Abstract
Introduction: Metastatic cancers are frequently detected on fine-needle aspiration (FNA) cytology, and confirmation of metastatic breast cancer often requires immunocytochemistry. Tissue provisioning for FNA specimens is important. In this study, GATA3, gross cystic disease fluid protein-15 (GCDFP15), mammaglobin (MMG), and SOX10 were performed on cell block preparations from aspirates of histologically confirmed metastatic breast cancers. The diagnostic performance of single markers and combinations of these markers were investigated with the aim to construct a tissue-efficient immunopanel. Methodology: Aspirates of metastatic breast cancer with corresponding histology and biomarker (estrogen receptor (ER), progesterone receptor (PR), HER2 and ki67) profile were retrieved. ER, GATA3, GCDFP15, MMG and SOX10 immunostains were performed on cell block sections and their expressions were assessed and compared. Results: Immunostaining was performed on a total of 115 aspirates. GATA3 showed the highest expression, followed by MMG, GCDFP15 and SOX10. Twenty-three, five and five cases expressed GATA3, MMG and SOX10 only. The five cases expressing SOX10 only were ER negative, and SOX10 expression was negatively associated with ER (p = 0.001), MMG (p = 0.001), GCDFP15 (p = 0.010) and GATA3 (p = 0.002), whereas GATA3 expression showed positive correlation with ER positivity (p < 0.001). MMG and GCDFP15 showed association with high Ki67 (p < 0.05), and no correlations were found with HER2 expression. Conclusion: In this cohort, GATA3 was the most sensitive single marker. The addition of MMG and SOX10 increases the sensitivity for detection of ER positive and ER negative breast cancers, respectively. These findings support the use of a combination of GATA3/MMG/SOX10 for confirmation of metastatic breast cancer.
Keywords:
aspiration cytology; breast cancer; GATA3; GCDFP15; mammaglobin; SOX10; immunocytochemistry 1. Introduction
Metastatic cancers are frequently detected on fine-needle aspiration (FNA) cytology [1], in which breast cancer is not uncommonly encountered. Breast cancers are biologically diverse, and confirmation of breast origin often necessitates the use of a panel of immunocytochemistry [2]. As biomarker testing is mandatory for breast cancers [3], tissue provision has to be made. For small volume specimens such as FNA, the cytopathologist must be prudent in selecting immunostains to be performed. In this study, GATA3, gross cystic disease fluid protein-15 (GCDFP15), mammaglobin (MMG) and SOX10 were performed on cell block preparations from aspirates of metastatic breast cancer with histologic confirmation. The expression pattern of each immunostain was compared to investigate the performance of each single marker and different combinations of these markers for the diagnosis of metastatic breast cancer.
2. Methodology
2.1. Case Collection
This study was approved by the Joint Chinese University of Hong Kong—New Territories East Cluster clinical research ethics committee. A computerized search of the cytology archives of the institute, from the year 2008 to 2021, was performed. Aspirates of metastatic carcinomas with a hospital diagnosis code of breast cancer were retrieved. Case notes, radiology and pathology reports were reviewed to confirm cases of metastatic breast carcinoma and record the biomarker status (estrogen receptor (ER), progesterone receptor (PR), HER2 and ki67) in the reference biopsy or excision specimen. Cases without histologic confirmation of breast carcinoma were excluded. Cases with cell block preparation available were retrieved and reviewed for the presence of tumor cells by two authors (JJXL and JKMN), and unstained sections were prepared for immunocytochemistry.
2.2. Immunocytochemistry
ER (Cell Margue, Rocklin, CA, USA, 249R-16, 1:60), GATA3 (Biocare, Pacheco, CA, USA, CM405B, 1:150), GCDFP15 (Novocastra, Newcastle upon Tyne, UK, NCL-GCDFP15. 1:200), mammaglobin (Cell Marque, Rocklin, CA, USA, 280R-16, 1:400), and SOX10 (Biocare, AC13099C, 1:100) immunocytochemistry were performed and scored by intensity (negative, weak, moderate and strong) and proportion (0 to 100% staining of tumor cells). Scoring was performed by two authors (JJXL and JKMN). Discrepant cases were reviewed with a third author (GMT) under a multiheaded microscope until a consensus was reached.
2.3. Fine Needle Aspiration Cytology Procedure
FNAC was performed free-hand or endoscopically with/without imaging guidance. Cell block preparations were generated with/without smears or cytospin preparations.
2.4. Statistical Analysis
The statistical software SPSS for Windows (version 23; IBM Corporation, Armonk, NY, USA) was used for statistical analysis. Chi-square analysis was used to test the association between immunocytochemistry expression and clinicopathological parameters as categorical variables. A p-value of <0.05 was considered significant.
3. Results
A total of 115 cell block preparations with tumor cells from 107 patients were retrieved. The most common histological type of breast cancer in the cohort was invasive breast carcinoma, no special type (n = 77/107, 72.0%). Other included histological types were invasive lobular carcinoma, metaplastic carcinoma, neuroendocrine carcinoma, micropapillary carcinoma and mucinous carcinoma. The site of aspiration included lymph node (n = 89/115, 77.4%), chest wall (n = 14/115, 12.2%), lung (n = 7/115, 6.1%), soft tissue (n = 4/115, 3.8%) and the thyroid (n = 1/115, 0.9%) (Table 1).

Table 1.
Demographics of the cohort.
Staining could be assessed in 107 cases for GATA3, MMG and SOX10, in 102 cases for GCDFP15 and in 101 cases for ER. GATA3 showed the highest expression at all cut-offs, followed by MMG, GCDFP15 and SOX10 (Table 2). The proportion of tumor cell expression was also the greatest for GATA3 (mean = 76.6/100), followed by SOX10 (mean = 60.8/100), MMG (mean = 29.4/100) and GCDFP15 (mean = 20.4/100). As for ER negative cases, proportion of expression was high in GATA3 (mean = 87.2/100) and SOX10 (mean = 60.8/100) while low in MMG (mean = 27.5) and GCDFP15 (mean = 15.4) (Table 2).

Table 2.
The expression of GATA3, gross cystic disease fluid protein-15 (GCDFP15), mammaglobin (MMG) and SOX10 in aspirates of metastatic breast cancer.
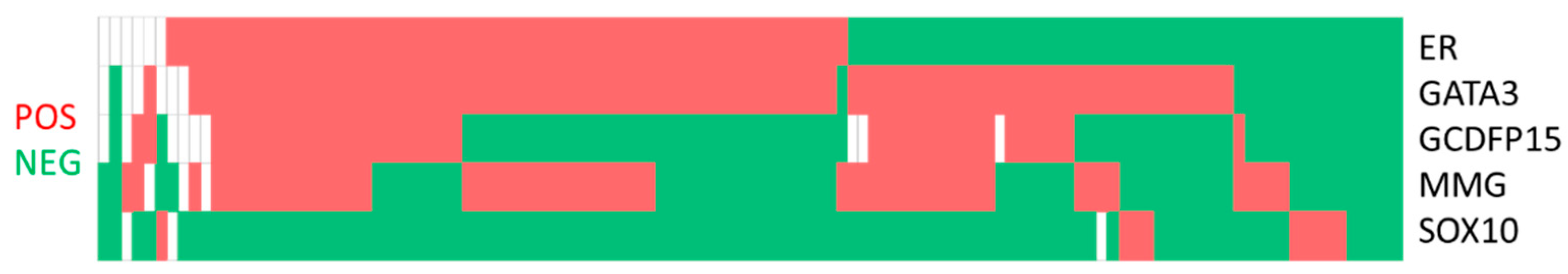
For co-expression patterns (Figure 1), the most common pattern was GATA3+/GCDFP15−/MMG−/SOX10−. Twenty-three, five and five cases only expressed GATA3, MMG and SOX10, respectively (Table 3). SOX10 expression was negatively associated with ER (p = 0.001), MMG (p = 0.001), GCDFP15 (p = 0.010) and GATA3 (p = 0.002) expression. In terms of positive correlations, GCDFP15 expression was associated with MMG (p = 0.012), and GATA3 expression was associated with ER (p < 0.001) and GCDFP15 (p = 0.002) (Table 4). When the comparison was repeated with the biomarker status of the corresponding primary breast cancer, a negative association between SOX10 and hormone markers (ER, p = 0.002, and PR, p = 0.020) and a positive association between GATA3 and hormone markers (ER, p < 0.001, and PR, p < 0.001) were demonstrated. GCDFP15 (p = 0.017) and MMG (p = 0.042) showed association with a high Ki67 score, and none of the immunostains was correlated with HER2 expression (Table 5). Comparing the ER expression of the cell block with the reference ER status in the reference biopsy or excision specimen, there were 83 (87.4%) concordant cases and 12 (12.6%) discordant cases. All the discordant cases were ER positive in the reference specimen and negative on cell block immunocytochemistry (Table 6).
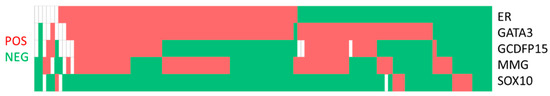
Figure 1.
Co-expression pattern of estrogen receptor (ER), GATA3, gross cystic disease fluid protein-15 (GCDFP15), mammaglobin (MMG) and SOX10 in metastatic breast cancer.

Table 3.
Co-expression pattern of GATA3, gross cystic disease fluid protein-15 (GCDFP15), mammaglobin (MMG) and SOX10 at cut-offs of (a) ≥1% (any intensity), (b) ≥5% (any intensity) and (c) ≥5% (moderate to strong intensity).

Table 4.
Comparison of co-expression pattern of estrogen receptor (ER), GATA3, gross cystic disease fluid protein-15 (GCDFP15), mammaglobin (MMG) and SOX10.

Table 5.
Comparison of estrogen receptor (ER), GATA3, gross cystic disease fluid protein-15 (GCDFP15), mammaglobin (MMG) and SOX10 expression with biomarker status.

Table 6.
Comparison of estrogen receptor (ER) expression in cell block material and reference biopsy or excision specimen.
4. Discussion
Breast cancer is one of the leading causes of global mortality, of which the majority of breast cancer-related deaths are attributable to metastatic disease [4]. Breast cancer is among one of the most common types of metastatic cancer [1,5]. FNA is minimally invasive with a very low risk of complications [6]. Due to these advantages, FNA is a favored modality for obtaining tissue diagnosis in clinically suspected metastatic lesions, particularly in lymph nodes and solid organs such as the lung [7]. It is not uncommon to encounter FNA specimens of metastatic breast cancers in routine clinical practice [8]. Although clinical correlation and cytomorphologic assessment are central to diagnosis of metastatic cancers, immunocytochemistry is performed for confirmation, or is even necessary for diagnosis when clinical information is incomplete [8]. For metastatic breast cancers, immunocytochemistry is often required, as late recurrences are not infrequent in breast cancers [9], and the histotypes and corresponding cytomorphology of breast cancers are diverse [10].
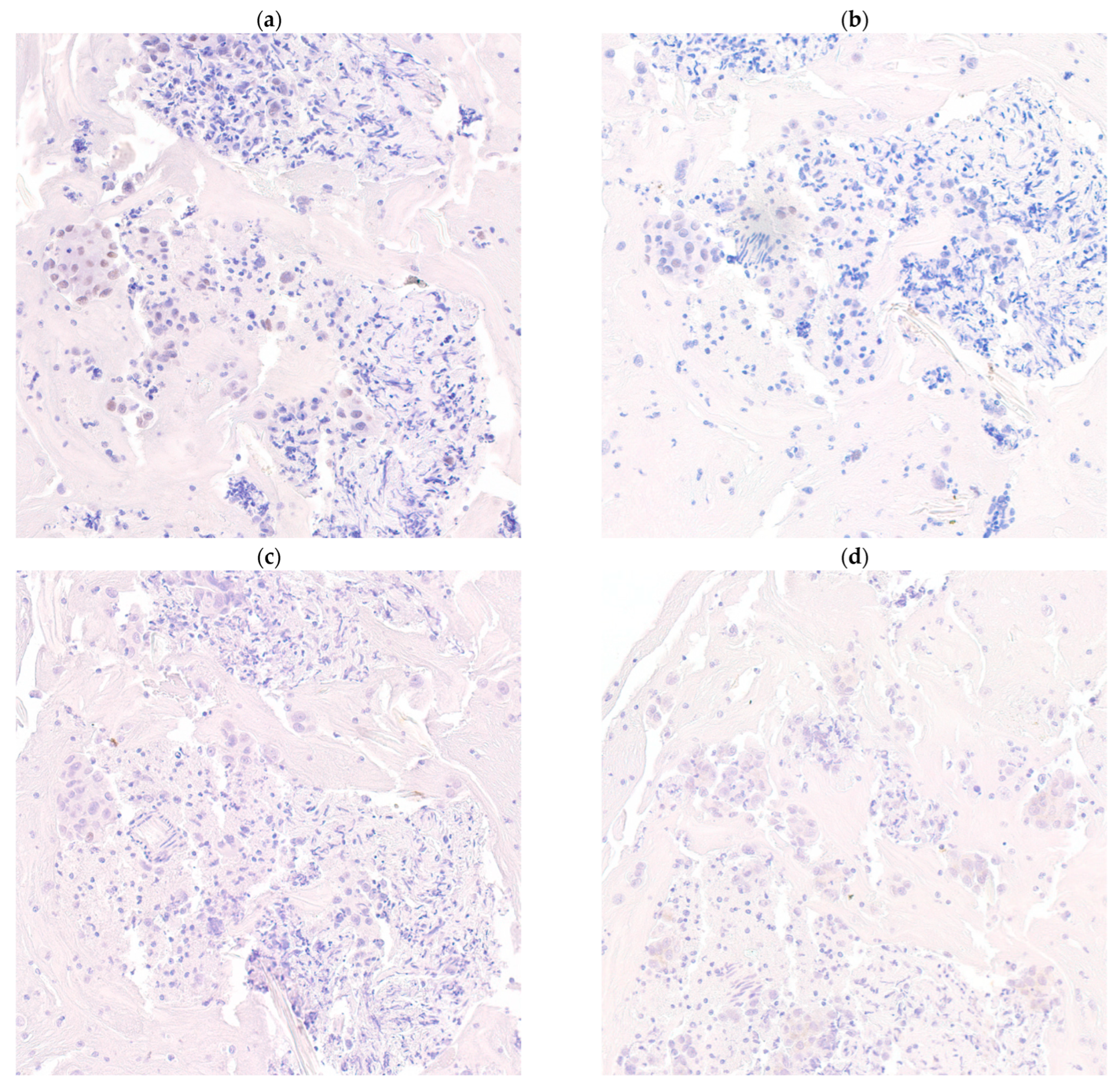
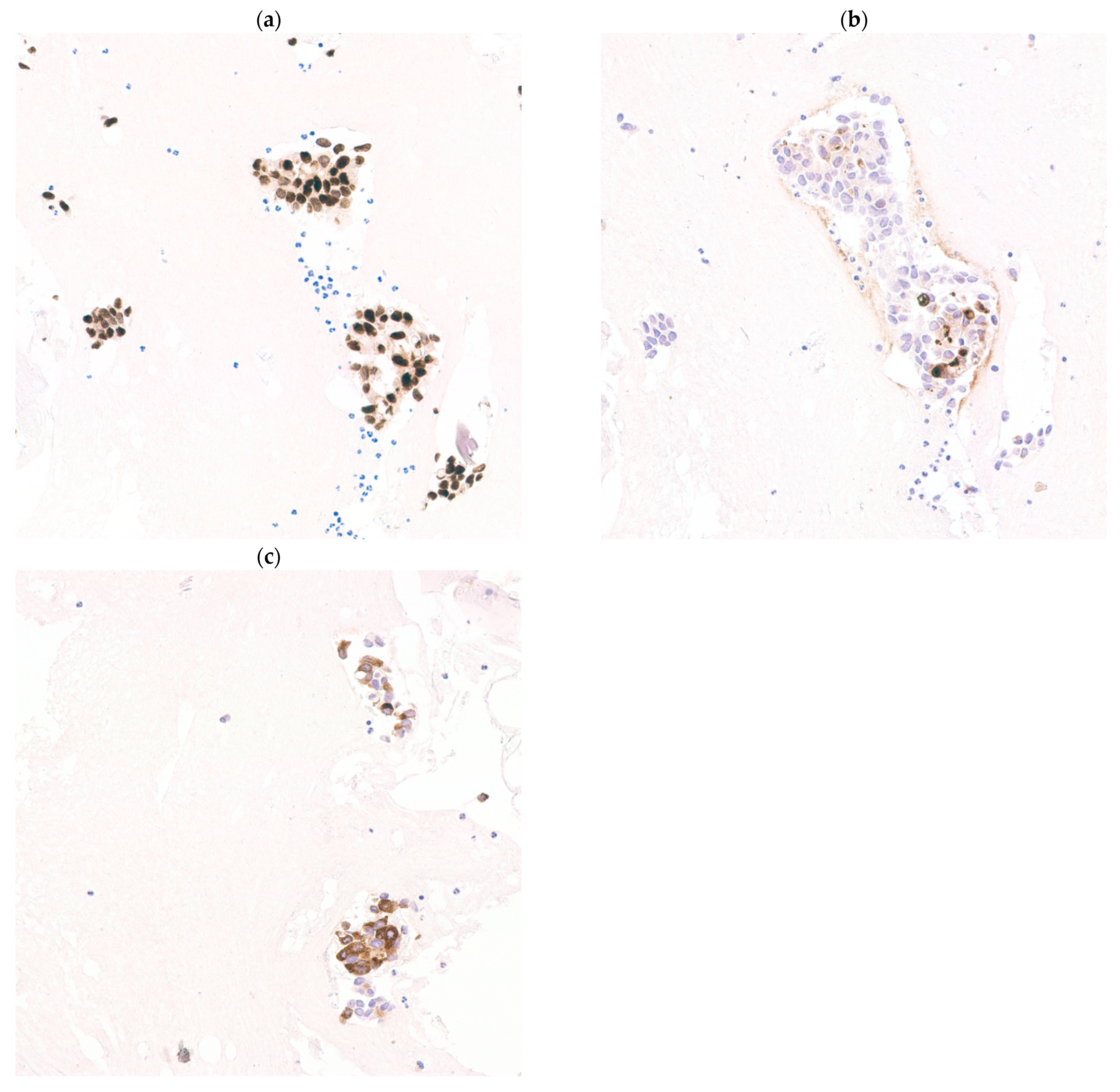
Breast-specific immunocytochemical stains that are commonly used include GATA3, GCDFP15 and MMG [11]. Hormone negative breast cancers display a different immunoprofile with SOX10 being a sensitive marker [12]. Similar associations have been demonstrated in this cohort, with SOX10 negatively correlated with expression of GATA3, GCDFP15, MMG and ER (p < 0.05) (Figure 2), whereas positive correlations between GATA3 and ER (p < 0.001), and also between MMG, GCDP15 and GATA3 (p < 0.05), were found (Figure 3). These markers have been extensively verified in formalin-fixed paraffin-embedded (FFPE) biopsy and excision tissue [13,14]. The current study investigates the performance of each single stain and combinations of these immunocytochemical stains in cell block material.
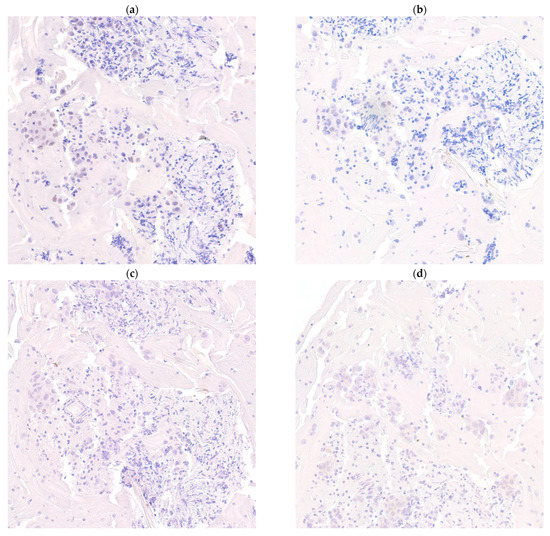
Figure 2.
SOX10 positivity is negatively correlated to GATA3, gross cystic disease fluid protein-15 (GCDFP15) and mammaglobin expression. An estrogen receptor metastatic breast cancer showing weak SOX10 expression (a) (SOX10, 200× magnification) while negative to GATA3 (b) (GATA3, 200× magnification), GCDFP15 (c) (GCDFP15, 200× magnification) and mammaglobin (d) (mammaglobin, 200× magnification).
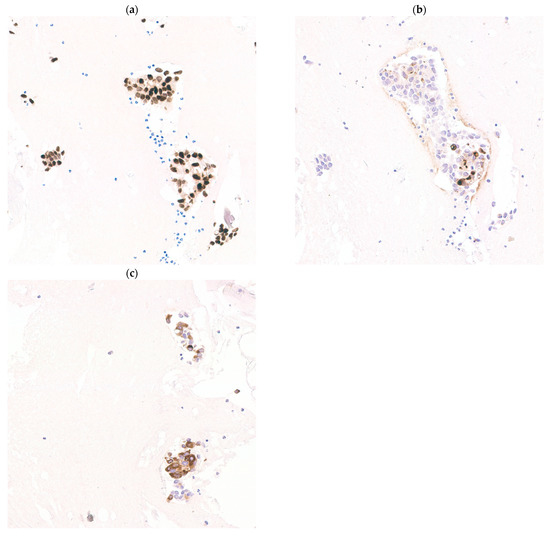
Figure 3.
Expressions of GATA3, gross cystic disease fluid protein-15 (GCDFP15) and mammaglobin are positively correlated. Metastatic breast cancer showing GATA3 (a) (GATA3, 200× magnification), GCDFP15 (b) (GCDFP15, 200× magnification) and mammaglobin (c) (mammaglobin, 200× magnification) co-expression.
GATA3 was positive in the greatest number of cases (Table 2), up to 91 out of 107 cases (85.0%) at a cut-off of ≥1% expression, and exceeded that of all other markers (GCDFP15, MMG and SOX10) in other cut-offs (Table 2). The proportion of tumor cell expression, in both estrogen positive and estrogen negative breast cancers, is also the highest for GATA3. In cases that were only positive to one immunostain, the most frequent positive immunostain was GATA3 (23 to 39, at different cut-offs). There were also cases positive only to MMG and SOX10 but the addition of GCDFP15 to all combinations of immunostaining does not increase detection of metastatic breast cancer. Depending on the cut-off for positivity, 5.9% (n = 6/102) to 15.7% (n = 16/102) of cases were negative to all immunostains (Table 2).
These findings suggest that GATA3 may be the most sensitive marker among GATA3, GCDFP15, MMG and SOX10. However, it has to be taken into consideration that GATA3 can be expressed in carcinomas of different primaries, including endometrial, salivary glandular and urothelial carcinomas [15]. MMG was demonstrated to be specific for breast carcinomas in FFPE material [16], and was positive in up to 25.8% (n = 8/31) of GATA3 negative breast cancers in this cohort (Table 3). GCDFP15 is known to be positive in a plethora of other human cancers [17], and data from this cohort suggest that GCDFP15 is less useful in complementing other immunostains. However, neither GATA3, GCDFP15, MMG nor SOX10 is entirely specific for breast cancers. Hence, the role of GCDFP15 in increasing specificity of an immunostain panel, or for resolving cases of conflicting/equivocal GATA3, MMG or SOX10 staining, cannot be disregarded.
The associations between GATA3 and SOX10 between the hormone status of the cell block preparation and reference biopsy or excision specimen were the same. As for HER2 and ki67, GCDFP15 and MMG were associated with a high (≥20%) ki67 index. Thirteen cases showed “loss” of ER expression in cell block preparation compared to the reference biopsy or excision specimen, which is explained by changes induced by endocrine therapy [18].
5. Conclusions
Immunocytochemistry is indispensable for assessment of metastatic cancers. For metastatic breast cancers, GATA3, GCDFP15, MMG and SOX10 are useful immunostains. In terms of a single marker, GATA3 is the most sensitive and showed the highest positive rate in both ER positive and negative breast cancers. The addition of MMG and SOX10 to GATA3 increases the sensitivity for detection of ER positive and ER negative breast cancers, respectively. However, GCDFP15 appears to be less useful for complementing other immunostains in terms of sensitivity for breast cancers. GATA3 expression is associated with hormone positivity whereas SOX10 is associated with a hormone negative status. These findings support the use of a combination of GATA3 and MMG for confirmation of a hormone positive breast cancer, and when a hormone negative breast cancer is suspected or when the hormone status is unknown, the addition of SOX10 is recommended.
Author Contributions
J.J.X.L.—conceptualization, data curation, investigation, methodology, formal analysis, writing—original draft; J.K.M.N.—investigation, methodology, formal analysis; C.H.C.L.—data curation, resources; C.-Y.T.—validation, visualization; J.Y.S.T.—formal analysis, resources, validation; G.M.T.—conceptualization, investigation, methodology, supervision, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
The authors have no funding to declare.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Joint Chinese University of Hong Kong—New Territories East Cluster Clinical Research Ethics Committee (reference number: 2020.289).
Informed Consent Statement
Patient consent was waived by the Joint Chinese University of Hong Kong—New Territories East Cluster Clinical Research Ethics Committee (reference number: 2020.289).
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article.
Conflicts of Interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
References
- Poon, I.K.; Chan, R.C.K.; Choi, J.S.H.; Ng, J.K.M.; Tang, K.T.; Wong, Y.Y.H.; Chan, K.P.; Yip, W.H.; Tse, G.M.; Li, J.J.X. A comparative study of diagnostic accuracy in 3026 pleural biopsies and matched pleural effusion cytology with clinical correlation. Cancer Med. 2022, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Tsang, J.Y.S.; Tse, G.M. Molecular Classification of Breast Cancer. Adv. Anat. Pathol. 2020, 27, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Allison, K.H.; Hammond, M.E.H.; Dowsett, M.; McKernin, S.E.; Carey, L.A.; Fitzgibbons, P.L.; Hayes, D.F.; Lakhani, S.R.; Chavez-MacGregor, M.; Perlmutter, J.; et al. Estrogen and Progesterone Receptor Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Guideline Update. Arch. Pathol. Lab. Med. 2020, 144, 545–563. [Google Scholar] [CrossRef]
- Riggio, A.I.; Varley, K.E.; Welm, A.L. The lingering mysteries of metastatic recurrence in breast cancer. Br. J. Cancer 2021, 124, 13–26. [Google Scholar] [CrossRef] [PubMed]
- López, F.; Rodrigo, J.P.; Silver, C.E.; Haigentz, M., Jr.; Bishop, J.A.; Strojan, P.; Hartl, D.M.; Bradley, P.J.; Mendenhall, W.M.; Suárez, C.; et al. Cervical lymph node metastases from remote primary tumor sites. Head Neck 2016, 38 (Suppl. S1), E2374–E2385. [Google Scholar] [CrossRef] [PubMed]
- Cappelli, C.; Pirola, I.; Agosti, B.; Tironi, A.; Gandossi, E.; Incardona, P.; Marini, F.; Guerini, A.; Castellano, M. Complications after fine-needle aspiration cytology: A retrospective study of 7449 consecutive thyroid nodules. Br. J. Oral Maxillofac. Surg. 2017, 55, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Jhala, D.; Wee, A.; Tse, G.; Baloch, Z. Fine-Needle Aspiration Cytology: An Advancing Horizon. Pathol. Res. Int. 2011, 2011, 281930. [Google Scholar] [CrossRef][Green Version]
- Chan, R.C.K.; Lee, A.L.S.; To, C.C.K.; Cheung, T.L.H.; Ho, C.T.; Choi, J.S.H.; Li, J.J.X. The role of cytokeratin 7/20 coordination revisited-Machine learning identifies improved interpretative algorithms for cell block immunohistochemistry in aspirates of metastatic carcinoma. Cancer Cytopathol. 2022, 130, 455–468. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, R.N.; Esen, B.Ö.; Mellemkjær, L.; Christiansen, P.; Ejlertsen, B.; Lash, T.L.; Nørgaard, M.; Cronin-Fenton, D. The Incidence of Breast Cancer Recurrence 10-32 Years After Primary Diagnosis. JNCI J. Natl. Cancer Inst. 2021, 114, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Weigelt, B.; Geyer, F.C.; Reis-Filho, J.S. Histological types of breast cancer: How special are they? Mol. Oncol. 2010, 4, 192–208. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.X.; Tse, G.M. Marker assessments in ER-positive breast cancers: Old markers, new applications? Histopathology 2022. [Google Scholar] [CrossRef] [PubMed]
- Jamidi, S.K.; Hu, J.; Aphivatanasiri, C.; Tsang, J.Y.; Poon, I.K.; Li, J.J.; Chan, S.K.; Cheung, S.Y.; Tse, G.M. Sry-related high-mobility-group/HMG box 10 (SOX10) as a sensitive marker for triple-negative breast cancer. Histopathology 2020, 77, 936–948. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.B.; Tsang, J.Y.S.; Shao, M.M.; Chan, S.K.; Cheung, S.Y.; Tong, J.; To, K.F.; Tse, G.M. GATA-3 is superior to GCDFP-15 and mammaglobin to identify primary and metastatic breast cancer. Breast Cancer Res. Treat. 2018, 169, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Yoon, E.C.; Wang, G.; Parkinson, B.; Huo, L.; Peng, Y.; Wang, J.; Salisbury, T.; Wu, Y.; Chen, H.; Albarracin, C.T.; et al. TRPS1, GATA3, and SOX10 expression in triple-negative breast carcinoma. Hum. Pathol. 2022, 125, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Aphivatanasiri, C.; Li, J.; Chan, R.; Jamidi, S.K.; Tsang, J.Y.; Poon, I.K.; Shao, Y.; Tong, J.; To, K.-F.; Chan, S.-K.; et al. Combined SOX10 GATA3 is most sensitive in detecting primary and metastatic breast cancers: A comparative study of breast markers in multiple tumors. Breast Cancer Res. Treat. 2020, 184, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, E.; Tsunoda, N.; Hatanaka, Y.; Mori, N.; Iwata, H.; Yatabe, Y. Breast-specific expression of MGB1/mammaglobin: An examination of 480 tumors from various organs and clinicopathological analysis of MGB1-positive breast cancers. Mod. Pathol. 2007, 20, 208–214. [Google Scholar] [CrossRef]
- Wick, M.R.; Lillemoe, T.J.; Copland, G.T.; Swanson, P.E.; Manivel, J.C.; Kiang, D.T. Gross cystic disease fluid protein-15 as a marker for breast cancer: Immunohistochemical analysis of 690 human neoplasms and comparison with alpha-lactalbumin. Hum. Pathol. 1989, 20, 281–287. [Google Scholar] [CrossRef]
- Kuukasjärvi, T.; Kononen, J.; Helin, H.; Holli, K.; Isola, J. Loss of estrogen receptor in recurrent breast cancer is associated with poor response to endocrine therapy. J. Clin. Oncol. 1996, 14, 2584–2589. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).