Abstract
Ricinine is an alkaloid of Ricinus communis that displays numerous biological properties. Ricinine (4-methoxy-1-methyl-2-oxo-dihydropyridine-3-carbonitrile)) and new N-analogues of Ricinine were obtained by the reaction of ethyl α-ethoxyethylidenecyanoacetate with various amines. Earlier, ethyl α-ethoxyethylidenecyanoacetate was easily prepared from ethyl cyanoacetate. Biologically, amines such as tryptamine and histamine were used in order to introduce a second pharmacophore on the target molecule.
1. Introduction
Ricinine (4-methoxy-1-methyl-2-oxo-dihydropyridine-3-carbonitrile) is a simple pyridinone alkaloid that was isolated by Tuson in 1864 [1] from castor-oil seed (Ricinus communis). Ricinine is very poorly toxic in comparison to the protein Ricin, which is also present in castor-oil seed. Ricinine exhibits insecticidal properties and is used against leaf-cutting ant (Atta sexdens rubropilosa) [2,3,4]. In the field of medicine, it inhibits the cellular entry of calcium ions and displays cardiotonic properties [5], in addition to analgesic [6] and anti-leukemic ones [7,8].
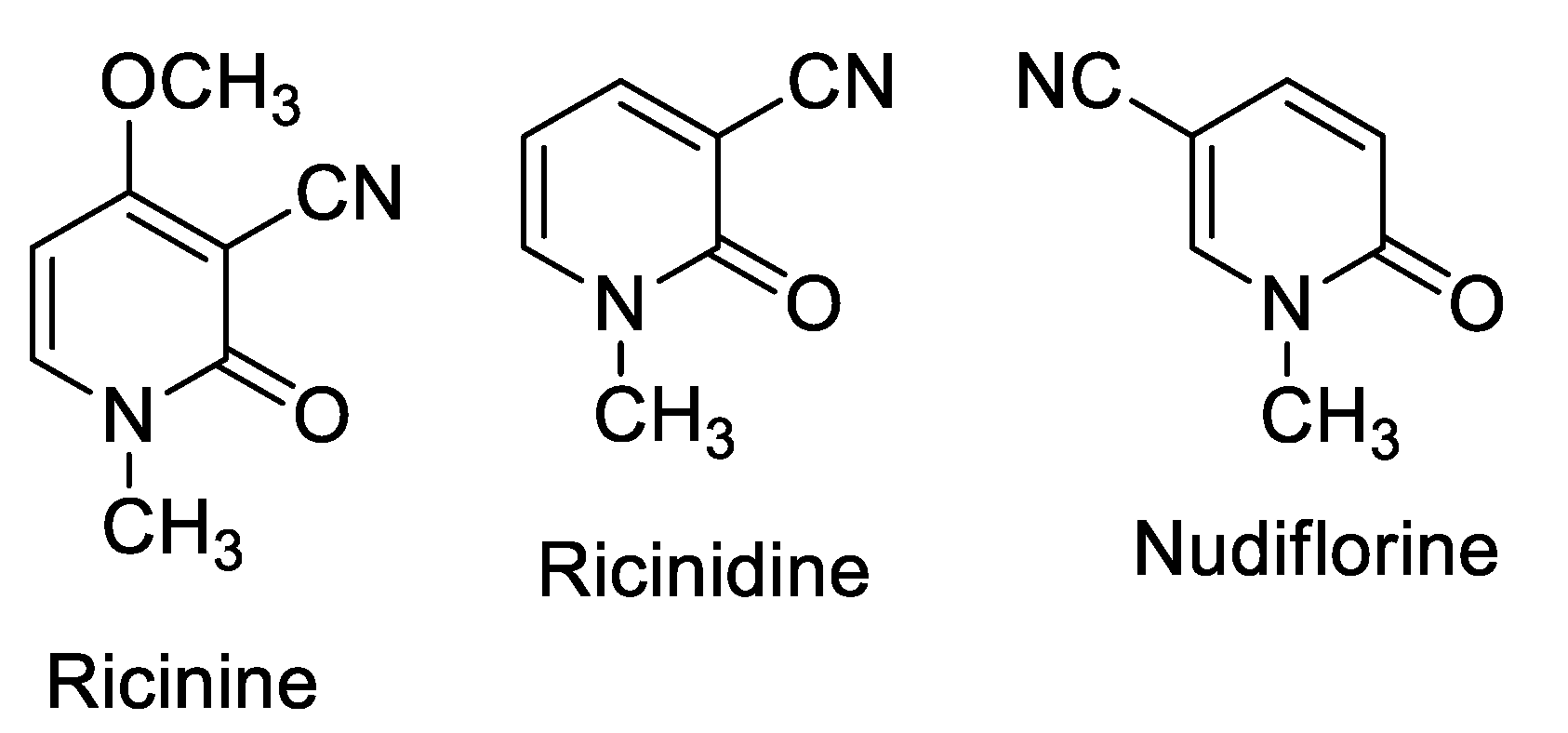
Ricinine is a cyanopyridinone, according to Späth and Köler [9]; many other cyanopyridinone alkaloids are well-known, such as Nudiflorine and Ricinidine [10] (Figure 1).
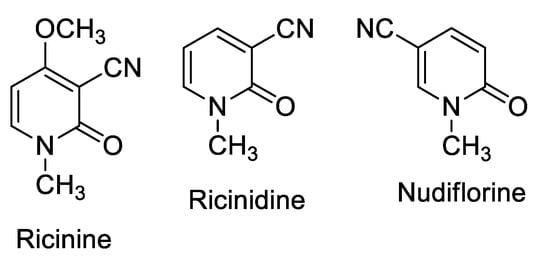
Figure 1.
Structure of Ricinine and two natural cyanopyridines, Ricinidine and Nudiflorine.
Many syntheses of Ricinine are described in the literature [11]; the earliest syntheses were based on the pyridine ring transformation in many steps. Moreover, cyanopyridin-2(1H)-one analogues of Ricinine, which are important chemical intermediates, were the subject of many synthetic approaches [11]. On the other hand, the N-analogues of Ricinine are poorly described in the literature. However, Cid et al. [12,13] have recently synthesized some alkyl derivatives by alkylation of 2-hydroxy-4-methoxynicotinonitrile, according to the method described by Juneck [11] for the synthesis of Ricinine. However, the synthesis of 2-hydroxy-4-methoxynicotinonitrile requires multistep reactions (>3), and the alkyl halides used are not easily commercially available, especially when heterocycles are involved such as histidine or tryptamine.
On the other side, we have previously described the formation of the pyridinone cycle in the synthesis of Cerpegin, by the reaction of amines with (dimethylamino)pentadienoate, obtained by a sequence involving the reactant DMF-DMA (dimethylformamide acetals) [14]. Other analogous syntheses of cyanopyridinones, using DMF-DMA, were described in the literature [15], but not the synthesis of Ricinine.
2. Results and Discussion
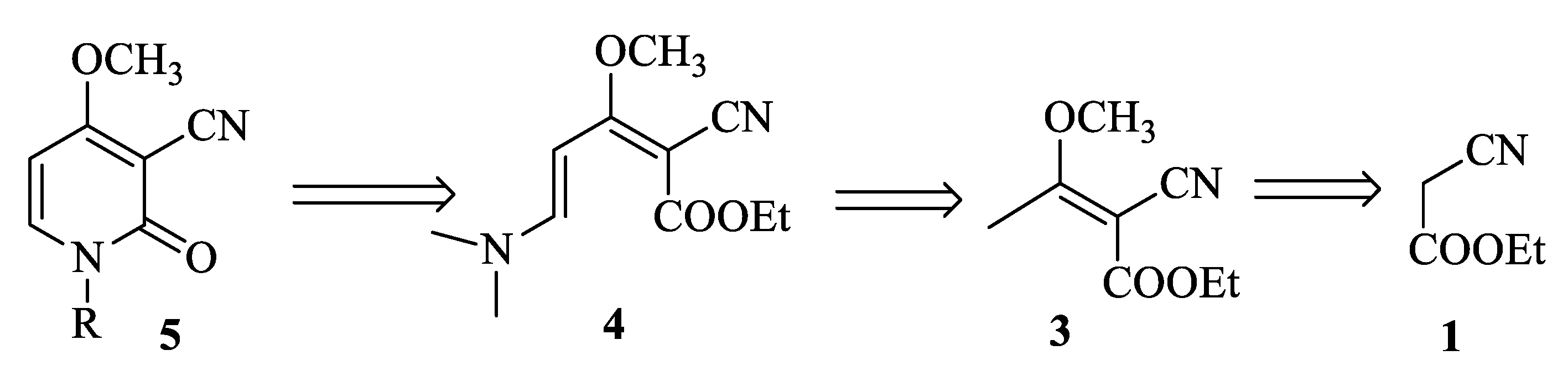
We propose herein the synthesis of Ricinidine and N-derivatives according to the retrosynthetic Scheme 1.
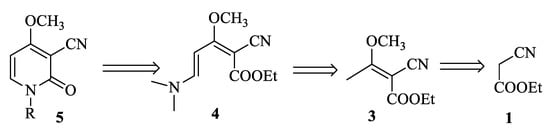
Scheme 1.
Retrosynthesis of N-derivatives of Ricinine.
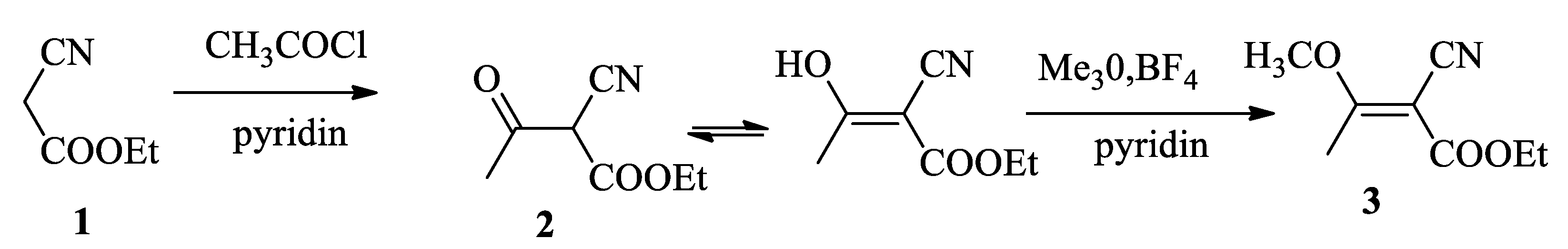
Initially, our attempts to synthesize 2-cyano-3-methoxybut-2-enoate (3) by acylation of ethyl cyanoacetate (1) according to the former literature [16] were unsuccessful. We have obtained a mixture of enol and acetyl derivatives which was methylated by trimethyloxonium fluoroborate into (3) (Scheme 2).
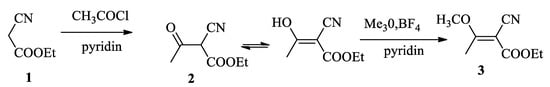
Scheme 2.
Synthesis of 2-cyano-3-methoxybut-2-enoate (3) by acylation of ethyl cyanoacetate followed by methylation with Me3O, BF4.
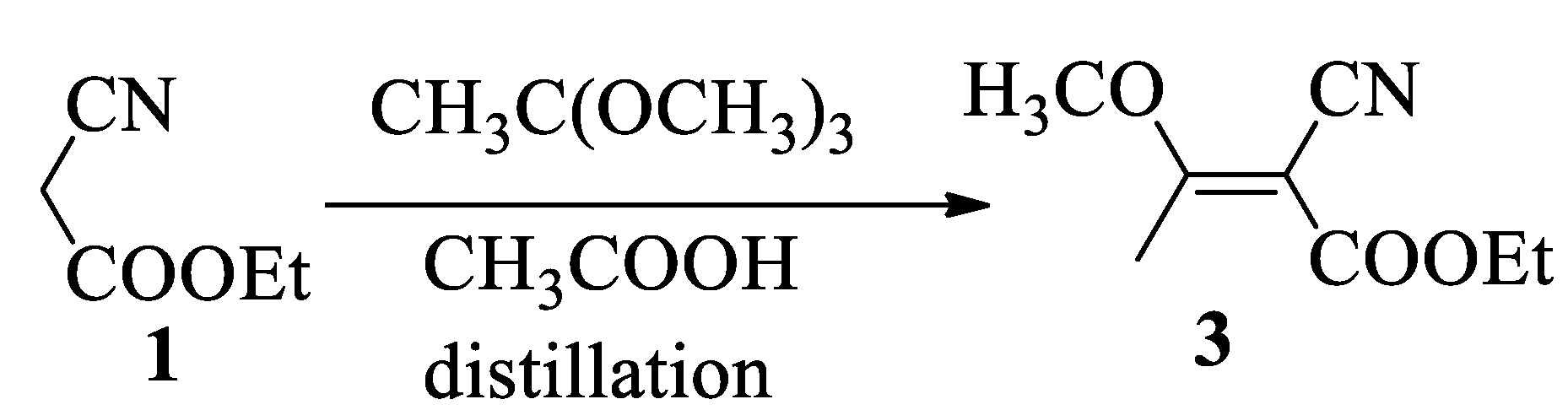
A better method to obtain the cyanobutenoate (3) was to apply the one-step reaction of trimethoxyethane with ethyl cyanoacetate (1) catalyzed by acetic acid, according to a modified process described by Nicholl with malonitrile [17], see Scheme 3. This approach leads to compound (3) with a yield of 70%.
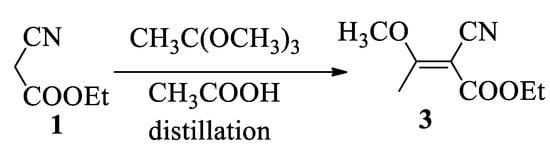
Scheme 3.
Synthesis of 2-cyano-3-methoxybut-2-enoate (3) by reaction with trimethoxyethane.
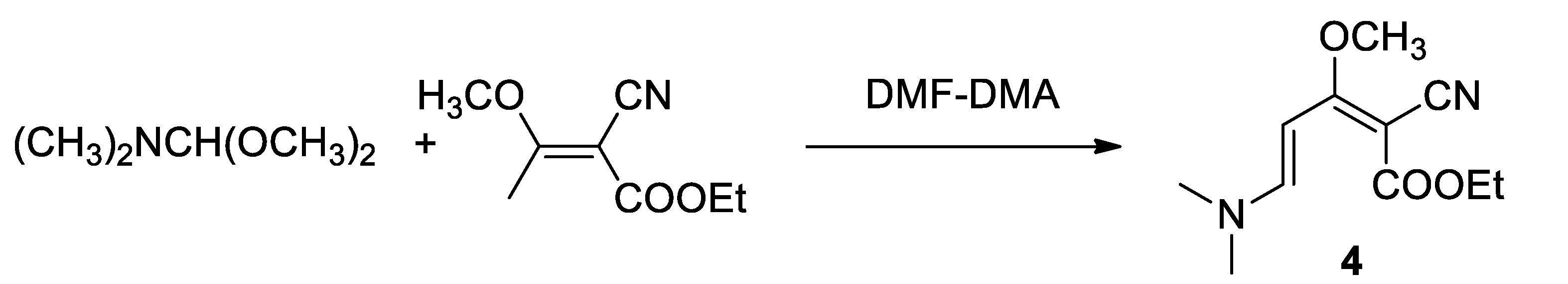
By the reaction of the commercially available dimethyl formamide dimethylacetal (DMFDMA), the cyanobutenoate (3) was then converted into (4), according to Kasum and Prager in the synthesis of Perloline [18] according to the Scheme 4.
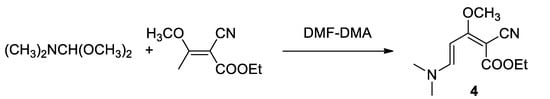
Scheme 4.
Synthesis of (dimethylamino)pentadienoate (4) with DMF-DMA.
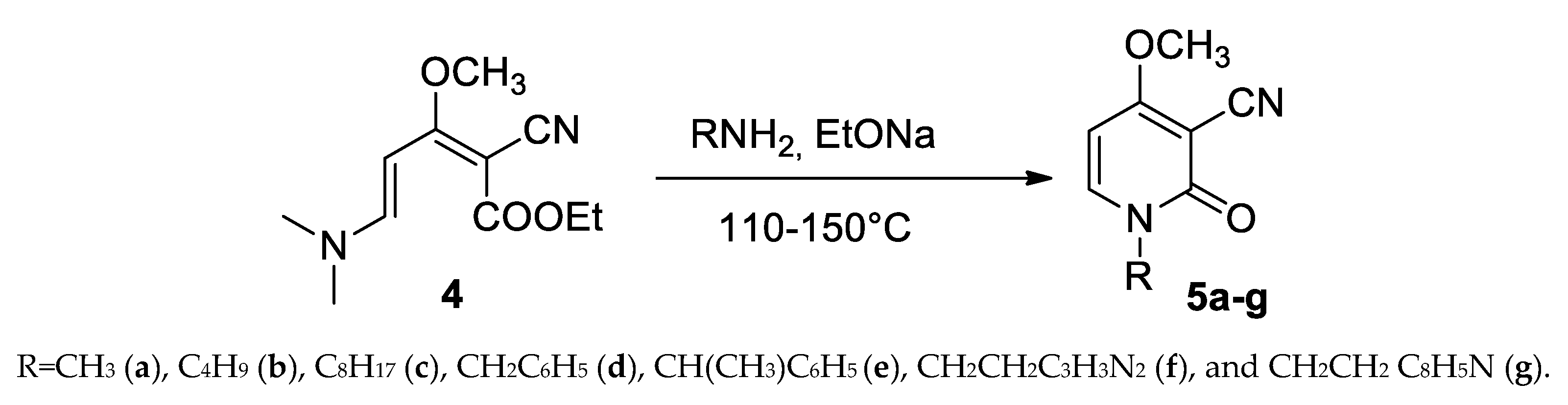
The reaction of (dimethylamino)pentadienoate (4) in the presence of sodium ethoxide as the catalyst affords the synthesis of Ricinine with methylamine or N-derivatives with other primary amines, according to the Scheme 5.
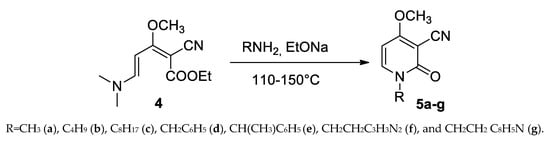
Scheme 5.
Synthesis of Ricinine (5a) and N-analogues of Ricinine (5b–g).
This method allows for the preparation of the butyl, octyl, benzyl, methylbenzyl, and the derivatives of biologically active tryptamine and histidine, permitting the introduction of a second pharmacophore group in the target molecule.
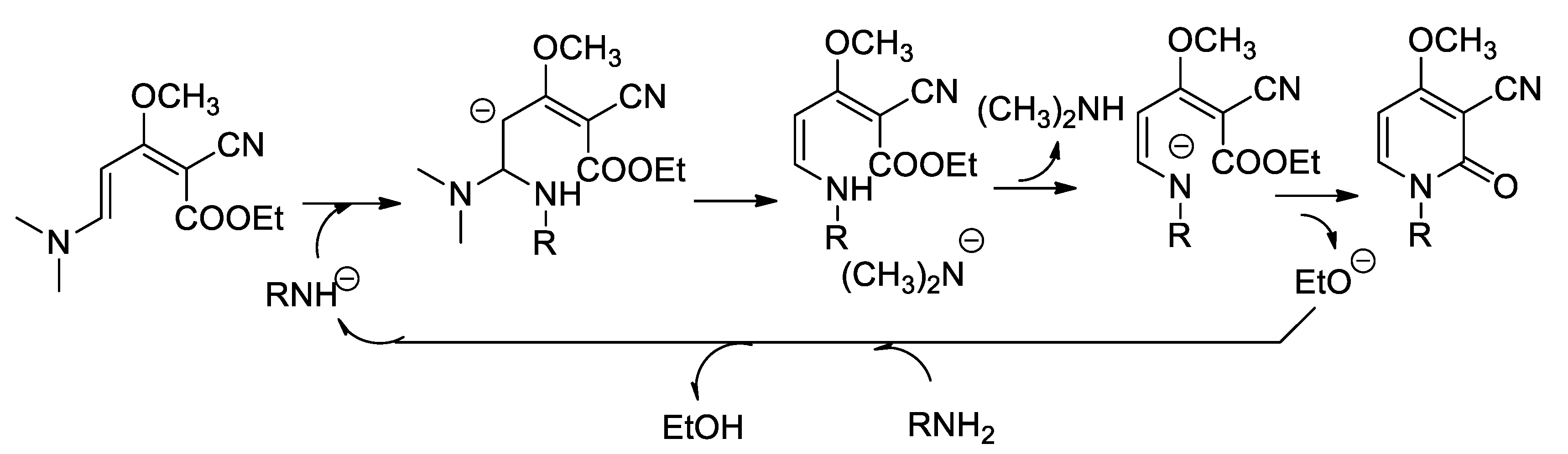
On the other side, the formation of 2-pyridinone by ester-amine cyclization is well documented [19]. We propose the mechanism of formation of the pyridinone cycle by a basic catalysis according to Scheme 6:
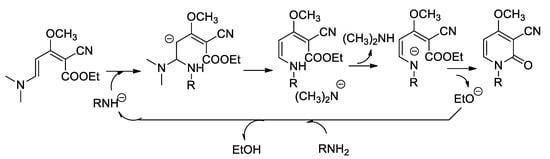
Scheme 6.
Mechanism of formation of the pyridinones by basic catalysis.
The results that were obtained are reported in Table 1. New compounds were characterized by 1H and 13C NMR and mass spectroscopy.

Table 1.
Synthesis of Ricinine (5a) and N-analogues of Ricinine (5b–g).
As the two final steps of the sequence are thermal reaction, the multicomponent approach from ethyl cyanomalonate (3) (1 eq), DMF-DMA (1.2 eq), and benzylamine (1 eq) was investigated, but, in these conditions, a mixture of different products with only a very poor yield of Ricinine derivative (5d) was obtained.
In conclusion, the reaction of primary amines with the ethyl 2-cyano-1-methoxy-5-(dimethylamino)pentadienoate in the presence of sodium ethoxide produced N-derivatives of Ricinine. This reaction allows for a simple and easy synthesis of a variety of N-substituted Ricinine derivatives. The biological properties of these new compounds (5b–g) are being tested.
3. Experimental
Melting points were measured on a Koffler apparatus and are reported uncorrected. IR spectra were obtained with a Fourier-transform Perkin-Elmer Spectrum One with ATR accessory. The frequencies of absorption are given in cm−1. Only significant absorptions are listed. 1H NMR (400 MHz) and 13C NMR (100 MHz) spectra were recorded while using CDCl3 with TMS as an internal standard on a Bruker DPX 400 NMR spectrometer. Chemical shifts are reported in ppm. Mass spectra were recorded on a Xevo G2- XS QTof WATERS, with a mass range of (50–1000 m/z), a source temperature of 120 °C, and a desolvatation temperature of 500 °C.
- (1)
- Synthesis of ethyl 2-cyano-3-methoxybut-2-enoate (3)
A mixture of 1,1,1-trimethoxyethane (0.3 mol, 37.5 mL), ethyl cyanoacetate (0.2 mol, 21 mL), and acetic acid (0.5 mL) was stirred and distillated. Three portions of 0.5 mL of acetic acid were added when approximately 6.9 and 12 mL of ethanol were collected. After the recovery of ethanol, the solution was cooled and evaporated under vaccuo. The mixture was crystallized in ethyl acetate.
Yellow solid, 1H NMR (400 MHz, CDCl3): 4.20 (q, J = 6.8 Hz, 2H), 4.01 (s, 3H), 2.61 (s, 3H), 1.30 (t, J = 6.8 Hz, 3H). 13C NMR (100 MHz, CDCl3): 184.5, 163.8, 115.1, 85.8, 58.1, 15.1, 15.1.
- (2)
- Synthesis of ethyl 2-cyano-5-(dimethylamino)-3-methoxypenta-2,4-dienoate (4)
Under a stream of nitrogen, a mixture of ethyl 2-cyano-3-methoxybut-2-enoate (29.6 mmol, 5.02 g) and dimethylformamide dimethyl acetal (44.4 mmol, 4 mL) was stirred and refluxed for 1 h. The reaction was followed by TLC (eluent 50% diethylether/50% ethyl acetate). After cooling under a stream of nitrogen, a viscous red solution was obtained by evaporation under vaccuo. The mixture was crystallized in ethyl acetate/diethylether. Yield = 64%. Mp = 97 °C (lit = 98–100 °C [16]).
1H NMR (400 MHz, CDCl3): 7.21–7.72 (m, 2H, CH=CH), 4.10 (q, J = 6.8 Hz, 2H), 4.02 (s, 3H), 3.11 (s, 3H), 2.90 (s, 3H), 1.22 (t, J = 6.8 Hz, 3H). 13C NMR (100 MHz, CDCl3): 181.8, 157.5, 153.1, 119.7, 91.7, 62.6, 60.9, 45.7, 14.5.
- (3)
- Synthesis of 2-cyano-3-methoxy-2-pyridones
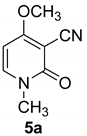
1-methyl-2-oxo-1,2-dihydropyridine-3-carbonitrile (5a) (Ricinine)
A mixture of enamine (1.12 g, 5 mmol), methylamine in ethanol (33%) (21 mmol), and sodium ethoxide (0.1 mmol) in dry DMF under argon was stirred and refluxed for 15 min. A viscous red solution was obtained. Purification using preparative thin-layer chromatography on silica with CHCl3/n-butanol/acetic acid (25/10/1) as eluant (Rf = 0.20, pale red) visualized in UV light was performed.
White crystals, Mp = 196–197 °C (ethanol) (Lit Mp = 197 °C [9]). IR: 2220 cm−1 (CN). 1H NMR (CDCl3) δ: 7.39 (d, 1 H, J = 8.0 Hz, CH); 5.94 (d, 1 H, J = 8.0 Hz, CH); 3.86 (s, 3H, OCH3); 3.43 (s, 3H, NCH3). 13C NMR (CDCl3) δ 186.3; 157.4 (CO); 134.2; 115.8 (CN); 99.7; 67.9; 57.7 (CH3); 38.9 (CH3)
HRMS (ESI-QTOF):calcd for C8H9N2O2 (M + H) 165.0664; found 165.0666.
General procedure for N-Ricinine derivatives: A mixture of enamine (2 mmol), primary amine (2.1 mmol), and sodium ethoxide (0.1 mmol) in dry DMF under argon was stirred and refluxed for 15 min. The viscous red-brown solution was chromatographed on silica with a mixture of diethyl ether/ethyl acetate/methanol = 1/1/0 to 1/1/0.5.
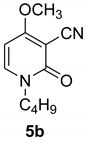
1-butyl-4-methoxy-2-oxo-1,2-dihydropyridine-3-carbonitrile (5b)
With 1-butylamine
White crystals, Mp = 249 °C. 1H NMR (CDCl3) δ: 7.39 (d, 1 H, J = 8.0 Hz, CH); 5.94 (d, 1 H, J = 8.0 Hz, CH); 3.86 (s, 3H, OCH3); 3.30 (t, 2H, NCH2);1.70 (m, 2H, CH2); 1.37 (m, 2H, CH2); 0.98 (t, 3H, CH3). 13C NMR (CDCl3) δ: 186.3; 157.4 (CO);134.2; 115.8 (CN); 99.7; 67.9; 57.7 (CH3); 47.6; 30.1; 20.2; 13.8. HRMS (ESI-QTOF): calcd for C11H15N2O2 (M + H) 207.2490; found 207.2501.
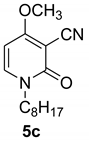
4-methoxy-1-octyl-2-oxo-1,2-dihydropyridine-3-carbonitrile (5c)
With 1-octylamine
White crystals, Mp = 319 °C. 1H NMR (CDCl3) δ: 7.39 (d, 1 H, J = 8.0 Hz, CH); 5.93 (d, 1 H, J = 8.0 Hz, CH); 3.86 (s, 3H, OCH3); 3.30 (t, 2H, NCH2); 1.75–1.48 (m, 10H, (CH2)5); 1.38 (m, 2H, CH2); 1.08 (t, 3H, CH3). 13C NMR (CDCl3) δ: 186.3; 157.4 (CO); 134.2; 115.8 (CN); 99.7; 67.8; 54.7 (CH3); 47.9; 31.9; 30.8; 29.3; 27.2; 22.8; 14.1. HRMS (ESI-QTOF): calcd for C15H23N2O2 (M + H) 263.1759; found 263.1756.
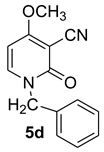
1-benzyl-4-methoxy-2-oxo-1,2-dihydropyridine-3-carbonitrile (5d)
With benzylamine
Mp = 325 °C (dec). 1H NMR (CDCl3) δ: 7.39 (d, 1H, J = 8.0 Hz, CH); 7.28–7.12 (m, 5H, Haro); 5.94 (d, 1H, J = 8.0 Hz, CH); 4.68 (s, 2H, CH2); 3.86 (s, 3H, OCH3). 13C NMR (CDCl3) δ: 186.3; 157.4 (CO); 136.5; 134.2; 128.9; 128.5; 126.7; 115.8 (CN); 99.7; 67.9; 57.7 (CH3); 50.4 (CH2). HRMS (ESI-QTOF): calcd for C14H13N2O2 (M + H) 241.0977; found 241.0980.
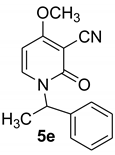
α-Methyl-1-benzyl-4-methoxy-2-oxo-1,2-dihydropyridine-3-carbonitrile (5e)
With methylbenzylamine
White crystals, Mp > 300 °C (dec). 1H NMR (CDCl3) δ: 7.39 (d, 1H, J = 8.0 Hz, CH); 7.28–7.12 (m, 5H, Haro); 5.94 (d, 1H, J = 8.0 Hz, CH); 6.24 (q, J = 8.0 Hz, CH); 3.86 (s, 3H, OCH3; 1.30 (d, J = 8.0 Hz, CH3). 13C NMR (CDCl3) δ: 186.3; 157.4 (CO); 140.0; 134.2; 128.9; 128.5; 126.7; 115.8 (CN); 99.7; 67.9; 57.7 (CH3); 50.4; 18.3 (CH3). HRMS (ESI-QTOF) calcd for C15H15N2O2 (M + H) 255.1133. Found 255.1132.
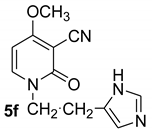
1-(21H-imidazol-5-yl)ethyl-4-methoxy-2-oxo-1,2-dihydropyridine-3-carbonitrile (5f)
With histidine
White crystals, Mp > 240 °C (dec). 1H NMR (CDCl3) δ: 8.45 (1H, broad s, NH); 7.44 (1H, s, NCHN); 7.39 (d, 1H, J = 8.0 Hz, CH); 5.94 (d, 1H, J = 8.0 Hz, CH); 6.86 (1H, CH=C); 3.86 (s, 3H, OCH3); 3.58 (t, J = 6.4 Hz, 2H, NCH2); 3.22 (t, J = 6.4 Hz, 2H, CH2). 13C NMR (CDCl3) δ: 186.3; 157.1 (CO); 135.5; 134.2; 133.5; 118.6; 115.8 (CN); 99.7; 67.9; 57.7; 48.5; 26.5. HRMS (ESI-QTOF) calcd for C12H13N4O2 (M + H) 245.1038. Found 245.1037.
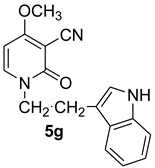
1-(2-(1H-indol-3-yl)ethyl-4-methoxy-2-oxo-1,2-dihydropyridine-3-carbonitrile (5g)
With tryptamine
White crystals, Mp > 250 °C (dec). 1H NMR (CDCl3) δ: 10.11 (broad s, 1H, NHindol); 7.39 (d, 1H, J = 8.0 Hz, CH); 7.25–7.14 (m, 4H, Haro); 6.44 (s, 1H, CH); 5.94 (d, 1H, J = 8.0 Hz, CH); 3.87 (t, J = 6.4 Hz, 2H, NCH2); 3.86 (s, 3H, OCH3); 3.13 (t, J = 6.4 Hz, 2H, CH2). 13C: δ: 186.3; 157.1 (CO); 136.5; 134.2; 127.4; 122.9; 121.7; 115.9; 115.8 (CN); 111.1; 99.7; 67.9; 57.7; 51.9 (CH2); 26.7 (CH2). HRMS (ESI-QTOF) calcd for C17H16N3O2 (M + H) 294.12425; found 294.1242.
4. Conclusions
In conclusion, the reaction of primary amines with the ethyl 2-cyano-1-methoxy-5-(dimethylamino)pentadienoate in the presence of sodium ethoxide produced N-derivatives of Ricinine. This reaction constitutes a convenient and versatile synthesis of a variety of N-substituted Ricinine derivatives from commercial primary amines. These compounds are much more available than the alkyl halides sometimes used. The biological properties of the new compounds (5b–5g) are currently being tested.
Author Contributions
F.F. and Z.K., experiments; N.B., review and editing; D.V., supervision, writing, and editing; N.C.-B., review. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tuson, R.V. Note on an alkaloïd contained in the seeds of the Ricinus communis, or castor-oil plant. J. Chem. Soc. 1864, 17, 195–197. [Google Scholar] [CrossRef] [Green Version]
- Siegler, E.H.; Schechter, M.S.; Haller, H.L. Toxicity of Ricin, Ricinine, and Related Compounds to Codling Moth Larvae. J. Econ. Èntomol. 1944, 37, 416–418. [Google Scholar] [CrossRef]
- Abadi, A.; Al-Deeb, O.; Al-Afify, A.; El-Kashef, H. Synthesis of 4-alkyl (aryl)-6-aryl-3-cyano-2(1H)-pyridinones and their 2-imino isosteres as nonsteroidal cardiotonic agents. Farmaco 1999, 54, 195–201. [Google Scholar] [CrossRef]
- Wheeler, T.N.; Shaffer, J.E.; Kenakin, T. Preparation of 3-cyano-6-methylpyridine-2-ones for Treating Cardiovascular Disease. E.P. Patent 419286, 27 March 1991. [Google Scholar]
- Manna, F.; Chimenti, F.; Bolasco, A.; Rossi, F.; Cenicola, M.; D’Amico, M.; Parrillo, C. Antiinflammatory, Analgesic and Antipyfuztic 4,6-Disubstituted 3-Cyanopyridine-2-ones and 3-Cyano- 2-aminopyridines. Pharmacol. Res. 1992, 26, 267–277. [Google Scholar] [CrossRef]
- Abadi, A.H.; Al-Khamees, H.A. 3-Cyano-4,6-disubstituted-2(1H)-imino or oxopyridines: New Antineoplastic Agents with High Selectivity Towards Leukemia Cell Lines. Arch. Pharm. (Weinheim) 1998, 331, 319–324. [Google Scholar] [CrossRef]
- Cocco, M.T.; Congiu, C.; Onnis, V. Synthesis and antitumour activity of 4-hydroxy-2-pyridone derivatives. Eur. J. Med. Chem. 2000, 35, 545–552. [Google Scholar] [CrossRef]
- Abadi, A.H.; Ibrahim, T.; Abouzid, K.M.; Lehmann, J.; Tinsley, H.N.; Gary, B.D.; Piazza, G. Design, synthesis and biological evaluation of novel pyridine derivatives as anticancer agents and phosphodiesterase 3 inhibitors. Bioorgan. Med. Chem. 2009, 17, 5974–5982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Späth, E.; Koller, G. Die Synthese des Ricinins. Berichte Dtsch. Chem. Ges. 1923, 56, 2454–2460. [Google Scholar] [CrossRef]
- Marion, L. The Alkaloids: Chemistry and Physiology; Manske, R.H.E., Holmes, H.G., Eds.; Academic Press: New York, NY, USA, 1950. [Google Scholar]
- Mittelbach, M.; Kastner, G.; Junek, H. Ricinin—Einfach synthetisiert. Monatsh. Chem. 1984, 115, 1467–1470. [Google Scholar] [CrossRef]
- Cid, J.M.; Duvey, G.; Tresadern, G.; Nhem, V.; Furnari, R.; Cluzeau, P.; Vega, J.A.; de Lucas, A.I.; Matesanz, E.; Alonso, J.M.; et al. Discovery of 1,4-Disubstituted 3-Cyano-2-pyridones: A New Class of Positive Allosteric Modulators of the Metabotropic Glutamate 2 Receptor. J. Med. Chem. 2012, 55, 2388–2405. [Google Scholar] [CrossRef] [PubMed]
- Cid, J.M.; Tresadern, G.; Duvey, G.; Lütjens, R.; Finn, T.; Rocher, J.P.; Poli, S.; Vega, J.A.; de Lucas, A.I.; Matesanz, E.; et al. Discovery of 1-Butyl-3-chloro-4-(4-phenyl-1-piperidinyl)- (1H)-pyridone (JNJ-40411813): A Novel Positive Allosteric Modulator of the Metabotropic Glutamate 2 Receptor. J. Med. Chem. 2014, 57, 6495–6512. [Google Scholar] [CrossRef]
- Kibou, Z.; Cheikh, N.; Villemin, D.; Choukchou-Braham, N.; Mostefa-Kara, B.; Benabdallah, M. A simple and Efficient Procedure for a 2-Pyridones Synthesis under Solvent-Free Conditions. Int. J. Org. Chem. 2011, 1, 242–249. [Google Scholar] [CrossRef] [Green Version]
- Al-Mousawi, S.M.; Moustafa, M.S.; Abdelkhalik, M.M.; Elnagdi, M.H. Enaminones as building blocks in organic syntheses: On the reaction of 3-dimethylamino-2-propenones with malononitrile. Arkivoc 2009, 2009, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Michael, A.; Eckstein, O. Ueber die Bildung von C-Acylderivaten aus Cyanessigester durch Anwendung von Pyridin und Chinolin. Ber. Dtsch. Chem. Ges. 1905, 38, 50–53. [Google Scholar] [CrossRef] [Green Version]
- Nicholl, L.; Tarsio, P.; Point, S.; Blohm, H. Process for Preparation of Oxy Alkylidene Compounds. U.S. Patent 2824121A, 18 February 1958. [Google Scholar]
- Kasum, B.; Prager, R.H. A classical approach to the synthesis of Perloline. Aust. J. Chem. 1983, 36, 1455–1467. [Google Scholar] [CrossRef]
- Torres, M.; Gil, S.; Parra, M. New Synthetic Methods to 2-Pyridone Rings. Curr. Org. Chem. 2005, 9, 1757–1779. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).