Binary and Ternary Oxide Nanostructured Multisystems for Gas Sensors †
Abstract
:1. Introduction
2. Experiment
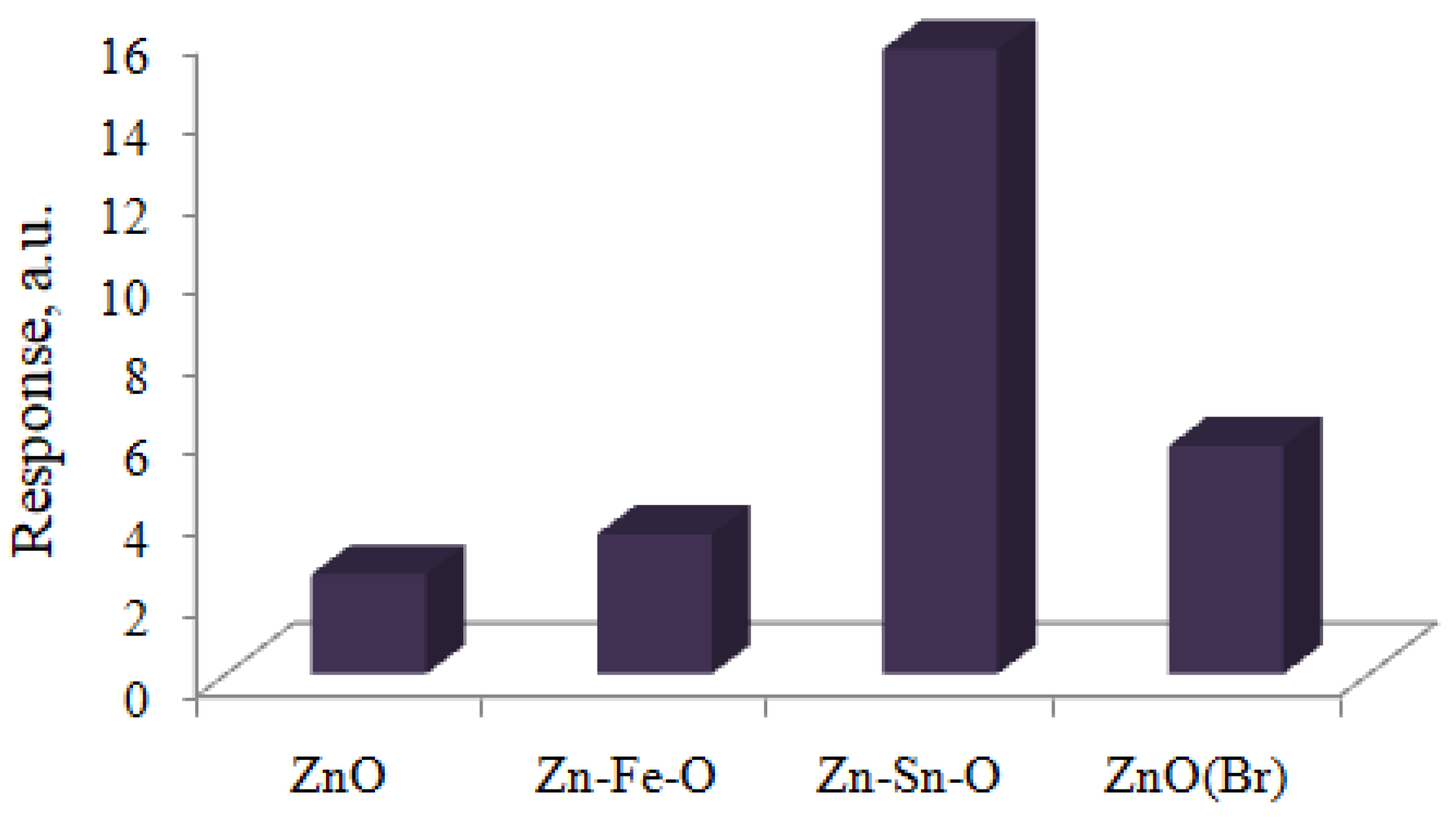
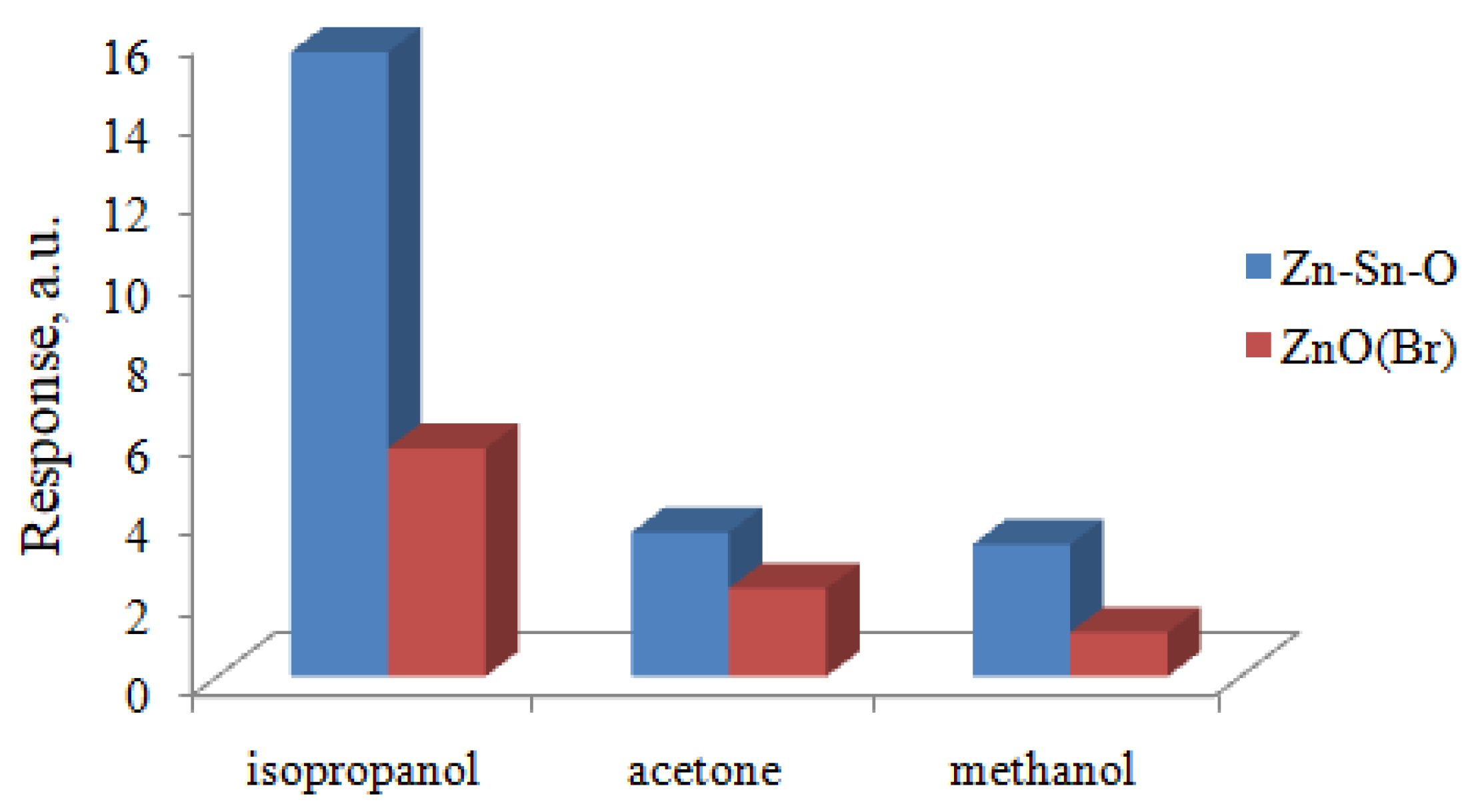
3. Results and Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Korotcenkov, G. Metal oxides for solid-state gas sensors: What determines our choice? Mater. Sci. Eng. B 2007, 139, 1–23. [Google Scholar] [CrossRef]
- Behera, B.; Chandra, S. An innovative gas sensor incorporating ZnO–CuO nanoflakes in planar MEMS technology. Sens. Actuators B 2016, 229, 414–424. [Google Scholar] [CrossRef]
- Kim, K.; Choi, P.; Itoh, T.; Masuda, I. Catalyst-free Highly Sensitive SnO2 Nanosheet Gas Sensors for Parts per Billion-Level Detection of Acetone. ACS Appl. Mater. Interfaces 2020, 12, 51637–51644. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhu, Y.; Meng, D.; Wang, K.; Wang, C. A novel room temperature ethanol gas sensor based on 3D hierarchical flower-like TiO2 microstructures. Mater. Lett. 2020, 277, 128372. [Google Scholar] [CrossRef]
- Choi, M.S.; Kim, M.Y.; Mirzaei, A.; Kim, H.S.; Kim, S.; Baek, S.H.; Chun, D.W.; Jin, C.; Lee, K.H. Selective, sensitive, and stable NO2 gas sensor based on porous ZnO nanosheets. Appl. Surf. Sci. 2021, 568, 150910. [Google Scholar] [CrossRef]
- Umar, A.; Alduraibi, M.; Al-Dossary, O. Development of Ethanol Gas Sensor Using α-Fe2O3Nanocubes Synthesized by Hydrothermal Process. J. Nanoelectron. Optoelectron. 2020, 15, 59–64. [Google Scholar] [CrossRef]
- Sui, N.; Zhang, P.; Zhou, T.; Zhang, T. Selective ppb-level ozone gas sensor based on hierarchical branch-like In2O3 nanostructure. Sens. Actuators B 2021, 336, 129612. [Google Scholar] [CrossRef]
- Hjiri, M.; Bahanan, F.; Aida, M.S.; El Mir, L.; Neri, G. High Performance CO Gas Sensor Based on ZnO Nanoparticles. J. Inorg. Organomet. Polym. 2020, 30, 4063–4071. [Google Scholar] [CrossRef]
- Wang, T.; Kou, X.; Zhao, L.; Sun, P.; Liu, C.; Wang, Y.; Shimanoe, K.; Yamazoe, N.; Lu, G. Flower-like ZnO hollow microspheres loaded with CdO nanoparticles as high performance sensing material for gas sensors. Sens. Actuators B 2017, 250, 692–702. [Google Scholar] [CrossRef]
- Caicedo, N.; Leturcq, R.; Raskin, J.-P.; Flandre, D.; Lenoble, D. Detection mechanism in highly sensitive ZnO nanowires network gas sensors. Sens. Actuators B 2019, 297, 126602. [Google Scholar] [CrossRef]
- Ananthi, S.; Kavitha, M.; Ranjith Kumar, E.; Prakash, T.; Vandamar Poonguzhali, R.; Ranjithkumar, B.; Balamurugan, A.; Srinivas, C.; Sastry, D.L. Investigation of physicochemical properties of ZnO nanoparticles for gas sensor applications. Inorg. Chem. Commun. 2022, 146, 110152. [Google Scholar] [CrossRef]
- Kirtay, H.; Omur, B.C.; Altindal, A.; Arsu, N. One step facile in-situ photochemical synthesis of hollow, doughnut-like ZnO nanoparticles and their alcohol vapor sensing properties. Mater. Res. Bull. 2020, 122, 110661. [Google Scholar] [CrossRef]
- Li, Q.; Chen, D.; Miao, J.; Lin, S.; Yu, Z.; Cui, D.; Yang, Z.; Chen, X. Highly sensitive sensor based on ordered porous ZnO nanosheets for ethanol detecting application. Sens. Actuators B 2021, 326, 128952. [Google Scholar] [CrossRef]
- Fan, C.; Sun, F.; Wang, X.; Huang, Z.; Keshvardoostchokami, M.; Kumar, P.; Liu, B. Synthesis of ZnO Hierarchical Structures and Their Gas Sensing Properties. Nanomaterials 2019, 9, 1277. [Google Scholar] [CrossRef] [PubMed]
- Cao, P.; Yang, Z.; Navale, S.T.; Han, S.; Liu, X.; Liu, W.; Lu, Y.; Stadler, F.J.; Zhu, D. Ethanol sensing behavior of Pd-nanoparticles decorated ZnO-nanorod based chemiresistive gas sensors. Sens. Actuators B 2019, 298, 126850. [Google Scholar] [CrossRef]
- Sankar Ganesh, R.; Patil, V.L.; Durgadevi, E.; Navaneethan, M.; Ponnusamy, S.; Muthamizhchelvan, C.; Kawasaki, S.; Patil, P.S.; Hayakawa, Y. Growth of Fe doped ZnOnanoellipsoids for selective NO2 gas sensing application. Chem. Phys. Lett. 2019, 734, 136725. [Google Scholar] [CrossRef]
- Kasapoğlu, A.E.; Habashyani, S.; Baltakesmez, A.; İskenderoğlu, D.; Gür, E. The effect of the change in the amount of Sb doping in ZnO nanorods for hydrogen gas sensors. Int. J. Hydrog. Ener. 2021, 46, 21715–21725. [Google Scholar] [CrossRef]
- Zheng, X.; Fan, H.; Wang, H.; Yan, B.; Ma, J.; Wang, W.; Yadav, A.K.; Dong, W.; Wang, S. ZnO–SnO2 nano-heterostructures with high-energy facets for high selective and sensitive chlorine gas sensor. Ceram. Int. 2020, 46, 27499–27507. [Google Scholar] [CrossRef]
- Arockiam, C.H.; Ananthanarayanan, R.; Srinivasan, P.; Krishnakumar, A. Room temperature selective sensing of benzene vapor molecules using mixed oxide thin film of zinc oxide and cadmium oxide. Mater. Sci. Semicond. Proc. 2021, 132, 105930. [Google Scholar] [CrossRef]
- Morandi, S.; Ghiotti, G.; Chiorino, A.; Bonelli, B.; Comini, E.; Sberveglieri, G. MoO3–WO3 mixed oxide powder and thin films for gas sensing devices: A spectroscopic characterization. Sens. Actuators B 2005, 111–112, 8–35. [Google Scholar] [CrossRef]
- Cheng, P.; Lv, L.; Wang, Y.; Zhang, B.; Zhang, Y.; Zhang, Y.; Lei, Z.; Xu, L. SnO2/ZnSnO3 double-shelled hollow microspheres based high-performance acetone gas sensor. Sens. Actuators B 2021, 332, 129212. [Google Scholar] [CrossRef]
- Zheng, L.; Zhang, C.; He, L.; Zhang, K.; Zhang, J.; Jin, L.; Asiri, A.M.; Alamry, K.A.; Chu, X. ZnFe2O4/ZnO nanosheets assembled microspheres for high performance trimethylamine gas sensing. J. Alloys Comp. 2020, 849, 156461. [Google Scholar] [CrossRef]
- Aubekerov, K.; Punegova, K.N.; Nalimova, S.S.; Moshnikov, V.A.; Sergeenko, R.; Kuznetsov, A.; Kondratev, V.M.; Kadinskaya, S.A. Synthesis and study of gas sensitive ZnFe2O4-modified ZnO nanowires. J. Phys. Conf. Ser. 2022, 2227, 012014. [Google Scholar] [CrossRef]
- Nalimova, S.S.; Moshnikov, V.A.; Bobkov, A.A.; Ryabko, A.A.; Shomakhov, Z.V.; Kalazhokov, Z.K. An X-ray photoelectron spectroscopy study of zinc stannate layer formation. Tech. Phys. 2020, 65, 1087–1090. [Google Scholar] [CrossRef]
- Kondratev, V.M.; Bolshakov, A.D.; Nalimova, S.S. Technologically feasible ZnO nanostructures for carbon monoxide gas sensing. In Proceedings of the 2021 IEEE Conferenceof Russian Young Researchers in Electrical and Electronic Engineering, ElConRus 2021, St. Perersburg, Moscow, Russia, 26–29 January 2021. [Google Scholar]
- Anikina, M.A.; Ryabko, A.A.; Nalimova, S.S.; Maximov, A.I. Synthesis and study of zinc oxide nanorods for semiconductor adsorption gas sensors. J. Phys. Conf. Ser. 2021, 1851, 012010. [Google Scholar] [CrossRef]
- Ryabko, A.A.; Bobkov, A.A.; Nalimova, S.S.; Maksimov, A.I.; Levitskii, V.S.; Moshnikov, V.A.; Terukov, E.I. Gas sensitivity of nanostructured coatings based on zinc oxide nanorods under combined activation. Tech. Phys. 2022, 67, 644–649. [Google Scholar] [CrossRef]
- Punegova, K.N.; Nalimova, S.S.; Arkhipenko, V.A.; Ryabko, A.A.; Kondratev, V.M.; Shomakhov, Z.V.; Guketlov, A.M. Zinc stannate nanostructures for low-temperature gas sensors with improved response and performance. St. Petersburg State Polytech. Univ. J. Phys. Math. 2023, 16, 229–235. [Google Scholar]
- Aubekerov, K.; Guketlov, A.M.; Gagarina, A.Y.; Ryabko, A.A.; Shomakhov, Z.V.; Nalimova, S.S. Enhanced gas sensing performances of ZnO-based composite nanostructures. In Proceedings of the 2022 Conference of Russian Young Researchers in Electrical and Electronic Engineering, ElConRus 2022, St. Petersburg, Moscow, Russia, 25–28 January 2022. [Google Scholar]
- Nalimova, S.; Shomakhov, Z.; Bobkov, A.; Moshnikov, V. Sacrificial Doping as an Approach to Controlling the Energy Properties of Adsorption Sites in Gas-Sensitive ZnO Nanowires. Micro 2023, 3, 591–601. [Google Scholar] [CrossRef]
- Wlodek, S.; Golbow, K.; Consadori, F. Kinetic model of thermally cycled tin oxide gas sensor. Sens. Actuators B 1991, 3, 123–127. [Google Scholar] [CrossRef]
- Shomakhov, Z.V.; Nalimova, S.S.; Shurdumov, B.Z.; Maximov, A.I.; Moshnikov, V.A. Zinc stannate nanostructures for fast response gas sensors. Phys.-Chem. Asp. Study Clust. Nanostruct. Nanomater. 2022, 14, 726–735. [Google Scholar] [CrossRef]
- Nalimova, S.S.; Shomakhov, Z.V.; Gerasimova, K.V.; Punegova, K.N.; Guketlov, A.M.; Kalmykov, R.M. Gas-sensitive composite nanostructures based on zinc oxide for detecting organic solvent vapors. Phys.-Chem. Asp. Study Clust. Nanostruct. Nanomater. 2022, 14, 678–687. [Google Scholar] [CrossRef]
- Karpova, S.S.; Moshnikov, V.A.; Mjakin, S.V.; Kolovangina, E.S. Surface functional composition and sensor properties of ZnO, Fe2O3, and ZnFe2O4. Semiconductors 2013, 47, 392–395. [Google Scholar] [CrossRef]
- Karpova, S.S.; Moshnikov, V.A.; Maksimov, A.I.; Mjakin, S.V.; Kazantseva, N.E. Study of the effect of the acid-base surface properties of ZnO, Fe2O3 and ZnFe2O4 oxides on their gas sensitivity to ethanol vapor. Semiconductors 2013, 47, 1026–1030. [Google Scholar] [CrossRef]
- Shomakhov, Z.V.; Nalimova, S.S.; Kondratev, V.M.; Maksimov, A.I.; Ryabko, A.A.; Moshnikov, V.A.; Molokanov, O.A. Changes in the Energy of Surface Adsorption Sites of ZnO Doped with Sn. J. Surf. Investig. X-ray Synchrotron Neutron Tech. 2023, 17, 898–902. [Google Scholar] [CrossRef]
- Wang, T.; Wang, X.; Wang, Y.; Yi, G.; Shi, C.; Yang, Y.; Sun, G.; Zhang, Z. Construction of Zn2SnO4 decorated ZnO nanoparticles for sensing triethylamine with dramatically enhanced performance. Mater. Sci. Semicond. Proc. 2022, 140, 106403. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nalimova, S.; Shomakhov, Z.; Moshnikov, V. Binary and Ternary Oxide Nanostructured Multisystems for Gas Sensors. Eng. Proc. 2023, 48, 47. https://doi.org/10.3390/CSAC2023-14880
Nalimova S, Shomakhov Z, Moshnikov V. Binary and Ternary Oxide Nanostructured Multisystems for Gas Sensors. Engineering Proceedings. 2023; 48(1):47. https://doi.org/10.3390/CSAC2023-14880
Chicago/Turabian StyleNalimova, Svetlana, Zamir Shomakhov, and Vyacheslav Moshnikov. 2023. "Binary and Ternary Oxide Nanostructured Multisystems for Gas Sensors" Engineering Proceedings 48, no. 1: 47. https://doi.org/10.3390/CSAC2023-14880
APA StyleNalimova, S., Shomakhov, Z., & Moshnikov, V. (2023). Binary and Ternary Oxide Nanostructured Multisystems for Gas Sensors. Engineering Proceedings, 48(1), 47. https://doi.org/10.3390/CSAC2023-14880





