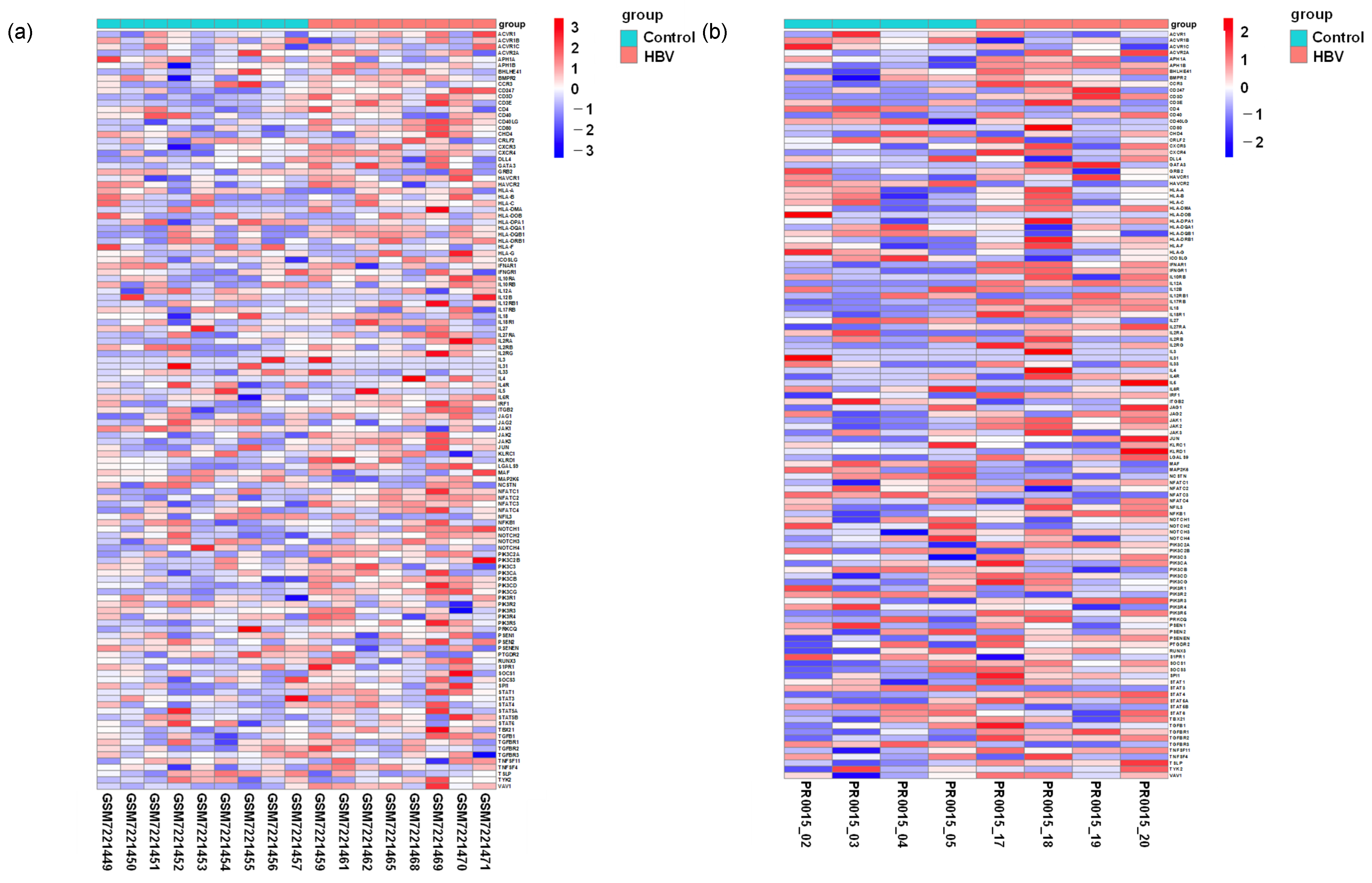
Comparison of the expression level of genes associated with Th1 and Th2 cell differentiation pathways. (a) Heatmap depicting genes associated with the Th1/Th2 activation pathway in healthy individuals relative to HBV carriers. (b) Heatmap showing genes associated with Th1/Th2 activation pathway-associated genes in uninfected control relative to HBV-infected mice.
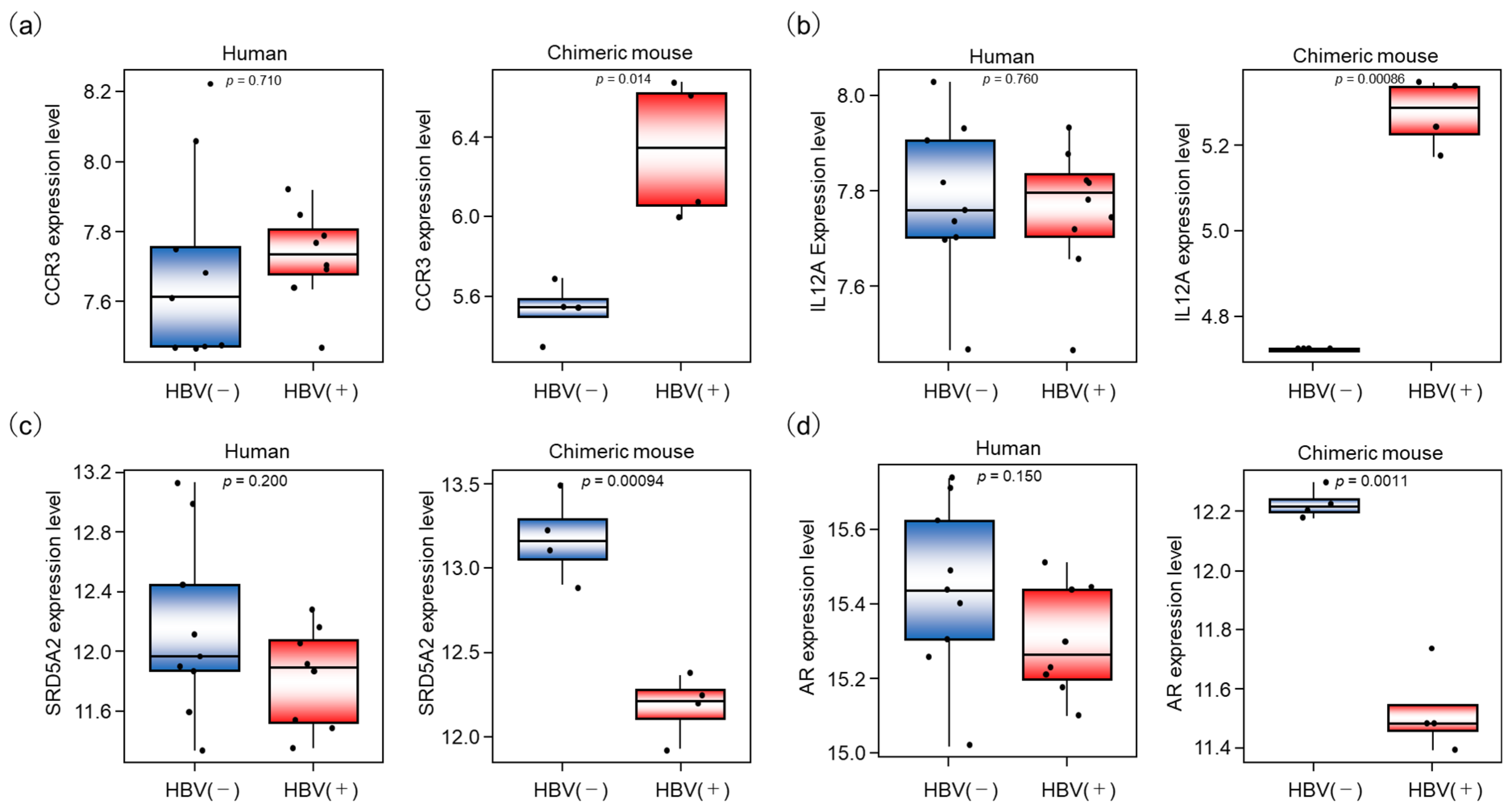
To further assess these differences, we compared the expression of each gene in the clinical and chimeric mouse samples. Although CCR3 and IL12A were not significantly different in the clinical samples, both genes were significantly upregulated in the chimeric mouse livers following HBV infection (Table 1 and Figure 6b). On the other hand, the genes SRDSA2 and AR, which negatively regulate IL12A transcription, were downregulated in the chimeric mouse livers following HBV infection but were not altered in the clinical samples (Figure 6c,d).

Table 1.
Comparison of differential gene expression in genes in the Th1/Th2 activation pathway following HBV infection in human and chimeric mice.
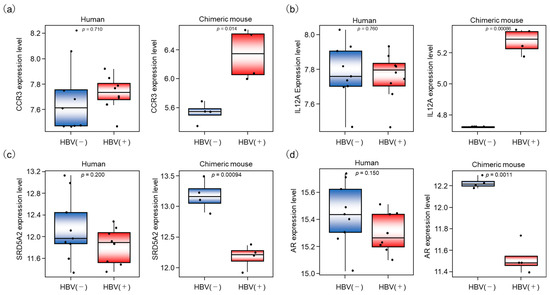
Figure 6.
Differential gene expressions associated with HBV infection in human and chimeric mouse livers. (a–d) The expression of 4 genes, CCR3, IL12A, SRD5A2, and AR, was compared between HBV-infected and uninfected livers in human and chimeric mouse livers, respectively.
4. Discussion
We previously established a human hepatocyte chimeric mouse model capable of supporting chronic hepatitis B and C virus infection [17,18,19]. This model allows us to analyze viral infection effects and drug responses under immunodeficient conditions. In this study, we used next-generation sequencing to investigate how HBV infection directly influences gene expression in human hepatocytes.
To minimize contamination from mouse tissue, we utilized chimeric mice in which the mouse liver has been predominantly (>90%) repopulated by transplanted human hepatocytes. Human albumin levels in mouse serum served as an indicator of repopulation efficiency. However, tissue samples from the explanted mouse livers still contained small amounts of mouse-derived cells, including interstitial, bile duct, and vascular cells. Due to the high homology between human and mouse genomes, cross-hybridization with mouse mRNA could affect sequencing data. Nonetheless, a previous study validated gene expression analysis in chimeric mice using a functional genomics approach [20]. Ideally, HBV-negative serum-injected mice would be used as a negative control, but completely removing HBV particles from the inoculum is challenging. Using healthy human serum as a control is also problematic due to differences in human-derived components. However, as the chimeric mice were derived from SCID mice, the immune response to foreign human components was expected to be minimal. For these reasons, mice that had not been infected with HBV were established as a negative control, although we note these caveats as potential limitations of the study. To ensure that the inoculated HBV had enough time to spread widely throughout the mouse liver, we sacrificed the chimeric mice 8 weeks after HBV inoculation, at which time the serum HBV DNA level had reached a plateau, indicating that all human hepatocytes had likely been infected by that point.
In the previous study, we demonstrated that the chimeric mouse model is useful for studying HBV virology as well as for evaluating the influence of HBV infection on intracellular signaling under immunodeficient conditions [19]. However, as noted above, it was unclear whether gene expression profiles obtained from the severely immunodeficient HBV-infected chimeric mouse livers would accurately reflect HBV- HBV-associated gene expression changes in human livers. Therefore, we decided to compare gene expression profiles from both human and chimeric mouse livers. To minimize the influence of active host immune responses to HBV infection, we selected a GEO dataset containing gene expression profiles obtained from HBV genotype C carriers in an immunotolerant state, as well as from healthy uninfected individuals that could serve as an appropriate control. Thus, we could demonstrate that similar pathways, such as the p53, T cell activation, inflammation mediated by chemokine, and cytokine signaling pathways, clustered as significantly enriched pathways associated with HBV infection (Figure 3a,b).
On the other hand, we also revealed that expression levels of several genes differed in the response to HBV infection in human livers compared to chimeric mouse livers. Although DEGs enriched in mediating T cell activation and immune response were upregulated after HBV infection, genes involved in inflammatory signaling were extracted in human samples, while genes enriched in immune responses were extracted in chimeric mouse samples. The genes associated with the Th1 and Th2 activation pathways were upregulated in both human and chimeric mouse livers; however, the alteration of IL12A expression after HBV infection differed between human and chimeric mouse livers (Figure 6b).
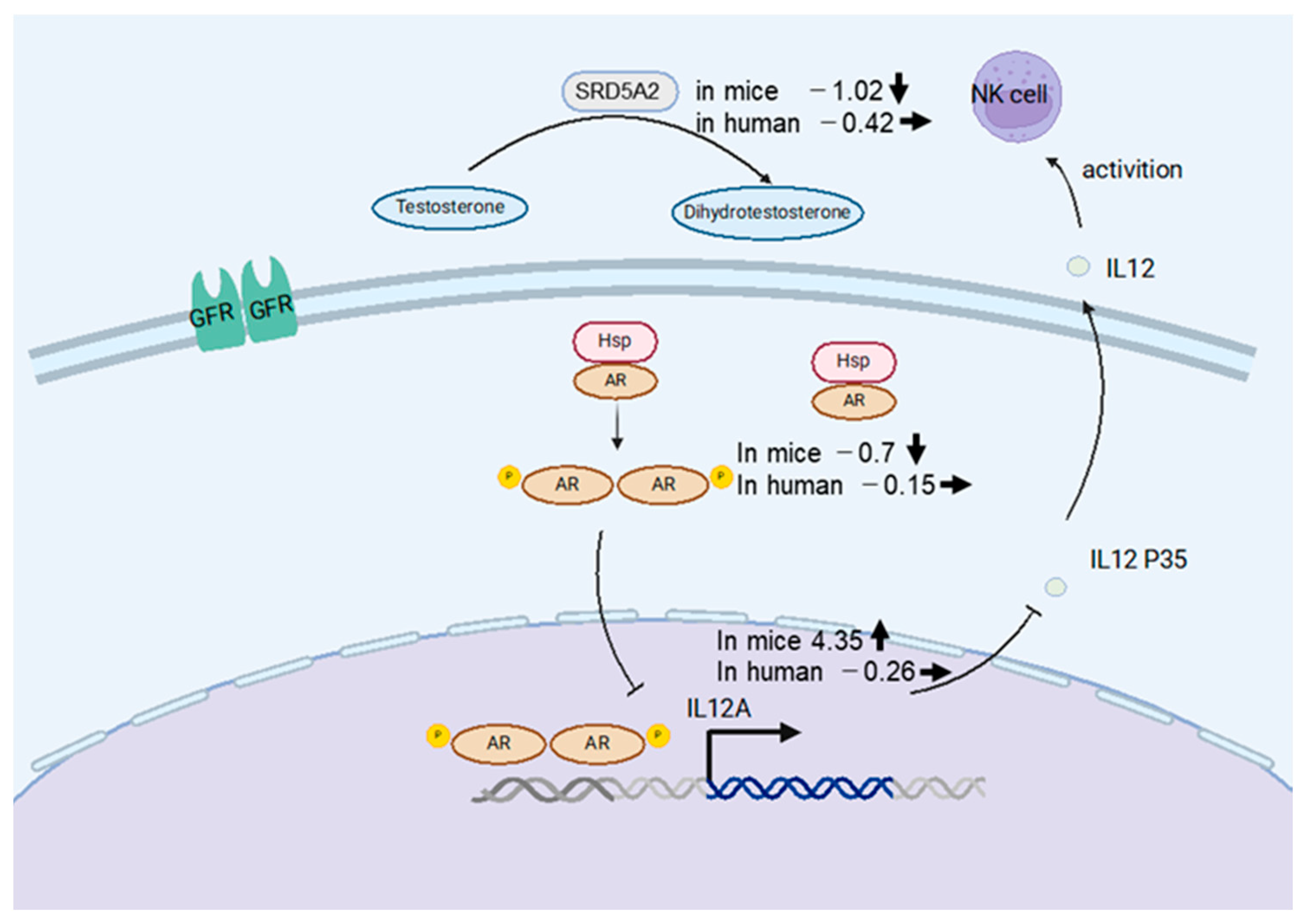
IL12A, a member of the IL12 family, plays an important role in eradicating tumor cells and virus-infected cells via activation of NK cells and cytotoxic T cells [21]. To identify the mechanism of IL12A regulation, we searched the hTFtarget database for upstream regulatory factors. The transcription factors AR and Steroid 5-alpha-reductase type 2 (SRD5A2) were identified as regulators of IL12A transcription. Previous studies have shown that AR suppresses IL12A expression by directly binding to its promoter region, thereby inhibiting NK cell cytotoxicity against HCC [22] (Figure 7). As shown in Figure 6b,d, IL12A was significantly upregulated in the chimeric mouse livers by HBV infection, while AR was negatively regulated. On the contrary, IL12A expression was not altered in HBV-infected human livers relative to livers from healthy volunteers. These results indicate that immune tolerance to HBV was induced not only in immune cells but also in human hepatocytes in asymptomatic HBV carriers, whereas human hepatocytes in chimeric mouse livers are not in an immune-tolerant state and may react to eradicate HBV from hepatocytes. Furthermore, when we searched for upstream regulators of AR, we found that SRD5A2 may positively regulate AR expression. SRD5A2 is a crucial enzyme in androgen metabolism, converting testosterone into the more potent dihydrotestosterone (DHT) [22,23]. In the present study, SRD5A2 expression was downregulated by HBV infection in the chimeric mouse livers, resulting in the downregulation of AR expression.
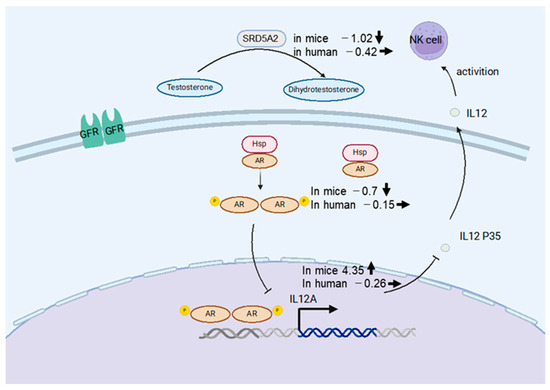
Figure 7.
Schematic of the NK cell activation mechanism in human hepatocytes (Created with Biorender). SRD5A2 converts testosterone into dihydrotestosterone (DHT), which is released into hepatocytes, leading to AR phosphorylation and activation of AR-mediated transcription, thereby promoting NK cell secretion. The numbers written at the left of gene names represent fold changes by HBV infection, and bold arrows represent the direction of alterations in gene expression. Thin lines indicate activating or inhibitory interaction.
Molecular-targeted anti-cancer drugs, such as tyrosine kinase inhibitors and immune checkpoint inhibitors, have been developed to treat unresectable HCC, but no drug is currently available that targets the highlighted genes AR, IL12A, and SRD5A2. Only AR has been reported to be associated with hepatocarcinogenesis via increased cellular oxidative stress and DNA damage and has been considered as a new therapeutic target [24]. However, in the present study, we did not observe AR upregulation in either HBV-infected human livers or HBV-infected chimeric mouse livers. Therefore, we suggest that hepatocarcinogenesis promoted by HBV infection might be activated by alteration of genes other than AR.
We acknowledge several limitations in this study. First, although we integrated a number of bioinformatics analyses and compared gene expression levels between human and chimeric mouse livers, the amount of experimental data available was small, and we could not verify the gene expression profiles sufficiently. Secondly, the biological activity of the identified genes and the connections among these genes have not been fully studied. Previous studies have indicated that IL12 stimulates NK cells, inducing the production of IFN- γ [25] and leading to the optimal generation of T helper 1 (Th1) cells [23]. Although we observed the upregulation of IL12A expression in the chimeric mouse livers, we could not demonstrate an association between IL12A expression and NK cell or cytotoxic T cell activation. Thirdly, AR is the male hormone receptor known as a major oncogenic driver of prostate cancer [26], but we were unable to examine differences in AR expression with respect to gender. Finally, we performed gene expression analysis using human and chimeric mouse livers infected with HBV genotype C. As we have demonstrated in a previous study, gene expression profiles differ in several ways in response to HBV genotype A and C infection [13]. Therefore, analysis based on infection with other HBV genotypes might yield different results, suggesting that further studies are needed.
5. Conclusions
In the present study, we demonstrated several differences in intracellular responses after HBV infection in human and chimeric mouse livers. Our study revealed that immune responses after HBV infection were similar in human and chimeric mouse livers, especially with respect to the Th1 and Th2 activation pathways. Although current antiviral therapies involving long-term treatment with nucleotide or nucleoside analogs can strongly suppress HBV replication and hepatic inflammation, it is difficult to fully eliminate HBV from hepatocytes, resulting in the possibility of relapses if the antiviral therapy is discontinued. Therefore, effectively suppressing hepatocarcinogenesis through complete elimination of HBV will require the development of novel drugs to activate and target host immune responses. A deeper investigation of gene expression profiles, as in the present study, could help to clarify the mechanism by which immune tolerance is induced following HBV infection and inflammation, leading to the discovery of novel drugs capable of eradicating HBV from hepatocytes using therapeutic immune targets.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/livers5020018/s1, Table S1: DEGs in the GSE230397 datasets, Table S2: DEGs in the PR0015 datasets, Table S3: KEGG analysis of DEGs in GSE230397 datasets, Table S4: KEGG analysis of DEGs in PR0015 datasets, Table S5: Go analysis of DEGs in the cellular component category (CC) using GSR230397 datasets, Table S6: Go analysis of DEGs in the cellular component category (CC) using PR0015 datasets, Table S7: Go analysis of DEGs in the molecular function (MF) ontology using GSR230397 datasets, Table S8: Go analysis of DEGs in the molecular function (MF) ontology using PR0015 datasets.
Author Contributions
Concept and design: H.B. and M.T.; experiments and procedures: H.B., S.M., H.F., A.O., E.M., C.N.H. and M.T.; project administration: S.U., Y.F., H.F., A.O., E.M., T.K., D.M., M.T. and S.O.; resources: M.T.; writing of the article: H.B., M.T. and C.N.H. All authors have read and agreed to the published version of the manuscript.
Funding
Japan Agency for Medical Research and Development (AMED; Grant number: JP24fk0210143s0501). Japanese Ministry of Education, Culture, Sports, Science, and Technology (MEXT; Grant number: 24K11112).
Institutional Review Board Statement
The experimental protocol meets the ethical guidelines of the Declaration of Helsinki and was approved by the Hiroshima University Ethical Committee (Approval ID: E2015-0103).
Informed Consent Statement
Informed consent was obtained from all the subjects involved in this study.
Data Availability Statement
Absence of shared data.
Conflicts of Interest
The authors declare no conflict of interest.
References
- World Health Organization. Hepatitis B. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-b (accessed on 30 January 2025.).
- Sarin, S.K.; Kumar, M.; Lau, G.K.; Abbas, Z.; Chan, H.L.; Chen, C.J.; Chen, D.S.; Chen, H.L.; Chen, P.J.; Chien, R.N.; et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: A 2015 update. Hepatol. Int. 2016, 10, 1–98. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J. Hepatol. 2017, 67, 370–398. [Google Scholar] [CrossRef] [PubMed]
- Drafting Committee for Hepatitis Management Guidelines, the Japan Society of Hepatology. Japan Society of Hepatology Guidelines for the Management of Hepatitis B Virus Infection: 2019 update. Hepatol. Res. 2020, 50, 892–923. [Google Scholar] [CrossRef] [PubMed]
- Terrault, N.A.; Lok, A.S.F.; McMahon, B.J.; Chang, K.M.; Hwang, J.P.; Jonas, M.M.; Brown, R.S., Jr.; Bzowej, N.H.; Wong, J.B. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 2018, 67, 1560–1599. [Google Scholar] [CrossRef]
- Mercer, D.F.; Schiller, D.E.; Elliott, J.F.; Douglas, D.N.; Hao, C.; Rinfret, A.; Addison, W.R.; Fischer, K.P.; Churchill, T.A.; Lakey, J.R.; et al. Hepatitis C virus replication in mice with chimeric human livers. Nat. Med. 2001, 7, 927–933. [Google Scholar] [CrossRef]
- Tateno, C.; Yoshizane, Y.; Saito, N.; Kataoka, M.; Utoh, R.; Yamasaki, C.; Tachibana, A.; Soeno, Y.; Asahina, K.; Hino, H.; et al. Near completely humanized liver in mice shows human-type metabolic responses to drugs. Am. J. Pathol. 2004, 165, 901–912. [Google Scholar] [CrossRef]
- Bao, H.; Murakami, S.; Tsuge, M.; Uchida, T.; Uchikawa, S.; Fujino, H.; Ono, A.; Murakami, E.; Kawaoka, T.; Miki, D.; et al. Alteration of Gene Expression After Entecavir and Pegylated Interferon Therapy in HBV-Infected Chimeric Mouse Liver. Viruses 2024, 16, 1743. [Google Scholar] [CrossRef]
- Tsuge, M.; Hiraga, N.; Zhang, Y.; Yamashita, M.; Sato, O.; Oka, N.; Shiraishi, K.; Izaki, Y.; Makokha, G.N.; Uchida, T.; et al. Endoplasmic reticulum-mediated induction of interleukin-8 occurs by hepatitis B virus infection and contributes to suppression of interferon responsiveness in human hepatocytes. Virology 2018, 525, 48–61. [Google Scholar] [CrossRef]
- Tsuge, M.; Takahashi, S.; Hiraga, N.; Fujimoto, Y.; Zhang, Y.; Mitsui, F.; Abe, H.; Kawaoka, T.; Imamura, M.; Ochi, H.; et al. Effects of hepatitis B virus infection on the interferon response in immunodeficient human hepatocyte chimeric mice. J. Infect. Dis. 2011, 204, 224–228. [Google Scholar] [CrossRef]
- Uchida, T.; Imamura, M.; Hayes, C.N.; Hiraga, N.; Kan, H.; Tsuge, M.; Abe-Chayama, H.; Zhang, Y.; Makokha, G.N.; Aikata, H.; et al. Persistent Loss of Hepatitis B Virus Markers in Serum without Cellular Immunity by Combination of Peginterferon and Entecavir Therapy in Humanized Mice. Antimicrob. Agents Chemother. 2017, 61, 10–1128. [Google Scholar] [CrossRef]
- Takeuchi, Y.; Tsuge, M.; Tsushima, K.; Suehiro, Y.; Fujino, H.; Ono, A.; Yamauchi, M.; Makokha, G.N.; Nakahara, T.; Murakami, E.; et al. Signal Activation of Hepatitis B Virus-Related Hepatocarcinogenesis by Up-regulation of SUV39h1. J. Infect. Dis. 2020, 222, 2061–2070. [Google Scholar] [CrossRef]
- Tsushima, K.; Tsuge, M.; Hiraga, N.; Uchida, T.; Murakami, E.; Makokha, G.N.; Kurihara, M.; Nomura, M.; Hiyama, Y.; Fujino, H.; et al. Comparison of intracellular responses between HBV genotype A and C infection in human hepatocyte chimeric mice. J. Gastroenterol. 2019, 54, 650–659. [Google Scholar] [CrossRef]
- Montanari, N.R.; Ramirez, R.; Aggarwal, A.; van Buuren, N.; Doukas, M.; Moon, C.; Turner, S.; Diehl, L.; Li, L.; Debes, J.D.; et al. Multi-parametric analysis of human livers reveals variation in intrahepatic inflammation across phases of chronic hepatitis B infection. J. Hepatol. 2022, 77, 332–343. [Google Scholar] [CrossRef]
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.; Dai, Z.; Feng, T.; Zhou, L.; Tang, W.; Zhan, L.; et al. clusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innovation 2021, 2, 100141. [Google Scholar] [CrossRef]
- Tang, D.C.M.; Huang, X.; Zhang, G.; Zeng, L.; Zhang, G.; Wu, S.; Wang, Y. SRplot: A free online platform for data visualization and graphing. PLoS ONE 2023, 18, e0294236. [Google Scholar] [CrossRef]
- Hiraga, N.; Imamura, M.; Tsuge, M.; Noguchi, C.; Takahashi, S.; Iwao, E.; Fujimoto, Y.; Abe, H.; Maekawa, T.; Ochi, H.; et al. Infection of human hepatocyte chimeric mouse with genetically engineered hepatitis C virus and its susceptibility to interferon. FEBS Lett. 2007, 581, 1983–1987. [Google Scholar] [CrossRef]
- Kimura, T.; Imamura, M.; Hiraga, N.; Hatakeyama, T.; Miki, D.; Noguchi, C.; Mori, N.; Tsuge, M.; Takahashi, S.; Fujimoto, Y.; et al. Establishment of an infectious genotype 1b hepatitis C virus clone in human hepatocyte chimeric mice. J. Gen. Virol. 2008, 89 Pt 9, 2108–2113. [Google Scholar] [CrossRef]
- Tsuge, M.; Hiraga, N.; Takaishi, H.; Noguchi, C.; Oga, H.; Imamura, M.; Takahashi, S.; Iwao, E.; Fujimoto, Y.; Ochi, H.; et al. Infection of human hepatocyte chimeric mouse with genetically engineered hepatitis B virus. Hepatology 2005, 42, 1046–1054. [Google Scholar] [CrossRef]
- Walters, K.A.; Joyce, M.A.; Thompson, J.C.; Proll, S.; Wallace, J.; Smith, M.W.; Furlong, J.; Tyrrell, D.L.; Katze, M.G. Application of functional genomics to the chimeric mouse model of HCV infection: Optimization of microarray protocols and genomics analysis. Virol. J. 2006, 3, 37. [Google Scholar] [CrossRef][Green Version]
- Orange, J.S.; Biron, C.A. Characterization of early IL-12, IFN-alphabeta, and TNF effects on antiviral state and NK cell responses during murine cytomegalovirus infection. J. Immunol. 1996, 156, 4746–4756. [Google Scholar] [CrossRef]
- Shi, L.; Lin, H.; Li, G.; Jin, R.A.; Xu, J.; Sun, Y.; Ma, W.L.; Yeh, S.; Cai, X.; Chang, C. Targeting Androgen Receptor (AR)→IL12A Signal Enhances Efficacy of Sorafenib plus NK Cells Immunotherapy to Better Suppress HCC Progression. Mol. Cancer Ther. 2016, 15, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Heufler, C.; Koch, F.; Stanzl, U.; Topar, G.; Wysocka, M.; Trinchieri, G.; Enk, A.; Steinman, R.M.; Romani, N.; Schuler, G. Interleukin-12 is produced by dendritic cells and mediates T helper 1 development as well as interferon-gamma production by T helper 1 cells. Eur. J. Immunol. 1996, 26, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.L.; Hsu, C.L.; Wu, M.H.; Wu, C.T.; Chang, C. Androgen receptor is a new potential therapeutic target for the treatment of hepatocellular carcinoma. Gastroenterology 2008, 135, 947–955. [Google Scholar] [CrossRef]
- Trinchieri, G.; Gerosa, F. Immunoregulation by interleukin-12. J. Leukoc. Biol. 1996, 59, 505–511. [Google Scholar] [CrossRef]
- Zhang, H.; Spencer, K.; Burley, S.K.; Zheng, X.F.S. Toward improving androgen receptor-targeted therapies in male-dominant hepatocellular carcinoma. Drug Discov. Today 2021, 26, 1539–1546. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).