Evaluation of Antioxidant, Antibacterial and Enzyme-Inhibitory Properties of Dittany and Thyme Extracts and Their Application in Hydrogel Preparation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Plant Extraction
2.2.2. Chemical Characterization of the Plant Extracts
Total Phenolic Content and Flavonoid Content of Plant Extracts
Liquid Chromatography–MSn Analysis
Quantitative Measurements of the Main Compounds in Dittany and Thyme Extracts
2.2.3. Biological Evaluation of the Extracts and Molecular Docking Studies for the Main Contained Polyphenolic Compounds
Determination of Lipase Inhibitory Activity
Determination of Tyrosinase Inhibitory Activity
In Silico Analysis of Thymol, Carvacrol and Rosmarinic Acid against Candida rugosa Lipase and Tyrosinase from Mushrooms
Determination of Antioxidant Activity
Determination of Antibacterial Activity
2.2.4. Preparation and Characterization of Gelatin Hydrogels Containing Extracts
DES Preparation
Preparation of Gelatin Hydrogels Containing Extracts
Determination of the Antioxidant Activity of Gelatin Hydrogels Containing Extracts
Determination of Antibacterial Activity of Extracts and Gelatin Hydrogels Containing Extracts
2.2.5. Statistical Analysis
3. Results and Discussion
3.1. Chemical Characterization of the Prepared Extracts through Analytical and Spectrophotometric Methods
3.2. Biological Evaluation of the Extracts and Molecular Docking Studies of Their Main Phenolic Compounds
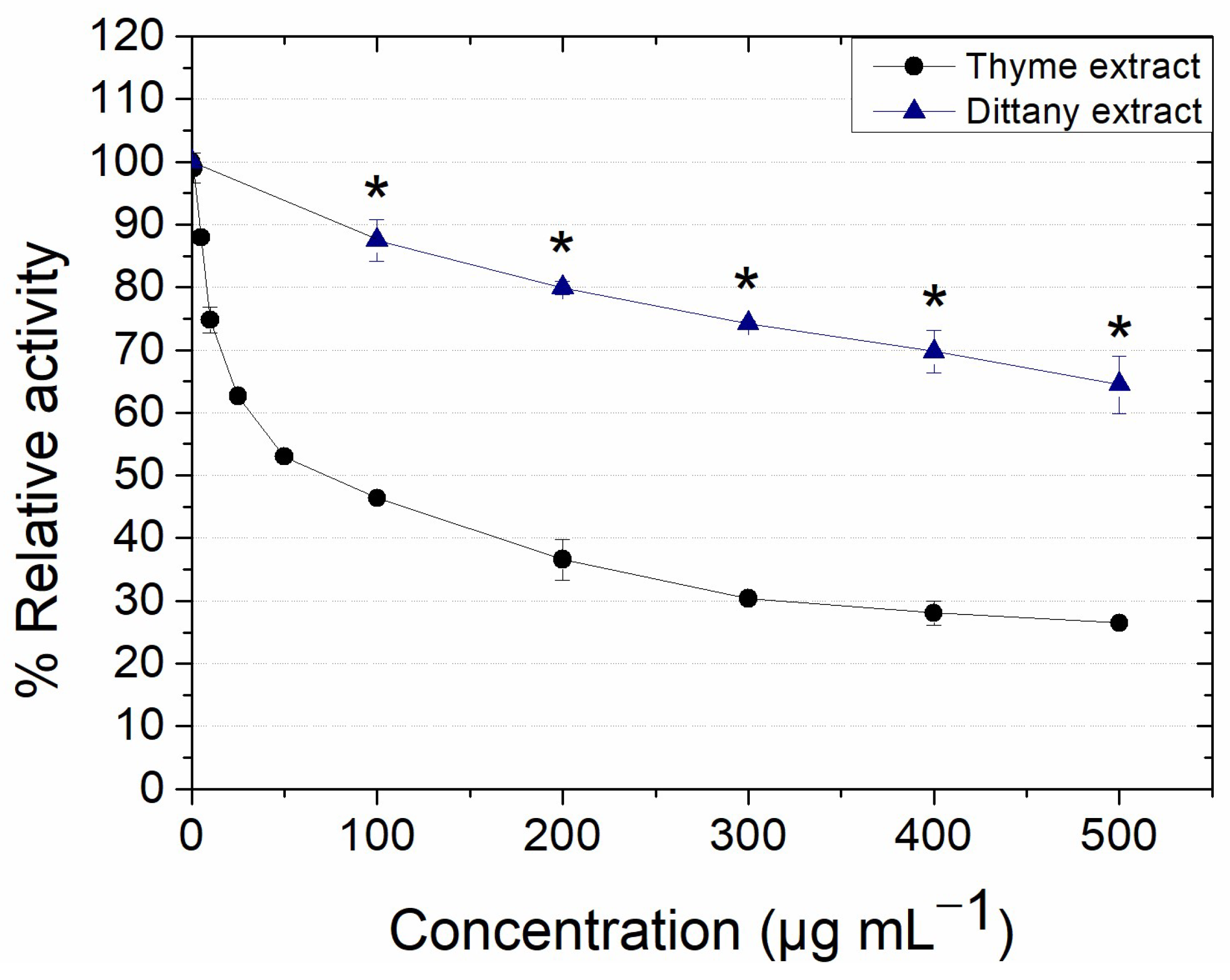
3.2.1. Effect on the Activity of Lipase from Candida rugosa
3.2.2. Effect on the Activity of Tyrosinase from Mushrooms
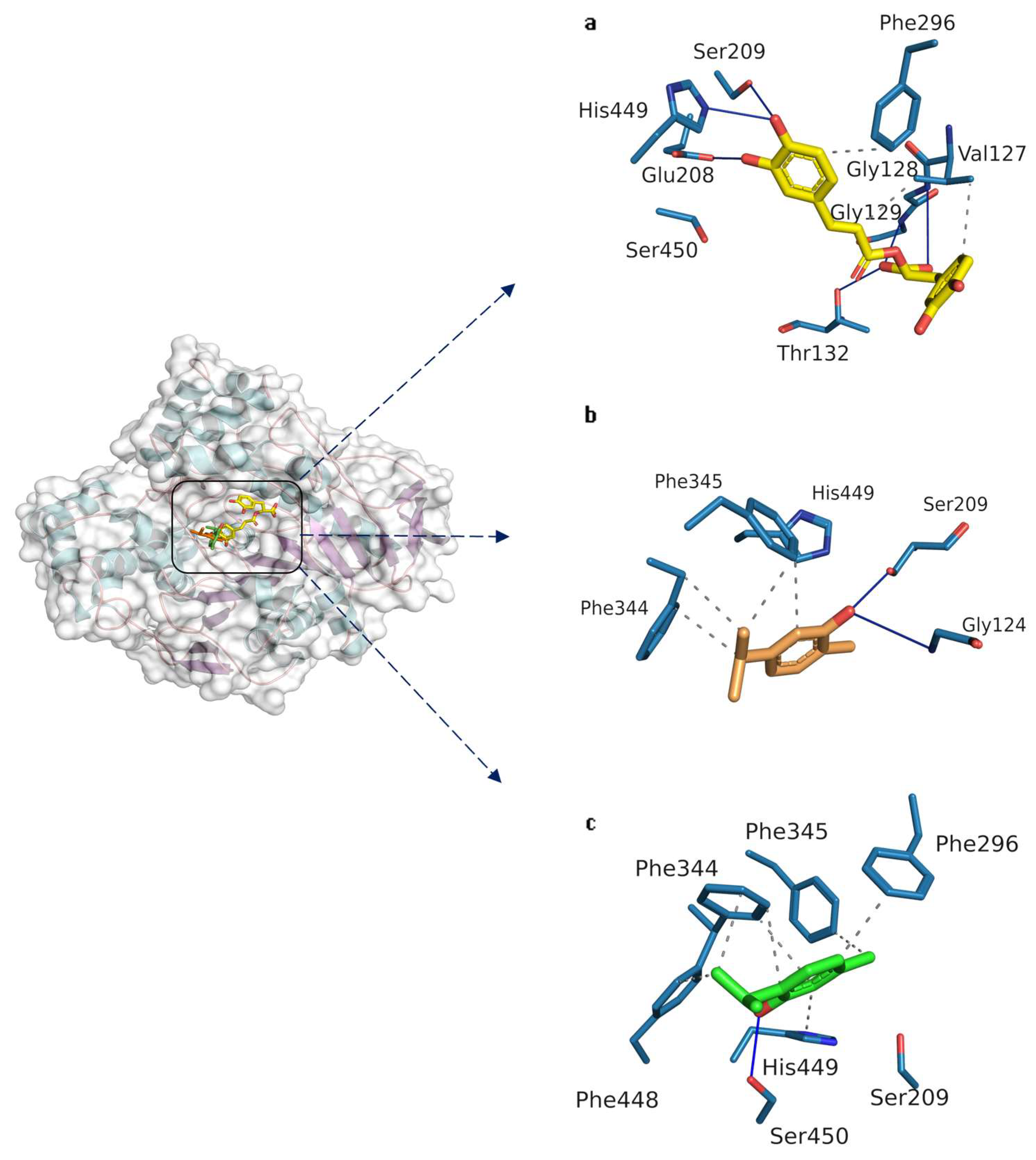
3.2.3. Docking Studies on Candida rugosa Lipase
3.2.4. Docking Studies on Tyrosinase from Mushroom
3.2.5. Evaluation of the Antioxidant Activity of the Extracts
3.2.6. Evaluation of the Antibacterial Activity of the Extracts
3.3. Preparation and Biological Assessment of Gelatin Hydrogels Containing Extracts
3.3.1. Preparation of Gelatin Hydrogels Containing Extracts
3.3.2. Evaluation of the Antioxidant Activity of Gelatin Hydrogels Containing Extracts
3.3.3. Evaluation of the Antibacterial Activity of Gelatin Hydrogels Containing Extracts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Petrovska, B. Historical Review of Medicinal Plants’ Usage. Pharmacogn. Rev. 2012, 6, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Venkateshappa, S.M.; Sreenath, K.P. Potential Medicinal Plants of Lamiaceae. Am. Int. J. Res. Form. Appl. Nat. Sci. 2013, 3, 82–87. [Google Scholar]
- Reddy, P.; Kandisa, R.V.; Varsha, P.V.; Satyam, S. Review on Thymus Vulgaris Traditional Uses and Pharmacological Properties. Med. Aromat. Plants 2014, 3, 167. [Google Scholar] [CrossRef]
- Dauqan, E.M.A.; Abdullah, A. Medicinal and Functional Values of Thyme (Thymus vulgaris L.) Herb. J. Appl. Biol. Biotechnol. 2017, 5, 17–22. [Google Scholar] [CrossRef]
- Patil, S.M.; Ramu, R.; Shirahatti, P.S.; Shivamallu, C.; Amachawadi, R.G. A Systematic Review on Ethnopharmacology, Phytochemistry and Pharmacological Aspects of Thymus Vulgaris Linn. Heliyon 2021, 7, e07054. [Google Scholar] [CrossRef] [PubMed]
- Liolios, C.C.; Graikou, K.; Skaltsa, E.; Chinou, I. Dittany of Crete: A Botanical and Ethnopharmacological Review. J. Ethnopharmacol. 2010, 131, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Mitropoulou, G.; Fitsiou, E.; Stavropoulou, E.; Papavassilopoulou, E.; Vamvakias, M.; Pappa, A.; Oreopoulou, A.; Kourkoutas, Y. Composition, Antimicrobial, Antioxidant, and Antiproliferative Activity of Origanum dictamnus (dittany) Essential Oil. Microb. Ecol. Health Dis. 2015, 26, 26543. [Google Scholar] [CrossRef] [PubMed]
- Solomou, A.D.; Fountouli, A.; Molla, A.; Petrakis, M.; Manolikaki, I.; Skoufogianni, E. Ecology, Cultivation, and Utilization of the Dittany of Crete (Origanum dictamnus L.) from Ancient Times to the Present: A Short Review. Agronomy 2024, 14, 1066. [Google Scholar] [CrossRef]
- Uyama, H. Artificial Polymeric Flavonoids: Synthesis and Applications. Macromol. Biosci. 2007, 7, 410–422. [Google Scholar] [CrossRef]
- Sauceda, A.E.Q.; Sáyago-Ayerdi, S.G.; Ayala-Zavala, J.F.; Wall-Medrano, A.; De La Rosa, L.A.; González-Aguilar, G.A.; Álvarez-Parrilla, E. Biological Actions of Phenolic Compounds. Fruit. Veg. Phytochem. 2017, 2, 125–138. [Google Scholar] [CrossRef]
- Nabavi, S.M.; Marchese, A.; Izadi, M.; Curti, V.; Daglia, M.; Nabavi, S.F. Plants Belonging to the Genus Thymus as Antibacterial Agents: From Farm to Pharmacy. Food Chem. 2015, 173, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Aslam, M.; Alsagaby, S.A.; Saeed, F.; Ahmad, I.; Afzaal, M.; Arshad, M.U.; Abdelgawad, M.A.; El-Ghorab, A.H.; Khames, A.; et al. Therapeutic Application of Carvacrol: A Comprehensive Review. Food Sci. Nutr. 2022, 10, 3544–3561. [Google Scholar] [CrossRef] [PubMed]
- Chatzopoulou, A.; Karioti, A.; Gousiadou, C.; Lax Vivancos, V.; Kyriazopoulos, P.; Golegou, S.; Skaltsa, H. Depsides and Other Polar Constituents from Origanum dictamnus L. and Their in Vitro Antimicrobial Activity in Clinical Strains. J. Agric. Food Chem. 2010, 58, 6064–6068. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.M.; Valentão, P.; Pereira, J.A.; Andrade, P.B. Phenolics: From Chemistry to Biology. Molecules 2009, 14, 2202–2211. [Google Scholar] [CrossRef]
- Kouri, G.; Tsimogiannis, D.; Bardouki, H.; Oreopoulou, V. Extraction and Analysis of Antioxidant Components from Origanum dictamnus. Innov. Food Sci. Emerg. Technol. 2007, 8, 155–162. [Google Scholar] [CrossRef]
- Roby, M.H.H.; Sarhan, M.A.; Selim, K.A.H.; Khalel, K.I. Evaluation of Antioxidant Activity, Total Phenols and Phenolic Compounds in Thyme (Thymus vulgaris L.), Sage (Salvia officinalis L.), and Marjoram (Origanum majorana L.) Extracts. Ind. Crops Prod. 2013, 43, 827–831. [Google Scholar] [CrossRef]
- Gortzi, O.; Lalas, S.; Chinou, I.; Tsaknis, J. Evaluation of the Antimicrobial and Antioxidant Activities of Origanum dictamnus Extracts before and after Encapsulation in Liposomes. Molecules 2007, 12, 932–945. [Google Scholar] [CrossRef]
- Habashy, N.H.; Abu Serie, M.M.; Attia, W.E.; Abdelgaleil, S.A.M. Chemical Characterization, Antioxidant and Anti-Inflammatory Properties of Greek Thymus Vulgaris Extracts and Their Possible Synergism with Egyptian Chlorella Vulgaris. J. Funct. Foods 2018, 40, 317–328. [Google Scholar] [CrossRef]
- Paloukopoulou, C.; Govari, S.; Soulioti, A.; Stefanis, I.; Angeli, A.; Matheeussen, A.; Capasso, C.; Cos, P.; Supuran, C.T.; Karioti, A. Phenols from Origanum dictamnus L. and Thymus vulgaris L. and Their Activity against Malassezia globosa Carbonic Anhydrase. Nat. Prod. Res. 2022, 36, 1558–1564. [Google Scholar] [CrossRef]
- Olszewska, M.A.; Gędas, A.; Simões, M. Antimicrobial Polyphenol-Rich Extracts: Applications and Limitations in the Food Industry. Food Res. Int. 2020, 134, 109214. [Google Scholar] [CrossRef]
- Guimarães, I.; Baptista-Silva, S.; Pintado, M.; Oliveira, A.L. Polyphenols: A Promising Avenue in Therapeutic Solutions for Wound Care. Appl. Sci. 2021, 11, 1230. [Google Scholar] [CrossRef]
- Li, Z.; Chen, Z.; Chen, H.; Chen, K.; Tao, W.; Ouyang, X.; Mei, L.; Zeng, X. Polyphenol-Based Hydrogels: Pyramid Evolution from Crosslinked Structures to Biomedical Applications and the Reverse Design. Bioact. Mater. 2022, 17, 49–70. [Google Scholar] [CrossRef]
- Alipal, J.; Mohd Pu’ad, N.A.S.; Lee, T.C.; Nayan, N.H.M.; Sahari, N.; Basri, H.; Idris, M.I.; Abdullah, H.Z. A Review of Gelatin: Properties, Sources, Process, Applications, and Commercialisation. Mater. Today Proc. 2021, 42, 240–250. [Google Scholar] [CrossRef]
- Rather, J.A.; Akhter, N.; Ashraf, Q.S.; Mir, S.A.; Makroo, H.A.; Majid, D.; Barba, F.J.; Khaneghah, A.M.; Dar, B.N. A Comprehensive Review on Gelatin: Understanding Impact of the Sources, Extraction Methods, and Modifications on Potential Packaging Applications. Food Packag. Shelf Life 2022, 34, 100945. [Google Scholar] [CrossRef]
- Montazerian, H.; Sampath, R.R.; Annabi, N.; Khademhosseini, A.; Weiss, P.S. Polyphenolic Gelatin-Based Bioadhesives. Acc. Mater. Res. 2023, 4, 627–640. [Google Scholar] [CrossRef]
- Kavoosi, G.; Dadfar, S.M.M.; Mohammadi Purfard, A.; Mehrabi, R. Antioxidant and Antibacterial Properties of Gelatin Films Incorporated with Carvacrol. J. Food Saf. 2013, 33, 423–432. [Google Scholar] [CrossRef]
- Khan, M.R.; Sadiq, M.B.; Mehmood, Z. Development of Edible Gelatin Composite Films Enriched with Polyphenol Loaded Nanoemulsions as Chicken Meat Packaging Material. CYTA J. Food 2020, 18, 137–146. [Google Scholar] [CrossRef]
- Hu, X.; Yuan, L.; Han, L.; Li, S.; Song, L. Characterization of Antioxidant and Antibacterial Gelatin Films Incorporated with: Ginkgo biloba Extract. RSC Adv. 2019, 9, 27449–27454. [Google Scholar] [CrossRef]
- Gómez-Estaca, J.; Bravo, L.; Gómez-Guillén, M.C.; Alemán, A.; Montero, P. Antioxidant Properties of Tuna-Skin and Bovine-Hide Gelatin Films Induced by the Addition of Oregano and Rosemary Extracts. Food Chem. 2009, 112, 18–25. [Google Scholar] [CrossRef]
- Wu, J.; Chen, S.; Ge, S.; Miao, J.; Li, J.; Zhang, Q. Preparation, Properties and Antioxidant Activity of an Active Film from Silver Carp (Hypophthalmichthys molitrix) Skin Gelatin Incorporated with Green Tea Extract. Food Hydrocoll. 2013, 32, 42–51. [Google Scholar] [CrossRef]
- Limpisophon, K.; Schleining, G. Use of Gallic Acid to Enhance the Antioxidant and Mechanical Properties of Active Fish Gelatin Film. J. Food Sci. 2017, 82, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Kontogianni, V.G.; Kasapidou, E.; Mitlianga, P.; Mataragas, M.; Pappa, E.; Kondyli, E.; Bosnea, L. Production, Characteristics and Application of Whey Protein Films Activated with Rosemary and Sage Extract in Preserving Soft Cheese. LWT 2022, 155, 112996. [Google Scholar] [CrossRef]
- Kontogianni, V.G.; Tomic, G.; Nikolic, I.; Nerantzaki, A.A.; Sayyad, N.; Stosic-Grujicic, S.; Stojanovic, I.; Gerothanassis, I.P.; Tzakos, A.G. Phytochemical Profile of Rosmarinus Officinalis and Salvia Officinalis Extracts and Correlation to Their Antioxidant and Anti-Proliferative Activity. Food Chem. 2013, 136, 120–129. [Google Scholar] [CrossRef]
- Spyrou, S.; Chatzikonstantinou, A.; Giannakopoulou, A.; Fotiadou, R.; Priska, S.; Simos, Y.; Tsakni, A.; Peschos, D.; Houhoula, D.; Voutsas, E.; et al. Fungal Laccase-Mediated Enhancement of the Bioactivity of Green Algae Extracts. Catal. Res. 2023, 3, 1–29. [Google Scholar] [CrossRef]
- Di Petrillo, A.; González-Paramás, A.M.; Era, B.; Medda, R.; Pintus, F.; Santos-Buelga, C.; Fais, A. Tyrosinase Inhibition and Antioxidant Properties of Asphodelus Microcarpus Extracts. BMC Complement. Altern. Med. 2016, 16, 453. [Google Scholar] [CrossRef] [PubMed]
- Forli, S.; Huey, R.; Pique, M.E.; Sanner, M.F.; Goodsell, D.S.; Olson, A.J. Computational Protein–Ligand Docking and Virtual Drug Screening with the AutoDock Suite. Nat. Protoc. 2016, 11, 905–919. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.J.; Headd, J.J.; Moriarty, N.W.; Prisant, M.G.; Videau, L.L.; Deis, L.N.; Verma, V.; Keedy, D.A.; Hintze, B.J.; Chen, V.B.; et al. MolProbity: More and Better Reference Data for Improved All-Atom Structure Validation. Protein Sci. 2018, 27, 293–315. [Google Scholar] [CrossRef] [PubMed]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An Advanced Semantic Chemical Editor, Visualization, and Analysis Platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef]
- Adasme, M.F.; Linnemann, K.L.; Bolz, S.N.; Kaiser, F.; Salentin, S.; Haupt, V.J.; Schroeder, M. PLIP 2021: Expanding the Scope of the Protein–Ligand Interaction Profiler to DNA and RNA. Nucleic Acids Res. 2021, 49, W530–W534. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in Vitro Evaluating Antimicrobial Activity: A Review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
- Parvekar, P.; Palaskar, J.; Metgud, S.; Maria, R.; Dutta, S. The Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) of Silver Nanoparticles against Staphylococcus aureus. Biomater. Investig. Dent. 2020, 7, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Chatzikonstantinou, A.V.; Bellou, M.G.; Spyrou, S.; Papanikolaou, A.; Simos, Y.V.; Peschos, D.; Stamatis, H. Enhancement of the Biological Activity of Hydroxytyrosol through Its Oxidation by Laccase from Trametes Versicolor. J. Biotechnol. 2024, 385, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Jafari, M.; Afkhami, R.; Sedaghat, N. Preparation and Characterization of Active Cirish Fructans–Fish Gelatin Film: Physicochemical, Antioxidant, and Antimicrobial Properties. Food Sci. Nutr. 2023, 11, 709–721. [Google Scholar] [CrossRef] [PubMed]
- Vujicic, M.; Nikolic, I.; Kontogianni, V.G.; Saksida, T.; Charisiadis, P.; Orescanin-Dusic, Z.; Blagojevic, D.; Stosic-Grujicic, S.; Tzakos, A.G.; Stojanovic, I. Methanolic Extract of Origanum vulgare Ameliorates Type 1 Diabetes through Antioxidant, Anti-Inflammatory and Anti-Apoptotic Activity. Br. J. Nutr. 2015, 113, 770–782. [Google Scholar] [CrossRef] [PubMed]
- Bendif, H.; Peron, G.; Miara, M.D.; Sut, S.; Dall’Acqua, S.; Flamini, G.; Maggi, F. Total Phytochemical Analysis of Thymus munbyanus subsp. coloratus from Algeria by HS-SPME-GC-MS, NMR and HPLC-MSn Studies. J. Pharm. Biomed. Anal. 2020, 186, 113330. [Google Scholar] [CrossRef]
- Ahmad, S.; Biosci, I.J.; Mehmood, T.; Shafique, S.; Tabassam, Q.; Afzal, M.; Author, C. Variation in Antioxidant Attributes, Individual Phenolic Acids Composition and Biological Activities of Thymus Vulgaris: Effects of Extraction Solvents. Int. J. Biosci. 2015, 6, 73–86. [Google Scholar] [CrossRef]
- Lagouri, V.; Alexandri, G. Antioxidant Properties of Greek O. dictamnus and R. officinalis Methanol and Aqueous Extracts - HPLC Determination of Phenolic Acids. Int. J. Food Prop. 2013, 16, 549–562. [Google Scholar] [CrossRef]
- Zengin, G.; Atasagun, B.; Zakariyyah Aumeeruddy, M.; Saleem, H.; Mollica, A.; Babak Bahadori, M.; Mahomoodally, M.F. Phenolic Profiling and in Vitro Biological Properties of Two Lamiaceae Species (Salvia Modesta and Thymus Argaeus): A Comprehensive Evaluation. Ind. Crops Prod. 2019, 128, 308–314. [Google Scholar] [CrossRef]
- Lemonis, I.; Tsimogiannis, D.; Louli, V.; Voutsas, E.; Oreopoulou, V.; Magoulas, K. Extraction of Dittany (Origanum dictamnus) Using Supercritical CO2 and Liquid Solvent. J. Supercrit. Fluids 2013, 76, 48–53. [Google Scholar] [CrossRef]
- Grippa, E.; Valla, R.; Battinelli, L.; Mazzanti, G.; Saso, L.; Silvestrini, B. Inhibition of Candida Rugosa Lipase by Berberine and Structurally Related Alkaloids, Evaluated by High-Performance Liquid Chromatography. Biosci. Biotechnol. Biochem. 1999, 63, 1557–1562. [Google Scholar] [CrossRef]
- Ruiz, C.; Falcocchio, S.; Xoxi, E.; Villo, L.; Nicolosi, G.; Pastor, F.I.J.; Diaz, P.; Saso, L. Inhibition of Candida Rugosa Lipase by Saponins, Flavonoids and Alkaloids. J. Mol. Catal. B Enzym. 2006, 40, 138–143. [Google Scholar] [CrossRef]
- Benarous, K.; Bombarda, I.; Iriepa, I.; Moraleda, I.; Gaetan, H.; Linani, A.; Tahri, D.; Sebaa, M.; Yousfi, M. Harmaline and Hispidin from Peganum Harmala and Inonotus Hispidus with Binding Affinity to Candida Rugosa Lipase: In Silico and in Vitro Studies. Bioorganic Chem. 2015, 62, 1–7. [Google Scholar] [CrossRef]
- Santana, C.C.; Silva-Júnior, E.F.; Santos, J.C.N.; Rodrigues, E.E.D.S.; da Silva, I.M.; Araújo-Júnior, J.X.; do Nascimento, T.G.; Barbosa, L.A.O.; Dornelas, C.B.; Figueiredo, I.M.; et al. Evaluation of Guanylhydrazone Derivatives as Inhibitors of Candida Rugosa Digestive Lipase: Biological, Biophysical, Theoretical Studies and Biotechnological Application. Bioorganic Chem. 2019, 87, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Gatto, M.T.; Falcocchio, S.; Grippa, E.; Mazzanti, G.; Battinelli, L.; Nicolosi, G.; Lambusta, D.; Saso, L. Antimicrobial and Anti-Lipase Activity of Quercetin and Its C2-C16 3-O-Acyl-Esters. Bioorganic Med. Chem. 2002, 10, 269–272. [Google Scholar] [CrossRef] [PubMed]
- Serseg, T.; Benarous, K.; Yousfi, M. The Inhibitory Effect of Three Essential Oils on Candida Rugosa Lipase: In Vitro and In Silico Studies. Nat. Prod. J. 2020, 10, 208–215. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, F.Q.; Gao, J.L. A Simple and Portable Personal Glucose Meter Method Combined with Molecular Docking for Screening of Lipase Inhibitors. Evid. -Based Complement. Altern. Med. 2022, 2022, 4430050. [Google Scholar] [CrossRef]
- Benguechoua, M.; Nia, S.; Benarous, K.; Khacheba, I.; Yousfi, M. Inhibition of Candida Rugosa Lipase by Different Extracts of Five Algerian Plants and Their Antioxidant Activities. Curr. Enzym. Inhib. 2014, 10, 121–128. [Google Scholar] [CrossRef]
- Slanc, P.; Doljak, B.; Kreft, S.; Lunder, M.; Janes, D.; Ítrukelj, B. Screening of Selected Food and Medicinal Plant Extracts for Pancreatic Lipase Inhibition. Phytother. Res. 2009, 23, 874–877. [Google Scholar] [CrossRef] [PubMed]
- Jaradat, N.; Zaid, A.; Hussein, F.; Zaqzouq, M.; Aljammal, H.; Ayesh, O. Anti-Lipase Potential of the Organic and Aqueous Extracts of Ten Traditional Edible and Medicinal Plants in Palestine; a Comparison Study with Orlistat. Medicines 2017, 4, 89. [Google Scholar] [CrossRef]
- Panzella, L.; Napolitano, A. Natural and Bioinspired Phenolic Compounds as Tyrosinase Inhibitors for the Treatment of Skin Hyperpigmentation: Recent Advances. Cosmetics 2019, 6, 57. [Google Scholar] [CrossRef]
- Lee, Y.S.; Park, J.H.; Kim, M.H.; Seo, S.H.; Kim, H.J. Synthesis of Tyrosinase Inhibitory Kojic Acid Derivative. Arch. Pharm. 2006, 339, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Zhang, H.; He, J.; Zhang, J.; Yu, Q.; Luo, C.; Li, S. Synthesis and Biological Evaluation of Novel Hydroxybenzaldehyde-Based Kojic Acid Analogues as Inhibitors of Mushroom Tyrosinase. Bioorganic Med. Chem. Lett. 2017, 27, 530–532. [Google Scholar] [CrossRef] [PubMed]
- Brotzman, N.; Xu, Y.; Graybill, A.; Cocolas, A.; Ressler, A.; Seeram, N.P.; Ma, H.; Henry, G.E. Synthesis and Tyrosinase Inhibitory Activities of 4-Oxobutanoate Derivatives of Carvacrol and Thymol. Bioorganic Med. Chem. Lett. 2019, 29, 56–58. [Google Scholar] [CrossRef]
- Lin, L.; Dong, Y.; Zhao, H.; Wen, L.; Yang, B.; Zhao, M. Comparative Evaluation of Rosmarinic Acid, Methyl Rosmarinate and Pedalitin Isolated from Rabdosia Serra (MAXIM.) HARA as Inhibitors of Tyrosinase and α-Glucosidase. Food Chem. 2011, 129, 884–889. [Google Scholar] [CrossRef]
- Zuo, A.R.; Dong, H.H.; Yu, Y.Y.; Shu, Q.L.; Zheng, L.X.; Yu, X.Y.; Cao, S.W. The Antityrosinase and Antioxidant Activities of Flavonoids Dominated by the Number and Location of Phenolic Hydroxyl Groups. Chin. Med. 2018, 13, 51. [Google Scholar] [CrossRef] [PubMed]
- Sarikurkcu, C.; Zengin, G.; Oskay, M.; Uysal, S.; Ceylan, R.; Aktumsek, A. Composition, Antioxidant, Antimicrobial and Enzyme Inhibition Activities of Two Origanum vulgare Subspecies (subsp. vulgare and subsp. hirtum) Essential Oils. Ind. Crops Prod. 2015, 70, 178–184. [Google Scholar] [CrossRef]
- Duletić-Laušević, S.; Aradski, A.A.; Kolarević, S.; Vuković-Gačić, B.; Oalde, M.; Živković, J.; Šavikin, K.; Marin, P.D. Antineurodegenerative, Antioxidant and Antibacterial Activities and Phenolic Components of Origanum majorana L. (Lamiaceae) Extracts. J. Appl. Bot. Food Qual. 2018, 91, 126–134. [Google Scholar] [CrossRef]
- El Aanachi, S.; Gali, L.; Rammali, S.; Bensouici, C.; Aassila, H.; Dari, K. In Vitro Study of the Antioxidant, Photoprotective, Anti-Tyrosinase, and Anti-Urease Effects of Methanolic Extracts from Leaves of Six Moroccan Lamiaceae. J. Food Meas. Charact. 2021, 15, 1785–1795. [Google Scholar] [CrossRef]
- Güven, L.; Behbudbayli, U.; Ertürk, A.; Hancı, H.; Yılmaz, B.; Kaya, Y.; Gülçin, İ. Determination of Antioxidant, Antimicrobial, Anticholinesterase, Antityrosinase, Antidiabetic and Antiglaucoma Activities of Essential Oils from Three Different Thymus Species and Their Chemical Characterization by GC-MS Analysis. J. Essent. Oil-Bear. Plants 2023, 26, 1424–1446. [Google Scholar] [CrossRef]
- Küçükaydın, S.; Çayan, F.; Tel-Çayan, G.; Duru, M.E. HPLC-DAD Phytochemical Profiles of Thymus Cariensis and T. Cilicicus with Antioxidant, Cytotoxic, Anticholinesterase, Anti-Urease, Anti-Tyrosinase, and Antidiabetic Activities. S. Afr. J. Bot. 2021, 143, 155–163. [Google Scholar] [CrossRef]
- Sethi, A.; Joshi, K.; Sasikala, K.; Alvala, M. Molecular Docking in Modern Drug Discovery: Principles and Recent Applications. In Drug Discovery and Development—New Advances; IntechOpen: London, UK, 2020. [Google Scholar]
- Gagic, Z.; Ruzic, D.; Djokovic, N.; Djikic, T.; Nikolic, K. In Silico Methods for Design of Kinase Inhibitors as Anticancer Drugs. Front. Chem. 2020, 7, 873. [Google Scholar] [CrossRef] [PubMed]
- Pantsar, T.; Poso, A. Binding Affinity via Docking: Fact and Fiction. Molecules 2018, 23, 1899. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, D.; Caballero, J. Is It Reliable to Take the Molecular Docking Top Scoring Position as the Best Solution without Considering Available Structural Data? Molecules 2018, 23, 1038. [Google Scholar] [CrossRef] [PubMed]
- Naz, F.; Khan, I.; Islam, A.; Khan, L.A. Interaction of Fungal Lipase with Potential Phytotherapeutics. PLoS ONE 2022, 17, e0264460. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, A.P.; De Silva, N.F.; Andrade, E.H.A.; Gratieri, T.; Setzer, W.N.; Maia, J.G.S.; Da Silva, J.K.R. Tyrosinase Inhibitory Activity, Molecular Docking Studies and Antioxidant Potential of Chemotypes of Lippia Origanoides (Verbenaceae) Essential Oils. PLoS ONE 2017, 12, e0175598. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, D.-Y.; Yin, S.-J.; Jiang, H.; Lu, M.; Yang, F.-Q.; Hu, Y.-J. Screening of Potential Thrombin and Factor Xa Inhibitors from the Danshen–Chuanxiong Herbal Pair through a Spectrum–Effect Relationship Analysis. Molecules 2021, 26, 7293. [Google Scholar] [CrossRef]
- Kim, Y.J.; Uyama, H. Tyrosinase Inhibitors from Natural and Synthetic Sources: Structure, Inhibition Mechanism and Perspective for the Future. Cell. Mol. Life Sci. 2005, 62, 1707–1723. [Google Scholar] [CrossRef] [PubMed]
- Ha, T.J.; Lee, M.H.; Kwon, H.S.; Lee, B.W.; Park, C.H.; Pae, S.B.; Jung, C.S.; Park, K.Y. Oxidation of Rosmarinic Acid Catalyzed by Mushroom Tyrosinase. J. Korean Soc. Appl. Biol. Chem. 2011, 54, 619–622. [Google Scholar] [CrossRef]
- Satooka, H.; Kubo, I. Effects of Thymol on Mushroom Tyrosinase-Catalyzed Melanin Formation. J. Agric. Food Chem. 2011, 59, 8908–8914. [Google Scholar] [CrossRef]
- Si, Y.-X.; Yin, S.-J.; Park, D.; Chung, H.Y.; Yan, L.; Lü, Z.-R.; Zhou, H.-M.; Yang, J.-M.; Qian, G.-Y.; Park, Y.-D. Tyrosinase Inhibition by Isophthalic Acid: Kinetics and Computational Simulation. Int. J. Biol. Macromol. 2011, 48, 700–704. [Google Scholar] [CrossRef]
- Wong, S.P.; Leong, L.P.; William Koh, J.H. Antioxidant Activities of Aqueous Extracts of Selected Plants. Food Chem. 2006, 99, 775–783. [Google Scholar] [CrossRef]
- Mensor, L.L.; Menezes, F.S.; Leitão, G.G.; Reyes, A.S.; dos Santos, T.C.; Fit, C.S.; Leitão, S.G. Screening of Brazilian Plant Extracts for Antioxidant Activity by the Use of DPPH Free Radical Method. Phytother. Res. 2001, 15, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yang, J.; Ma, L.; Li, J.; Shahzad, N.; Kim, C.K. Structure-Antioxidant Activity Relationship of Methoxy, Phenolic Hydroxyl, and Carboxylic Acid Groups of Phenolic Acids. Sci. Rep. 2020, 10, 2611. [Google Scholar] [CrossRef] [PubMed]
- Topal, M.; Gulcin, İ. Evaluation of the in Vitro Antioxidant, Antidiabetic and Anticholinergic Properties of Rosmarinic Acid from Rosemary (Rosmarinus officinalis L.). Biocatal. Agric. Biotechnol. 2022, 43, 102417. [Google Scholar] [CrossRef]
- Gavaric, N.; Mozina, S.S.; Kladar, N.; Bozin, B. Chemical Profile, Antioxidant and Antibacterial Activity of Thyme and Oregano Essential Oils, Thymol and Carvacrol and Their Possible Synergism. J. Essent. Oil-Bear. Plants 2015, 18, 1013–1021. [Google Scholar] [CrossRef]
- Gedikoğlu, A.; Sökmen, M.; Çivit, A. Evaluation of Thymus vulgaris and Thymbra spicata Essential Oils and Plant Extracts for Chemical Composition, Antioxidant, and Antimicrobial Properties. Food Sci. Nutr. 2019, 7, 1704–1714. [Google Scholar] [CrossRef] [PubMed]
- Shan, B.; Cai, Y.Z.; Sun, M.; Corke, H. Antioxidant Capacity of 26 Spice Extracts and Characterization of Their Phenolic Constituents. J. Agric. Food Chem. 2005, 53, 7749–7759. [Google Scholar] [CrossRef] [PubMed]
- Pizzale, L.; Bortolomeazzi, R.; Vichi, S.; Überegger, E.; Conte, L.S. Antioxidant Activity of Sage (Salvia officinalis and S fruticosa) and Oregano (Origanum onites and O indercedens) Extracts Related to Their Phenolic Compound Content. J. Sci. Food Agric. 2002, 82, 1645–1651. [Google Scholar] [CrossRef]
- Daglia, M. Polyphenols as Antimicrobial Agents. Curr. Opin. Biotechnol. 2012, 23, 174–181. [Google Scholar] [CrossRef]
- Zgurskaya, H.I.; Rybenkov, V.V. Permeability Barriers of Gram-negative Pathogens. Ann. N. Y. Acad. Sci. 2020, 1459, 5–18. [Google Scholar] [CrossRef]
- Anastasaki, E.; Zoumpopoulou, G.; Astraka, K.; Kampoli, E.; Skoumpi, G.; Papadimitriou, K.; Tsakalidou, E.; Polissiou, M. Phytochemical Analysis and Evaluation of the Antioxidant and Antimicrobial Properties of Selected Herbs Cultivated in Greece. Ind. Crops Prod. 2017, 108, 616–628. [Google Scholar] [CrossRef]
- Eguale, T.; Tilahun, G.; Debella, A.; Feleke, A.; Makonnen, E. In Vitro and in Vivo Anthelmintic Activity of Crude Extracts of Coriandrum Sativum against Haemonchus Contortus. J. Ethnopharmacol. 2007, 110, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Mogana, R.; Adhikari, A.; Tzar, M.N.; Ramliza, R.; Wiart, C. Antibacterial Activities of the Extracts, Fractions and Isolated Compounds from Canarium Patentinervium Miq. against Bacterial Clinical Isolates. BMC Complement. Med. Ther. 2020, 20, 55. [Google Scholar] [CrossRef] [PubMed]
- Xing, Q.; Yates, K.; Vogt, C.; Qian, Z.; Frost, M.C.; Zhao, F. Increasing Mechanical Strength of Gelatin Hydrogels by Divalent Metal Ion Removal. Sci. Rep. 2014, 4, 4706. [Google Scholar] [CrossRef]
- Qin, H.; Owyeung, R.E.; Sonkusale, S.R.; Panzer, M.J. Highly Stretchable and Nonvolatile Gelatin-Supported Deep Eutectic Solvent Gel Electrolyte-Based Ionic Skins for Strain and Pressure Sensing. J. Mater. Chem. C Mater. 2019, 7, 601–608. [Google Scholar] [CrossRef]
- Wan, H.; Zhu, Z.; Sun, D.W. Deep Eutectic Solvents (DESs) Films Based on Gelatin as Active Packaging for Moisture Regulation in Fruit Preservation. Food Chem. 2024, 439, 4706. [Google Scholar] [CrossRef]
- Bhatia, S.; Al-Harrasi, A.; Jawad, M.; Shah, Y.A.; Al-Azri, M.S.; Ullah, S.; Anwer, M.K.; Aldawsari, M.F.; Koca, E.; Aydemir, L.Y. A Comparative Study of the Properties of Gelatin (Porcine and Bovine)-Based Edible Films Loaded with Spearmint Essential Oil. Biomimetics 2023, 8, 172. [Google Scholar] [CrossRef]
- López, D.; Márquez, A.; Gutiérrez-Cutiño, M.; Venegas-Yazigi, D.; Bustos, R.; Matiacevich, S. Edible Film with Antioxidant Capacity Based on Salmon Gelatin and Boldine. LWT 2017, 77, 160–169. [Google Scholar] [CrossRef]
- Chang, O.K.; Ha, G.E.; Jeong, S.G.; Seol, K.H.; Oh, M.H.; Kim, D.W.; Jang, A.; Kim, S.H.; Park, B.Y.; Ham, J.S. Antioxidant Activity of Porcine Skin Gelatin Hydrolyzed by Pepsin and Pancreatin. Korean J. Food Sci. Anim. Resour. 2013, 33, 493–500. [Google Scholar] [CrossRef]
- Lu, Y.; Luo, Q.; Chu, Y.; Tao, N.; Deng, S.; Wang, L.; Li, L. Application of Gelatin in Food Packaging: A Review. Polymers 2022, 14, 436. [Google Scholar] [CrossRef]
- Bouhdid, S.; Abrini, J.; Zhiri, A.; Espuny, M.J.; Manresa, A. Investigation of Functional and Morphological Changes in Pseudomonas aeruginosa and Staphylococcus aureus Cells Induced by Origanum Compactum Essential Oil. J. Appl. Microbiol. 2009, 106, 1558–1568. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, L.A.; Cardeira, M.; Leonardo, I.C.; Gaspar, F.B.; Radojčić Redovniković, I.; Duarte, A.R.C.; Paiva, A.; Matias, A.A. Deep Eutectic Systems from Betaine and Polyols—Physicochemical and Toxicological Properties. J. Mol. Liq. 2021, 335, 116201. [Google Scholar] [CrossRef]
- Maietta, M.; Colombo, R.; Corana, F.; Papetti, A. Cretan Tea (Origanum dictamnus L.) as a Functional Beverage: An Investigation on Antiglycative and Carbonyl Trapping Activities. Food Funct. 2018, 9, 1545–1556. [Google Scholar] [CrossRef] [PubMed]
- Miron, T.L.; Herrero, M.; Ibáñez, E. Enrichment of Antioxidant Compounds from Lemon Balm (Melissa Officinalis) by Pressurized Liquid Extraction and Enzyme-Assisted Extraction. J. Chromatogr. A 2013, 1288, 1–9. [Google Scholar] [CrossRef]
- Hossain, M.B.; Rai, D.K.; Brunton, N.P.; Martin-Diana, A.B.; Barry-Ryan, A.C. Characterization of Phenolic Composition in Lamiaceae Spices by LC-ESI-MS/MS. J. Agric. Food Chem. 2010, 58, 10576–10581. [Google Scholar] [CrossRef] [PubMed]
- Elshamy, S.; Abdel Motaal, A.; Abdel-Halim, M.; Medhat, D.; Handoussa, H. Potential Neuroprotective Activity of Mentha Longifolia L. in Aluminum Chloride-Induced Rat Model of Alzheimer’s Disease. J. Food Biochem. 2021, 45, 1770. [Google Scholar] [CrossRef]
- Sut, S.; Poloniato, G.; Malagoli, M.; Dall’acqua, S. Fragmentation of the Main Triterpene Acids of Apple by LC- APCI-MSn. J. Mass Spectrom. 2018, 53, 882–892. [Google Scholar] [CrossRef]
- Martins, N.; Barros, L.; Santos-Buelga, C.; Silva, S.; Henriques, M.; Ferreira, I.C.F.R. Decoction, Infusion and Hydroalcoholic Extract of Cultivated Thyme: Antioxidant and Antibacterial Activities, and Phenolic Characterisation. Food Chem. 2015, 167, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Parejo, I.; Jauregui, O.; Sánchez-Rabaneda, F.; Viladomat, F.; Bastida, J.; Codina, C. Separation and Characterization of Phenolic Compounds in Fennel (Foeniculum Vulgare) Using Liquid Chromatography-Negative Electrospray Ionization Tandem Mass Spectrometry. J. Agric. Food Chem. 2004, 52, 3679–3687. [Google Scholar] [CrossRef]
- Rainis, G.; Ternes, W. Identification and Characterization of Dimeric Oxidation Products of P-Cymene-2,3-Diol Isolated from Thymus vulgaris L. J. Agric. Food Chem. 2014, 62, 235–243. [Google Scholar] [CrossRef]





| Compound | [M-H]− (m/z) (%) | Rt (min) | Dittany Extract (mg g−1 Dry Extract) | Thyme Extract (mg g−1 Dry Extract) |
|---|---|---|---|---|
| Rosmarinic acid-O- hexoside | 521 (100), 463 (21), 447 (21) | 10.8 | - | 11.6 ± 1.3 |
| Salvianolic acid K | 555 (100), 445 (34), 493 (21) | 12.2 | - | 4.8 ± 0.2 |
| Rosmarinic acid | 359 (100), 719 (82) | 12.6 | 8.8 ± 0.3 | 57.5 ± 2.2 |
| Lithospermic acid | 537( 100), 493 (28), 359 (13) | 13.3 | 4.4 ± 0.1 | 8.5 ± 0.7 |
| Salvianolic acid A | 493 (100), 359 (8) | 14.6 | 3.9 ± 0.1 | - |
| Carvacrol | * | 23.8 | 110.2 ± 2.1 | - |
| Thymol | * | 24.3 | - | 37.2 ± 0.0 |
| Extract | MIC | MBC | MBC/MIC |
|---|---|---|---|
| Dittany | 0.5 | >10 | >20 |
| Thyme | 2 | >10 | >5 |
| Extract | MIC | MBC | MBC/MIC |
|---|---|---|---|
| Dittany | 2.5 | 8 | 3.2 |
| Thyme | 8 | 8 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spyrou, S.; Bellou, M.G.; Papanikolaou, A.; Nakou, K.; Kontogianni, V.G.; Chatzikonstantinou, A.V.; Stamatis, H. Evaluation of Antioxidant, Antibacterial and Enzyme-Inhibitory Properties of Dittany and Thyme Extracts and Their Application in Hydrogel Preparation. BioChem 2024, 4, 166-188. https://doi.org/10.3390/biochem4030009
Spyrou S, Bellou MG, Papanikolaou A, Nakou K, Kontogianni VG, Chatzikonstantinou AV, Stamatis H. Evaluation of Antioxidant, Antibacterial and Enzyme-Inhibitory Properties of Dittany and Thyme Extracts and Their Application in Hydrogel Preparation. BioChem. 2024; 4(3):166-188. https://doi.org/10.3390/biochem4030009
Chicago/Turabian StyleSpyrou, Stamatia, Myrto G. Bellou, Angelos Papanikolaou, Konstantina Nakou, Vasiliki G. Kontogianni, Alexandra V. Chatzikonstantinou, and Haralambos Stamatis. 2024. "Evaluation of Antioxidant, Antibacterial and Enzyme-Inhibitory Properties of Dittany and Thyme Extracts and Their Application in Hydrogel Preparation" BioChem 4, no. 3: 166-188. https://doi.org/10.3390/biochem4030009
APA StyleSpyrou, S., Bellou, M. G., Papanikolaou, A., Nakou, K., Kontogianni, V. G., Chatzikonstantinou, A. V., & Stamatis, H. (2024). Evaluation of Antioxidant, Antibacterial and Enzyme-Inhibitory Properties of Dittany and Thyme Extracts and Their Application in Hydrogel Preparation. BioChem, 4(3), 166-188. https://doi.org/10.3390/biochem4030009





_Stamatis.png)

