Abstract
Thymol and carvacrol (isopropylmethylphenols) are natural phenolic monoterpenoids with antibacterial, antifungal, insecticidal, and antioxidant properties. Their dose-dependent antioxidant effect requires control in real samples. Various modes of voltammetry have been successfully developed for thymol and carvacrol quantification. A glassy carbon electrode (GCE) modified with multi-walled carbon nanotubes (MWCNTs) and electropolymerized thymolphthalein has been developed for this purpose. The conditions of thymolphthalein electropolymerization (monomer concentration, number of cycles, and parameters of electrolysis) providing the best response to thymol have been found. The scanning electron microscopy and electrochemical methods confirm the effectivity of the sensor developed. In differential pulse mode, the sensor gives a linear response in the ranges of 0.050–25 and 25–100 μM for thymol and 0.10–10 and 10–100 μM for carvacrol, with detection limits of 0.037 and 0.063 μM, respectively, that are significantly improved compared to those reported earlier. The sensor developed is selective to isopropylmethylphenols in the presence of typical interferences (inorganic ions, saccharides, and ascorbic acid) and other phenolics (caffeic, chlorogenic, gallic and rosmarinic acids, quercetin, and rutin). A sensor has been applied for the evaluation of total isopropylmethylphenols in oregano and thyme spices using single sonication-assisted extraction with methanol. The voltammetric sensor data agreed well with the independent spectrophotometric quantification.
1. Introduction
Thymol and carvacrol (isopropylmethylphenols) are phenolic monoterpenoids with antibacterial, antifungal, insecticidal, and antioxidant properties [1,2,3,4]. Thyme, oregano, and other culinary and medicinal herbs are their natural sources [5]. Similar to other natural phenolics, isopropylmethylphenols show prooxidant activity at high concentrations [6] that leads to the formation of free radicals that able to provide harmful effects on living cells. Therefore, the control of the thymol and carvacrol contents in real samples is required.
The presence of phenolic hydroxyl groups in the thymol and carvacrol structures makes them active at the electrode surface, allowing the application of voltammetry for their quantification. Similarity of the structures leads to almost the same oxidation potentials of both compounds. Therefore, the total isopropylphenols determination is usually performed.
Traditional carbon-based electrodes [7,8,9] and boron-doped diamond electrodes [10,11] are applied for these purposes. Recently, chemically modified electrodes have been developed for the determination of isopropylmethylphenols, improving its sensitivity. Multi- [12,13] and single-walled carbon nanotubes [14], graphene oxide nanosheets [15], monodisperse Ag@C@Ag core–double shell spheres [16], MnY nanozeolite [17], and CeO2 nanoparticles in combination with graphene [18] and Brij® 35 micellar medium [19] are successfully used as sensing layers for thymol determination. Carvacrol is less studied in electroanalysis. To date, only two electrochemical sensors for carvacrol based on the multi-walled carbon nanotubes (MWCNTs) [20] and La2O3/Co3O4 nanocomposite [21] have been reported.
Thus, the further development of electrochemical sensors for isopropylmethylphenols is of practical interest. Polymer-modified electrodes that are widely developed for other natural phenolic compounds [22] are fully out of consideration in application to thymol and carvacrol. The current work is focused on the development of a sensitive voltammetric sensor for isopropylmethylphenols determination using an electrode modified with MWCNTs and electropolymerized thymolphthalein.
2. Materials and Methods
Thymol (99.5% purity) and thymolphthalein (95%) from Sigma (Steinheim, Germany) and carvacrol (98%) from Aldrich (Steinheim, Germany) were used. Their standard 10 mM solutions were prepared in methanol (c.p. grade) in 5.0 mL flasks. Ascorbic (99%), gallic (99%), caffeic (98%), and rosmarinic (98%) acids and quercetin dihydrate (95%) from Sigma (Steinheim, Germany) and chlorogenic acid (95%) from Aldrich (Steinheim, Germany) were used in the interference study. Their 10 mM stock solutions in methanol were prepared in 5.0 mL flasks. Less-concentrated solutions were obtained by the exact dilution.
MWCNTs (outer diameter 40–60 nm, inner diameter 5–10 nm, and 0.5–500 μm length) from Aldrich (Steinheim, Germany) were used as a platform for the further electrodeposition of polythymolphthalein. A homogeneous 0.5 mg mL−1 suspension of MWCNTs was prepared in 1% sodium dodecylsulfate (Panreac, Barcelona, Spain) by 30 min of sonication in an ultrasonic bath (WiseClean WUC-A03H (DAIHAN Scientific Co., Ltd., Wonju-si, Republic of Korea)).
All reagents were c.p. grade. Distilled water was used for the measurements. The laboratory temperature was 25 ± 2 °C.
Electrochemical measurements were conducted on the potentiostat/galvanostat Autolab PGSTAT 302N with the FRA 32M module (Eco Chemie B.V., Utrecht, the Netherlands) and NOVA 1.10.1.9 software. A glassy electrochemical cell of 10 mL volume was used. The tree–electrode system consisted of the working GCE of 3 mm diameter (CH Instruments, Inc., Bee Cave, TX, USA) or a modified electrode, an Ag/AgCl reference electrode, and a platinum wire as the auxiliary electrode.
pH measurements were carried out using the “Expert-001” pH meter (Econix-Expert Ltd., Moscow, Russia) with a glassy electrode.
Spectrophotometric measurements were performed on the spectrophotometer PE-5300 (NPO Ecros, Saint Petersburg, Russia).
3. Results
3.1. Electrodeposition of Polythymolphthalein at the MWNT-Modified Electrode
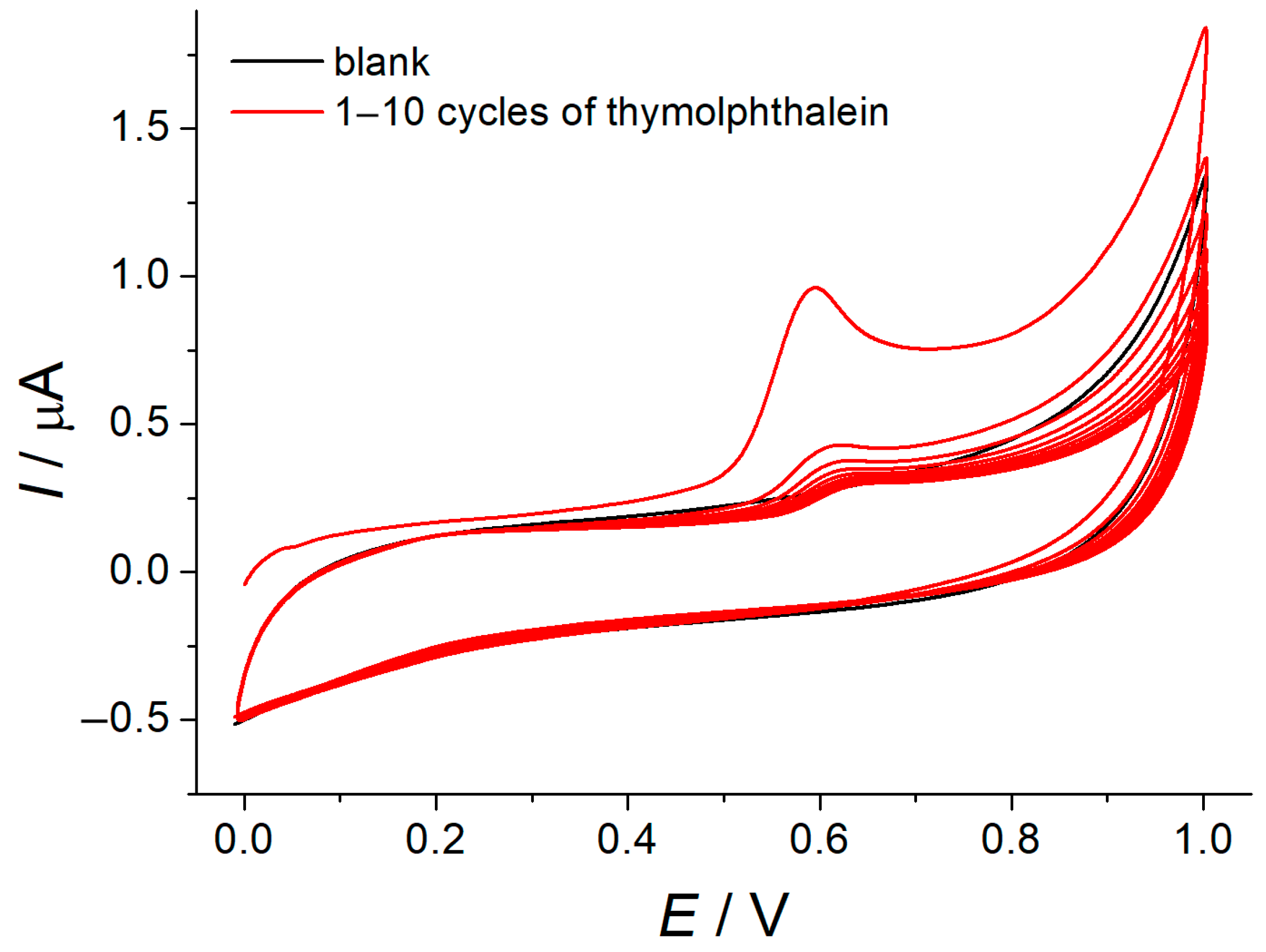
GCE was modified with MWCNTs by using the drop casting method. Then, the electrochemical deposition of polythymolphthalein was performed in the potentiodynamic mode. Irreversible single-step electrooxidation of thymolphthalein at 0.594 V was observed in neutral medium (Figure 1). Thymolphthalein underwent one electron and one proton oxidation with the participation of thymol fragments forming a phenoxyl radical that can further dimerize and polymerize that agrees well with the reported data for thymolphthalein [23] and bromothymol blue [24]. The dramatic decrease of the oxidation current with the increase of the number of cycles confirms the formation of insulating polymeric coverage that is typical for the electropolymerization of phenolics [25].
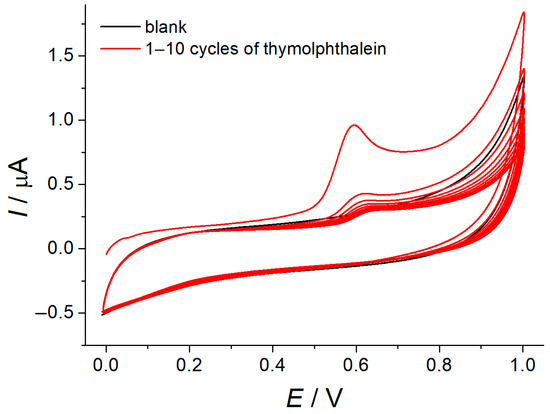
Figure 1.
Electropolymerization of 10 μM thymolphthalein at the MWNT/GCE in phosphate buffer pH 7.0. υ = 100 mV s−1.
The electropolymerization conditions of thymolphthalein were optimized on the basis of the voltammetric response of 10 μM thymol. The best characteristics of the analyte were registered on the polymer-modified electrode obtained by 10-fold potential cycling from 0.0 to 1.0 V with a scan rate of 100 mV s−1 in the 10 μM monomer solution in phosphate buffer pH 7.0. Comparison of the voltammetric characteristics of thymol on bare GCE, MWCNTs/GCE, and polythymolphthalein/MWCNTs/GCE (Table 1) clearly indicates the effectivity of the developed electrode. Furthermore, capacitive currents are significantly less on the polymer-modified electrode vs. MWCNTs/GCE.

Table 1.
Voltammetric characteristics of 10 μM thymol at the bare and modified GCE in Britton–Robinson buffer pH 7.0.
The polymer-modified electrode shows a significant increase in the effective surface area (88 ± 5 mm2 vs. 8.9 ± 0.3 mm2 for GCE) and electron transfer rate (electron transfer resistance is 9.9-fold less than for the GCE).
3.2. Electrooxidation of Isopropylmethylphenols at the Polythymolphthalein/MWCNTs/GCE
Varying supporting electrolyte pH in the range of 2.0–12.0, the voltammetric characteristics of thymol and carvacrol, were studied under conditions of cyclic voltammetry. The decrease of the oxidation potentials of both compounds at pH 2.0–11.0 confirms the participation of protons in the electrode reaction. Then, pH-independent oxidation occurs that agrees well with the thymol and carvacrol ionization constants [26]. Oxidation currents are also decreased with the increase of the Britton–Robinson buffer pH. Therefore, pH 2.0 were chosen for further investigations.
Diffusion-controlled two-electron oxidation of thymol and carvacrol was confirmed on the basis of cyclic voltammetry data at various potential scan rates. An irreversible process was observed for both compounds, and anodic transfer coefficients of 0.40 and 0.44 were calculated for thymol and carvacrol, respectively.
3.3. Quantification of Isopropylmethylphenols Using a Polythymolphthalein-Based Sensor
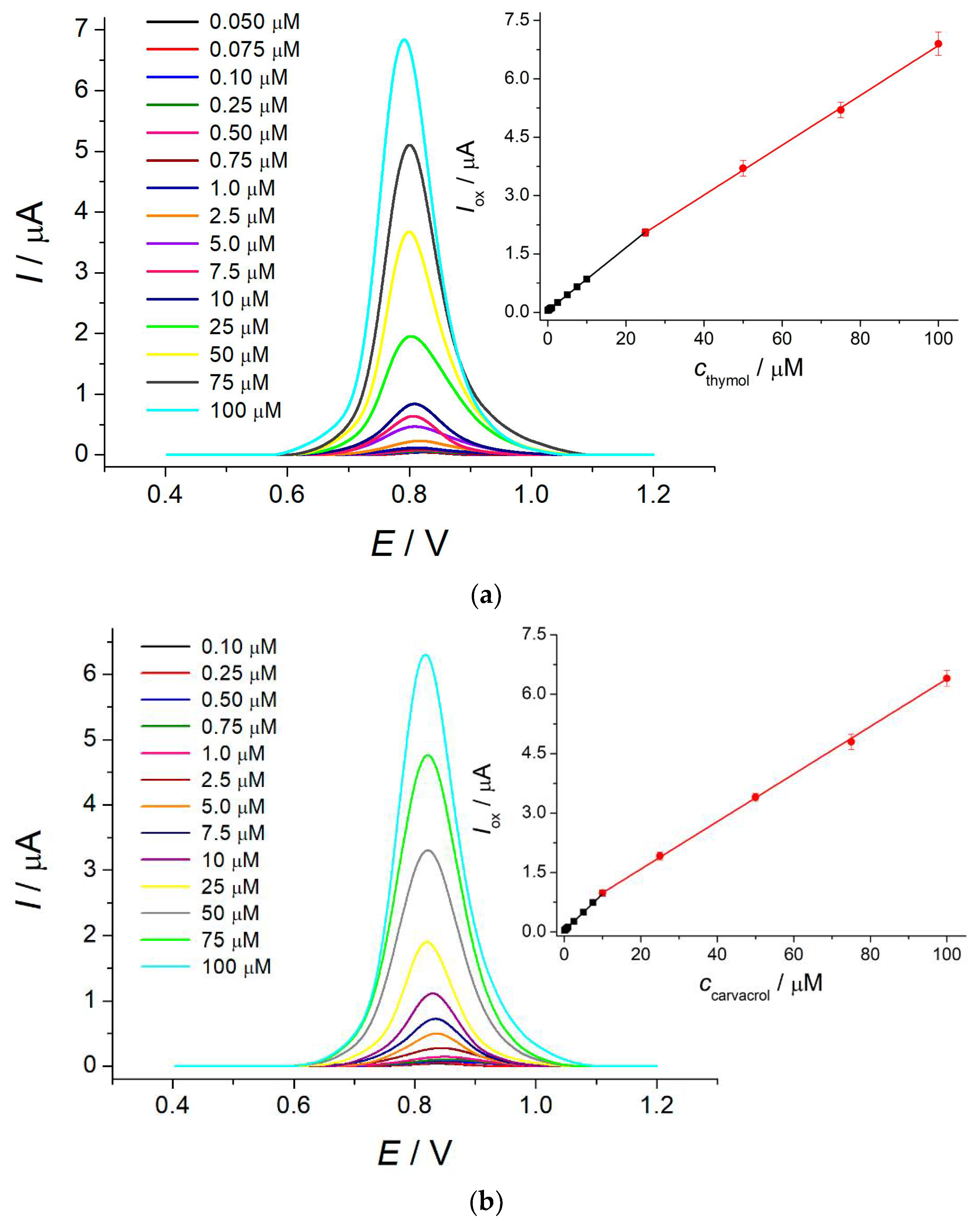
The electrode created was used as a voltammetric sensor for thymol and carvacrol. The differential pulse voltammetric response of the sensor (Figure 2) increases linearly with the growth of the isopropylmethylphenol concentration in the ranges of 0.050–25 and 25–100 μM for thymol and 0.10–10 and 10–100 μM for carvacrol with detection limits of 0.037 and 0.063 μM, respectively. The oxidation potentials of thymol and carvacrol are 0.81 and 0.83 V, respectively, making impossible their simultaneous determination. Similar to other electrochemical approaches, the total contents of isopropylmethylphenols can be evaluated. The analytical characteristics obtained are improved vs. those reported for other voltammetric sensors [12,13,14,15,16,17,18,19,20,21]. The high precision of the developed sensor has been confirmed by recovery of 99–100.1% for the determination of thymol and carvacrol in the model solutions. The relative standard deviation is less than 5%, which indicates good reproducibility of the sensor response as far as the electrode surface was renewed after each measurement.
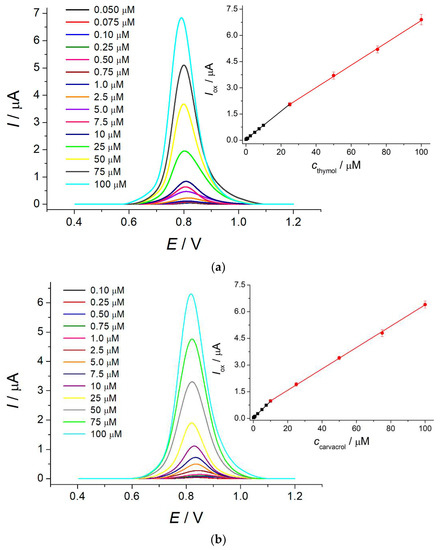
Figure 2.
Differential pulse voltammograms of isopropylmethylphenols at the polythymolphthalein/MWCNTs/GCE in Britton–Robinson buffer pH 2.0: (a) thymol; (b) carvacrol. ΔEpulse = 100 mV, tpulse = 25 ms, and υ = 20 mV s−1. Inserts are the corresponding calibration graphs.
The sensor response to isopropylmethylphenols is selective in the presence of typical interferences (1000-fold excesses of K+, Mg2+, Ca2+, NO3−, Cl−, and SO42− and 100-fold excesses of saccharides and ascorbic acid) and other phenolics (50-fold excesses of caffeic, chlorogenic, and rosmarinic acids and 1.0 μM of gallic acid, and 25-fold excess of quercetin and rutin). The data obtained allow to use the sensor in the analysis of plant materials.
3.4. Quantification of Total Isopropylmethylphenols in Thyme and Oregano Spices
The sensor developed was tested on thyme and oregano spices, being the major sources of isopropylmethylphenols. Single sonication-assisted extraction with methanol for 5 min was used. The spice/methanol ratio providing the highest yield of isopropylmethylphenols is 1:40 for oregano and 1:30 for thyme.
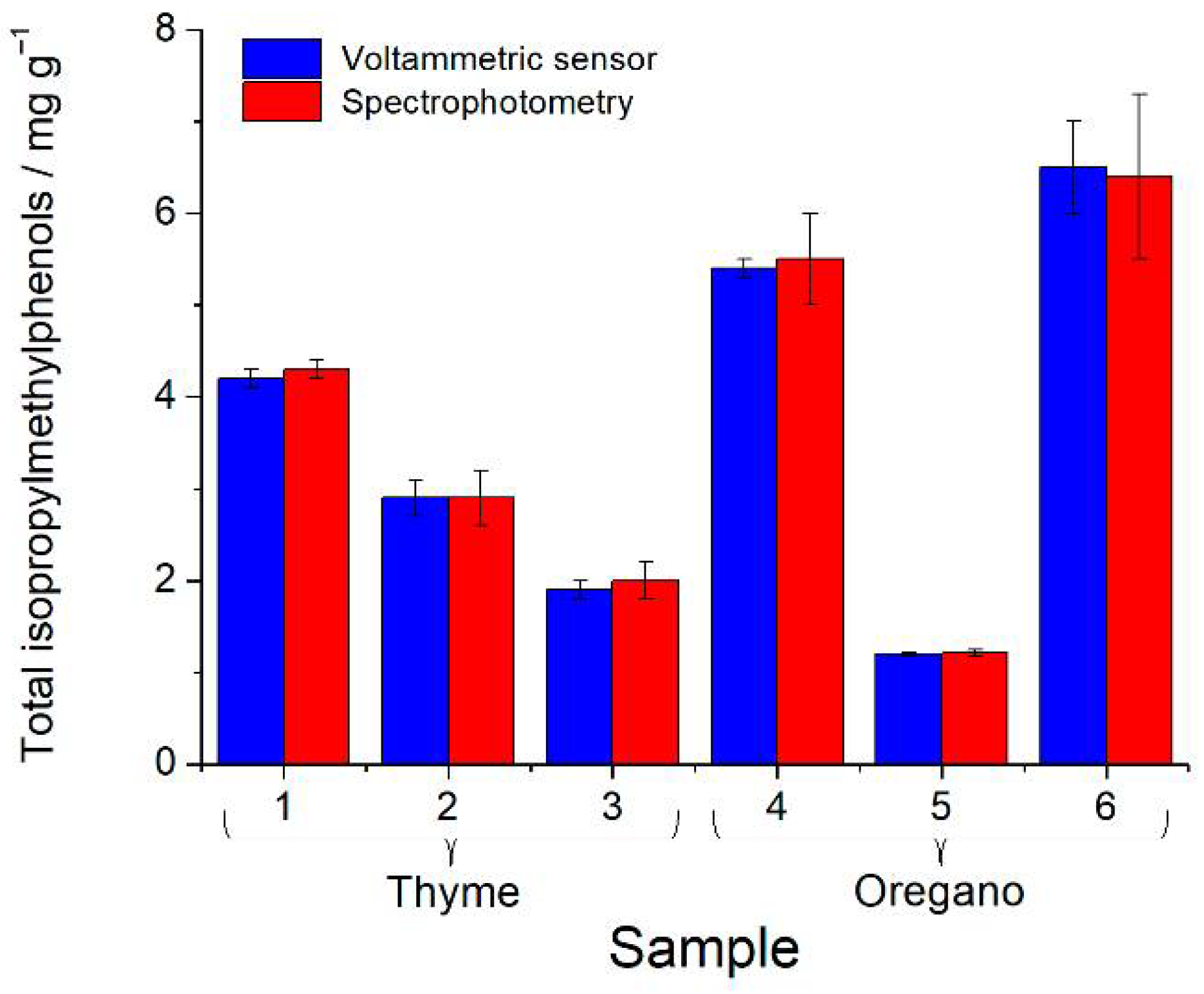
The voltammetric determination of the total isopropylmethylphenols was compared with the independent spectrophotometric method [27] (Figure 3). The results obtained agree well, and the F-test confirms a similar accuracy of the methods (F-criterium values of 1.75–6.09 are less than the critical value 6.59).
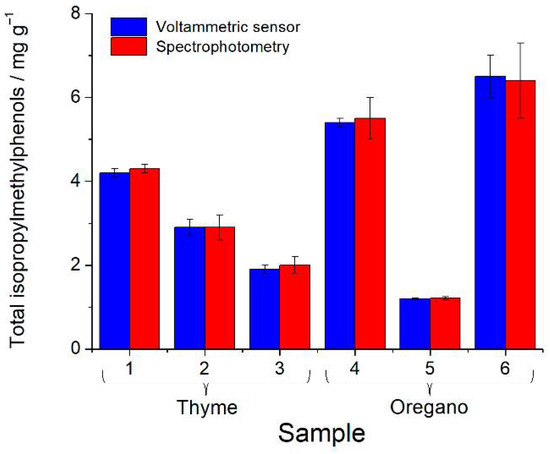
Figure 3.
Quantification of the total isopropylmethylphenols in thyme and oregano spices.
4. Conclusions
A sensitive and selective voltammetric sensor based on MWCNTs and electropolymerized thymolphthalein was developed for the determination of thymol and carvacrol. The application of MWCNTs significantly increases the conductivity of the electrode and its surface area, providing a higher load of polymeric coverage. The polymeric film provides structural similarity to the analytes and the porous structure, leading to the increase of the determination sensitivity. The analytical characteristics achieved are the best ones reported to date. The sensor is simple, reliable, cost-effective, and can be applied for a routine analysis for the fast screening of plant materials.
Author Contributions
Conceptualization, G.Z.; methodology, G.Z.; validation, G.Z.; investigation, N.C. and G.Z.; writing—original draft preparation, G.Z.; writing—review and editing, G.Z.; visualization, N.C. and G.Z.; and supervision, G.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fachini-Queiroz, F.C.; Kummer, R.; Estevão-Silva, C.F.; de Barros Carvalho, M.D.; Cunha, J.M.; Grespan, R.; Bersani-Amado, C.A.; Cuman, R.K.N. Effects of thymol and carvacrol, constituents of Thymus vulgaris L. essential oil, on the inflammatory response. Evid. Based Complement. Alternat. Med. 2012, 2012, 657026. [Google Scholar] [CrossRef]
- Falcone, P.; Speranza, B.; Del Nobile, M.A.; Corbo, M.R.; Sinigaglia, M.J. A study on the antimicrobial activity of thymol intended as a natural preservative. Food Pro. 2005, 68, 1664–1670. [Google Scholar] [CrossRef]
- Nostro, A.; Papalia, T. Antimicrobial activity of carvacrol: Current progress and future prospectives. Recent Pat. Antiinfect. Drug Discov. 2012, 7, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Yanishlieva, N.V.; Marinova, E.M.; Gordon, M.H.; Raneva, V.G. Antioxidant activity and mechanism of action of thymol and carvacrol in two lipid systems. Food Chem. 1999, 64, 59–66. [Google Scholar] [CrossRef]
- Naghdi Badi, H.; Abdollahi, M.; Mehrafarin, A.; Ghorbanpour, M.; Tolyat, S.; Qaderi, A.; Ghiaci Yekta, M. An overview on two valuable natural and bioactive compounds, thymol and carvacrol, in medicinal plants. J. Med. Plants. 2017, 16, 1–32. [Google Scholar]
- Ziyatdinova, G.; Budnikov, H. Natural phenolic antioxidants in bioanalytical chemistry: State of the art and prospects of development. Russ. Chem. Rev. 2015, 84, 194–224. [Google Scholar] [CrossRef]
- Michelitsch, A.; Rittmannsberger, A.; Hüfner, A.; Rückert, U.; Likussar, W. Determination of isopropylmethylphenols in black seed oil by differential pulse voltammetry. Phytochem. Anal. 2004, 15, 320–324. [Google Scholar] [CrossRef]
- Robledo, S.N.; Pierini, G.D.; Nieto, C.H.D.; Fernández, H.; Zon, M.A. Development of an electrochemical method to determine phenolic monoterpenes in essential oils. Talanta 2019, 196, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Lau, O.-W.; Luk, S.-F.; Wong, W.-C. Simultaneous determination of methyl salicylate and thymol in various pharmaceutical formulations by differential-pulse voltammetry using a glassy carbon electrode. Analyst 1988, 113, 865–868. [Google Scholar] [CrossRef]
- Kowalcze, M.; Jakubowska, M. Voltammetric determination of carvacrol on boron doped diamond electrode. Anal. Chim. Acta 2019, 1045, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Stanković, D.M. Sensitive voltammetric determination of thymol in essential oil of Carum copticum seeds using boron-doped diamond electrode. Anal. Biochem. 2015, 486, 1–4. [Google Scholar] [CrossRef]
- Piech, R.; Paczosa-Bator, B. Application of glassy carbon electrode modified with Nafion/MWCNTs for sensitive voltammetric determination of thymol. Acta Pol. Pharm. 2015, 72, 1081–1088. [Google Scholar]
- Ziyatdinova, G.K.; Romashkina, S.A.; Ziganshina, E.R.; Budnikov, H.C. Voltammetric determination of thymol on an electrode modified by coimmobilized carboxylated multiwalled carbon nanotubes and surfactants. J. Anal. Chem. 2018, 73, 63–70. [Google Scholar] [CrossRef]
- Fuentes, F.G.; Gil, M.Á.L.; Mendoza, S.; Escarpa, A. Electrochemical screening of biomarkers in chemotype Mexican oregano oils on single-walled carbon nanotubes screen-printed electrodes. Electroanalysis 2011, 23, 2212–2216. [Google Scholar] [CrossRef]
- Behpour, M.; Masouma, S.; Meshkia, M. Determination of trace amounts of thymol and caffeic acid in real samples using a graphene oxide nanosheet modified electrode application of experimental design in voltammetric studies. RSC Adv. 2014, 4, 14270–14280. [Google Scholar] [CrossRef]
- Gan, T.; Lv, Z.; Deng, Y.; Sun, J.; Shi, Z.; Liu, Y. Facile synthesis of monodisperse Ag@C@Ag core-double shell spheres for application in the simultaneous sensing of thymol and phenol. New J. Chem. 2015, 39, 6244–6252. [Google Scholar] [CrossRef]
- Aghamohseni, B.; Hassaninejad-Darzi, S.K.; Asadollahi-Baboli, M. A new sensitive voltammetric determination of thymol based on MnY nanozeolite modified carbon paste electrode using response surface methodology. Microchem. J. 2019, 145, 819–832. [Google Scholar] [CrossRef]
- Zhao, X.; Du, Y.; Ye, W.; Lu, D.; Xia, X.; Wang, C. Sensitive determination of thymol based on CeO2 nanoparticle-decorated graphene hybrid film. New J. Chem. 2013, 37, 4045–4051. [Google Scholar] [CrossRef]
- Ziyatdinova, G.; Ziganshina, E.; Cong, P.N.; Budnikov, H. Voltammetric determination of thymol in oregano using CeO2-modified electrode in Brij® 35 micellar medium. Food Anal. Methods 2017, 10, 129–136. [Google Scholar] [CrossRef]
- Ziyatdinova, G.; Budnikov, H. MWNT-based electrode for the voltammetric quantification of carvacrol. Food Anal. Methods 2021, 14, 401–410. [Google Scholar] [CrossRef]
- Mohammadi, S.Z.; Beitollahi, H.; Rohani, T.; Allahabadi, H. Carvacrol electrochemical reaction characteristics on screen printed electrode modified with La2O3/Co3O4 nanocomposite. J. Electrochem. Sci. Eng. 2019, 9, 113–123. [Google Scholar] [CrossRef]
- Ziyatdinova, G.K.; Zhupanova, A.S.; Budnikov, H.C. Electrochemical sensors for the simultaneous detection of phenolic antioxidants. J. Anal. Chem. 2022, 77, 155–172. [Google Scholar] [CrossRef]
- Guss, E.V.; Ziyatdinova, G.K.; Zhupanova, A.S.; Budnikov, H.C. Voltammetric determination of quercetin and rutin in their simultaneous presence on an electrode modified with polythymolphthalein. J. Anal. Chem. 2020, 75, 526–535. [Google Scholar] [CrossRef]
- Chandrashekar, B.N.; Swamy, B.E.K.; Mahesh, K.R.V.; Chandra, U.; Sherigara, B.S. Electrochemical studies of bromothymol blue at surfactant modified carbon paste electrode by using cyclic voltammetry. Int. J. Electrochem. Sci. 2009, 4, 471–480. [Google Scholar]
- Ziyatdinova, G.; Guss, E.; Yakupova, E. Electrochemical sensors based on the electropolymerized natural phenolic antioxidants and their analytical application. Sensors 2021, 21, 8385. [Google Scholar] [CrossRef] [PubMed]
- Mastelić, J.; Jerković, I.; Blazević, I.; Poljak-Blazi, M.; Borović, S.; Ivancić-Baće, I.; Smrecki, V.; Zarković, N.; Brcić-Kostic, K.; Vikić-Topić, D.; et al. Comparative study on the antioxidant and biological activities of carvacrol, thymol, and eugenol derivatives. J. Agric. Food Chem. 2008, 56, 3989–3996. [Google Scholar] [CrossRef]
- Tonello, N.V.; D’Eramo, F.; Marioli, J.M.; Crevillen, A.G.; Escarpa, A. Extraction-free colorimetric determination of thymol and carvacrol isomers in essential oils by pH-dependent formation of gold nanoparticles. Microchim. Acta 2018, 185, 352. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).