Analysis of the Pyrolysis of Methane Reaction over Molten Metals for CO2-Free Hydrogen Production: An Application of DFT and Machine Learning †
Abstract
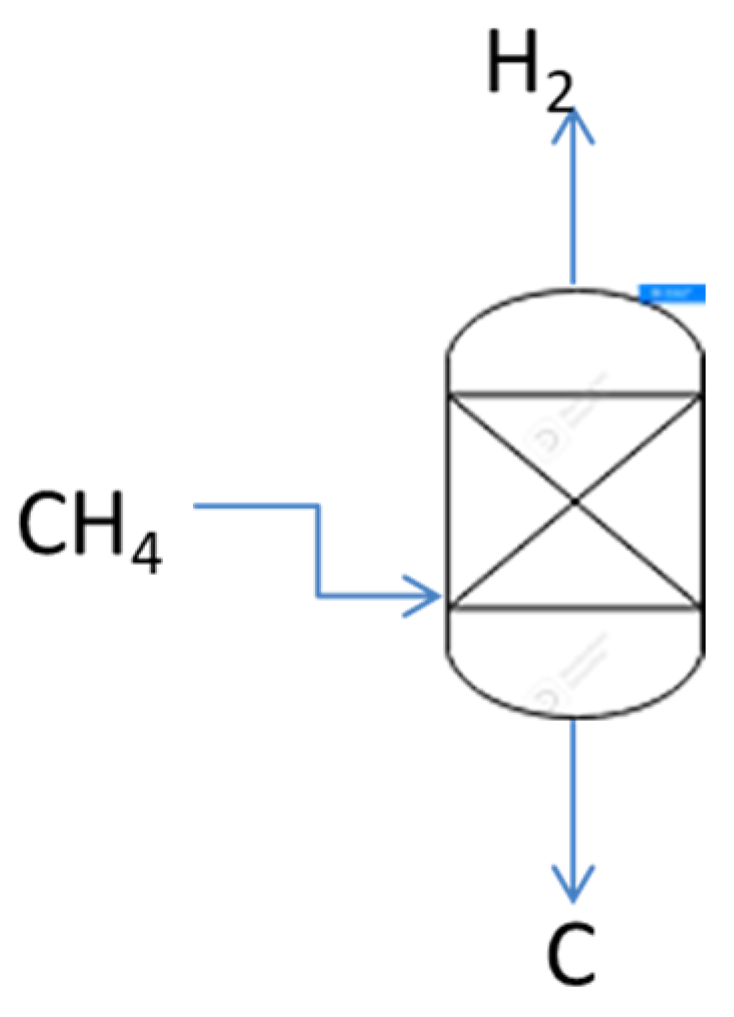
1. Introduction
2. Methodology
- Determine the kinetic and wavefunction cut-offs for the separate metal and metal alloy systems.
- Perform variable cell relaxation for separate active (Ni, Pt, Pd, Cu, Ag, Au), inactive (In, Ga, Sn, and Pb), and molten metal alloy systems containing different concentrations.
- Determination of density of states (DOS), projected DOS, D-band, etc., to characterize the metal systems.
- Calculate the pair distribution functions, the local concentrations, and Bader charges for the constituent metal atoms as one approaches the surface of the liquid.
- Calculate the activation parameters for the dissociative adsorption of methane using a nudged elastic band (NEB).
- Calculate the adsorption energies of the adsorbate on the catalyst surface.
- Correlation of parameters such as concentrations with turnover frequency (TOF).
- Simulate the effect of temperature and pressure on the molten catalyst systems for the pyrolysis of methane.
- Predict catalytic performance and optimum operating conditions using machine learning models.
- Generation and optimization of catalyst constituents’ crystal structures.
- Variable cell relaxation SCF calculation for Bismuth base metal doped with varying proportions (0%, 5%, 10%, 15%, 25%) of Ni, Pt, and Pd.
- Variable cell relaxation SCF calculation for Bismuth base metal doped with varying proportions (0%, 5%, 10%, 15%, 25%) of Ni, Pt, Pd, Cu, Ag, and Au using the PBE form of the generalized gradient approximation for the electron exchange and correlation and the electron core interaction described using projector augmented wave (PAW), ultrasoft (US) pseudopotentials (PPs), and norm-conserving (NC) methods.
- Cell Molecular Dynamics was calculated using the Car-Parinello Method for thermostat-varied temperatures with a cell vacuum thickness of 12A for the simulation. Electron and ion dynamics were simulated using the Verlet algorithm.
- Computation of adsorption energies with the formulae:
- 6.
- The transition state will be found via a two-step approach using the nudged elastic band (NEB).
- 7.
- Computation of catalyst TOF/TON
- 8.
- Creation of and comparison of ML models for the prediction of efficient catalyst compositions and optimum operating conditions using the computed adsorption energies, binding energies, and reaction kinetics. The models will be compared on the basis of their MAE, MSE, and R2 for the selection of the most accurate models for the prediction
3. Results and Discussion
3.1. Computational Parameters Optimization
3.2. Molecular Dynamics of Bismuth Base Metal
4. Conclusions
- Bi crystal with 64 atoms relaxes within 90 ps with a ground state energy of 210 Ry, which is a suitable base metal for pyrolysis of methane, as confirmed by an NVE simulation.
- NVT simulation is indicative that in connection with a heat bath, the optimum temperature for MD is 872.58 K, which is above the melting point of Bi. Hence, its suitability as a molten base metal carrier for active metals for CH4 pyrolysis.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giddey, S.; Badwal, S.P.S.; Kulkarni, A. Review of electrochemical ammonia production technologies and materials. Int. J. Hydrog. Energy 2013, 38, 14576–14594. [Google Scholar] [CrossRef]
- Chen, L.; Qi, Z.; Zhang, S.; Su, J.; Somorjai, G.A. Catalytic hydrogen production from methane: A review on recent progress and prospect. Catalysts 2020, 10, 858. [Google Scholar] [CrossRef]
- Angeli, S.D.; Turchetti, L.; Monteleone, G.; Lemonidou, A.A. Catalyst development for steam reforming of methane and model biogas at low temperature. Appl. Catal. B Environ. 2016, 181, 34–46. [Google Scholar] [CrossRef]
- Upham, D.C.; Agarwal, V.; Khechfe, A.; Snodgrass, Z.R.; Gordon, M.J.; Metiu, H.; McFarland, E.W. Catalytic molten metals for the direct conversion of methane to hydrogen and separable carbon. Science 2017, 358, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Palmer, C.; Tarazkar, M.; Kristoffersen, H.H.; Gelinas, J.; Gordon, M.J.; McFarland, E.W.; Metiu, H. Methane Pyrolysis with a Molten Cu-Bi Alloy Catalyst. ACS Catal. 2019, 9, 8337–8345. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ugwu, L.; Morgan, Y.; Ibrahim, H. Analysis of the Pyrolysis of Methane Reaction over Molten Metals for CO2-Free Hydrogen Production: An Application of DFT and Machine Learning. Eng. Proc. 2024, 76, 97. https://doi.org/10.3390/engproc2024076097
Ugwu L, Morgan Y, Ibrahim H. Analysis of the Pyrolysis of Methane Reaction over Molten Metals for CO2-Free Hydrogen Production: An Application of DFT and Machine Learning. Engineering Proceedings. 2024; 76(1):97. https://doi.org/10.3390/engproc2024076097
Chicago/Turabian StyleUgwu, Lord, Yasser Morgan, and Hussameldin Ibrahim. 2024. "Analysis of the Pyrolysis of Methane Reaction over Molten Metals for CO2-Free Hydrogen Production: An Application of DFT and Machine Learning" Engineering Proceedings 76, no. 1: 97. https://doi.org/10.3390/engproc2024076097
APA StyleUgwu, L., Morgan, Y., & Ibrahim, H. (2024). Analysis of the Pyrolysis of Methane Reaction over Molten Metals for CO2-Free Hydrogen Production: An Application of DFT and Machine Learning. Engineering Proceedings, 76(1), 97. https://doi.org/10.3390/engproc2024076097






