Abstract
The data obtained by the authors in the field of carbon cluster chemistry, namely, the catalytic cycloaddition of diazo compounds of modern pharmacologically significant and natural compounds to C60-fullerene under the action of complex Pd–catalysts, are summarized. Cycloaddition reactions of diazoacetates, diazoamides, and diazoketones with C60-fullerene, catalyzed by Pd(acac)2–PPh3–Et3Al, with the selective formation of methano– and pyrazolinofullerenes, are new and promising classes of biologically active derivatives of C60-fullerenes.
1. Introduction
The discovery of fullerenes, a new allotropic form of carbon, is recognized as one of the amazing and most important discoveries in the science of the XX century. The interest in fullerenes and its derivatives is due to the possibility of their wide application in various fields of technology and science. At the same time, fullerene derivatives are of particular practical value for medicine. Thus, according to the literature data, functionally substituted fullerenes have high antioxidant, antitumor, and antiviral properties, and are also of interest as X-ray reducing agents [1,2,3,4,5,6,7,8,9,10,11,12]. At the time of the beginning of our research, one of the popular directions in the synthesis of organic derivatives of C60-fullerene was the Bingel–Hirsch reaction [13,14] based on the cycloaddition of in situ generated α–halocarbanions to C60-fullerene with the formation of corresponding methanofullerenes. As well as an alternative method for obtaining functionally substituted fullerenes, C60 was considered to be the cycloaddition of diaz compounds followed by thermolysis or photolysis of the resulting fullerenopyrazolines [15,16,17]. However, there was practically no information in the literature concerning the selective cycloaddition of diazo compounds of complex structure synthesized on the basis of pharmacologically significant compounds, including natural ones, to C60-fullerene in the presence of metal complex catalysts.
2. Results and Discussion
To date, we have accumulated considerable experience in the catalytic method of selective citation of C60-fullerene under the action of Pd catalysts [18,19,20,21,22]. As a result of the research, effective preparative methods for the synthesis of functionally substituted fullerenes containing known natural and biologically active compounds as a substitute have been proposed.
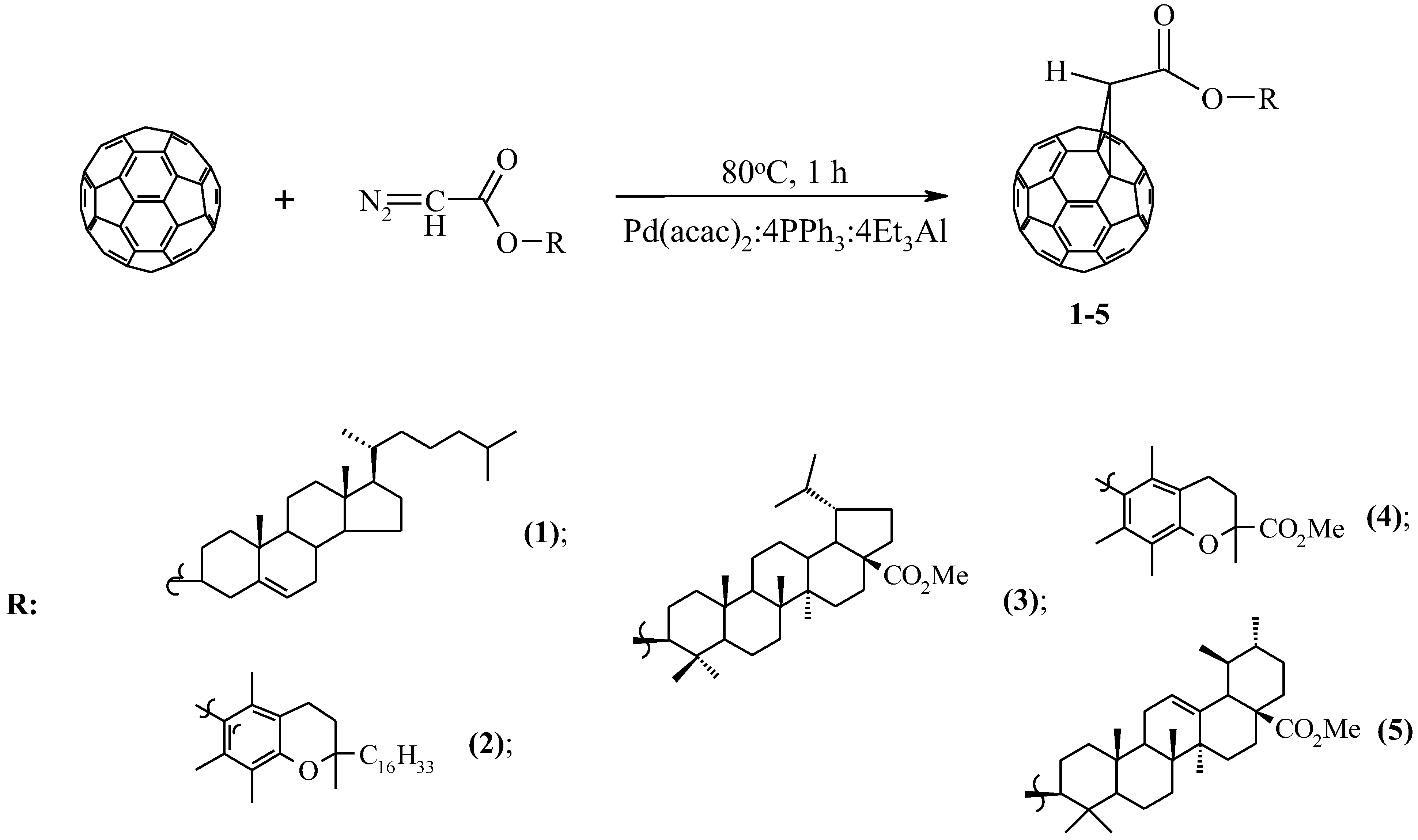
Diazoacetates containing cholesterol in the ester group [23], α–tocopherol, Trolox methyl esters, 20.29–dehydrobetulic, and ursolic acids [24], which have antioxidant, antitumor, and antiviral properties, were used as the initial pharmacological objects of the study. It has been shown that the above diazo compounds interact with C60-fullerene (molar ratio 5:1) in the presence of 20 mol.% of the three–component catalyst Pd(acac)2–PPh3–Et3Al, taken in a ratio of 1:4:4 at 80 °C, for 1 h in 1,2–dichlorobenzene, the corresponding methanofullerenes 1–5 are selectively formed with a yield of 60–75% (Scheme 1).
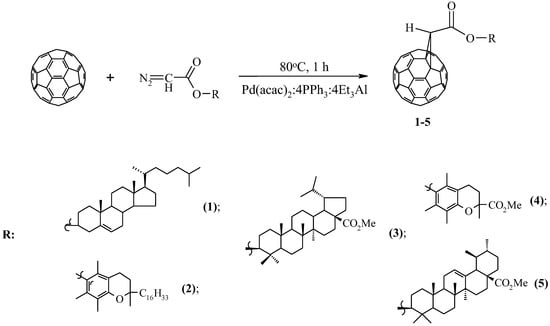
Scheme 1.
Catalytic cycloaddition of diazoacetates to C60-fullerene.
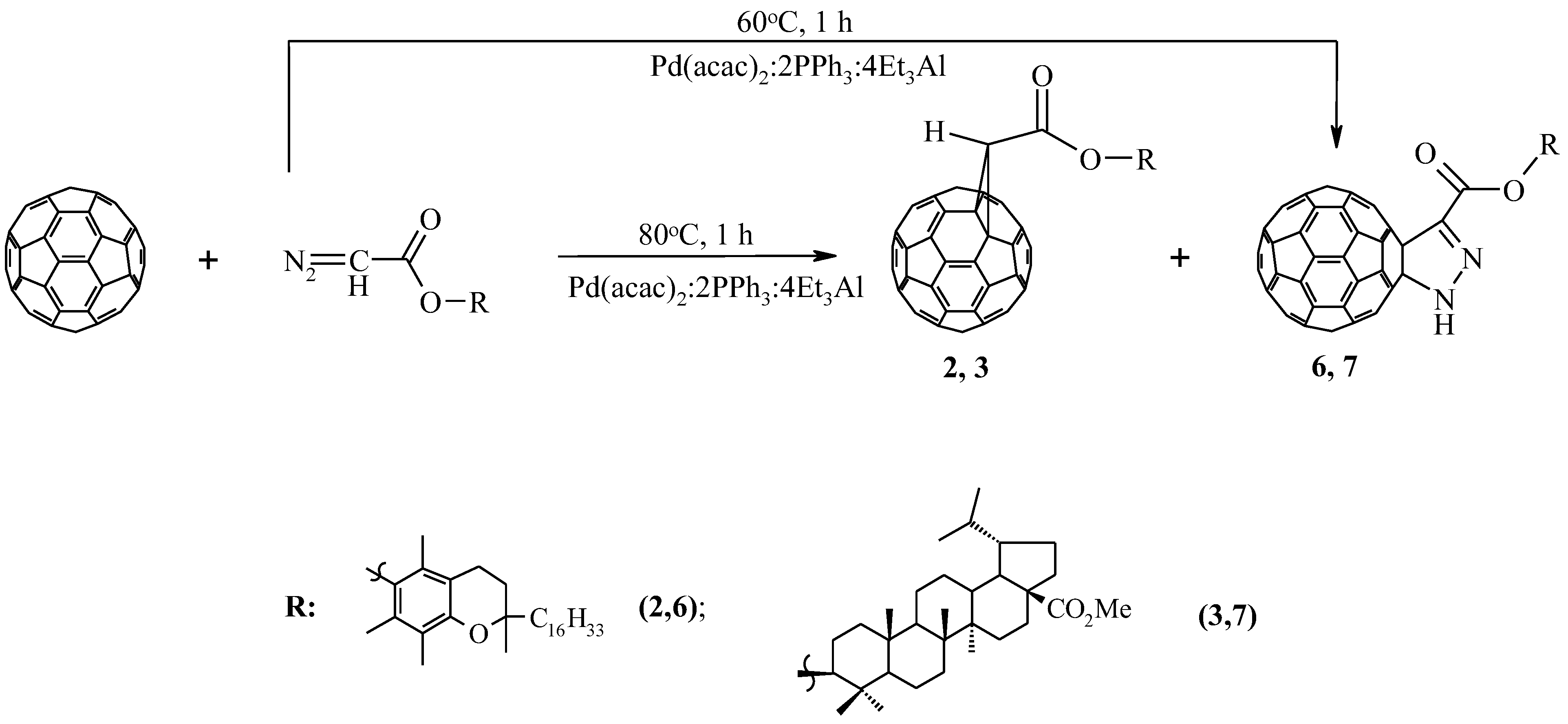
Previously, we found that a change in the ratio of the components of the Pd(acac)2–PPh3–Et3Al catalytic system, taken in a ratio of 1:4:4 to 1:2:4 in the reactions of cyclopreduction of diazoacetates to fullerene C60, leads to the predominant formation of 5,6-open cycloadducts [18,19,20,21,22]. However, in our experiments under these conditions, the interaction of diaz-derived α-tocopherol and methyl ester 20,29–dehydrobetulin with C60-fullerene, instead of the expected homofullerenes, obtained the corresponding methanofullerenes 2,3 and pyrazolinofullerenes 6 and 7 with a total yield of 58 and 65%. When the reaction temperature drops to 60 °C, pyrazollinofullerenes 6 and 7 are predominantly formed with yields of 35 and 41%, respectively (Scheme 2).
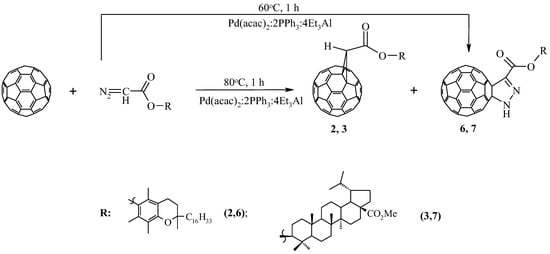
Scheme 2.
Synthesis of [2+3]-cycloadducts of C60-fullerene.
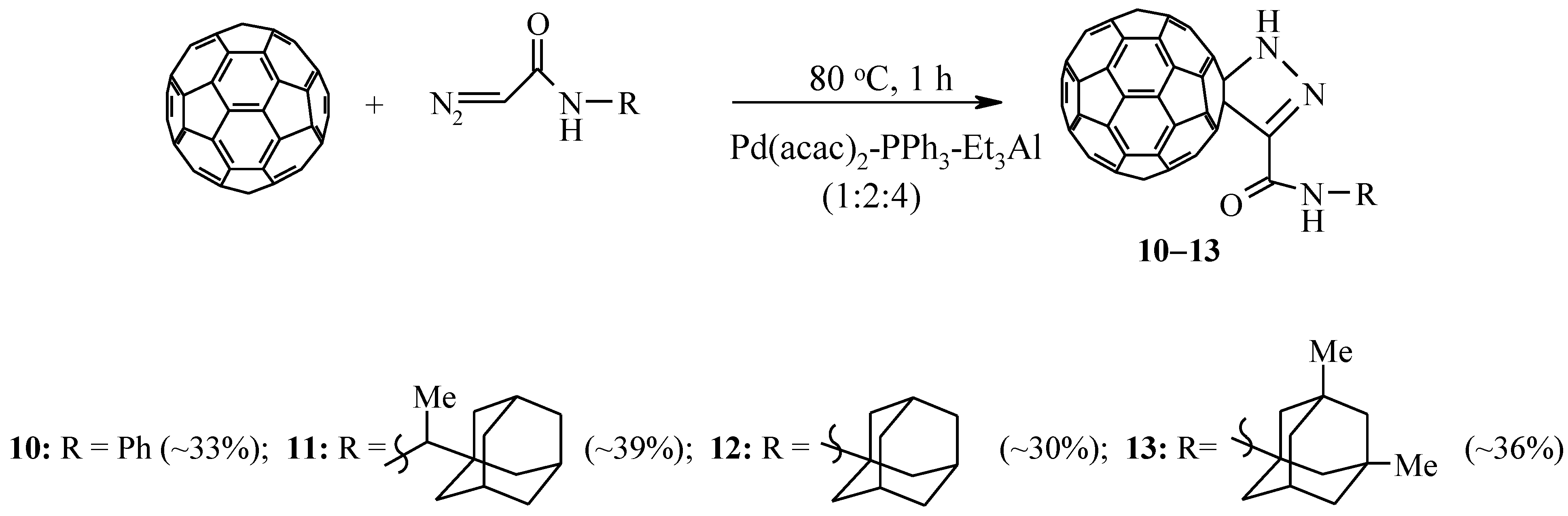
In the development of our research, and also taking into account the lack of information in the literature on the catalytic cycloaddition of diazoamides to fullerenes, we studied the possibility of catalytic cycloaddition to C60-fullerene of diazoamides synthesized on the basis of glycine and cyclohexylamine, aniline, or adamantane–containing amines [25]. As a result of the reactions, we isolated individual pyrazolinofullerenes 9–13. In the absence of a catalyst, this reaction proceeds with the formation of a mixture of methane and stereoisomerichomofullerenes [26] (Scheme 3).
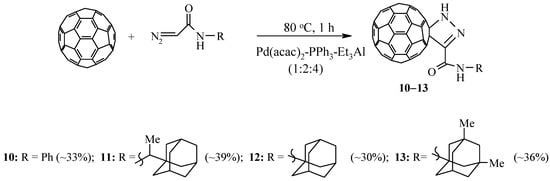
Scheme 3.
Cycloaddition of diazoamides to C60-fullerene.
It is known from the literature that pyrazolinofullerenes thermally transformed into the corresponding methanofullerenes [27,28]. By boiling the synthesized [2+3]–cycloadducts 9–13 in 1,2–dichlorobenzene for 100 h, the formation of the corresponding methanofullerenes not was found.
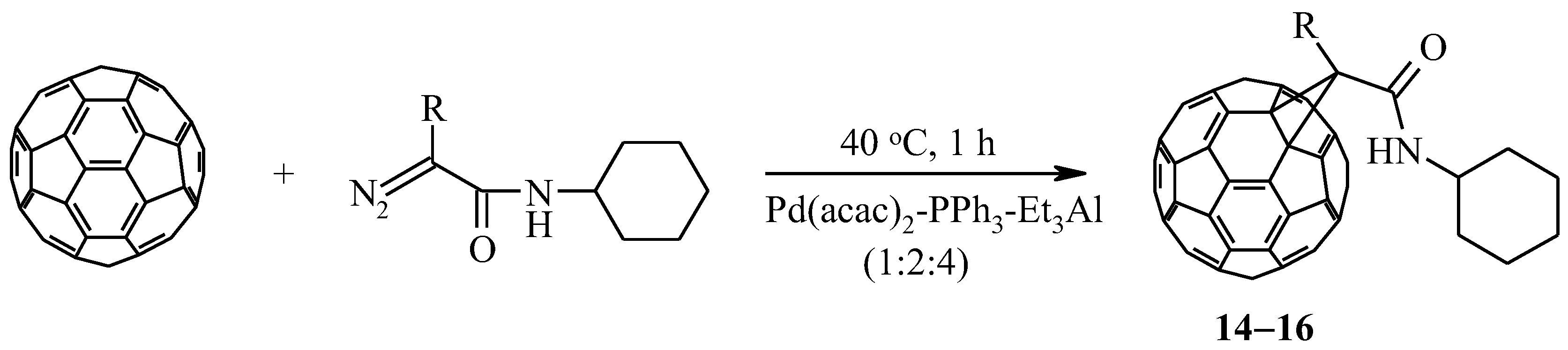
It is known [28] that the presence of a substituent in the α–position to the diazo group of the initial diazocompound leads to the destabilization of the pyrazolinofullerene and the formation of the corresponding [2+1]–cycloadducts. To this end, we have been involved in the reaction catalytic cycloaddition of diazoamides, synthesized from α–alanine, α–leucine, or α–methionine and cyclohexylamine. It was found that these diazoamides react with C60-fullerene in the developed conditions (40 °C, 1 h) in the presence of 20 mol.% of a three–component catalyst Pd(acac)2–PPh3–Et3Al (1:2:4) to form exclusively methanofullerenes 14–16 with a yield of 40–50% (Scheme 4).
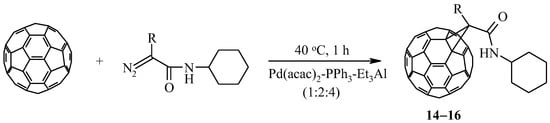
Scheme 4.
Cycloaddition of α–substituted diazoamides to C60-fullerene under the action of the [Pd] complex.
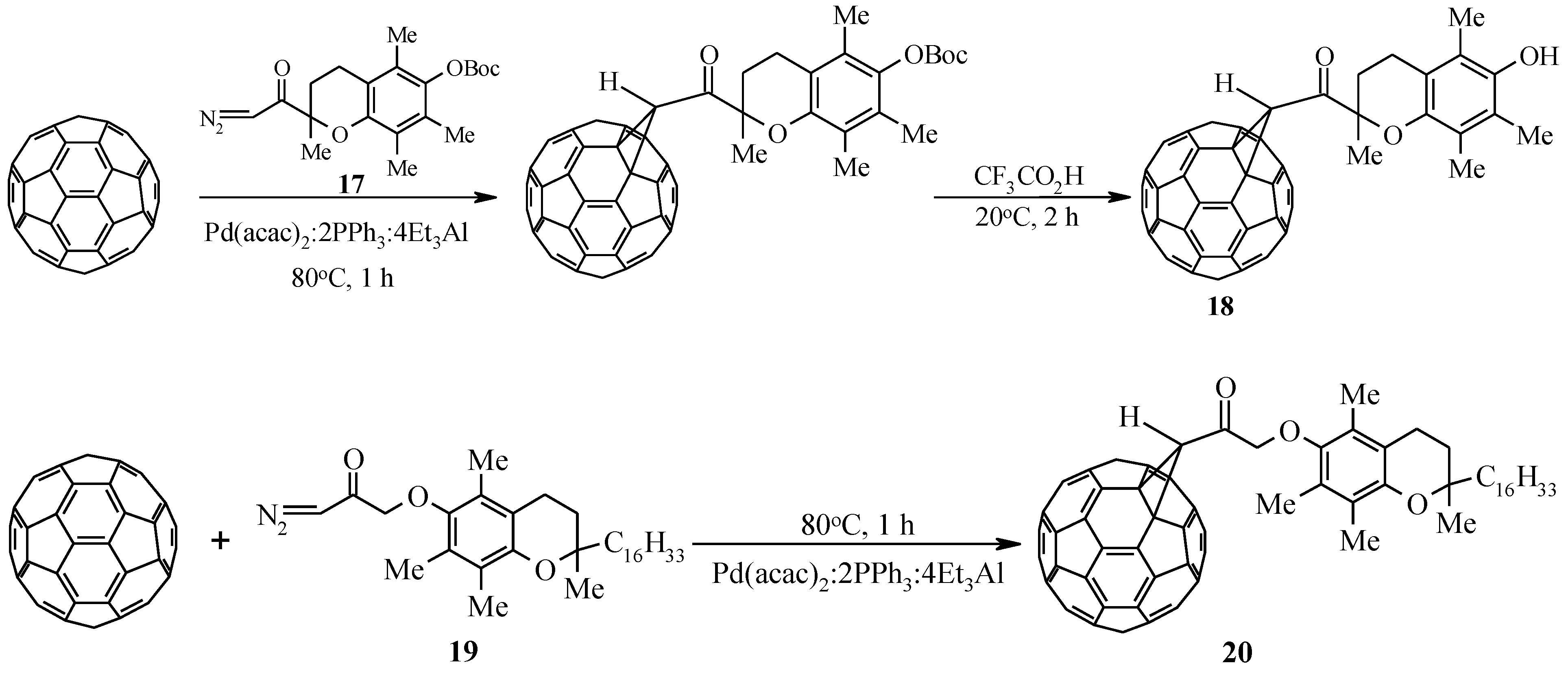
In the development of ongoing research aimed at developing effective methods for covalent binding of C60-fullerene with modern pharmacologically significant compounds, we studied the catalytic cycloaddition to the latter of diazoketones synthesized on the basis of biologically active carboxylic acids. Trolox, a synthetic analogue of α–tocopherol, and tocopherylacetic acid, were chosen as model pharmacons. As a result of the interaction of C60-fullerene with diazoketones 17 and 19, individual methanofullerenes 18 and 20 were obtained in ~40% yield and 37%, respectively (Scheme 5).
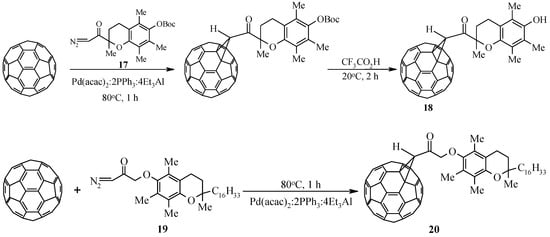
Scheme 5.
Catalytic cycloaddition of diazoketones to C60-fullerene.
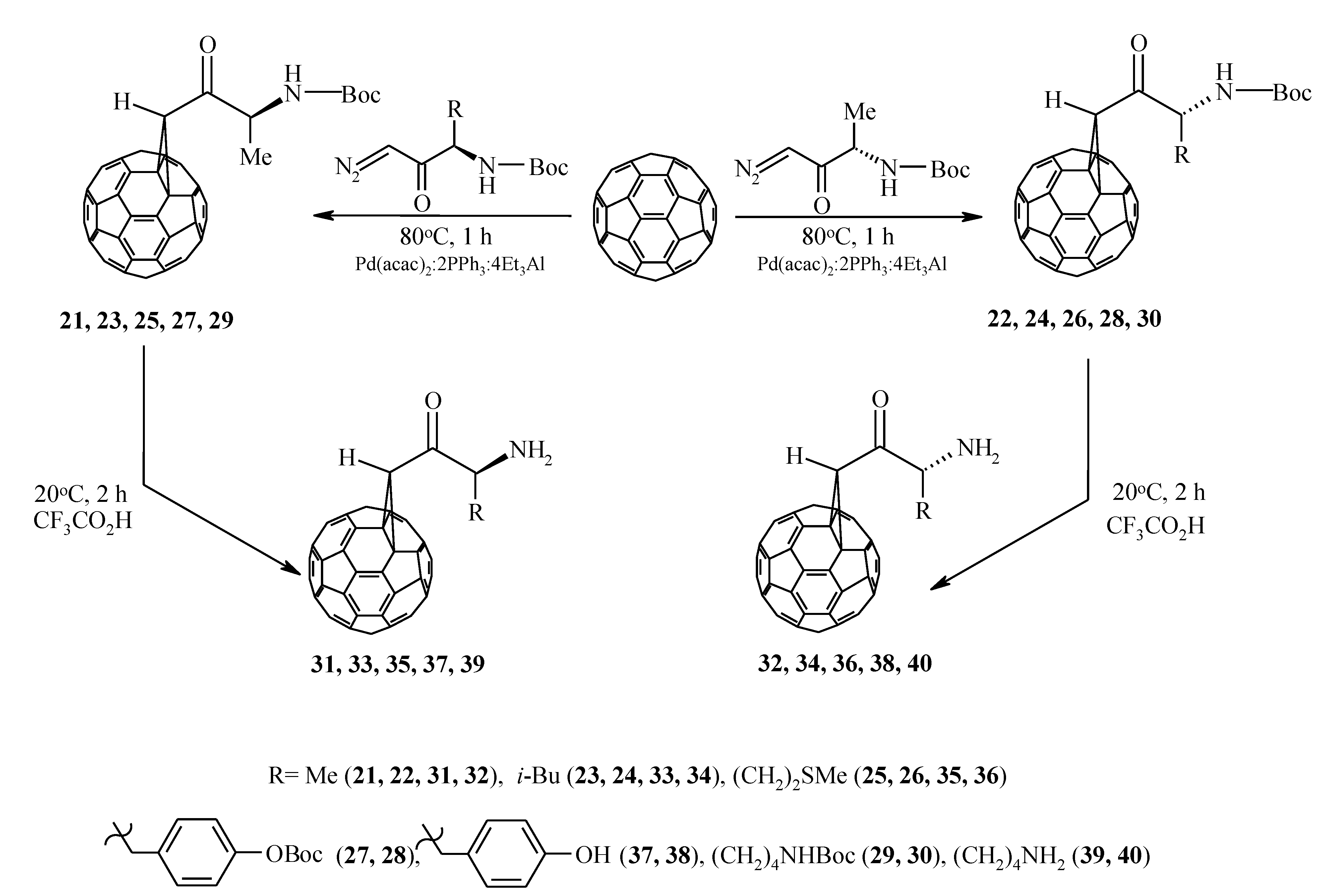
In order to obtain previously undescribed optically active methanofullerenes [29], we implemented a catalytic cycloaddition of C60-fullerene with optically active diazoketones synthesized from L– and D– α–amino acids: alanine, leucine, methionine, tyrosine, and lysine, in which the amino group is protected with the butyloxycarbonyl (Boc) group (Scheme 6).
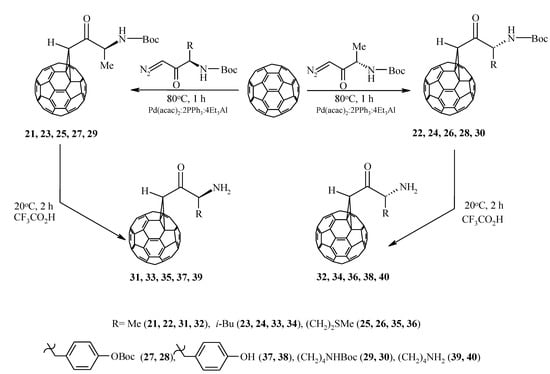
Scheme 6.
Catalytic cycloaddition of optically active diazoketones to C60-fullerene.
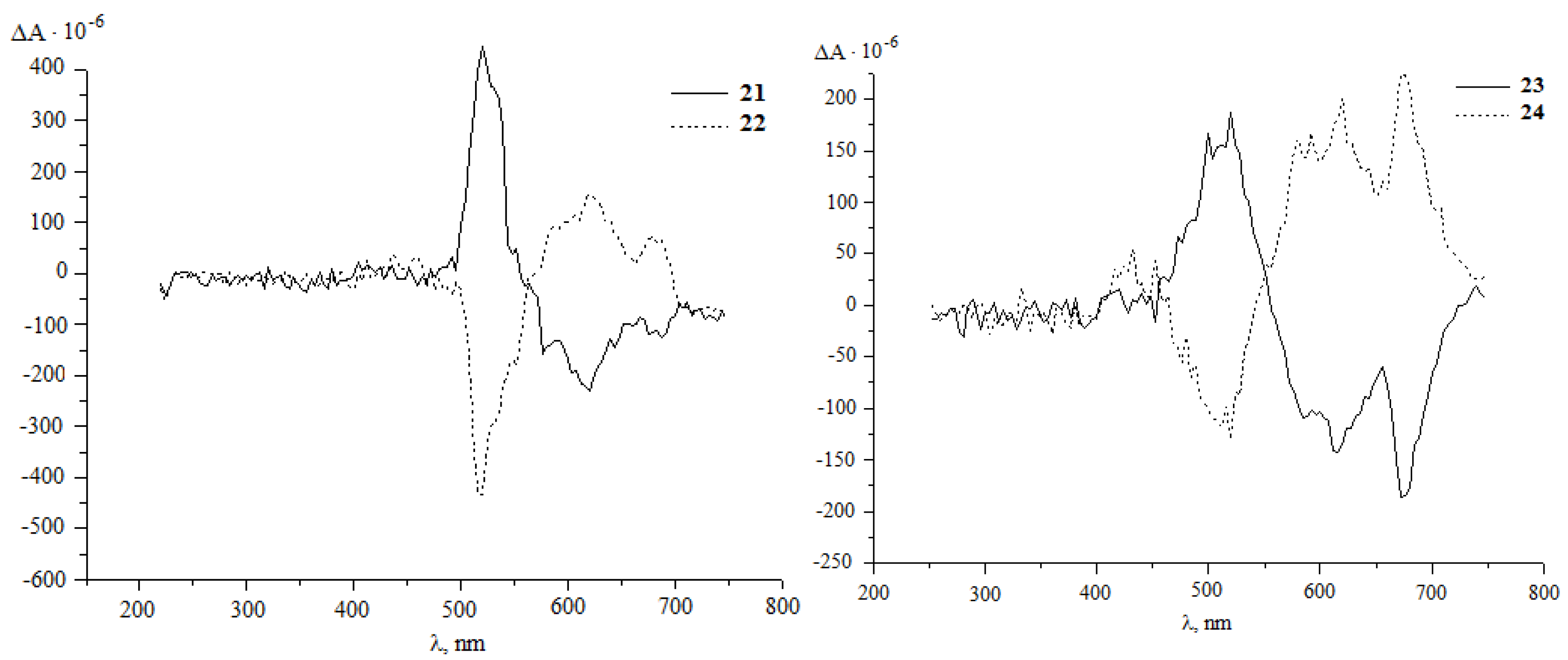
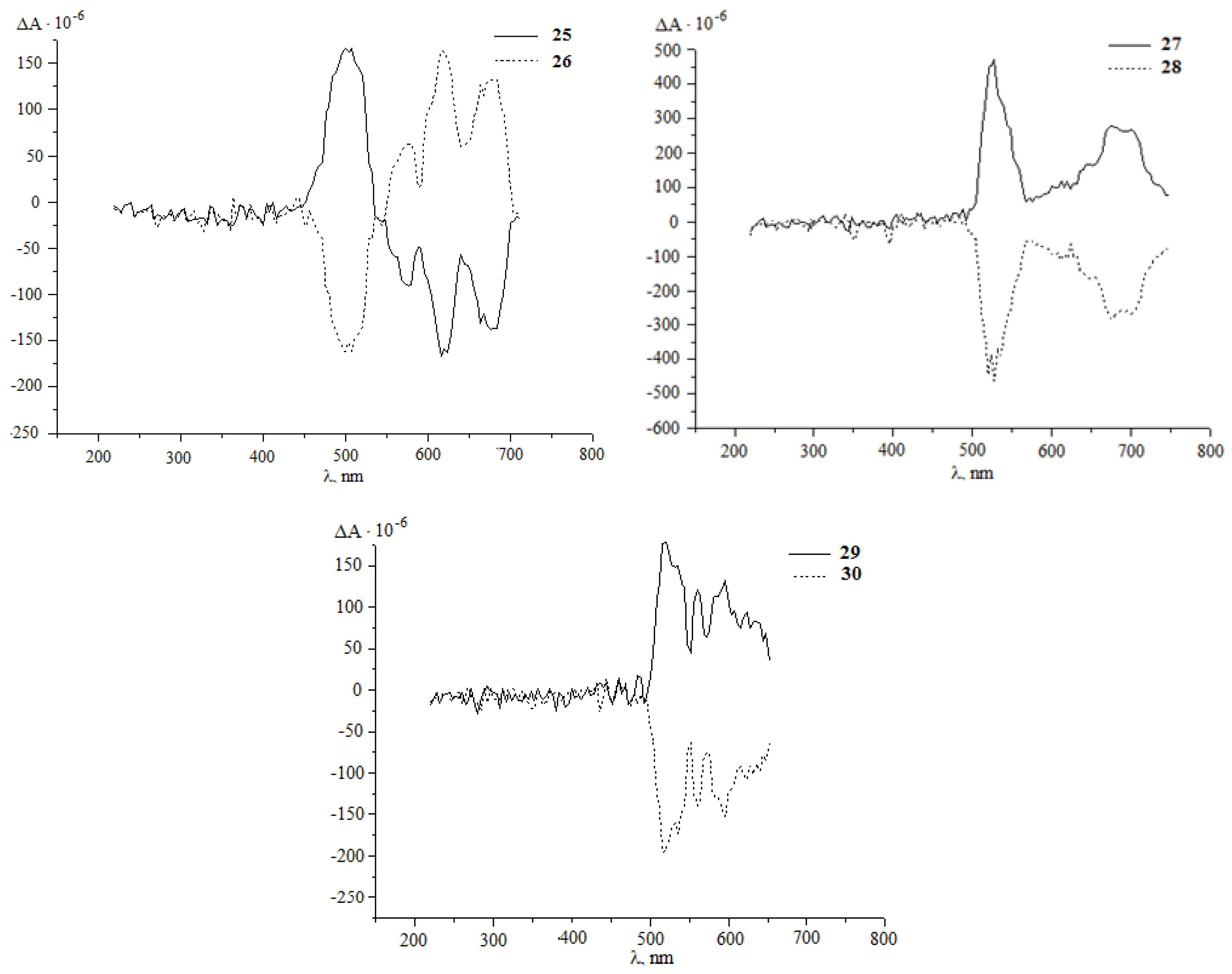
It was established that under the developed conditions (80 °C, 1 h, chlorobenzene) C60 interacts with diazoketones of the indicated amino acids (molar ratio 1:5) under the action of 20 mol.%Pd(acac)2–2PPh3–4Et3Al, selectively forming the corresponding methanoful-lerenes 21–30 with yields of 30–77%. Unfortunately, all attempts to measure the optical angles of rotation of the polarization plane of synthesized methanofullerenes with protected amino groups were unsuccessful. Therefore, for a more reliable proof of the stereochemistry of optically active methanofullerenes, we turned to the circular dichroism (CD) method [30,31]. As expected, in the CD spectra for the synthesized enantiomers 21–30, a mirror image of the cotton effects (EC) was obtained (Figure 1).
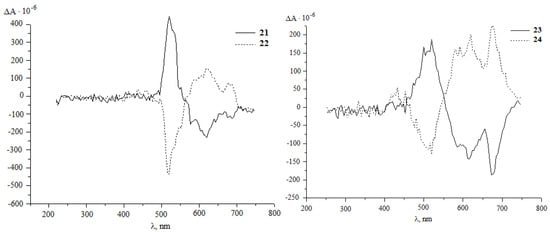
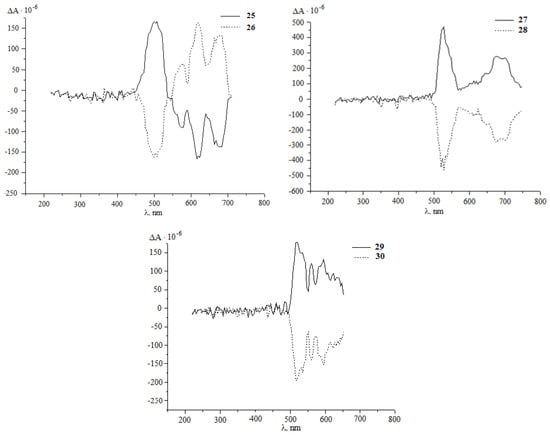
Figure 1.
CD spectra of methanofullerenes 21–30 in chloroform with = 1.0 g/L.
Using the derivatizing shift reagent (tris [3–(heptafluorobutyryl)–L–camphorato]europium (III)), using the example of compound 21, we established a high enantiomeric purity (more than 98%).
Deprotection of functional groups in methanofullerenes 21–30 with CF3CO2H leads to the formation of cycloadducts 31–40 in the form of a solid powder, hardly soluble in traditional solvents for fullerenes and its derivatives (toluene, chlorobenzene, 1,2–dichlorobenzene, chloroform, carbon disulfide). In the case of compounds 31–40, we failed to record the CD spectra of their solutions in pyridine, but the saponification of the amino group associated with the chiral center made it possible to measure the optical rotation angles of the polarization plane of these methanofullerenes.
The development and implementation of new highly effective drugs is one of the priority areas of modern medicine and pharmacology. In connection with the foregoing, within the framework of this work, antioxidant activity was studied for adducts 2 and 19, as well as antitumor and anti-inflammatory activity for derivatives 3, 7, and 20.
3. Conclusions
Thus, based on the results obtained, it can be concluded that the hybrid molecules synthesized by us based on C60-fullerene and pharmacologically significant compounds are of exceptional interest for the development of a new generation of targeted drugs.
Author Contributions
Conceptualization, U.M.D., A.R.T. and L.L.K.; methodology, validation, and execution of chemistry experiments, L.L.K.; manuscript preparation, A.R.T., U.M.D. and L.L.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Ministry of Science and Higher Education within the State Assignments of the Institute of Petrochemistry and Catalysis of RAS (FMRS–2022–0075).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The structural studies of the synthesized compounds were performed with the use of the Collective Usage Centre “Agidel” at the Institute of Petrochemistry and Catalysis of RAS.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sidorov, L.N.; Yurovskaya, M.A. Fullerenes; Ekzamen: Moscow, Russia, 2005; 688p. [Google Scholar]
- Tokyjama, H.; Yamago, S.; Nakamura, E. Photoinduced biochemical activity of fullerene carboxylic acid. J. Am. Chem. Soc. 1993, 115, 7918–7919. [Google Scholar] [CrossRef]
- Ikeda, A.; Hatano, T.; Kawaguchi, M.; Shinkai, S.; Suenaga, H. Water–soluble [60]fullerene–cationic homooxacalix [3]arene complex which is applicable to the photocleavage of DNA. Chem. Commun. 1999, 1403–1404. [Google Scholar] [CrossRef]
- Osterodt, J.; Zett, A.; Vortle, F. Fullerenes by Pyrolysis of Hydrocarbons and Synthesis of Isomeric Methanofullerenes. Tetrahedron 1996, 52, 4949–4962. [Google Scholar] [CrossRef]
- Tsao, N.; Luh, T.-Y.; Chou, C.-K.; Chang, T.-Y.; Wu, J.-J.; Liu, C.-C.; Lei, H.-Y. In vitro action of carboxyfullerene. J. Antimicrob. Chemother. 2002, 49, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Krusic, P.J.; Wasserman, E.; Keizer, P.N.; Morton, J.R.; Preston, K.F. Radical reactions in C60. Science. 1991, 254, 1183–1185. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Takada, H.; Gana, X.; Miwa, N. The water–soluble fullerene derivate “Radical Sponge” exerts cytoprotective action against UVA irradiation but not visible–ligth–catalysedcytotoxity in human skin keratinocytes. Bioorg. Med. Chem. Lett. 2006, 16, 1590–1595. [Google Scholar] [CrossRef]
- Dugan, L.L.; Turelsky, D.M.; Lobner, C.; Du, D.; Wheler, M.; Almli, R.; Shen, C.K.; Luh, T.Y.; Choi, D.; Lin, T.S. Carboxylfullerenes as neuroprotective agents. Proc. Natl. Acad. Sci. USA 1997, 94, 9434–9439. [Google Scholar] [CrossRef] [PubMed]
- Friedman, S.H.; DeCamp, D.L.; Sijbesma, R.P.; Srdanov, G.; Wudl, F.; Kenyon, G.L. Inhibition of the HIV–I Protease by Fullerene Derivatives: Model Building Studies and Experimental Verification. J. Am. Chem. Soc. 1993, 115, 6506–6509. [Google Scholar] [CrossRef]
- Mashino, T.; Okuda, K.; Hirota, T.; Hirobe, M.; Nagano, T.; Mochizuki, M. Inhibition of E. coli growth by fullerene derivatives and inhibition mechanism. Bioorg. Med. Chem. Lett. 1999, 9, 2959–2962. [Google Scholar] [CrossRef]
- Bosi, S.; Da Ros, T.; Castellano, S.; Banfi, E.; Prato, M. Antimycobacterial Activity of Ionic Fullerene Derivatives. Bioorg. Med. Chem. Lett. 2000, 10, 1043–1045. [Google Scholar] [CrossRef] [PubMed]
- Mashino, T.; Nishikawa, D.; Takanashi, K.; Usui, N.; Yamori, T.; Seki, M.; Endo, T.; Mochizuki, M. Antibacterial and antiproliferative activity of cationic fullerene derivatives. Bioorg. Med. Chem. Lett. 2003, 13, 4395–4397. [Google Scholar] [CrossRef]
- Bingel, C. Cyclopropanierung von Fullerenen. Chem. Ber. 1993, 126, 1957–1959. [Google Scholar] [CrossRef]
- Camps, X.; Hirsch, A. Efficient cyclopropanation of C60 starting from malonates. J. Chem. Soc. Perkin Trans. 1997, 1, 1595–1596. [Google Scholar] [CrossRef]
- Suzuki, T.; Li, Q.; Khemani, K.C.; Wudl, F. Dihydrofulleroid H2C61: Synthesis and properties of the parent fulleroid. J. Am. Chem. Soc. 1992, 114, 7301–7302. [Google Scholar] [CrossRef]
- Smith, A.B., III; Strongin, R.M.; Brard, L.; Furst, G.T.; Romanov, W. 1,2–Methanobuckminsterfullerene (C61H2), the parent fullerene cyclopropane: Synthesis and structure. J. Am. Chem. Soc. 1993, 115, 5829–5830. [Google Scholar] [CrossRef]
- Smith, A.B., III; Strongin, R.M.; Brard, L.; Furst, G.T.; Romanov, W.J.; Owens, K.G.; Goldschmidt, R.J.; King, R.C. Synthesis of Prototypical Fullerene Cyclopropanes and Annulenes.Isomer Differentiation via NMR and UV Spectroscopy. J. Am. Chem. Soc. 1995, 117, 5492–5502. [Google Scholar] [CrossRef]
- Tuktarov, A.R.; Akhmetov, A.R.; Kamalov, R.F.; Khalilov, L.M.; Pudas, M.; Ibragimov, A.G.; Dzhemilev, U.M. Catalytic [2+1]-cycloaddition of ethyl diazoacetate to fullerene [60]. Russ. J. Org. Chem. 2009, 45, 1168–1174. [Google Scholar] [CrossRef]
- Tuktarov, A.R.; Akhmetov, A.R.; Sabirov, D.S.; Khalilov, L.M.; Ibragimov, A.G.; Dzhemilev, U.M. Catalytic [2+1] –cycloaddition of diazo compounds to fullerene [60]. Russ. Chem. Bull. 2009, 8, 1724–1730. [Google Scholar] [CrossRef]
- Tuktarov, A.R.; Korolev, V.V.; Dzhemilev, U.M. Catalytic cycloaddition of diazoalkanes generated in situ to C60-fullerene. J. Org. Chem. 2010, 46, 595–596. [Google Scholar] [CrossRef]
- Tuktarov, A.R.; Korolev, V.V.; Tulyabaev, A.R.; Yanybin, V.M.; Khalilov, L.M.; Dzhemilev, U.M. Cycloaddition of cyclic diazo compounds to C60 fullerene in the presence of a Pd–containing complex catalyst. Russ. Chem. Bull. 2010, 5, 977–993. [Google Scholar] [CrossRef]
- Tuktarov, A.R.; Akhmetov, A.R.; Khalilov, L.M.; Dzhemilev, U.M. Catalytic cycloaddition of diazoketones to C60 fullerene. Russ. Chem. Bull. 2010, 3, 598. [Google Scholar]
- Tuktarov, A.R.; Akhmetov, A.R.; Korolev, V.V.; Khuzin, A.A.; Khasanova, L.L.; Popod’ko, N.R.; Khalilov, L.M. Palladium–catalyzed selective cycloaddition of diazo compounds to [60]fullerene. Arkivoc 2011, 8, 54–66. [Google Scholar] [CrossRef]
- Tuktarov, A.R.; Khuzina, L.L.; Dzhemilev, U.M. Covalent binding of fullerene C60 to pharmacologically important compounds. Russ. Chem. Bull. 2011, 4, 662–666. [Google Scholar] [CrossRef]
- Tuktarov, A.R.; Khuzina, L.L.; Popod’ko, N.R.; Zhemilev, U.M.D. Catalytic cycloaddition of diazo amides to fullerene 60. Tetrahedron Lett. 2013, 54, 2146–2148. [Google Scholar] [CrossRef]
- Skiebe, A.; Hirsch, A. A facile method for the synthesis of amino acid and amido derivatives of C60. J. Chem. Soc. Chem. Commun. 1994, 3, 335–336. [Google Scholar] [CrossRef]
- Wang, G.-W.; Li, Y.-J.; Peng, R.-F.; Liang, Z.-H.; Liu, Y.-C. Are the pyrazolines formed from the reaction of [60]fullerene with alkyl diazoacetates unstable? Tetrahedron 2004, 60, 3921–3925. [Google Scholar] [CrossRef]
- Tuktarov, A.R.; Khuzin, A.A.; Popod’ko, N.R.; Dzhemilev, U.M. Cycloaddition of diazothioates to [60] fullerene. Tetrahedron Lett. 2012, 53, 3123–3125. [Google Scholar] [CrossRef]
- Tuktarov, A.R.; Khuzina, L.L.; Dzhemilev, U.M. Catalytic synthesis of optically active functionally substituted fullerenes. In Proceedings of the All–Russian Conference: “Organic synthesis: Chemistry and technology”, Ekaterinburg, Russia, 4–8 June 2012; p. 105. [Google Scholar]
- Krantz, A.; Copp, L.J.; Coles, P.J.; Smith, R.A.; Heard, S.B. Peptidyl(acyloxy)methyl ketones and the quiescent affinity label concept: The departing group as a variable structural element in the design of inactivators of cysteine proteinases. Biochemistry 1991, 30, 4678–4687. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, C.F.; Li, L.; Zou, W.; Huang, Y.Q.; Gao, J.X. Synthesis and CD Spectra of Chiral Molybdenum–fullerenyl Complexes with Pineno–bipyridine Ligands. ChineseChem. Lett. 2004, 15, 1411–1414. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).