Abstract
Chemical phosphorylation of hyaluronic acid (HA) remains an unresolved problem for the chemistry of this unique polysaccharide, since convenient phosphorylating reagents are not reactive enough to obtain HA phosphates (HA-P) with a satisfactory degree of esterification of hydroxyl groups. The synthesis of phosphates of low-molecular-weight (43 kDa) and high-molecular-weight (0.5–0.7 MDa) HA was undertaken using such reagents as sodium trimetaphosphate Na3P3O9, H3PO4, NaH2PO4/Na2HPO4, and anhydride P2O5. Solid-phase HA esterification with P2O5 was found to be the most convenient and efficient method. The HA-P samples were characterized by XRF and NMR spectroscopy (31P and 1H-31P) and contained, depending on the HA/P2O5 ratio, 0.30–6.25% P wt., in the form of disubstituted mono-, di-, and polyphosphates.
1. Introduction
Chemical phosphorylation of hyaluronic acid (HA) with several phosphorylating reagents was recently undertaken by Bojarski et al. [1]. Trimetaphosphate sodium salt Na3P3O9 (STMP), P2O5/H3PO4/Et3PO4 (in hexanol), polyphosphoric acid/tributylamine (in DMSO), POCl3/DMF (in DMSO), and P2O5/methanesulfonic acid in diethyl ether were used. However, all these reagents, including STMP, were found to be insufficiently effective, and obtaining HA phosphates (HA-P) with a satisfactory degree of esterification of hydroxyl groups was not possible. Interestingly, the STMP method was previously patented and characterized as effective [2]. Taking into account the contradictory data regarding STMP’s reactivity, we decided to repeat the experiment with it once again. In addition to STMP, orthophosphoric acid H3PO4 (85%), a mixture of NaH2PO4/Na2HPO4 salts [3], and P2O5 were tested as HA-phosphorylating agents. Reactions with salts and anhydride were carried out in the solid phase.
2. Materials and Methods
Samples of low-molecular-weight (LMW, 43 kDa, Leko Style, St.-Petersburg) and high-molecular-weight (HMW, 0.5–0.7 MDa, Contipro, Czech Republic) HA were used. Phosphorous (V) oxide was purchased from Acros Organics. D2O was bought from Eurisotop. Other chemicals were of analytical reagent grade.
NMR spectra (31P and 1H-31P HMBC) were recorded on a Bruker Avance II 400 MHz (400.13 MHz for 1H and 161.90 MHz for 31P) spectrometer. Samples were analyzed as solutions in D2O (5–20 mg/mL) at room temperature (δ 0 ppm for H3PO4). The total content of P in HA-P samples was analyzed with the help of an XRF spectrometer EDX-7000 (Shimadzu, Kyoto, Japan).
3. Results and Discussion
The reactions of LMW and HMW HA with STMP were carried out under conditions close to those of the patent [2], at various HA concentrations, HA/STMP ratios, alkaline reagents (K2CO3 or NaOH), and reaction times (Table 1). Samples were purified by dialysis for three days. However, even after such purification, the 31P NMR spectra of all samples contained a signal at (−21)–(−21.5) ppm characteristic for STMP, as well as signals at 2.44, −5.43, −5.54, and −6.47 ppm, most likely corresponding to the decomposition products of STMP under the action of alkali. Evidence for the covalent binding of P to HA was obtained using two-dimensional 1H-31P HMBC spectra, in which cross-peaks were detected at 3.84/0.02 (C-6 in GlcNAc), 3.77/0.02 (C-6 in GlcNAc), and 3.46/0.02 ppm (C-3 in GlcA, minor intensity) and corresponded to disubstituted monophosphates of HA (dMP); phosphate residue was connected mainly with the HA primary hydroxyl groups. As can be seen from Table 1, the conditions of protocols 2 (0.3% P, K2CO3, 48 h) and 3 (0.27% P, NaOH, 3 h) were found to be the best. The long reaction time in protocol 4 apparently caused the hydrolytic elimination of phosphate groups. HMW HA reacted with STMP (entry 7) only under the conditions of experience 2, but less efficiently than LMW HA.

Table 1.
Conditions for HA reactions with Na3P3O9, H3PO4 (85%), and NaH2PO4/Na2HPO4 and characteristics of the products.
Phosphorylation of LMW HA with H3PO4 and NaH2PO4/Na2HPO4 was found to be unsuccessful (Table 1, entries 8, 9).
The reactions of LMW HA and HMW HA with P2O5 at different ratios were carried out by intensive grinding of dry HA and P2O5 in a porcelain mortar at room temperature for 20–30 min. Next, the samples were kept for ~2 h, and then purified and dried. The total P content found by XRF analysis was 1.28–6.25% for LMW HA-P and 0.30–2.55% for HMW HA-P and varied depending on the HA/P2O5 ratio (Table 2). As can be seen from these data, dry HA phosphorylation under the action of P2O5 is much more efficient than other methods of obtaining HA-P [1].

Table 2.
Characteristics of LMW HA-P (entries 1–4) and HMW HA-P (entries 5–8) samples depending on the HA/P2O5 ratio.
It is known from the literature that various types of phosphate residues can be formed in the process of phosphorylation of polysaccharides (PSs): mono- and disubstituted monophosphates (mMPs and dMPs), mono- and disubstituted diphosphates (mDPs and dDPs), polyphosphates (PPs) both with a terminal phosphate group (mPPs) and in the form of disubstituted derivatives (dPPs). The characteristic signals in the 31P NMR spectra for each of the listed structures are shown in Table 3.

Table 3.
Characteristic signals of atom P in 31P NMR spectra depending on the structure of phosphates.
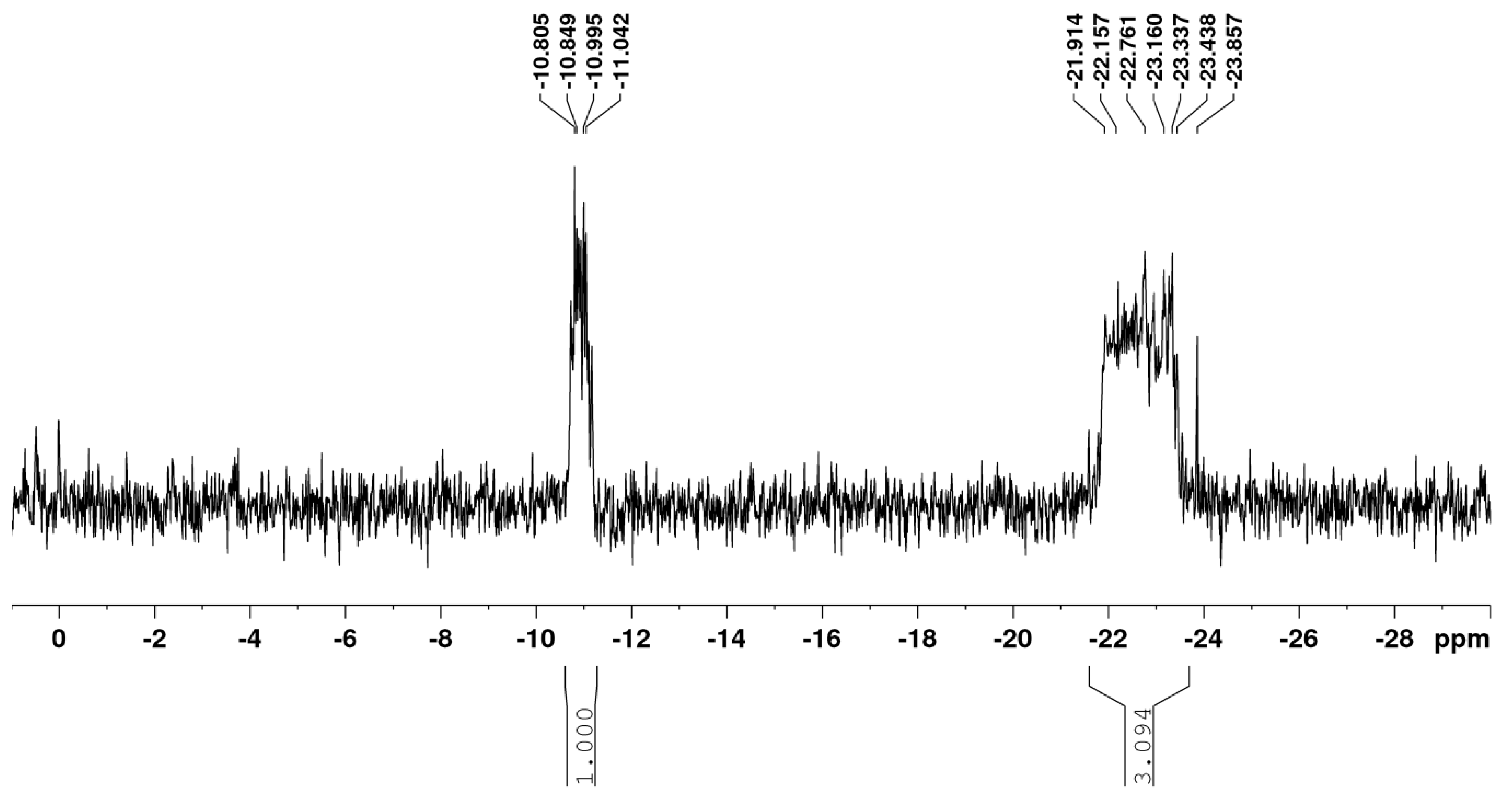
According to these data (Table 3), the obtained HA-P samples contain only three types of phosphate residues: dMF (0.02 ppm), dDP (−10.8 ppm), and dPP ((−22.2)–(−23.8) ppm). Their distribution in HA-P, calculated from the XRF data and the integral intensity of each of the corresponding signals in the 31P NMR spectra, is represented by the P content and is given in Table 2.
It is interesting to note the high content of polyphosphate sequences in samples 7 and 8. The 31P NMR spectrum of sample 8 is shown in Figure 1.
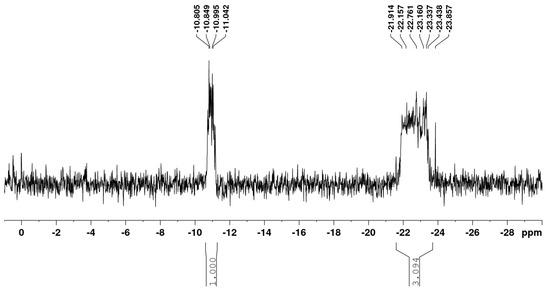
Figure 1.
31P NMR spectrum of sample 8 (Table 3).
Polyphosphates (PPs) can be considered as inorganic fragments included in the HA macromolecular chains. According to modern concepts, inorganic PPs are a source of phosphate in the process of bone mineralization. PPs can also be used in regenerative medicine. Firstly, they have shown morphogenetic activity, i.e., take part in cell differentiation through gene induction; secondly, they act as an accumulator and energy donor in the intercellular space. In addition, adenosine diphosphate and adenosine triphosphate (ADP/ATP) are formed from PPs under the combined action of alkaline phosphatase and adenylate kinase. For example, inorganic PPs added externally to mammalian cells lead to a 3-fold increase in ATP [4].
4. Conclusions
HMW and LMW HA phosphorylated derivatives with a high content of phosphate residues were first obtained by solid-phase reaction with P2O5. One feature of the phosphorylation process was formation of disubstituted mono-, di-, and polyphosphates in the structure of HA macromolecules.
Author Contributions
Conceptualization, I.Y.P. and E.A.K.; methodology, validation, and execution of chemistry experiments, E.A.K., I.Y.P., S.V.K. and T.V.T.; manuscript preparation, I.Y.P., E.A.K. and T.V.T. All authors have read and agreed to the published version of the manuscript.
Funding
The work was conducted within the parameters of the approved plans for research projects at the IPC RAS State Registration No. FMRS-2022-0081.
Acknowledgments
The studies were performed with the use of equipment from the Collective Usage Centre “Agidel” of Ufa Research of the Russian Academy of Science at the Institute Petrochemistry and Catalysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bojarski, K.K.; Becher, J.; Riemer, T.; Lemmnitzer, K.; Möller, S.; Schiller, J.; Schnabelrauch, M.; Samsonov, S.A. Synthesis and in silico characterization of artificially phosphorylated glycosaminoglycans. J. Mol. Struct. 2019, 1197, 401–416. [Google Scholar] [CrossRef]
- Magnani, A.; Consumi, M.; Rossi, C.; Greco, G. Phosphated Derivatives of Polysaccharides and Uses Thereof. Patent WO2008090583, 31 July 2008. [Google Scholar]
- Sitohy, M.Z.; Labib, S.M.; El-Saadany, S.S.; Ramadan, M.F. Optimizing the conditions for starch dry phosphorylation with sodium mono- and dihydrogen orthophosphate under heat and vacuum. Starch-Stärke 2000, 52, 95–100. [Google Scholar] [CrossRef]
- Müller, W.E.G.; Schröder, H.C.; Wang, X.H. Inorganic polyphosphates as storage for and generator metabolic energy in the extracellular matrix. Chem. Rev. 2019, 119, 12337–12374. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).