Abstract
Specific structural modifications in eugenol molecules can simultaneously improve the biological activity and reduce side effects of the respective analogues. The esterification of eugenol by two different experimental procedures, and subsequently the conversion of one of the esters into the corresponding oxirane, was carried out. All derivatives obtained were then evaluated for their effect on the viability of Sf9 (Spodoptera frugiperda) cells. In addition, a structured-based inverted virtual screening protocol was employed to identify the potential proteins associated with the observed insecticidal activity. The encouraging results obtained allowed us to establish a preliminary structure–activity relationship.
1. Introduction
Due to the exponential increase in the world population, it is necessary to ensure agricultural production that meets the actual food requirements. The improvement in the productivity of agricultural crops implies an incessant need to prevent, control and destroy the pests that affect them, achieved through the extensive use of synthetic pesticides. Although synthetic pesticides represent a plausible approach, they present a serious threat because their uncontrolled use causes negative impacts on the environment (pollution and loss of biodiversity) and on human health [1,2].
Natural products are good alternatives, due to the structural diversity and associated biological activity, making them a rich source of inspiration in the design and optimization of active principles in the development of formulations, highlighting the crucial role of plant extracts [3,4]. In this category, essential oils fit perfectly, exhibiting a broad spectrum of actions, including antibacterial, antifungal, insecticidal, and antioxidant activities, for example, eugenol [5,6].
Considering these facts, and as a continuation of our recent interests in alternative pesticides, eugenol derivatives were obtained through esterification and epoxidation reactions and evaluated for their effect on the viability of Sf9 cells. A structure-based inverted virtual screening protocol was employed to identify the potential proteins associated with the observed insecticidal activity.
2. Results and Discussion
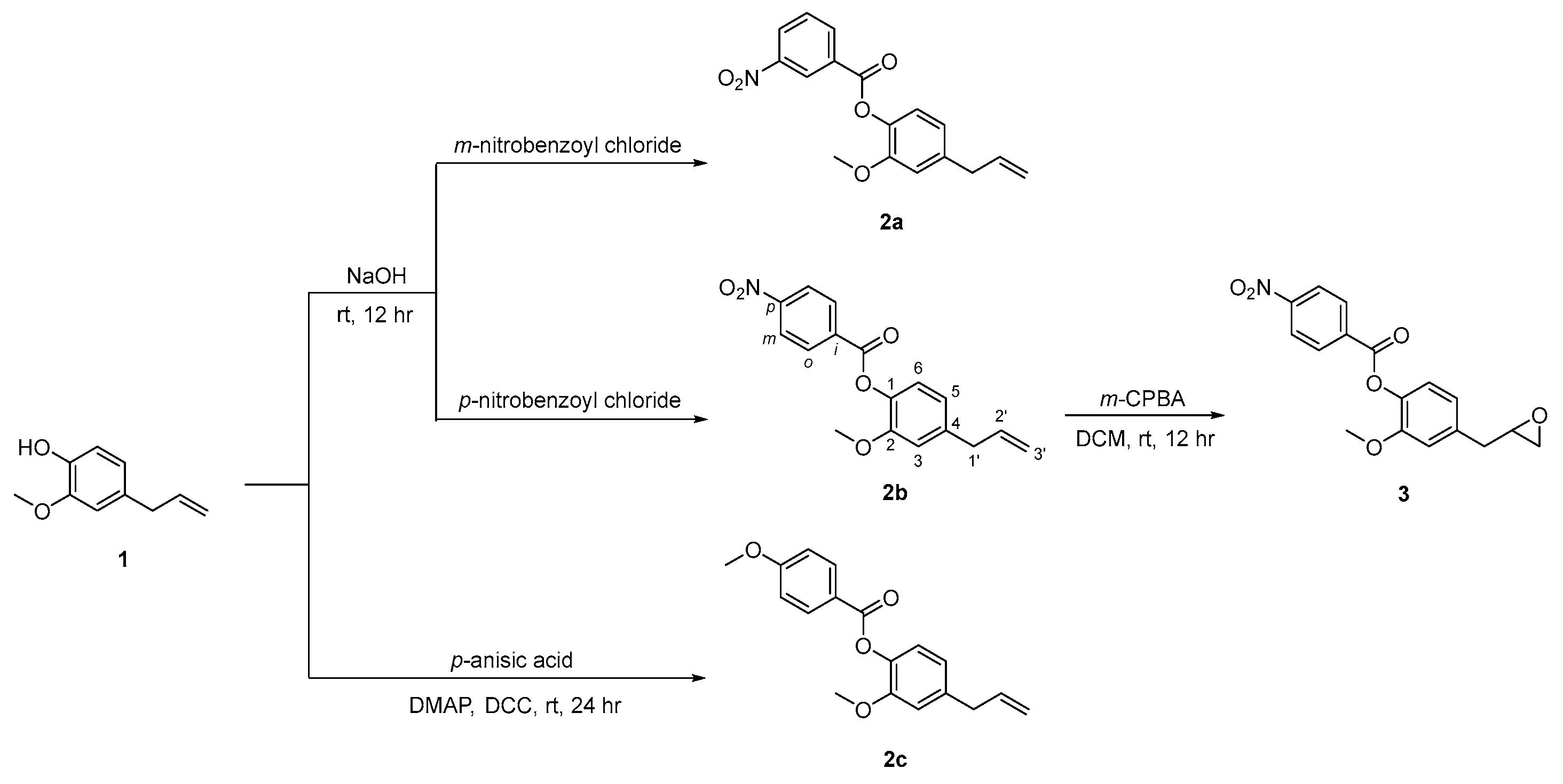
2.1. Synthesis of Eugenol Derivatives 2a–c and 3
The compound 4-allyl-2-methoxyphenol, eugenol 1, was the lead compound used in the synthesis of three O-esterified derivatives 2a–c, of which compound 2b was then converted in the respective oxirane 3 as shown in Scheme 1. The esterification of 4-allyl-2-methoxyphenol 1 in basic conditions with m-nitrobenzoyl chloride and p-nitrobenzoyl chloride gave 4-allyl-2-methoxyphenyl 3-nitrobenzoate 2a and 4-allyl-2-methoxyphenyl 4-nitrobenzoate 2b, as solids in 72 and 49% yields, respectively. In addition, compound 1 was also esterified with p-anisic acid, by using dicyclohexylcarbodiimide (DCC) and 4-(dimethylamino)pyridine (DMAP), in dichloromethane, at room temperature, resulting in the 4-allyl-2-methoxyphenyl 4-methoxybenzoate 2c as a solid material in 69% yield.
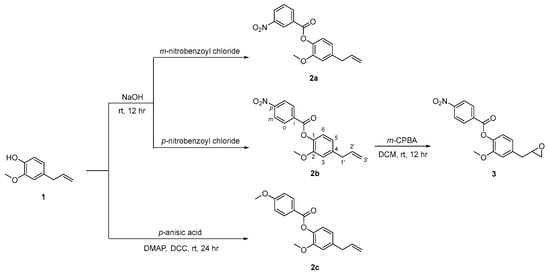
Scheme 1.
Synthesis of eugenol derivatives 2a–c and 3.
Epoxidation of the double bond of compound 2b with m-perchloroperbenzoic acid in dichloromethane at room temperature, resulted in 2-methoxy-4-(oxiran-2-ylmethyl)phenyl 4-nitrobenzoate 3, isolated with 31% yield. Compounds 2a–c and 3 were fully characterized by the usual analytical techniques. The 1H NMR showed the signals of aromatic protons derived from the eugenol unity (δ 6.81–7.12 ppm), in addition to the protons of the nitro- or methoxyphenyl rings, highlighting H-2 and H-5 displayed as triplets or multiplets (δ 8.34–9.06 ppm, H-2; δ 6.84–7.75 ppm, H-5) for compounds 2a and 2b, respectively, and as dublets (δ 8.18 ppm, H-2; δ 6.99 ppm, H-5) for compound 2c. The alkene protons are shown as multiplets (δ 5.10–6.03 ppm) in compounds 2a–c, and are absent in compound 3, giving way to the oxirane ring protons, shown as quartet and multiplets (δ 2.58–3.23 ppm). The 13C NMR also confirm the presence of the ester bond (δ 162.96–164.62 ppm) in all compounds, as well as the oxirane ring in compound 3 (δ 46.79–52.28 ppm).
2.2. Biological Activity of Compounds 2a–c and 3 in Sf9 Insect Cells
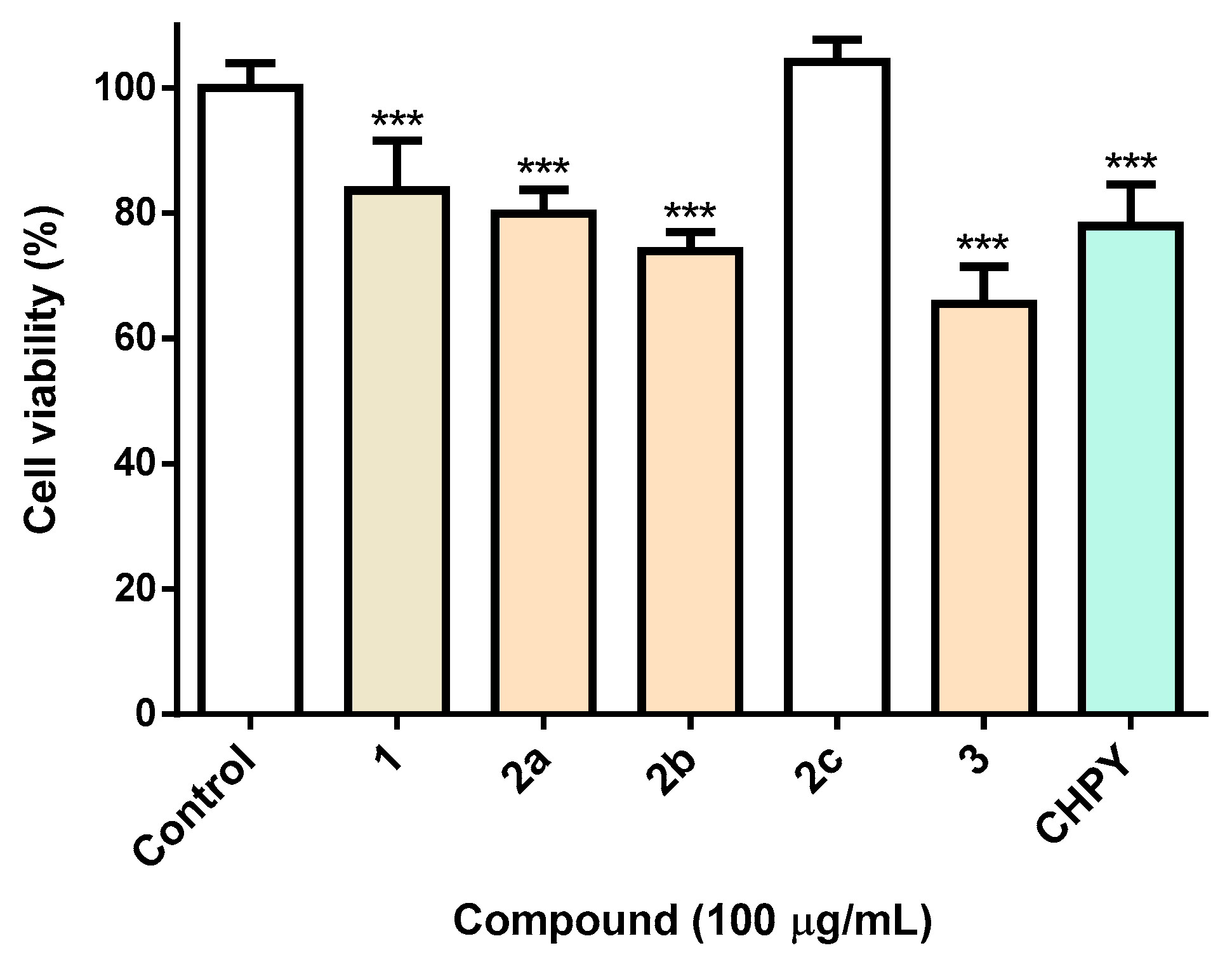
Aiming at the evaluation of the insecticidal activity of the synthesized eugenol derivatives 2a–c and 3, studies were carried out in Spodoptera frugiperda (Sf9) cells, a common pest widely used in the screening of insecticides. For benchmarking purposes, the insecticide chlorpyrifos (CHPY) was used at the same concentration (100 µg/mL). As can be seen in Figure 1, it is clear that the esterification of eugenol with a nitrobenzene group pontentiate eugenol toxicity, derivatives under study displaying equivalent (compound 2a) or even higher (compound 2b and 3) toxicity than the commercial insecticide, CHPY (Figure 1). It is noteworthy that when the nitro group linked to the benzene ring (compound 2b) is replaced by a methoxy group (compound 2c), the cytotoxicity is completely lost. On the other hand, oxirane formation (compound 3) lead to a slight increase in toxicity (Figure 1).
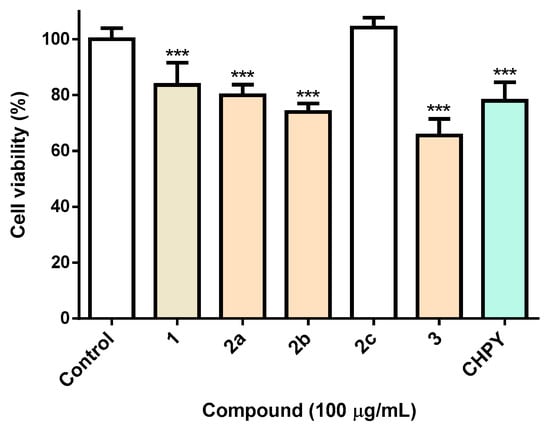
Figure 1.
Viability of Sf9 insect cells exposed to the molecules under study 2a–c and 3 (100 µg/mL), or medium (control) or the reference insecticide chlorpyrifos (CHPY). *** p < 0.001.
2.3. Inverted Virtual Screening Results
Table 1 presents the average scores obtained for the four eugenol derivatives for each potential target calculated with each SFs. Regarding the difference in the values, it must be stated that different SFs are based on different scales and metrics. The score for all the GOLD scoring functions is dimensionless with a higher score yielding a better binding affinity. Vina, on the other hand, uses a metric that is a more precise approximation of binding free energy, meaning that a more negative value is equivalent to better affinity.

Table 1.
Average eugenol derivate scores obtained for all PDB structures with the six different scoring functions.
Generally, the results show good consistency between SFs, with odorant-binding proteins, acetylcholinesterases, chitinases, and beta-N-acetyl-D-hexosaminidase yielding better scores. On the other hand, targets such as octopamine receptor, N-acetylglucosamine-1-phosphate uridyltransferase (GlmU), and voltage-gated sodium channels, consistently present lower scores for across all the SFs.
From each set of targets, the structure with the best score was selected and ranked from the best target to worst, according to the predictions of the different SFs. The overall ranking is listed in Table 2. Globally, considering the results obtained with the several SFs, odorant-binding proteins are the most likely target with the highest affinity towards eugenol derivatives, followed closely by acetylcholinesterases. The discrepancy in some of the values of the different SFs, can be explained by the nature of each SF, as they consider different aspects of protein-ligand binding.

Table 2.
Average RMSD values (Å), average ligand RMSD (Å), average SASA (Å2), percentage of potential ligand SASA buried and an average number of hydrogen bonds for the ligands for the last 70 ns of the simulation of the OBP and AChE-ligand complexes.
The hypothesis formed is that eugenol and eugenol derivatives can be used as repellents because they can bind to odorant-binding proteins or as pesticides, inhibiting insect acetylcholinesterase.
Interestingly, in the PDB database there is a structure of an odorant-binding protein bound to eugenol Apis mellifera (PDB: 3S0E) [7]. This might be an important indicator of the increased affinity of eugenol derivatives against OBPs.
2.4. Molecular Dynamics Simulations and Free Energy Calculations Results
In order to validate the inverted VS predictions, molecular dynamics simulations were then performed for the eugenol derivatives complexes formed with the two groups of targets predicted at the inverted VS stage: odorant-binding proteins and acetylcholinesterases. The structure with the best score from each group was selected (3K1E for OBP and 1QON for acetylcholinesterases—AChE). The results are detailed in Table 2.
The OBP–eugenol derivatives complexes are very stable throughout the simulation and presented an average protein RMSD of around 2 Å. The prediction from the inverted VS were confirmed as the ligand RMSD is very low. For AChE–eugenol derivatives, however, the average RMSD is higher, indicating that the system shifted to a more stable conformation in the beginning of the simulation. Also, the inverted VS predictions were validated for this target, as the average ligand RMSD values are below or equal to 1 Å.
The average SASA and percentage of potential ligand SASA buried indicate the ligand exposure to solvent, and increased SASA and a lower percentage of ligand buried means more solvent exposure. Compounds 2c and 3 are the ones that are less exposed to the solvent and more buried in the binding pocket of OBP. Regarding AChE, the compounds that are less exposed and more buried in the active site are 2a and 3.
Generally, the Gibbs free energy of association was better for OBP–eugenol derivatives than for AChE–eugenol derivatives. Compounds 2a and 3 are the ones that present the strongest affinity toward OBP. Compound 3 is also the compound that presents the strongest affinity toward AChE compared with all the other eugenol derivatives studied.
When bound to OBP, the ligands are mainly stabilized by Trp105, Leu67 and Ile78. When bound to AChE, the main interacting residues are Trp81, Tyr69 and Tyr322.
3. Materials and Methods
3.1. Typical Procedure for the Preparation of Compounds 2a–c (Illustrated for 2b)
The compound 4-allyl-2-methoxyphenol 1 (0.500 g, 3.05 × 10−3 mol, 1 equiv.) was added dropwise to 2 M NaOH solution (3.37 mL) at room temperature. The mixture was kept under stirring until a homogeneous green solution was formed. To this mixture, 4-nitrobenzoyl chloride (0.622 g, 3.36 × 10−3 mol, 1.1 equiv.) was added and the reaction was kept under stirring for 12 hr. After this period, the obtained solid was filtered and recrystallized (ethyl acetate/n-hexane), giving 4-allyl-2-methoxyphenyl 4-nitrobenzoate 2b as a white solid (0.463 g, 49% yield). Rf = 0.62 (silica: dichloromethane), m.p. = 59–61 °C. 1H NMR (CDCl3, 400 MHz): δH 3.43 (2H, d, J = 6.4 Hz, CH2Ph), 3.82 (3H, s, OCH3), 5.12–5.20 (2H, m, CH=CH2), 5.95–6.03 (1H, m, CH=CH2), 6.83 (1H, d, J = 2.0 Hz, H-3), 6.86 (1H, dd, J = 4.4 and 2.0 Hz, H-5), 7.09 (1H, d, J = 8.0 Hz, H-6), 8.32–8.41 (4H, m, 4 × H Ph-NO2) ppm. 13C NMR (CDCl3, 100 MHz): δC 40.11 (CH2Ph), 55.48 (OCH3), 112.86 (C-5), 116.31 (CH=CH2), 120.79 (C-3), 122.32 (C-6), 123.62 (2 × H-Ph-NO2), 131.40 (2 × H Ph-NO2), 134.96 (C-1 Ph-NO2), 136.91 (CH=CH2), 137.73 (C-4), 139.64 (C-1), 150.77 (C-2), 150.81 (C-4 Ph-NO2), 163.02 (C=O) ppm.
3.2. Synthesis of Compound 2c
A mixture of 4-allyl-2-methoxyphenol 1 (0.500 g, 3.05 × 10−3 mol, 1 equiv.), DMAP (0.075 g, 6.1 × 10−4 mol. 0.2 equiv.), and DCC (0.944 g, 4.56 × 10−3 mol, 1.5 equiv.) was added to p-anisic acid (0.703 g, 4.58 × 10−3 mol, 1.5 equiv.) in dichloromethane (5 mL). The reaction mixture was stirred at room temperature for 24 hr. At the end of this period, the white suspension obtained was filtered and the liquid phases were washed successively with 5% (w/v) hydrochloric acid (2 × 5 mL), 5% sodium hydrogen carbonate (w/v; 3 × 5 mL), and water (3 × 5 mL). Finally, after drying with anhydrous sodium sulfate, the organic phases were evaporated under reduced pressure to give 4-allyl-2-methoxyphenyl 4-methoxybenzoate 2c as white solid (0.627 g, 69%). Rf = 0.49 (silica: dichloromethane), m.p. = 95–97 °C. 1H NMR (CDCl3, 400 MHz): δH 3.41 (2H, d, J = 6.8 Hz, CH2Ph), 3.81 (3H, s, OCH3), 3.90 (3H, s, Ph-OCH3), 5.10–5.16 (2H, m, CH=CH2), 5.95–6.03 (1H, m, CH=CH2), 6.81 (1H, d, J = 2.0 Hz, H-3), 6.83 (1H, dd, J = 4.4 and 2.0 Hz, H-5), 6.99 (2H, d, J = 8.0 Hz, H-3 and H-5 Ph-OCH3), 7.06 (1H, d, J = 8.0 Hz, H-6), 8.18 (2H, d, J = 9.2 Hz, H-2 and H-6 Ph-OCH3) ppm. 13C NMR (CDCl3, 100 MHz): δC 40.11 (CH2Ph), 55.48 (OCH3), 55.89 (Ph-OCH3), 112.85 (C-5), 113.74 (C-3 and C-5 Ph-OCH3), 116.08 (CH=CH2), 120.71 (C-3), 121.86 (C-1 Ph-OCH3), 122.74 (C-6), 132.39 (C-2 and C-6 Ph-OCH3), 137.15 (CH=CH2), 138.31 (C-1), 138.86 (C-4), 151.19 (C-2), 163.75 (C-4 Ph-OCH3), 164.62 (C=O) ppm.
3.3. Synthesis of Compound 3
The compound 4-allyl-2-methoxyphenyl 4-nitrobenzoate 2b (0.300 g, 9.58 × 10−4 mol, 1 equiv.) was dissolved in dichloromethane (5 mL) at room temperature. The resulting solution was added dropwise to a solution of m-chloroperbenzoic acid (0.236 g, 1.37 × 10−3 mol, 1 equiv.) in dichloromethane (5 mL) at 0 °C (ice bath). After stirring for 1 hr, m-chloroperbenzoic acid was again added (0.236 g, 1.37 × 10−3 mol, 1 equiv.), and the reaction mixture was stirred for more 12 h. A 10% aqueous solution of sodium sulfate (2 × 15 mL) was added, and the resulting mixture was washed with 5% aqueous solution of sodium hydrogen carbonate (2 × 15 mL). The organic phase was dried with anhydrous magnesium sulfate, the solvent was evaporated to give 2-methoxy-4-(oxiran-2-ylmethyl)phenyl 4-nitrobenzoate 3 as green solid (0.098 g, 31%). Rf = 0.71 (silica: dichloromethane), m.p. = 59–61 °C. 1H NMR (CDCl3, 400 MHz): δH 2.59 (1H, q, J = 2.8 Hz, CH2 oxirane), 2.74–2.92 (3H, m, CH2Ph and CH2 oxirane), 3.18–3.23 (1H, m, CH oxirane), 3.84 (s, 3H, OCH3), 6.91 (1H, dd, J = 8 and 2 Hz, H-5), 6.95 (1H, d, J = 2.0 Hz, H-3), 7.12 (1H, d, J = 8 Hz, H-6), 8.35–8.41 (4H, m, 4 × H Ph-NO2) ppm. 13C NMR (CDCl3, 100 MHz): δC 38.68 (CH2Ph), 46.79 (CH2 oxirane), 52.28 (CH oxirane), 55.91 (OCH3), 113.33 (C-3), 121.20 (C-5), 122.49 (C-6), 123.64 (2 × C Ph-NO2), 131.41 (2 × C Ph-NO2), 134.88 (C-1 Ph-NO2), 136.88 (C-4), 138.24 (C-1), 150.84 (C-2), 150.87 (C-4 Ph-NO2), 162.96 (C=O) ppm.
3.4. Evaluation of Viability in Sf9 Cells
As a model, the Spodoptera frugiperda Sf9 cell line was used. Cells were purchased from Sigma–Aldrich (St. Louis, MO, USA) and maintained in Grace’s insect medium enriched with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin (Pen–Strep) at 28 °C. Cells were routinely subcultured as a suspension culture and assays conducted in the exponential growth phase.
For the assessment of viability, Sf9 cells were plated at a density of 3.0 × 104 cells/well, followed by incubation for 24 h with the various compounds. After this period, a commercial solution of resazurin was added (Thermo Fisher A13261, final concentration: 1:10) and fluorescence was measured 60 min thereafter.
3.5. Inverted Virtual Screening Protocol Optimization
Considering the relevance of the target and year of publication, a search on Scopus was performed using the keywords: Virtual Screening (VS) and insecticide target. Seventeen studies were selected, and thirteen targets chosen for the inverted VS assays. The targets identified are listed in Table 3.

Table 3.
List of targets selected for the inverted virtual screening study.
Each structure was extracted from the PDB database [25] and was prepared for docking using the Autodock Vina plugin for Pymol [26] with the removal of crystallographic waters and the extraction of ligands to separate files. The saved ligands were later used for active site coordinates and as reference for root mean square deviation (RMSD) calculations. In the absence of crystallographic ligands, the active site coordinates were obtained by selecting the most important active site residues. Re-docking was used as a quality measure, to evaluate the ability of the docking software in reproducing the geometry and orientation of the crystallographic pose.
The docking programs/scoring functions (SF) used were AutoDock Vina [27] and GOLD [28] (PLP, ASP, ChemScore, GoldScore). The protocol was optimized for each protein target and each SF, to minimize the RMSD values.
The optimized parameters for each SF consisted of the coordinates for the docking region centre, docking box dimension or radius, exhaustiveness, search efficiency, and number of runs. Once the RMSD values between poses (crystallographic and docked) were satisfactory (below 2 Å), the optimized conditions were used for the subsequent stages. The molecules were prepared for docking using Datawarrior [29] and OpenBabel [30] and were docked into each structure with all the five SF in study. A ranked list was prepared based on the average scores of each target.
3.6. Molecular Dynamics Simulations and Free Energy Calculations
Molecular dynamics simulations were performed on the four eugenol derivatives in complex with the two most promising targets identified from the inverted VS study: Odorant-Binding Protein 1 (OBP—3K1E) and Acetylcholinesterase (AChE—1QON). The Amber18 software [31] was used throughout.
The complexes were treated with the Leap module of AMBER [32]. The protein targets were treated with the ff14SB force field [33], while the eugenol derivatives were parameterized using ANTECHAMBER, with RESP HF/6-31G(d) charges calculated with Gaussian [23,34] and the General Amber Force Field (GAFF) [35]. The complexes were placed in TIP3P water boxes with a minimum distance of 12 Å between the protein-surface and the side of the box and periodic boundary conditions were applied. Counter-ions (Na+) were added to neutralize the overall charge and the complete systems.
To remove clashes prior to the MD simulation, four consecutive minimization stages were performed with a maximum of 2500 steps. Subsequently, the minimized systems were then subject to an equilibration procedure, divided into two stages: in the first stage (50 ps), the systems were gradually heated to 298 K using a Langevin thermostat at constant volume (NVT ensemble); in the second stage (50 ps) the density of the systems was further equilibrated at 298 K. Lastly, the production runs were performed for 100 ns, in a NPT ensemble at constant temperature (298 K, Langevin thermostat) and pressure (1 bar, Berendsen barostat). A 10 Å cutoff for nonbonded interactions was used along with the SHAKE algorithm, to constrain all covalent bonds. An integration time of 2.0 fs was applied. The final trajectories were analyzed using the cpptraj tool [36] and VMD [37], to confirm that all the systems were well equilibrated. The last 70 ns of the simulation were considered for hydrogen bonding analysis, and cluster analysis of the conformations generated.
In order to estimate the binding free energies of the protein-eugenol derivatives complexes, the molecular Mechanics/Generalized Born Surface Area method [38] was applied using the MM/PBSA.py [39] script from amber. The salt concentration applied was 0.100 mol dm−3. From each MD trajectory, a total of 1400 conformations were taken from the last 70 ns and the contribution of the amino acid residues was estimated using the energy decomposition method.
4. Conclusions
In this work, three esters derived from eugenol and the corresponding oxirane from one of these esters were efficiently prepared. The obtained eugenol derivatives were subjected to biological activity evaluation in Sf9 cell line, in order to predict their potential as natural based insecticides. We identified that the three derivatives esterified with a nitrobenzene were those showing higher potency, in some cases higher than the benchmark used.
In the present study, we report the application of an integrated molecular modelling—inverted virtual screening protocol for a selection of four eugenol derivatives in order to find possible protein targets in which they present insecticidal activity. After the target selection and protocol optimization, the eugenol derivatives were docked into each of the thirteen targets with five different SFs (PLP, ASP, ChemScore, GoldScore, Vina). Eugenol derivates showed an increased binding affinity for odorant-binding proteins and acetylcholinesterases. The fact that there is, already, in the PDB database a structure of an OBP bound to eugenol, is a strong suggestion that eugenol derivates, could be used as repellents.
Author Contributions
Conceptualization, J.R.A.C., M.S.T.G. and S.F.S.; methodology, J.R.A.C., M.S.T.G., S.F.S., D.M.P.; formal analysis, J.R.A.C., M.S.T.G., M.J.G.F., D.M.P. and S.F.S.; investigation, J.R.A.C., T.F.V. and R.B.P.; writing—original draft preparation, J.R.A.C., M.S.T.G., M.J.G.F., R.B.P. and S.F.S.; writing—review and editing, M.S.T.G., M.J.G.F., R.B.P., D.M.P., S.F.S. and E.M.S.C.; supervision, M.S.T.G., M.J.G.F. and A.G.F.; project administration, M.S.T.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by FCT under project PTDC/ASP-AGR/30154/2017 (PO-CI-01-0145-FEDER-030154) of COMPETE 2020, co-financed by FEDER and EU. FCT- Portugal and FEDER-COMPETE/QREN-EU also gave a financial support to the research centres CQ/UM (UIDB/00686/2020), CF-UM-UP (UIDB/04650/2020) and REQUIMTE (UIDB/50006/2020). The NMR spectrometer Bruker Avance III 400 (part of the National NMR Network) was financed by FCT and FEDER.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lengai, G.M.W.; Muthomi, J.W.; Mbega, E.R. Phytochemical activity and role of botanical pesticides in pest management for sustainable agricultural crop production. Sci. Afr. 2020, 7, e00239. [Google Scholar] [CrossRef]
- Oliveira, J.L.; Campos, E.V.R.; Fraceto, L.F. Recent developments and challenges for nanoscale formulation of botanical pesticides for use in sustainable agriculture. J. Agric. Food Chem. 2018, 66, 8898–8913. [Google Scholar] [CrossRef] [PubMed]
- Salman, M.; Abbas, R.Z.; Israr, M.; Abbas, A.; Mehmood, K.; Khan, M.K.; Sindhu, Z.D.; Hussaind, R.; Saleemie, M.K.; Shaha, S. Repellent and acaricidal activity of essential oils and their components against Rhipicephalus ticks in cattle. Vet. Parasitol. 2020, 283, 109178. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.Y. Essential oils as repellents against arthropods. Biomed. Res. Int. 2018, 2018, 6860271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandes, M.J.G.; Pereira, R.B.; Pereira, D.M.; Fortes, A.G.; Castanheira, E.M.S.; Gonçalves, M.S.T. New eugenol derivatives with enhanced insecticidal activity. Int. J. Mol. Sci. 2020, 21, 9257. [Google Scholar] [CrossRef]
- Ju, J.; Xie, Y.; Yu, H.; Guo, Y.; Cheng, Y.; Qian, H.; Yao, W. Analysis of the synergistic antifungal mechanism of eugenol and citral. LWT Food Sci. Technol. 2020, 123, 109128. [Google Scholar] [CrossRef]
- Spinelli, S.; Lagarde, A.; Iovinella, I.; Legrand, P.; Tegoni, M.; Pelosi, P.; Cambillau, C. Crystal structure of Apis mellifera OBP14, a C-minus odorant-binding protein, and its complexes with odorant molecules. Insect Biochem. Mol. Biol. 2012, 42, 41–50. [Google Scholar] [CrossRef]
- Ramos, R.S.; Costa, J.S.; Silva, R.C.; Costa, G.V.; Rodrigues, A.B.L.; Rabelo, E.M.; Souto, R.N.P.; Taft, C.A.; Silva, C.H.T.P.; Rosa, J.M.C.; et al. Identification of potential inhibitors from Pyriproxyfen with insecticidal activity by virtual screening. Pharmaceuticals 2019, 12, 20. [Google Scholar] [CrossRef] [Green Version]
- Riva, C.; Suzanne, P.; Charpentier, G.; Dulin, F.; Halm-Lemeille, M.-P.; Santos, J.S.-O. In silico chemical library screening and experimental validation of novel compounds with potential varroacide activities. Pestic. Biochem. Physiol. 2019, 160, 11–19. [Google Scholar] [CrossRef]
- Correy, G.J.; Zaidman, D.; Harmelin, A.; Carvalho, S.; Mabbitta, P.D.; Calaora, V.; Peter, J.; James, P.J.; Kotzeg, A.C.; Jackson, C.J.; et al. Overcoming insecticide resistance through computational inhibitor design. Proc. Natl. Acad. Sci. USA 2019, 116, 21012–21021. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Liu, M.; Yao, Y.; Wang, J.; Li, Y.; Li, G.; Wang, Y. Identification of novel potential β-N-Acetyl-D-Hexosaminidase inhibitors by virtual screening, molecular dynamics simulation and MM-PBSA calculations. Int. J. Mol. Sci. 2012, 13, 4545–4563. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Shen, S.; Xu, Y.; Wang, L.; Yang, Q.; Zhang, J.; Lu, H. Identification of novel insect β-N-acetylhexosaminidase OfHex1 inhibitors based on virtual screening, biological evaluation, and molecular dynamics simulation. J. Biomol. Struct. Dyn. 2021, 39, 1735–1743. [Google Scholar] [CrossRef]
- Dong, Y.; Jiang, X.; Liu, T.; Ling, Y.; Yang, Q.; Zhang, L.; He, X. Structure-based virtual screening, compound synthesis, and bioassay for the design of chitinase inhibitors. J. Agric. Food Chem. 2018, 66, 3351–3357. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Yin, B.; Cappelle, K.; Swevers, L.; Smagghe, G.; Yang, X.; Zhang, L. Identification of novel agonists and antagonists of the ecdysone receptor by virtual screening. J. Mol. Graph Model. 2018, 81, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Harada, T.; Nakagawa, Y.; Ogura, T.; Yamada, Y.; Ohe, T.; Miyagawa, H. Virtual screening for ligands of the insect molting hormone receptor. J. Chem. Inf. Model. 2011, 51, 296–305. [Google Scholar] [CrossRef]
- Min, J.; Lin, D.; Zhang, Q.; Zhang, J.; Yu, Z. Structure-based virtual screening of novel inhibitors of the uridyltransferase activity of Xanthomonas oryzae pv. oryzae GlmU. Eur. J. Med. Chem. 2012, 53, 150–158. [Google Scholar] [CrossRef]
- Offermann, L.R.; Chan, S.L.; Osinski, T.; Tan, Y.W.; Chew, F.T.; Sivaraman, J.; Mok, Y.-K.; Minor, W.; Chruszcz, M. The major cockroach allergen Bla g 4 binds tyramine and octopamine. Mol. Immunol. 2014, 60, 86–94. [Google Scholar] [CrossRef] [Green Version]
- Laughlin, J.D.; Ha, T.S.; Jones, D.N.M.; Smith, D.P. Activation of pheromone-sensitive neurons is mediated by conformational activation of pheromone-binding protein. Cell 2008, 133, 1255–1265. [Google Scholar] [CrossRef] [Green Version]
- Oliferenko, P.V.; Oliferenko, A.A.; Poda, G.I.; Osolodkin, D.I.; Pillai, G.G.; Bernier, U.R.; Tsikolia, M.; Agramonte, N.M.; Clark, G.G.; Linthicum, K.J.; et al. Promising aedes aegypti repellent chemotypes identified through integrated QSAR, virtual ccreening, synthesis, and bioassay. PLoS ONE 2013, 8, e64547. [Google Scholar] [CrossRef]
- Joshi, T.; Joshi, T.; Sharma, P.; Chandra, S.; Pande, V. Molecular docking and molecular dynamics simulation approach to screen natural compounds for inhibition of Xanthomonas oryzae pv. Oryzae by targeting peptide deformylase. J. Biomol. Struct. Dyn. 2021, 39, 823–840. [Google Scholar] [CrossRef]
- Fu, Y.; Liu, Y.-X.; Kang, T.; Sun, Y.-N.; Li, J.-Z.; Ye, F. Identification of novel inhibitors of p-hydroxyphenylpyruvate dioxygenase using receptor-based virtual screening. J. Taiwan Inst. Chem. Eng. 2019, 103, 33–43. [Google Scholar] [CrossRef]
- Fattouch, S.; Raboudi-Fattouch, F.; Ponce, J.V.G.; Forment, J.V.; Lukovic, D.; Marzouki, N.; Vidal, D.R. Concentration dependent effects of commonly used pesticides on activation versus inhibition of the quince (Cydonia Oblonga) polyphenol oxidase. Food Chem. Toxicol. 2010, 48, 957–963. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Du, X.; Wang, C.; Lin, J.; Du, X. Identification of Potential Helicoverpa armigera (Lepidoptera: Noctuidae) Sterol Carrier Protein-2 Inhibitors through High-Throughput Virtual Screening. J. Econ. Entomol. 2017, 110, 1779–1784. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Li, Z.; Jiang, Y.; Pan, X.; Wu, J.; Cristofori-Armstrong, B.; Smith, J.J.; Chin, Y.K.Y.; Lei, J.; Zhou, Q.; et al. Structural basis for the modulation of voltage-gated sodium channels by animal toxins. Science 2018, 362, eaau2596. [Google Scholar] [CrossRef] [Green Version]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The protein data bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [Green Version]
- Seeliger, D.; de Groot, B.L. Ligand docking and binding site analysis with PyMOL and Autodock/Vina. J. Comput. Aided Mol. Des. 2010, 24, 417–422. [Google Scholar] [CrossRef] [Green Version]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2009, 31, 455–461. [Google Scholar] [CrossRef] [Green Version]
- Jones, G.; Willett, P.; Glen, R.C.; Leach, A.R.; Taylor, R. Development and validation of a genetic algorithm for flexible docking Edited by F. E. Cohen. J. Mol. Biol. 1997, 267, 727–748. [Google Scholar] [CrossRef] [Green Version]
- Sander, T.; Freyss, J.; von Korff, M.; Rufener, C. DataWarrior: An open-source program for chemistry aware data visualization and analysis. J. Chem. Inf. Model. 2015, 55, 460–473. [Google Scholar] [CrossRef]
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open babel: An open chemical toolbox. J. Cheminform. 2011, 3, 33. [Google Scholar] [CrossRef] [Green Version]
- Case, D.A.; Cheatham, T.E., III; Darden, T.; Gohlke, H.; Luo, R.; Merz, K.M., Jr.; Onufriev, A.; Simmerling, C.; Wang, B.; Woods, R.J. The Amber biomolecular simulation programs. J. Comput. Chem. 2005, 26, 1668–1688. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Wolf, R.M.; Caldwell, J.W.; Kollman, P.A.; Case, D.A. Development and testing of a general amber force field. J. Comp. Chem. 2004, 25, 1157–1174. [Google Scholar] [CrossRef] [PubMed]
- Maier, J.A.; Martinez, C.; Kasavajhala, K.; Wickstrom, L.; Hauser, K.E.; Simmerling, C. ff14SB: Improving the accuracy of protein side chain and backbone parameters from ff99SB. J. Chem. Theory Comput. 2015, 11, 3696–3713. [Google Scholar] [CrossRef] [Green Version]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian16; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Wang, J.; Wang, W.; Kollman, P.A.; Case, D.A. Automatic atom type and bond type perception in molecular mechanical calculations. J. Mol. Graph Model. 2006, 25, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Roe, D.R.; Cheatham, T.E. PTRAJ and CPPTRAJ: Software for processing and analysis of molecular dynamics trajectory data. J. Chem. Theory Comput. 2013, 9, 3084–3095. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Wang, E.; Sun, H.; Wang, J.; Wang, Z.; Liu, H.; Zhang, J.Z.H.; Hou, T. End-point binding free energy calculation with MM/PBSA and MM/GBSA: Strategies and applications in drug design. Chem. Rev. 2019, 119, 9478–9508. [Google Scholar] [CrossRef]
- Miller, B.R.; McGee, T.D.; Swails, J.M.; Homeyer, N.; Gohlke, H.; Roitberg, A.E. MMPBSA.py: An efficient program for end-state free energy calculations. J. Chem. Theory Comput. 2012, 8, 3314–3321. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).