Abstract
Members of the Cinnamomum genus have been utilized for medicinal treatment for millennia. In recent years, particular attention has been given to the bioactive metabolites involved in the medicinal properties of natural products and their extracts. Cinnamon is particularly interesting due to the presence of both terpenoid and polyphenol moieties, both of which have been extensively studied for their medicinal applications in the treatment of a wide range of conditions, from bacterial infection, obesity and diabetes to cancer and cardiovascular pathologies. Here, we reviewed some of the properties of cinnamon and its derivatives cinnamic acid, trans-cinnamaldehyde and beta-caryophyllene. In addition, recent advancements in the application of cinnamon and its derivatives in cancer, particularly focusing on gynecological and breast cancers, which present unique challenges to treatment due to late diagnosis, have been discussed. Current advancements to further enhance the delivery of cinnamon and its derivatives through nanoencapsulation and nanoparticulate strategies as well as the development of novel conjugates and hybrids are also discussed. Additionally, the use of cinnamon and its derivatives as adjuvants with chemotherapeutics that can work synergistically was also touched upon. Overall, biotechnological innovations have enhanced the delivery of natural products such as cinnamon and its derivatives and may pave the path for novel therapeutic strategies with fewer side effects and higher potency. Cinnamon represents a valuable source of developing novel anticancer materials that warrant additional research for development as potential interventions or combination treatments.
1. Introduction
Natural products have been considered an enormous resource for medicinal purposes for centuries [1]. In particular, small molecules derived from natural sources present a veritable horde of bioactive compounds, many of which have diverse biological roles in their native ecosystems. While the original extracts themselves are effective, the secondary metabolites produced by these organisms, in some cases, are more effective than the precursors [2]. Many of the metabolites from terrestrial and marine organisms have been found to display antimicrobial, immunosuppressive, antitumor or antiherbivore roles in nature and present a phenomenal resource for the discovery of novel bioactive materials. For example, polycyclic polyethers, such as bryostatin-1 derived from the marine species Bugula neritina, are undergoing clinical trials as an anticancer drug [3]. It has also been shown that certain naturally derived extracts, such as those from mesopelagic species like lanternfish Myctophum punctatum and the Mediterranean krill Meganyctiphanes norvegica, exhibit both antimicrobial and anticancer properties [4,5], leading to the advent of the target hopping approach for drug discovery. It has been reported that such dual targeting is likely a result of shared mechanisms and targeting features. For example, many antibiotics function through preferential topoisomerase inhibition in bacterial cells, and this mechanism can be carried over to tumor cells, leading to antineoplastic antibiotics [6]. In addition, the shared negative charge of bacterial cell membranes and that of mammalian cells due to phospholipid components has led to the development of positively charged cell-penetrating peptides that have dual antimicrobial and anticancer properties [7]. For example, the cell-penetrating antitumor TAT-RasGAP317-326 peptide was recently shown to demonstrate potent antimicrobial activity [8]. Such peptides derived from natural species have garnered major traction and are being developed for a wide array of novel approaches for targeted tumor treatments [9].
Among natural products, terpenoids and polyphenolic flavonoids are of particular interest due to their anticancer properties. Polyphenols and their derivatives are secondary metabolites with multiple phenolic groups, which have been extensively characterized and explored for the development of anticancer drugs [10,11,12,13,14,15,16]. These include coumarins, ellagitannins, hesperetin, rosmarinic acid and apigenin, to name a few. In addition to antitumor activity, many display potent antioxidant and anti-inflammatory activity. The mechanisms implicated in anticancer activity involve signaling pathway modifications, such as p53 phosphorylation and the inhibition of MMP-2, an enzyme involved in angiogenesis and metastasis, as well as cell cycle interference and apoptosis induction [17,18,19,20,21,22], in addition to antioxidant activity [23,24,25,26]. It is theorized that apoptotic properties result from the inhibition of NF-kB and activator protein (AP-1) [27,28]. Due to the presence of multiple hydroxyl groups, polyphenols are able to act as hydrogen donors in the quenching of free radicals, causing them to display anti-inflammatory and antioxidant properties [29]. Polyphenols are particularly attractive due to their relative biocompatibility and safety compared to synthetic chemotherapeutics [30,31].
Over the years, researchers have also been exploring the biochemical effects of terpenoids as similar benefits as those of polyphenols have been observed, and thus, terpenoids encompass a promising area of current and future research. Terpenoids are a class of secondary metabolites derived from plants and marine organisms that contain multiple isoprene units with a wide range of pharmacological activities [32,33]. Several terpenoids are also able to act as free radical scavengers yielding significant antioxidant and anti-inflammatory properties that enhance their activity against several neuropathological diseases as well as cancers [34,35]. The radical scavenging activity of several terpenes has often been examined using the reagent 1,1-diphenyl-2-picrylhydrazyl-2,2-diphenyl-1-picrylhydrazyl (DPPH), where the antioxidant activity is attributed to the presence of conjugated π bonds that lead to chain cleavage in terpenes [36].
In the realm of cancer, terpenoids have shown effectiveness against numerous cancer cell lines, from early-stage tumorigenesis suppression to cancer cell differentiation interference and apoptotic activation among cancer cells. Specifically, for disproportionately late-diagnosed cancers such as ovarian cancer, of note is the ability of terpenoids such as sesquiterpene lactones, as well as thymoquinone and artesunate, which have been shown to inhibit angiogenesis and metastasis [37,38,39].
Among various secondary metabolites, plant-derived cinnamon and clove oil stand out due to their extensive array of both polyphenolic and terpenoid components, leading to their remarkable range of proposed medicinal applications [40,41,42]. Both clove and cinnamon oil and their extracts have been shown to present significant anti-inflammatory, antimicrobial and anticancer properties [43,44,45,46,47]. Over the years, cinnamon and its derivatives have been used for a diverse range of medicinal applications [48,49,50,51,52,53,54,55,56]. Due to the pressing need for novel treatments for cancers, there has been increasing interest in the potential of cinnamon extracts and their metabolites for developing naturally derived therapeutics for combating cancer [57]. This rapidly expanding field of study is highly promising and aims to develop innovative treatments that leverage a variety of cinnamon-derived bioactive metabolites as a relatively untapped source of anticancer therapeutics.
2. Cinnamon Extract as an Anticancer Intervention in Breast and Gynecologic Cancers
Of the various types of cancers, gynecological cancers as a group are disproportionately diagnosed late and are often complicated due to hormonal involvement (in some cases), making them difficult to treat and generating a pressing need for novel interventions. Additionally, while breast cancer does not exclusively occur in the female populace (male cases make up less than 1% of annual diagnoses), in general, breast cancers are also classified as a primarily female pathology that is subject to many of the same challenges as gynecological carcinomas [58]. Due to their multifaceted anticancer properties, cinnamon extracts and their metabolites are a uniquely intriguing frontier for potential therapies and adjuvants in the treatment of such cancers. Additionally, breast and ovarian cancers are estimated to lead the incidence of new cases in the world [59]. In this review, we have focused on current studies being carried out to investigate the efficacy of cinnamon and its derivatives, including novel hybrid conjugates and nanotherapeutics that are being developed for potential treatment of breast, ovarian and cervical cancers. In addition to tumors, cinnamon and its extracts have also been shown to clinically improve symptoms related to polycystic ovary syndrome (PCOS) by enhancing the antioxidant levels in the serum, as well as lowering LDL cholesterol levels [60]. Several investigators aim to leverage whole cinnamon extract (CE) or cinnamon essential oil (CEO) in targeting cancer. This approach allows for the synergistic application of multiple metabolites while also minimizing the degree of processing required to achieve anticancer effects. However, the molecular mechanism of action is more difficult to glean due to the lack of clarity with respect to the active components involved in treatment.
There have been numerous investigations aiming to elucidate the anticancer ability of cinnamon extracts. For example, in vitro experiments exploring the impacts of CE on the proliferation of tumor cells have demonstrated IC50 values < 1 µg/mL for both non-Hodgkin lymphoma and HeLa cervical cancer cell lines [61], a highly promising result that is comparable to market chemotherapeutics such as Doxorubicin [62] and Topotecan [63]. This is particularly of note, as cervical cancer is the fourth leading cause of cancer death in women, presenting unique challenges as a gynecological carcinoma [64] and driving the need for novel interventions. In another study, Kaul-Ghanekar and colleagues also showed the antiproliferative activity of CE against cervical cancer, utilizing the SiHA cell line [65]. Furthermore, they also showed that CE impeded cell migration and thus may have potential antimetastatic benefits. Interestingly, this study identified the loss of mitochondrial membrane potential as the key mechanism, as evaluated using a JC-1 fluorescence assay. These results show promise, particularly because CE was shown to be active against both HeLa cells (originating from adenocarcinoma) and SiHa cells (which originate from squamous cell carcinoma) [66].
In addition to cervical cancer, breast cancer is the most common cancer globally, making it another pathology with a high need for novel innovations [67]. In a study conducted by Husain and co-workers, ethanolic extracts of cinnamon zeylancium were shown to display IC50 values of 25 µg/mL against MDA-MB-231 triple-negative breast (TNB) cancer cells. Specifically, the authors quantified the proapoptotic properties of CE through DNA fragmentation, identifying the degree of fragmented DNA as an indicator of apoptosis. These authors established differential cytotoxicity against breast cancer to the exclusion of kidney epithelial non-cancer cells [68]. This is particularly promising given that TNBC is one of the most complex types of cancers that have very few treatments available, particularly when detected at a late stage [69].
To further explore the activity of CE, Makinoshima and colleagues confirmed the antimetastatic properties of CE in zebrafish embryos, complementing earlier works and further characterizing the potential of CE [70]. Makinoshima leveraged epiboly inhibition in zebrafish embryos as an indicator for antimetastatic properties, an interesting method that takes advantage of the similarity in the mechanism of embryo gastrulation and cancer metastasis [71]. Interestingly, this work further characterized a mechanism of antimetastatic activity of CE in MDA-MB-231 TNBC cells. The authors found that glycolytic metabolism interruption played a role in antimetastatic activity. To establish this, they utilized an extracellular acidification rate (ECAR) experiment to quantify glycolytic activity, in tandem with GC–MS measurements of glucose levels over time. This two-pronged approach provided a method for validation and confirmed the comparative impacts of CE on the rates of glycolysis. After identifying the inhibition of glycolysis by CE in cancer cells, it was shown that hexokinase 2 expression was lowered upon CE treatment, implicating this enzyme in the observed glycolysis inhibition. Finally, the authors were able to emulate the observed antimetastatic properties of CE using a targeted hexokinase 2 inhibitor, thereby confirming that the mechanism of CE was the inhibition of this enzyme and, thus, glycolysis.
Interestingly, in addition to TNBC, studies have also shown that CE extracts derived from cassia bark are potent against the estrogen-positive breast cancer cell line (MCF-7). Mechanistically it was found that CE successfully inhibited the activity of caspase-9 through stimulation of AKT1 in MCF-7 cells; however, this was not seen in MDA-MB-231 cells [72]. This study is indicative that the mechanism of activity of CE varies broadly depending on the type of tumor cell line within the realm of breast cancer. In another study conducted, CE was shown to reduce the proliferation of MCF-7 cancer cells through apoptosis. Specifically, the authors demonstrated the upregulation of pro-apoptotic genes such as BAX, BAD and BIM, while also downregulating antiapoptotic genes, including (Bcl-2 and Bcl-Xl) [73].
One of the hallmarks of metastasis is the circulation of tumor cells and uncontrolled angiogenesis, among many other traits [74]. Thus, the antiangiogenic ability of CE has also been explored in breast cancer. In 2009, Wen and co-workers showed that CE displayed antiangiogenic activity through the inhibition of Vascular Endothelial Factor Receptor 2 (VEGFR2), a potent tyrosine kinase crucial to angiogenesis [75,76]. The authors established this inhibitory activity using ELISA. More recently, in 2022, the group further characterized the antiangiogenic properties of CE in MDA-MB-231 breast cancer cell lines as well as SKOV3 ovarian cancer cells, with highly promising and informative results [77]. In addition to validating VEGF expression inhibition by CE, the authors demonstrated the foundational mechanism of this effect. They demonstrated that CE suppressed STAT3 and AKT expression and phosphorylation, thereby suppressing HIF-1α, which in turn inhibited angiogenesis. The authors elucidated the mechanism of inhibition by quantifying the expression of HIF-1α (a positive regulator of VEGF expression) through Western blotting, which showed a decrease in HIF-1α expression. This implicates either direct gene suppression, protein degradation, or translation inhibition as a likely mechanism of the antiangiogenic activity of CE. By systematically inhibiting each of these processes, the authors were able to specify that CE inhibits HIF-1α protein synthesis as well as the transcription of the associated gene.
Taken together, these investigations provide an overview of the potential of cinnamon extracts against both TNBC and estrogen-positive breast cancer as well as other types of women-centric cancers. In addition to in vitro studies with CE, Kubataka and colleagues evaluated the impact of dietary cinnamon powder on mouse and rat models of breast carcinoma [78]. Their work revealed that cinnamon reduced the incidence and improved the prognosis of mammary carcinomas in rodent models. In particular, the authors elucidated the effect of dietary cinnamon on the Bax/Bcl-2/caspase-3 signaling pathway, observing an increase in the Bax/Bcl-2 ratio, leading to an increase in caspase-3 expression and thereby inducing apoptosis in tumor cells [79]. The authors also postulated that it is a likely mechanism of the observed mitochondria-induced apoptosis in rodent tumor models. The group additionally observed these pathway effects and proapoptotic impacts in vitro in both MCF-7 and MDA-MB-231 breast cancer cells using flow cytometry. Additionally, the authors confirmed the decrease in VEGF expression in vivo as found, with implications for antiangiogenic properties in vivo.
This body of research provides robust support for a multifaceted mechanism of action for cinnamon against breast cancer, as well as the potential to treat other cancers.
3. Cinnamon Metabolites in Gynecological and Breast Carcinomas
In addition to investigations of the holistic activity of cinnamon extract, several investigations have focused on particular metabolites derived from cinnamon. Investigating individual metabolites allows for greater precision and is more directly transferable to clinical applications but it lacks the possible synergistic effects of complementary natural products in whole extracts and oils. While the precise composition of CEO varies among Cinnamomum species and is not consistent in the literature, the main characteristic components of CEO have been found to be trans-cinnamaldehyde, cinnamic acid, and beta-caryophyllene oxide [80,81,82,83] among others. While other molecules such as eugenol have also been identified in CEO, these three molecules encompass the significant constituents of CEO and contribute to its unique bioactive properties.
3.1. Trans-cinnamaldehyde (CAL)
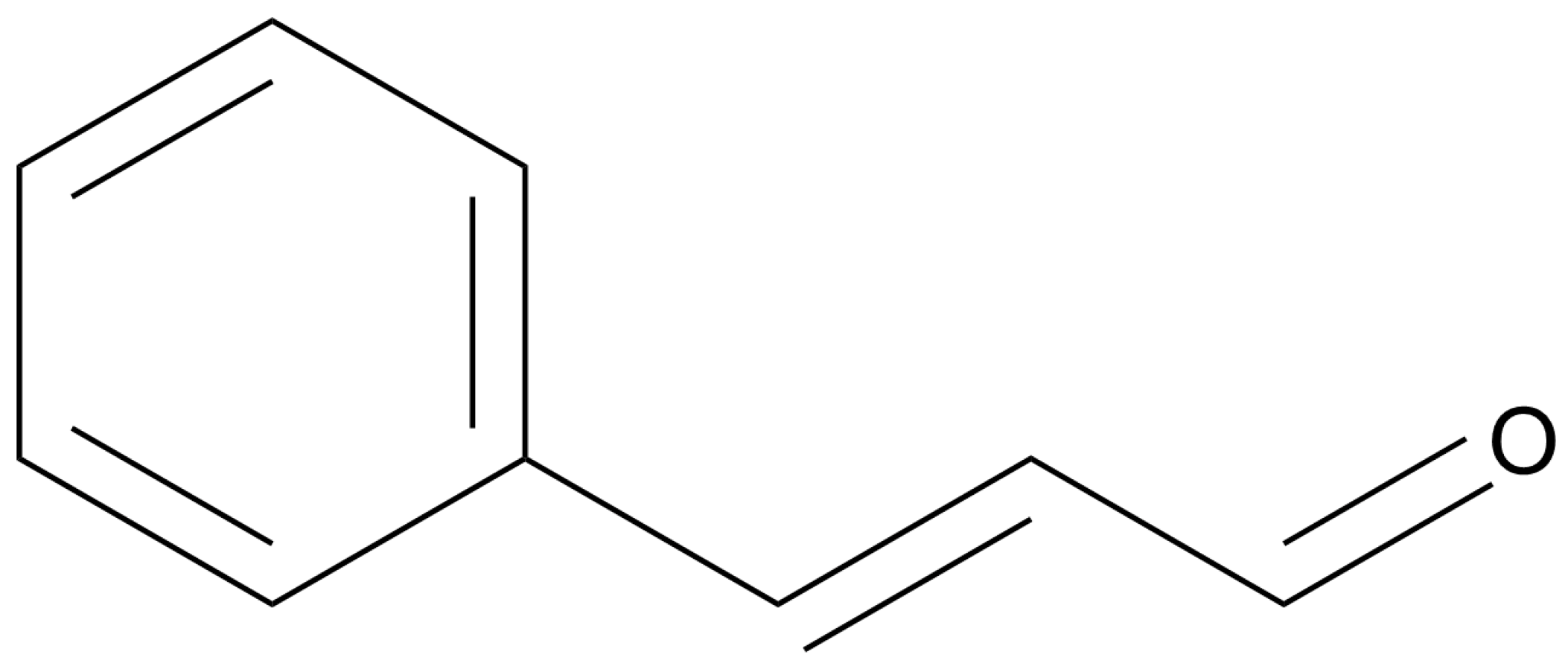
Of the metabolites isolated from cinnamon, trans-cinnamaldehyde (CAL) (Figure 1), is by far the most characterized and studied. CAL has noted antimicrobial, anti-inflammatory and anticancer properties [84], making it a highly interesting candidate for clinical applications. CAL is the major component of CEO [85]. Studies related to CAL against gynecological and breast cancers have been reported in an array of recent works. Multiple cytotoxicity evaluations have been conducted against estrogen-positive MCF-7 breast cancer cells, showing cytotoxicity and growth inhibition [86,87]. For example, Liu and colleagues found that CAL shows transcriptional interference abilities against 59 different target biochemical pathways involved in breast cancer [88]. In their investigation, the authors confirmed the cytotoxicity of CAL against the TNBC cell line MDA-MB-231, with an IC50 value of 16.9 µg/mL. Additionally, they confirmed specific proapoptotic activity using flow cytometry and evaluated the inhibition of cell migration. Furthermore, their computational work revealed the most likely molecular targets for the anticancer activity of various cinnamon components, with an emphasis on CAL. They used the traditional Chinese medicine system pharmacology database and analysis platform (TCMSP) to identify targets of CAL and other cinnamon metabolites. Subsequently, the authors limited the targets to those related to breast cancer to identify the most important cellular pathways. Following statistical analysis, the twenty most important pathways were listed. Pursuant to the previously discussed works, the PI3K/AKT pathway was found to be one of the most crucial in this analysis. The cAMP pathway, NF-kappa B pathway and HIF-1 pathway were also among the top five pathways. These pathways align closely with analyses for CE and CEO, emphasizing the significant role of CAL in the bioactive properties of CE and CEO. Taken together with strong cytotoxicity, it is clear that CAL acts as an antimetastatic and antiproliferative agent against breast cancer cells by interfering in multiple carcinogenic pathways, likely with a synergistic impact.
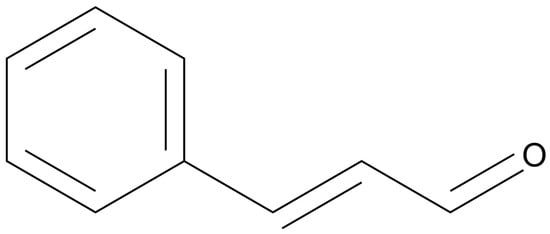
Figure 1.
Chemical structure of trans-cinnamaldehyde (drawn using ChemDraw 23.1.1).
In addition to breast cancer, CAL also shows efficacy against ovarian cancer. For example, CAL was shown to inhibit cell proliferation and migration in both SKOV3 and A2780 cell lines in a recent work by Yue Wang and colleagues [89]. This is particularly significant as A2780 and SKOV3 cell lines are non-serous epithelial carcinomas [90]. Furthermore, non-serous carcinoma cells have a higher migratory ability and drug resistance to traditional chemotherapeutic drugs such as cisplatin, Adriamycin and Paclitaxel [91,92]. It was shown that CAL successfully reversed epidermal growth factor (EGF)-induced epithelial/mesenchymal transition in both cell lines in vitro. Of particular note was the decreased phosphorylation of the PI3K and AKT with the resulting inhibition of the PI3K/AKT pathway. This corresponds well with the computational work conducted by Liu and coworkers for breast cancer, in which this pathway was also implicated. In addition, caspase-3 cleavage was observed following CAL treatment, implicating the associated pathway in proapoptotic effects. This pathway was also identified for CE and CEO, once again confirming the potential role of CAL in CE and CEO bioactivity. In addition to in vitro studies, the authors further confirmed the anticancer activity in vivo using mouse xenograft tumor models. Pursuant to in vitro data, decreased metastasis and decreasing tumor size were observed with CAL treatment. Overall, this work demonstrates the anticancer activity and mechanism of CAL in ovarian cancer. The potential of CAL in ovarian cancer treatment in vivo has been further established by Penchalaneni and co-workers, who established a clinically relevant dosage of CAL to re-establish hormonal equilibrium in ovarian cancer in rats [93]. Hormonal factors play a major role in ovarian carcinogenesis and treatment failure, making this finding especially relevant and addressing a major source of difficulty in modern ovarian cancer treatment. The authors were able to identify effective dosages for CAL in mouse models, a vital step toward clinical trials for this intervention.
Thus, in both breast and ovarian cancers, CAL shows high potential as a novel cancer therapeutic. In isolation, many of the same pathways implicated in CE and CEO treatments are shown to be impacted. By isolating this active compound, consistency and replicability are enhanced across the extracts.
3.2. Trans-Cinnamic Acid
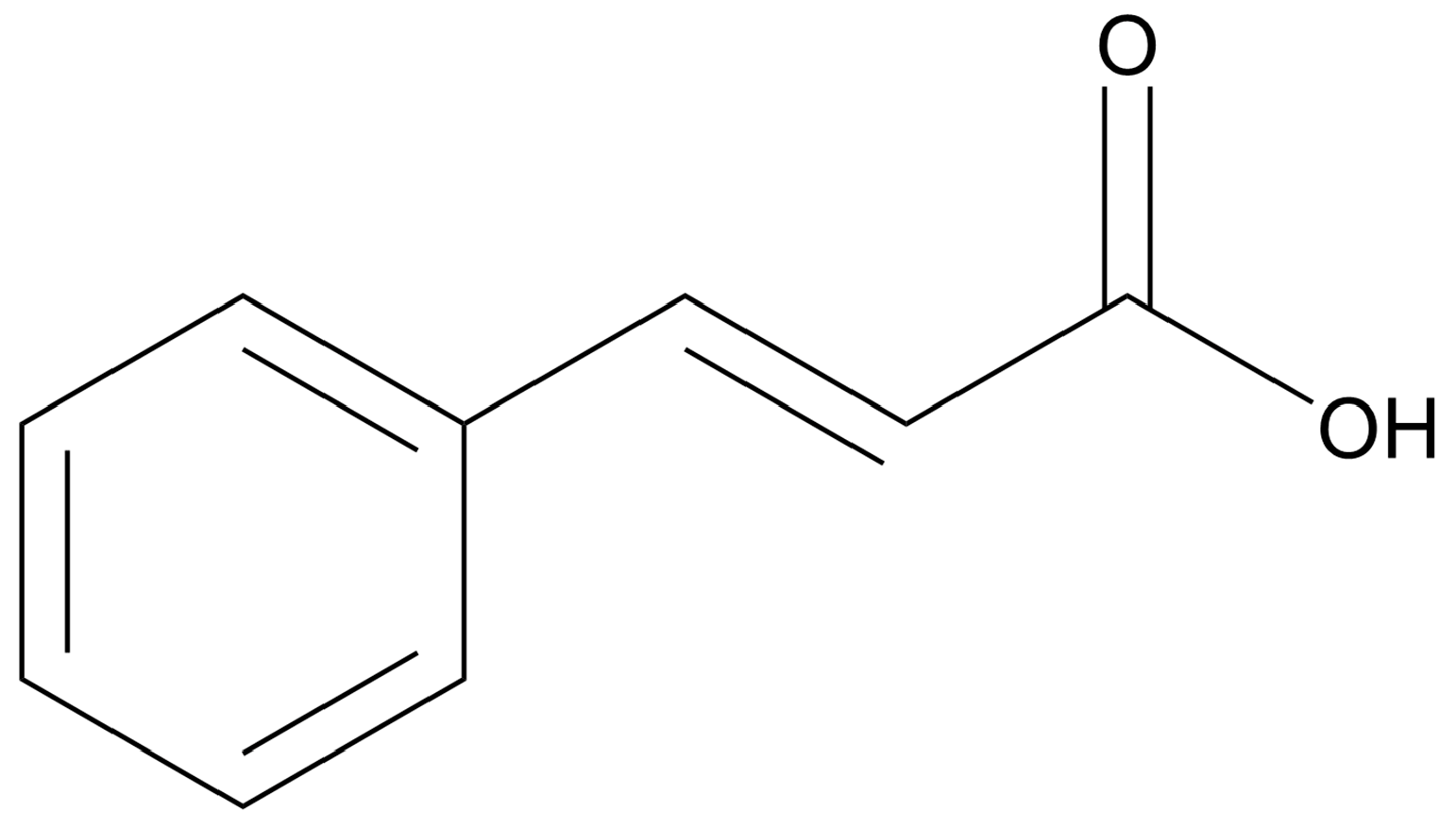
Trans-cinnamic acid (CA) (Figure 2), also known as 2-phenyl-2-propenoic acid, comprises a carboxylic acid side chain (instead of an aldehyde, as seen in CAL) formed biosynthetically. Like CAL, CA has also been well characterized for its antioxidant, antimicrobial and anticancer properties [94]. CA in particular has gained importance due to its activity against breast cancer. In 2021, Pal and colleagues examined its cytotoxicity against the MDA-MB-231 breast cancer cell line, with the results indicating selective cytotoxicity against the cancer cells compared to non-cancer fibroblasts [95].
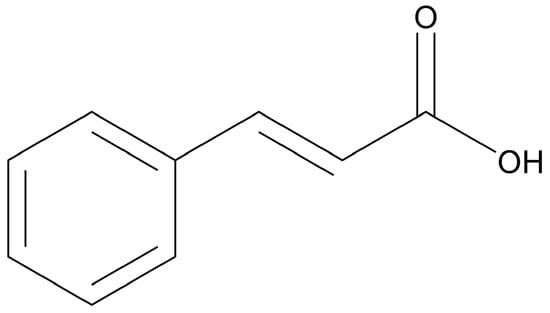
Figure 2.
Chemical structure of trans-cinnamic acid (drawn using ChemDraw 23.1.1).
Subsequently, they characterized DNA damage using Comet analysis. The stages of apoptosis were quantified using flow cytometry, confirming the proapoptotic effects of CA. Immunoblotting studies revealed an increased expression of Tumor Necrosis Factor A (TNFA) and TNF Receptor 1 (TNFR1), both implicated in necrosis induction. In addition, increased levels of caspase-3 and caspase-8 were observed, confirming apoptosis induction upon treatment. The authors concluded that the upregulation of the TNFα/TNF1 mediated apoptotic pathway is likely a key mechanism of action for the proapoptotic properties of CA. In a separate study, Hunke and co-workers demonstrated the anticancer activity of CA against MCF-7 breast cancer cells as well. A unique aspect of their study was that they also probed the activity of CA dimer molecules [96]. Cytotoxicity against both MCF-7 and MDA-MB-231 breast cancer cells was established, and the authors observed improved cytotoxicity for 3-fluoro cinnamic acid dimer and the 3,4-difluoro cinnamic acid dimer over the corresponding monomeric CA. Of those, the 3-fluoro cinnamic acid dimer was found to be the most potent and induced apoptosis and cell cycle arrest. This is an interesting study that demonstrates the potential of developing dimers of cinnamic acid and its derivatives for possible enhancement in anti-cancer activity against breast cancer.
In addition to breast cancer, the potential of CA to target ovarian cancer was investigated by Huang and colleagues in a recent study utilizing the network pharmacology approach [97]. This computational method utilizes databases such as the Comparative Toxicogenomics Database and SwissTarget Prediction to identify optimal molecular targets of the therapeutic being studied for the disease being investigated. In this case, the overlap between ovarian cancer-associated targets and optimal CA targets was analyzed to identify ovarian cancer targets that could be susceptible to CA. The major identified targets were cytochrome P450 proteins, catalase and caspase-3. In addition, the authors quantified the cytotoxicity of CA against four ovarian cancer cell lines (H08910, A2780, OVCAR3 and SKOV-3) using a Cell Counting Kit-8 assay (CCK-8), yielding IC50 values consistently below 10 µmol/mL from 12 to 72 h after treatment. These findings are promising and implicate the antiproliferative effects of CA for ovarian cancer cells through interference with metabolism modulation and immune microenvironment regulation pathways. However, further validation studies are needed to elucidate the particular pathways involved, as well as any antimetastatic or antiangiogenic properties of CA.
In addition to trans-cinnamic acid, its dihydroxy derivative (3,4-dihydroxy cinnamic acid, caffeic acid) has also shown promising results against HeLa cervical cancer cells due to its oxidative properties in tumor cells that cause DNA damage and trigger a cascade of apoptotic pathways. Furthermore, it has also been shown to lead to an increase in lipid peroxidative markers in HeLa cells [98]. Caffeic acid has also been functionalized with long-chain ester groups to enhance its potency. For example, the tri-hydroxycinnamic acid decyl ester developed by Imai and co-workers was found to be more potent compared to cinnamic acid against both the MCF-7 breast cancer cell line and PC3 prostate cancer cells through cell cycle arrest as a result of apoptosis [99]. Another derivative of caffeic acid, caffeic acid phenethyl ester (also known as CAPE), has been shown to be highly effective against SKOV-3 ovarian tumor cells both in vivo and in vitro. The work conducted by Liu and co-workers [100] showed that CAPE not only inhibited SKOV-3 cell proliferation, migration and invasion but also reduced the expression of ki67 and PCNA in mouse models. Additionally, CAPE also repressed the NF/κB pathway along with NF-κB-p65 DNA binding activity. These results are promising and may provide a new strategy for developing therapeutics against ovarian tumors.
3.3. Caryophyllenes
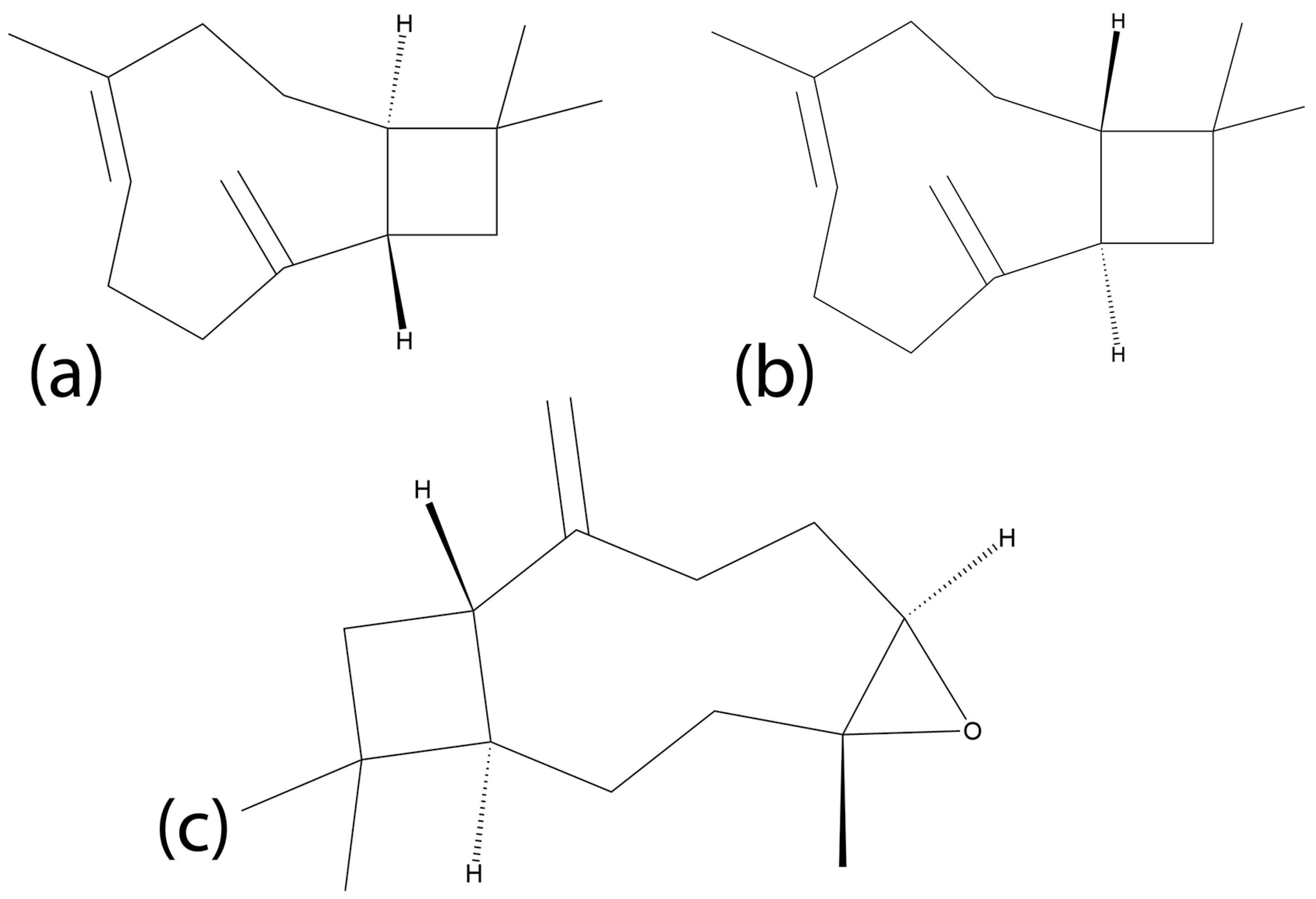
Beta-caryophyllene oxide (BCPO) has been identified as a minor component of certain CEOs [101,102]. This compound is of particular interest due to its unique targeting mechanism compared to other cinnamon metabolites, particularly involving cannabinoid receptors. BCPO and its reduced form, trans-β-caryophyllene (BCP), are sesquiterpenes that have recently been investigated for anticancer properties in various cancers. Sesquiterpenes are a remarkably wide family of natural products consisting of a 15-atom backbone, often with a cyclic moiety [103] generating an enormous diversity of structures (Figure 3) and biological properties. BCPO has been found in plants in a diverse range of families outside of Lauraceae, such as Cannabaceae and Myrtaceae, among others [104]. While BCPO belongs to the cannabinoid family, it has selective activity as an agonist of Cannabinoid receptor 2 (CBR-2) to the exclusion of Cannabinoid receptor 1 (CBR-1), preventing any psychoactive properties shared by other cannabinoids.
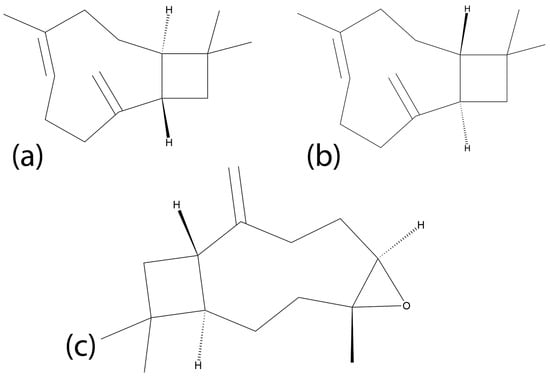
Figure 3.
Chemical structures of three sesquiterpenes: (a) trans-caryophyllene, (b) beta-cis-caryophyllene and (c) beta-caryophyllene oxide.
Compared to the reduced form, BCPO possesses stronger anticancer activity, particularly owing to the methylene and epoxide functional groups, which allow for covalent binding with DNA and proteins [105]. In breast cancer, BCPO alone is not as highly cytotoxic as it has been shown to be in other cancer types [106]. Nonetheless, it has been shown to demonstrate antiproliferative activity associated with intracellular ROS generation and the loss of mitochondrial membrane potential, leading to apoptosis [107]. For instance, Park and colleagues showed the cytotoxicity of BCPO against MCF-7 breast cancer cells and PC3 prostate cancer cells and elucidated the proapoptotic effects mediated by intracellular ROS generation. In addition to the upregulation of this pathway, the authors observed the suppression of the PI3K/AKT pathway as a likely contributing mechanism of proapoptotic activity. In addition, BCPO treatment induced the expression of p54 and p21 cell cycle regulators, as well as active caspase-3. Finally, potential antiangiogenic properties were indicated by interference with VEGF expression. Their work, overall, established a diverse range of mechanisms of anticancer activity against MCF-7 and PC3 cancer cells for BCPO. It is likely that this activity is due to the synergistic inhibition of several pathways involved in cell proliferation.
In addition to its use as an isolated treatment, BCPO has been investigated as a chemo-sensitizing agent. Given its relatively lower cytotoxicity against MCF-7 cells, this application presents a potential avenue for utilizing and amplifying its biochemical impacts. In their study, Hanušová and coworkers treated both MDA-MB-231 and MCF-7 breast cancer cells in in vivo mice models with a combination of BCPO and the chemotherapeutic drug Doxorubicin, identifying strong synergistic effects [108]. The authors utilized a neutral red uptake test to measure cytotoxicity in both cell lines, observing significant increases in cytotoxicity particularly against the MDA-MB-231 cell line. In addition, while tumor size in vivo was not significantly altered with combination treatment, BCPO with Doxorubicin was shown to increase apoptosis in tumor samples, nonetheless.
In addition to breast cancer, BCP has been studied with ovarian cancer cell lines with highly promising results. In a study conducted by Shahwar and colleagues, the activity of multiple cinnamon alkaloids against A2780 ovarian cancer cells was explored. Their studies noted an IC50 value for BCP of 8.94 µg/mL [109]. More recently, Arul and coworkers confirmed the cytotoxicity of BCP against PA-1 (from primary peritoneal serous papillary carcinoma) and OAW 42 (from serous cystadenocarcinoma) ovarian cancer cell lines. In addition, they further established proapoptotic properties using flow cytometry. Based on protein expression evaluations, they further characterized the upregulation of caspase-3 as the likely mechanism of proapoptotic properties. There is further work to be conducted in developing BCP as an anticancer therapeutic, especially given these strong in vitro results. However, current studies to date look promising. The potency of BCP against H8 cervical cancer cells [110] was also confirmed, particularly noting the proapoptotic and antiproliferative properties of BCP. The authors further characterized the molecular mechanism of apoptosis induction, identifying Wnt/beta-catenin pathway inhibition. This pathway is involved in inducing cell proliferation and is often abnormally activated in cervical cancer [111].
Overall, BCPO and BCP represent highly promising cinnamon metabolites for the treatment of gynecological carcinomas and breast cancer. While some work has been conducted to characterize the cytotoxicity and mechanism thereof in these cancers, there is a need for further characterization and in vivo examinations of anticancer activity.
4. Nanoformulations of Cinnamon Extract and Its Derivatives
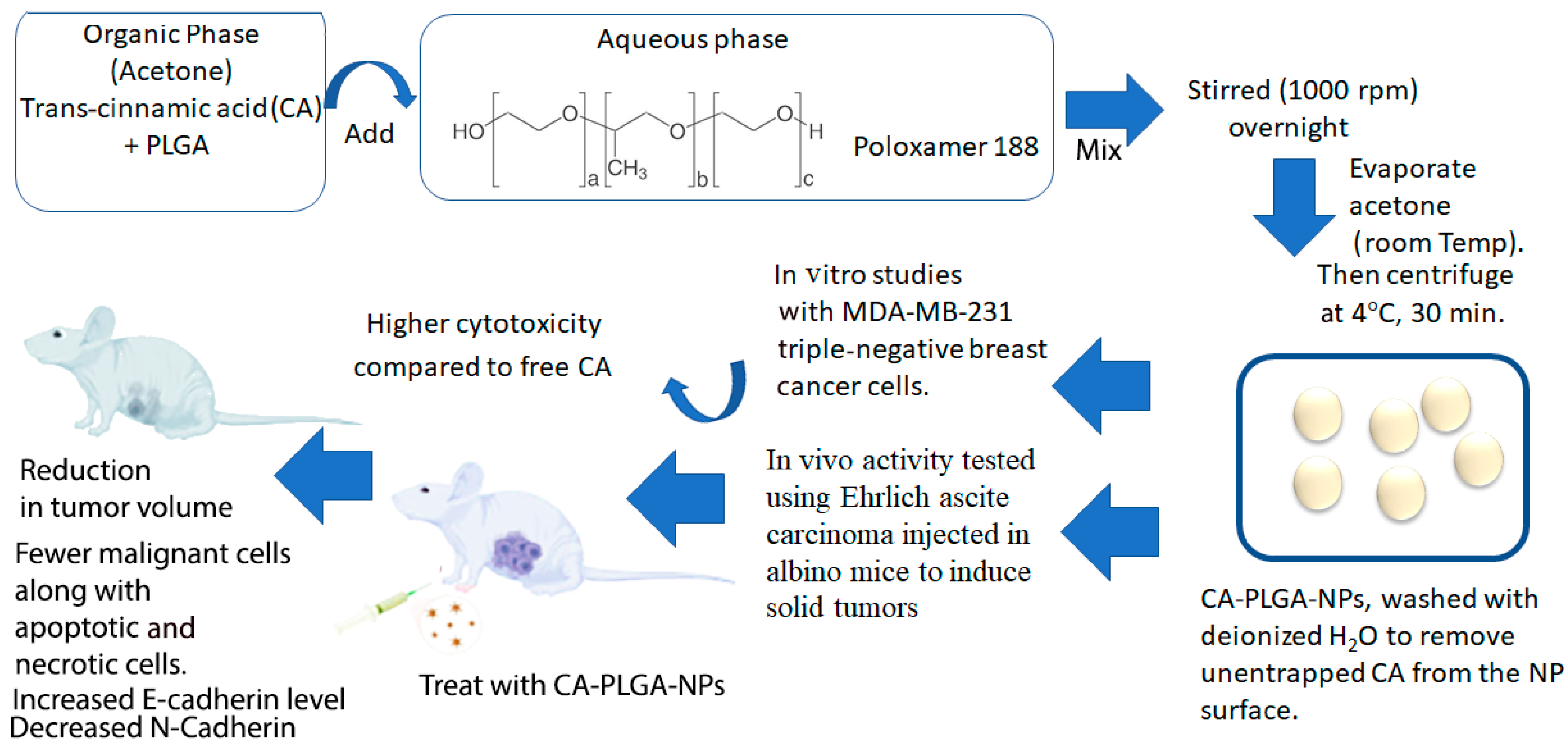
While the medicinal properties of cinnamon extract and its derivatives are highly desirable, often, their bioavailability may not be as high due to poor solubility, making it difficult to utilize them as a drug. Thus, nanoformulations have been developed to enhance the delivery of these materials for treatment. In a study conducted by Khalifa and coworkers, trans-cinnamic acid was encapsulated in poly(lactic-co-glycolic acid) (PLGA) nanoparticles to optimize delivery and distribution to MDA-MB-231 breast cancer cells [112]. Furthermore, it was shown that the CA-loaded nanoparticles showed a 6-fold decrease in the IC50 value compared to free CA, as well as improved antimetastatic activity. Additionally, CA-loaded nanoparticles also demonstrated a reduction in tumor size in the mouse tumor models studied (Figure 4).
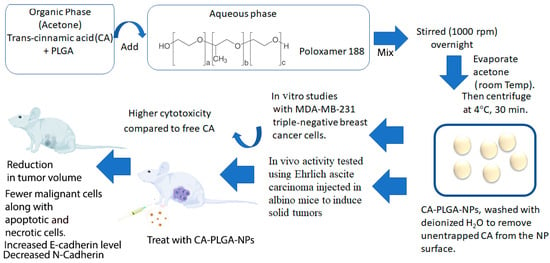
Figure 4.
Schematic diagram showing the methodology for developing CA-PLGA nanoparticles and the results summarizing their in vitro and in vivo activity. Schematic was created based on the methodology given in reference [112].
In a separate study, the CA derivative sinapic acid (SA) (3,5 dimethoxy-4-hydroxycinnamic acid) was loaded into PLGA nanoparticles in order to enhance the solubility of SA. The nanoparticles were found to show a significant drop in PCNA levels in MCF-7 cells, concomitant with increased apoptosis. Furthermore, no cytotoxicity was seen for MCF-10A (non-tumorigenic) cells under the concentrations tested (100–200 µg/mL). Interestingly, the levels of malondialdehyde (MDA) were found to increase in the first 24 h after treatment, but after 48 h, a significant decrease in MDA levels was observed, implying an increase in ROS levels within 24 h, causing lipid peroxidation, followed by a decrease. The authors also found that after 48 h of incubation with the SA-loaded NPs at concentrations between 100 and 200 µg/mL, the cells showed an increase in the activity of the enzyme catalase [113]. In addition to polymeric nanoparticles, citrate-stabilized gold nanoparticles have been tethered with cinnamic acid and have shown greater efficacy in inhibiting the proliferation of MCF-7 breast cancer cells and improved apoptosis compared to free CA, once again proving the effectiveness of nanoformulations in drug delivery to breast tumor cells [114]. In another study, taking advantage of the antitumorigenic properties of the spice saffron, Hassan and co-workers developed a nanoformulation consisting of cinnamon/saffron extracts, which resulted in decreased cell migration and promoted apoptosis in oral squamous cell carcinoma [115]. Although the authors did not study ovarian or breast cancer cells, this formulation may potentially be applied for treatment against ovarian or breast cancer cells.
More recently, the disruption of redox and thermal balances in cancer cells was investigated for enhancing delivery to HeLa cervical cancer cells. To do so, Li and co-workers developed a novel lipid-based nanoparticle encapsulated with cinnamaldehyde (CAL) and indocyanine green (IG) dye. The goal was to simultaneously increase the formation of ROS (due to the presence of CAL), while IG would cause photothermal damage to cancer cells upon irradiation with near-infrared light. Their results were promising, and the nanoparticles successfully reduced the viability of HeLa cells through oxidative and thermal stress caused in the cancer cells [116].
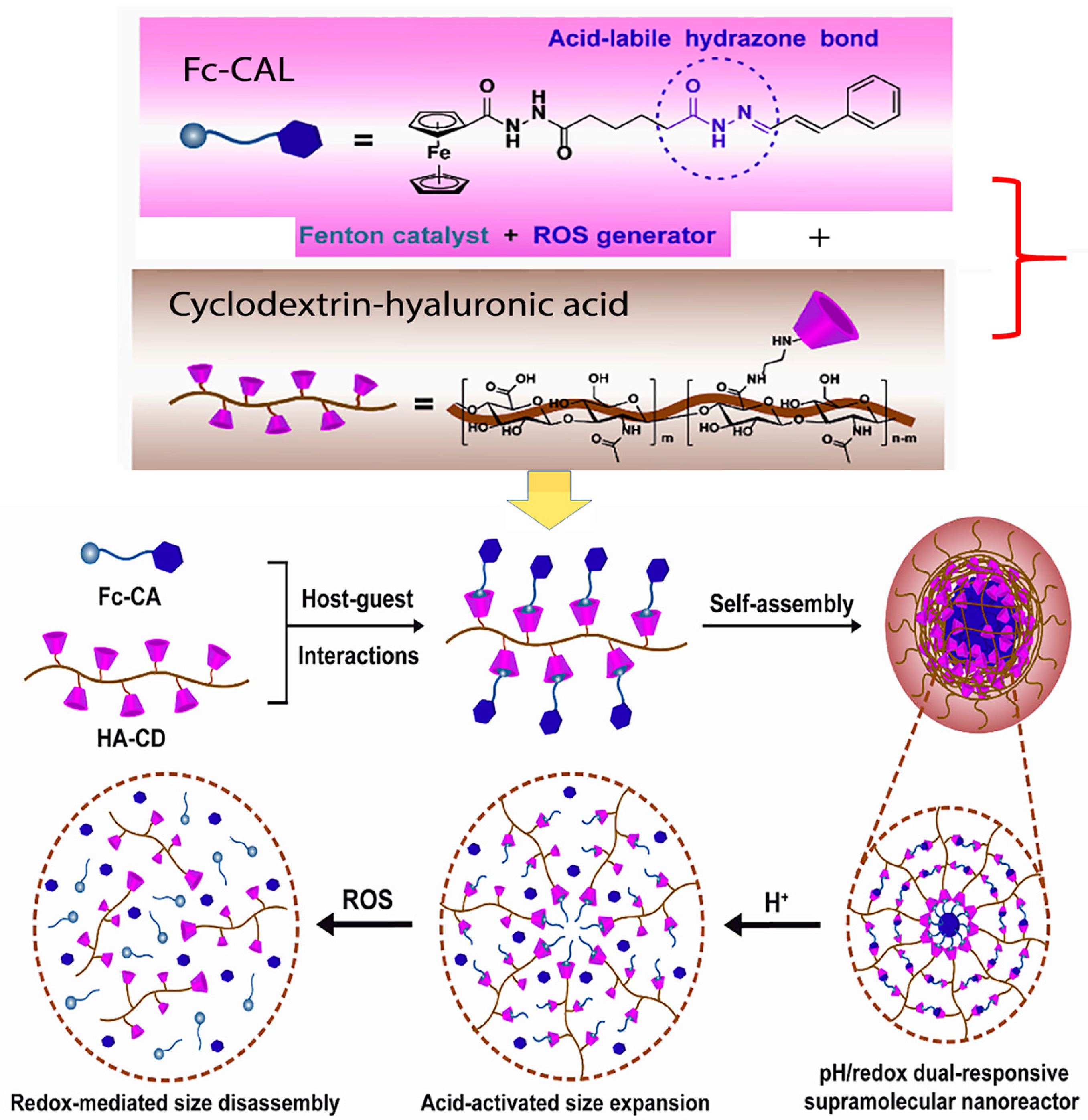
In an innovative study by Zhao and co-workers, the authors developed an acid-labile ferrocene-modified CAL (Fc-CAL) pro-drug molecule in order to blend the characteristic properties of the Fenton catalyst ferrocene with the ROS-generating properties of CAL. They then entrapped the hydrophobic Fc-CAL pro-drug in hydrophilic cyclodextrin-modified hyaluronic acid nanoparticles, which rendered the hybrid nanoparticles both pH and redox-responsive. Their results showed that not only were the nanoparticles highly stable but the drug-loaded nanoparticles accumulated primarily in tumor tissues of mice via an EPR effect and were endocytosed through CD44 receptor. Both in vivo and in vitro results showed that this strategy to enhance the Fenton reaction, thereby increasing the generation of cytotoxic amounts of hydroxyl free radicals to upsurge the effects in cancer cells, was promising. A scheme depicting this strategy is shown in Figure 5 [117].
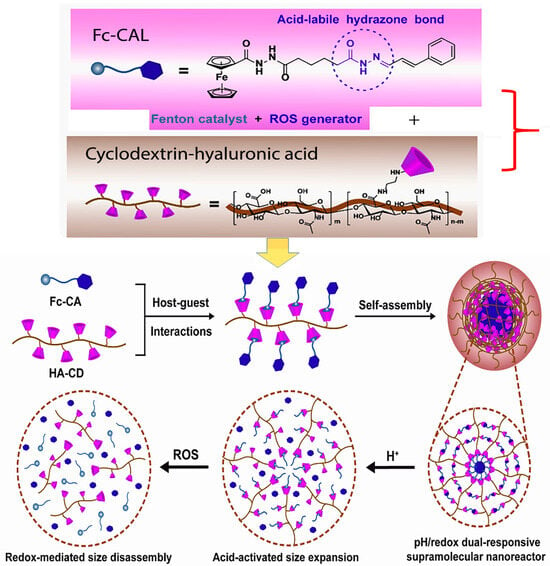
Figure 5.
Scheme showing the preparation of tumor-targeted dual-responsive hyaluronic acid-cyclodextrin/ferrocene-modified CAL nanoparticles. Adapted from Zhao et al. (reference [117]). Reproduced with permission from Elsevier.
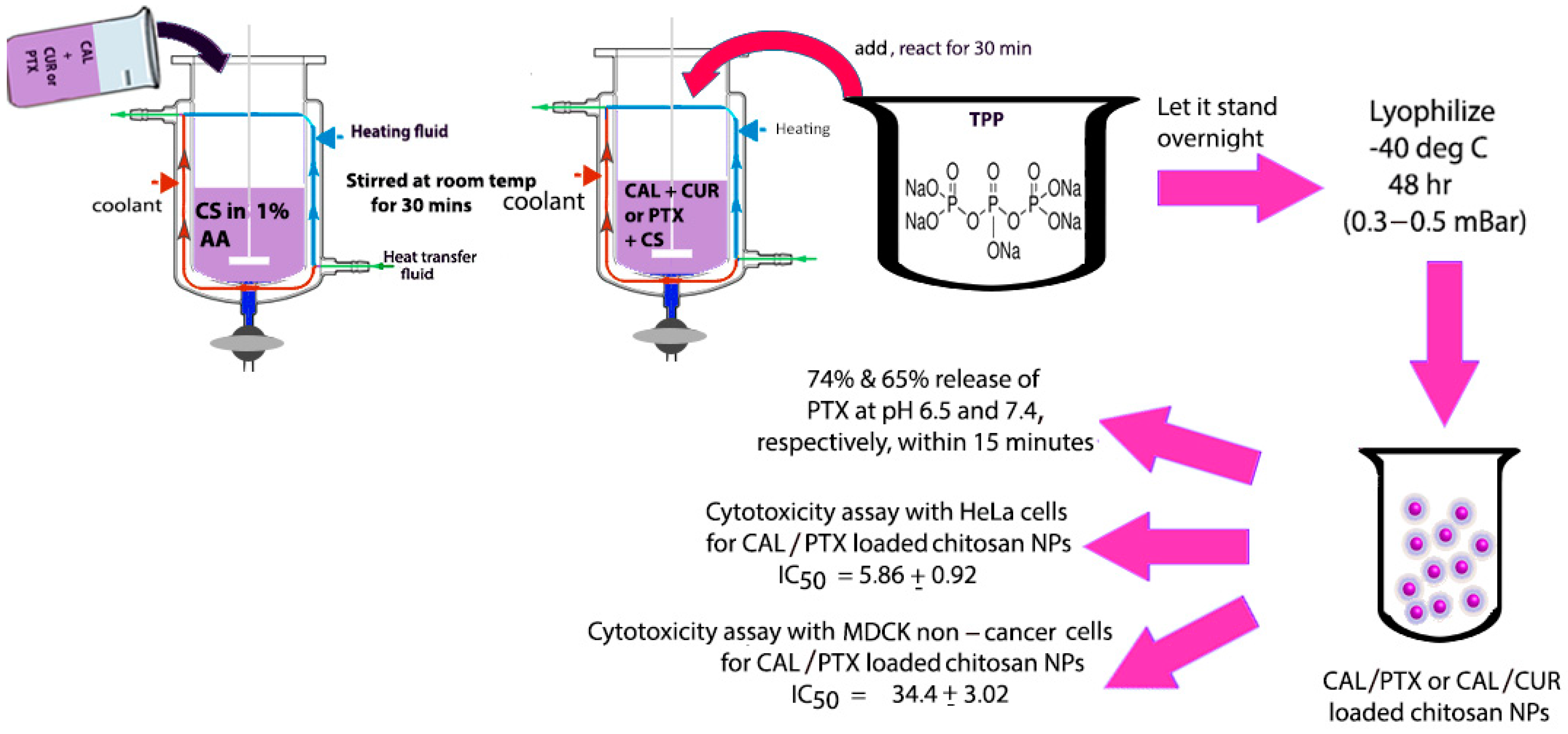
Chang and co-workers developed a new CAL derivative by reacting CAL with anthraniloyl hydrazine, which was then conjugated with BSA (bovine serum albumin) to form CAL-derivative BSA nanoparticles that were found to penetrate cancer cells and inhibit the growth of laryngeal cancer cells [118]. We believe that given the composition of these nanoparticles, they may also be tested against breast, cervical or ovarian cancer cell lines. In a separate study, Barrera-Martinez and co-workers loaded chitosan microparticles with trans-CAL in combination with either the chemotherapeutic Paclitaxel (PTX) or the natural antitumor polyphenol curcumin (CUR). The group utilized an “in situ” entrapment method for encapsulating CAL, a schematic for which is shown in Figure 6. Although the group observed a burst release, the cytotoxicity was found to be selective and the results showed that the pH-responsive microparticles demonstrated preferential cytotoxicity toward HeLa cervical cancer cells in comparison to non-cancer cells [119]. Overall, a significantly higher release was observed for PTX compared to CUR. The authors attributed the lower release of CUR to its hydrophobicity and its tendency to crystallize. In a pioneering study by Zong and co-workers, a thioacetal/CAL/DOX/PEG amphiphilic polymer was synthesized and self-assembled into nanoparticles under aqueous conditions. Those nanoparticles not only accumulated in the tumor tissue but it was also found that endogenous ROS from the cancer cells acted as a triggering agent that promoted the cleavage of thioacetal and the release of CAL, which produced more ROS through mitochondrial dysfunction and polymer degradation and resulted in the further release of DOX that allowed for enhanced chemo-immunotherapy in mice [120].
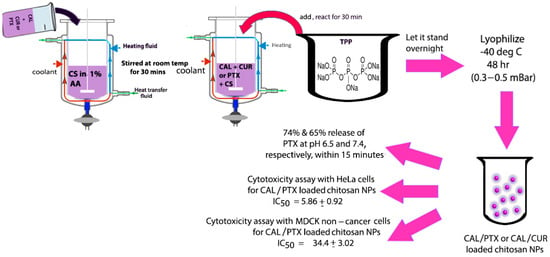
Figure 6.
Scheme showing the methodology for developing CAL/chitosan microparticles loaded with Paclitaxel (PTX) or curcumin (CUR) and the results summarizing pH-sensitive drug release and preferential cytotoxicity toward HeLa cells. Diagram was created based on methodology explained in reference [119].
In addition to CAL and CA, Khojah and colleagues have reported the investigation of the potential of CEO nanosuspensions to act as a nanocarrier for the chemotherapeutic bleomycin, with results indicating the enhancement of cytotoxicity for the CEO nanosuspension [121]. CEO nanosuspensions present a novel method of cancer targeting that leverages the biophysical properties of CEO nanosuspensions as well as the bioactivity of associated terpenoids and polyphenols. However, further studies are necessary to characterize the bioactivity and properties of cinnamon-based nanomaterials. In another particularly interesting recent study, CEO was loaded into chitosan nanoparticles for drug delivery in breast cancer cells [122]. This work revealed an IC50 value of 52 µg/mL for CEO nanosuspensions, an enhancement over the CEO alone when compared. Antiproliferative and pro-apoptosis properties were noted; through a mechanism of upregulation of the expression of caspase-3 and Apoptosis-Inducing Factor, proteins that were involved in cell cycle regulation and apoptosis induction. This finding corresponds with the discussed work by Kubataka and co-workers in which increased caspase-3 expression was also implicated as a mechanism of pro-apoptotic properties.
To increase cytotoxicity and decrease the effective dosage, a liposomal BCPO formulation using soy phosphatidylcholine (SPC) was developed by Di Sotto and colleagues [123]. Liposomes are a well-established drug delivery material utilized to improve the solubility and distribution of hydrophobic drug molecules. Due to their amphiphilic nature, liposomes allow for increased membrane permeability and therefore increased cell penetrance. With BCPO-loaded liposomes, the authors examined cytotoxicity against the triple-negative MDA-MB-468 breast cancer cells. The results showed a decreased IC50 value for loaded liposomes compared to free BCPO against MDA-MB-468 breast cancer cells, which is also another line of TNBC [124]. The effect is theorized to be a result of increased cell penetrance. Furthermore, nanoliposomes were also developed by co-encapsulating zein hydrosylate, a storage protein obtained from corn seeds with cinnamon extracts that were derived from cinnamon bark. The resultant nanoliposomes demonstrated enhanced antioxidant activity [125]. While the authors did not conduct anticancer studies, such co-loaded nanoliposomes may potentially be further developed for drug delivery and anti-tumor studies.
In addition, Sabouri and co-workers recently developed water-soluble cinnamon nanoparticles directly using hydrothermal methods. While the authors did not conduct tests with tumor cells, they found that the formed nanoparticles demonstrated antidiabetic properties and glucose-lowering effects in HepG2 cells [126]. Their study opens a new avenue where self-assembled cinnamon-based nanoparticles may be further probed for anticancer applications.
5. CE and Its Derivatives as Adjuvants
In recent years, marked attention has also been paid to the potential application of CEO, CE and their derivatives as chemotherapy adjuvants, allowing for enhancement of anticancer activity while leveraging the anti-inflammatory and antioxidant properties of cinnamon to reduce non-target toxicity. For example, two recent works showed the ability of CE and CEO to act as co-chemotherapeutics with Doxorubicin (DOX) treatment, thereby reducing off-target impacts and improving treatment impacts [127,128]. Aliyah and coworkers targeted 4T1 cells from BALB/c mice, which are known to closely mimic human breast tumors and metastatic spread using a combination of CEO and DOX. In addition to increased cytotoxicity against the cancer cells with an IC50 value of 25 µg/mL, the authors also showed that the coupled use of CEO with DOX improved antimetastatic activity. It is well known that long-term treatment with Doxorubicin may induce cardiotoxicity [129], thus making the antioxidant characteristics of CEO especially important and potentially useful. The authors also investigated the impacts on Matrix Metalloproteinase-9 (MMP-9) as metrics for antimetastatic activity. MMP-9 has a complicated regulation pathway for expression, synthesis and activation, and it has high homology with beneficial and necessary MMP proteins, making it difficult to target despite its noted contributions to carcinogenic processes, including metastasis [130]. The authors demonstrated a reduction in MMP-9 expression using a gelatin zymography assay, revealing that the combination treatment successfully inhibited MMP9 expression.
In another study, folate-functionalized monoolein cubosomes encapsulated with poly(ethyleneimine) PEI, cinnamic acid and DOX were utilized for targeted delivery, specifically to KB tumor cells, which are known to originate from the cervix and overexpress the folate receptor. Furthermore, the cubosomes were stabilized with a hydrophobically modified gelatin/folate conjugate and Pluronic F127. The results demonstrated higher delivery into the KB tumor cells compared to free DOX [131].
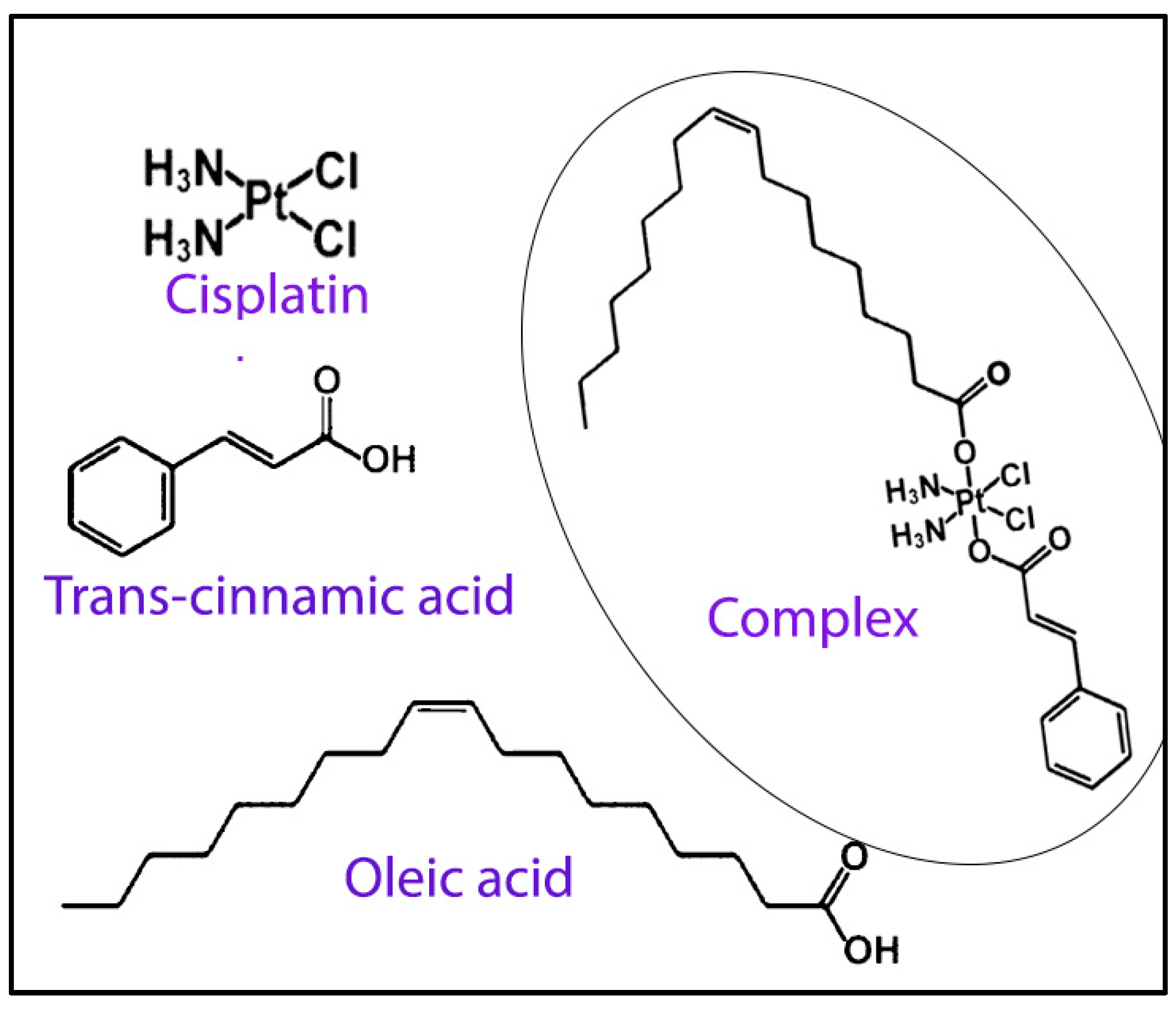
In a fascinating study conducted by Kasparkova and co-workers, a new cisplatin-based Pt (IV) complex containing oleate and trans-cinnamate as axial ligands, cis, trans, cis-[Pt-(NH3)2(t-cinnamate)(oleate)Cl2] (Figure 7) [132] was developed efficiently for potential treatment against aggressive HER2-positive breast tumor cells. The results showed a significantly higher accumulation of the complex in tumor cells compared to free cisplatin due to its higher lipophilicity. In addition, the complex was found to be significantly more sensitive and selective toward SKBR-3 breast cancer cells, which are HER2-positive, compared to MCF-7 cells, which are HER2-negative. Furthermore, the complex was also found to show promising results against SKBR-3 cells by cancer stem cells, thus showing its potential activity to curb recurrence and drug resistance. The authors also successfully showed that the complex demonstrated increasing antiproliferative effects in 3D spheroid models with higher HER2 expression.
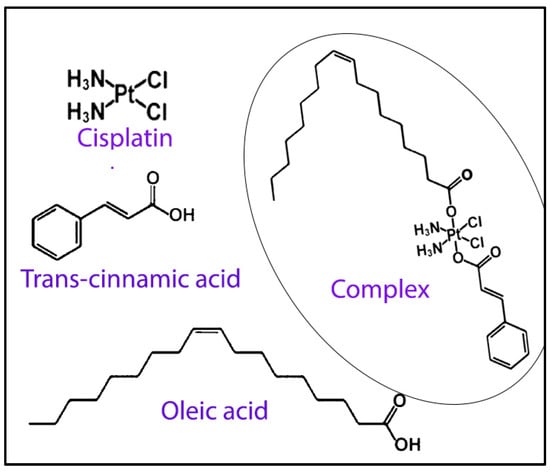
Figure 7.
Scheme showing the components of Pt (IV) complex containing oleate, cisplatin and trans-cinnamate as axial ligands. The formed complex is shown within the circle. Reproduced and adapted with permission from Wiley (reference [132]).
In addition to CAL, CE and CEO, another bioactive component of cinnamon, eugenol, a phenylpropanoid derived from guaiacol, was recently examined as an adjuvant with the chemotherapeutic drug cisplatin against two ovarian cancer cell lines (SKOV3 and OV2774) and in mouse models. The authors reported that the inclusion of eugenol with cisplatin worked synergistically to inhibit tumor growth through the initiation of apoptosis. A critical finding of their work was that, biochemically, it was shown that while levels of Hes1 in the Notch/Hes1 signaling pathway were increased, resulting in increased resistance to neat cisplatin treatment, the cisplatin/eugenol combination inhibited the Notch/Hes1 pathway and downregulated the drug resistance ABC transporter genes, thereby indicating that eugenol as an adjuvant with chemotherapeutic drugs may aid in targeting ovarian cancer stem cells, and may potentially improve prognosis [133]. Additionally, it has been reported that β-caryophyllene and β-caryophyllene oxide are efficient chemosensitizers and reverse drug resistance in tumor cells [134]. Other studies have shown that hepatotoxicity induced by DOX can be mitigated upon treatment with cinnamaldehyde-narigin (a natural flavonoid) co-encapsulated zein nanoparticles that were coated with lactoferrin and sodium caseinate. The authors found that the nanoparticles demonstrated stability and hemocompatibility in mice, in addition to significantly reducing oxidative stress induced by DOX [135].
6. Hybrid Scaffolds of Cinnamic Acid and Its Derivatives
More recently, hybrids of cinnamon and its derivatives with additional bioactive molecules have been developed. In a pioneering study, taking advantage of the anti-cancer properties of curcumin, a naturally occurring polyphenol, Regasini and co-workers synthesized a series of cinnamaldehyde/curcumin hybrids through aldol condensation reactions and examined their antiproliferative effects on a wide variety of breast, ovarian, vulvar and cervical cancer cell lines. Their results indicated anti-neoplastic effects and higher efficacy against breast, cervical and vulvar cancer cells compared to non-tumorigenic cells. The authors attributed the higher efficacy of the formed hybrids to the presence of the electrophilic β-carbon in the compounds [136]. In a recent study by Sreekanth and co-workers, substituted N-(4)-substituted thiosemicarbazones that were derived from cinnamaldehyde were prepared with ruthenium (II)-(η6-p-cymene)/(η6-benzene) complexes for targeting cervical (HeLa) and breast cancer (MDA-MB-231 and MCF-7) cells. They found that the complexes successfully generated apoptosis in the cancer cells selectively due to the bioactivity of the thiosemicarbazones [137]. In a separate study, Bulakowska and colleagues developed novel N–(4–chloro–2–mercapto–5–methyl phenylsulfonyl) cinnamamide derivatives, containing cinnamic moiety and benzene sulfonamide as the two specific pharmacophores. They demonstrated that the designed compounds not only showed antimicrobial activity and antioxidant activity but also showed potent activity against cervical (HeLa), ovarian (SKOV-3) and breast MCF-7 cancer cell lines. In particular, two of the derivatives of N–{[4–chloro–5-methyl–2–(naphthalen–1–ylmethylthio)phenyl]sulfonyl}cinnamamide showed selective high potency against SKOV-3 ovarian cancer cell proliferation [138]. Hybrids of CA and artemisinin, a natural compound that contains an endoperoxide bridge, have also been developed [139]. For example, mono and disubstituted dihydroartemisinin-cinnamon acid ester hybrids have been developed by Yu and co-workers [140]. However, dihydroartemisinin hybrids were found to show significantly higher activity against four different cancer cell lines. The authors showed that apoptosis was induced by ROS and ferrous ion accumulation, resulting in significant anticancer activity against A549 cells. In an interesting study by Abourehab and co-workers, pyrrolizine/indolizine/cinnamaldehyde Schiff bases were developed through condensation with CAL. The authors found that two of the compounds in particular showed high cytotoxicity against MCF-7 cell lines. Molecular docking studies revealed that the compounds showed high binding affinity toward p38 MAP kinase as well as COX-2 proteins [141].
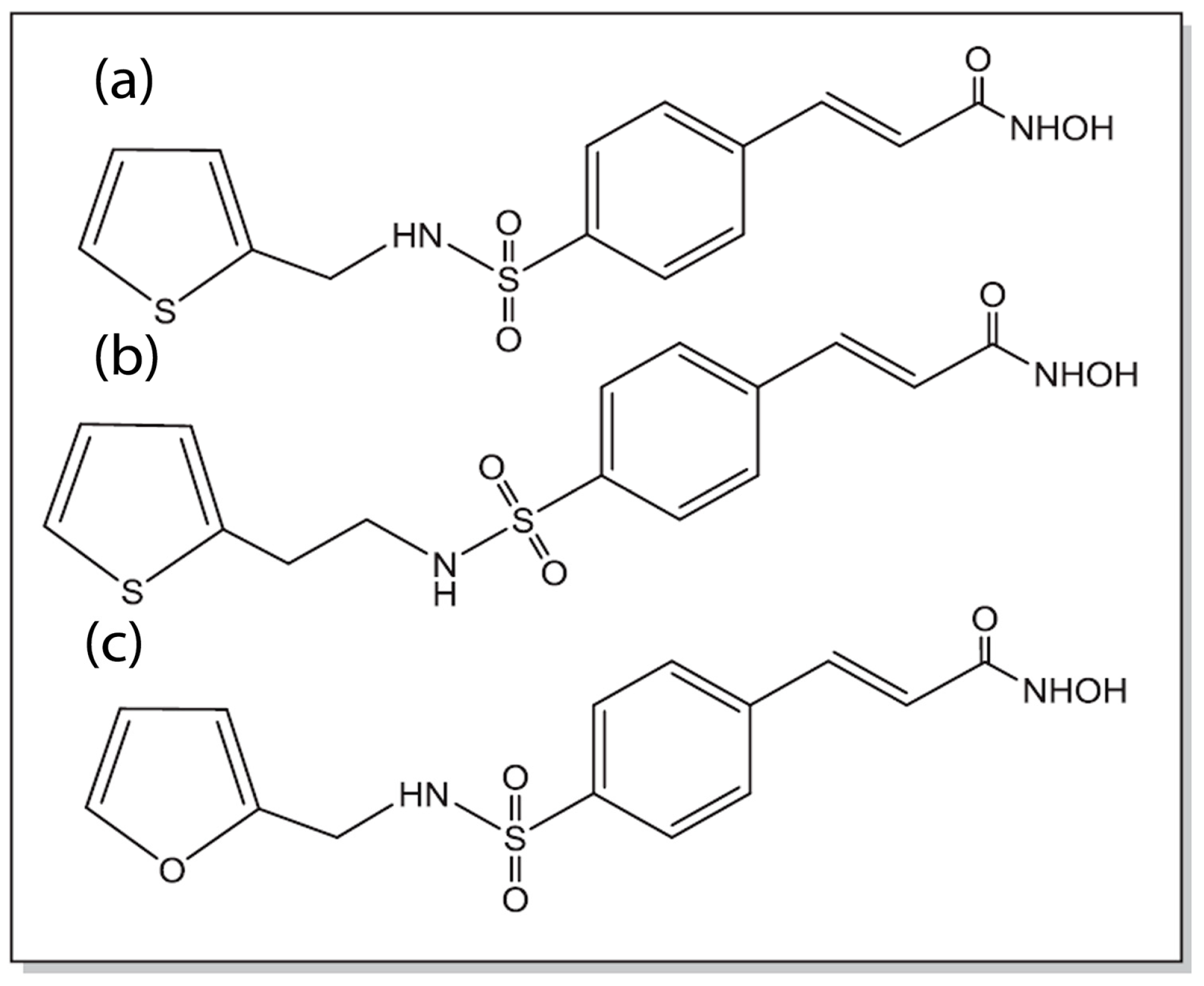
Natural products such as plant-derived triterpenoids like oleanolic acid (OA) and glycyrrhetinic acid (GA) have also been conjugated with cinnamic acid to develop cinnamic acid ester derivatives. The combination of OA or GA with cinnamic acid allows for the incorporation of two antioxidant and antitumor compounds within the same molecule for enhancing potency against MCF-7 tumor cells. The authors indicated that the IC50 values obtained for the conjugates were close to the known FDA-approved drug gefitinib, which is promising [142]. In a study conducted by Rao and co-workers, cinnamyl sulfonamide hydroxamate derivatives (Figure 8) were developed that were found to be potent histone deacetylase (HDAC) inhibitors and demonstrated strong cytotoxicity against MCF-7 breast cancer cells as well as A549 lung cancer cells. Furthermore, the derivatives showed antiangiogenic activity (as demonstrated through the reduction in the expression of HIF-1α) as well as antimetastatic activity (as shown by reduction in cell migration and inhibition of MMP-2 and MMP-9 expression) [143].
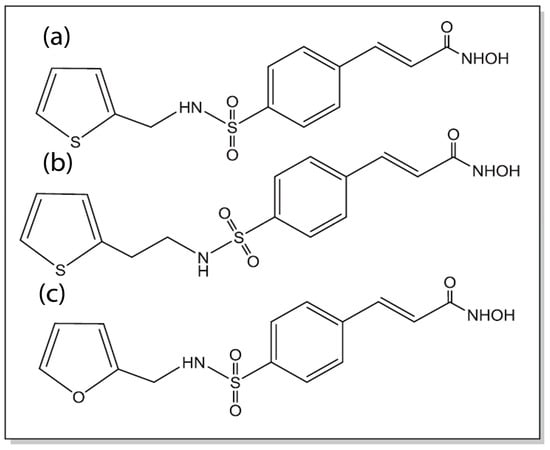
Figure 8.
Chemical structures of bioisosteres of sulfonylamide/hydroxamate derivatives of cinnamic acid (compounds a–c). Compound b was found to exhibit the highest potency against MCF-7 and A549 cells on par with the known HDAC inhibitor Vorinostat (adapted from reference [143] with permission from Elsevier).
In another interesting study, the 8-amino quinoline-derived primaquine (a known antimalarial drug) was conjugated with various derivatives of cinnamic acid, such as including α-methyl cinnamic acid or methoxy, dimethoxy, trimethoxy, methylene dioxy, chloro, fluoro, trifluoromethyl and bis-trifluoromethyl cinnamic acid and tested against HeLa, MCF-7 and the SW 620 cell lines. The authors found that all of their designed compounds showed high potency against breast MCF-7 cell lines. However, the (E)-3-(2-fluorophenyl)-N-(4-(6-methoxyquinolin-8-ylamino) pentyl) acrylamide compound showed significantly high selectivity and cytotoxicity against HeLa and MCF-7 cell lines, as well as the SW 620 cells [144]. Studies have also shown that the conjugation of cinnamic acid with amino acid building blocks such as those with leucine, alanine, glycine and methionine can enhance cytotoxicity against various tumor cells, including the MCF-7 cell line. Furthermore, the authors found that the potency of the derivatives induced DNA damage in the tumor cells [145]. In a recent study, a series of chimeric compounds of the naturally derived chalcone 2′,4′-dihydroxychalcone derivatized with cinnamic acid were examined by Ventura-Salazar and co-workers using QSAR modeling studies, and then the most optimal compounds were synthesized. Of those, the 2′-hydroxy, 4′ cinnamate chalcone was predicted to show highly potent cytotoxicity against MCF-7 cancer cell lines in addition to activity against lung A-549, cervical (CasKi) and liver (HepG2, Hep3B) cancer cells [146]. In an interesting study by Ogunalakin and collaborators, acrylhyrazone cinnamate derivatives were designed and synthesized. Molecular docking and simulation studies revealed that the lead compounds demonstrated molecular interactions with the kinase domain of HER2 and human aromatase cytochrome P450. Furthermore, the compound N’-(2,4-dichlorobenzylidene)-3-(4-methoxyphenyl) acrylohydrazide (which contained two chloride ions on the aromatic system) inhibited the proliferation of both CHO-I cancer cells (IC50 value of 4.2 ± 0.6 µg/mL) and HeLa cervical cancer cells (IC50 value of 21.4 ± 2.1 µg/mL), while N′-(2, 6-dimethoxybenzylidene)-3-(4-methoxyphenyl) acrylohydrazide showed slightly lower inhibition, demonstrating an IC50 value of 18.4 ± 4.1 µg/mL with CHO-1 cells and an IC50 value of 22.4 ± 1.5 µg/ mL with HeLa cell lines [147]. Overall, these studies suggest that while there have been a wide variety of cinnamoyl conjugates that have been synthesized and shown to be viable as potent anticancer agents, the field still remains wide open, where there is a potential to develop more potent anticancer agents toward gynecological cancer treatment by combining multiple bioactive motifs (both natural and synthetic) within a single molecule.
7. Conclusions and Future Challenges
The molecular mechanisms involved in the antioxidant, antimetastatic and antiangiogenic properties of cinnamon extract and its derivatives continue to be an important topic of current research. Because CE and CEO contain multiple bioactive plant metabolites, it is likely that several synergistic mechanisms are involved in the cumulative effects observed. The research indicates that CE, CA, CAL and CEO impact numerous pathways at multiple cell cycle stages, from alteration of gene expression to pathway inhibition [148]. The potential of CE and CEO, as well as other major constituent compounds, as novel anticancer therapeutics has been investigated in numerous studies. In particular, the ability of these cinnamon-based compounds to treat gynecological and breast cancers is a promising area of ongoing research that has been discussed in this review. These materials exhibit antimetastatic, antiangiogenic and proapoptotic properties that make them particularly valuable in the treatment of such cancers. Despite the progress in this area, there are several challenges that remain to be resolved. As oil components, most of these metabolites are hydrophobic and face solubility challenges. To enhance bioavailability, nanoscale delivery methods are being pursued. Additionally, CE and CEO-based nanoparticles or nanoemulsions and hybrids are also being explored. Furthermore, these nanoparticles can be further utilized for targeted therapeutics through appropriate functionalization. A particularly promising approach is the synthesis and development of novel hybrid drug compounds with cinnamon derivatives and other bioactive components that can synergistically enhance the anti-cancer effects. While most of the work reported in this study focused primarily on breast, cervical and ovarian tumor cells, cinnamon and its derivatives have been found to be effective against a multitude of other types of cancers. An aspect of CE that needs to be addressed includes the standardization of CE and CEO, which remains a gap, as noted by the differences in measured CEO composition across the literature. Future works should focus on the further development of hybrid materials and nanoscale-targeted therapeutics either directly with CA and its derivatives or as adjuvants. In addition, further in vivo and patient-specific studies will be necessary.
Author Contributions
M.A.B. wrote a multitude of drafts of the article; I.A.B. edited and wrote the final draft of the article. All authors have read and agreed to the published version of the manuscript.
Funding
M.B thanks the Blavatnik Family Foundation, the Henry Luce Foundation and the Barry Goldwater Scholarship for financial support. I.B. thanks Fordham University research grants.
Data Availability Statement
No new data were generated in this review article. All studies and data reported are available publicly from the references cited.
Acknowledgments
M.A.B and I.A.B. thank the Fordham University Department of Chemistry and Biochemistry.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ji, H.; Lix, X.; Zhang, H.Y. Natural products and drug discovery: Can thousands of years of ancient medical knowledge lead us to new and powerful drug combinations in the fight against cancer and dementia? EMBO Rep. 2009, 10, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Reshi, Z.; Ahmad, W.; Lukatkin, A.; Javed, S. From nature to lab: A review of secondary metabolite biosynthetic pathways, environmental influences and in vitro approaches. Metabolites 2023, 13, 895. [Google Scholar] [CrossRef] [PubMed]
- Rinehart, K. Secondary metabolites from marine organisms. Ciba Found. Symp. 1992, 171, 236–249. [Google Scholar]
- Chifiriuc, M.C.; Filip, R.; Constantin, M.; Pircalabioru, G.G.; Bleotu, C.; Burlibasa, L.; Ionica, E.; Corcionivoschi, N.; Mihaescu, G. Common Themes in Antimicrobial and Anticancer Drug Resistance. Front. Microbiol. 2022, 13, 960693. [Google Scholar] [CrossRef]
- Lauritano, C.; Martinez, K.; Battaglia, P.; Granata, A.; de la Cruz, M.; Cautain, B.; Martin, J.; Reyes, F.; Ianora, A.; Guglielmo, L. First evidence of anticancer and antimicrobial activity in mediterranean mesopelagic species. Sci. Rep. 2020, 10, 4929. [Google Scholar] [CrossRef]
- Pommier, Y.; Leo, E.; Zhang, H.; Marchand, C. DNA Topoisomerases and Their Poisoning by Anticancer and Antibacterial Drugs. Chem. Biol. 2010, 17, 421–433. [Google Scholar] [CrossRef]
- Tornesello, A.L.; Borrelli, A.; Buonaguro, L.; Buonaguro, F.M.; Tornesello, M.L. Antimicrobial Peptides as Anticancer Agents: Functional Properties and Biological Activities. Molecules 2020, 25, 2850. [Google Scholar] [CrossRef] [PubMed]
- Heulot, M.; Jacquier, N.; Aeby, S.; Le Roy, D.; Roger, T.; Trofimenko, E.; Barras, D.; Greub, G.; Widmann, C. The anticancer peptide TAT-RasGAP317-326 exerts broad antimicrobial activity. Front. Microbiol. 2017, 8, 994. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Zou, X.; Cheng, K.; Zhong, S.; Su, Y.; Wu, T.; Tao, Y.; Long, L.; Yan, B.; Jiang, Y. The role of cell-penetrating peptides in potential anti-cancer therapy. Clin. Trans. Med. 2022, 12, e822. [Google Scholar] [CrossRef]
- Rizvi, A.; Farhan, M.; Nabi, F.; Khan, R.H.; Adil, M.; Ahmad, A. Transcriptional Control of the Oxidative Stress Response and Implications of Using Plant Derived Molecules for Therapeutic Interventions in Cancer. Curr. Med. Chem. 2021, 28, 8480–8495. [Google Scholar] [CrossRef]
- Ávila-Gálvez, M.Á.; González-Sarrías, A.; Martínez-Díaz, F.; Abellán, B.; Martínez-Torrano, A.J.; Fernández-López, A.J.; Giménez-Bastida, J.A.; Espín, J.C. Disposition of Dietary Polyphenols in Breast Cancer Patients’ Tumors, and Their Associated Anticancer Activity: The Particular Case of Curcumin. Mol. Nutr. Food Res. 2021, 65, 2100163. [Google Scholar] [CrossRef]
- Li, H.; Yang, B.; Huang, J.; Xiang, T.; Yin, X.; Wan, J.; Luo, F.; Zhang, L.; Li, H.; Ren, G. Naringin Inhibits Growth Potential of Human Triple-Negative Breast Cancer Cells by Targeting β-Catenin Signaling Pathway. Toxicol. Lett. 2013, 220, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Spatafora, C.; Tringali, C. Natural-Derived Polyphenols as Potential Anticancer Agents. Anticancer Agents Med. Chem. 2012, 12, 902–918. [Google Scholar] [CrossRef] [PubMed]
- Piao, L.; Mukherjee, S.; Chang, Q.; Xie, X.; Li, H.; Castellanos, M.R.; Banerjee, P.; Iqbal, H.; Ivancic, R.; Wang, X.; et al. TriCurin, a Novel Formulation of Curcumin, Epicatechin Gallate, and Resveratrol, Inhibits the Tumorigenicity of Human Papillomavirus-Positive Head and Neck Squamous Cell Carcinoma. Oncotarget 2016, 8, 60025–60035. [Google Scholar] [CrossRef]
- Han, Y.; Huang, M.; Li, L.; Cai, X.; Gao, Z.; Li, F.; Rakariyatham, K.; Song, M.; Fernández Tomé, S.; Xiao, H. Non-Extractable Polyphenols from Cranberries: Potential Anti-Inflammation and Anti-Colon-Cancer Agents. Food Funct. 2019, 10, 7714–7723. [Google Scholar] [CrossRef]
- Gollucke, A.P.B.; Aguiar, O.; Barbisan, L.F.; Ribeiro, D.A. Use of Grape Polyphenols against Carcinogenesis: Putative Molecular Mechanisms of Action Using in Vitro and in Vivo Test Systems. J. Med. Food 2013, 16, 199–205. [Google Scholar] [CrossRef]
- Huang, S.F.; Horng, C.T.; Hsieh, Y.S.; Hsieh, Y.H.; Chu, S.C.; Chen, P.N. Epicatechin-3-Gallate Reverses TGF-β1-Induced Epithelial-To-Mesenchymal Transition and Inhibits Cell Invasion and Protease Activities in Human Lung Cancer Cells. Food Chem. Toxicol. 2016, 94, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Flores-Pérez, A.; Marchat, L.A.; Sánchez, L.L.; Romero-Zamora, D.; Arechaga-Ocampo, E.; Ramírez-Torres, N.; Chávez, J.D.; Carlos-Reyes, Á.; Astudillo-de la Vega, H.; Ruiz-García, E.; et al. Differential Proteomic Analysis Reveals That EGCG Inhibits HDGF and Activates Apoptosis to Increase the Sensitivity of Non-Small Cells Lung Cancer to Chemotherapy. Proteom. Clin. Appl. 2015, 10, 172–182. [Google Scholar] [CrossRef]
- Sidhar, H.; Giri, R.K. Induction of Bex Genes by Curcumin Is Associated with Apoptosis and Activation of P53 in N2a Neuroblastoma Cells. Sci. Rep. 2017, 7, 41420. [Google Scholar] [CrossRef]
- Hernandez-Valencia, J.; Garcia-Villa, E.; Arenas-Hernandez, A.; Garcia-Mena, J.; Diaz-Chavez, J.; Gariglio, P. Induction of P53 Phosphorylation at Serine 20 by Resveratrol Is Required to Activate P53 Target Genes, Restoring Apoptosis in MCF-7 Cells Resistant to Cisplatin. Nutrients 2018, 10, 1148. [Google Scholar] [CrossRef]
- Sun, X.-X.; Dai, M.-S. Deubiquitinating Enzyme Regulation of the P53 Pathway: A Lesson from Otub1. World J. Biol. Chem. 2014, 5, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Zhang, X. Stimulatory Effects of Curcumin and Quercetin on Posttranslational Modifications of P53 during Lung Carcinogenesis. Hum. Exp. Toxicol. 2017, 37, 618–625. [Google Scholar] [CrossRef]
- Minnelli, C.; Moretti, P.; Fulgenzi, G.; Mariani, P.; Laudadio, E.; Armeni, T.; Galeazzi, R.; Mobbili, G. A Poloxamer-407 Modified Liposome Encapsulating Epigallocatechin-3-Gallate in the Presence of Magnesium: Characterization and Protective Effect against Oxidative Damage. Int. J. Pharm. 2018, 552, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Dilkalal, A.; Annapurna, A.S.; Umesh, T.G. In Vitro Antioxidant, Anticancer and in Silico Studies of Polyphenol Enriched Leaf Extract of Asystasia Gangetica. Sci. Rep. 2024, 14, 28374. [Google Scholar] [CrossRef]
- Leopoldini, M.; Russo, N.; Toscano, M. The Molecular Basis of Working Mechanism of Natural Polyphenolic Antioxidants. Food Chem. 2011, 125, 288–306. [Google Scholar] [CrossRef]
- Oyenihi, A.B.; Smith, C. Are Polyphenol Antioxidants at the Root of Medicinal Plant Anti-Cancer Success? J. Ethnopharmacol. 2019, 229, 54–72. [Google Scholar] [CrossRef]
- Cabello, C.M.; Bair, W.B.; Lamore, S.D.; Ley, S.; Bause, A.S.; Azimian, S.; Wondrak, G.T. The Cinnamon-Derived Michael Acceptor Cinnamic Aldehyde Impairs Melanoma Cell Proliferation, Invasiveness, and Tumor Growth. Free Radic. Biol. Med. 2009, 46, 220–231. [Google Scholar] [CrossRef]
- Lee, C.W.; Lee, S.H.; Lee, J.W.; Ban, J.O.; Lee, S.Y.; Yoo, H.S.; Jung, J.K.; Moon, D.C.; Oh, K.W.; Hong, J.T. 2-Hydroxycinnamaldehyde Inhibits SW620 Colon Cancer Cell Growth through AP-1 Inactivation. J. Pharmacol. Sci. 2007, 104, 19–28. [Google Scholar] [CrossRef]
- Tsao, R. Chemistry and Biochemistry of Dietary Polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef]
- Karimi, A.; Majlesi, M.; Rafieian-Kopaei, M. Herbal versus Synthetic Drugs; Beliefs and Facts. J. Nephropharmacol. 2015, 4, 27–30. [Google Scholar]
- Vladu, A.; Ficai, D.; Ene, A.; Ficai, A. Combination Therapy Using Polyphenols: An Efficient Way to Improve Antitumoral Activity and Reduce Resistance. Int. J. Mol. Sci. 2022, 23, 10244. [Google Scholar] [CrossRef]
- Pichersky, E.; Raguso, R. Why do plants produce so many terpenoid compounds. New Phytol. 2018, 220, 692–702. [Google Scholar] [CrossRef]
- Avila, C. Terpenoids in marine heterobranch molluscs. Mar. Drugs. 2020, 18, 162. [Google Scholar] [CrossRef]
- Gutiérrez-del-Río, I.; López-Ibáñez, S.; Magadán-Corpas, P.; Fernández-Calleja, L.; Pérez-Valero, Á.; Tuñón-Granda, M.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Terpenoids and Polyphenols as Natural Antioxidant Agents in Food Preservation. Antioxidants 2021, 10, 1264. [Google Scholar] [CrossRef]
- Alam, W.; Ahmed, I.; Ali, M.; Khan, F.; Khan, H. Neuroprotective effects of terpenoids. In Phytonutrients and Neurological Disorders; Academic Press: Cambridge, MA, USA, 2023; Chapter 8; pp. 227–244. [Google Scholar]
- Wojtunik, K.A.; Ciesla, L.M.; Waksmundzka-Hajnos, M. Model studies on the antioxidant activity of common terpenoid constituents of essential oils by means of the 2,2 diphenyl-1-picrylhydrazyl method. J. Agric. Food Chem. 2014, 62, 9088–9094. [Google Scholar] [CrossRef]
- Talib, W.H.; Abukhader, M.M. Combinatorial Effects of Thymoquinone on the Anticancer Activity and Hepatotoxicity of the Prodrug CB 1954. Sci. Pharm. 2013, 81, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Shoaib, M.; Shah, I.; Ali, N.; Adhikari, A.; Tahir, M.N.; Shah, S.W.A.; Ishtiaq, S.; Khan, J.; Khan, S.; Umer, M.N. Sesquiterpene lactone! a promising antioxidant, anticancer and moderate antinociceptive agent from Artemisia macrocephala jacquem. BMC Complement. Altern. Med. 2017, 17, 27. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Sun, W.-J.; Wang, W.-M.; Chen, K.; Zheng, J.-H.; Lu, M.-D.; Li, P.-H.; Zheng, Z.-Q. Artesunate inhibits the growth of gastric cancer cells through the mechanism of promoting oncosis both in vitro and in vivo. Anticancer Drugs 2013, 24, 920–927. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, R.K. Therapeutic and Pharmaceutical Potential of Cinnamomum Tamala. Res. Rev. Pharm. Pharm. Sci. 2017, 6, 18–28. [Google Scholar]
- Plata-Rueda, A.; Campos, J.M.; da Silva Rolim, G.; Martínez, L.C.; Dos Santos, M.H.; Fernandes, F.L.; Serrão, J.E.; Zanuncio, J.C. Terpenoid Constituents of Cinnamon and Clove Essential Oils Cause Toxic Effects and Behavior Repellency Response on Granary Weevil, Sitophilus Granarius. Ecotoxicol. Environ. Saf. 2018, 156, 263–270. [Google Scholar] [CrossRef]
- Rao, P.V.; Gan, S.H. Cinnamon: A Multifaceted Medicinal Plant. eCAM 2014, 2014, 642942. [Google Scholar] [CrossRef] [PubMed]
- Akonoso, E.; Latifah, A.; Suwanti, L.; Haq, K.; Pertiwi, H. Clove flower extract (Syzygium aromaticum) has anticancer potential effect analyzed by molecular docking and brine shrimp lethality test (BSLT). Vet. Med. Int. 2022, 5113742. [Google Scholar]
- Mohanty, D.; Padhee, S.; Sahoo, C.; Jena, S.; Sahoo, A.; Panda, P.C.; Nayak, S.; Ray, A. Integrating Network Pharmacology and Experimental Verification to Decipher the Multitarget Pharmacological Mechanism of Cinnamomum Zeylanicum Essential Oil in Treating Inflammation. Heliyon 2024, 10, e24120. [Google Scholar] [CrossRef] [PubMed]
- Mutlu, M.; Bingol, Z.; Mehtap Uc, E.; Köksal, E.; Gören, A.C.; Alwasel, S.H.; Gülçin, İ. Comprehensive Metabolite Profiling of Cinnamon (Cinnamomum Zeylanicum) Leaf Oil Using LC-HR/MS, GC/MS, and GC-FID: Determination of Antiglaucoma, Antioxidant, Anticholinergic, and Antidiabetic Profiles. Life 2023, 13, 136. [Google Scholar] [CrossRef] [PubMed]
- Khedkar, S.; Khan, M.A. An in Vitro Study Elucidating the Synergistic Effects of Aqueous Cinnamon Extract and an Anti-TNF-α Biotherapeutic: Implications for a Complementary and Alternative Therapy for Non-Responders. BMC Complement. Med. Ther. 2024, 24, 131. [Google Scholar] [CrossRef]
- Rad, S.K.; Movafagh, A. Study of Antioxidant, Antiproliferative and DNA Damage Protecting Activities of Cinnamomum Cassia Extracts Obtained by Sequential Extraction. Recent Pat. Food Nutr. Agric. 2020, 11, 45–57. [Google Scholar] [CrossRef]
- Ribeiro-Santos, R.; Andrade, M.; Madella, D.; Martinazzo, A.P.; de Aquino Garcia Moura, L.; de Melo, N.R.; Sanches-Silva, A. Revisiting an Ancient Spice with Medicinal Purposes: Cinnamon. Trends Food Sci. Technol. 2017, 62, 154–169. [Google Scholar] [CrossRef]
- Haw, S.G. Cinnamon, Cassia and Ancient Trade. J. Anc. Hist. Archaeol. 2017, 4, 5–18. [Google Scholar] [CrossRef]
- Bronkhorst, J. Early Interactions Between South and Southeast Asia: Reflections on Cross-Cultural Exchange; Manguin, P.-Y., Mani, A., Wade, G., Eds.; Institute of Southeast Asian Studies: Singapore, 2011; pp. 263–275. [Google Scholar]
- Wang, Y.-H.; Avula, B.; Nanayakkara, N.P.D.; Zhao, J.; Khan, I.A. Cassia Cinnamon as a Source of Coumarin in Cinnamon-Flavored Food and Food Supplements in the United States. J. Agric. Food Chem. 2013, 61, 4470–4476. [Google Scholar] [CrossRef]
- Ngoc, T.M.; Lee, I.; Ha, D.T.; Kim, H.; Min, B.; Bae, K. Tyrosinase-Inhibitory Constituents from the Twigs of Cinnamomum Cassia. J. Nat. Prod. 2009, 72, 1205–1208. [Google Scholar] [CrossRef]
- Liao, S.-G.; Yuan, T.; Zhang, C.; Yang, S.-P.; Wu, Y.; Yue, J.-M. Cinnacassides A–E, Five Geranylphenylacetate Glycosides from Cinnamomum Cassia. Tetrahedron 2009, 65, 883–887. [Google Scholar] [CrossRef]
- Khan, A.; Safdar, M.; Ali Khan, M.M.; Khattak, K.N.; Anderson, R.A. Cinnamon Improves Glucose and Lipids of People with Type 2 Diabetes. Diabetes Care 2003, 26, 3215–3218. [Google Scholar] [CrossRef]
- Allen, R.W.; Schwartzman, E.; Baker, W.L.; Coleman, C.I.; Phung, O.J. Cinnamon Use in Type 2 Diabetes: An Updated Systematic Review and Meta-Analysis. Ann. Fam. Med. 2013, 11, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Wariyapperuma, W.A.N.M.; Kannangara, S.; Wijayasinghe, Y.S.; Subramanium, S.; Jayawardena, B. In Vitro Anti-Diabetic Effects and Phytochemical Profiling of Novel Varieties of Cinnamomum Zeylanicum (L.) Extracts. PeerJ 2020, 8, e10070. [Google Scholar] [CrossRef]
- Morsi, D.; El-Nabi, S.; Elmaghraby, M.; Ali, O.; Fayad, E.; Khalifa, S.; El-Seedi, H.; El-Garawani, I.M. Antiproliferative and immunomodulatory potencies of cinnamon oil in Ehrlich ascites carcinoma bearing mice. Sci. Rep. 2022, 12, 11839. [Google Scholar] [CrossRef] [PubMed]
- Ly, D.; Forman, D.; Ferlay, J.; Brinton, L.A.; Cook, M.B. An International Comparison of Male and Female Breast Cancer Incidence Rates. Int. J. Cancer 2012, 132, 1918–1926. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information (US). Genes and Disease [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 1998-. Breast and Ovarian Cancer. Available online: https://www.ncbi.nlm.nih.gov/books/NBK22168/ (accessed on 30 December 2024).
- Borzoei, A.; Rafraf, M.; Niromanesh, S.; Farzadi, L.; Narimani, F.; Doostan, F. Effects of cinnamon supplementation on antioxidant status and serum lipids in women in polycystic ovary syndrome. J. Tradit. Complement. Med. 2018, 8, 128–133. [Google Scholar] [CrossRef]
- Kallel, I.; Hadrich, B.; Gargouri, B.; Chaabane, A.; Lassoued, S.; Gdoura, R.; Bayoudh, A.; Ben Messaoud, E. Optimization of Cinnamon (Cinnamomum Zeylanicum Blume) Essential Oil Extraction: Evaluation of Antioxidant and Antiproliferative Effects. eCAM 2019, 2019, 6498347. [Google Scholar] [CrossRef]
- Yang, T.; Xu, L.; Li, B.; Li, W.; Ma, X.; Fan, L.; Lee, R.; Xu, C.; Xiang, G. Antitumor Activity of a Folate Receptor-Targeted Immunoglobulin G-Doxorubicin Conjugate. Int. J. Nanomed. 2017, 12, 2505–2515. [Google Scholar] [CrossRef]
- Wang, Y.; Peng, R.-Q.; Li, D.-D.; Ding, Y.; Wu, X.-Q.; Zeng, Y.-X.; Zhu, X.-F.; Zhang, X.-S. Chloroquine Enhances the Cytotoxicity of Topotecan by Inhibiting Autophagy in Lung Cancer Cells. Chin. J. Cancer 2011, 30, 690–700. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Koppikar, S.J.; Choudhari, A.S.; Suryavanshi, S.A.; Kumari, S.; Chattopadhyay, S.; Kaul-Ghanekar, R. Aqueous Cinnamon Extract (ACE-c) from the Bark of Cinnamomum Cassiacauses Apoptosis in Human Cervical Cancer Cell Line (SiHa) through Loss of Mitochondrial Membrane Potential. BMC Cancer 2010, 10, 210. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.-M.; Chou, C.-J.; Tseng, S.-H.; Hung, C.-F. Bioinformatics and in vitro experimental analyses identify the selective therapeutic potential of interferon gamma and apigenin against cervical squamous cell carcinoma and adenocarcinoma. Oncotarget 2017, 8, 46145–46162. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Morgan, E.; Rumgay, H.; Mafra, A.; Singh, D.; Laversanne, M.; Vignat, J.; Gralow, J.R.; Cardoso, F.; Siesling, S.; et al. Current and Future Burden of Breast Cancer: Global Statistics for 2020 and 2040. Breast 2022, 66, 15–23. [Google Scholar] [CrossRef]
- Husain, I.; Ahmad, R.; Chandra, A.; Raza, S.T.; Shukla, Y.; Mahdi, F. Phytochemical Characterization and Biological Activity Evaluation of Ethanolic Extract of Cinnamomum Zeylanicum. J. Ethnopharmacol. 2018, 219, 110–116. [Google Scholar] [CrossRef]
- Wagner, K.-U. Know Thy Cells: Commonly Used Triple-Negative Human Breast Cancer Cell Lines Carry Mutations in RAS and Effectors. Breast Cancer Res. 2022, 24, 44. [Google Scholar] [CrossRef]
- Nakayama, J.; Konno, Y.; Maruyama, A.; Tomita, M.; Makinoshima, H. Cinnamon Bark Extract Suppresses Metastatic Dissemination of Cancer Cells through Inhibition of Glycolytic Metabolism. J. Nat. Med. 2022, 76, 686–692. [Google Scholar] [CrossRef]
- Nakayama, J.; Tan, L.; Li, Y.; Goh, B.C.; Wang, S.; Makinoshima, H.; Gong, Z. A Zebrafish Embryo Screen Utilizing Gastrulation Identifies the HTR2C Inhibitor Pizotifen as a Suppressor of EMT-Mediated Metastasis. eLife 2021, 10, e70151. [Google Scholar] [CrossRef]
- Rad, S.K.; Kanthimathi, M.; Malek, N.; Lee, G.; Looi, C.; Wong, W. Cinnamomum cassia suppresses Caspase-9 through stimulation of AKT1 in MCF-7 cells but not MDA-MB-231 cells. PLoS ONE 2015, 10, e0145216. [Google Scholar]
- Singh, S.; Shrivastava, R.; Goswami, B.; Koner, B.C. The apoptosis modulating effect of hydroethanolic cinnamon extract on breast cancer cell line. J. Herbal Med. 2024, 44, 100847. [Google Scholar] [CrossRef]
- Nagy, J.; Armbruster, D. Evolution of uncontrolled proliferation and angiogenic switch in cancer. Math. Biosci. Eng. 2012, 9, 843–876. [Google Scholar]
- Lu, J.; Zhang, K.; Nam, S.; Anderson, R.A.; Jove, R.; Wen, W. Novel Angiogenesis Inhibitory Activity in Cinnamon Extract Blocks VEGFR2 Kinase and Downstream Signaling. Carcinogenesis 2010, 31, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Duffy, A.M.; Bouchier-Hayes, D.J.; Harmey, J.H. Vascular Endothelial Growth Factor (VEGF) and Its Role in Non-Endothelial Cells: Autocrine Signaling by VEGF. In Madame Curie Bioscience Database; Landes Bioscience: Austin, TX, USA, 2013. Available online: https://www.ncbi.nlm.nih.gov/books/NBK6482/ (accessed on 28 December 2024).
- Zhang, K.; Han, E.S.; Dellinger, T.H.; Lu, J.; Nam, S.; Anderson, R.A.; Yim, J.H.; Wen, W. Cinnamon Extract Reduces VEGF Expression via Suppressing HIF-1α Gene Expression and Inhibits Tumor Growth in Mice. Mol. Carcinog. 2016, 56, 436–446. [Google Scholar] [CrossRef]
- Kubatka, P.; Kello, M.; Kajo, K.; Samec, M.; Jasek, K.; Vybohova, D.; Uramova, S.; Liskova, A.; Sadlonova, V.; Koklesova, L.; et al. Chemopreventive and Therapeutic Efficacy of Cinnamomum Zeylanicum L. Bark in Experimental Breast Carcinoma: Mechanistic in Vivo and in Vitro Analyses. Molecules 2020, 25, 1399. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, L.; Yuan, X.; Ou, Y.; Zhu, X.; Cheng, Z.; Zhang, P.; Wu, X.; Meng, Y.; Zhang, L. The Relationship between the Bcl-2/Bax Proteins and the Mitochondria-Mediated Apoptosis Pathway in the Differentiation of Adipose-Derived Stromal Cells into Neurons. PLoS ONE 2016, 11, e0163327. [Google Scholar] [CrossRef]
- Li, Y.; Kong, D.; Wu, H. Analysis and Evaluation of Essential Oil Components of Cinnamon Barks Using GC–MS and FTIR Spectroscopy. Ind. Crops Prod. 2013, 41, 269–278. [Google Scholar] [CrossRef]
- Alizadeh Behbahani, B.; Falah, F.; Lavi Arab, F.; Vasiee, M.; Tabatabaee Yazdi, F. Chemical Composition and Antioxidant, Antimicrobial, and Antiproliferative Activities of Cinnamomum Zeylanicum Bark Essential Oil. eCAM 2020, 2020, 5190603. [Google Scholar] [CrossRef]
- Shahina, Z.; Molaeitabari, A.; Sultana, T.; Dahms, T.E.S. Cinnamon Leaf and Clove Essential Oils Are Potent Inhibitors of Candida Albicans Virulence Traits. Microorganisms 2022, 10, 1989. [Google Scholar] [CrossRef] [PubMed]
- Bahramsoltani, R.; Shahpiri, Z.; Farzaei, M.H.; Hosseinzadeh, L.; Rezazadeh, D.; Pourfarzam, M.; Rahimi, R. Effects of Cinnamon Oil and Its Main Constituents, Cinnamic Acid and Cinnamaldehyde, on 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine-Induced Neurodegeneration in PC-12 Cells. Boletín Latinoam. Caribe Plantas Med. Aromáticas 2023, 22, 837–847. [Google Scholar] [CrossRef]
- Armando, B.; Awwad, F.; Desgagné-Penix, I. Cinnamaldehyde in Focus: Antimicrobial Properties, Biosynthetic Pathway, and Industrial Applications. Antibiotics 2024, 13, 1095. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Dey, A.; Koirala, N.; Shaheen, S.; El Omari, N.; Salehi, B.; Goloshvili, T.; Cirone Silva, N.C.; Bouyahya, A.; Vitalini, S.; et al. Cinnamomum Species: Bridging Phytochemistry Knowledge, Pharmacological Properties and Toxicological Safety for Health Benefits. Front. Pharmacol. 2021, 12, 600139. [Google Scholar] [CrossRef] [PubMed]
- Wahab, W.A.; Adzmi, A.N. The Investigation of Cytotoxic Effect of Cinnamomum zeylanicum Extracts on Human Breast Cancer Cell Line (MCF-7). Sci. Herit. J. 2017, 1, 23–28. [Google Scholar] [CrossRef]
- Vangalapati, M.; Nandam, S.S.; Prakash, D.V.S. In-Vitro Anti-Cancer Studies of Cinnamaldehyde on Breast Cancer Cell Line (MCF-7). Tsbt-BioTechnology 2013, 7, 81–84. [Google Scholar]
- Liu, Y.; An, T.; Wan, D.; Yu, B.; Fan, Y.; Pei, X. Targets and Mechanism Used by Cinnamaldehyde, the Main Active Ingredient in Cinnamon, in the Treatment of Breast Cancer. Front. Pharmacol. 2020, 11, 582719. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Y.; Wang, L.; Chen, B.; Zhu, M.; Ma, C.; Mu, C.; Tao, A.; Li, S.; Luo, L.; et al. Cinnamaldehyde Suppressed EGF-Induced EMT Process and Inhibits Ovarian Cancer Progression through PI3K/AKT Pathway. Front. Pharmacol. 2022, 13, 779608. [Google Scholar] [CrossRef]
- Hallas-Potts, A.; Dawson, J.; Herrington, C. Ovarian cancer cell lines derived from non-serous carcinomas migrate and invade more aggressively than those derived from high-grade serous carcinomas. Sci. Rep. 2019, 9, 5515. [Google Scholar] [CrossRef]
- Lamendola, D.; Duan, Z.; Yusuf, R.; Seiden, M. Molecular description of evolving paclitaxel resistance in the SKOV-3 human ovarian carcinoma cell line. Cancer Res. 2003, 63, 2200–2205. [Google Scholar]
- Yan, X.D.; Li, M.; Yuan, Y.; Mao, N.; Pan, L.Y. Biological comparison of ovarian cancer resistant cell lines to cisplatin and Taxol by two different administrations. Oncol. Rep. 2007, 17, 1163–1169. [Google Scholar] [CrossRef] [PubMed]
- Boggiti, M. Effect of Cinnamaldehyde on Female Reproductive Hormones in 7, 12 Dimethyl Benzanthracene Induced Ovarian Cancer Rats. Int. J. Res. Appl. Sci. Eng. Technol. 2021, 9, 346–350. [Google Scholar] [CrossRef]
- Ruwizhi, N.; Aderibigbe, B.A. Cinnamic Acid Derivatives and Their Biological Efficacy. Int. J. Mol. Sci. 2020, 21, 5712. [Google Scholar] [CrossRef]
- Pal, A.; Tapadar, P.; Pal, R. Exploring the Molecular Mechanism of Cinnamic Acid-Mediated Cytotoxicity in Triple Negative MDA-MB-231 Breast Cancer Cells. Anticancer Agents Med. Chem. 2020, 20, 1141–1150. [Google Scholar] [CrossRef]
- Hunke, M.; Martinez, W.; Kashyap, A.; Bokoskie, T.; Pattabiraman, M.; Chandra, S. Antineoplastic Actions of Cinnamic Acids and Their Dimers in Breast Cancer Cells: A Comparative Study. Anticancer Res. 2018, 38, 4469–4474. [Google Scholar] [CrossRef]
- Fan, R.; Liang, Z.; Wang, Q.; Chen, S.; Huang, S.; Liu, J.; Huang, R.; Chen, J.; Zhao, F.; Huang, W. Beneficial Action of Cinnamic Acid against Ovarian Cancer via Network Pharmacology Analysis and the Pharmacological Activity Assessment. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2023, 397, 2987–2994. [Google Scholar] [CrossRef] [PubMed]
- Kanimozhi, G.; Prasad, N. Chp. 73 Anticancer effect of caffeic acid on human cervical cancer cells. In Coffee in Health and Disease Prevention; Academic Press: San Diego, CA, USA, 2015; pp. 655–661. [Google Scholar]
- Imai, M.; Yokoe, H.; Tsubuki, M.; Takahasi, N. Growth inhibition of human breast and prostate cancer cells by cinnamic acid derivatives and their mechanism of action. Biol. Pharm. Bull. 2019, 42, 1134–1139. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.-L.; Han, N.-Z.; Liu, S.-S. Caffeic acid phenethyl ester inhibits the progression of ovarian cancer by regulating NF-κb signaling. Biomed. Pharmacother. 2018, 99, 825–831. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; Alagawany, M.; Abdel-Moneim, A.M.E.; Mohammed, N.G.; Khafaga, A.F.; Bin-Jumah, M.; Othman, S.I.; Allam, A.A.; Elnesr, S.S. Cinnamon (Cinnamomum zeylanicum) Oil as a Potential Alternative to Antibiotics in Poultry. Antibiotics 2020, 9, 210. [Google Scholar] [CrossRef] [PubMed]
- Tung, Y.-T.; Chua, M.-T.; Wang, S.-Y.; Chang, S.-T. Anti-Inflammation Activities of Essential Oil and Its Constituents from Indigenous Cinnamon (Cinnamomum osmophloeum) Twigs. Bioresour. Technol. 2008, 99, 3908–3913. [Google Scholar] [CrossRef]
- Francomano, F.; Caruso, A.; Barbarossa, A.; Fazio, A.; La Torre, C.; Ceramella, J.; Mallamaci, R.; Saturnino, C.; Iacopetta, D.; Sinicropi, M.S. β-Caryophyllene: A Sesquiterpene with Countless Biological Properties. Appl. Sci. 2019, 9, 5420. [Google Scholar] [CrossRef]
- Nuutinen, T. Medicinal Properties of Terpenes Found in Cannabis sativa and Humulus lupulus. Eur. J. Med. Chem. 2018, 157, 198–228. [Google Scholar] [CrossRef]
- Fidyt, K.; Fiedorowicz, A.; Strządała, L.; Szumny, A. β-Caryophyllene and β-Caryophyllene Oxide—Natural Compounds of Anticancer and Analgesic Properties. Cancer Med. 2016, 5, 3007–3017. [Google Scholar] [CrossRef]
- Dahham, S.S.; Tabana, Y.M.; Iqbal, M.A.; Ahamed, M.B.K.; Ezzat, M.O.; Majid, A.S.A.; Majid, A.M.S.A. The Anticancer, Antioxidant and Antimicrobial Properties of the Sesquiterpene β-Caryophyllene from the Essential Oil of Aquilaria Crassna. Molecules 2015, 20, 11808–11829. [Google Scholar] [CrossRef]
- Park, K.-R.; Nam, D.; Yun, H.-M.; Lee, S.-G.; Jang, H.-J.; Sethi, G.; Cho, S.K.; Ahn, K.S. β-Caryophyllene Oxide Inhibits Growth and Induces Apoptosis through the Suppression of PI3K/AKT/MTOR/S6K1 Pathways and ROS-Mediated MAPKs Activation. Cancer Lett. 2011, 312, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Hanušová, V.; Caltová, K.; Svobodová, H.; Ambrož, M.; Skarka, A.; Murínová, N.; Králová, V.; Tomšík, P.; Skálová, L. The Effects of β-Caryophyllene Oxide and Trans-Nerolidol on the Efficacy of Doxorubicin in Breast Cancer Cells and Breast Tumor-Bearing Mice. Biomed. Pharmacother. 2017, 95, 828–836. [Google Scholar] [CrossRef]
- Shahwar, D.; Ullah, S.; Khan, M.A.; Ahmad, N.; Saeed, A.; Ullah, S. Anticancer Activity of Cinnamon Tamala Leaf Constituents towards Human Ovarian Cancer Cells. Pak. J. Pharm. Sci. 2015, 28, 969–972. [Google Scholar] [PubMed]
- Fei, M.; Fan, W. Beta-Caryophyllene Can Inhibit the Proliferation and Autophagy of HPV16-Infected Immortalized Cervical Epithelial Cells. Eur. J. Gynaecol. Oncol. 2024, 45, 93–100. [Google Scholar]
- Li, L.; Yang, W.-T.; Zheng, P.-S.; Liu, X.-F. SOX17 Restrains Proliferation and Tumor Formation by Down-Regulating Activity of the Wnt/β-Catenin Signaling Pathway via Trans-Suppressing β-Catenin in Cervical Cancer. Cell Death Dis. 2018, 9, 741. [Google Scholar] [CrossRef]
- Badawi, N.M.; Attia, Y.M.; El-Kersh, D.M.; Hammam, O.; Khalifa, M.K.A. Investigating the Impact of Optimized Trans-Cinnamic Acid-Loaded PLGA Nanoparticles on Epithelial to Mesenchymal Transition in Breast Cancer. Int. J. Nanomed. 2022, 17, 733–750. [Google Scholar] [CrossRef]
- Poyraz, F.; Akbas, G.; Duranoglu, D.; Acar, S.; Mansuroglu, B.; Ersoz, M. Sinapic-acid-loaded nanoparticles optimized via experimental design methods: Cytotoxic, antiapoptotic, antiproliferative and antioxidant activity. ACS Omega 2024, 9, 40329–40345. [Google Scholar] [CrossRef]
- Subramanian, K.; Ponnuchamy, K. Gold nanoparticles tethered cinnamic acid: Preparation, characterization, and cytotoxic effects on MCF-7 breast cancer cell lines. Appl. Nanosci. 2018, 8, 1133–1138. [Google Scholar] [CrossRef]
- Hassan, P.; Elshamy, I.; Abo Azma, N. Anticancer effect of combined cinnamon-saffron versus its nanoparticles on oral squamous cell carcinoma cell line. Tanta Dental. J. 2024, 21, 229–236. [Google Scholar] [CrossRef]
- Li, Q.-Q.; Li, B.-L. Cinnamaldehyde and indocyanine green loaded lipid nanoparticles induced intracellular oxidative / thermal stress damage for effective cervical cancer therapy. Micro Nano Lett. 2022, 17, 175–180. [Google Scholar]
- Xu, X.; Zeng, Z.; Chen, J.; Huang, B.; Guan, Z.; Huang, Y.; Huang, Z.; Zhao, C. Tumor-targeted supramolecular catalytic nanoreactor for synergistic chemo/chemodynamic therapy via oxidative stress amplification and cascaded Fenton Reaction. Chem. Eng. J. 2020, 390, 124628. [Google Scholar] [CrossRef]
- Chang, S.; Qin, D.; Wang, L.; Zhang, M.; Yan, R.; Zhao, C. Preparation of novel cinnamaldehyde derivative-BSA nanoparticles with high stability, good cell penetrating ability and promising anticancer activity. Coll. Surfaces. A 2021, 624, 126765. [Google Scholar] [CrossRef]
- Barrera-Martinez, C.; Melendez-Ortiz, H.; Padilla-Vaca, F.; Atanase, L.; Peralta-Rodriguez, R.; Liakos, I. Dual loading of trans-cinnamaldehyde and either paclitaxel or curcumin in chitosan nanoparticles: Physicochemical characterization and biological evaluation against MDCK and HeLa cells. Polymers 2024, 16, 3087. [Google Scholar] [CrossRef] [PubMed]
- Zong, Q.; Li, S.; Xiao, X.; Du, X.; Yuan, Y. Self-amplified chain-shattering cinnamaldehyde-based poly(thioacetal) boosts cancer chemo-immunotherapy. Acta Biomater. 2022, 154, 97–107. [Google Scholar] [CrossRef]
- Alkhatib, M.H.; Alghamdi, R.S.; Balamash, K.S.; Khojah, S.M. Cinnamon Oil Nanoemulsion as a Novel Nanocarrier for Bleomycin Amplifies Its Apoptotic Effect on SKOV-3 Ovarian Cancer Cells. Indian J. Exp. Biol. 2022, 59, 10. [Google Scholar]
- Xu, X.; Li, Q.; Dong, W.; Zhao, G.; Lu, Y.; Huang, X.; Liang, X. Cinnamon Cassia Oil Chitosan Nanoparticles: Physicochemical Properties and Anti-Breast Cancer Activity. Int. J. Biol. Macromol. 2023, 224, 1065–1078. [Google Scholar] [CrossRef]
- Di Sotto, A.; Paolicelli, P.; Nardoni, M.; Abete, L.; Garzoli, S.; Di Giacomo, S.; Mazzanti, G.; Ma, C.; Petralito, S. SPC Liposomes as Possible Delivery Systems for Improving Bioavailability of the Natural Sesquiterpene β-Caryophyllene: Lamellarity and Drug-Loading as Key Features for a Rational Drug Delivery Design. Pharmaceutics 2018, 10, 274. [Google Scholar] [CrossRef]
- Wojtowciz, W.; Wrobel, A.; Pyziak, K.; Tarkowski, R.; Balcerzak, A.; Bebenek, M.; Mlynarz, P. Evaluation of MDA-MB-468 cell culture media analysis in predicting triple-negative breast cancer patient sera metabolic profiles. Metabolites 2020, 10, 173. [Google Scholar] [CrossRef]
- Imani, S.; Alizadeh, A.; Tabibiazar, M.; Hamishekar, H.; Roufergarinejad, L. Nanoliposomal co-encapsulation of cinnamon extract and zein hydrosylates with synergistic antioxidant activity for nutraceutical applications. Chem. Pap. 2022, 76, 2059–2069. [Google Scholar] [CrossRef]
- Sabouri, Z.; Shakour, N.; Sabouri, M.; Moghaddas, S.S.T.H. Biochemical, structural characterization and assessing biological effects of cinnamon nanoparticles. Biotechnol. Bioprocess Eng. 2024, 29, 165–175. [Google Scholar] [CrossRef]
- Aliyah, A.N.; Lintangsari, G.; Maran, G.G.; Hermawan, A.; Meiyanto, E. Cinnamon Oil as a Co-Chemotherapy Agent through Inhibition of Cell Migration and MMP-9 Expression on 4T1 Cells. J. Altern. Complement. Med. 2021, 19, 921–928. [Google Scholar] [CrossRef]
- Sandamali, J.A.N.; Hewawasam, R.P.; Jayatilaka, K.A.P.W.; Mudduwa, L.K.B. Cinnamomum zeylanicum Blume (Ceylon Cinnamon) Bark Extract Attenuates Doxorubicin Induced Cardiotoxicity in Wistar Rats. Saudi Pharm. J. 2021, 29, 820–832. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Zhou, D.; Yang, J.; Zhang, D. Doxorubicin Promotes Breast Cancer Cell Migration and Invasion via DCAF13. FEBS Open Bio. 2021, 12, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Augoff, K.; Hryniewicz-Jankowska, A.; Tabola, R.; Stach, K. MMP9: A Tough Target for Targeted Therapy for Cancer. Cancers 2022, 14, 1847. [Google Scholar] [CrossRef]
- Park, D.; Park, S.; Kim, J.-C. In vitro anti-cancer efficacy and cellular interaction of cubic phases of containing cinnamic acid, poly(ethyleneimine) and doxorubicin. Biotechnol. Bioprocess Eng. 2020, 25, 235–245. [Google Scholar] [CrossRef]
- Kostrhunova, H.; Zajac, J.; Markova, L.; Brabec, V.; Kasparkova, J. A multi-action Pt-IV conjugate with oleate and cinnamate ligands targets human epithelial growth factor receptor HER2 in aggressive breast cancer cells. Angew. Chem. Int. Ed. 2020, 59, 21157–21162. [Google Scholar] [CrossRef]
- Islam, S.; Aboussekhra, A. Sequential combination of cisplatin with eugenol targets ovarian cancer stem cells through Notch-Hes1 signalling pathway. J. Exp. Clin. Cancer Res. 2019, 38, 382. [Google Scholar] [CrossRef] [PubMed]
- Di Giacomo, S.; Di Sotto, A.; Mazzanti, G.; Wink, M. Chemosensitizing properties of β-Caryophyllene and β-Caryophyllene oxide in combination with doxorubicin in human cancer cells. Anticancer Res. 2017, 37, 1191–1196. [Google Scholar]
- Mohamed, S.; Helmy, M.; Mahmoud, H.; Embaby, A.; Haroun, M.; Sabra, S.A. Cinnamaldehyde/naringin co-loaded into lactoferrin/casienate-coated zein. J. Drug Del. Sci. Technol. 2024, 96, 105688. [Google Scholar] [CrossRef]
- Anselmo, D.B.; Polaquini, C.; Marques, B.; Ayusso, G.; Assis, L.; Torrezan, G.; Rahal, P.; Fachin, A.; Calmon, M.; Marins, M.; et al. Curcumin-cinnamaldehdye hybrids as antiproliferative agents against women’s cancer cells. Med. Chem. Res. 2021, 30, 2007–2015. [Google Scholar]
- Namboothiri, V.; Palakkezhillam, V.; Haribabu, J.; Kumar, V.; Manakkadan, V.; Rasin, P.; Muena, J.P.; Dharmasivam, M.; Sreekanth, A. Cinnamaldehyde-derived thiosemicarbazone ligands: Analysis combining in silico and in vitro approaches. ACS Appl. Bio Mater. 2024, 7, 5622–5639. [Google Scholar]
- Bulakowska, A.; Slawinski, J.; Halasa, R.; Hering, A.; Gucwa, M.; Ochocka, J.; Stefanowicz-Hajduk, J. An in vitro antimicrobial, anticancer and antioxidant activity of N-[(2-arylmethylthio)phenylsulfonyl] cinnamamide derivatives. Molecules 2023, 28, 3087. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, P.; Barton, V.; Ward, S. The molecular mechanism of action of artemisinin—The debate continues. Molecules 2010, 15, 1705–1721. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.-C.; Deng, T.; Fan, M.-L.; Lv, W.-B.; Liu, J.-H.; Yu, B.-Y. Synthesis and in vitro antitumor evaluation of dihydroartemesisinin-cinnamic ester derivatives. Eur. J. Med. Chem. 2016, 107, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Abourehab, M.; Alqahtani, A.; Almalki, F.; Abdalla, A.; Gouda, A.M. Pyrrolizine/indolizine-cinnamaldehyde Schiff bases: Design, synthesis, biological evaluation, ADME, molecular docking study. Eur. J. Med. Chem. Rep. 2022, 4, 100036. [Google Scholar] [CrossRef]
- Wang, S.R.; Yang, W.; Fan, Y.; Dehaen, W.; Li, Y.; Li, H.; Wang, W.; Zheng, Q.; Huai, Q. Design and synthesis of the novel oleanolic acid-cinnamic acid ester derivatives and glycyrrhetinic acid-cinnamic acid derivatives with cytotoxic properties. Bioorg. Chem. 2019, 88, 102951. [Google Scholar] [CrossRef]
- Reddy, N.D.; Shoja, M.H.; Biswas, S.; Nayak, P.G.; Kumar, N.; Rao, C.M. An appraisal of cinnamyl sulfonamide hydroxamate derivatives (HDAC inhibitors) for anti-cancer, anti-angiogenic and anti-metastatic activities in human cancer cells. Chem. Biol. Interact. 2016, 253, 112–124. [Google Scholar] [CrossRef]
- Pavić, K.; Perković, I.; Gilja, P.; Kozlina, F.; Ester, K.; Kralj, M.; Schols, D.; Hadjipavlou-Litina, D.; Ponitiki, E.; Zorc, B. Design, synthesis and biological evaluation of novel primaquine cinnamic acid conjugates of the amide and acylsemicarbazide type. Molecules 2016, 21, 1629. [Google Scholar] [CrossRef]
- Çalişkan, E.; Özturk, D.; Koran, K.; Tekin, S.; Sandal, S.; Erkan, S.; Gorgulu, A.; Çetin, A. Synthesis of new cinnamoyl-amino acid conjugates and in vitro cytotoxicity and genotoxicity studies. Chem. Biodivers. 2022, 19, e202200426. [Google Scholar] [CrossRef]
- Ventura-Salzar, I.; Palacios-Can, F.; Gonzalez-Maya, L.; Sanchez-Carranza, J.; Antunez-Mojica, M.; Razo-Hernandez, R.; Alvarez, L. Finding a novel chalcone-cinnamic acid chimeric compound with antiproliferative activity against MCF-7 cell line using Free-Wilson type approach. Molecules 2023, 28, 5486. [Google Scholar] [CrossRef] [PubMed]
- Ogunalakin, A.; Sonibare, M.; Yeye, O.; Jabeen, A.; Shah, S.; Ojo, O.; Gyebi, G.; Ayokunle, D. Design, synthesis and characterization of cinnamic acid derivatives with two novel acrylohydrazones on HeLa and CHO-1 cancer cell lines: The experimental and computational perspective. Chem. Afr. 2023, 7, 583–604. [Google Scholar] [CrossRef]
- Aggarwal, S.; Bhadana, K.; Singh, B.; Rawat, M.; Mohammad, T.; Al-Keridis, L.A.; Alshammari, N.; Hassan, M.I.; Das, S.N. Cinnamomum Zeylanicum Extract and Its Bioactive Component Cinnamaldehyde Show Anti-Tumor Effects via Inhibition of Multiple Cellular Pathways. Front. Pharmacol. 2022, 13, 918479. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).