Developing a Benzimidazole-Silica-Based Hybrid Sol–Gel Coating with Significant Corrosion Protection on Aluminum Alloys 2024-T3 †
Abstract
:1. Introduction
2. Experimental Procedures
2.1. Sol–Gel Preparation
2.2. Substrate Preparation and Film Deposition
2.3. Coating Testing and Characterization
3. Results and Discussion
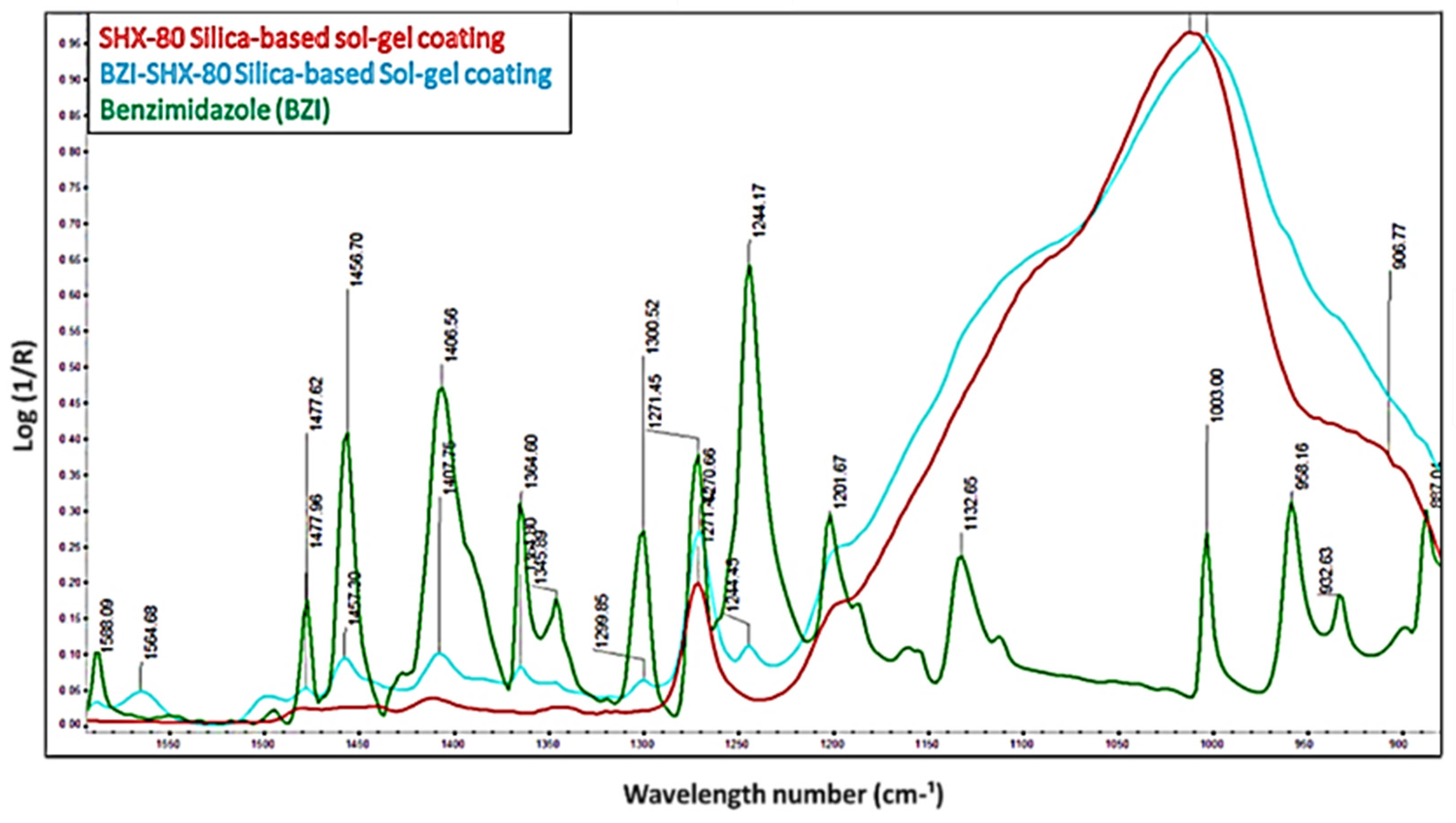
3.1. ATR-FTIR for the BZI-SBX-80 Sol–Gel Chemical Composition
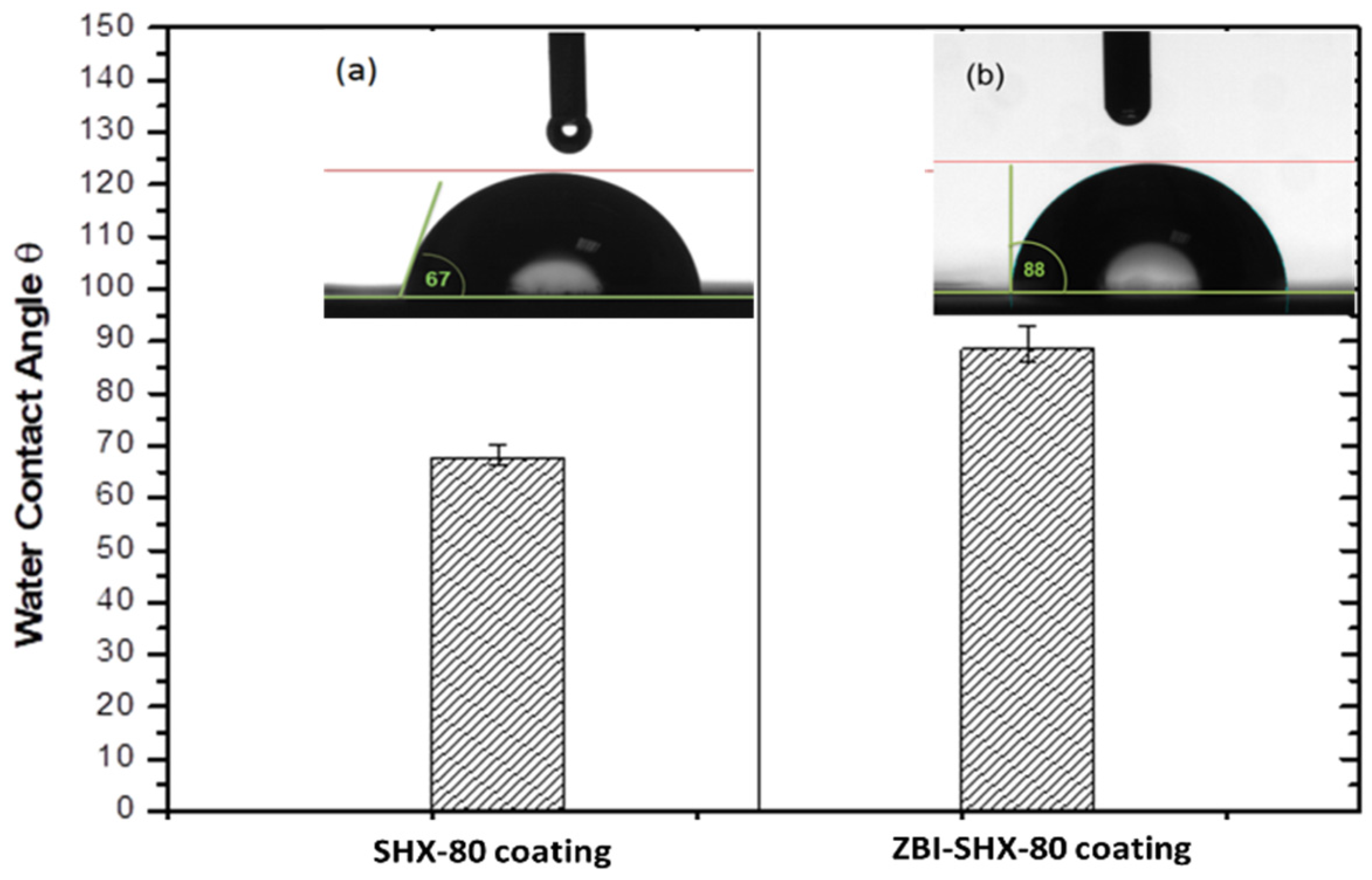
3.2. Water Contact Angle of the SBX and BZI-SBX Coatings
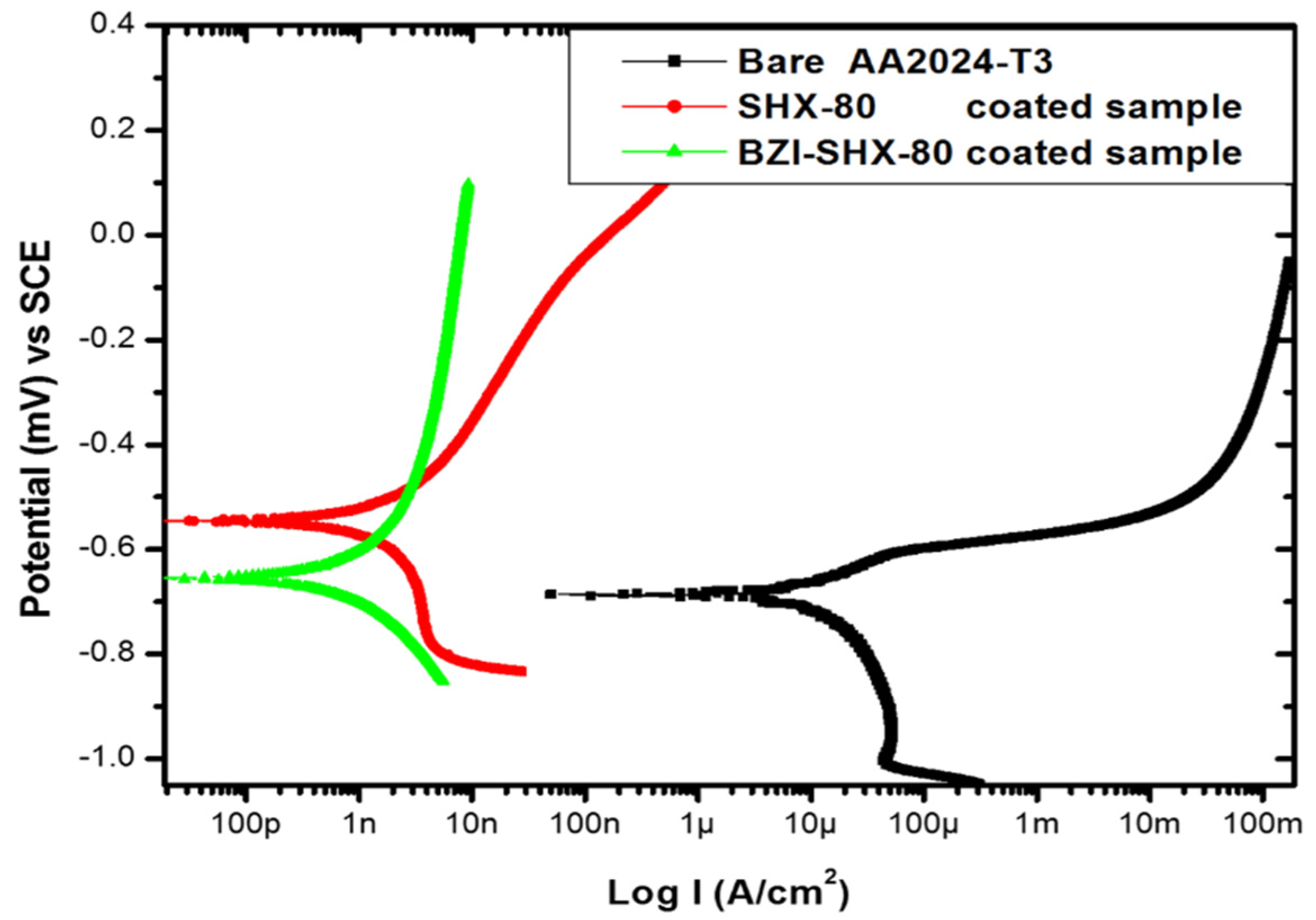
3.3. Potentiodynamic Polarization Scanning
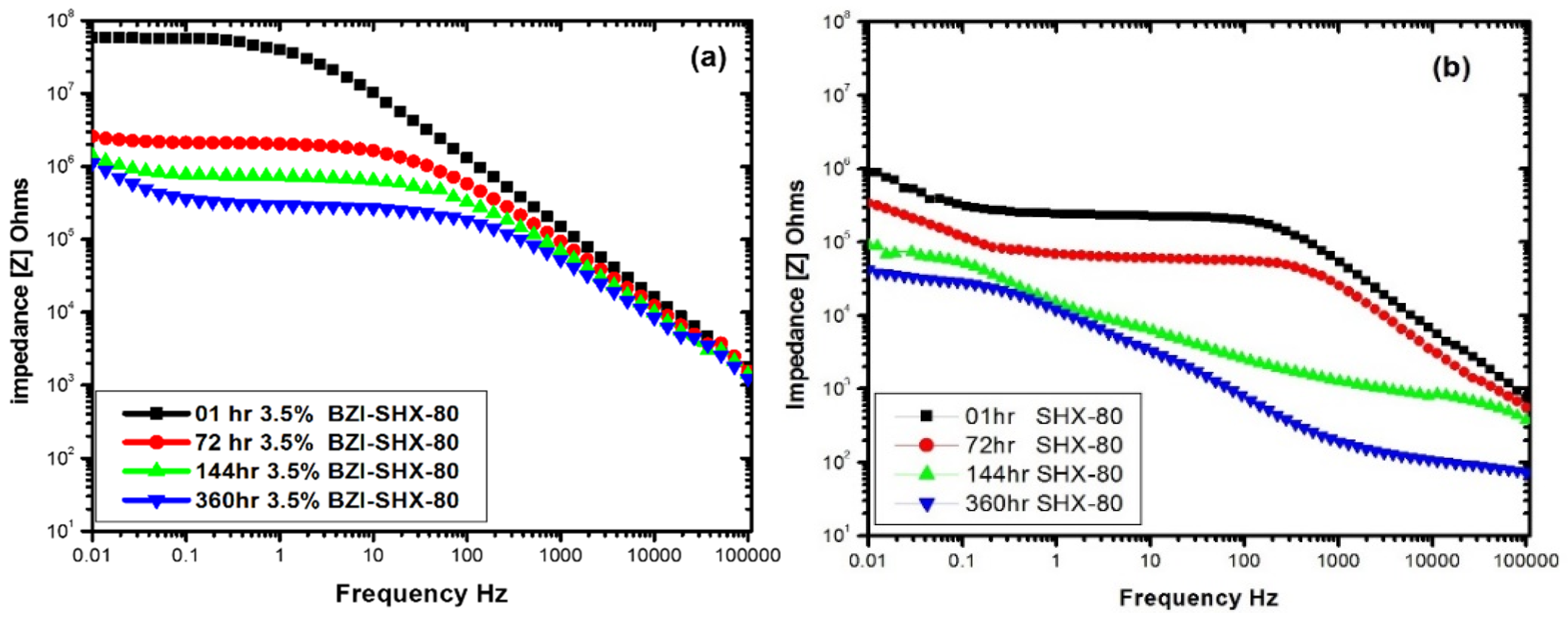
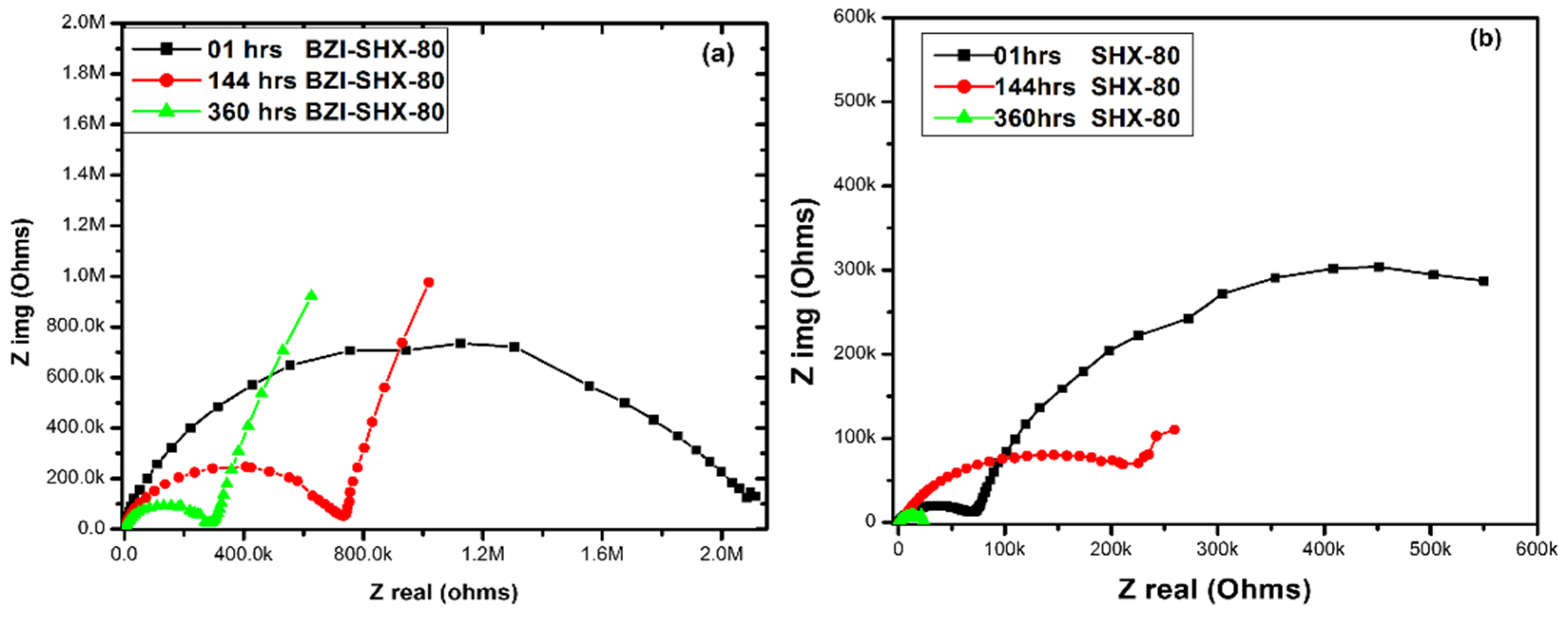
3.4. Electrochemical Impedance Spectroscopy
3.4.1. Impedance Magnitude Bode Plots
3.4.2. Using Nyquist Plots to Investigate the Corrosion-Protective Behavior
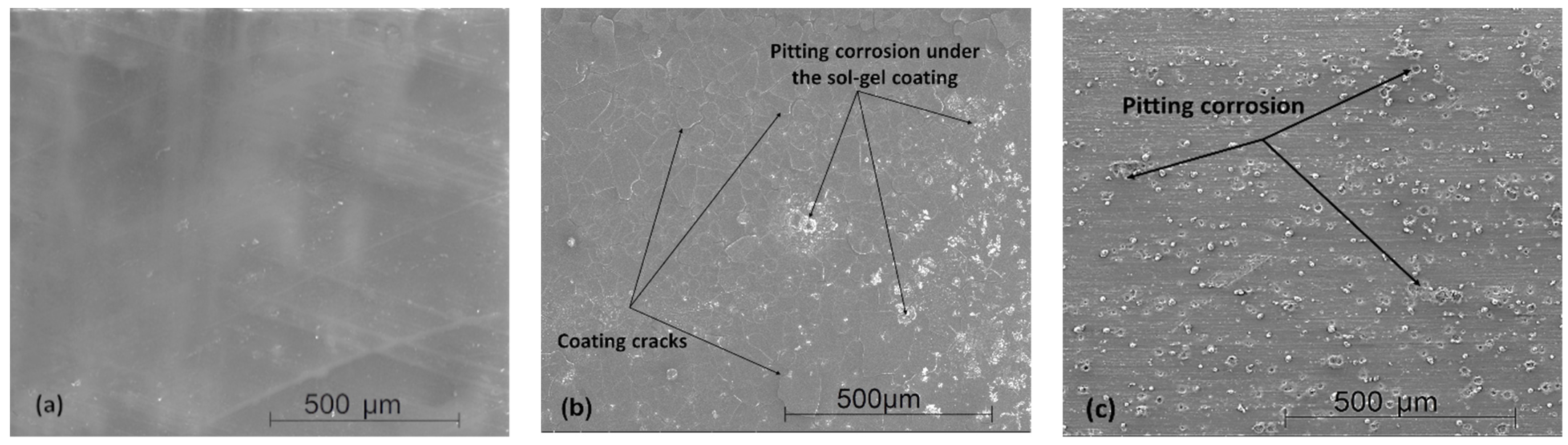
3.5. Scanning Electron Microscopy Images after Immersion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Antonijevic, M.M.; Petrovic, M.B. Copper corrosion inhibitors. A review. Int. J. Electrochem. Sci. 2008, 3, 1–28. [Google Scholar]
- Antonijevic, M.; Milic, S.; Petrovic, M.; Radovanovic, M.; Stamenkovic, A. The Influence of pH and Chlorides on Electrochemical Behavior of Copper in the Presence of Benzotriazole. Int. J. Electrochem. Sci. 2009, 4, 962–979. [Google Scholar]
- Wright, J.B. The Chemistry of the Benzimidazoles. Chem. Rev. 1951, 48, 397–541. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.K. Herbicides and fungicides. In Biomarkers in Toxicology; Elsevier Inc.: Amsterdam, The Netherlands, 2014; ISBN 9780124046306. [Google Scholar]
- Gutiérrez, E.; Rodríguez, J.A.; Cruz-Borbolla, J.; Alvarado-Rodríguez, J.G.; Thangarasu, P. Development of a predictive model for corrosion inhibition of carbon steel by imidazole and benzimidazole derivatives. Corros. Sci. 2016, 108, 23–35. [Google Scholar] [CrossRef]
- Obot, I.B.; Madhankumar, A.; Umoren, S.A.; Gasem, Z.M. Surface protection of mild steel using benzimidazole derivatives: Experimental and theoretical approach. J. Adhes. Sci. Technol. 2015, 29, 2130–2152. [Google Scholar] [CrossRef]
- Grillo, F.; Tee, D.W.; Francis, S.M.; Früchtl, H.A.; Richardson, N.V. Passivation of copper: Benzotriazole films on Cu(111). J. Phys. Chem. C 2014, 118, 8667–8675. [Google Scholar] [CrossRef]
- Colreavy, J.; Duffy, B.; Varma, P.C.R.; Hayden, H.; Oubaha, M. Organosilane Coating Compositions and Use Thereof. United States US20100330380A1, 30 December 2010. [Google Scholar]
- Vijaykumar, S.; Prakash, O.; Raghavan, S.; Ramachandra, K.; Reddy, D. Sol-Gel Coating Compositions Including Corrosion Inhibitor-Encapsulated Layered Metal Phosphates and Related Processes. United States US10421869B2, 24 September 2019. [Google Scholar]
- Mussa, M.H.; Rahaq, Y.; Takita, S.; Farmilo, N. Study the Enhancement on Corrosion Protection by Adding PFDTES to Hybrid Sol-Gel on AA2024-T3 Alloy in 3.5% NaCl Solutions. Albahit. J. Appl. Sci. 2021, 2, 61–68. [Google Scholar]
- ASTM International ASTM Code B209—14 Standard Specification for Aluminum and Aluminum-Alloy Sheet and Plate; ASTM International: West Conshohocken, PA, USA, 2016; Volume 25, p. 16.
- Tait, W.S. Electrochemical Impedance Spectroscopy Fundamentals, an Introduction to Electrochemical Corrosion Testing for Practicing Engineers and Scientists; Tait, W.S., Ed.; PairODocs Publications: Racine, WI, USA, 1994; ISBN 13-978-0966020700. [Google Scholar]
- Mohan, S.; Sundaraganesan, N.; Mink, J. FTIR and Raman studies on benzimidazole. Spectrochim. Acta Part A Mol. Spectrosc. 1991, 47, 1111–1115. [Google Scholar] [CrossRef]
- Kumar, D.; Wu, X.; Fu, Q.; Ho, J.W.C.; Kanhere, P.D.; Li, L.; Chen, Z. Development of durable self-cleaning coatings using organic-inorganic hybrid sol-gel method. Appl. Surf. Sci. 2015, 344, 205–212. [Google Scholar] [CrossRef]
- Yabuki, A.; Yamagami, H.; Noishiki, K. Barrier and self-healing abilities of corrosion protective polymer coatings and metal powders for aluminum alloys. Mater. Corros. 2007, 58, 497–501. [Google Scholar] [CrossRef]

| No. | Identifier | Formula Base Composite | (BZI) v/v% | Curing Temperature |
|---|---|---|---|---|
| 1- | SHX-80 | TEOS + MTMS + PSX | - | 80 °C |
| 2- | ZBI-SHX-80 | TEOS + MTMS + PSX | 3.5% | 80 °C |
| 3- | Bare AA2024-T3 | - | - | - |
| Circuit | Element | Immersion Time (H) | ||
|---|---|---|---|---|
| 01 | 48 | 144 | ||
| R(Q(R(QR))) | R(Q(R(Q(RW)))) | R(Q(R(Q(RW)))) | ||
| Rs | 105 | 160 | 201 | |
| Qct | 1.471 × 10−9 | 4.549 × 10−9 | 9.148 × 10−9 | |
| n | 0.9626 | 0.887 | 0.8406 | |
| Rct | 1.326 × 107 | 1.301 × 106 | 6.991 × 105 | |
| QiL | 3.453 × 10−9 | 6.406 × 10−8 | 9.016 × 10−8 | |
| n | 0.800 | 0.650 | 0.800 | |
| RiL | 4.697 × 107 | 5.885 × 105 | 1.778 × 103 | |
| W | - | 4.349 × 10−8 | 7.044 × 10−10 | |
| Circuit | Element | Immersion Time (H) | ||
|---|---|---|---|---|
| 01 | 48 | 144 | ||
| R(Q(R(QR))) | R(Q(R(Q(R(QR))))) | R(Q(R(Q(R(QR))))) | ||
| Rs | 100 | 205 | 225 | |
| Qct | 1.079 × 10−7 | 2.850 × 10−7 | 4.771 × 10−6 | |
| n | 0.800 | 0.627 | 0.490 | |
| Rct | 7.287 × 104 | 815 | 253 | |
| QiL | 4.933 × 10−6 | 1.151 × 10−6 | 3.912 × 10−6 | |
| n | 0.850 | 0.694 | 0.896 | |
| RiL | 7.793 × 105 | 4.022 × 105 | 1.221 × 105 | |
| Qp | - | 7.364 × 10−5 | 4.835 × 10−5 | |
| n | - | 0.900 | 0.455 | |
| Rp | - | 1.369 × 106 | 1 × 1020 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mussa, M.H.; Zahoor, F.D.; Lewis, O.; Farmilo, N. Developing a Benzimidazole-Silica-Based Hybrid Sol–Gel Coating with Significant Corrosion Protection on Aluminum Alloys 2024-T3. Eng. Proc. 2021, 11, 3. https://doi.org/10.3390/ASEC2021-11124
Mussa MH, Zahoor FD, Lewis O, Farmilo N. Developing a Benzimidazole-Silica-Based Hybrid Sol–Gel Coating with Significant Corrosion Protection on Aluminum Alloys 2024-T3. Engineering Proceedings. 2021; 11(1):3. https://doi.org/10.3390/ASEC2021-11124
Chicago/Turabian StyleMussa, Magdi H., F. Deeba Zahoor, Oliver Lewis, and Nicholas Farmilo. 2021. "Developing a Benzimidazole-Silica-Based Hybrid Sol–Gel Coating with Significant Corrosion Protection on Aluminum Alloys 2024-T3" Engineering Proceedings 11, no. 1: 3. https://doi.org/10.3390/ASEC2021-11124






