Abstract
Thiosemicarbazones are a versatile type of organic compounds which are known for their coordination ability with different types of analytes, due to the presence of sulfur and nitrogen heteroatoms. Therefore, the functionalization of thiosemicarbazones with heterocyclic moieties can be a promising route to developing new optical chemosensors. Tributyltin (TBT) is an antifouling component of paints that is acutely toxic to aquatic environments, being quickly absorbed by microorganisms and causing problems such as imposex. Herein, the synthesis of a novel heterocyclic thiosemicarbazone, functionalized with a quinoline moiety, is reported to assess the potential of this recognition moiety for TBT optical chemosensing. A preliminary chemosensory study in acetonitrile solution was performed showing that 50 equivalents of TBT were needed to induce a change of color from colorless to yellow. Spectrophotometric titration was performed to assess the concentration of TBT necessary for a maximum optical signal, revealing that 100 equivalents of TBT were necessary to reach maximum absorbance, although it was able to respond with a detectable color change to a TBT concentration as low as 10 µM.
1. Introduction
Optical chemosensors are pursued worldwide due to advantages over other types of sensors such as low cost and selectivity []. Within this field, colorimetric chemosensors show the possibility of “naked eye” detection [,], which is extremely relevant and advantageous to reach a preliminary qualitative detection of the desired analyte. Besides, this type of sensor may also allow fast and simple quantitative detection []. Colorimetric optical chemosensors are often based on organic chromophores, that can be modified toward the detection of a particular analyte.
Thiourea derivatives, particularly thiosemicarbazones, are molecules of particular interest in the sensing field due to the conjugation of heteroatoms with electronic properties that can be tuned by the presence of electron donor/withdrawing groups [,,]. The combination of this core with π-conjugated bridges can yield selective and sensitive optical chemosensors for different ions. Particularly, the functionalization of a thiosemicarbazone with a heterocyclic ring such as quinoline can be interesting for the development of colorimetric chemosensors, due to the combination of the coordination ability of sulfur and nitrogen heteroatoms from the thiosemicarbazone with a π-conjugated heterocyclic group.
Tributyltin (TBT) is an antifouling component of paints used on ships, vessels, and submersed structures to avoid biofouling []. This compound is a biocide that prevents accumulation of microorganisms on the abovementioned structures. However, it was found that TBT is extremely toxic to aquatic organisms such as bacteria, fish, or algae [,,,,,]. TBT is quickly absorbed by microorganisms and induces long-term problems in aquatic living beings such as imposex i.e., superimposition of male sexual characteristics on female marine gastropods [,]. The bioaccumulation of TBT in gastropods results in higher testosterone levels causing an endocrine-disrupting effect [].
Currently, TBT monitoring is only detected and quantified by sampling and analysis using chromatographic techniques, such as Liquid Chromatography-Mass Spectrometry (LC-MS) and Gas Chromatography-Mass Spectrometry (GC-MS). However, these techniques require expensive equipment, expert operators, and long procedures with several steps, such as extraction, preconcentration, and derivatization []. Other attempts to monitor TBT include analysis by graphite furnace atomic absorption spectrometry with Zeeman correction (ZGFAAS), nevertheless this method shares the same disadvantages []. Therefore, TBT optical chemosensors would be a huge and interesting advantage to building devices capable of in situ TBT detection.
This work reports, for the first time, the synthesis of a novel heterocyclic thiosemicarbazone, functionalized with a quinoline moiety, to assess the potential of this recognition moiety for TBT optical chemosensing. The new compound was obtained by a condensation reaction, between quinoline-2-carboxyaldehyde and N-phenylhydrazinecarbothioamide, and its chemosensory ability was studied in the presence of TBT in acetonitrile solution. A spectrophotometric titration was also performed to assess the concentration of TBT necessary for a maximum optical signal.
2. Experimental Section
2.1. Methods and Materials
Melting points were measured on a Stuart SMP3 melting point apparatus (Barloworld Scientific Ltd, Staffordshire, UK). TLC analysis was carried out on 0.20 mm thick precoated silica plates (Macherey-Nagel), and spots were observed under UV light on a CN-15 camera (Vilber Lourmat, Marne-la-Vallée, France). UV-Vis absorption spectra (200–700 nm) were obtained using Shimadzu UV/3101PC spectrophotometer (Shimadzu Europa GmbH, Duisburg, Germany). Nuclear Magnetic Resonance (NMR) spectra were obtained on a Bruker Avance III 400 (Bruker Corporation, Massachusetts, USA) at an operating frequency of 400 MHz for 1H using the solvent peak as internal reference at 25 °C. All chemical shifts are given in ppm using δH Me4Si = 0 ppm as reference and J values are given in Hz. Assignments were supported by spin decoupling-double resonance and bidimensional heteronuclear correlation techniques. All commercial reagents and solvents were used as received.
2.2. Synthesis of N-phenyl-2-(quinolin-2-ylmethylene)hydrazine-1-carbothioamide 1
Quinoline-2-carboxaldehyde 2 and N-phenylhydrazinecarbothioamide 3 in equal amounts (0.318 mmol) were dissolved in 10 mL of MeOH at room temperature. The reaction mixture was stirred for 8 h, and then for 6 h more at 60 °C. After cooling, the precipitated compound was filtered, dried, and obtained as a brown solid in 17% yield (0.017 g).
Mp: 160 °C. 1H NMR (DMSO-d6, 400MHz): δ = 7.24 (t, J = 7.6 Hz, 1H, H4), 7.40 (dt, J = 7.6 and 1.6 Hz, 2H, H3 + H5), 7.55 (dd, J = 8.0 and 0.4 Hz, 2H, H2 + H6), 7.62 (dt, J = 7.2 and 1.2 Hz, 1H, H6’), 7.78 (dt, J = 7.2 and 1.2 Hz, 1H, H7’), 7.99 (dd, J = 8.0 and 0.4 Hz, 1H, H5’), 8.02 (d, J = 8.4 Hz, 1H, H8’), 8.33 (s, 1H, N=CH), 8.38 (d, J = 8.8 Hz, 1H, H4′), 8.59 (d, J = 8.4 Hz, 1H, H3′), 10.38 (s, 1H, NH-C=S), 12.17 (s, 1H, NH-N) ppm.
2.3. Preliminary Chemosensory Studies
For the preliminary test, 50 equivalents of TBT (50 µL, 1 × 10−1 M) were added to an acetonitrile (ACN) solution of the new thiosemicarbazone 1 (1 mL, 1 × 10−4 M). The color/fluorescence changes were assessed by visual inspection and in a UV-vis chamber under ultraviolet light at 312 nm. Spectrophotometric titration was performed with sequential addition of TBT (10−2 M) to an ACN solution of compound 1 (3 mL, 1 × 10−5 M). Absorbance spectra were collected until a plateau was reached.
3. Results and Discussion
3.1. Synthesis of N-phenyl-2-(quinolin-2-ylmethylene)hydrazine-1-carbothioamide 1
The synthesis of the new thiosemicarbazone 1 was performed with a mixture of quinoline-2-carboxaldehyde 2 and N-phenylhydrazinecarbothioamide 3 in equal amounts (0.318 mmol). The two precursors were dissolved in MeOH (10 mL) and stirred at room temperature for 8 h. However, TLC analysis showed that the reaction did not progress, so it was stirred for further 6 h at 60 °C. After this time, the pure product 1 was obtained as a brown solid, which was filtered from the cold reaction mixture in 17% yield (Scheme 1). The novel thiosemicarbazone 1 was characterized by 1H NMR.
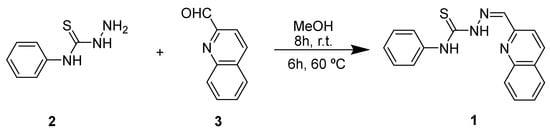
Scheme 1.
Synthesis of thiosemocarbazone 1.
1H NMR spectrum shows the characteristic signals for NH protons at 12.17 and 10.38 ppm. Imine N=CH proton appears as a singlet at 8.33 ppm. Phenyl group protons appear at a smaller chemical shift at 7.24 (H4), 7.40 (H3 and H5), and 7.55 (H2 and H6) ppm, while quinoline protons show higher chemical shifts with H6’ at 7.62 ppm, H7’ at 7.78 ppm, H5’ at 7.99 ppm, H8’ at 8.02 ppm, H4’ at 8.38 ppm, and H3’ at 8.59 ppm.
3.2. Studies of the Chemosensory Ability of Thiosemocarbazone 1 for TBT
The chemosensory ability of the new thiosemicarbazone 1 was studied in the presence of TBT. To an ACN solution of the compound (1 mL, 10−4 M), 50 equivalents of TBT (50 µL, 10−1 M) were added. The optical response was analyzed by visual inspection and in a UV-vis chamber under ultraviolet light at 312 nm. A color change from colorless to yellow was observed (see Figure 1). No changes in fluorescence were found.

Figure 1.
Preliminary TBT chemosensory test for the new thiosemicarbazone 1: left—compound 1; right—compound 1 + 50 equivalents of TBT.
A spectrophotometric titration was performed to assess the number of equivalents necessary to reach the maximum optical signal (see Figure 2). A sequential addition of a TBT solution (10−2 M) to an ACN solution of the compound (10−5 M) was conducted and the absorbance spectra were collected. It was observed that 100 equivalents were necessary to reach the absorbance plateau. However, the addition of 1 equivalent (10 µM) was enough to detect a change in the optical signal, showing this compound has a large range of concentrations in which TBT can be detected.
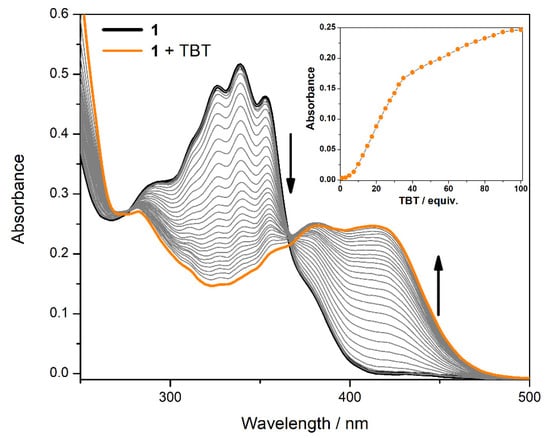
Figure 2.
Spectrophotometric titration of thiosemicarbazone 1 with TBT.
4. Conclusions
In this work, a new thiosemicarbazone was successfully synthesized by a condensation reaction between quinoline-2-carboxyaldehyde and N-phenylhydrazinecarbothioamide. The compound was tested in the presence of the biocide TBT and the preliminary test revealed a color change from colorless to yellow. A spectrophotometric titration was performed to assess the number of equivalents necessary to reach the plateau of the absorbance spectra. It was found that 100 equivalents of TBT were necessary to reach the maximum optical signal. However, this colorimetric probe was able to detect a change in the optical signal with a concentration as low as 10 µM. The wide range of concentrations that can result in optical changes, allows to conclude that compound 1 shows potential to be used as a TBT chemosensor.
Author Contributions
Conceptualization, S.P.G.C., M.M.M.R.; methodology, R.P.C.L.S., S.P.G.C., M.M.M.R.; formal analysis, R.P.C.L.S., S.P.G.C., M.M.M.R.; investigation, R.P.C.L.S.; resources, S.P.G.C., R.B.F., M.M.M.R.; writing—original draft preparation, R.P.C.L.S.; writing—review and editing, S.P.G.C., R.B.F., M.M.M.R.; supervision, S.P.G.C., R.B.F., M.M.M.R.; project administration, S.P.G.C., R.B.F., M.M.M.R.; funding acquisition, S.P.G.C., R.B.F., M.M.M.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Fundação para a Ciência e Tecnologia (FCT) and FEDER (European Fund for Regional Development)-COMPETE-QRENEU for financial support through the Chemistry Research Centre of the University of Minho (Ref. CQ/UM UID/QUI/00686/2020), and a PhD grant to R. P. C. L. Sousa (SFRH/BD/145639/2019).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kaur, B.; Kaur, N.; Kumar, S. Colorimetric metal ion sensors—A comprehensive review of the years 2011–2016. Coord. Chem. Rev. 2018, 358, 13–69. [Google Scholar] [CrossRef]
- Wang, B.; Anslyn, E. Colorimetric sensor design. In Chemosensors: Principles, Strategies, and Applications; Wiley: Hoboken, NJ, USA, 2011; pp. 275–295. [Google Scholar]
- Raposo, M.M.M.; García-Acosta, B.; Ábalos, T.; Calero, P.; Martínez-Máñez, R.; Ros-Lis, J.V.; Soto, J. Synthesis and study of the use of heterocyclic thiosemicarbazones as signaling scaffolding for the recognition of anions. J. Org. Chem. 2010, 75, 2922–2933. [Google Scholar] [CrossRef] [PubMed]
- Santos-Figueroa, L.E.; Moragues, M.E.; Raposo, M.M.M.; Batista, R.M.F.; Costa, S.P.G.; Ferreira, R.C.M.; Sancenón, F.; Martínez-Máñez, R.; Ros-Lis, J.V.; Soto, J. Synthesis and evaluation of thiosemicarbazones functionalized with furyl moieties as new chemosensors for anion recognition. Org. Biomol. Chem. 2012, 10, 7418–7428. [Google Scholar] [CrossRef] [PubMed]
- Santos-Figueroa, L.E.; Moragues, M.E.; Raposo, M.M.M.; Batista, R.M.; Ferreira, R.C.M.; Costa, S.P.; Sancenón, F.; Martínez-Máñez, R.; Soto, J.; Ros-Lis, J.V. Synthesis and evaluation of fluorimetric and colorimetric chemosensors for anions based on (oligo)thienyl-thiosemicarbazones. Tetrahedron 2012, 68, 7179–7186. [Google Scholar] [CrossRef]
- Sousa, R.P.C.L.; Figueira, R.B.; Costa, S.P.G.; Raposo, M.M.M. Optical fiber sensors for biocide monitoring: Examples, transduction materials, and prospects. ACS Sens. 2020, 5, 3678–3709. [Google Scholar] [CrossRef] [PubMed]
- Antizar-Ladislao, B. Environmental levels, toxicity and human exposure to tributyltin (TBT)-contaminated marine environment. A review. Environ. Int. 2008, 34, 292–308. [Google Scholar] [CrossRef] [PubMed]
- Fent, K. Ecotoxicology of organotin compounds. Crit. Rev. Toxicol. 1996, 26, 3–117. [Google Scholar] [CrossRef]
- Cooke, G.M. Toxicology of tributyltin in mammalian animal models. Immunol. Endocr. Metab. Agents Med. Chem. 2006, 6, 63–71. [Google Scholar] [CrossRef]
- Boyer, I.J. Toxicity of dibutyltin, tributyltin and other organotin compounds to humans and to experimental animals. Toxicology 1989, 55, 253–298. [Google Scholar] [CrossRef] [PubMed]
- Tornero, V.; Hanke, G. Chemical contaminants entering the marine environment from sea-based sources: A review with a focus on European seas. Mar. Pollut. Bull. 2016, 112, 17–38. [Google Scholar] [CrossRef] [PubMed]
- Cima, F.; Bragadin, M.; Ballarin, L. Toxic effects of new antifouling compounds on tunicate haemocytes: I. Sea-Nine 211™ and chlorothalonil. Aquat. Toxicol. 2008, 86, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Artifon, V.; Castro, B.; Fillmann, G. Spatiotemporal appraisal of TBT contamination and imposex along a tropical bay (Todos os Santos Bay, Brazil). Environ. Sci. Pollut. Res. 2016, 23, 16047–16055. [Google Scholar] [CrossRef] [PubMed]
- Laranjeiro, F.; Sánchez-Marín, P.; Oliveira, I.B.; Galante-Oliveira, S.; Barroso, C. Fifteen years of imposex and tributyltin pollution monitoring along the Portuguese coast. Environ. Pollut. 2018, 232, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Matthiessen, P.; Gibbs, P.E. Critical appraisal of the evidence for tributyltin-mediated endocrine disruption in mollusks. Environ. Toxicol. Chem. 1998, 17, 37–43. [Google Scholar] [CrossRef]
- Cole, R.F.; Mills, G.A.; Parker, R.; Bolam, T.; Birchenough, A.; Kröger, S.; Fones, G.R. Trends in the analysis and monitoring of organotins in the aquatic environment. Trends Environ. Anal. Chem. 2015, 8, 1–11. [Google Scholar] [CrossRef]
- Oliver, A.L.-S.; Sanz-Landaluze, J.; Muñoz-Olivas, R.; Guinea, J.; Cámara, C. Zebrafish larvae as a model for the evaluation of inorganic arsenic and tributyltin bioconcentration. Water Res. 2011, 45, 6515–6524. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).