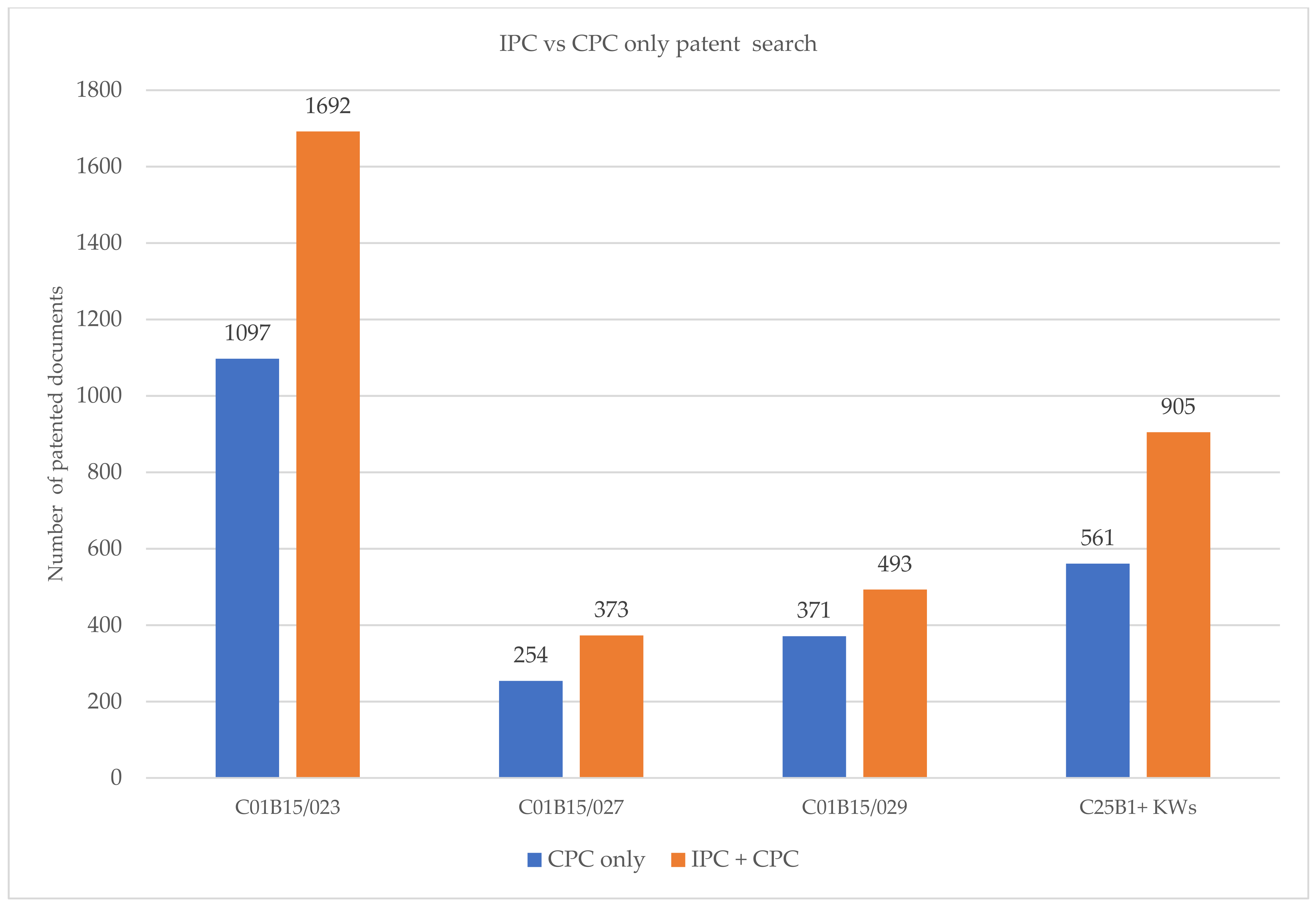Abstract
The aim of this study is to present an overview of patented industrial hydrogen peroxide production processes. Searches were conducted using two databases, namely, Espacenet and Orbit, using mainly IPC and CPC classification symbols, sometimes combined with keywords. An analysis of the data from the Orbit database reveals that the anthraquinone auto-oxidation (AO) process and electrochemical methods have the highest number of active patents. When examining patent applications filed from 2020 onwards, electrochemical methods are found to be the most prevalent, followed by the auto-oxidation of anthraquinone. The data suggest that companies are investing less in the anthraquinone auto-oxidation process compared to electrochemical methods. Research is now focused on synthesizing hydrogen peroxide from water by means of photocatalytic methods, instead of direct synthesis using H2 and O2.
1. Introduction
Hydrogen peroxide is a colorless liquid that is completely miscible in water. It is employed as an oxidant in organic synthesis, as a disinfectant in wastewater treatment, and as a bleaching agent in the pulp and paper industry.
It is industrially produced through the process of the reduction and oxidation of an alkylated anthraquinone, using hydrogen (from the steam reforming of methane) and air, with Pd/Al2O3 as a catalyst, at a temperature of 45 °C. [1]
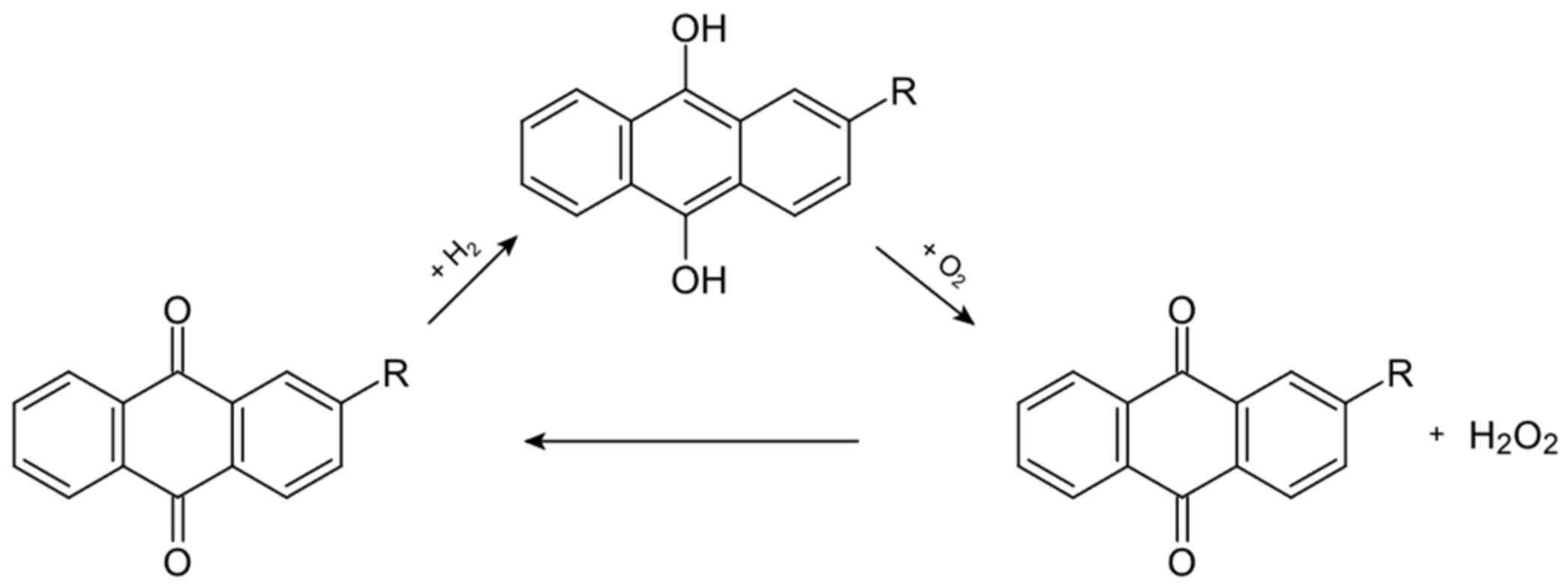
The anthraquinone auto-oxidation process is schematized in Figure 1.
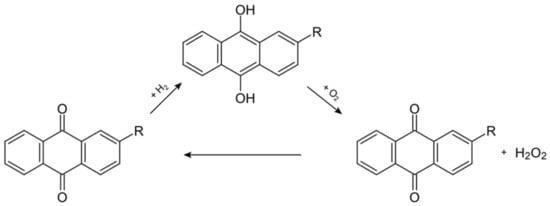
Figure 1.
This scheme illustrates the chemical reactions involved in the anthraquinone process [2].
Various methods can be used to produce the desired product, including direct synthesis from hydrogen and oxygen, electrochemical, photochemical, and enzymatic synthesis, and the partial oxidation of primary or secondary alcohols resulting in the formation of aldehydes or ketones as by-products. Another method involves synthesizing the product from water, carbon monoxide, and oxygen [3,4] in the presence of a catalyst, for example, a soluble palladium compound (such as palladium nitrate, palladium 2,4-pentanedionate, palladium sulfate, or palladium acetate) associated with phosphine or arsine ligands.
2. Materials and Methods
Patent searches were carried out using two databases:
Espacenet [5], a free-of-charge patent database provided by the European Patent Office) and Orbit Intelligence platform [6], a fee-based IP software v2.0.0 managed by Questel and consisting of three search systems: FamPat, FullPat, and FullText.
All patent searches were performed using the FamPat database, using a combination of keywords and classification symbols [7].
Classification symbols can be retrieved using a variety of tools provided by the EPO (e.g., the CPC Text Categorizer and the Classification search tool available on Espacenet) or by WIPO (IPCCAT tool [8]), using simple text queries.
Hydrogen peroxide synthesis is classified into four main typologies:
- Synthesis from organic compounds (mainly by the alkyl-anthraquinone process);
- Synthesis from water (photocatalyst processes and by the reaction of water, carbon monoxide, and oxygen);
- Synthesis from hydrogen and oxygen (direct synthesis);
- Synthesis from inorganic peroxy compounds.
The electrochemical synthesis methods for hydrogen peroxide are not completely classified by the C01B 15/01 subgroups but rather by C25B 1/30 (Electrolytic production of peroxides).
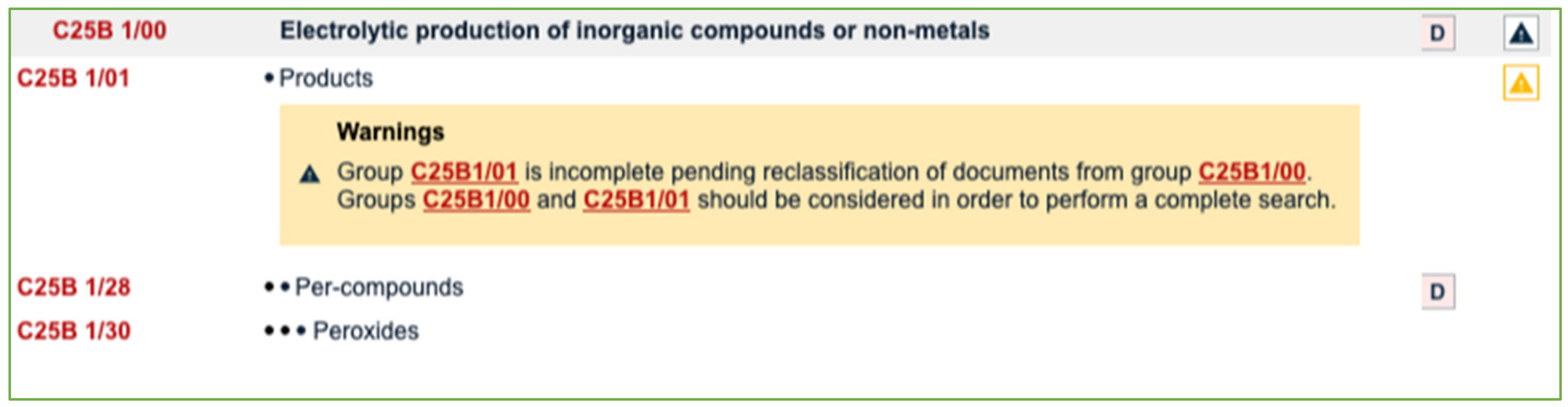
In order to perform a comprehensive search, it is necessary to consider the main groups C25B 1/00 and C25B 1/01 (see Figure 2), since the reclassification of documents is still pending.

Figure 2.
Screenshot of the main group C25B 1/00 and corresponding subgroups (Source: Espacenet).
All classification symbols were referred to as “peroxides” in general, and the terms “hydrogen peroxide” and “H2O2” were used in combination with the classification symbols in order to obtain a precise search. In order to retrieve the patented electrolytic processes, the following search query was employed on Espacenet:
(cpc = “C25B1/30/low” OR cpc = “C25B1/00” OR cpc = “C25B1/01”) AND ctxt =
(“hydrogen” prox/ordered “peroxide?”)
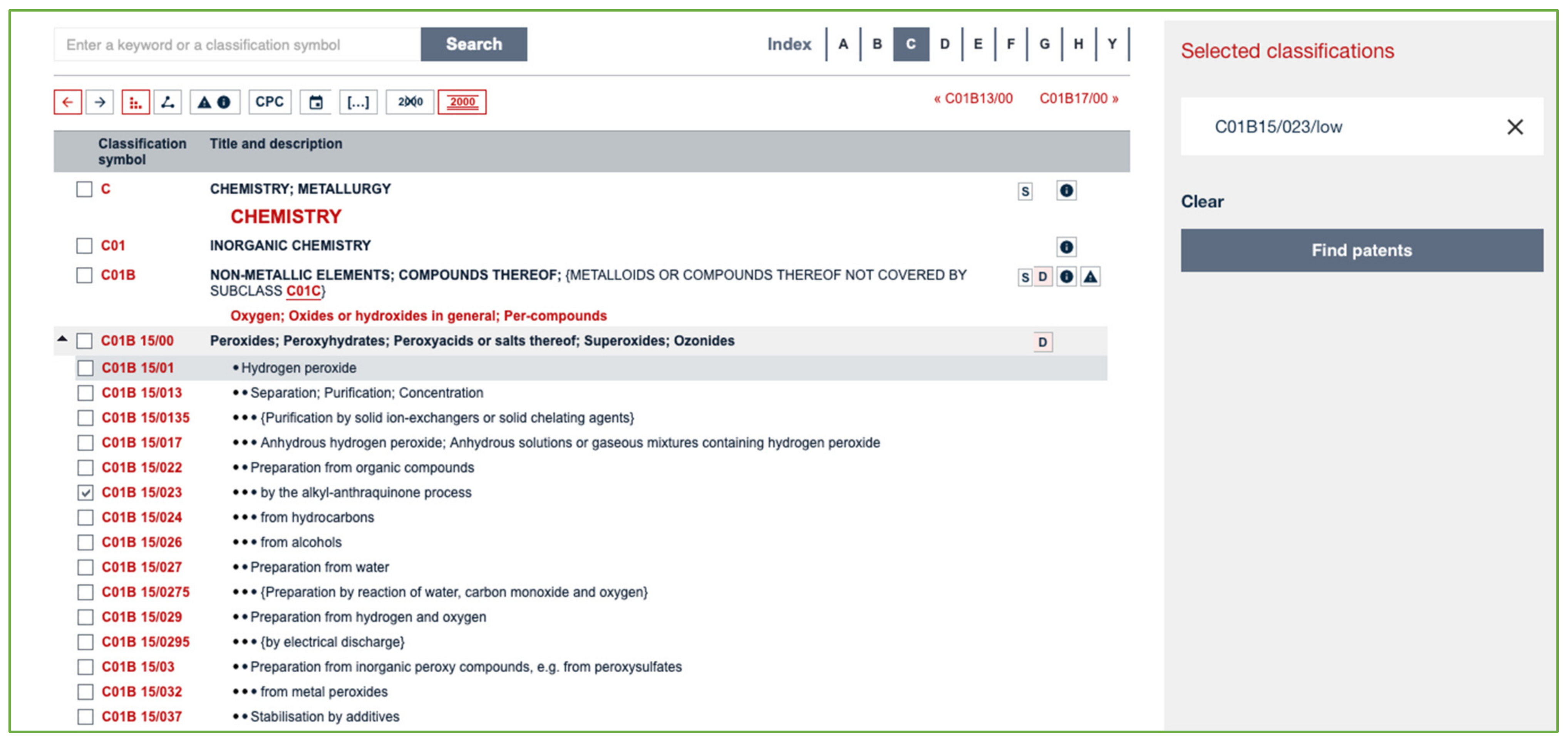
A very quick search may be carried out on Espacenet by selecting the specific code and clicking on the button labeled “Find patents” (see Figure 3).
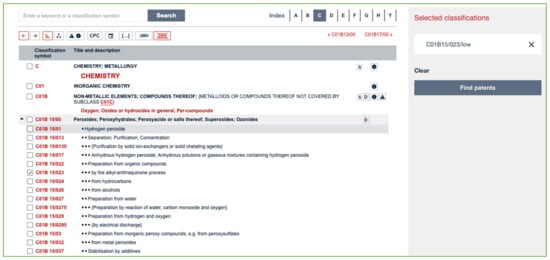
Figure 3.
Screenshot of the Espacenet Classification tool.
However, this method of searching only encompasses CPC codes.
In order to obtain more comprehensive search results, it is also necessary to consider IPC codes.
The graph in Figure 4 presents the results obtained with Espacenet using only CPC and IPC codes, in addition to CPC codes. The number of retrieved results is greater for an IPC + CPC search than for a CPC-only search. Consequently, in order to obtain a complete search, both classification symbols should be considered [9].
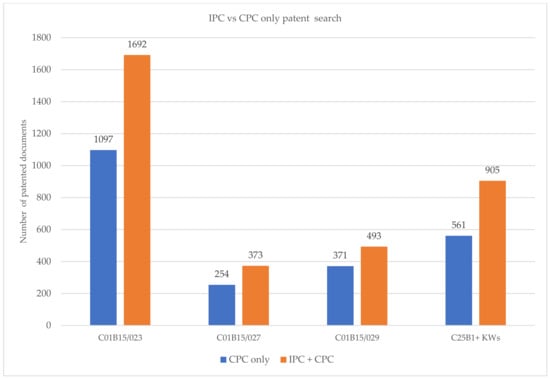
Figure 4.
Comparison between the number of results obtained with IPC + CPC and CPC classification.
Table 1 provides the complete list of classification codes (IPC and CPC) used in the search queries.

Table 1.
List of classification symbols used in the patent search.
For electrolytic production, where the classification symbol has a broad definition, the keywords (‘H2O2’ and ‘hydrogen peroxide’) were used in the title, abstract, and claims search fields with a proximity operator.
Other classification codes found in Espacenet do not relate to the production of hydrogen peroxide but rather to its uses.
For example, C02F 1/722 is related to wastewater treatment processes, where contaminants are oxidized by peroxides. Therefore, these codes were not considered in our research.
Photochemical (a) and enzymatic methods (b) were retrieved with the following search queries:
- (a)
- cl = “C01B15/027” AND (ftxt = “photo*” OR cl = “B01J19/12” OR cl = “B01J35/39”)
- (b)
- cl = “C01B15/01/low” AND (cl = “C12P” OR cl = “C12Y” OR cl = “C12M” OR cl = “C12N”)
3. Results
The anthraquinone auto-oxidation process and electrochemical methods have the highest number of active patents (see Table 2).

Table 2.
List of results derived from the patent search in Espacenet and Orbit (Accessed 20 May 2024).
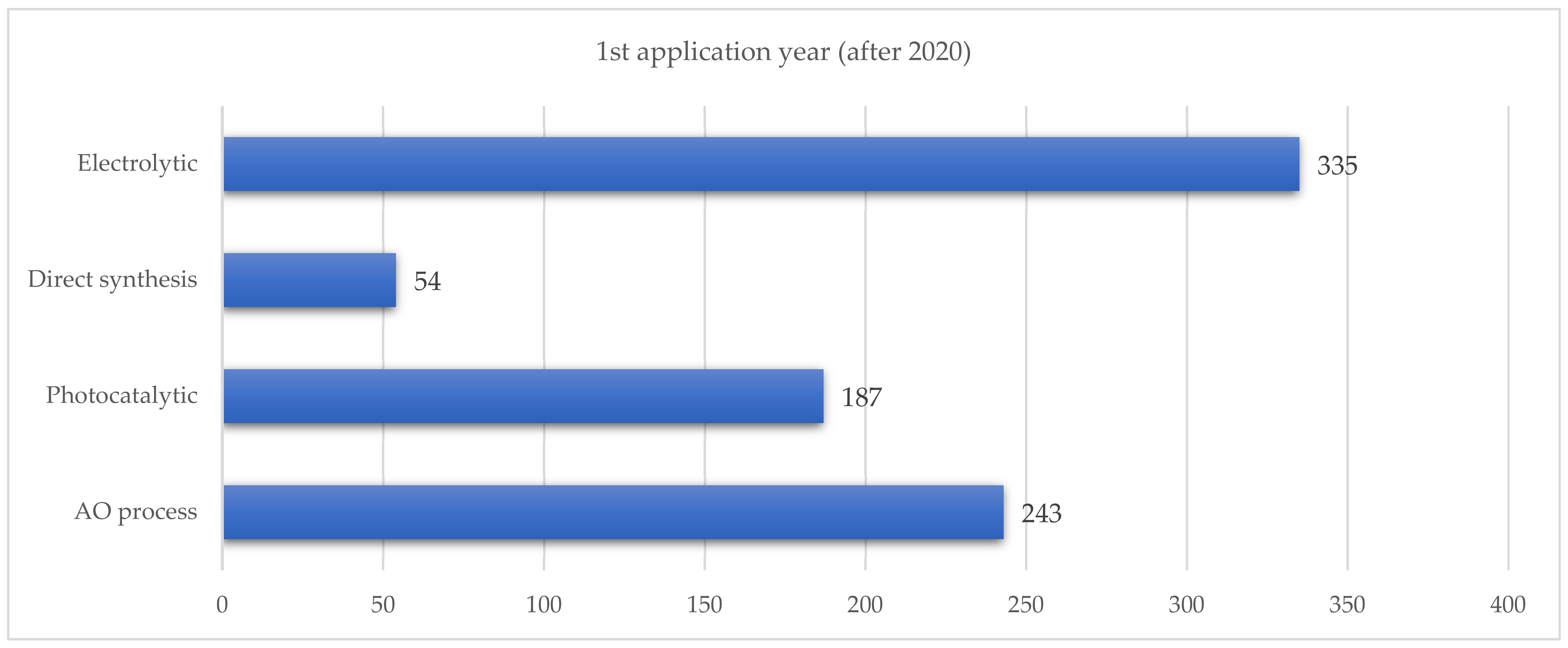
When examining patent applications filed from 2020 onwards, electrochemical methods were found to be the most prevalent, followed by the auto-oxidation of anthraquinone (see Figure 5).
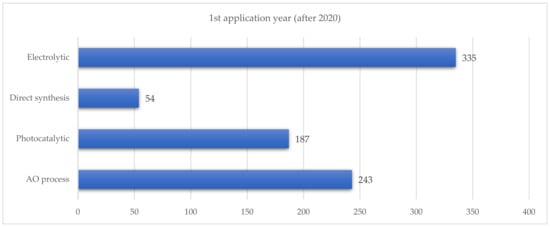
Figure 5.
Number of patented applications filed after 2020.
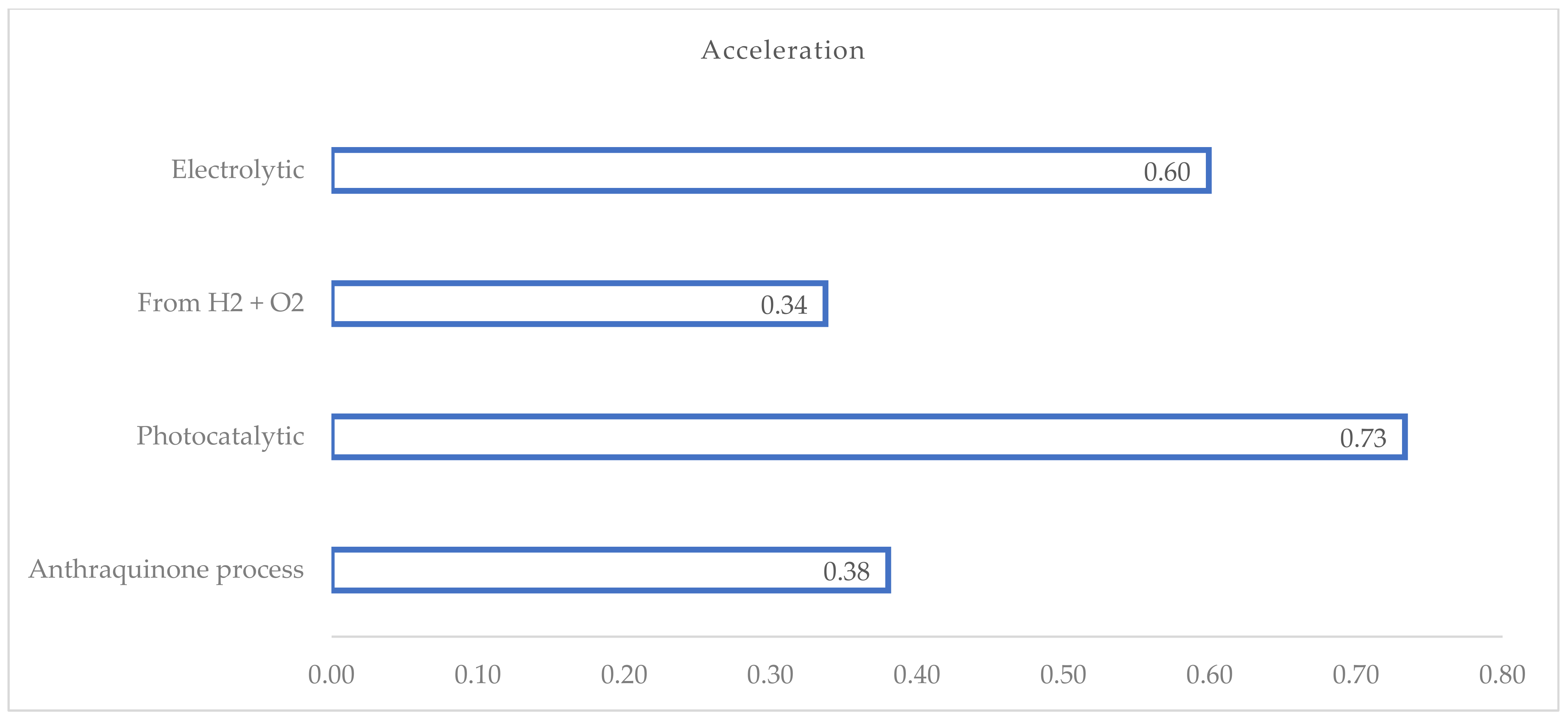
The ‘acceleration’ indicator, defined as the ratio of the number of patents with a first application year after 2020 to the number of active patents/patent applications, allows for the identification of patented technologies that have experienced the greatest increase in the number of filings over time (see Figure 6).
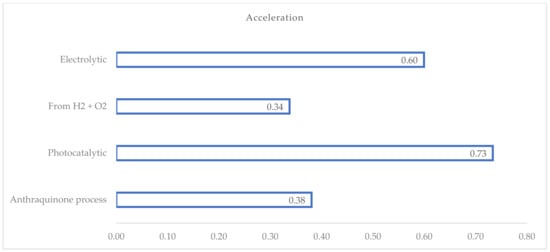
Figure 6.
Acceleration factor calculated for each relevant H2O2 process.
4. Discussions
Currently, the anthraquinone method represents the predominant industrial production method for hydrogen peroxide. In comparison to other preparation methods, it exhibits the advantages of a low production cost and the capacity for large-scale production and to operate in a continuous way.
The AO process encompasses four principal stages [10], as depicted in the subsequent flowchart:
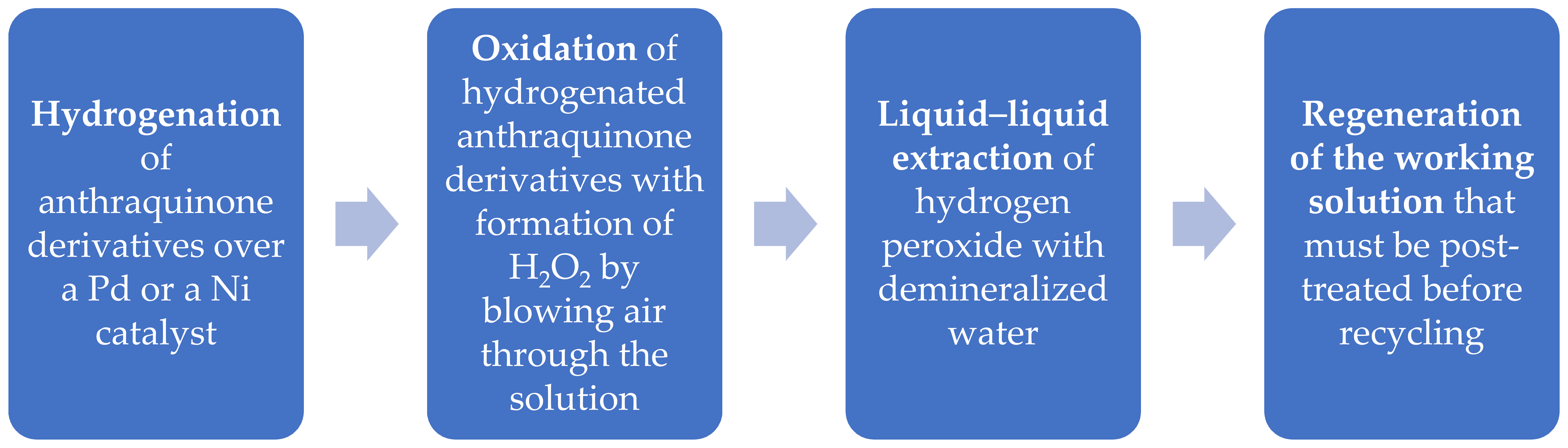

Palladium is the most frequently used catalyst in combination with other elements (Pd-Zn-Ba/SBA-15, Pd-Ru alloy, Pd-alumina, Pd-phosphorous, Pd-Co alloy, Ni hydroxide-Pd, Pt-Pd-Ag, Pd-Fe SiO2, Pd-Au).
The aim of recently patented research projects is to develop novel and innovative catalytic systems that will facilitate the acceleration of the hydrogenation process while simultaneously enhancing the efficiency of the regeneration process. Some examples of these materials include nickel and ruthenium, platinum and rare earths, and iron or copper in combination with noble metals.
The most commonly employed reactors are fixed-bed, fluidized-bed, and slurry-bed reactors. A fixed-bed reactor, which consists of a cylindrical column filled with catalyst pellets, is subject to a number of limitations, including a high pressure drop, low catalyst utilization, and insufficient heat removal.
A fluidized-bed reactor, a type of continuous flow reactor that combines the characteristics of a stirred tank and a packed-bed reactor, exhibits a notable enhancement in heat transfer.
The efficiency of the hydrogenation process in a fluidized-bed reactor is enhanced due to the increased productivity of the catalyst. Additional benefits of a fluidized-bed reactor over a fixed-bed reactor include a reduction in catalyst consumption per ton of hydrogen peroxide and the simplicity of the online removal/addition of the catalyst, which extends the operational lifespan of the reactor [11].
A slurry bed is a multiphase flow reactor in which a reactant gas is bubbled through a solution containing solid catalyst particles [12]. The difficulty in separating the catalyst and the handling of the slurry represent significant limitations in the application of slurry reactors in continuous processes. Nevertheless, slurry bed reactors offer numerous advantages, including a relatively low initial investment cost and a straightforward construction process [13].
The direct synthesis of hydrogen peroxide represents an alternative and eco-friendly route, although it has not been widely adopted at an industrial level due to a number of drawbacks. These include the formation of water, the risk of explosion, and the instability of the H2O2 produced.
The most commonly used catalysts are platinum, palladium, and gold. Fixed-bed, slurry-bed, and trickle-bed reactors are the reactors that are most frequently employed.
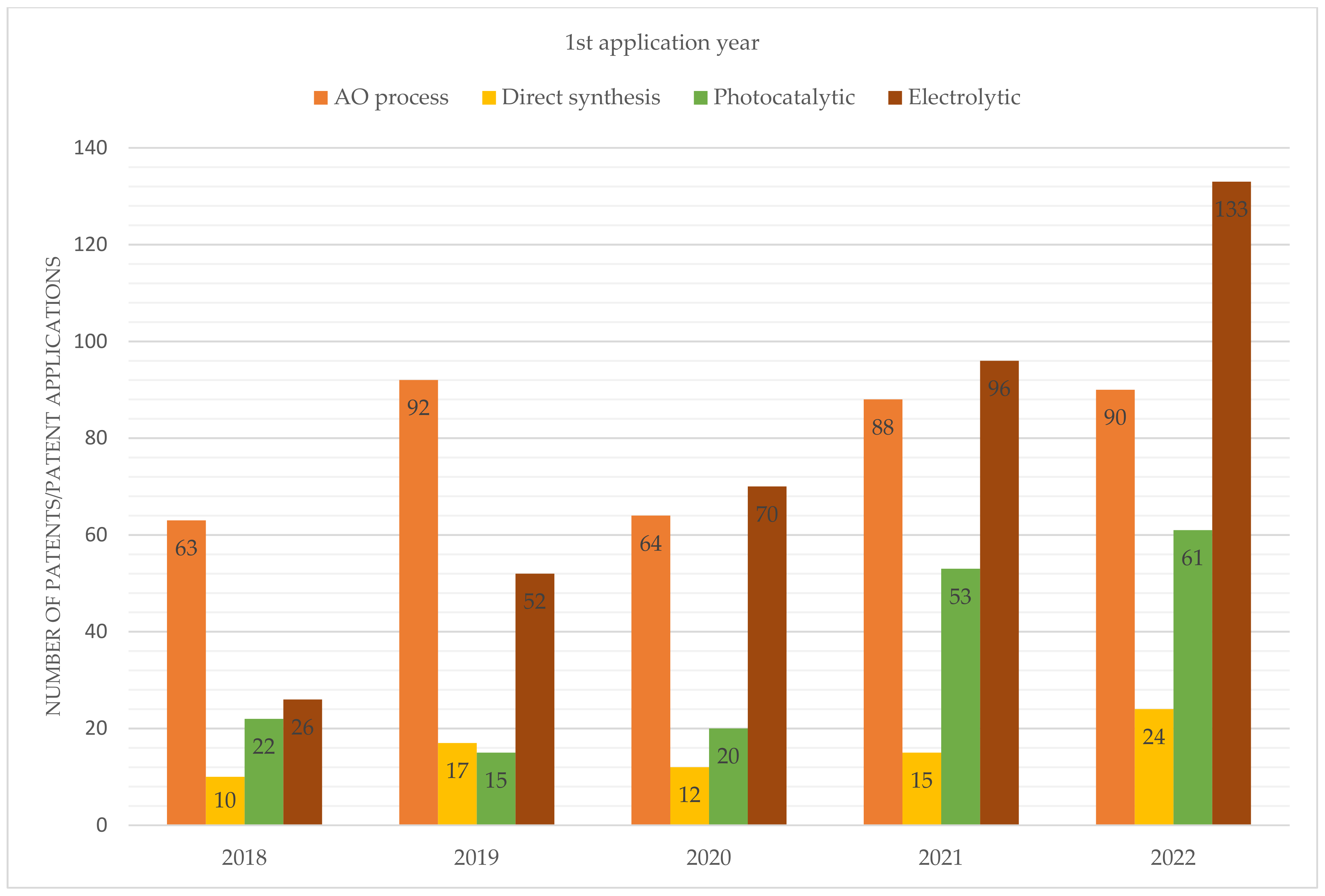
From the perspective of intellectual property, there has been a growing interest in photocatalytic and electrolytic processes in recent years, as evidenced by the results presented in Figure 7.
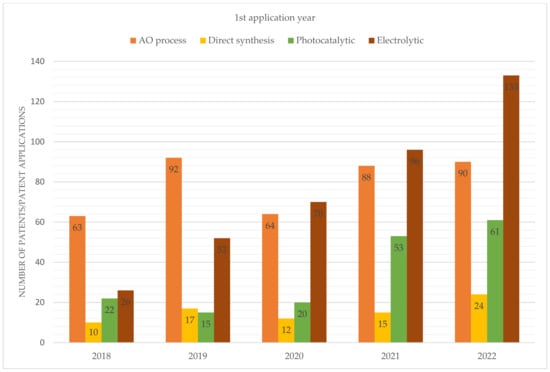
Figure 7.
Number of patent applications filed during the period from 2018 to 2022.
The photocatalytic process has recently attracted considerable interest due to its potential to utilize water and oxygen as raw materials, thereby eliminating the need for hydrogen. Furthermore, the process can be powered by solar energy, offering a sustainable alternative to traditional methods [14].
The most frequently patented catalytic systems contain nitrogen compounds, in particular, graphite-like carbon nitride (g-C3N4), titanium dioxide (TiO2) polyoxometalates, metal–organic materials, supramolecular coordination complexes (i.e., metal–organic frameworks), and metal-free polymers.
In the field of electrolytic processes, carbon-based materials (containing other elements such as boron or transition and noble metals) are the most frequently cited catalytic systems in patents and patent applications, followed by nitrogen compounds and nickel.
5. Conclusions
The data suggest that companies are investing less in the anthraquinone auto-oxidation process compared to electrochemical and photocatalytic methods.
Research is now focused on synthesizing hydrogen peroxide from water by photocatalytic processes instead of direct synthesis using H2 and O2.
China is the leading country in terms of the number of patent applications filed across all fields, with the exception of direct synthesis, where the USA is the country of origin with the highest number of filed applications. With regard to the European continent, Germany occupies the leading position in terms of the number of patents filed.
This paper can be a valuable resource for both researchers and technology transfer practitioners, as it provides a comprehensive overview of the patent landscape in the field of H2O2 production. For example, based on the findings, a research team may be engaged to investigate the hydrogenation step in the AO process or directed towards the electrolytic production of H2O2.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is available upon request.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Ciriminna, R.; Albanese, L.; Meneguzzo, F.; Pagliaro, M. Hydrogen Peroxide: A Key Chemical for Today’s Sustainable Development. ChemSusChem 2016, 9, 3374–3381. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://commons.wikimedia.org/wiki/File:Anthrachinonverfahren.svg (accessed on 7 January 2025).
- Gao, G.; Tian, Y.; Gong, X.; Pan, Z.; Yang, K.; Zong, B. Advances in the production technology of hydrogen peroxide. Chin. J. Catal. 2020, 41, 1039–1046. [Google Scholar] [CrossRef]
- Garcia-Munoz, P.; Valenzuela, L.; Wegstein, D.; Schanz, T.; Lopez, G.E.; Ruppert, A.M.; Remita, H.; Bloh, J.Z.; Keller, N. Photocatalytic Synthesis of Hydrogen Peroxide from Molecular Oxygen and Water. Top. Curr. Chem. 2023, 381, 15. [Google Scholar] [CrossRef] [PubMed]
- Espacenet. Available online: https://worldwide.espacenet.com (accessed on 20 May 2024).
- Orbit Intelligence. Available online: https://www.orbit.com (accessed on 20 May 2024).
- Barbieri, M. Patent Prior Art Searches: Basic Principles and Strategies. Preprints 2022, 2022050054. [Google Scholar] [CrossRef]
- WIPO. IPCCAT. Available online: https://ipcpub.wipo.int/ (accessed on 29 December 2023).
- Barbieri, M.; Andreoni, G. Carbon Allotrope-Based Textile Biosensors: A Patent Landscape Analysis. Eng. Proc. 2023, 58, 107. [Google Scholar] [CrossRef]
- Ranganathan, S.; Sieber, V. Recent Advances in the Direct Synthesis of Hydrogen Peroxide Using Chemical Catalysis—A Review. Catalysts 2018, 8, 379. [Google Scholar] [CrossRef]
- Li, H.; Zheng, B.; Pan, Z.; Zong, B.; Qiao, M. Advances in the slurry reactor technology of the anthraquinone process for H2O2 production. Front. Chem. Sci. Eng. 2018, 12, 124–131. [Google Scholar] [CrossRef]
- Kayode Coker, A. Chapter 21—Industrial and Laboratory Reactors—Chemical Reaction Hazards and Process Integration of Reactors. In Ludwig’s Applied Process Design for Chemical and Petrochemical Plants, 4th ed.; Kayode Coker, A., Ed.; Gulf Professional Publishing: Houston, TX, USA, 2015; pp. 1095–1208. ISBN 9780750685245. [Google Scholar] [CrossRef]
- Meng, F.; Nawaz, M.A. Review of Slurry Bed Reactor for Carbon One Chemistry. In Advances in Slurry Technology; Jones, T.F., Ed.; Intech Open: London, UK, 2022; ISBN 978-1-80356-669-6. [Google Scholar] [CrossRef]
- Hou, H.; Zeng, X.; Zhang, X. Production of Hydrogen Peroxide by Photocatalytic Processes. Angew. Chem. Int. Ed. 2020, 59, 17356–17376. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).