Comparative Life Cycle Assessment of Recyclable Polyhydroxyurethanes Synthesized from Five- and Six-Membered Carbonates
Abstract
:1. Introduction
2. Methods
2.1. Definitions and System Boundaries
2.2. LCA Methodology
2.3. Life Cycle Inventory LCI
2.4. Chemicals and Instrument
2.5. PHU-CAN Synthesis
2.6. Energy Calculations for Organic Molecule Synthesis and for PHU-CAN Synthesis
2.7. E-Factor Definition and Calculation
3. Results and Discussion
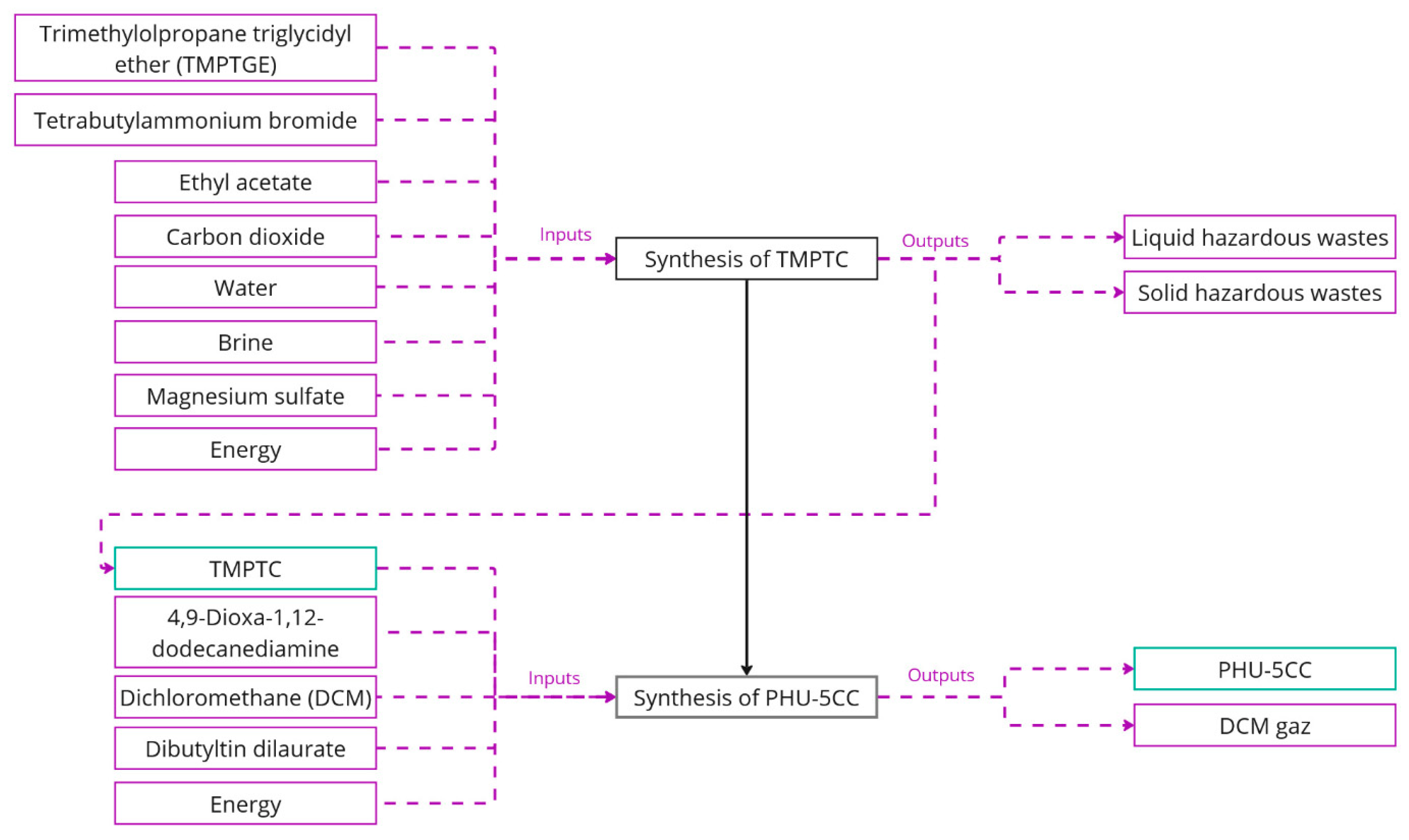
3.1. PHU-5CC-Inventories
3.2. PHU-6CC-Inventories
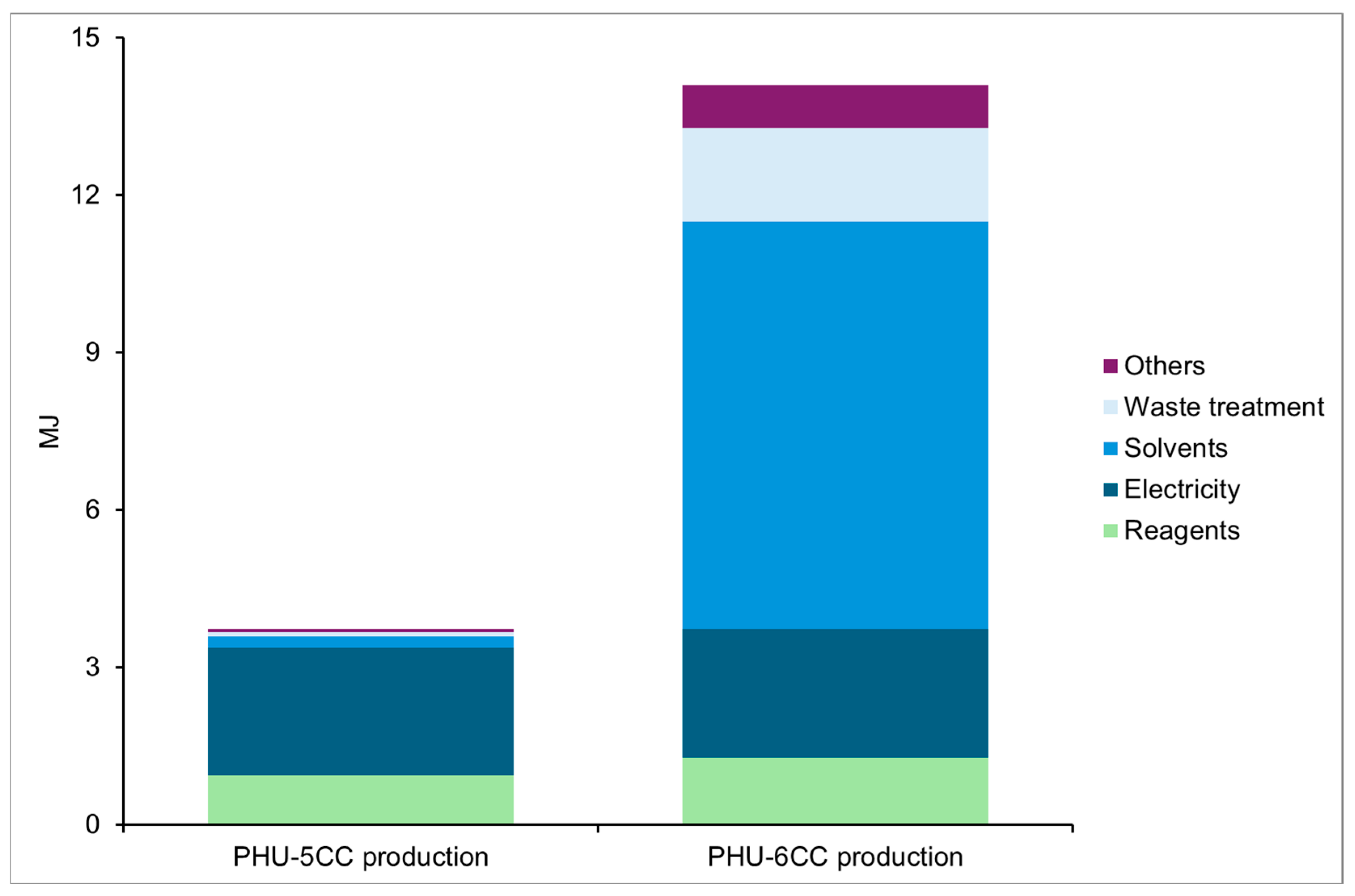
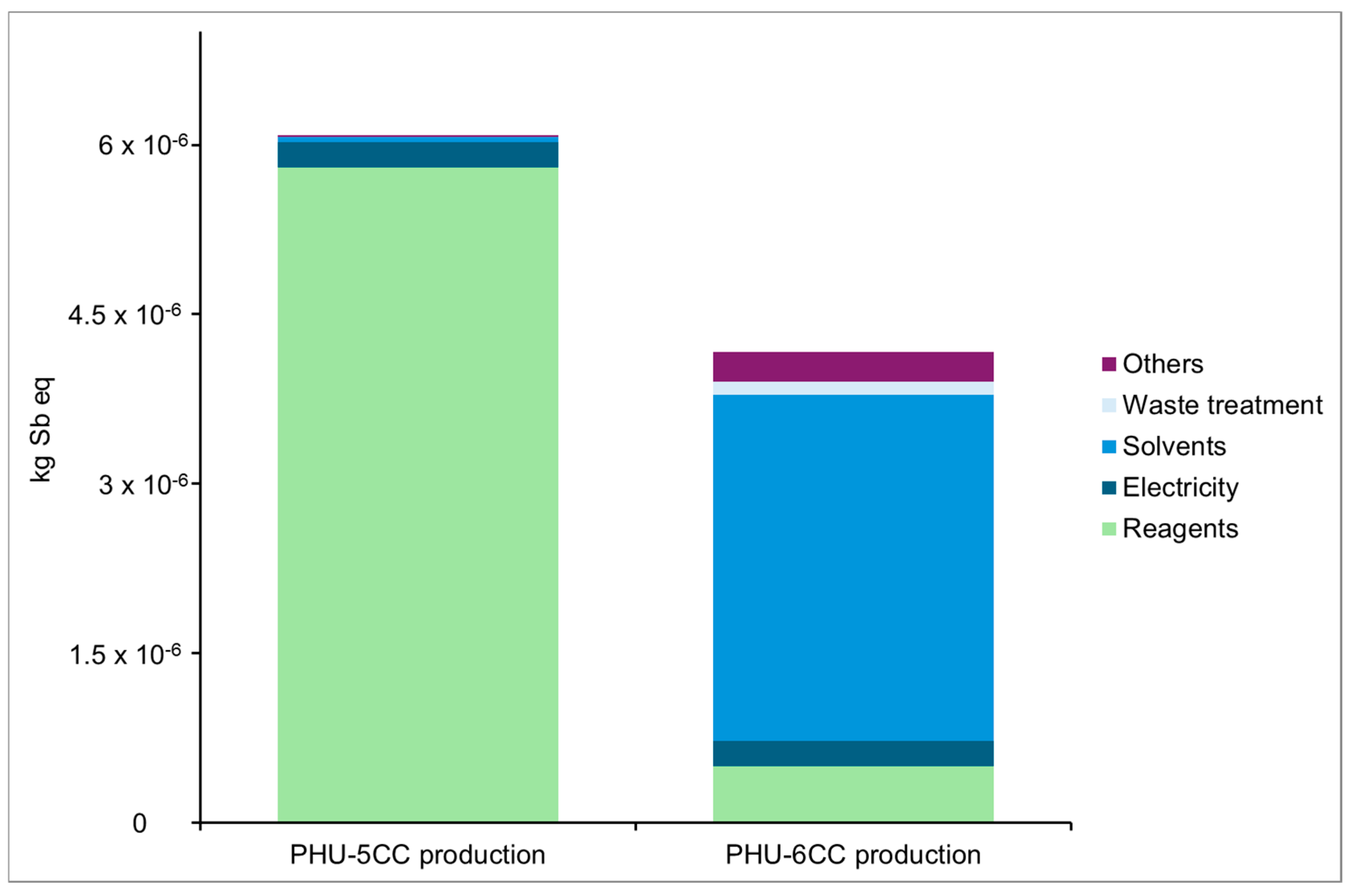
4. Impact Assessment
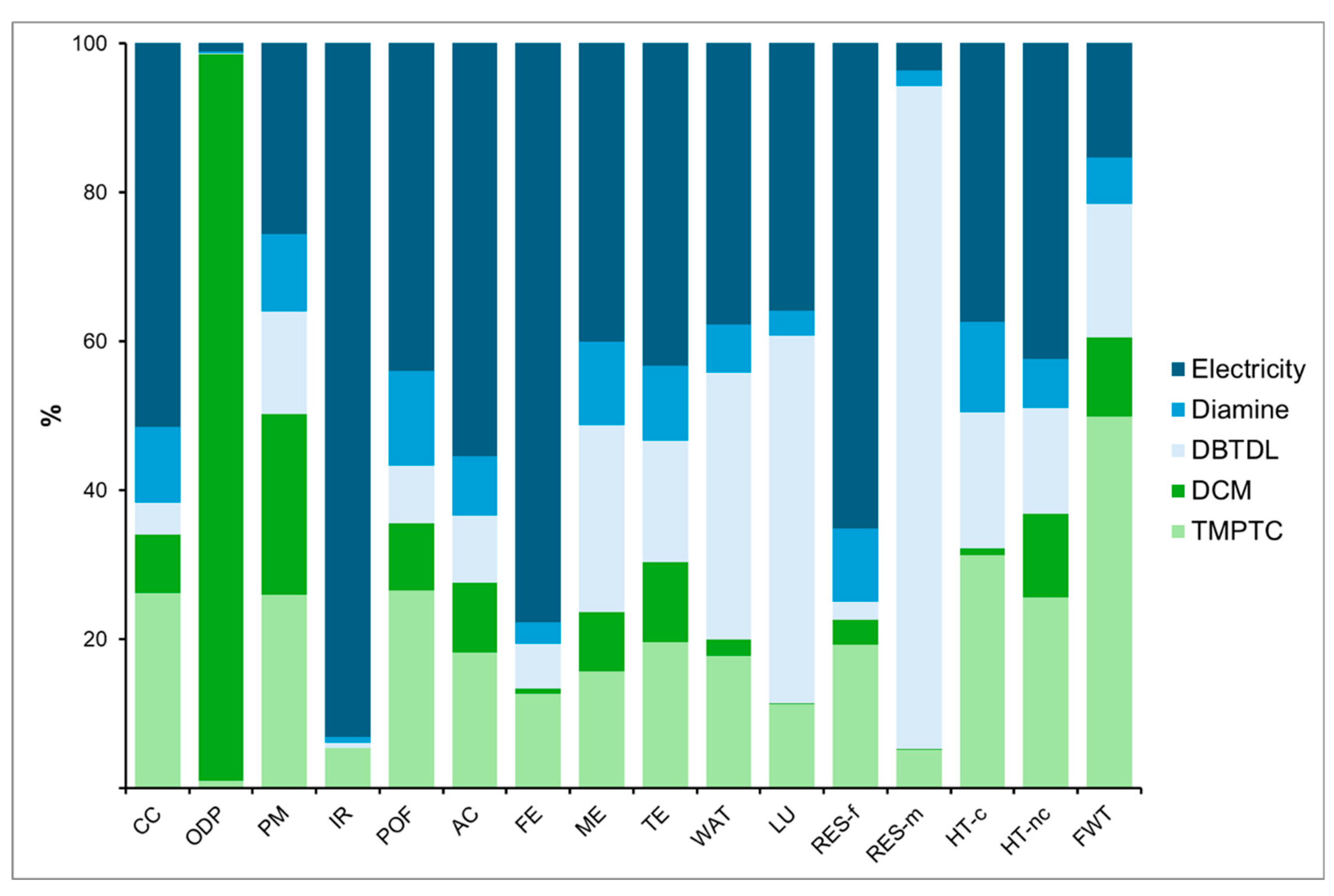
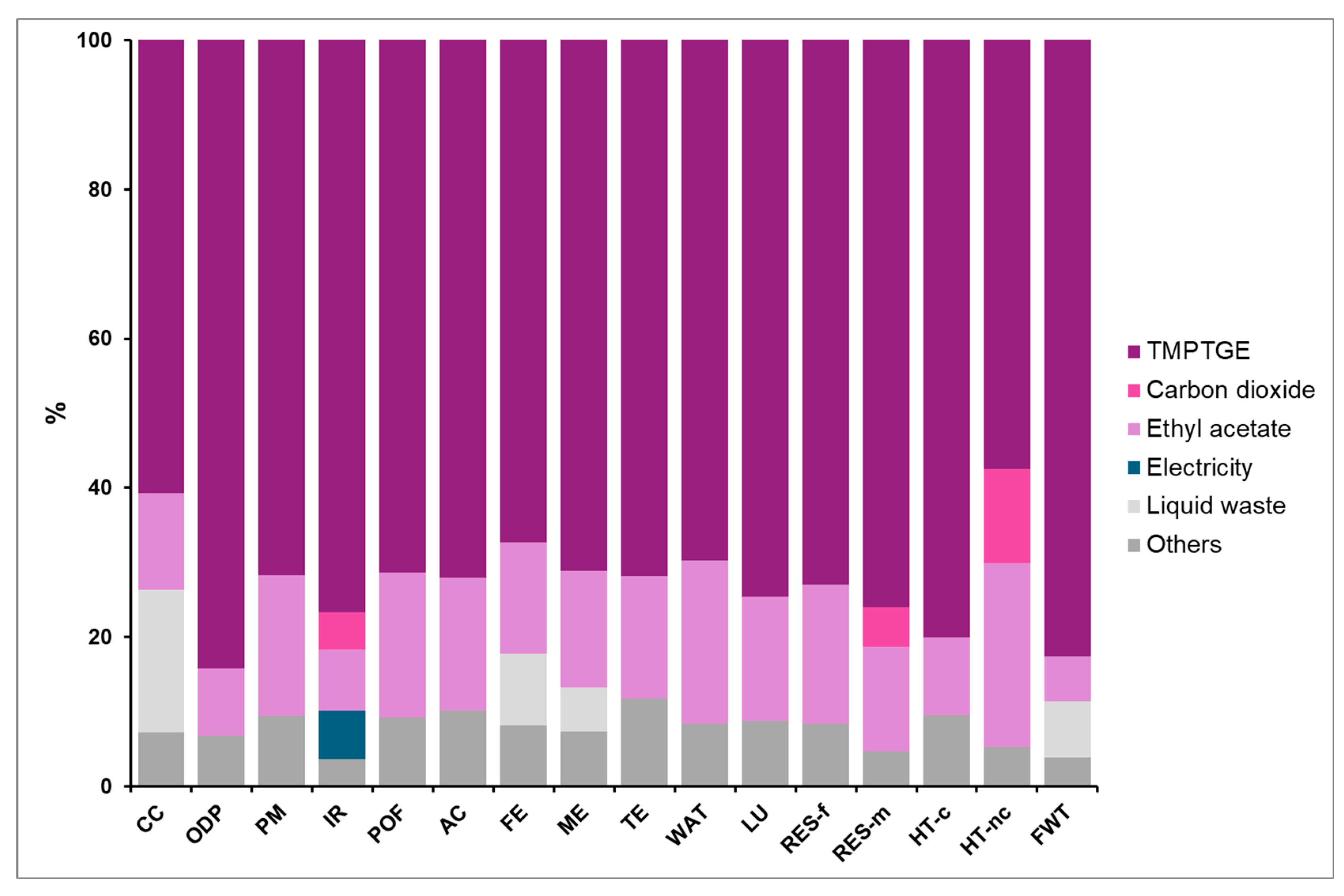
4.1. PHU-5CC Synthesis
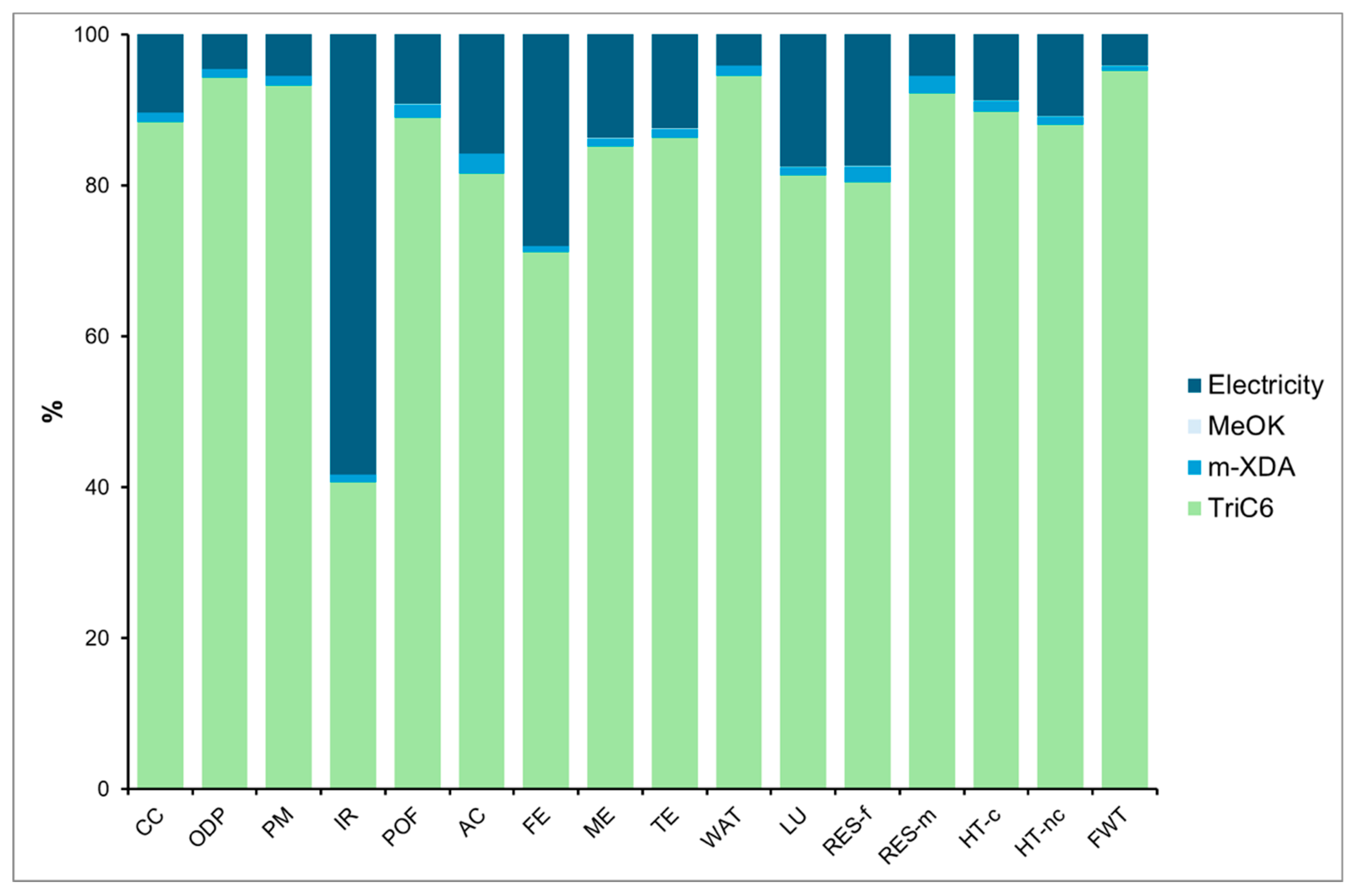
4.2. PHU-6CC Synthesis
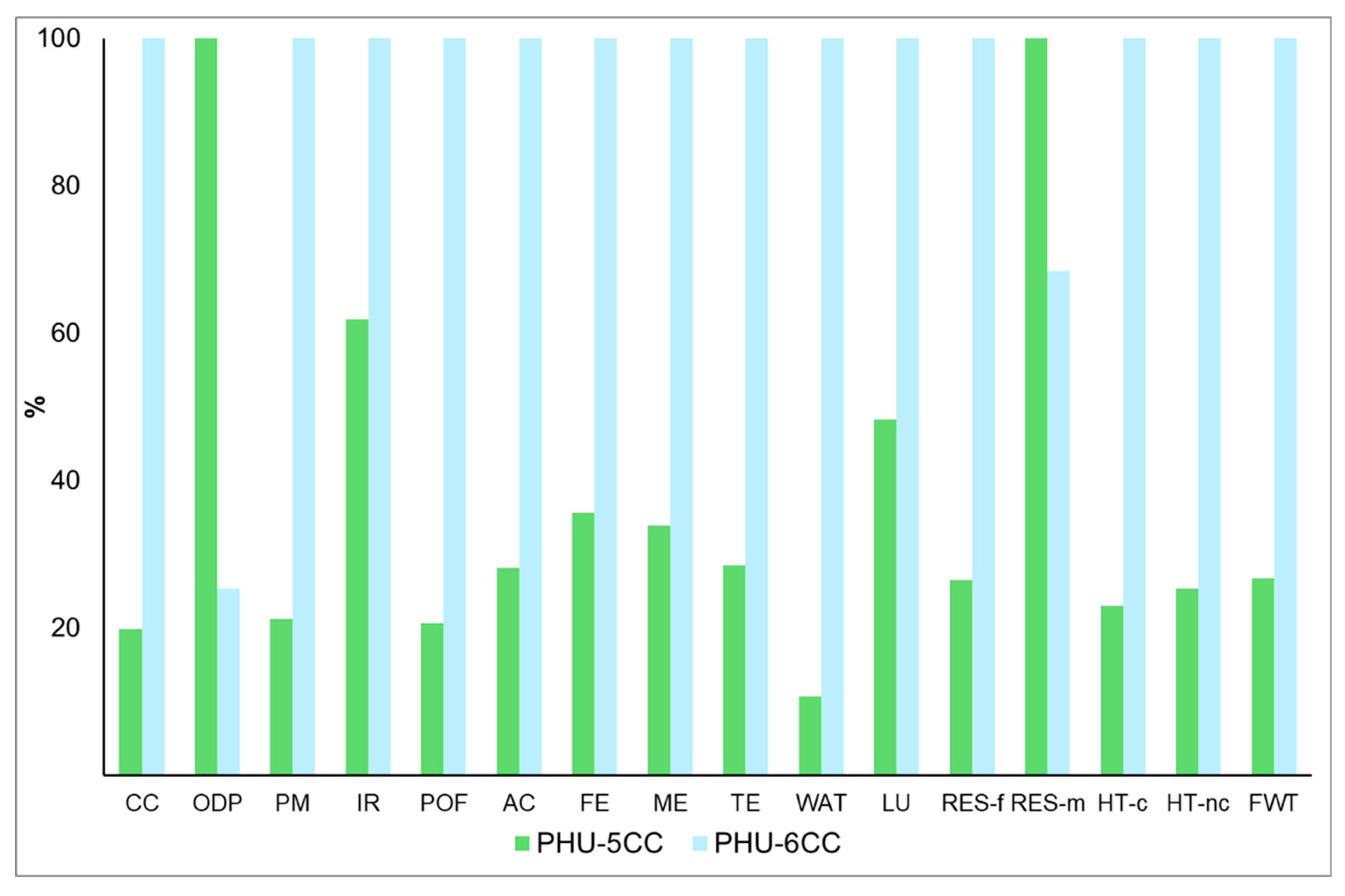
4.3. Comparison of PHU-5CC Vs. PHU-6CC Synthesis
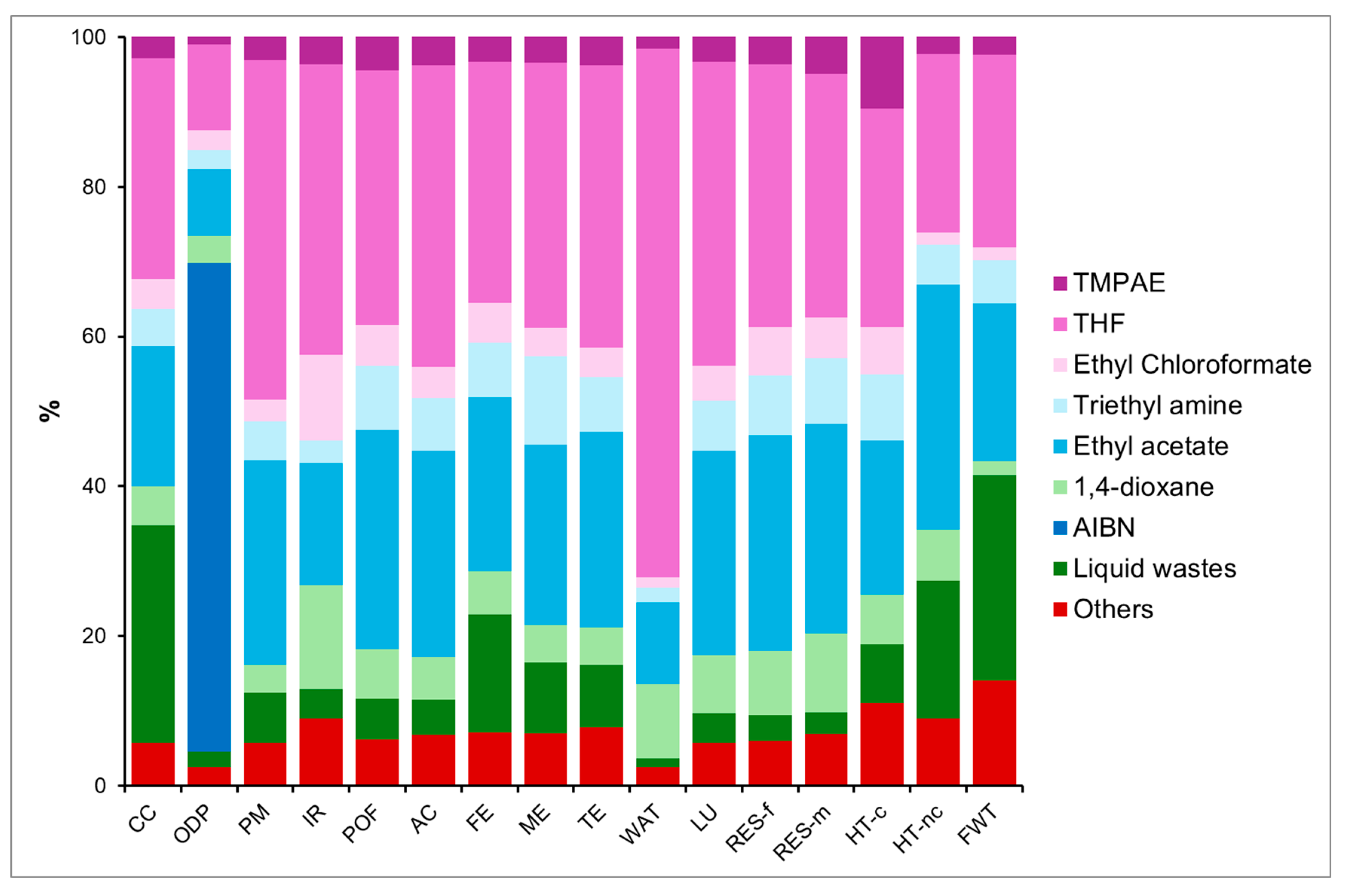
4.3.1. Global Vision of Impacts of Both Syntheses
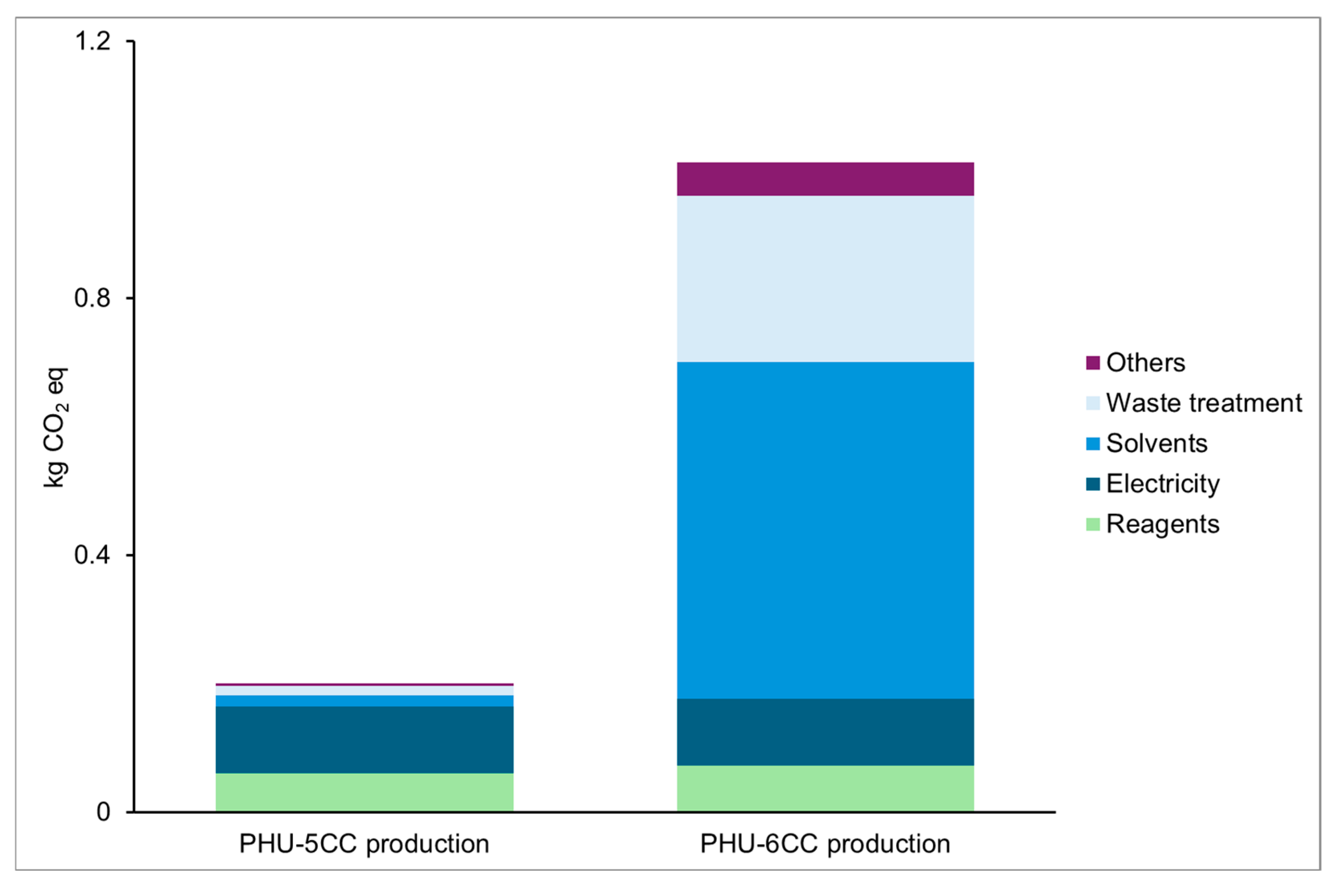
Climate Change
Particulate Matter
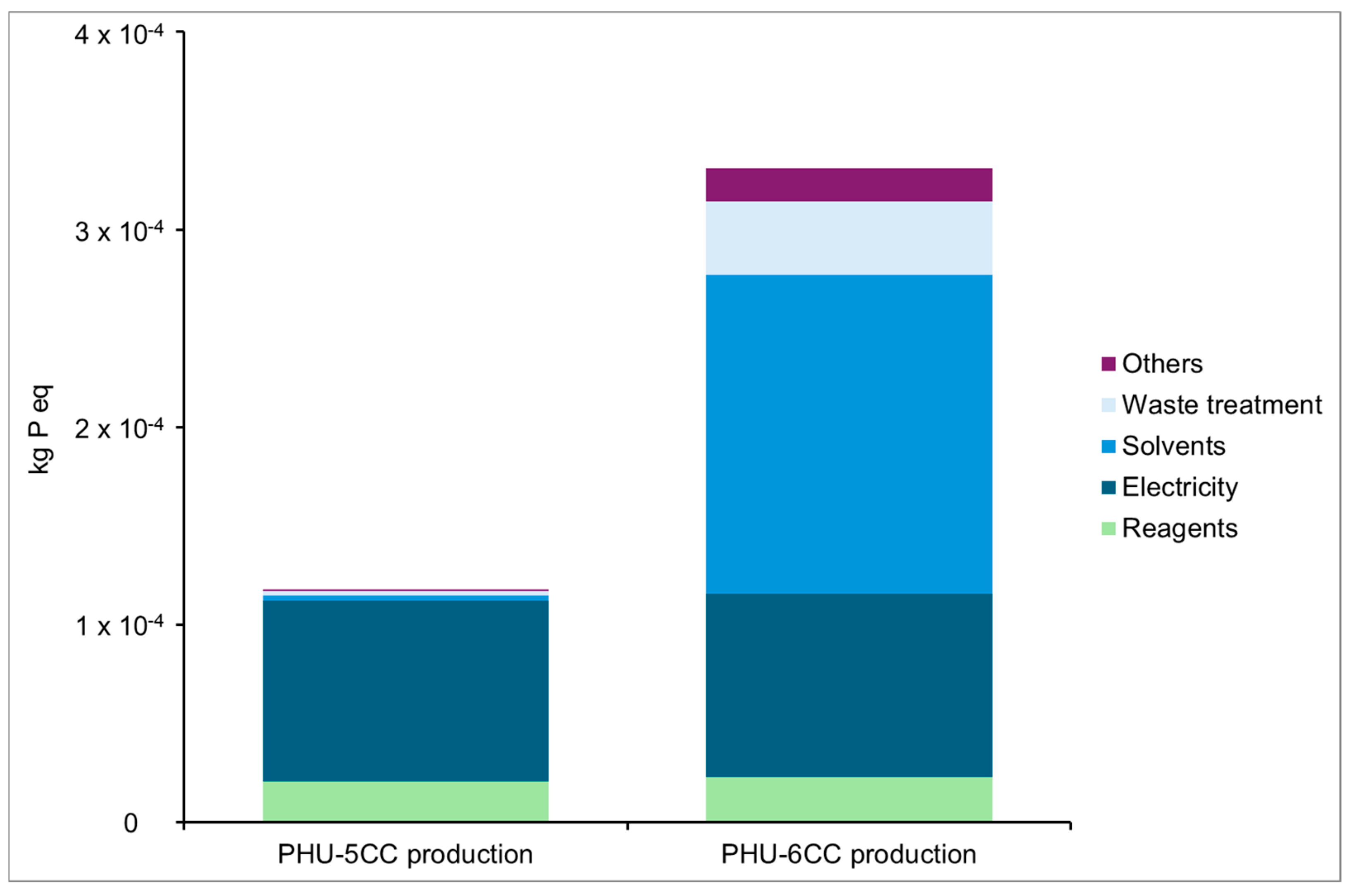
Eutrophication, Freshwater
Resource Use, Fossil
Resource Use, Minerals and Metals
Preliminary Conclusions
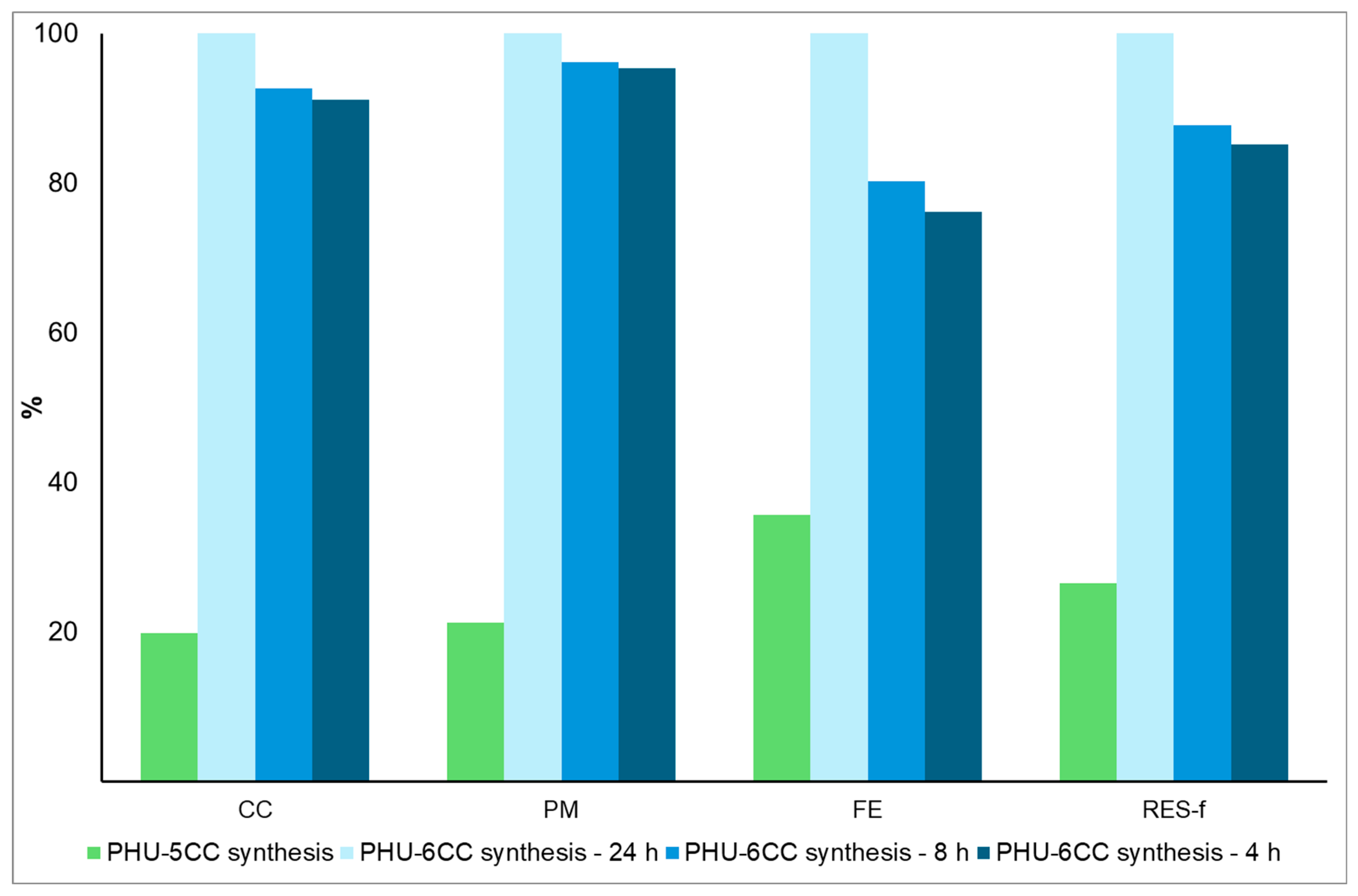
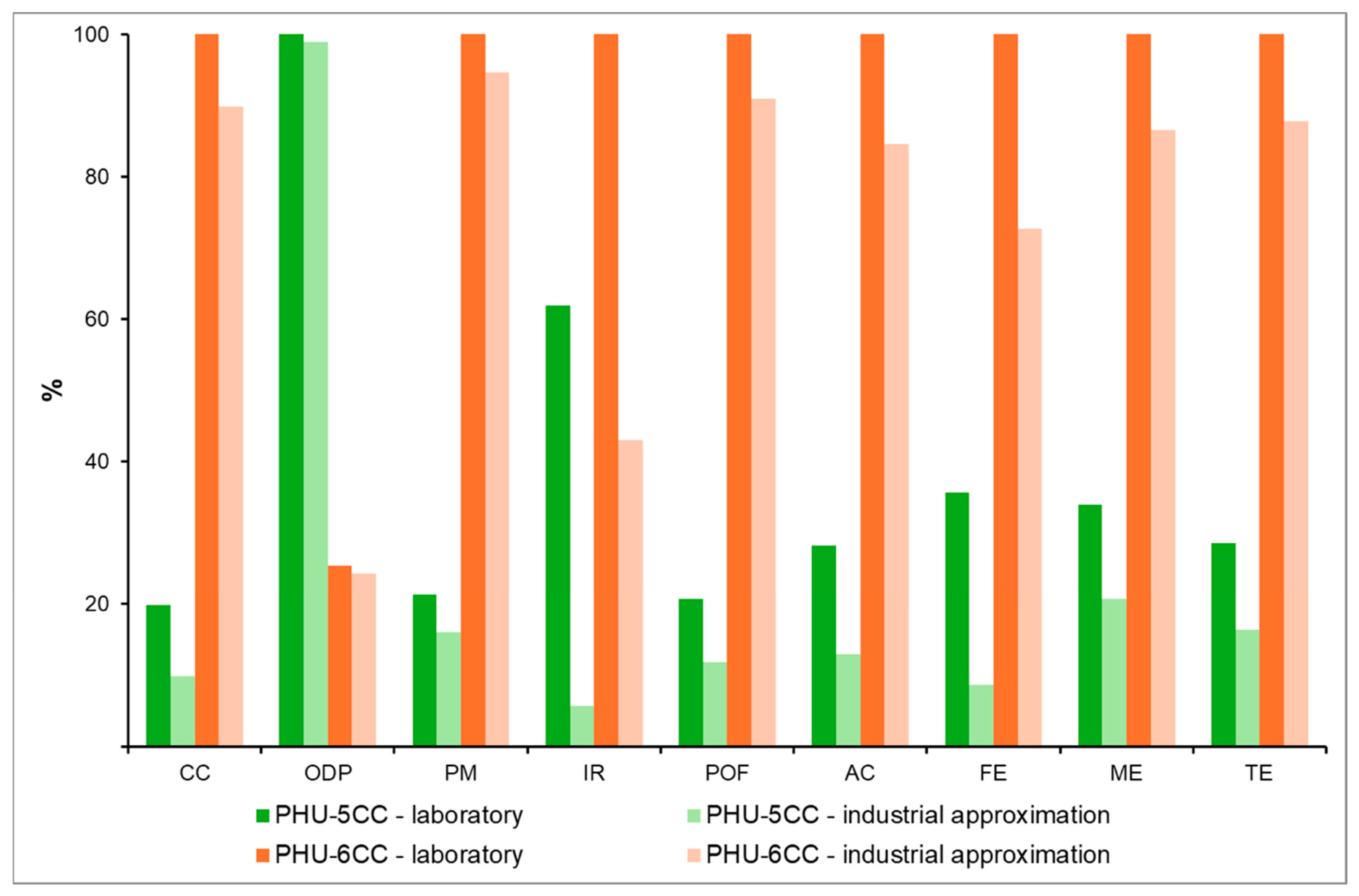
5. Sensitivity Studies
5.1. Variation in Synthesis Time
5.2. Industrial-Scale Optimization
5.3. E-Factor Calculation for Both Syntheses, and Comparison with LCA
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AC | Acidification |
| AIBN | Azobisisobutyronitrile |
| CAN | Covalent adaptable network |
| CC | Climate change |
| CFC | Chlorofluorocarbon |
| CO2 | Carbon dioxide |
| DBTDL | Dibutyltin dilaurate |
| DCM | Dichloromethane |
| Diamine | 4,9-dioxa-1,12-dodecanediamine |
| DMAP | 4-dimethylaminopyridine |
| FE | Eutrophication, freshwater |
| FU | Functional unit |
| FWT | Ecotoxicity, freshwater |
| GHG | Greenhouse gas |
| GLO | Dataset refers to global worldwide relevant data |
| HT-c | Human toxicity, cancer |
| HT-nc | Human toxicity, non-cancer |
| ILCD | International Life Cycle Data System |
| IR | Ionizing radiation |
| LCA | Life cycle assessment |
| LCI | Life cycle inventory |
| LU | Land use |
| MC6 | 5-((allyloxy)methyl)-5-ethyl-1,3-dioxan-2-one |
| ME | Eutrophication, marine |
| MeOK | Potassium methoxide |
| m-XDA | m-xylylenediamine |
| NIPU | Non-isocyanate polyurethane |
| ODP | Ozone depletion |
| P | Phosphorus |
| PHU | Polyhydroxyurethane |
| PM | Particulate matter |
| PM2.5 | Particles with a diameter of less than 2.5 μm |
| POF | Photochemical ozone formation |
| PU | Polyurethane |
| RER | Datasets refer to Europe-relevant data |
| RES-f | Resource use, fossils |
| RES-m | Resource use, minerals and metals |
| RoW | Rest-of-World-relevant data |
| SI | Supplementary Information |
| TE | Eutrophication, terrestrial |
| TEBAC | Benzyltriethylammonium chloride |
| THF | Tetrahydrofuran |
| TMPAE | Trimethylolpropane allyl ether |
| TMPTC | Trimethylolpropane triglycidyl carbonate |
| TMPTGE | Trimethylolpropane triglycidyl ether |
| TMPTM | Trimethylolpropane tris(3-mercaptoproionate) |
| TriC6 | 2-ethyl-2-(((3-((3-((5-ethyl-2-oxo-1,3-dioxan-5-yl)methoxy)propyl)thio)propanoyl)oxy) me-thyl)propane-1,3-diyl bis(3-((3-((5-ethyl-2-oxo-1,3-dioxan-5-yl)methoxy)propyl)thio)propanoate) |
| VOC | Volatile organic compound |
| WAT | Water use |
| 5CC | Five-membered cyclic carbonate |
| 6CC | Six-membered cyclic carbonate |
References
- OECD. Global Plastics Outlook: Economic Drivers, Environmental Impacts and Policy Options; OECD: Paris, France, 2022. [Google Scholar] [CrossRef]
- Delavarde, A.; Savin, G.; Derkenne, P.; Boursier, M.; Morales-Cerrada, R.; Nottelet, B.; Pinaud, J.; Caillol, S. Sustainable Polyurethanes: Toward New Cutting-Edge Opportunities. Prog. Polym. Sci. 2024, 151, 101805. [Google Scholar] [CrossRef]
- Ghosh, B.; Urban, M.W. Self-Repairing Oxetane-Substituted Chitosan Polyurethane Networks. Science 2009, 323, 1458–1460. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Fei, G.; Xia, H.; Zhao, Y. Ultrasound Healable Shape Memory Dynamic Polymers. J. Mater. Chem. A 2014, 2, 16051–16060. [Google Scholar] [CrossRef]
- Fang, Y.; Du, X.; Yang, S.; Wang, H.; Cheng, X.; Du, Z. Sustainable and Tough Polyurethane Films with Self-Healability and Flame Retardance Enabled by Reversible Chemistry and Cyclotriphosphazene. Polym. Chem. 2019, 10, 4142–4153. [Google Scholar] [CrossRef]
- Ma, S.; Webster, D.C. Degradable Thermosets Based on Labile Bonds or Linkages: A Review. Prog. Polym. Sci. 2018, 76, 65–110. [Google Scholar] [CrossRef]
- Elling, B.R.; Dichtel, W.R. Reprocessable Cross-Linked Polymer Networks: Are Associative Exchange Mechanisms Desirable? ACS Cent. Sci. 2020, 6, 1488–1496. [Google Scholar] [CrossRef]
- Morici, E.; Dintcheva, N.T. Recycling of Thermoset Materials and Thermoset-Based Composites: Challenge and Opportunity. Polymers 2022, 14, 4153. [Google Scholar] [CrossRef]
- Li, X.; Bai, R.; McKechnie, J. Environmental and Financial Performance of Mechanical Recycling of Carbon Fibre Reinforced Polymers and Comparison with Conventional Disposal Routes. J. Clean. Prod. 2016, 127, 451–460. [Google Scholar] [CrossRef]
- Alves, S.M.C.; da Silva, F.S.; Donadon, M.V.; Garcia, R.R.; Corat, E.J. Process and Characterization of Reclaimed Carbon Fiber Composites by Pyrolysis and Oxidation, Assisted by Thermal Plasma to Avoid Pollutants Emissions. J. Compos. Mater. 2018, 52, 1379–1398. [Google Scholar] [CrossRef]
- 4,4′-Diisocyanate de Diphénylméthane (FT 129). Généralités—Fiche Toxicologique—INRS. Available online: https://www.inrs.fr/publications/bdd/fichetox/fiche.html?refINRS=FICHETOX_129 (accessed on 25 May 2024).
- Diisocyanate de Tolylène (FT 46). Généralités—Fiche Toxicologique—INRS. Available online: https://www.inrs.fr/publications/bdd/fichetox/fiche.html?refINRS=FICHETOX_46 (accessed on 25 May 2024).
- Bello, D.; Herrick, C.A.; Smith, T.J.; Woskie, S.R.; Streicher, R.P.; Cullen, M.R.; Liu, Y.; Redlich, C.A. Skin Exposure to Isocyanates: Reasons for Concern. Environ. Health Perspect. 2007, 115, 328–335. [Google Scholar] [CrossRef]
- Kathalewar, M.S.; Joshi, P.B.; Sabnis, A.S.; Malshe, V.C. Non-Isocyanate Polyurethanes: From Chemistry to Applications. RSC Adv. 2013, 3, 4110. [Google Scholar] [CrossRef]
- Kloxin, C.J.; Scott, T.F.; Adzima, B.J.; Bowman, C.N. Covalent Adaptable Networks (CANs): A Unique Paradigm in Cross-Linked Polymers. Macromolecules 2010, 43, 2643–2653. [Google Scholar] [CrossRef] [PubMed]
- Bowman, C.N.; Kloxin, C.J. Covalent Adaptable Networks: Reversible Bond Structures Incorporated in Polymer Networks. Angew. Chem. Int. Ed. 2012, 51, 4272–4274. [Google Scholar] [CrossRef]
- Kloxin, C.J.; Bowman, C.N. Covalent Adaptable Networks: Smart, Reconfigurable and Responsive Network Systems. Chem. Soc. Rev. 2013, 42, 7161–7173. [Google Scholar] [CrossRef]
- Fortman, D.J.; Brutman, J.P.; Cramer, C.J.; Hillmyer, M.A.; Dichtel, W.R. Mechanically Activated, Catalyst-Free Polyhydroxyurethane Vitrimers. J. Am. Chem. Soc. 2015, 137, 14019–14022. [Google Scholar] [CrossRef] [PubMed]
- Scott, T.F.; Schneider, A.D.; Cook, W.D.; Bowman, C.N. Photoinduced Plasticity in Cross-Linked Polymers. Science 2005, 308, 1615–1617. [Google Scholar] [CrossRef] [PubMed]
- García, F.; Pelss, J.; Zuilhof, H.; Smulders, M.M.J. Multi-Responsive Coordination Polymers Utilising Metal-Stabilised, Dynamic Covalent Imine Bonds. Chem. Commun. 2016, 52, 9059–9062. [Google Scholar] [CrossRef]
- Amamoto, Y.; Kamada, J.; Otsuka, H.; Takahara, A.; Matyjaszewski, K. Repeatable Photoinduced Self-Healing of Covalently Cross-Linked Polymers through Reshuffling of Trithiocarbonate Units. Angew. Chem. Int. Ed. 2011, 50, 1660–1663. [Google Scholar] [CrossRef]
- Montarnal, D.; Capelot, M.; Tournilhac, F.; Leibler, L. Silica-Like Malleable Materials from Permanent Organic Networks. Science 2011, 334, 965–968. [Google Scholar] [CrossRef]
- Dannecker, P.-K.; Meier, M.A.R. Facile and Sustainable Synthesis of Erythritol Bis(Carbonate), a Valuable Monomer for Non-Isocyanate Polyurethanes (NIPUs). Sci. Rep. 2019, 9, 9858. [Google Scholar] [CrossRef]
- Hu, S.; Chen, X.; Torkelson, J.M. Biobased Reprocessable Polyhydroxyurethane Networks: Full Recovery of Crosslink Density with Three Concurrent Dynamic Chemistries. ACS Sustain. Chem. Eng. 2019, 7, 10025–10034. [Google Scholar] [CrossRef]
- Nohra, B.; Candy, L.; Blanco, J.-F.; Guerin, C.; Raoul, Y.; Mouloungui, Z. From Petrochemical Polyurethanes to Biobased Polyhydroxyurethanes. Macromolecules 2013, 46, 3771–3792. [Google Scholar] [CrossRef]
- Blattmann, H.; Fleischer, M.; Bähr, M.; Mülhaupt, R. Isocyanate- and Phosgene-Free Routes to Polyfunctional Cyclic Carbonates and Green Polyurethanes by Fixation of Carbon Dioxide. Macromol. Rapid Commun. 2014, 35, 1238–1254. [Google Scholar] [CrossRef]
- Rokicki, G.; Parzuchowski, P.G.; Mazurek, M. Non-Isocyanate Polyurethanes: Synthesis, Properties, and Applications: Non-Isocyanate Polyurethanes: Synthesis, Properties, and Applications. Polym. Adv. Technol. 2015, 26, 707–761. [Google Scholar] [CrossRef]
- North, M.; Pasquale, R.; Young, C. Synthesis of Cyclic Carbonates from Epoxides and CO2. Green Chem. 2010, 12, 1514. [Google Scholar] [CrossRef]
- Mundo, F.; Caillol, S.; Ladmiral, V.; Meier, M.A.R. On Sustainability Aspects of the Synthesis of Five-Membered Cyclic Carbonates. ACS Sustain. Chem. Eng. 2024, 12, 6452–6466. [Google Scholar] [CrossRef]
- Tomita, H.; Sanda, F.; Endo, T. Reactivity Comparison of Five- and Six-Membered Cyclic Carbonates with Amines: Basic Evaluation for Synthesis of Poly(Hydroxyurethane). J. Polym. Sci. Part Polym. Chem. 2001, 39, 162–168. [Google Scholar] [CrossRef]
- Fortman, D.J.; Brutman, J.P.; Hillmyer, M.A.; Dichtel, W.R. Structural Effects on the Reprocessability and Stress Relaxation of Crosslinked Polyhydroxyurethanes. J. Appl. Polym. Sci. 2017, 134, 44984. [Google Scholar] [CrossRef]
- Wu, H.; Jin, B.; Wang, H.; Wu, W.; Cao, Z.; Wu, J.; Huang, G. A Degradable and Self-Healable Vitrimer Based on Non-Isocyanate Polyurethane. Front. Chem. 2020, 8, 585569. [Google Scholar] [CrossRef]
- Chen, X.; Li, L.; Jin, K.; Torkelson, J.M. Reprocessable Polyhydroxyurethane Networks Exhibiting Full Property Recovery and Concurrent Associative and Dissociative Dynamic Chemistry via Transcarbamoylation and Reversible Cyclic Carbonate Aminolysis. Polym. Chem. 2017, 8, 6349–6355. [Google Scholar] [CrossRef]
- Adeel, M.; Zhao, B.; Li, L.; Zheng, S. Nanocomposites of Poly(Hydroxyurethane)s with Multiwalled Carbon Nanotubes: Synthesis, Shape Memory, and Reprocessing Properties. ACS Appl. Polym. Mater. 2020, 2, 1711–1721. [Google Scholar] [CrossRef]
- Liu, J.; Miao, P.; Leng, X.; Che, J.; Wei, Z.; Li, Y. Chemically Recyclable Biobased Non-Isocyanate Polyurethane Networks from CO2-Derived Six-Membered Cyclic Carbonates. Macromol. Rapid Commun. 2023, 44, 2300263. [Google Scholar] [CrossRef]
- Zhang, T.; Xue, B.; Yan, Q.; Yuan, Y.; Tan, J.; Guan, Y.; Wen, J.; Li, X.; Zhao, W. New Kinds of Lignin/Non-Isocyanate Polyurethane Hybrid Polymers: Facile Synthesis, Smart Properties and Adhesive Applications. Ind. Crops Prod. 2023, 199, 116706. [Google Scholar] [CrossRef]
- Miao, P.; Leng, X.; Liu, J.; Xu, X.; Li, Y. Chemically Recyclable and Mechanically Robust Non-Isocyanate Polyurethanes from Resveratrol. Polym. Chem. 2023, 14, 4216–4226. [Google Scholar] [CrossRef]
- Saeed, M.U.; Hang, G.; Gao, Y.; Hu, J.; Li, L.; Zhang, T.; Zheng, S. Reprocessing, Shape Recovery and Dielectric Properties of Nanocomposites of Polyhydroxyurethane with Barium Titanate. React. Funct. Polym. 2024, 202, 105986. [Google Scholar] [CrossRef]
- He, Y.; Keul, H.; Möller, M. Synthesis, Characterization, and Application of a Bifunctional Coupler Containing a Five- and a Six-Membered Ring Carbonate. React. Funct. Polym. 2011, 71, 175–186. [Google Scholar] [CrossRef]
- Maisonneuve, L.; Wirotius, A.-L.; Alfos, C.; Grau, E.; Cramail, H. Fatty Acid-Based (Bis) 6-Membered Cyclic Carbonates as Efficient Isocyanate Free Poly(Hydroxyurethane) Precursors. Polym. Chem. 2014, 5, 6142–6147. [Google Scholar] [CrossRef]
- Chesterman, J.P.; Chen, F.; Brissenden, A.J.; Amsden, B.G. Synthesis of Cinnamoyl and Coumarin Functionalized Aliphatic Polycarbonates. Polym. Chem. 2017, 8, 7515–7528. [Google Scholar] [CrossRef]
- Tomita, H.; Sanda, F.; Endo, T. Polyaddition Behavior of Bis(Five- and Six-Membered Cyclic Carbonate)s with Diamine. J. Polym. Sci. Part Polym. Chem. 2001, 39, 860–867. [Google Scholar] [CrossRef]
- Tomita, H.; Sanda, F.; Endo, T. Self-Polyaddition of Six-Membered Cyclic Carbonate Having Fmoc-Protected Amino Group: Novel Synthetic Method of Polyhydroxyurethane. Macromolecules 2001, 34, 7601–7607. [Google Scholar] [CrossRef]
- Asgari, A.; Parak, M.; Nourian, Y.H.; Ghanei, M. Phosgene Toxicity Clinical Manifestations and Treatment: A Systematic Review. Cell J. Yakhteh 2024, 26, 91. [Google Scholar] [CrossRef]
- Matsukizono, H.; Endo, T. Phosgene-Free Syntheses and Hydrolytic Properties of Water-Soluble Polyhydroxyurethanes with Ester–Carbonate–Ether Structures in Their Main Chains. Macromol. Chem. Phys. 2017, 218, 1700043. [Google Scholar] [CrossRef]
- Matsukizono, H.; Endo, T. Reworkable Polyhydroxyurethane Films with Reversible Acetal Networks Obtained from Multifunctional Six-Membered Cyclic Carbonates. J. Am. Chem. Soc. 2018, 140, 884–887. [Google Scholar] [CrossRef]
- Matsukizono, H.; Endo, T. Synthesis and Hydrolytic Properties of Water-Soluble Poly(Carbonate–Hydroxyurethane)s from Trimethylolpropane. Polym. Chem. 2016, 7, 958–969. [Google Scholar] [CrossRef]
- Matsukizono, H.; Endo, T. Ring-Opening Polymerization of Six-Membered Cyclic Carbonates Initiated by Ethanol Amine Derivatives and Their Application to Protonated or Quaternary Ammonium Salt-Functionalized Polycarbonate Films. J. Polym. Sci. Part Polym. Chem. 2016, 54, 487–497. [Google Scholar] [CrossRef]
- Matsukizono, H.; Endo, T. Mono- and Bifunctional Six-Membered Cyclic Carbonates Synthesized by Diphenyl Carbonate toward Networked Polycarbonate Films. J. Appl. Polym. Sci. 2015, 132, 41956. [Google Scholar] [CrossRef]
- Matsukizono, H.; Endo, T. Synthesis of Polyhydroxyurethanes from Di(Trimethylolpropane) and Their Application to Quaternary Ammonium Chloride-Functionalized Films. RSC Adv. 2015, 5, 71360–71369. [Google Scholar] [CrossRef]
- Jehanno, C.; Demarteau, J.; Mantione, D.; Arno, M.C.; Ruipérez, F.; Hedrick, J.L.; Dove, A.P.; Sardon, H. Synthesis of Functionalized Cyclic Carbonates through Commodity Polymer Upcycling. ACS Macro Lett. 2020, 9, 443–447. [Google Scholar] [CrossRef]
- Bakkali-Hassani, C.; Berne, D.; Bron, P.; Irusta, L.; Sardon, H.; Ladmiral, V.; Caillol, S. Polyhydroxyurethane Covalent Adaptable Networks: Looking for Suitable Catalysts. Polym. Chem. 2023, 14, 3610–3620. [Google Scholar] [CrossRef]
- Wernet, G.; Conradt, S.; Isenring, H.P.; Jiménez-González, C.; Hungerbühler, K. Life Cycle Assessment of Fine Chemical Production: A Case Study of Pharmaceutical Synthesis. Int. J. Life Cycle Assess. 2010, 15, 294–303. [Google Scholar] [CrossRef]
- Lee, C.K.; Khoo, H.H.; Tan, R.B.H. Life Cyle Assessment Based Environmental Performance Comparison of Batch and Continuous Processing: A Case of 4-d-Erythronolactone Synthesis. Org. Process Res. Dev. 2016, 20, 1937–1948. [Google Scholar] [CrossRef]
- Liang, C.; Jadidi, Y.; Chen, Y.; Gracida-Alvarez, U.; Torkelson, J.M.; Hawkins, T.R.; Dunn, J.B. Techno-Economic Analysis and Life Cycle Assessment of Biomass-Derived Polyhydroxyurethane and Nonisocyanate Polythiourethane Production and Reprocessing. ACS Sustain. Chem. Eng. 2024, 12, 12161–12170. [Google Scholar] [CrossRef] [PubMed]
- Sanfelix, J.; Poussard, L.; Mariage, J.; Grignard, B.; Talon, O. Checking the Expected Environmental Benefits of a R&D Project by LCA A Biobased NIPU Foam Case Study. In Proceedings of the avniR Conference 2017—Life Cycle Management, Lille, France, 8–9 November 2017. [Google Scholar] [CrossRef]
- Finkbeiner, M.; Inaba, A.; Tan, R.; Christiansen, K.; Klüppel, H.-J. The New International Standards for Life Cycle Assessment: ISO 14040 and ISO 14044. Int. J. Life Cycle Assess. 2006, 11, 80–85. [Google Scholar] [CrossRef]
- European Platform on LCA|EPLCA. Available online: https://eplca.jrc.ec.europa.eu/ilcd.html (accessed on 13 August 2024).
- Joint Research Centre (European Commission); Andreasi Bassi, S.; Biganzoli, F.; Ferrara, N.; Amadei, A.; Valente, A.; Sala, S.; Ardente, F. Updated Characterisation and Normalisation Factors for the Environmental Footprint 3.1 Method; Publications Office of the European Union: Luxembourg, 2023. [Google Scholar]
- Coste, G.; Denis, M.; Sonnier, R.; Caillol, S.; Negrell, C. Synthesis of Reactive Phosphorus-Based Carbonate for Flame Retardant Polyhydroxyurethane Foams. Polym. Degrad. Stab. 2022, 202, 110031. [Google Scholar] [CrossRef]
- Coste, G.; Berne, D.; Ladmiral, V.; Negrell, C.; Caillol, S. Non-Isocyanate Polyurethane Foams Based on Six-Membered Cyclic Carbonates. Eur. Polym. J. 2022, 176, 111392. [Google Scholar] [CrossRef]
- Piccinno, F.; Hischier, R.; Seeger, S.; Som, C. From Laboratory to Industrial Scale: A Scale-up Framework for Chemical Processes in Life Cycle Assessment Studies. J. Clean. Prod. 2016, 135, 1085–1097. [Google Scholar] [CrossRef]
- Muegge. Estimation de l’énergie Nécessaire au Séchage des Matériaux par Micro-Ondes. Muegge. Available online: https://muegge-group.com/fr/estimation-de-lenergie-necessaire-au-sechage-des-materiaux-par-micro-ondes/ (accessed on 9 August 2024).
- Sheldon, R.A. The E Factor at 30: A Passion for Pollution Prevention. Green Chem. 2023, 25, 1704–1728. [Google Scholar] [CrossRef]
- Sheldon, R.A. The E Factor: Fifteen Years On. Green Chem. 2007, 9, 1273–1283. [Google Scholar] [CrossRef]
- Roth, M.; Wolleb, H.; Truffer, M.-A. Process for the Preparation of Glycidyl Ethers. US5162547A, 10 November 1992. Available online: https://patents.google.com/patent/US5162547A/en (accessed on 29 July 2024).
- Santiago, D.; Guzmán, D.; Ferrando, F.; Serra, À.; De la Flor, S. Bio-Based Epoxy Shape-Memory Thermosets from Triglycidyl Phloroglucinol. Polymers 2020, 12, 542. [Google Scholar] [CrossRef]
- Chen, B.; Li, J.; Yu, G.; Xu, R.; Dai, C. Environmental Sustainability of π-Electron Donor-Based Deep Eutectic Solvents for Toluene Absorption: A Life-Cycle Perspective. ChemSusChem 2024, 17, e202301310. [Google Scholar] [CrossRef]
- Glotz, G.; Waniek, K.; Schöggl, J.-P.; Cantillo, D.; Stueckler, C.; Arzt, A.; Gollner, A.; Schipfer, R.; Baumgartner, R.J.; Kappe, C.O. Continuous Flow Synthesis of a Blocked Polyisocyanate: Process Intensification, Reaction Monitoring Via In-Line FTIR Analysis, and Comparative Life Cycle Assessment. Org. Process Res. Dev. 2021, 25, 2367–2379. [Google Scholar] [CrossRef]
- Rozsa, L.; Meszaros, L.; Mogyorodi, F. Method for Producing Chloroformates. US3966786A, 29 June 1976. Available online: https://patents.google.com/patent/US3966786A/en (accessed on 7 August 2024).
- Ang, P.; Mothe, S.R.; Chennamaneni, L.R.; Aidil, F.; Khoo, H.H.; Thoniyot, P. Laboratory-Scale Life-Cycle Assessment: A Comparison of Existing and Emerging Methods of Poly(ε-Caprolactone) Synthesis. ACS Sustain. Chem. Eng. 2021, 9, 669–683. [Google Scholar] [CrossRef]
- Lavery, N.P.; Jarvis, D.J.; Brown, S.G.R.; Adkins, N.J.; Wilson, B.P. Life Cycle Assessment of Sponge Nickel Produced by Gas Atomisation for Use in Industrial Hydrogenation Catalysis Applications. Int. J. Life Cycle Assess. 2013, 18, 362–376. [Google Scholar] [CrossRef]
- Gagnon, L.; Bélanger, C.; Uchiyama, Y. Life-Cycle Assessment of Electricity Generation Options: The Status of Research in Year 2001. Energy Policy 2002, 30, 1267–1278. [Google Scholar] [CrossRef]
- Spellman, F.R. Environmental Impacts of Renewable Energy; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Dinc, C.; Badea, A.; Apostol, T. Life Cycle Impact Assessment Of Fossil Fuels. Nat. Gas 2010, 9, 116–126. [Google Scholar]
- Nian, V.; Chou, S.K.; Su, B.; Bauly, J. Life Cycle Analysis on Carbon Emissions from Power Generation—The Nuclear Energy Example. Appl. Energy 2014, 118, 68–82. [Google Scholar] [CrossRef]
- Motuzienė, V.; Čiuprinskas, K.; Rogoža, A.; Lapinskienė, V. A Review of the Life Cycle Analysis Results for Different Energy Conversion Technologies. Energies 2022, 15, 8488. [Google Scholar] [CrossRef]
- Caetano, N.S.; Martins, F.F.; Oliveira, G.M. 2—Life Cycle Assessment of Renewable Energy Technologies. In The Renewable Energy-Water-Environment Nexus; Jafarinejad, S., Beckingham, B.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 37–79. [Google Scholar] [CrossRef]
- Fthenakis, V.; Kim, H.C. Land Use and Electricity Generation: A Life-Cycle Analysis. Renew. Sustain. Energy Rev. 2009, 13, 1465–1474. [Google Scholar] [CrossRef]
- Su, Z.; Zhang, Y.; Liu, B.; Lu, M.; Li, G.; Jiang, T. Extraction and Separation of Tin from Tin-Bearing Secondary Resources: A Review. JOM 2017, 69, 2364–2372. [Google Scholar] [CrossRef]
- Liu, Y.J.; Li, Y. Synthesis Method of Dibutyltin Dilaurate. CN102838631A, 26 December 2012. Available online: https://patents.google.com/patent/CN102838631A/en (accessed on 27 August 2024).
- Dibutyltin Dilaurate|Cameo Chemicals|NOAA. Available online: https://cameochemicals.noaa.gov/chemical/20133 (accessed on 27 August 2024).
- Guinee, J.B. Handbook on Life Cycle Assessment Operational Guide to the ISO Standards. Int. J. Life Cycle Assess. 2002, 7, 311–313. [Google Scholar] [CrossRef]
- Poste, A.E.; Grung, M.; Wright, R.F. Amines and Amine-Related Compounds in Surface Waters: A Review of Sources, Concentrations and Aquatic Toxicity. Sci. Total Environ. 2014, 481, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Bansod, Y.; Pawanipagar, P.; Ghasemzadeh, K.; D’Agostino, C. Environmental Sustainability Evaluation of Glycerol and Propylene-Based Pathways to Acrylic Acid via Different Intermediates. Green Chem. 2024, 26, 9840–9858. [Google Scholar] [CrossRef]
- Tserpes, K.; Tzatzadakis, V. Life-Cycle Analysis and Evaluation of Mechanical Properties of a Bio-Based Structural Adhesive. Aerospace 2022, 9, 64. [Google Scholar] [CrossRef]
- Amelio, A.; Genduso, G.; Vreysen, S.; Luis, P.; Bruggen, B.V. der. Guidelines Based on Life Cycle Assessment for Solvent Selection during the Process Design and Evaluation of Treatment Alternatives. Green Chem. 2014, 16, 3045–3063. [Google Scholar] [CrossRef]
- Capello, C.; Wernet, G.; Sutter, J.; Hellweg, S.; Hungerbühler, K. A Comprehensive Environmental Assessment of Petrochemical Solvent Production. Int. J. Life Cycle Assess. 2009, 14, 467–479. [Google Scholar] [CrossRef]
- Committee on Acute Exposure Guideline Levels and National Academies of Sciences, Engineering, and Medicine. Chloroformates Acute Exposure Guideline Levels. In Acute Exposure Guideline Levels for Selected Airborne Chemicals: Volume 20; National Academies Press (US): Cambridge, MA, USA, 2016. [Google Scholar]
- Crenna, E.; Secchi, M.; Benini, L.; Sala, S. Global Environmental Impacts: Data Sources and Methodological Choices for Calculating Normalisation Factors for LCA. Int. J. LIFE CYCLE Assess. 2019, 24, 1851–1877. [Google Scholar] [CrossRef]
- Sala, S.; Crenna, E.; Secchi, M.; Pant, R. Global Normalisation Factors for the Environmental Footprint and Life Cycle Assessment; JRC Publications Repository: Luxembourg, 2017. [Google Scholar] [CrossRef]
- Available online: https://eplca.jrc.ec.europa.eu/uploads/ILCD-Handbook-Recommendations-for-Life-Cycle-Impact-Assessment-in-the-European-context.pdf (accessed on 13 August 2024).
- Barros, M.V.; Salvador, R.; Piekarski, C.M.; de Francisco, A.C.; Freire, F.M.C.S. Life Cycle Assessment of Electricity Generation: A Review of the Characteristics of Existing Literature. Int. J. Life Cycle Assess. 2020, 25, 36–54. [Google Scholar] [CrossRef]
- Morelli, B.; Hawkins, T.R.; Niblick, B.; Henderson, A.D.; Golden, H.E.; Compton, J.E.; Cooter, E.J.; Bare, J.C. Critical Review of Eutrophication Models for Life Cycle Assessment. Environ. Sci. Technol. 2018, 52, 9562–9578. [Google Scholar] [CrossRef]
- Petrescu, L.; Burca, S.; Fermeglia, M.; Mio, A.; Cormos, C.-C. Process Simulation Coupled with LCA for the Evaluation of Liquid—Liquid Extraction Processes of Phenol from Aqueous Streams. J. Water Process Eng. 2021, 41, 102077. [Google Scholar] [CrossRef]
- Zaib, Q.; Eckelman, M.J.; Yang, Y.; Kyung, D. Are Deep Eutectic Solvents Really Green?: A Life-Cycle Perspective. Green Chem. 2022, 24, 7924–7930. [Google Scholar] [CrossRef]
- Chapter 2: Emissions Trends and Drivers. Available online: https://www.ipcc.ch/report/ar6/wg3/chapter/chapter-2/ (accessed on 17 September 2024).
- Protocol to Abate Acidification, Eutrophication and Ground-Level Ozone|UNECE. Available online: https://unece.org/environment-policy/air/protocol-abate-acidification-eutrophication-and-ground-level-ozone (accessed on 17 September 2024).
- Drielsma, J.A.; Russell-Vaccari, A.J.; Drnek, T.; Brady, T.; Weihed, P.; Mistry, M.; Simbor, L.P. Mineral Resources in Life Cycle Impact Assessment—Defining the Path Forward. Int. J. Life Cycle Assess. 2016, 21, 85–105. [Google Scholar] [CrossRef]
- Nuss, P.; Eckelman, M.J. Life Cycle Assessment of Metals: A Scientific Synthesis. PLoS ONE 2014, 9, e101298. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.A.L.; Firmino, A.S.; Ferro, F.S.; Christoforo, A.L.; Leite, F.R.; Lahr, F.A.R.; Kellens, K. Life Cycle Assessment of a Hot-Pressing Machine to Manufacture Particleboards: Hotspots, Environmental Indicators, and Solutions. Int. J. Life Cycle Assess. 2020, 25, 1059–1077. [Google Scholar] [CrossRef]
- Pyo, S.-H.; Persson, P.; Lundmark, S.; Hatti-Kaul, R. Solvent-Free Lipase-Mediated Synthesis of Six-Membered Cyclic Carbonates from Trimethylolpropane and Dialkyl Carbonates. Green Chem. 2011, 13, 976. [Google Scholar] [CrossRef]
- Sakakura, T.; Kohno, K. The Synthesis of Organic Carbonates from Carbon Dioxide. Chem. Commun. 2009, 11, 1312. [Google Scholar] [CrossRef]




| Impact Category | Acronym | Recommended Methods | Method Classification |
|---|---|---|---|
| Climate change | CC | Bern model—Global Warming Potentials (GWOs) over a 100-year time horizon, IPCC | I |
| Ozone depletion | ODP | EDIP model based on the ODPs of the World Meteorological Organization (WMO) over an infinite time horizon | I |
| Particulate matter | PM | UNEP-recommended model | I |
| Ionizing radiation | IR | Human health effect model | II |
| Photochemical ozone formation | POF | LOTOS-EUROS as applied in ReCiPe | II |
| Acidification | AC | Accumulated Exceedance | II |
| Eutrophication, freshwater | FE | EUTREND model as implemented in ReCiPe | II |
| Eutrophication, marine | ME | EUTREND model as implemented in ReCiPe | II |
| Eutrophication, terrestrial | TE | Accumulated Exceedance | II |
| Water use | WAT | Available Water Remaining (AWARE) | III |
| Land use | LU | Soil quality index based on LANCA (EC-JRC) | III |
| Resource use, fossils | RES-f | CML 2002—Abiotic resource depletion, fossil fuels | III |
| Resource use, minerals and metals | RES-m | CML 2002—Abiotic resource depletion, ultimate reserves | III |
| Human toxicity, cancer | HT-c | USEtox 2.1 model | III |
| Human toxicity, non-cancer | HT-nc | USEtox 2.1 model | III |
| Ecotoxicity, freshwater | FWT | USEtox 2.1 model | III |
| Name | Process Unit/Emitted Substances | Input | Output | Unit |
|---|---|---|---|---|
| Production of TMPTC | ||||
| TMPTC | PHU—TMPTC | - | 387.7 | g |
| Trimethylolpropane triglycidyl ether | PHU—trimethylolpropane triglycidyl ether | 300 | - | g |
| Tetrabutylammonium bromide | Ethylene bromide {RER} | market (Ecoinvent) | 9.596 | - | g |
| Ethyl acetate | Ethyl acetate {GLO} | market (Ecoinvent) | 108.2 | - | g |
| Carbon dioxide | Carbon dioxide, liquid {RER} | market (Ecoinvent) | 131 | - | g |
| Water | Water, deionized {Europe without Switzerland} | market (Ecoinvent) | 320 | - | g |
| Brine solution | Sodium chloride, brine solution {GLO} | market (Ecoinvent) | 8 | - | g |
| Magnesium sulfate | Magnesium sulfate {GLO} | market (Ecoinvent) | 20 | - | g |
| Electricity | Electricity, medium voltage {RER} | market (Ecoinvent) | 0.07871962 | - | kWh |
| Heat | Heat, district or industrial, natural gas {RoW} | market (Ecoinvent) | 0.0372 | - | MJ |
| Liquid wastes | Spent solvent mixture {Europe without Switzerland} | market (Ecoinvent) | - | 436.2 | g |
| Solid wastes | Hazardous waste, for incineration {Europe without Switzerland} | market (Ecoinvent) | - | 20 | g |
| Production of PHU-5CC | ||||
| PHU-5CC | PHU-5CC | - | 17 | g |
| TMPTC | PHU—TMPTC | 10 | - | g |
| 4,9-dioxa-1,12-dodecanediamine | Hexamethylenediamine {GLO} | market (Ecoinvent) | 5.71 | - | g |
| Dichloromethane | Dichloromethane {RER} | market (Ecoinvent) | 5.32 | - | g |
| Dibutyltin dilaurate | PHU—dibutyltin dilaurate | 1.71 | - | g |
| Electricity | Electricity, medium voltage {RER} | market (Ecoinvent) | 0.54 | - | kWh |
| Liquid wastes | Spent solvent mixture {Europe without Switzerland} | market (Ecoinvent) | - | 5.31 | g |
| Air emissions | ||||
| Methane, dichloro-, HCC-30 | - | 0.01064 | g | |
| Name | Process Unit/Emitted Substances | Input | Output | Unit |
|---|---|---|---|---|
| Production of MC6 | ||||
| PHU-MC6 | PHU-MC6 | - | 45.6 | g |
| Trimethylolpropane allyl ether | Dimethyl hexanediol {GLO} | market (Ecoinvent) | 43.6 | - | g |
| Tetrahydrofuran | Tetrahydrofuran {GLO} | market (Ecoinvent) | 248.6 | - | g |
| Ethyl chloroformate | PHU—ethyl chloroformate | 78.7 | - | g |
| Triethylamine | Triethylamine {GLO} | market (Ecoinvent) | 75.9 | - | g |
| Ethyl acetate | Ethyl acetate {GLO} | market (Ecoinvent) | 144.3 | - | g |
| Hydrochloric acid solution (0,5M) | Hydrochloric acid, without water, in 30% solution state {RER}|market (Ecoinvent) | 8.6 | - | g |
| Sodium bicarbonate solution | Sodium bicarbonate {RER} | market (Ecoinvent) | 11.2 | - | g |
| Brine solution | Sodium chloride, brine solution {GLO} | market (Ecoinvent) | 8.0 | - | g |
| Water | Water, deionized {Europe without Switzerland} | market (Ecoinvent) | 472.8 | - | g |
| Magnesium sulfate | Magnesium sulfate {GLO} | market (Ecoinvent) | 20 | - | g |
| Electricity | Electricity, medium voltage {RER} | market (Ecoinvent) | 0.052192745 | - | kWh |
| Liquid wastes | Spent solvent mixture {Europe without Switzerland} | market (Ecoinvent) | - | 893.5 | g |
| Solid wastes | Hazardous waste, for incineration {Europe without Switzerland} | market (Ecoinvent) | - | 51.24 | g |
| Production of TriC6 | ||||
| TriC6 | PHU-TriC6 | - | 56 | g |
| MC6 | PHU-MC6 | 40.05 | - | g |
| 1,4-dioxane | Dioxane {RER} | market (Ecoinvent) | 66.1 | - | g |
| Azobisisobutyronitrile | PHU—azobisisobutyronitrile | 1.64 | - | g |
| Trimethylolpropane tris(3-mercaptopropionate) | Chemical, organic {GLO} | chemical production, organic (Ecoinvent) | 24.43 | - | g |
| Ethyl acetate | Ethyl acetate {GLO} | market (Ecoinvent) | 144.3 | - | g |
| Water | Water, deionized {Europe without Switzerland} | market (Ecoinvent) | 160 | - | g |
| Magnesium sulfate | Magnesium sulfate {GLO}| market (Ecoinvent) | 20 | - | g |
| Electricity | Electricity, medium voltage {RER}| market (Ecoinvent) | 0.037221292 | - | kWh |
| Liquid wastes | Spent solvent mixture {Europe without Switzerland}| market (Ecoinvent) | - | 370.5 | g |
| Solid wastes | Hazardous waste, for incineration {Europe without Switzerland}|market (Ecoinvent) | - | 20 | g |
| Production of PHU-6CC | ||||
| PHU-6CC | PHU-6CC | - | 8.4 | g |
| TriC6 | PHU-TriC6 | 7 | - | g |
| m-xylylenediamine | PHU-m-xylylenediamine | 1.4 | - | g |
| Potassium methoxyde | PHU—potassium methoxyde | 0.0637 | - | g |
| Electricity | Electricity, medium voltage {RER}| market (Ecoinvent) | 0.27 | - | kWh |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bron, P.; Talon, O.; Bakkali-Hassani, C.; Irusta, L.; Sardon, H.; Ladmiral, V.; Caillol, S. Comparative Life Cycle Assessment of Recyclable Polyhydroxyurethanes Synthesized from Five- and Six-Membered Carbonates. Macromol 2025, 5, 12. https://doi.org/10.3390/macromol5010012
Bron P, Talon O, Bakkali-Hassani C, Irusta L, Sardon H, Ladmiral V, Caillol S. Comparative Life Cycle Assessment of Recyclable Polyhydroxyurethanes Synthesized from Five- and Six-Membered Carbonates. Macromol. 2025; 5(1):12. https://doi.org/10.3390/macromol5010012
Chicago/Turabian StyleBron, Pauline, Olivier Talon, Camille Bakkali-Hassani, Lourdes Irusta, Haritz Sardon, Vincent Ladmiral, and Sylvain Caillol. 2025. "Comparative Life Cycle Assessment of Recyclable Polyhydroxyurethanes Synthesized from Five- and Six-Membered Carbonates" Macromol 5, no. 1: 12. https://doi.org/10.3390/macromol5010012
APA StyleBron, P., Talon, O., Bakkali-Hassani, C., Irusta, L., Sardon, H., Ladmiral, V., & Caillol, S. (2025). Comparative Life Cycle Assessment of Recyclable Polyhydroxyurethanes Synthesized from Five- and Six-Membered Carbonates. Macromol, 5(1), 12. https://doi.org/10.3390/macromol5010012










