Abstract
Nanotechnology has facilitated various applications in food and agricultural industries due to the unique characteristics of nanoparticles (NPs), such as their large surface area, reactivity, tendency to agglomerate, ability to penetrate, and specific size and structure. The exploration of nanoparticles in agriculture is gaining interest because of their ability to minimize the use of chemical fertilizers and significantly enhance plant growth. Several studies have demonstrated NPs’ effects on plants, depending on their size, shape, concentration, coatings, self-aggregate tendency, and stability. Here, we provide an overview of the applications of recently developed nanoparticles, including metallic and non-metallic nanoparticles and carbon-based nanomaterials.
1. Introduction
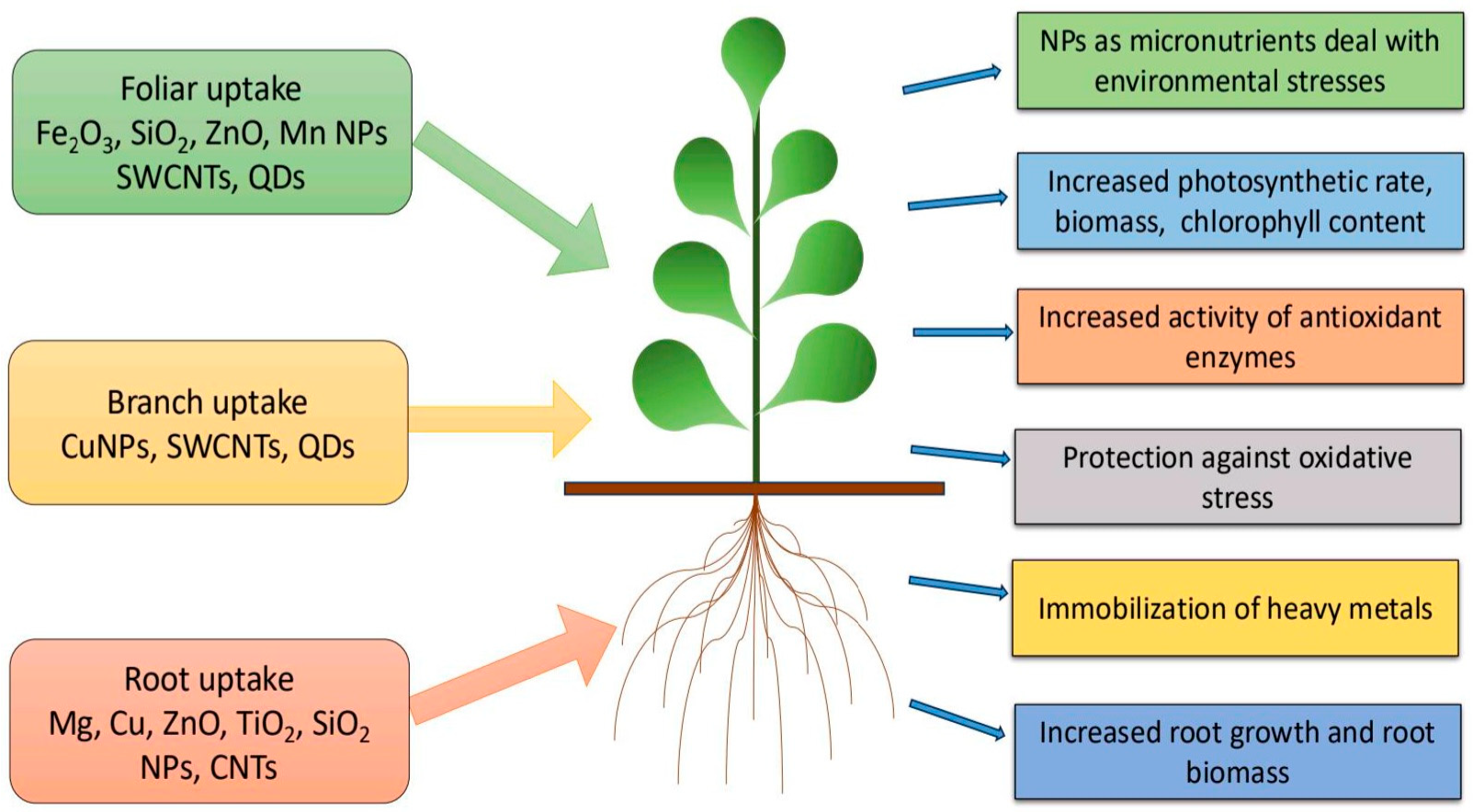
Nanotechnology plays a vital role in improving methods for monitoring ecological conditions, managing pathogenicity, managing crop capacity (for photosynthetic respiration), increasing nutrient or pesticide absorption, and accelerating flowering and seed germination. NPs have proven to be highly beneficial for plant development [1]. The initial stages of plant growth and development begin with seed germination, followed by root elongation and shoot emergence [2]. Seed germination forms the foundation for the productivity, growth, and overall development of plants. Significant improvements in both germination rates and overall yield have been achieved with NPs-treated seeds [3]. Various studies have revealed that controlled concentrations and moderate doses of NPs greatly promote seed germination [4] and facilitate robust plant growth. Additionally, they have been shown to enhance photosynthetic efficiency and increase chlorophyll content [5,6,7], as well as improve water and fertilizer utilization (Figure 1). In this study, we present a summary of the applications of recently developed NPs, including metallic, non-metallic, and carbon-based nanomaterials, which significantly enhance plant development by improving growth, stress resistance, and nutrient uptake. AgNPs boost growth and protect mustard plants from pathogens [8], while copper NPs enhance stress tolerance in Arabidopsis [9]. Fe2O3NPs improve iron uptake and photosynthesis in soybeans [10], while AuNPs promote seed germination and growth in wheat [11]. Silicon NPs increase drought tolerance in tomatoes [12], while ZnONPs enhance growth and nutrient uptake in maize [13]. Despite their benefits, NPs can also exhibit toxic effects under certain conditions. Their beneficial or harmful impacts significantly depend on dosage, exposure duration, and the specific type of NPs used.
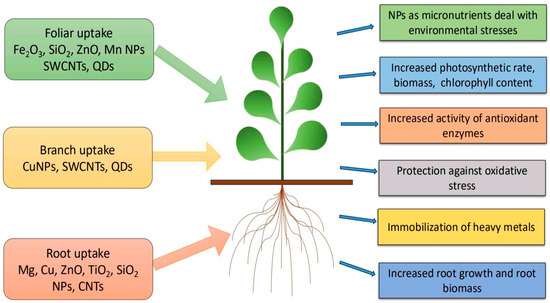
Figure 1.
Various NPs used as fertilizers for improvements of plant growth.
2. Nanoparticles for Enhancing Plant Growth and Development
2.1. Silver Nanoparticles (AgNPs)
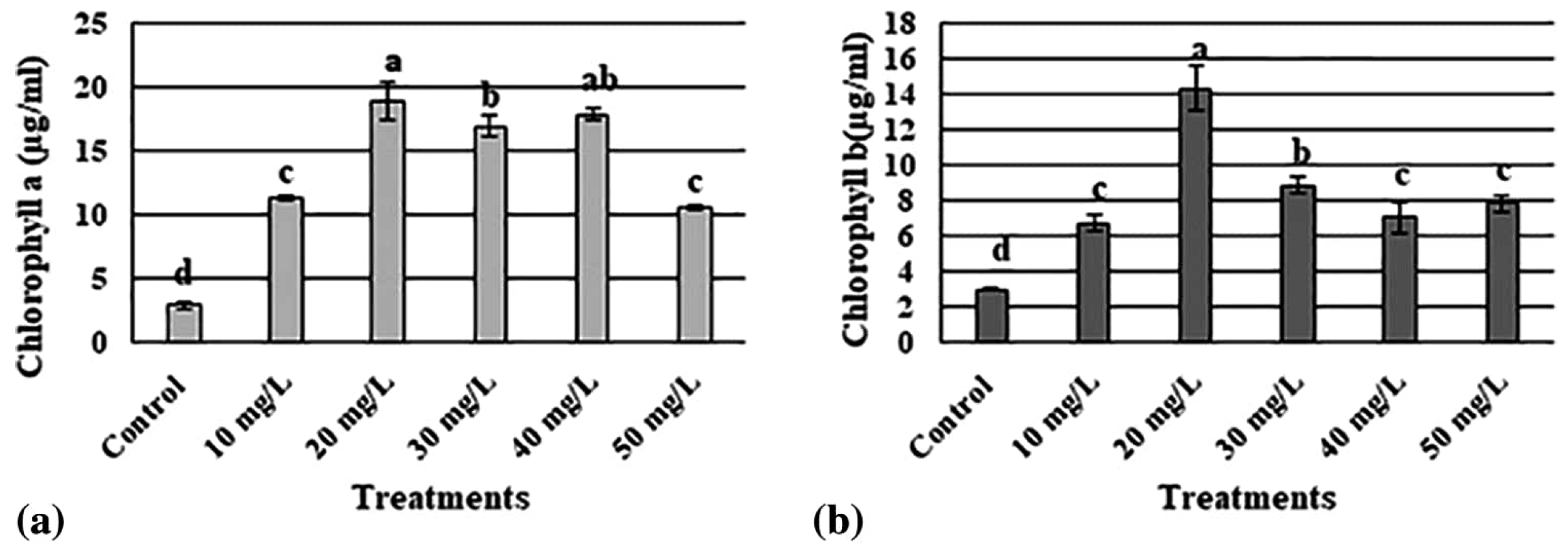
AgNPs are currently the most extensively manufactured nanomaterial, and they are incorporated into a wide range of agricultural applications. Moderate doses of AgNPs have been shown to increase the level of chlorophyll and carbohydrate contents, improve photosynthetic efficiency in different plant species, and improve water and fertilizer utilization [14,15]. Shaikhaldein et al. (2020) studied the morphophysiological effects of AgNPs on Maerua oblongifolia (M. oblongifolia) in vitro. Different concentrations of AgNPs exhibited distinct effects on the morphological characteristics like shoot number, shoot length, fresh weight, dry weight, and leaf number of M. oblongifolia compared to the control group. Notably, 20 mg/L concentration of AgNPs increased the shoot length, fresh weight, and dry weight, along with the highest levels of chlorophyll a and chlorophyll b (Figure 2). Moreover, concentrations of 20, 30, and 40 mg/L of AgNPs resulted in the highest number of shoots (Figure 3). This demonstrates the concentration-dependent impact of AgNPs on the growth and development of M. oblongifolia [16].
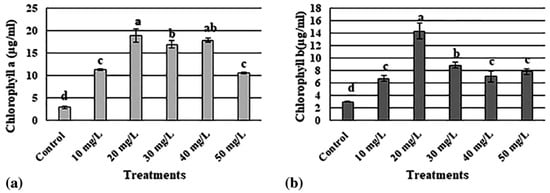
Figure 2.
Variation in (a) chlorophyll a and (b) chlorophyll b content of M. oblongifolia at different concentrations of AgNPs. The letters ‘a’–‘d’ represent significant differences between the treatments at p ≤ 0.05, based on Duncan’s test.

Figure 3.
Variation in number of shoots of M. oblongifolia at different concentrations of AgNPs.
Furthermore, in cowpea, Brassica sp. [17], fenugreek [18], and Lupinus termis [19], the germination percentage was notably higher in AgNP-treated plants compared to the control. AgNPs are also utilized as fungicides for preventing fungal diseases and are employed as agents to facilitate the ripening of fruits [20,21].
2.2. Zinc Oxide Nanoparticles (ZnONPs)
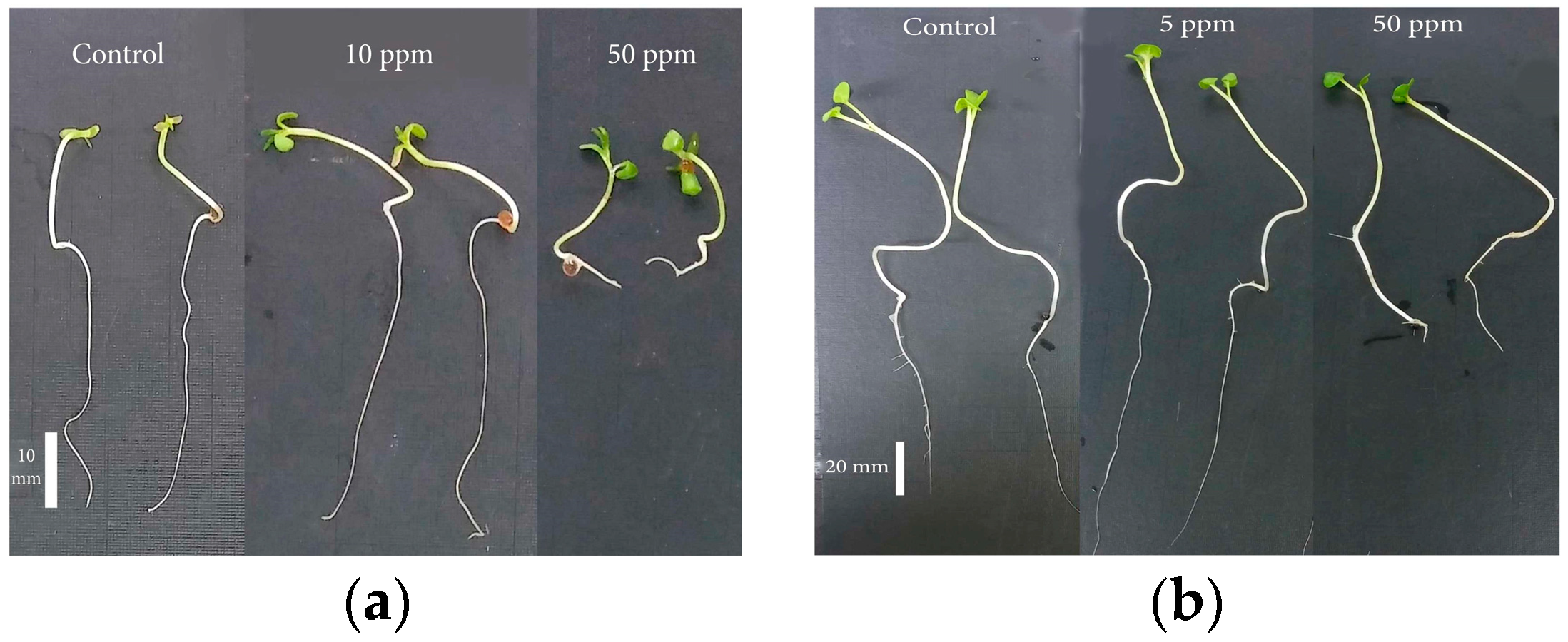
In agricultural practices, ZnONPs serve multiple functions, acting as fertilizers, pesticides, herbicides, and growth regulators [22,23]. Sarkhosh et al. (2022) investigated the effect of various concentrations of ZnONPs on the growth of Camelina sativa and Brassica napus L. (B. napus) crops. In the case of Camelina, the highest germination percentage was observed in seeds treated with 10 ppm ZnONPs compared to the control condition (Figure 4a), and for B. napus, the maximum germination percentage was recorded at a concentration of 5 ppm ZnONPs (Figure 4b) [24].
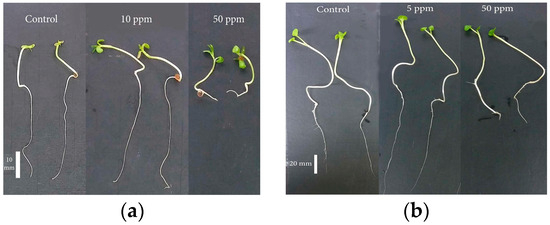
Figure 4.
The impact of ZnONPs on the growth of (a) Camelina seedlings and (b) B. napus seedlings.
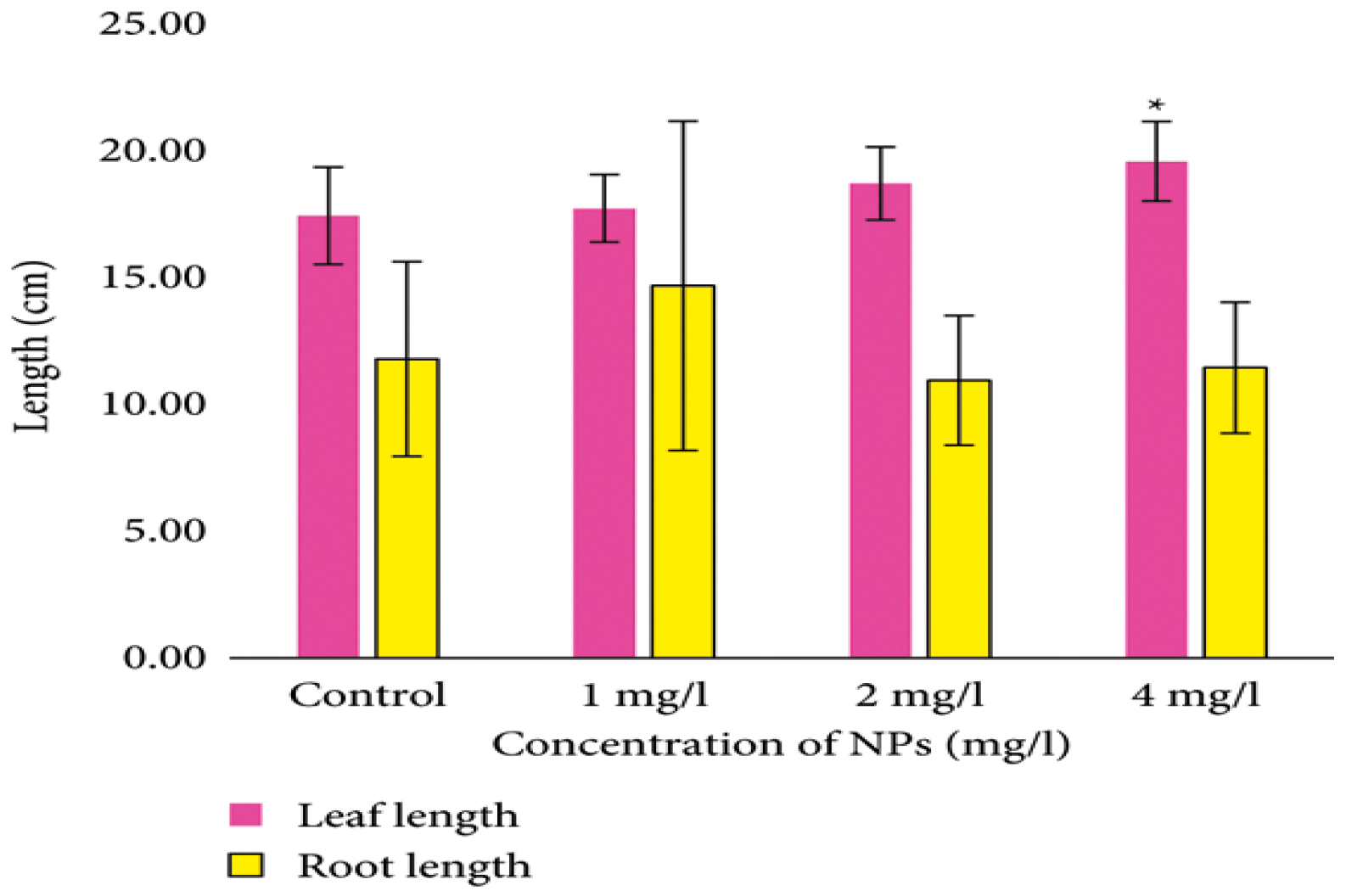
The application of ZnONPs demonstrated a significant enhancement in various physiological and growth factors of wheat [25]. Jankovskis et al. (2022) tested the effects of ZnONPs on wheat (Triticum aestivum L.), which significantly increased the chlorophyll b content at 1 mg/L and increased the leaf length at 4 mg/L; meanwhile, although root length increased at 1 mg/L, it decreased at 2 and 4 mg/L concentrations of ZnONPs (Figure 5) [26].
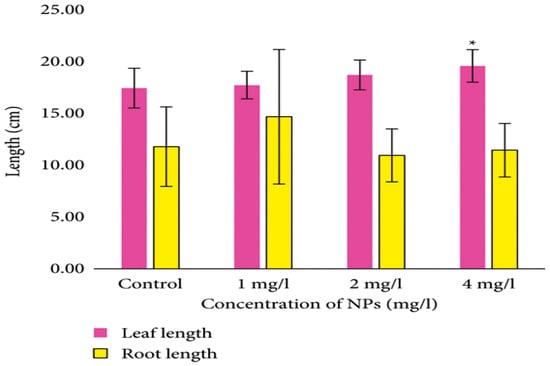
Figure 5.
Leaf and root lengths of wheat (Triticum aestivum L.) treated with ZnONPs. An asterisk (*) denotes a statistically significant difference compared to the control (p < 0.05).
2.3. Copper Nanoparticles (CuNPs) and Copper Oxide Nanoparticles (CuONPs)
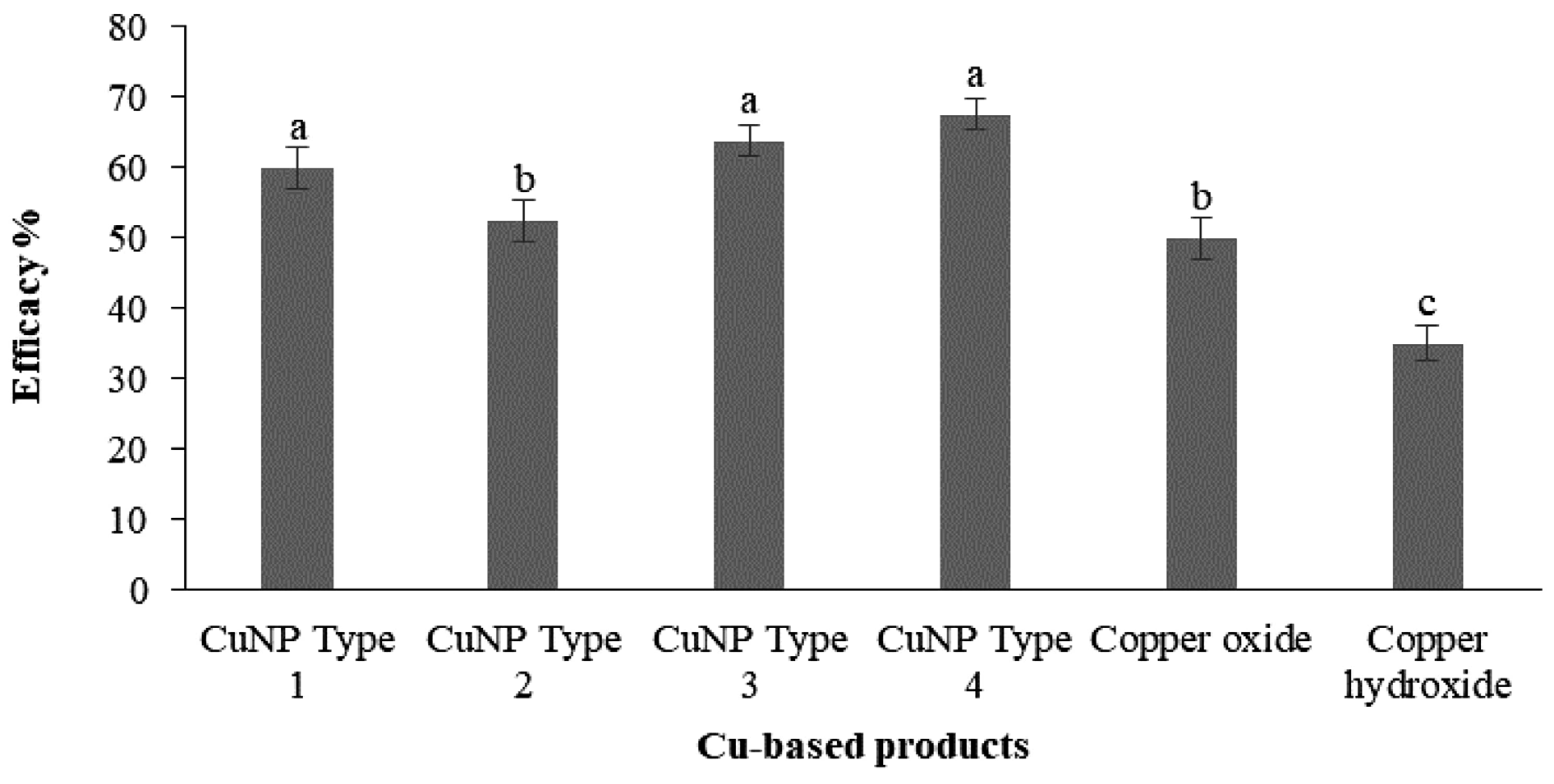
Various research studies have presented evidence demonstrating the antimicrobial application of CuNPs against a range of phytopathogens [27,28,29]. Ntasiou et al. (2021) synthesized and modified four CuNPs (CuNPs Type 1 to 4) by means of the wet chemistry approach. Among these, CuNPs Type 3 and CuNPs Type 4 demonstrated potent protective activity against both Fusicladium oleagineum and Colletotrichum acutatum. Their control efficacy values were notably higher as compared to the reference commercial compounds, copper oxide and copper hydroxide (Figure 6) [29].
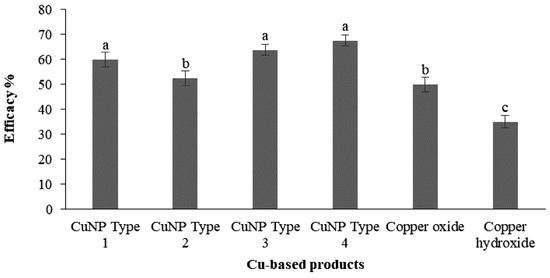
Figure 6.
Efficacy percentages of treatments using CuNPs and conventional copper-based products against Fusicladium oleagineum. The letters ‘a’–‘c’ represent significant differences between the treatments at p = 0.05, based on Fisher’s LSD test.
Badawy et al. (2021) discovered that CuONPs improve the growth metrics of wheat plants [30]. The impact of CuONPs on Indian mustard (Brassica juncea L.) differed based on the application method. Faraz et al. (2022) applied CuONPs as a foliar spray on Brassica juncea plants, leading to augmented growth, improved antioxidant capacity, and an elevated photosynthetic rate. However, at higher concentrations, the effect on growth was found to be minimal [31]. In another work, irrigation with CuONPs raised the levels of macro- and micronutrients in the roots of L. sativa plants [32].
2.4. Iron-Based Nanoparticles
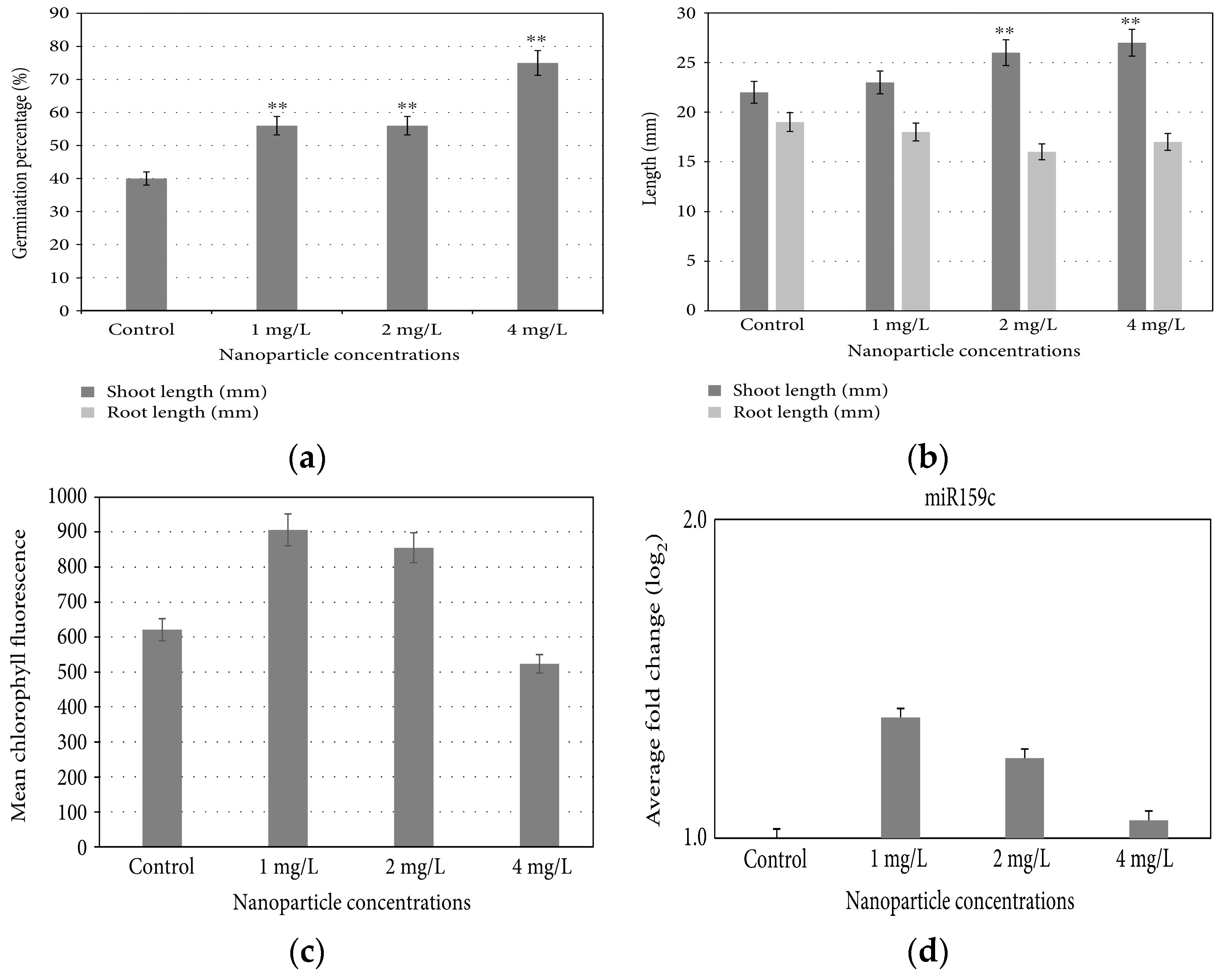
Iron-based NPs possess an intriguing characteristic in that they have the capacity to influence stomatal opening, germination, and seedling growth through various complex effects [33,34]. They also demonstrate the potential to shield plants from drought, potentially by mitigating the oxidative stress induced by water deficiency [35]. Additionally, treated plants exhibit heightened chlorophyll biosynthesis [36]. FeNPs have proven to be effective in the remediation of heavy metals in both soil and water [37,38]. Plaksenkova et al. (2019) studied the impact of Fe3O4NPs on rocket (Eruca sativa Mill.). They investigated the changes in morphology, chlorophyll contents, germination rate, genotoxicity, and level of miRNA expression at 1, 2, and 4 mg/L concentrations of Fe3O4NPs, which positively affected the growth and development of rocket plants. Fe3O4NPs significantly increased the germination percentage and shoot and root length. At a 4 mg/L concentration of Fe3O4NPs, the maximum germination rate (Figure 7a) and shoot length (Figure 7b) were observed. However, a decreased root length was observed at this concentration as compared to control. The chlorophyll a content and miRNA expression level increased at a 1 mg/L concentration (Figure 7c,d) [39].
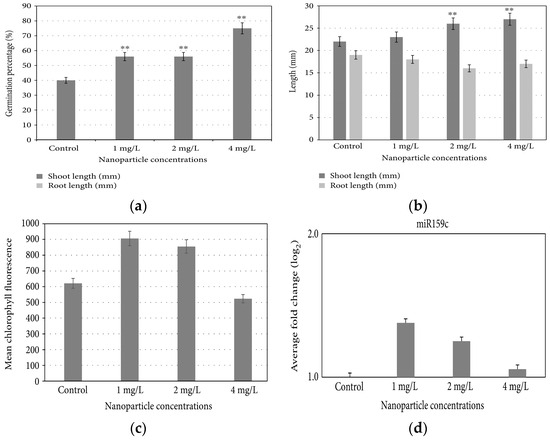
Figure 7.
Effect of exposure to Fe3O4NPs on (a) germination percentage, (b) shoot and root length, (c) chlorophyll content, and (d) miRNA expression of rocket plants. Double asterisk (**) indicates significant difference from the control at p < 0.01.
When rice seeds (cv. Gobindabhog) were exposed to FeNPs during germination, they displayed heightened seedling growth, cell viability, and cell membrane integrity, higher chlorophyll contents, and increased metabolic activity [40]. Furthermore, in a study conducted by Khan et al. (2020), the impact of Fe3O4NPs on rice plants subjected to arsenic stress was examined. They observed that a lower concentration of Fe3O4NPs significantly lowered the arsenic levels and facilitated plant growth. Conversely, a higher concentration failed to produce the same effects. These studies indicated that the effects of Fe3O4NPs varied depending on their dosage [41].
2.5. Titanium Dioxide Nanoparticles (TiO2NPs)
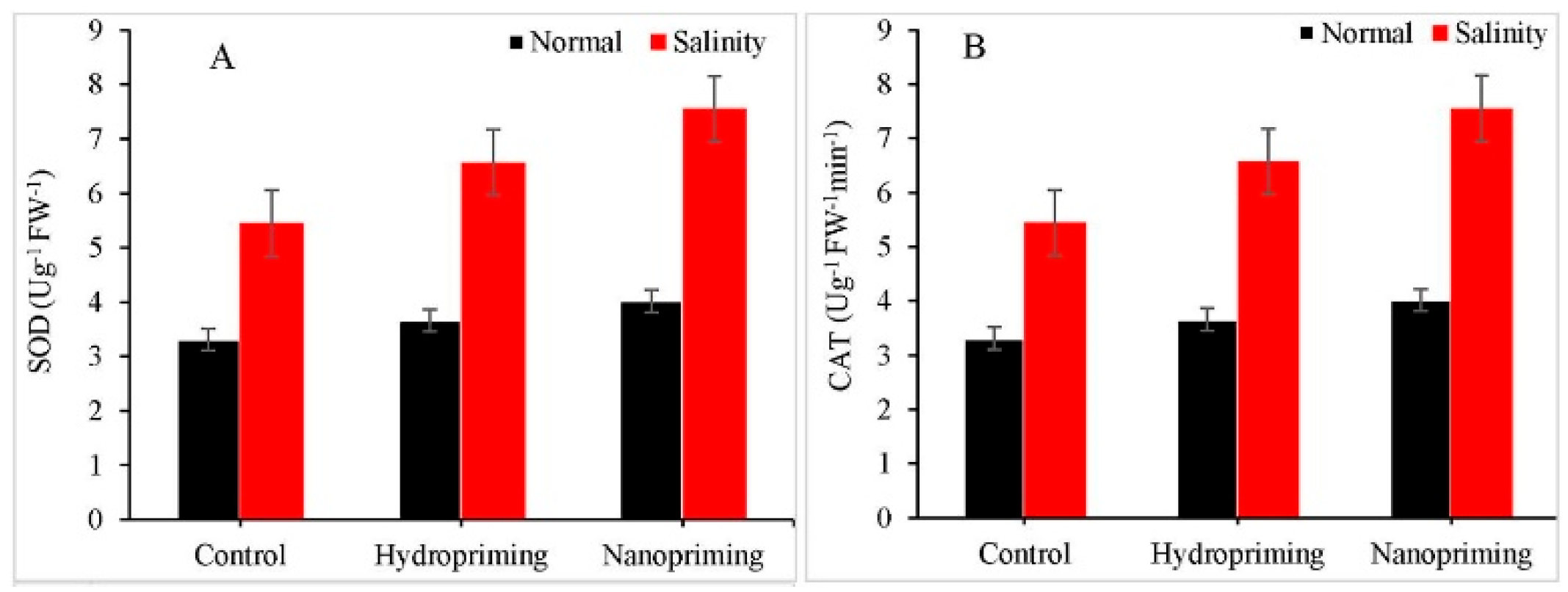
TiO2NPs have been shown to have an impact on various aspects of plants, including growth cell division, callus induction, cell size, and hormone levels (specifically Gibberellins and cytokinins), resulting in a significant increase in treated Rosmarinus officinalis plants [42]. TiO2NPs increased the levels of photosynthetic pigments in the wheat plants under drought stress [43]. The favourable effects of TiO2NPs on seed germination have also been observed in chickpea (Cicer arietinum) and in fodder crops like berseem and oat [44,45]. Recently, Shah et al. (2021) reported a significant impact of TiO2NP priming on seeds of maize (Zea mays L.) plants under salinity stress (Figure 8) [46].
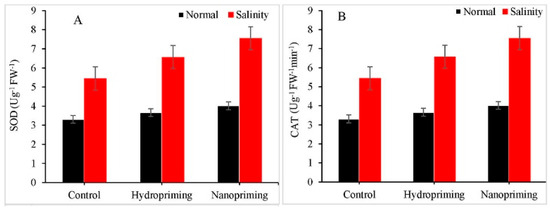
Figure 8.
Impact of TiO2NP nanopriming on the application of (A) SOD and (B) CAT enzymes in maize plants under saline and normal conditions.
2.6. Carbon-Based Nanomaterials
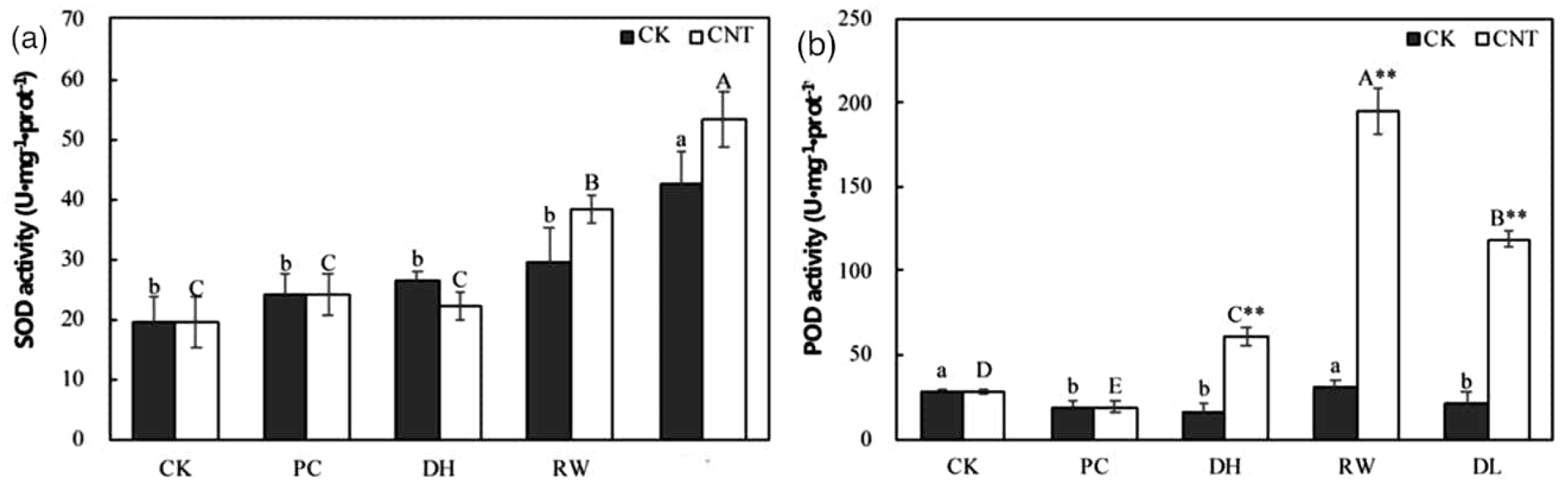
Carbon nanotubes (CNTs) have demonstrated a positive impact on tomato germination and seedling growth [47]. Additionally, research on six plant species including maize, soybean, rice, barley, tobacco, tomato and switchgrass demonstrated that single-walled carbon nanohorns (SWCNHs) enhanced the seed germination of these plant species [48]. Recently, Ren et al. (2020) discovered that the addition of single-walled carbon nanotubes (SWCNTs) to the plant vitrification solution significantly improved the relative survival rate in the cryopreservation of Agapanthus praecox embryogenic callus. The callus treated with SWCNTs exhibited elevated levels of antioxidants, including SOD, and POD, in comparison to the control group (Figure 9) [49].
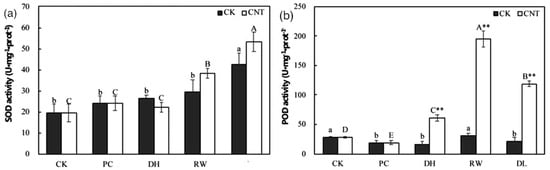
Figure 9.
Effect of SWCNTs on the (a) SOD and (b) POD of the cryopreservation system. (CK= cryopreservation without adding SWCNTs; CNT= cryopreservation with SWCNTs –added; PC = after pre-culture; DH= after dehydration; RW= after rapid cooling–warming; DL= after dilution). Values with different lowercase letters show significant differences among samples in the control group at the p = 0.05 level, while values with different uppercase letters indicate significant differences among samples in the SWCNTs group at the p = 0.05 level. Double asterisks (**) indicate highly significant differences between groups at the same stage, at the p = 0.01 level.
2.7. Other NPs
Various studies have demonstrated the positive effect of NPs on seed germination, plant growth, and plant development. Studies have shown that silica nanoparticles (SiO2NPs) enhanced the rate of germination of rice, thyme, broad bean, sunflower and lettuce [50,51,52,53,54]. Lemongrass (Cymbopogon flexuosus), known for its medicinal properties and essential oil production, experiences improved growth and yield with the application of silicon nanoparticles (SiNPs) [55]. Jayarambabu et al. (2016) demonstrated that magnesium nanoparticles (MgNPs) positively affect seed germination in maize plants [56]. Even though cerium is not considered an essential element for plants, it has been shown to have positive effects on various aspects including seed germination, plant growth, yield, as well as chlorophyll and saccharide content [57]. It inhibited the accumulation of cadmium [58]. Chitosan, a naturally occurring compound found in the exoskeleton of crustaceans, has been extensively studied for its effects on cell signalling molecules [59], film-forming ability, and antibacterial properties [60].
3. Toxic Effects of NPs
The above examples illustrate how NPs can positively influence plant growth and development, but NPs also show toxic effects. Both their toxic and beneficial impacts depend on factors like concentration, size, surface properties, and plant species. At low concentrations and under controlled conditions, NPs can enhance plant growth by improving nutrient uptake, increasing photosynthesis, and strengthening stress tolerance. However, at higher concentrations or with prolonged exposure, they may generate excessive reactive oxygen species (ROS), causing oxidative stress that damages cellular structures like membranes, proteins, and DNA. NPs can also interfere with water and nutrient absorption, disrupt enzyme activities, and release toxic metal ions, all of which impair plant growth [61,62,63].
To mitigate the toxic effects of NPs on plant growth and development, several strategies can be employed. Using NPs in controlled, low concentrations is essential to prevent overexposure. Surface modifications with biocompatible materials or the use of controlled-release systems can limit toxicity by reducing metal ion release and oxidative stress [64]. Soil amendments and phytoremediation plants can help to reduce NPs’ bioavailability in the soil. Additionally, antioxidant supplements can protect plants from ROS damage, while controlling NPs’ size and using biodegradable or eco-friendly NPs can further minimize long-term environmental effects [65]. These approaches can help to balance the positive uses of NPs in agriculture while reducing their negative effects on plant health and growth.
4. Conclusions
NPs offer several advantages over traditional fertilizers, making them a superior option for promoting plant growth through their targeted delivery, enhancing absorption and minimizing losses due to leaching. They also offer multifunctional capabilities by combining growth promotion, disease resistance, and stress tolerance, while improving soil quality by enhancing soil structure and microbial activity. This literature survey shows that controlled concentrations and moderate doses of NPs greatly promote plant growth. It is conceivable that the long-term use of NPs may impact the environment due to their accumulation in the soil [66,67,68]. Further exploration of NPs is required to enhance the growth of agricultural products and to minimize environmental challenges.
Author Contributions
Conceptualization, S.J., S. and T.K.; resources, S.J.; data curation, S., T.K. and S.J.; writing—original draft preparation, S., K.H., S.G., P.M., T.K. and S.J.; writing—review and editing, S., T.K. and S.J.; visualization, S., T.K. and S.J.; supervision, S.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data was generated during the study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Farooqui, A.R.; Tabassum, H.; Ahmad, A.; Mabood, A.; Ahmad, A.; Ahmad, I.Z. Role of nanoparticles in growth and development of plants: A review. Int. J. Pharm. Bio Sci. 2016, 7, 22–37. [Google Scholar] [CrossRef]
- Rico, C.M.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Chemistry, biochemistry of nanoparticles, and their role in antioxidant defense system in plants. Nanotechnol. Plant Sci. 2015, 1, 1–17. [Google Scholar]
- Ali, S.; Mehmood, A.; Khan, N. Uptake, translocation, and consequences of nanomaterials on plant growth and stress adaptation. J. Nanomater. 2021, 2021, 6677616. [Google Scholar] [CrossRef]
- Shelar, G.B.; Chavan, A.M. Myco-synthesis of silver nanoparticles from Trichoderma harzianum and its impact on germination status of oil seed. Biolife 2015, 3, 109–113. [Google Scholar]
- Sharma, P.; Bhatt, D.; Zaidi, M.G.H.; Saradhi, P.P.; Khanna, P.K.; Arora, S. Silver nanoparticle-mediated enhancement in growth and antioxidant status of Brassica juncea. Appl. Biochem. Biotechnol. 2012, 167, 2225–2233. [Google Scholar] [CrossRef]
- Vannini, C.; Domingo, G.; Onelli, E.; Prinsi, B.; Marsoni, M.; Espen, L.; Bracale, M. Morphological and proteomic responses of Eruca sativa exposed to silver nanoparticles or silver nitrate. PLoS ONE 2013, 8, e68752. [Google Scholar] [CrossRef]
- Hatami, M.; Ghorbanpour, M. Effect of nanosilver on physiological performance of pelargonium plants exposed to dark storage. J. Hortic. Res. 2013, 21, 15–20. [Google Scholar] [CrossRef]
- Giri, V.P.; Shukla, P.; Tripathi, A.; Verma, P.; Kumar, N.; Pandey, S.; Dimkpa, C.O.; Mishra, A. A review of sustainable use of biogenic nanoscale agro-materials to enhance stress tolerance and nutritional value of plants. Plants 2023, 12, 815. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Hernández, H.; Juárez-Maldonado, A.; Benavides-Mendoza, A.; Ortega-Ortiz, H.; Cadenas-Pliego, G.; Sánchez-Aspeytia, D.; González-Morales, S. Chitosan-PVA and copper nanoparticles improve growth and overexpress the SOD and JA genes in tomato plants under salt stress. Agronomy 2018, 8, 175. [Google Scholar] [CrossRef]
- Feng, Y.; Kreslavski, V.D.; Shmarev, A.N.; Ivanov, A.A.; Zharmukhamedov, S.K.; Kosobryukhov, A.; Yu, M.; Allakhverdiev, S.I.; Shabala, S. Effects of iron oxide nanoparticles (Fe3O4) on growth, photosynthesis, antioxidant activity and distribution of mineral elements in wheat (Triticum aestivum) Plants. Plants 2022, 11, 1894. [Google Scholar] [CrossRef]
- Manaf, A.; Wang, X.; Tariq, F.; Jhanzab, H.M.; Bibi, Y.; Sher, A.; Razzaq, A.; Fiaz, S.; Tanveer, S.K.; Qayyum, A. Antioxidant enzyme activities correlated with growth parameters of wheat sprayed with silver and gold nanoparticle suspensions. Agronomy 2021, 11, 1494. [Google Scholar] [CrossRef]
- Rea, R.S.; Islam, M.R.; Rahman, M.M.; Nath, B.; Mix, K. Growth, nutrient accumulation, and drought tolerance in crop plants with silicon application: A review. Sustainability 2022, 14, 4525. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Ahmad, A.; Alhammad, B.A.; Tola, E. Exogenous application of zinc oxide nanoparticles improved antioxidants, photosynthetic, and yield traits in salt-stressed maize. Agronomy 2023, 13, 2645. [Google Scholar] [CrossRef]
- Rani, P.U.; Yasur, J.; Loke, K.S.; Dutta, D. Effect of synthetic and biosynthesized silver nanoparticles on growth, physiology and oxidative stress of water hyacinth: Eichhornia crassipes (Mart) Solms. Acta Physiol. Plant. 2016, 38, 58. [Google Scholar] [CrossRef]
- Mohamed, A.K.S.; Qayyum, M.F.; Abdel-Hadi, A.M.; Rehman, R.A.; Ali, S.; Rizwan, M. Interactive effect of salinity and silver nanoparticles on photosynthetic and biochemical parameters of wheat. Arch. Agron. Soil Sci. 2017, 63, 1736–1747. [Google Scholar] [CrossRef]
- Shaikhaldein, H.O.; Al-Qurainy, F.; Nadeem, M.; Khan, S.; Tarroum, M.; Salih, A.M. Biosynthesis and characterization of silver nanoparticles using Ochradenus arabicus and their physiological effect on Maerua oblongifolia raised in vitro. Sci. Rep. 2020, 10, 17569. [Google Scholar] [CrossRef]
- Pallavi; Mehta, C.M.; Srivastava, R.; Arora, S.; Sharma, A.K. Impact assessment of silver nanoparticles on plant growth and soil bacterial diversity. 3 Biotech 2016, 6, 254. [Google Scholar] [CrossRef]
- Jasim, B.; Thomas, R.; Mathew, J.; Radhakrishnan, E.K. Plant growth and diosgenin enhancement effect of silver nanoparticles in Fenugreek (Trigonella foenum-graecum L.). Saudi Pharm. J. 2017, 25, 443–447. [Google Scholar] [CrossRef]
- Al-Huqail, A.A.; Hatata, M.M.; Al-Huqail, A.A.; Ibrahim, M.M. Preparation, characterization of silver phyto nanoparticles and their impact on growth potential of Lupinus termis L. seedlings. Saudi J. Biol. Sci. 2018, 25, 313–319. [Google Scholar] [CrossRef]
- Vinković, T.; Novák, O.; Strnad, M.; Goessler, W.; Jurašin, D.D.; Parađiković, N.; Vrček, I.V. Cytokinin response in pepper plants (Capsicum annuum L.) exposed to silver nanoparticles. Environ. Res. 2017, 156, 10–18. [Google Scholar] [CrossRef]
- Ibrahim, E.; Zhang, M.; Zhang, Y.; Hossain, A.; Qiu, W.; Chen, Y.; Wang, Y.; Wu, W.; Sun, G.; Li, B. Green-synthesization of silver nanoparticles using endophytic bacteria isolated from garlic and its antifungal activity against wheat Fusarium head blight pathogen Fusarium graminearum. Nanomaterials 2020, 10, 219. [Google Scholar] [CrossRef]
- Khot, L.R.; Sankaran, S.; Maja, J.M.; Ehsani, R.; Schuster, E.W. Applications of nanomaterials in agricultural production and crop protection: A review. Crop Prot. 2012, 35, 64–70. [Google Scholar] [CrossRef]
- Ditta, A.; Arshad, M. Applications and perspectives of using nanomaterials for sustainable plant nutrition. Nanotechnol. Rev. 2016, 5, 209–229. [Google Scholar] [CrossRef]
- Sarkhosh, S.; Kahrizi, D.; Darvishi, E.; Tourang, M.; Haghighi-Mood, S.; Vahedi, P.; Ercisli, S. Effect of Zinc Oxide Nanoparticles (ZnO-NPs) on Seed Germination Characteristics in Two Brassicaceae Family Species: Camelina sativa and Brassica napus L. J. Nanomater. 2022, 2022, 1892759. [Google Scholar] [CrossRef]
- Hussain, A.; Ali, S.; Rizwan, M.; ur Rehman, M.Z.; Javed, M.R.; Imran, M.; Chatha, S.A.; Nazir, R. Zinc oxide nanoparticles alter the wheat physiological response and reduce the cadmium uptake by plants. Environ. Pollut. 2018, 242, 1518–1526. [Google Scholar] [CrossRef]
- Jankovskis, L.; Kokina, I.; Plaksenkova, I.; Jermaļonoka, M. Impact of different nanoparticles on common wheat (Triticum aestivum L.) plants, course, and intensity of photosynthesis. Sci. World J. 2022, 2022, 3693869. [Google Scholar] [CrossRef] [PubMed]
- Pariona, N.; Mtz-Enriquez, A.I.; Sánchez-Rangel, D.; Carrión, G.; Paraguay-Delgado, F.; Rosas-Saito, G. Green-synthesized copper nanoparticles as a potential antifungal against plant pathogens. RSC Adv. 2019, 9, 18835–18843. [Google Scholar] [CrossRef]
- Malandrakis, A.A.; Kavroulakis, N.; Chrysikopoulos, C.V. Synergy between Cu-NPs and fungicides against Botrytis cinerea. Sci. Total Environ. 2020, 703, 135557. [Google Scholar] [CrossRef]
- Ntasiou, P.; Kaldeli Kerou, A.; Karamanidou, T.; Vlachou, A.; Tziros, G.T.; Tsouknidas, A.; Karaoglanidis, G.S. Synthesis and characterization of novel copper nanoparticles for the control of leaf spot and anthracnose diseases of olive. Nanomaterials 2021, 11, 1667. [Google Scholar] [CrossRef]
- Badawy, A.A.; Abdelfattah, N.A.; Salem, S.S.; Awad, M.F.; Fouda, A. Efficacy assessment of biosynthesized copper oxide nanoparticles (CuO-NPs) on stored grain insects and their impacts on morphological and physiological traits of wheat (Triticum aestivum L.) plant. Biology 2021, 10, 233. [Google Scholar] [CrossRef]
- Faraz, A.; Faizan, M.; Hayat, S.; Alam, P. Foliar application of copper oxide nanoparticles increases the photosynthetic efficiency and antioxidant activity in Brassica juncea. J. Food Qual. 2022, 2022, 5535100. [Google Scholar] [CrossRef]
- Kohatsu, M.Y.; Pelegrino, M.T.; Monteiro, L.R.; Freire, B.M.; Pereira, R.M.; Fincheira, P.; Lange, C.N. Comparison of foliar spray and soil irrigation of biogenic CuO nanoparticles (NPs) on elemental uptake and accumulation in lettuce. Environ. Sci. Pollut. Res. 2021, 28, 16350–16367. [Google Scholar] [CrossRef] [PubMed]
- Kasote, D.M.; Lee, J.H.; Jayaprakasha, G.K.; Patil, B.S. Seed priming with iron oxide nanoparticles modulate antioxidant potential and defense-linked hormones in watermelon seedlings. ACS Sustain. Chem. Eng. 2019, 7, 5142–5151. [Google Scholar] [CrossRef]
- Asadi-Kavan, Z.; Khavari-Nejad, R.A.; Iranbakhsh, A.; Najafi, F. Cooperative effects of iron oxide nanoparticle (α-Fe2O3) and citrate on germination and oxidative system of evening primrose (Oenthera biennis L.). J. Plant Interact. 2020, 15, 166–179. [Google Scholar] [CrossRef]
- Mohasseli, V.; Farbood, F.; Moradi, A. Antioxidant defense and metabolic responses of lemon balm (Melissa officinalis L.) to Fe-nano-particles under reduced irrigation regimes. Ind. Crop. Prod. 2020, 149, 112338. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Xu, M.; Xiao, L.; Dai, Z.; Li, J. The impacts of γ-Fe₂O₃ and Fe₃O₄ nanoparticles on the physiology and fruit quality of muskmelon (Cucumis melo) plants. Environ. Pollut. 2019, 249, 1011–1018. [Google Scholar] [CrossRef]
- Fajardo, C.; Costa, G.; Nande, M.; Martín, C.; Martín, M.; Sánchez-Fortún, S. Heavy metals immobilization capability of two iron-based nanoparticles (nZVI and Fe3O4): Soil and freshwater bioassays to assess ecotoxicological impact. Sci. Total Environ. 2019, 656, 421–432. [Google Scholar] [CrossRef]
- Litter, M.I. A short review on the preparation and use of iron nanomaterials for the treatment of pollutants in water and soil. Emerg. Mater. 2022, 5, 391–400. [Google Scholar] [CrossRef]
- Plaksenkova, I.; Jermaļonoka, M.; Bankovska, L.; Gavarāne, I.; Gerbreders, V.; Sledevskis, E.; Snikeris, J.; Kokina, I. Effects of Fe3O4 nanoparticle stress on the growth and development of rocket Eruca sativa. J. Nanomater. 2019, 2019, 2678247. [Google Scholar] [CrossRef]
- Guha, T.; Ravikumar, K.V.G.; Mukherjee, A.; Mukherjee, A.; Kundu, R. Nanopriming with zero valent iron (nZVI) enhances germination and growth in aromatic rice cultivar (Oryza sativa cv. Gobindabhog L.). Plant Physiol. Biochem. 2018, 127, 403–413. [Google Scholar] [CrossRef]
- Khan, S.; Akhtar, N.; Rehman, S.U.; Shujah, S.; Rha, E.S.; Jamil, M. Biosynthesized iron oxide nanoparticles (Fe₃O₄ NPs) mitigate arsenic toxicity in rice seedlings. Toxics 2020, 9, 2. [Google Scholar] [CrossRef] [PubMed]
- Golami, A.; Abbaspour, H.; Hashemi-Moghaddam, H.; Gerami, M. Photocatalytic Effect of TiO₂ Nanoparticles on Essential Oil of Rosmarinus officinalis. J. Biochem. Technol. 2018, 9, 50. [Google Scholar]
- Faraji, J.; Sepehri, A. Ameliorative effects of TiO2 nanoparticles and sodium nitroprusside on seed germination and seedling growth of wheat under PEG-stimulated drought stress. J. Seed Sci. 2019, 41, 309–317. [Google Scholar] [CrossRef]
- Hajra, A.; Mondal, N.K. Effects of ZnO and TiO₂ nanoparticles on germination, biochemical and morphoanatomical attributes of Cicer arietinum L. Energy Ecol. Environ. 2017, 2, 277–288. [Google Scholar] [CrossRef]
- Maity, A.; Natarajan, N.; Vijay, D.; Srinivasan, R.; Pastor, M.; Malaviya, D.R. Influence of metal nanoparticles (NPs) on germination and yield of oat (Avena sativa) and berseem (Trifolium alexandrinum). Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2018, 88, 595–607. [Google Scholar] [CrossRef]
- Shah, T.; Latif, S.; Saeed, F.; Ali, I.; Ullah, S.; Alsahli, A.A.; Jan, S.; Ahmad, P. Seed priming with titanium dioxide nanoparticles enhances seed vigor, leaf water status, and antioxidant enzyme activities in maize (Zea mays L.) under salinity stress. J. King Saud Univ. Sci. 2021, 33, 101207. [Google Scholar] [CrossRef]
- Khodakovskaya, M.; Dervishi, E.; Mahmood, M.; Xu, Y.; Li, Z.; Watanabe, F.; Biris, A.S. Carbon nanotubes are able to penetrate plant seed coat and dramatically affect seed germination and plant growth. ACS Nano 2009, 3, 3221–3227. [Google Scholar] [CrossRef] [PubMed]
- Lahiani, M.H.; Chen, J.; Irin, F.; Puretzky, A.A.; Green, M.J.; Khodakovskaya, M.V. Interaction of carbon nanohorns with plants: Uptake and biological effects. Carbon 2015, 81, 607–619. [Google Scholar] [CrossRef]
- Ren, L.; Deng, S.; Chu, Y.; Zhang, Y.; Zhao, H.; Chen, H.; Zhang, D. Single-wall carbon nanotubes improve cell survival rate and reduce oxidative injury in cryopreservation of Agapanthus praecox embryogenic callus. Plant Methods 2020, 16, 130. [Google Scholar] [CrossRef]
- Shah, V.; Belozerova, I. Influence of metal nanoparticles on the soil microbial community and germination of lettuce seeds. Water Air Soil Pollut. 2009, 197, 143–148. [Google Scholar] [CrossRef]
- Adhikari, T.; Kundu, S.; Rao, A.S. Impact of SiO2 and Mo nano particles on seed germination of rice (Oryza sativa L.). Int. J. Agric. Food Sci. Technol. 2013, 4, 809–816. [Google Scholar]
- Roohizadeh, G.; Majd, A.; Arbabian, S. The effect of sodium silicate and silica nanoparticles on seed germination and growth in the Vicia faba L. Trop. Plant Res. 2015, 2, 85–89. [Google Scholar]
- Abbasi Khalaki, M.; Ghorbani, A.; Moameri, M. Effects of silica and silver nanoparticles on seed germination traits of Thymus kotschyanus in laboratory conditions. J. Rangeland Sci. 2016, 6, 221–231. [Google Scholar]
- Janmohammadi, M.; Sabaghnia, N. Effect of pre-sowing seed treatments with silicon nanoparticles on germinability of sunflower (Helianthus annuus). Bot. Lith. 2015, 21, 13–21. [Google Scholar] [CrossRef]
- Mukarram, M.; Khan, M.M.A.; Corpas, F.J. Silicon nanoparticles elicit an increase in lemongrass (Cymbopogon flexuosus (Steud.) Wats) agronomic parameters with a higher essential oil yield. J. Hazard. Mater. 2021, 412, 125254. [Google Scholar] [CrossRef]
- Jayarambabu, N.; Kumari, S.B.; Rao, V.K.; Prabhu, Y.T. Enhancement of growth in maize by biogenic-synthesized MgO nanoparticles. Int. J. Pure Appl. Zool. 2016, 4, 262–270. [Google Scholar]
- Ramírez-Olvera, S.M.; Trejo-Téllez, L.I.; García-Morales, S.; Pérez-Sato, J.A.; Gómez-Merino, F.C. Cerium enhances germination and shoot growth, and alters mineral nutrient concentration in rice. PLoS ONE 2018, 13, e0194691. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, L.; Ma, C.; Wang, K.; Hao, Y.; Chen, Q.; Mo, Y.; Rui, Y. Effects of cerium oxide on rice seedlings as affected by co-exposure of cadmium and salt. Environ. Pollut. 2019, 252, 1087–1096. [Google Scholar] [CrossRef]
- Farhadihosseinabadi, B.; Zarebkohan, A.; Eftekhary, M.; Heiat, M.; Moosazadeh Moghaddam, M.; Gholipourmalekabadi, M. Crosstalk between chitosan and cell signaling pathways. Cell. Mol. Life Sci. 2019, 76, 2697–2718. [Google Scholar] [CrossRef]
- Ma, Y.; Fu, L.; Hussain, Z.; Huang, D.; Zhu, S. Enhancement of storability and antioxidant systems of sweet cherry fruit by nitric oxide-releasing chitosan nanoparticles (GSNO-CS NPs). Food Chem. 2019, 285, 10–21. [Google Scholar] [CrossRef]
- Szőllősi, R.; Molnár, Á.; Kondak, S.; Kolbert, Z. Dual effect of nanomaterials on germination and seedling growth: Stimulation vs. phytotoxicity. Plants 2020, 9, 1745. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Adeel, M.; Shakoor, N.; Guo, M.; Hao, Y.; Azeem, I.; Li, M.; Liu, M.; Rui, Y. Application of nanoparticles alleviates heavy metals stress and promotes plant growth: An overview. Nanomaterials 2020, 11, 26. [Google Scholar] [CrossRef] [PubMed]
- Kolesnikov, S.; Timoshenko, A.; Minnikova, T.; Tsepina, N.; Kazeev, K.; Akimenko, Y.; Zhadobin, A.; Shuvaeva, V.; Rajput, V.D.; Mandzhieva, S.; et al. Impact of metal-based nanoparticles on cambisol microbial functionality, enzyme activity, and plant growth. Plants 2021, 10, 2080. [Google Scholar] [CrossRef] [PubMed]
- Paramo, L.A.; Feregrino-Pérez, A.A.; Guevara, R.; Mendoza, S.; Esquivel, K. Nanoparticles in agroindustry: Applications, toxicity, challenges, and trends. Nanomaterials 2020, 10, 1654. [Google Scholar] [CrossRef]
- Maurer-Jones, M.A.; Gunsolus, I.L.; Murphy, C.J.; Haynes, C.L. Toxicity of engineered nanoparticles in the environment. Anal. Chem. 2013, 85, 3036–3049. [Google Scholar] [CrossRef]
- Satya; Hashmi, K.; Gupta, S.; Singh, N.; Khan, T.; Joshi, S. Nanofabrication of Metals and Their Compounds for Effective Medicinal and Environmental Applications (A Review). Russ. J. Gen. Chem. 2023, 93, 635–665. [Google Scholar] [CrossRef]
- Khan, T.; Rahman, Q.I.; Raza, S.; Zehra, S.; Ahmad, N.; Husen, A. Nanodimensional materials:an approach toward the biogenic synthesis. In Advances in Smart Nanomaterials and their Applications; Husen, A., Siddiqi, K.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 523–568. ISBN 978-0-323-99546-7. [Google Scholar]
- Veg, E.; Joshi, S.; Khan, T. Design and Fabrication of Heterojunctions of Thiosemicarbazones and Metal Oxide Nanoparticles in Search of Their Medicinal Activity. Eng. Proc. 2024, 67, 46. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).