Abstract
In this manuscript, we present the synthesis and structural identification of two novel glycodrugs derived from benzocaine and procaine. D-galactose was converted into a substrate suitable for the introduction of amino derivatives at the C6 position. The glycodrugs were obtained in good yields through a two-step process involving the formation of an imine followed by subsequent reduction.
1. Introduction
The need for new bioactive compounds warrants research in the field of organic synthesis to discover novel chemotherapeutics. The p-aminobenzoate moiety has been demonstrated as a potent substructure in medicinal chemistry. Several derivatives have been synthesized and their antibacterial and anticancer activities have been analyzed, yielding promising results [1,2,3].
In glycodrugs, the specific biological activity cannot be exclusively attributed to the aglycone moiety. It has been well demonstrated that the sugar residue may play a crucial role in therapeutic efficiency by modifying transport through various biological barriers or interacting with receptors or lectins on the cell surface [4,5].
As part of our ongoing efforts to synthesize novel glycosides [6,7,8,9,10], in the present work, we investigate the synthesis and structural analysis of novel benzocaine and procaine D-galactose derivatives. The molecule design incorporates two principles: the attachment of the bioactive aglycone at a non-anomeric position and, through a nitrogen atom, these structural features generate novel compounds that differ from classical O-glycosides, thereby creating molecular diversity to achieve more accurate structure–activity relationships [11,12].
2. Material and Methods
2.1. General
Unless otherwise noted, commercially available reagents were used without further purification. All reactions were performed in oven-dried glassware and monitored by thin layer chromatography (TLC) on silica gel (Merck 60 F254 plates, Merck, Darmstadt, Germany), using either UV light (254 nm) or sulfuric acid stain for compound detection. Flash chromatography was developed using silica gel (230–400 mesh).
1H and 13C NMR spectra were obtained at 25 °C using a Bruker UltraShield 14.1 T with shim Boss II (Probe multinuclear Bruker Smart Probe BBFO 5 mm, Bruker, Billerica, MA, USA) at 600.13 MHz and 150.91 MHz, respectively. HSQC and COSY spectra were unambiguously used to aid peak assignments in 1H and 13C NMR.
2.2. Synthesis of 1,2:3,4-Di-O-isopropylidene-α-D-galactohexodialdo-1,5-pyranose (1)
Compound 1 was prepared from 1,2:3,4-di-O-isopropylidene-α-D-galactopyranose in dimethyl sulfoxide and toluene with pyridine, phosphoric acid, and N,N′-dicyclohexylcarbodiimide according to already known procedures [13].
2.3. Synthesis of Glycodrugs
To a solution of 1 (1.2 mmol) in anhydrous DCM (14 mL) were added the arylamine (1 eq.) and molecular sieves (4 Å MS). The reaction mixture was stirred for 24 h at reflux. The mixture was filtered and concentrated under reduced pressure.
The residue (2) was dissolved in 7 mL of anhydrous isopropanol and NaBH4 was added (1 eq.). This suspension was stirred at room temperature for 24 h. After adding DCM (35 mL), the reaction mixture was filtered and washed with water (3 × 12 mL); the resulting organic phase was dried over NaSO4, filtered and concentrated under reduced pressure. The residue was purified by silica gel column chromatography using solvent mixtures yielding products 3a and 3b.
3. Results and Discussion
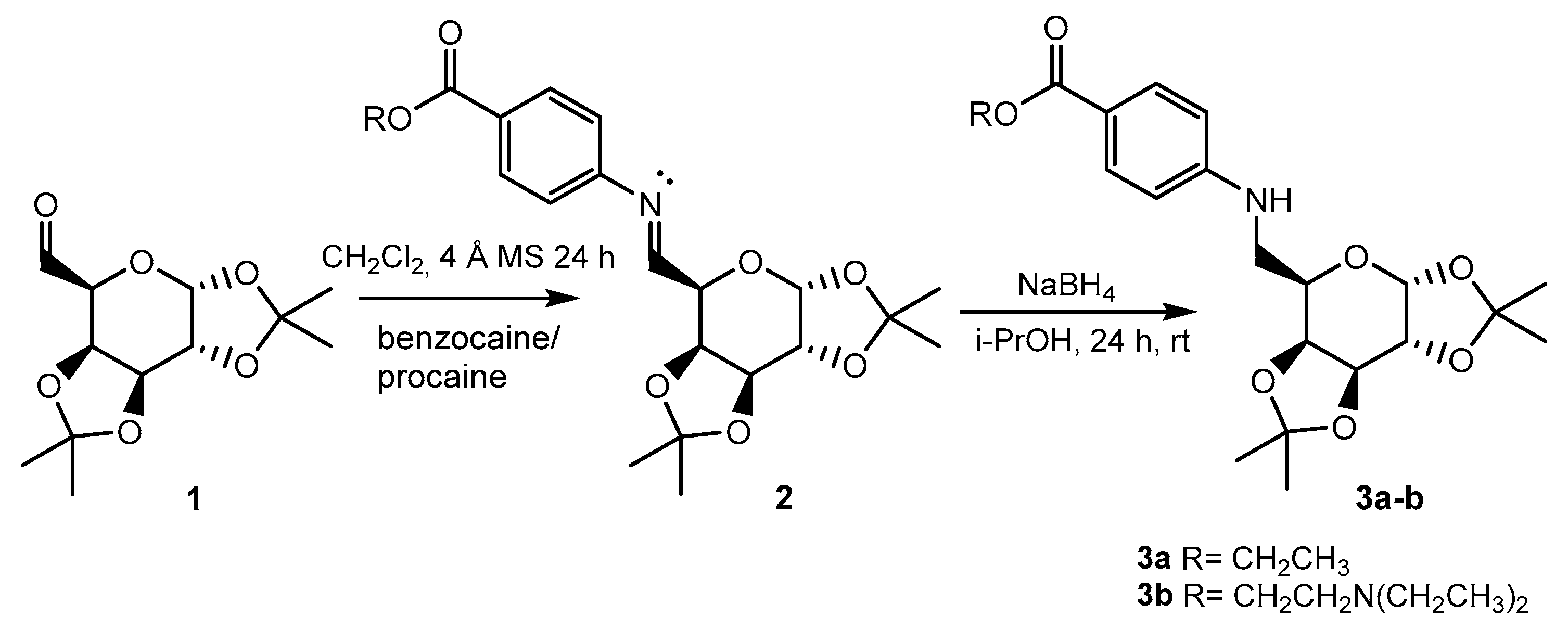
We synthesized 6-N-galactosyl derivatives of p-aminobenzoate using a two-step methodology with good yields (Figure 1).
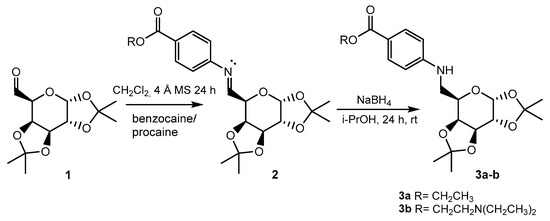
Figure 1.
Synthetic route developed for the synthesis of novel p-aminobenzoate glycodrugs.
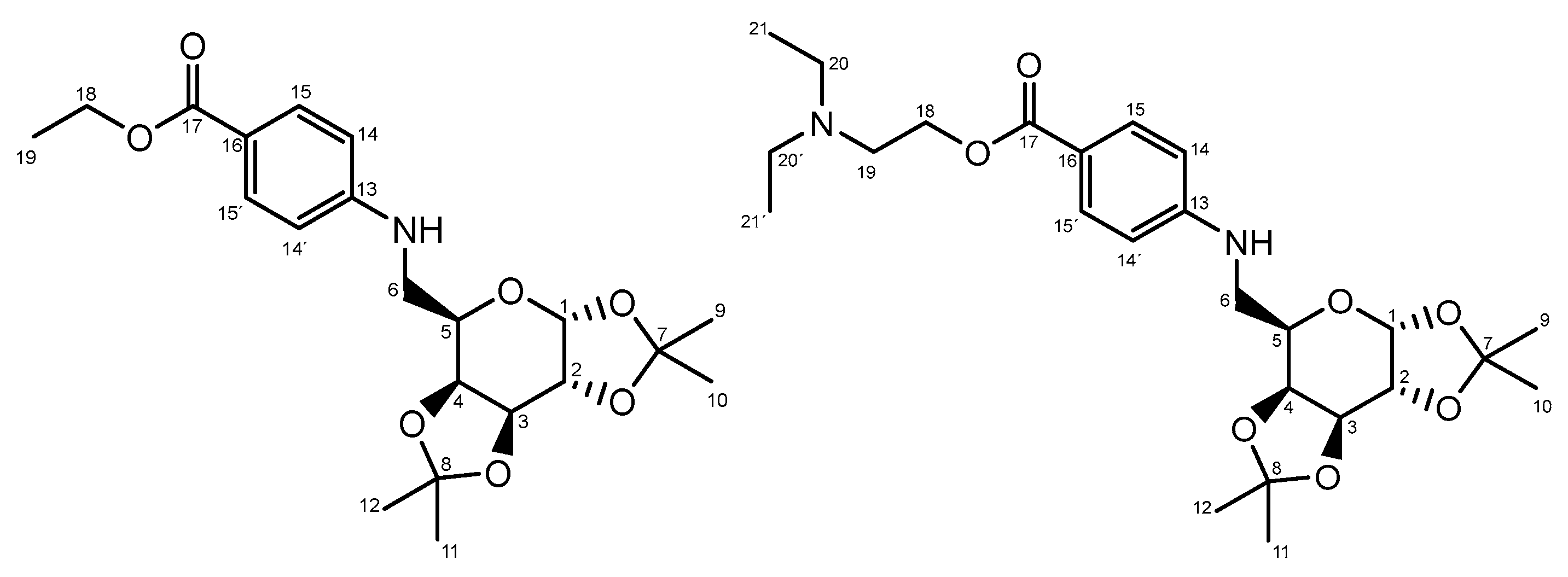
The oxidation of 1,2:3,4-di-O-isopropylidene-α-D-galactopyranose with DMSO/DCC yielded the corresponding galactosyl aldehyde. This aldehyde subsequently reacted with arylamines (benzocaine or procaine) in dichloromethane, forming the Schiff bases. The imine functional group was then reduced with NaBH4 in isopropanol. The resulting galactosyl derivatives of benzocaine (3a) and procaine (3b) were carefully purified (overall yields: 54 and 52%, respectively) and subsequently fully characterized using NMR spectroscopy (Figure 2).
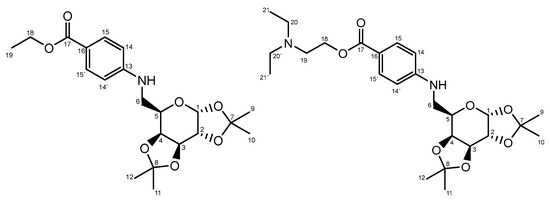
Figure 2.
Description and numbering of the novel products obtained: 3a and 3b.
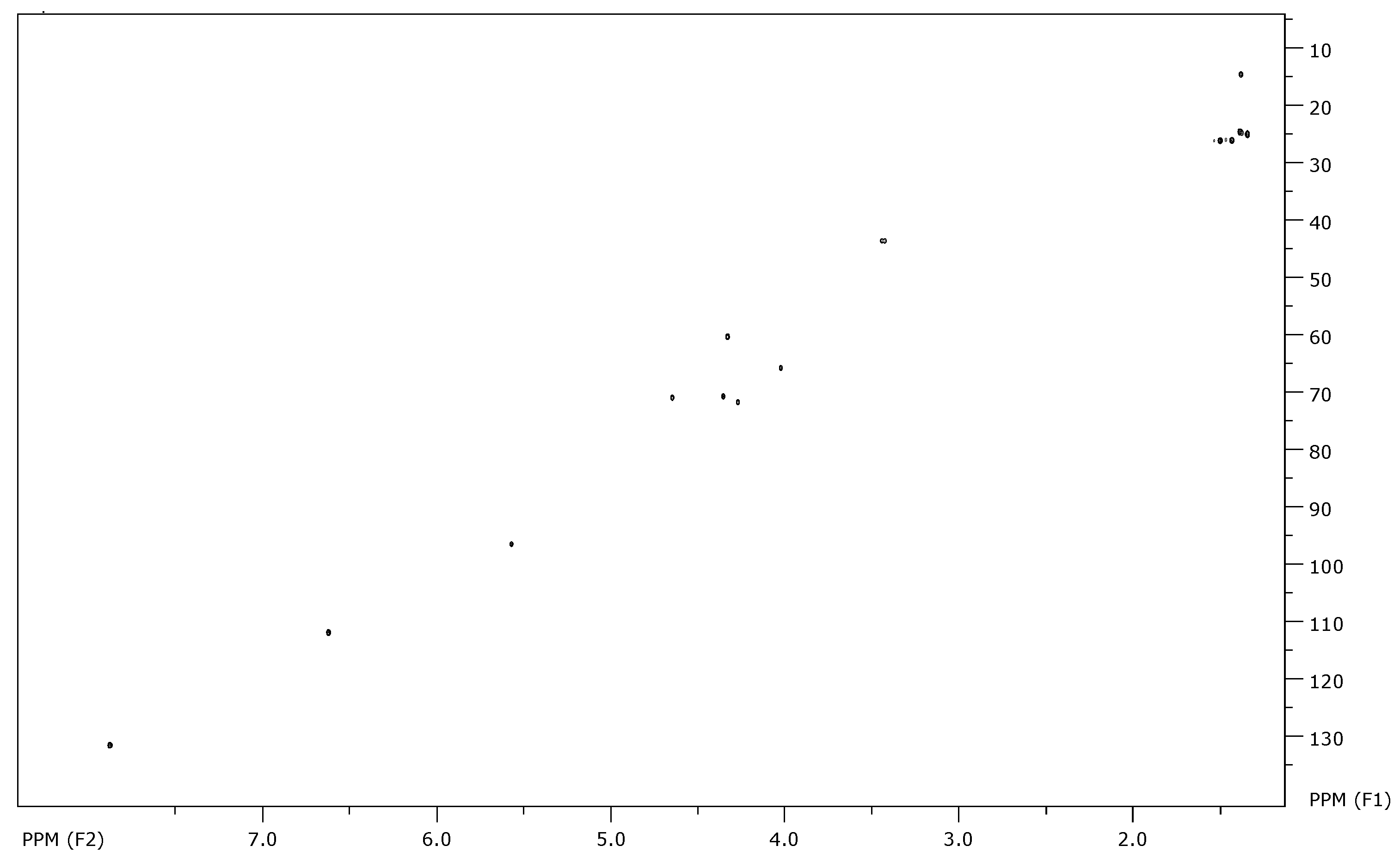
At first, the gHSQC NMR spectra enable the rapid identification of the obtained products. The characteristic signals of the molecule were easily identified (Figure 3).
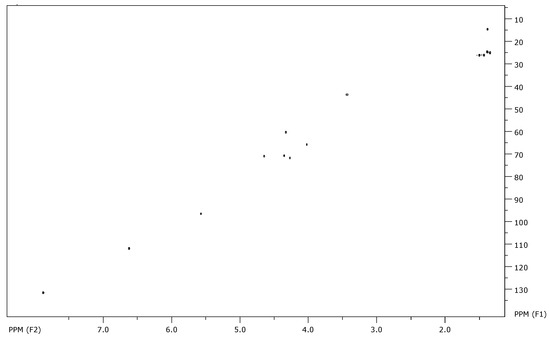
Figure 3.
gHSQC spectra of the benzocaine derived glycodrug (3a).
The signals of the sugar moiety were as expected, comprising four CH₃ signals corresponding to the acetal protecting groups, four CH signals from the carbohydrate ring, one signal from the anomeric center, and one CH₂-N methylene signal. Additionally, the presence of p-aminobenzoate derivatives in the glycodrug structure was confirmed by the aromatic and ethyl signals. A comprehensive description of the analytical data extracted from the 1H and 13C NMR spectra is provided herein.
3a. Rf: 0.55 (Hexane/ethyl acetate 7:3); 1H NMR (500 MHz, CDCl3) δ 7.88 (d, J = 8.7 Hz, 2H, H15 and 15′), 6.62 (d, J = 8.7 Hz, 2H, H14 and 14′), 5.57 (d, J = 5.0 Hz, 1H, H1), 4.65 (dd, J = 8.0, 2.4 Hz, 1H, H3), 4.37–4.31 (m, 3H, H2 and H18), 4.27 (dd, J = 7.9, 1.6 Hz, 1H, H4), 4.04–4.00 (m, 1H, H5), 3.43 (qdd, J = 13.2, 7.8, 4.6 Hz, 2H, H6), 1.50 (s, 3H, CH3), 1.43 (s, 3H, CH3), 1.38 (d, J = 3.3 Hz, 6H CH3 and H19), 1.34 (s, 3H, CH3); 13C NMR (126 MHz, CDCl3); δ 166.86 (C17), 151.81 (C13), 131.48 (C15 and C15′), 119.03 (C16), 111.83 (C14 and C14′), 109.57, 108.80 (C7, C8), 96.42 (C1), 71.63 (C4), 70.83 (C3), 70.59 (C2), 65.65 (C5), 60.21(C18), 43.48 (C6), 26.01, 25.96, 24.94, 24.43 (C9, C10, C11, C12) 14.46 (C19).
3b. Rf: 0.46 (Dichloromethane/methanol 10:1); 1H NMR (500 MHz, CDCl3) δ 7.86 (d, J = 8.4 Hz, 2H, H15 and 15′), 6.62 (d, J = 8.7 Hz, 2H, H14 and 14′), 5.57 (d, J = 5.0 Hz, 1H, H1), 4.65 (dd, J = 8.0, 2.2 Hz, 1H, H3), 4.42 (t, J = 5.7 Hz, 2H, H18), 4.35 (dd, J = 5.0, 2.4 Hz, 1H, H2), 4.29–4.25 (m, 1H, H4), 4.02 (m, 1H, H5), 3.43 (ddd, J = 12.6, 7.9, 4.9 Hz, 2H, H6), 2.96–2.92 (m, 2H, H19), 2.75–2.71 (m, 4H, H20 and H20′), 1.50 (s, 3H, CH3), 1.43 (s, 3H, CH3 ), 1.38 (s, 3H, CH3), 1.34 (s, 3H, CH3), 1.14 (t, J = 7.3 Hz, 6H, H21 and H21′); 13C NMR (126 MHz, CDCl3); δ 166.68 (C17), 151.99 (C13), 131.59 (C15 and C15′), 118.49 (C16), 111.86 (C14 and C14′), 109.58, 108.80 (C7, C8), 96.42 (C1), 71.62 (C4), 70.82 (C3), 70.58 (C2), 65.61 (C5), 62.03 (C18), 50.88 (C19), 47.75 (C20 and C20′), 43.45 (C6), 26.01, 25.94, 24.93, 24.43, (C9, C10, C11, C12), 11.64 (C21 and C21′).
4. Conclusions
We developed a synthetic methodology to prepare glycodrugs derived from benzocaine and procaine. D-galactose substituted at the C6 position with p-aminobenzoate derivatives was obtained in good yields following a two-step process. The products were identified and characterized by NMR spectroscopy. In the future, we plan to remove the acetal protective groups and conduct preliminary biological assays.
Author Contributions
Conceptualization, A.P.; methodology, N.B.R. and J.E.; formal analysis, N.B.R. and C.C.S.; investigation, N.B.R. and J.E.; resources, A.P.; writing—original draft preparation, N.B.R.; writing—review and editing, A.P. and C.C.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by UNLP grant number X988 and CONICET grant number PIP 2021-2023 of Argentina.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors wish to thank CICPBA for technical support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- El-Zahabi, H.S.; Nossier, E.S.; Mousa, S.M.; Hassan, H.; Shalaby, A.S.G.; Arafa, R.K. Antibacterial and anticancer profiling of new benzocaine derivatives: Design, synthesis, and molecular mechanism of action. Arch. Pharm. 2022, 355, 2100451. [Google Scholar] [CrossRef] [PubMed]
- Chrubasik, S.; Beime, B.; Magora, F. Efficacy of a benzocaine lozenge in the treatment of uncomplicated sore throat. Eur. Arch. Otorhinol. 2012, 269, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Villar-Garea, A.; Fraga, M.F.; Espada, J.; Esteller, M. Procaine is a DNA-demethylating agent with growth-inhibitory effects in human cancer cells. Cancer Res. 2003, 63, 4984–4989. [Google Scholar]
- Křen, V.; Martínková, L. Glycosides in medicine: “The role of glycosidic residue in biological activity”. Curr. Med. Chem. 2001, 8, 1303–1328. [Google Scholar] [CrossRef] [PubMed]
- Křen, V.; Řezanka, T. Sweet antibiotics–the role of glycosidic residues in antibiotic and antitumor activity and their randomization. FEMS Microbiol. Rev. 2008, 32, 858–889. [Google Scholar] [CrossRef] [PubMed]
- Rojas, A.H.; Lafuente, L.; Echeverría, G.A.; Piro, O.E.; Vetere, V.; Ponzinibbio, A. Structure investigation on a novel 2-halo-2, 3-unsaturated-N-galactoside, NMR and X-ray diffraction of a monoclinic multidomain crystal. Carbohydr. Res. 2021, 510, 108457. [Google Scholar] [CrossRef] [PubMed]
- Rojas, A.H.; Lafuente, L.; Echeverría, G.A.; Piro, O.E.; Vetere, V.; Ponzinibbio, A. Synthesis and structure of novel iodinated N-glycosyl-sulfonamides through Aza-Ferrier reaction of 2-substituted glycals. Tetrahedron Lett. 2020, 61, 152282. [Google Scholar] [CrossRef]
- Lafuente, L.; Rojas, A.H.; Piro, O.E.; Echeverría, G.A.; Ponzinibbio, A. Synthesis, NMR and X-ray studies on novel heteroaromatic aldoxime O-ether 2-and 2, 3-unsaturated glycosides. Tetrahedron Lett. 2020, 61, 152241. [Google Scholar] [CrossRef]
- Lafuente, L.; Santiago, C.C.; Rojas, A.H.; Piro, O.E.; Echeverría, G.A.; Ponzinibbio, A. Selective synthesis and molecular structure of novel aminooxyglycosyl derivatives bearing hydroxyphenyl moieties. ChemistrySelect 2020, 5, 864. [Google Scholar] [CrossRef]
- Bejarano, N.; Lafuente, L.; Esteche, J.; Santiago, C.C.; Rojas, A.H.; Ponzinibbio, A. Synthesis and Structure of Novel Potentially Bioactive Amphiphilic-O-(N)-Glycosides. Chem. Proc. 2021, 3, 100. [Google Scholar]
- Gerry, C.J.; Schreiber, S.L. Recent achievements and current trajectories of diversity-oriented synthesis. Curr. Opin. Chem. Biol. 2020, 56, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Scott, K.A.; Ropek, N.; Melillo, B.; Schreiber, S.L.; Cravatt, B.F.; Vinogradova, E.V. Stereochemical diversity as a source of discovery in chemical biology. Curr. Res. Chem. Biol. 2022, 2, 100028. [Google Scholar] [CrossRef]
- Butterworth, R.F.; Hanessian, S. Selected Methods of Oxidation in Carbohydrate Chemistry. Synthesis 1971, 1971, 70–88. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).