Abstract
Pharmaceutically important isoxazoles are within the wide range of heterocycles. The isoxazole ring, being five-membered, is also found in many bioactive natural products in addition to synthetic drugs. Many significant properties are exhibited by synthetically modified isoxazoles. Fused isoxazoles have widely shown their therapeutic potential as anticancer, insecticidal, antibacterial, antituberculosis, antifungal, antibiotic, antitumor, and antiulcerogenic agents. A variety of strategies are employed for the synthesis of these compounds, which are known for their pharmacological importance. Their synthesis is here reviewed. Synthesized isoxazoles have appeared as good forerunners for many other different molecules. This review summarizes the various synthesis approaches described so far for isoxazoles, providing a detailed study of their synthesis process. Substituted isoxazoles have been well discussed in the literature for their significant biological activities. This review is mainly focused on the synthesis of fused isoxazoles.
1. Introduction
A heterocyclic ring containing nitrogen along with oxygen has shown significance in pharmaceutical and medical chemistry. The preparation of molecules that are simple and possess contrasting functions because of the different heterocycles they contain is valuable in the field of heterocycles and their chemistry. Compounds containing nitrogen and oxygen include oxazole, isoxazoles, etc. Isoxazoles belong to an important class of heterocyclic compounds containing electronegative nitrogen and oxygen atoms and sp2 hybridized carbon atoms. Isoxazoles have been used as hydrogen bond donors or acceptors. Fused isoxazoles have displayed their utility in the pharmacological field, showing various biological activities, namely, anti-cancer, anti-microbial, antioxidant, antihypertensive, anti-inflammatory, and analgesic activities. An important antipsychotic activity was described for risperidone, and anticonvulsant activity was reported for monoamide. This has promoted a constant effort to optimize the synthesis of this kind of molecules.
Fused isoxazoles are a class of heterocyclic compounds that consist of an isoxazole ring fused with another aromatic or non-aromatic ring. Fused isoxazoles were found to show some unique structural properties, as their five-membered ring system can lead to the formation of specific angles in small molecules, promoting their interaction with the target, a property that can barely be observed for molecules containing six-membered rings. Medicinal chemists can modify different parts of the isoxazole molecule to fine-tune its properties, such as potency, selectivity, and pharmacokinetics, and optimize its therapeutic potential. The structural diversity and flexibility of fused isoxazoles allow for the design of compounds with high specificity for particular biological targets.
This is crucial for minimizing off-target effects and reducing potential side effects in patients. Interestingly, these properties were also found for naturally occurring ibetonic acid, along with some marketed drugs such as flucloxacillin, cloxacillin, valdecoxib, danazol, and dicloxacillin. In the last few years, fused isoxazole was reported as a bioactive compound with antibacterial (Figure 1A), antifungal (Figure 1B), anti-inflammatory (Figure 1C), and anti-tuberculosis activities (Figure 1D) [1]. Fused isoxazoles can be optimized to improve their absorption, distribution, metabolism, excretion, and toxicity (ADMET) properties. This would help in ensuring that a compound can be effectively administered and metabolized in the body. Libraries of fused isoxazole derivatives can be used in high-throughput screening programs to discover new lead compounds for various therapeutic targets. Their diversity expands the range of potential drug candidates. Fused isoxazoles can be used in combination therapies, where multiple drugs are used simultaneously to enhance their efficacy and reduce the likelihood of resistance development. In summary, fused isoxazoles represent an important class of compounds in pharmaceutical and medicinal research due to their diverse biological activities, their potential as precursors in drug design, and their relevance in treating a wide range of diseases. Their continued study and development hold promise for the discovery of novel therapeutic agents in the future.
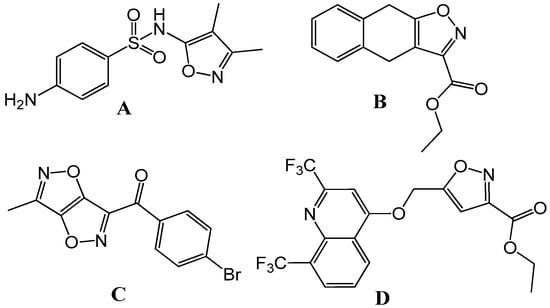
Figure 1.
Some promising isoxazole derivatives exhibiting superior biological activities.
Isoxazole is also found in various natural compounds, often as a part of more complex structures. For example, bilobalide and ginkgolides are neuroprotective and anti-inflammatory terpenes [2], pilocarpine is an alkaloid used in ophthalmology to treat glaucoma [3], lupine alkaloids have insecticidal activity and also play a role in plant–herbivore interactions [4], 2-phenyl-4-quinolone (PQ) plays a pivotal role in the virulence of bacteria [5], and isoginkgetin is a flavonoid with an isoxazole ring studied for its anti-inflammatory and anticancer properties [6]. These examples showcase the diverse range of natural compounds that contain the isoxazole ring. It is worth noting that while isoxazole is a relatively uncommon structural element in natural products, it plays important roles in the bioactivity of these compounds. The isolation and study of such natural products can lead to the development of novel drugs and provide insights into their ecological functions. Hence, to showcase the importance of fused isoxazole, a survey on the recent progress in the synthesis of fused isoxazoles, particularly over the last few years, is presented.
2. Results and Discussion
H. Ali Dondas et al. studied X=Y-ZH systems as potential 1,3-dipoles in the formation of cyclic nitrones from alkenyl oximes (Scheme 1). In their method, substituted alkenyl oxime (1) was cyclized using phenylselenyl chloride or phenyl-selenyl bromide in the presence of a silver salt to obtain cyclic nitrone salts that then were treated with anhydrous potassium carbonate and CH2Cl2 at 25 °C for 16 h to produce nitrones. Then, the obtained nitrones were treated with N-methylamine and acetonitrile at 80 °C for 9 h to obtain isoxazoles (2,3). The reactions involved in this process were regiospecific cyclization and stereospecific intramolecular cycloaddition [7].
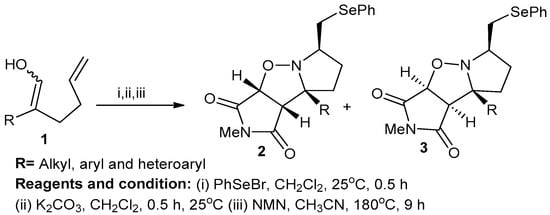
Scheme 1.
Synthesis of fused isoxazole derivatives.
Abutariq Taher et al. studied the reaction of an imidazo-[4,5-c]-isoxazole-6-carboxylate with dimethyl acetylene dicarboxylate leading to the formation of the first example of a [1,4]-diazepino-[2,3-c]-isoxazole (Scheme 2). Imidazo [4,5-c]-isoxazole-6-carboxylate (4), in the presence of DMAD and with an attack at C-2, produced a 1H-[1,4,4]diazepino [2,3-c] isoxazole (5) via a ring-opening reaction [8].

Scheme 2.
Synthesis of 1H-[1,4,4] diazepino[2,3-c]isoxazole.
Padmavathi et al. worked on the synthesis of some fused pyrazoles and isoxazoles (Scheme 3). Decarboxylation of 6-carbethoxy-3,5-diarylcyclohexenones (6) resulted in the formation of 3,5-diaryl-2-cyclohexenones through the Knoevenagel condensation of ethyl acetoacetate and 1, 3-diaryl-2-propen-1-one in the presence of sodium ethoxide. 3,5-diaryl-2-cyclohexenones along with ethyl formate in the presence of sodium chloride produced 6-hydroxymethylene-3,5-diaryl-2-cyclohexenones via the Claisen condensation reaction. This cyclocondensation reaction, followed by a reaction with hydrazine hydrate/hydroxylamine hydrochloride in AcOH, resulted in 4,5-dihydrobenzo-[3,4-d] pyrazole/isoxazole (7), with a 66% yield. Aromatization of pyrazole and isoxazole with DDQ produced fused benzopyrazole/isoxazole (8) [9].

Scheme 3.
Synthesis of fused benzo-isoxazole.
Tommo Matsuura et al. worked on the molecular sieve-promoted cyclocondensation of hindered, aromatic nitrile oxides and cyclic diketones under mild conditions (Scheme 4). The reaction, conducted using active nitrile oxide (9) and diketone (10) as an additive, proceeded at a high rate in mild conditions, resulting in the production of 3-(2,4,6-trimethylphenyl)-6,7-dihydro-1,2-benzoxazol-4(5H)-one (11) with a 79% yield [10].

Scheme 4.
Synthesis of benzene ring-fused isoxazole.
Jeffrey W. Bode et al. studied the facile construction and divergent transformation of polycyclic isoxazoles, obtaining direct access to polyketide architectures (Scheme 5). In this reaction, the cyclocondensation of nitrile oxides (12) and 1,3-diketones (13) produced polycyclic isoxazole (14) with a 77% yield in the presence of hydroxylamine hydrochloride [11].
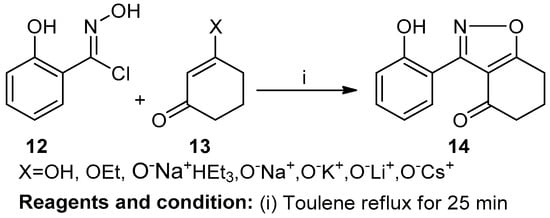
Scheme 5.
Synthesis of polycyclic isoxazoles.
Irini Akritopoulou-Zanze et al. studied the synthesis of novel fused isoxazoles and isooxazolines by sequential Ugi/INOC and [2+3] cycloaddition reactions (Scheme 6). A carboxylic acid-bearing nitro group, allyl or propargyl amine, and various isocyanides and aldehydes were used for the synthesis of fused isoxazoles (15). This reaction was conducted according to Mukaiyama in the presence of POCl3 and an excess of Et3N in CHCl3. The reaction only achieved a 50% conversion of the starting materials [12].
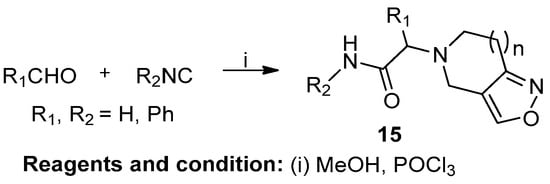
Scheme 6.
Synthesis of six- and seven-ring isoxazoles.
V. Padmavathi et al. prepared 5,7-diaryl-6,7,8-trihydrobenzo[3,4-d] isoxazolin-1-one (17) by the Knoevenagel reaction of ethyl acetoacetate and 1,3-diaryl -2-propen-1-one (16) (Scheme 7). This reaction was carried out with hydroxylamine hydrochloride/hydrazine/hydrate/phenyl hydrazine in 10% NaOH and ethanol and resulted in a 61% yield of the product (17) [13].

Scheme 7.
Synthesis of imine-based fused isoxazole.
Sarvesh Kumar et al. studied the heteroaromatic annulation of 10,11-dihydro-11-[bis(methylthio)methylin]dibenzoxepino[4,5]-fused heterocycles (Scheme 8). The reaction of 10,11-dihydro-11-one with sodium hydroxide with carbon disulfide at 0 °C in THF, along with alkylation with methyl iodide, produced an α-oxoketene dithioacetal (18). When compound (19) reacted with hydroxylamine hydrochloride in the presence of sodium ethoxide in refluxing ethanol, a 3-ethoxydibenzenopino[4,5-c] isoxazole (20) was obtained, whereas in the presence of a non-nucleophilic base like barium hydroxide, 3-(methylthio)dibenzoxepino-[4,5-c]isoxazole was produced with an 86% yield, and in the presence of sodium acetate–acetic acid buffer, a regioisomeric 3-(methylthio)-dibenzoxepino[4,5-d] isoxazole (21) was obtained [14].
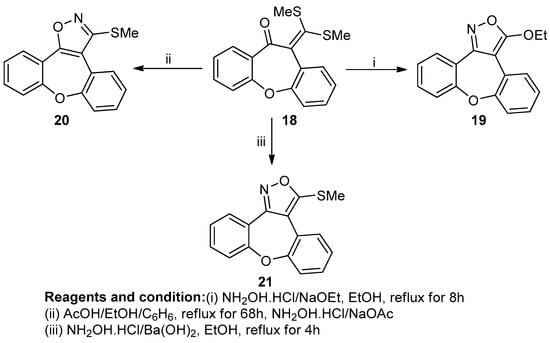
Scheme 8.
Synthesis of 3-(methylthio)-dibenzoxepino[4,5-d] isoxazole.
K. Karthileyan et al. studied the synthesis of fused isoxazoles (24, 25, 26), obtained from the respective aldehydes (22), by the 1,3-dipolar cyclocondensation of nitrile oxide (23) in the presence of hydroxylamine hydrochloride (Scheme 9) [15].
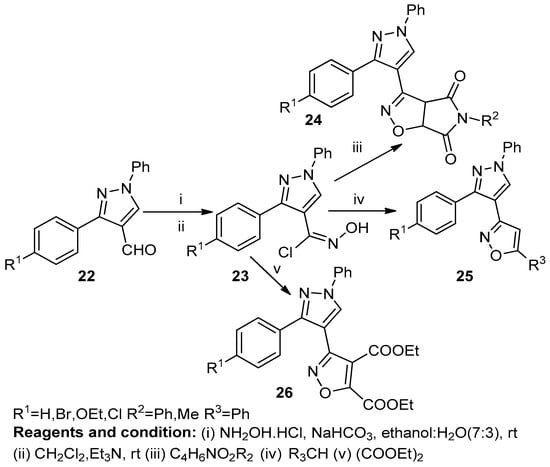
Scheme 9.
Synthesis of isoxazole derivatives.
Vijay V. Dabholkar and Faisal Y. Ansari worked on the synthesis and characterization of selected fused isoxazoles (Scheme 10). In this case, isoxazole was prepared by the reaction of 5,5-dimethylcyclohexane-1,3-dione (27) with aldehyde, obtaining 2-arylidene-5,5-dimethylcyclohexane-1,3-dione (28). In this reaction, nucleophilic reagents were used. Cyclocondensation of compound (28) with hydroxylamine hydrochloride in the presence of glacial acetic acid yielded 6,6-dimethyl-3-(substitutephenyl)-3,3a,6,7-tetrahydro-5H-2,1-benz-isoxazol-4-one (29) [16].
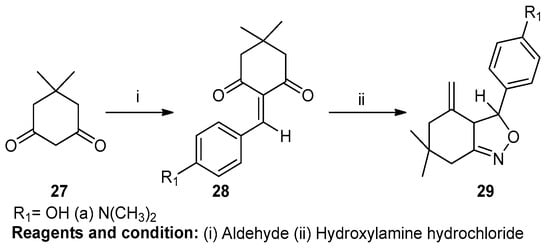
Scheme 10.
Synthesis of a fused isoxazole.
Chetan M. Bhalgat et al. worked on the synthesis of a new 3-(1-benzofuran-2-Yl)-5-(aryl-substituted) isoxazole based on the reaction of chalcone (30), hydroxylamine hydrochloride, and sodium acetate in refluxing ethanol for 6 h, which led to compound (31) (Scheme 11) [17].
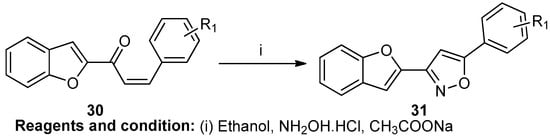
Scheme 11.
Synthesis of 3-(1-benzofuran-2-Yl)-5-(aryl-substituted)isoxazole.
Inga Cikotiene studied the intramolecular iodine-mediated oxygen transfer from nitro groups to C=C bonds (Scheme 12). Thieno[2,3-c] [1,2] isoxazole (33) was prepared using ortho-alkynyl nitrobenzene (32) in the presence of a gold bromide and iridium hydride complex [18].

Scheme 12.
Synthesis of thieno[2,3-c] [1,2] isoxazoles.
Mariappan Babu et al. worked on isoxazoles incorporating N-substituted decahydroquinolines (Scheme 13). 1-acetylcyclohexane (34), in a condensation reaction with benzylamine and benzaldehyde and piperidine, produced 1-benzyl-2-phenylocahydroquinolin-4(1H)-one. When this compound (35) was refluxed with hydroxylamine hydrochloride and sodium acetate, 3-aryl-4-phenyl-5-benzyl-decahydro-isoxazolo[4,3-c]quinolines (36) was obtained [19].

Scheme 13.
Synthesis of a fused isoxazole.
Zigmee T. Bhutia et al. worked on the in situ mechanochemical synthesis of nitrones followed by 1,3 dipolar cycloaddition, providing a catalyst-free, “green” route to obtain cis-fused chromano-[4,3-c]-isoxazole (37) (Scheme 14). Grinding an equimolar mixture of o-allyl salicylaldehyde and phenylhydroxylamine resulted in nitrones, and then intramolecular cycloaddition of the nitrones yielded compound (38) [20].
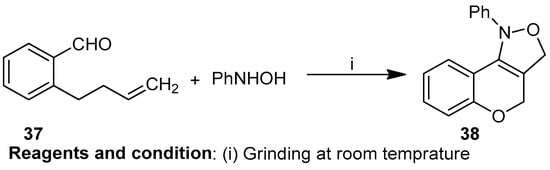
Scheme 14.
Synthesis of chromano-isoxazoles.
Yang Li et al. studied the facile synthesis of 5-bromotropono[c]-fused isoxazole (Scheme 15). The tropone-fused heterocycle 3-acetyl-5-bromotropolone (39) in the presence of hydroxylamine hydrochloride and methanol as a solvent produced the tropane-fused isoxazole 5-bromo-3-methyltropono[d] isoxazole (40) with a 70% yield [21].

Scheme 15.
Synthesis of 5-bromo-3-methyltropono[d] isoxazole.
Masashi Shigenobu et al. studied the pallidum-catalyzed directed C-H arylation of isoxazoles at the fifth position (Scheme 16). The C5 arylation of tetrahydrobenzo[c] isoxazole (41) and 4-idotoluene (42) in the presence of [PdCl2(MeCN)2 at 100 °C for 24 h resulted in the production of 3-phenylbenzoisoxazole (43) with an 86% yield [22].

Scheme 16.
Synthesis of a fused isoxazole.
Wen-Chang Chen et al. worked on the synthesis of fused isoxazoles and isoxazolines via intramolecular nitrile oxide cycloaddition (Scheme 17). Dimethyl 2-propargyl-2-(2-nitro-1-aryl/alkylethyl) malonates (44), treated with ter-butoxide and Yamaguchi reagents in DCM at −78 °C, produced a bicyclic isoxazole after 24 h with a yield of 65%. In optimum conditions, these bicyclic isoxazoles (45) were obtained with a 95% yield [23].

Scheme 17.
Synthesis of a bicyclic isoxazole.
Shakil N. Afraj et al. studied multicomponent coupling reactions and intramolecular nitrile oxide–alkyne cycloaddition towards isoxazole(3,4)-pyrrolidines (Scheme 18). Aldoxime, N-chlorosuccinimide (46), and triethylamine, reacted at room temperature for 2 h in DCM, resulted in (4S,8As)-3,4-diphenyl-6,7,8,8a-tetrahydro-4H-isoxazolo[3,4-a]pyrroli-zine (47) with a 72% yield [24].

Scheme 18.
Synthesis of isoxazole.
Victoria V. Grishko et al. worked on the preparation of novel fused azole derivatives (Scheme 19). Lupane-type betulone (48) yielded betulin by a one-step regioselective biotransformation. An oxime (49) treated with acetyl chloride under reflux with pyridine produced the compound 28-acetoxy-5′-methylisoxazolo [3′,4′:1,2]-1-oxolup-20(29)-ene (50), which yielded compound (51) after treatment with 5% KOH in EtOH [25].
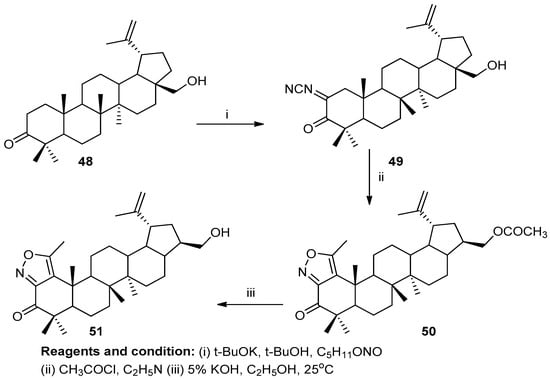
Scheme 19.
Synthesis of betulone-based isoxazoles.
Wei Xiao et al. studied regio- and diasterodivergent [4+2] cycloadditions with cyclic 2,4-dienones catalyzed by a cinchona-derived amine (Scheme 20). In this reaction, alkylidene isoxazole-5-(4H)-one was prepared using an aromatic aldehyde (53) and a heteroaryl or 2-styryl group in the presence of 2,4-dienone (52). The reaction was a [4+2] cycloaddition reaction. In the preparation of the diasterodivergent Z–4-alkylideneisoxazol-5(4H)-one (54), very simple starting materials were used [26].

Scheme 20.
Synthesis of alkylidene isoxazole-5-(4H)-one.
Mansour and co-workers studied the oxidative conversion of oximes (55) into 3-aryl isoxazoles (56) (Scheme 21). Unsaturated oximes treated with oxidants like manganese dioxide, copper chloride, and acetonitrile under reflux to get compound (56) with a 50% yield. Other attempts were performed using solvents like DMF or THF, which reported lower yields [27].
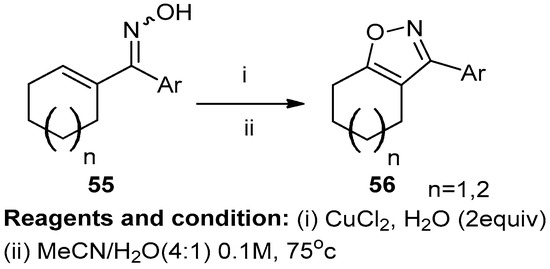
Scheme 21.
Synthesis of 3-aryl isoxazoles.
Alexander and co-workers studied the sterically facilitated intramolecular nucleophilic NMe2 group substitution for the synthesis of fused isoxazoles (Scheme 22). It is a SNAr aromatic nucleophilic substitution reaction. The compound 1,8-bis(dimethylamino)-2-naphthaldehyde oxime (57) was converted at 120 °C, and the substitution of the NMe2 group yielded the fused isoxazole N,N-dimethylnaphtho[2,1-d]isoxazole-9-amine (58) [28].
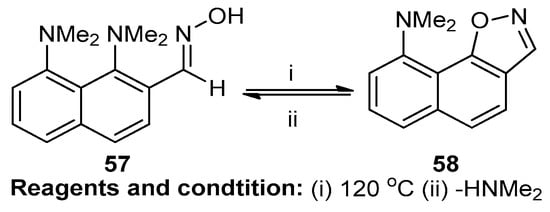
Scheme 22.
Synthesis of N,N-dimethylnaphtho[2,1-d]isoxazole-9-amine.
Overall, the synthetic methods employed for the synthesis of fused isoxazoles possess advantages such as the use of easily available starting materials, feasible reaction conditions, short reaction time, simple cyclization reactions, easy scale-up, and high yield. In few reported methods, green solvents such as water and mild solvents such as ethanol and methanol were used to obtain the target compounds. Also, in few examples, the reactions were conducted at room-to-moderate temperature. Regarding the disadvantages, a few of the target compounds were synthesized by using petroleum-based solvents such as benzene, toluene, dichloromethane, acetonitrile, and isopropyl alcohol. Hence, this review paper provides insights for selecting an ecofriendly approach for the design and synthesis of the desired fused isoxazole compounds.
3. Conclusions
This review article describes various synthesis methods and structural modifications of fused isoxazole derivatives. A general method for the generation of fused isoxazoles was reported. The present work shows that the main challenges are developing ecofriendly approaches that avoid toxic solvents and reagents, decreasing the reaction time, facilitating the reaction scale-up, and increasing the yield. This review also helps select the optimal method to solve problems associated with specific sites or positions within a molecule that possesses multiple active sites or functional groups.
Author Contributions
Manuscript preparation, N.N.M.; literature review, C.M. and V.B.; formal analysis, D.C.S.; Conceptualization, N.S.L. and S.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are grateful to the Principal and Management, Vidyavardhaka College of Engineering, Mysuru, for providing the necessary facility to carry out the research work.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zhu, J.; Mo, J.; Lin, H.Z.; Chen, Y.; Sun, H.P. The recent progress of isoxazole in medicinal chemistry. Bioorg. Med. Chem. 2018, 26, 3065–3075. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Sun, Q.; Chen, W.; Bai, Y.; Hu, D.; Xie, X. The neuroprotective mechanisms of ginkgolides and bilobalide in cerebral ischemic injury: A literature review. Mol. Med. 2019, 25, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Suleiman, O. Pilocarpine Alkaloid A Review. EAS J. Pharm. Pharmacol. 2020, 2, 161–164. [Google Scholar]
- Cakmak, M.; Mayer, P.; Trauner, D. An efficient synthesis of loline alkaloids. Nat. Chem. 2011, 3, 543–545. [Google Scholar] [CrossRef]
- Chen, Y.F.; Lawal, B.; Huang, L.J.; Kuo, S.C.; Sumitra, M.R.; Mokgautsi, N.; Lin, H.Y.; Huang, H.S. In Vitro and In Silico Biological Studies of 4-Phenyl-2-quinolone (4-PQ) Derivatives as Anticancer Agents. Molecules 2023, 28, 555–578. [Google Scholar] [CrossRef]
- Darrigrand, R.; Pierson, A.; Rouillon, M.; Renko, D.; Boulpicante, M.; Bouyssié, D.; Mouton-Barbosa, E.; Marcoux, J.; Garcia, C.; Ghosh, M.; et al. Isoginkgetin derivative IP2 enhances the adaptive immune response against tumor antigens. Commun. Biol. 2021, 4, 269–282. [Google Scholar] [CrossRef]
- Ali Dondas, H.; Ronal, G.; Maria, H.; Jasothra, M.W.; Anthony, T.; Peter, K. X=Y-ZH systems as potential 1,3-dipoles. Part 50: Phenylselenyl halide induced formation of cyclic nitrones from alkenyl oximes. Tetrahedron 2000, 56, 10087–10096. [Google Scholar] [CrossRef]
- Abutariq, T.; Alexandra, M.Z.S.; George, W.W. Reactions of an imidazo [4,5-c] isoxazole-6-carboxylate with dimethyl acetylene dicarboxylate: Formation of the first example of a [1,4] diazepino [2,3-c] isoxazole. Tetrahedron 2000, 41, 9319–9321. [Google Scholar]
- Venkatapuram, P.; Boggu, J.M.R.; Akula, B.; Katta, V.R.; Dandu, B.R. Synthesis of some fused pyrazole and isoxazole. Molecules 2000, 5, 1281–1286. [Google Scholar]
- Tommoo, M.; Jeffrey, W.B.; Yoshifumi, H.; Keisuke, S. Molecular sieve (MS4A) promoted cyclo-condensation of hindered, aromatic nitrile oxides and cyclic diketones under mild conditions. Synlett 2003, 11, 1746–1748. [Google Scholar]
- Jeffrey, W.B.; Yoshifumi, H.; Tomoo, M.; Keisuke, S. Facile construction and divergent transformation of polycyclic isoxazoles: Direct access to polyketide. Org. Lett. 2003, 5, 391–394. [Google Scholar]
- Irini, A.Z.; Vijaya, G.; Joel, D.M.; Stevan, W.D. Synthesis of novel fused isoxazoles and isooxazolines by sequential Ugi/INOC reactions. Tetrahedron Lett. 2004, 45, 3421–3423. [Google Scholar]
- Padmavathi, V.; Sharmia, K.A.; Baliah, A.; Reddy, S.; Reddy, B.D. Cyclohexanone carboxylates. A versatile source for fused isoxazoles and pyrazoles. Synth. Commun. 2006, 31, 2119–2126. [Google Scholar] [CrossRef]
- Sarvesh, K.; Hiriyakkanavar, I.; Hiriyakkanavar, J. Heteroaromatic annulation studies on 10,11-dihydro-11-[bis(methylthio)methylene] dibenzoxepin-10-one: A facile access to novel dibenzenzoxepin [4,5]-fused heterocycles. Tetrahedron 2007, 63, 10067–10076. [Google Scholar]
- Karthikeyan, K.T.; Veenus Seelan, K.G.; Lalitha, P.T.P. Synthesis and antinociceptive activity of pyrazolyl isoxazolines and pyrazolyl isoxazoles. Bioorg. Med. Chem. Lett. 2009, 19, 3370–3373. [Google Scholar] [CrossRef]
- Dabholakar, V.V.; Ansari, F.Y. Synthesis, and characterization of selected fused isoxazole and pyrazole derivatives and their antimicrobial activity. J. Serb. Chem. Soc. 2009, 74, 1219–1228. [Google Scholar] [CrossRef]
- Chetan, M.B.; Sachin, L.P.; Sandeep, K.C.; Krantisinha, R.; Kumar, G.P.; Santhosh, P. Synthesis of cytotoxic studies of newer 3- (1-benzofuran-2-Yl)-5- (substituted aryl) isoxazoles. Res. J. Pharm. Technol. 2011, 4, 247–251. [Google Scholar]
- Inga, C. Intramolecular iodine -mediated oxygen transfer from nitro groups to C=C bonds. Eur. J. Org. Chem. 2012, 14, 2766–2773. [Google Scholar]
- Mariappan, B.; Kasi, P.; Penugonda, R. Isoxazoles incorporated N -substituted decahydroquinolines: A precursor to the next generation antimicrobial drug. Eur. J. Med. Chem. 2012, 47, 608–614. [Google Scholar]
- Rajender, P.S.; Sridevi, G.; Reddy, K.K. Synthesis of novel isoxazole-fused heterocycles. Synth. Commun. 2012, 42, 2191–2200. [Google Scholar] [CrossRef]
- Yang, L.I.; Liangyu, X.U.; Wentao, G.A.O. Facile synthesis of 5- bromotropono[c]-fused pyrazoles and isoxazoles. Turk. J. Chem. 2014, 38, 470–476. [Google Scholar]
- Masashi, S.; Kazuhiro, T.; Hiroaki, S. Pallidum-catalyzed direct C-H arylation of isoxazoles at the 5-position. Angew. Chem. 2015, 127, 9708–9712. [Google Scholar]
- Chen, W.C.; Kavala, V.; Shih, Y.H.; Wang, Y.H.; Kuo, C.W.; Yang, T.H.; Huang, C.Y.; Chiu, H.H.; Yao, C.F. Synthesis of bicyclic isoxazoles and isoxazolines via intramolecular nitrile oxide cycloaddition. Molecules 2015, 20, 10910–10927. [Google Scholar] [CrossRef]
- Shakil, N.A.; Cut, N.; Chinpio, C.; Gene, H.L. Multicomponent coupling reaction and intramolecular nitrile oxide -alkyne cycloaddition towards isoxazole [3,4]-pyrrolizines. Asian J. Org. Chem. 2016, 5, 1015–1026. [Google Scholar]
- Victoria, V.G.; Irina, A.T.; Vladimir, O.N.; Natalia, V.G.; Alexei, V.N.; Maxim, V.D.; Irena, B.I. Preparation of novel ring—A fused azole derivatives of botulin and evaluation of their toxicity. Eur. J. Med. Chem. 2017, 125, 629–639. [Google Scholar]
- Xiao, W.; Yang, Q.-Q.; Chen, Z.; Ouyang, Q.; Du, W.; Chen, Y.-C. Regio- and diasterodivergent [4+2] cycloadditions with cyclic 2,4-dienones. Org. Lett. 2018, 20, 236–239. [Google Scholar] [CrossRef]
- Mansour, D.K.; Pakoupati, B.; Julian, G.; Laurent, E.K. Metal -free addition of boronic acids to silylnitronates. Synlett 2020, 31A-E, 1–8. [Google Scholar]
- Antonov, A.S.; Tupikina, E.Y.; Karpov, V.V.; Mulloyarova, V.V.; Bardakov, V.G. Sterically Facilitated Intramolecular Nucleophilic NMe2 Group Substitution in the Synthesis of Fused Isoxazoles: Theoretical Study. Molecules 2020, 25, 5977. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).