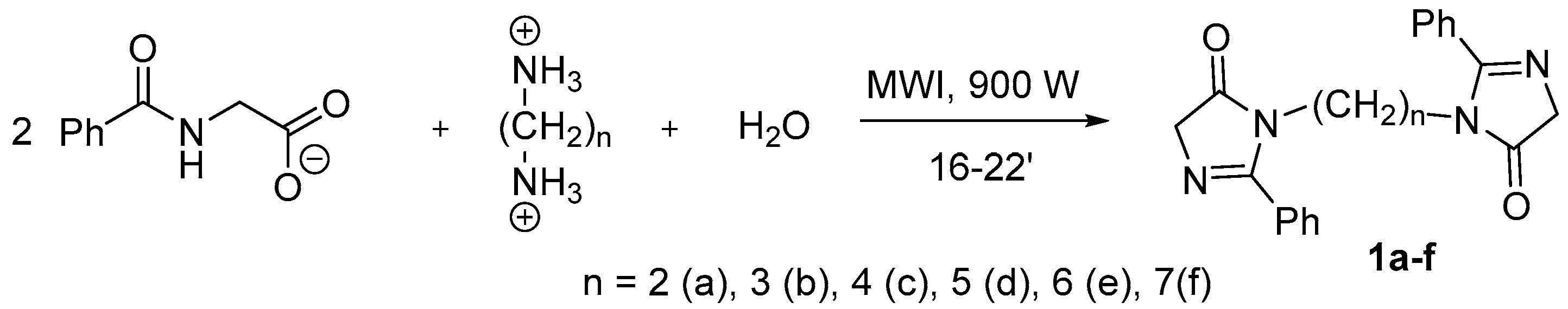Abstract
In this study, we investigated the conformational abilities of n,n’-(Alkanediyl)-bis(2-phenyl-3,5-dihydro-4H-imidazol-4-one)s (1) to gain insight into their biological potential as a platform for the generation of various QACs. Among the conformers of 1, due to the flexibility of the polymethylene fragment, the phenyl rings can take on different mutual arrangements, while there is a clear tendency for the formation of π–π interactions. From the point of view of the reactivity to alkylating agents, a preliminary conclusion can be drawn about the favorable mutual arrangement of imidazolone rings located at a sufficient distance to carry out both mono- and bisalkylation to obtain bis-QAC.
1. Introduction
Recently, 3,3’-(Alkandiyl)-bis(2-phenyl-3,5-dihydro-4H-imidazol-4-one)s are of interest not for their chemical structure, but also the properties of their N-alkylated derivatives. They represent a class of biogenic quaternary ammonium compounds (QACs) with antiseptic and disinfectant properties, which have become especially relevant over the past few years. There is a wide variety of structures for these compounds: mono-QAC, bis-QAC, poly-QAC and multi-QAC. The bis-QAC structures are a subclass of synthetic amphiphiles containing one or two cationic nitrogen atoms (head or core fragment), a “spacer” or “linker” connecting them, and two lipophilic alkyl substituents (“tails”), extending from the heterocycles—“heads” [1].
QACs are generally stable and soluble in water. The counterion included in these structures affects the solubility of the biocide without affecting the biological activity. Most registered QACs contain chlorides or bromides as anions. Due to their amphiphilic nature, QACs are capable of forming micelles.
The “tails” in the structure of a QAC are strong inducers of biological actions against pathogenic microorganisms [2]. By varying the lengths and nature of the “spacer” and “tails” in these structures, it is possible to obtain compounds with different types and degrees of biological activity. It is believed that with an increase in the number of heterocyclic “heads”, the biocidal properties of QACs increase and their penetrating ability toward bacterial membranes and protein capsids of viruses improves. In this regard, the search for platform compounds suitable for the preparation of QAC bis-structures is relevant, and the proposed 3,3’-(alkanediyl)-bis(2-phenyl-3,5-dihydro-4H-imidazole-4-one)s are convenient precursors for modification via alkylhalogenation for the synthesis of new bis-QACs.
In this study, we present the results of a conformational study of n,n’-(alkane-1,n-diyl)bis(2-phenyl-3,5-dihydro-4H-imidazol-4-one)s with different spacer lengths.
2. Materials and Methods
2.1. Physical Measurements
The FTIR transmission spectrum was collected in a KBr pellet with an FSM-1201 Fourier spectrometer (Infraspek, Saint-Petersburg, Russia) in the 4000–400 cm−1 range. The 1H (400 MHz) and 13C NMR (100 MHz) spectra in acetone-d 6 were recorded with a Varian (Agilent, Santa Clara, CA, USA) 400 spectrometer (Agilent Technologies, Santa Clara, CA, USA), and the internal standard was TMS. Chemical shifts (δ) are reported in ppm. Elemental analysis was performed on a CHNS analyzer “Elementar Vario MICRO cube” (Elementar Analysensysteme GmbH, Hanau, Germany). The melting point was determined on a Stuart™ SMP10 melting point apparatus (Cole-Parmer, Beacon Road, Stone, Staffordshire, ST15 OSA, UK). The progress of the reaction and the purity of the synthesized compound were monitored via TLC on ALUGRAM® SIL G UV254 plates (MACHEREY-NAGEL GmbH & Co. KG, Düren, Germany), and a hexane–ethyl acetate–acetone (2:2:1) mixture was the eluent.
2.2. Synthesis of the Bis-Imidazolones (1)
The series of bis-imidazolones (1) was obtained via the microwave irradiation (MWI) of alkyl diammonium dihippurates (Scheme 1) in good yields.
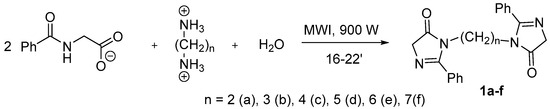
Scheme 1.
Synthesis of the bis-imidazolones (1a–f).
A portion of 0.4–1 g of substance (1a–f) is placed in a porcelain crucible and exposed to microwave radiation with a power of 900 W for 10–15 min. Then, a few drops of distilled water are added and the coloring observed. Then, it is exposed to microwave irradiation again for an additional 2–4 min. Upon completion, the reaction mixture is put in water and dried.
- 3,3’-(Ethan-1,2-diyl)bis(2-phenyl-3,5-dihydro-4H-imidazol-4-one) (1a): sand-colored solid; yield, 75%; m.p. 210–215 °C; 1H NMR, δ, ppm: 1.38 (s., 2H, -CH2-); 3.04–3.06 (d., J = 6 Hz, 2H, -CH2-); 3.80–3.82 (d., J = 6 Hz, 2H, -CH2-); 7.43–7.54, 7.80–7.92 (m., 5H, HAr).
- 3,3’-(Propan-1,3-diyl)bis(2-phenyl-3,5-dihydro-4H-imidazol-4-one) (1b): bright pink solid; yield 60%; m.p. 202–205 °C; 1H NMR, δ, ppm: 1.53 (s., 2H, -CH2-); 3.07–3.08 (J = 6 Hz, d., 2H, -CH2-); 3.81–3.82 (d., J = 6 Hz, 2H, -CH2-); 7.42–7.53, 7.85–7.90 (m., 5H, HAr).
- 3,3’-(Butan-1,4-diyl)bis(2-phenyl-3,5-dihydro-4H-imidazol-4-one) (1c): peach-colored solid; yield, 69%; m.p. 183–185 °C; 1H NMR, δ, ppm: 1.38 (s., 2H, -CH2-); 3.04–3.06 (J = 6 Hz, d., 2H, -CH2-); 3.80–3.82 (d., J = 6 Hz, 2H, -CH2-); 7.43–7.54, 7.80–7.92 (m., 5H, HAr).
- 3,3’-(Pentan-1,5-diyl)bis(2-phenyl-3,5-dihydro-4H-imidazol-4-one) (1d): light brown solid; yield, 64%; m.p. 172–177 °C. 1H NMR, δ, ppm: 3.05–3.06 (d., J = 6 Hz, 2H, -CH2-); 3.81–3.82 (J = 6 Hz, d., 2H, -CH2-); 7.41–7.53, 7.80–7.87 (m., 5H, HAr).
- 3,3’-(Hexan-1,6-diyl)bis(2-phenyl-3,5-dihydro-4H-imidazol-4-one) (1e): peach-colored solid; yield, 65%; m.p. 168–170 °C; 1H NMR, δ, ppm: 1.38 (s., 2H, -CH2-); 1.63–1.71 (d., J = 6 Hz, 2H, -CH2-); 3.04–3.06 (d., J = 6 Hz, 2H, -CH2-); 3.80–3.82 (d., J = 6 Hz, 2H, -CH2-); 7.43–7.54, 7.80–7.92 (m., 5H, HAr).
- 3,3’-(Heptan-1,7-diyl)bis(2-phenyl-3,5-dihydro-4H-imidazol-4-one) (1f): brick-red solid; yield, 77%; m.p. 163–165 °C; 1H NMR, δ, ppm: 3.80–3.82 (d., J = 6 Hz, 2H, -CH2-); 7.43–7.53, 7.80–7.87 (m., 5H, HAr).
2.3. DFT Calculations
All density functional theory calculations were carried out with the Gaussian 09 program using a high-performance computing cluster of the National Research Saratov State University and PC GAMESS/Firefly using a desktop workstation. After the full optimization, analysis of the matrices of the second derivatives of energy with respect to coordinates (Hessian) was performed to reveal the energy global minima on the potential energy surface.
2.4. Conformational Study
Conformational analysis of the synthesized compounds was carried out using the Frog2 program, optimized for searching for the most stable conformations of low-molecular compounds. Based on the structures obtained during optimization using the quantum chemical DFT method, the program generated 50 conformers, which were subsequently optimized employing the molecular mechanics method using AMMOS (automated molecular mechanics optimization tool for in silico screening). Conformations that were similar in structure were excluded from consideration. As a result, the 10 most stable conformers were obtained and considered for each of the synthesized compounds.
3. Results and Discussion
3.1. Spectral Characterization of the Synthesized bis-imidazolones (1)
The structure of products (1a–f) was established based on a set of 1H, 13C NMR spectroscopy data and using two-dimensional COSY, HSQC and HMBC methods. According to the spectral data, they represent a series of 3,3’-(alkandiyl)-bis(2-phenyl-3,5-dihydro-4H-imidazol-4-one)s with different alkyl chain lengths.
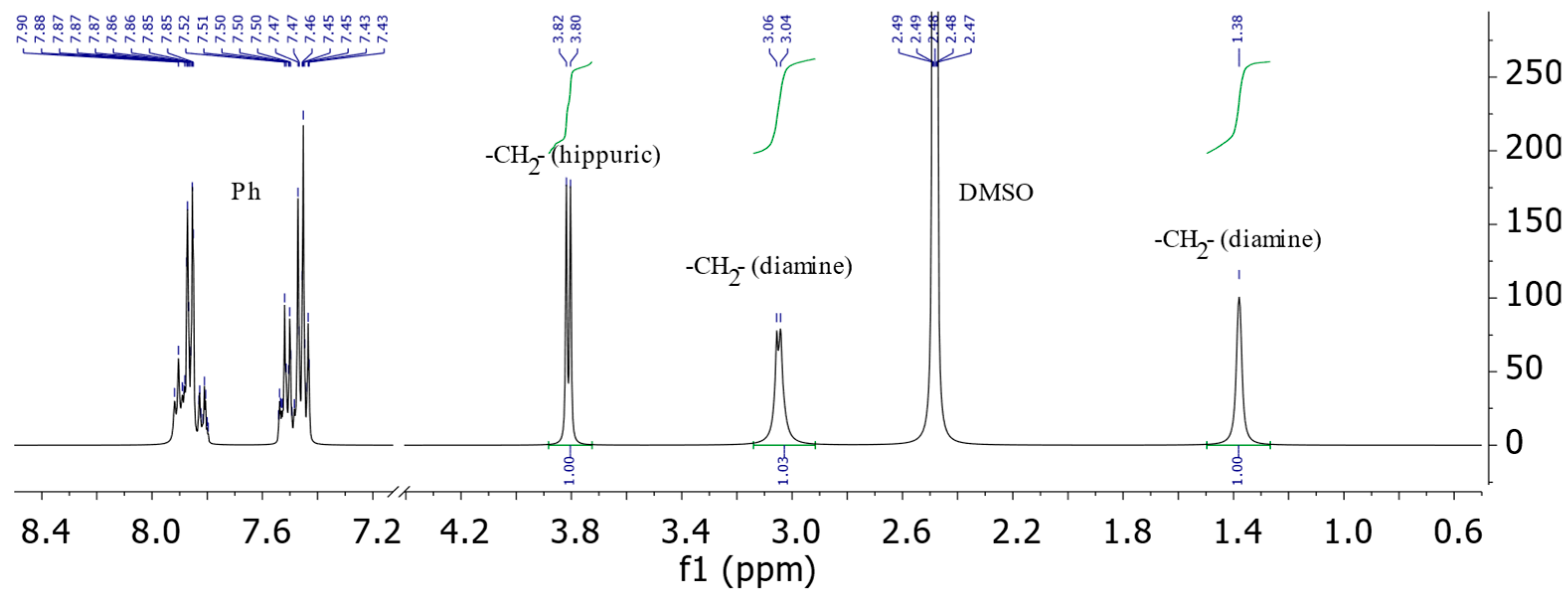
In particular, the 1H NMR spectrum of compound 1c contains a doublet of protons of the methyl group of the hippuric acid fragment at 3.73–3.88 ppm. At 1.38 ppm, a singlet is also observed and at 3.04–3.06 ppm, a doublet of protons corresponding to the aliphatic diamine fragment is observed. The multiplet of aromatic protons appears in the region of 7.43–7.54 ppm and 7.80–7.92 ppm (Figure 1).
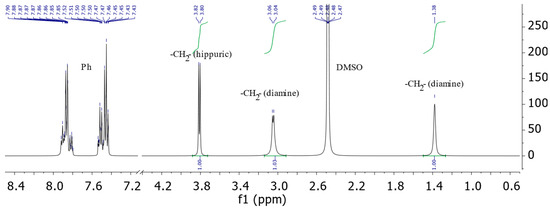
Figure 1.
The 1H NMR spectrum of 3,3’-(butan-1,4-diyl)bis(2-phenyl-,5-dihydro-4H-imidazol-4-one)a (1c), (solvent DMSO-d6).
The ratio of the integral intensities of the multiplets assigned to the methylene units of the diamine fragments and imidazolone rings was 1:1:1, which corresponds to the proposed bis-structure. In the 1H-1H COSY spectrum of the molecule 3,3’-(butan-1,4-diyl)bis(2-phenyl-3,5-dihydro-4H-imidazol-4-one)a 1c, the correlations of the protons are associated with the heteroatom of the imidazolone rings -CH2- of the “spacer” group with aromatic protons of the phenyl substituent at 3.05/7.89 ppm. The cross-peaks of the protons of the methylene unit further away from the heterocycle with the protons of the phenyl ring are detected with a significantly lower intensity, and the γ-unit does not show similar correlations at all. The absence of correlations of the γ-methylene unit with the protons of the benzene ring serves as evidence of the bis structure of the resulting compounds.
3.2. DFT Calculations
Calculations were carried out using the density functional theory (DFT) using the B3LYP hybrid functional and the 6–31G(d,p) basis set. Using the same starting geometric parameters of molecules and calculation parameters, PC GAMESS/Firefly returned flat structures as a result of optimization, and the analysis of the Hessian matrices of the second derivatives showed that they contain imaginary vibrational modes, that indicates local extrema (saddle points). This does not allow for using the results obtained for further conformational analysis; however, these results can be used to model the transition of a conformation with a co-directional arrangement of rings with a conformation with an anti-directional arrangement of rings.
The use of the Gaussian 09 optimization algorithm resulted in obtaining non-planar structures in the Hessians of which there were no imaginary frequencies. Initially, structures were obtained predominantly with a co-oriented arrangement of rings, with the exception of bis-imidazolone 1b, the anti-oriented arrangement of the phenyl rings of which prompted us to study both variants of conformations for each representative of the 1a–f series. We used these optimized non-planar structures for further analysis.
3.3. Conformational Study
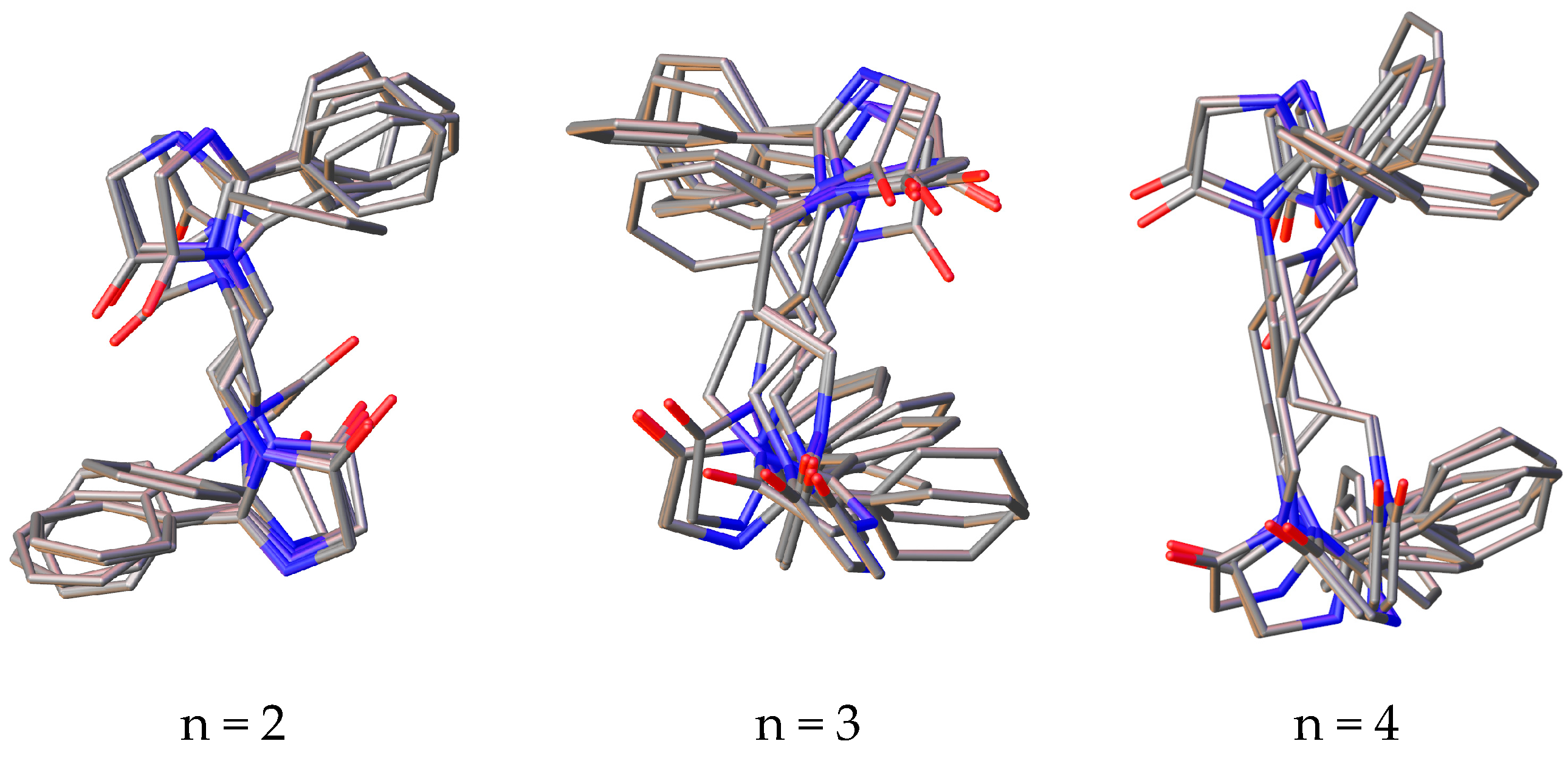
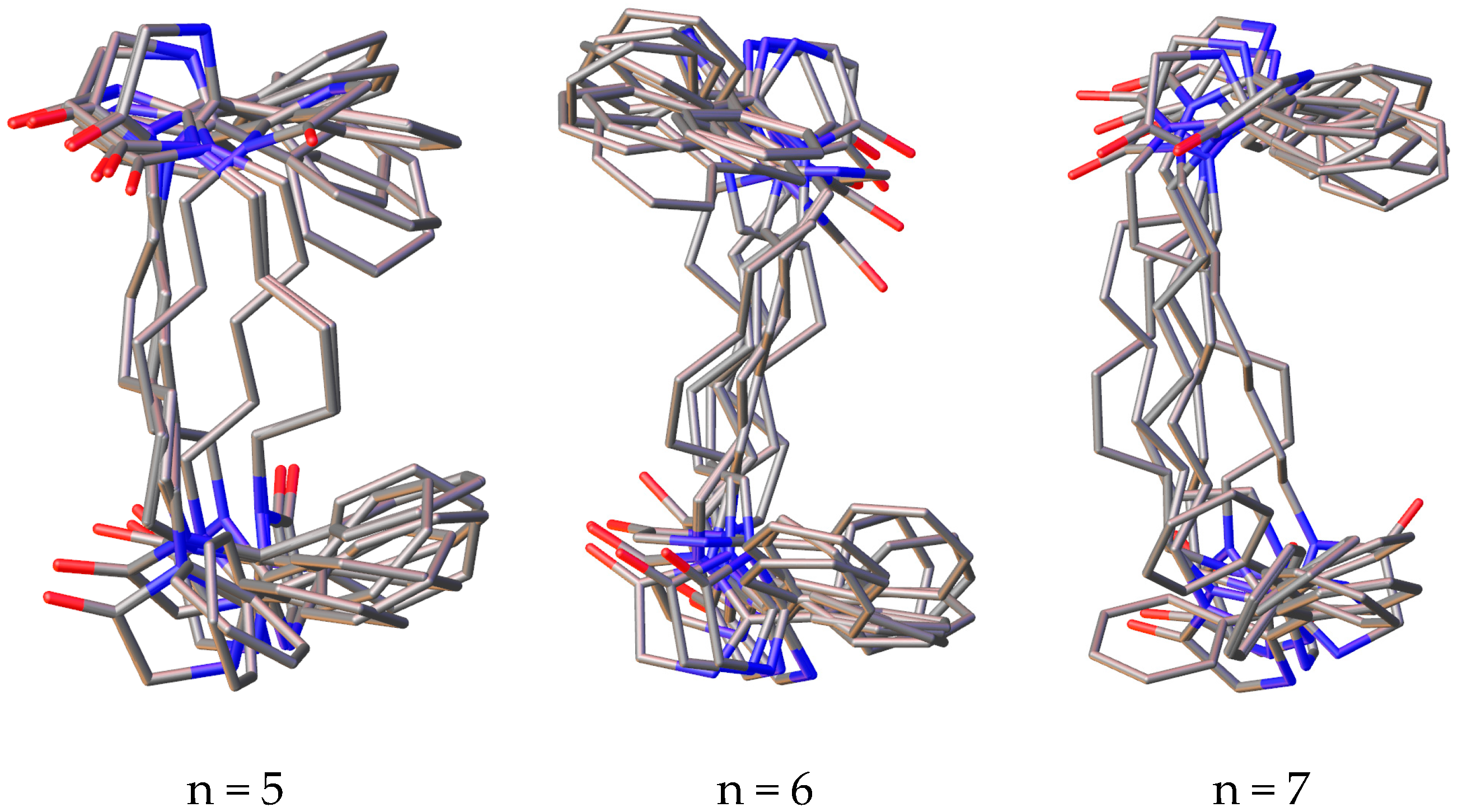
Among the conformers of 3,3’-(alkane-1,n-diyl)bisimidazolones (1a–f), due to the flexibility of the polymethylene fragment, phenyl rings can take on different mutual arrangements, and a clear tendency is observed for the formation of π–π interactions of the rings, and in a number of conformers, they may not be located coplanarly, but at a certain angle to each other, which, as is known, does not exclude the possibility of such π–π binding (Figure 2).
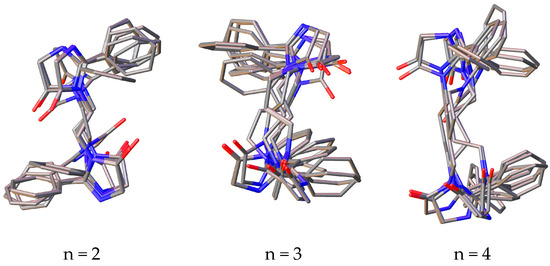
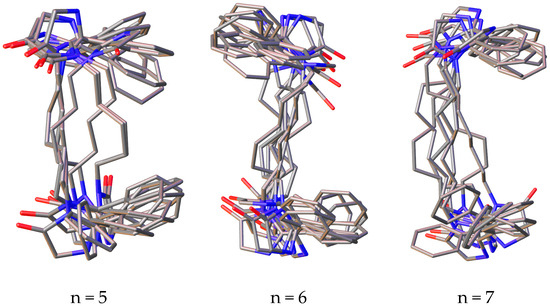
Figure 2.
Superposition of the 10 most stable conformations of compounds 1a–f with the number of methylene units in the “spacer” from 2 to 7.
It can also be noted that in a number of alkanediylbisimidazolones, the anti-oriented arrangement of the rings is more preferable (with n = 2, 3 and 6), while in other representatives of the series, there is a pronounced tendency toward the co-orientation of the phenyl rings. It should be noted that none of the representatives of a number of conformations is observed in which it would be possible for the protons of γ-methylene units or more distant ones to approach the phenyl rings, which is in agreement with the lack of correlations in the two-dimensional COSY NMR spectra.
For all studied conformers, an analysis of the key distances between the protons of the phenyl ring and protons in the α- and β-positions was carried out, the spread of which is given in Table 1.

Table 1.
Interatomic distances Ph-αCH2 and Ph-βCH2 (Å) in the conformations of compounds 1a–f.
As a result of the analysis of the data array, it turned out that regardless of the length of the alkyl chain, the average distance between the protons of the aromatic ring and the protons of the diamine fragment in the α-position is 2.050 Å, and in the β-position, 3.620 Å in space is consistent with the data obtained from two-dimensional NMR experiments, in which these long-range correlations were observed, confirming the interaction of protons through space.
4. Conclusions
Thus, using the activation of alkane-1,n-diammonium dihippurates via microwave radiation and an experimentally selected conversion scheme in the presence of catalytic amounts of water, it was possible to isolate the products of double heterocyclization, i.e., alkandiyl-bis(phenyldihydroimidazolones), in good yields, and characterize them spectrally to establish some features of their spatial structure and conformational potential, as well as identify characteristic structural features and their influence on the spectral characteristics, in particular, in two-dimensional NMR correlation spectroscopy.
Author Contributions
Conceptualization, V.S.G.; methodology, V.S.G.; software, V.S.G. and A.A.L.; validation, V.S.G. and A.Y.Y.; formal analysis, A.A.L.; investigation, A.A.L.; resources, V.S.G. and A.Y.Y.; writing—original draft preparation, V.S.G. and A.A.L.; writing—review and editing, V.S.G.; supervision, V.S.G. and A.Y.Y. All authors have read and agreed to the published version of the manuscript.
Funding
The work was financially supported by the Russian Science Foundation (grant no. 22-23-00171 to V.S. Grinev).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Menger, F.M.; Littau, C.A. Gemini surfactants: A new class of self–assembling molecules. J. Am. Chem. Soc. 1993, 115, 10083–10090. [Google Scholar] [CrossRef]
- Vereshchagin, A.N.; Frolov, N.A.; Egorova, K.S.; Seitkalieva, M.M.; Ananikov, V.P. Quaternary Ammonium Compounds (QACs) and Ionic Liquids (ILs) as Biocides: From Simple Antiseptics to Tunable Antimicrobials. Int. J. Mol. Sci. 2021, 22, 6793. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).