Enhanced Room-Temperature Hydrogen Physisorption in Zeolitic Imidazolate Frameworks and Carbon Nanotube Hybrids
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of Base Materials (ZIF-8, ZIF-67, and ZC-ZIF)
2.3. Synthesis of Hybrid Materials (ZIF-8-H, ZIF-67-H, and ZC-ZIF-H)
2.4. Analytical Techniques
2.5. Measurement of Physisorption Properties
3. Results and Discussion
3.1. Morphology, Particle Size, and Crystal Structure
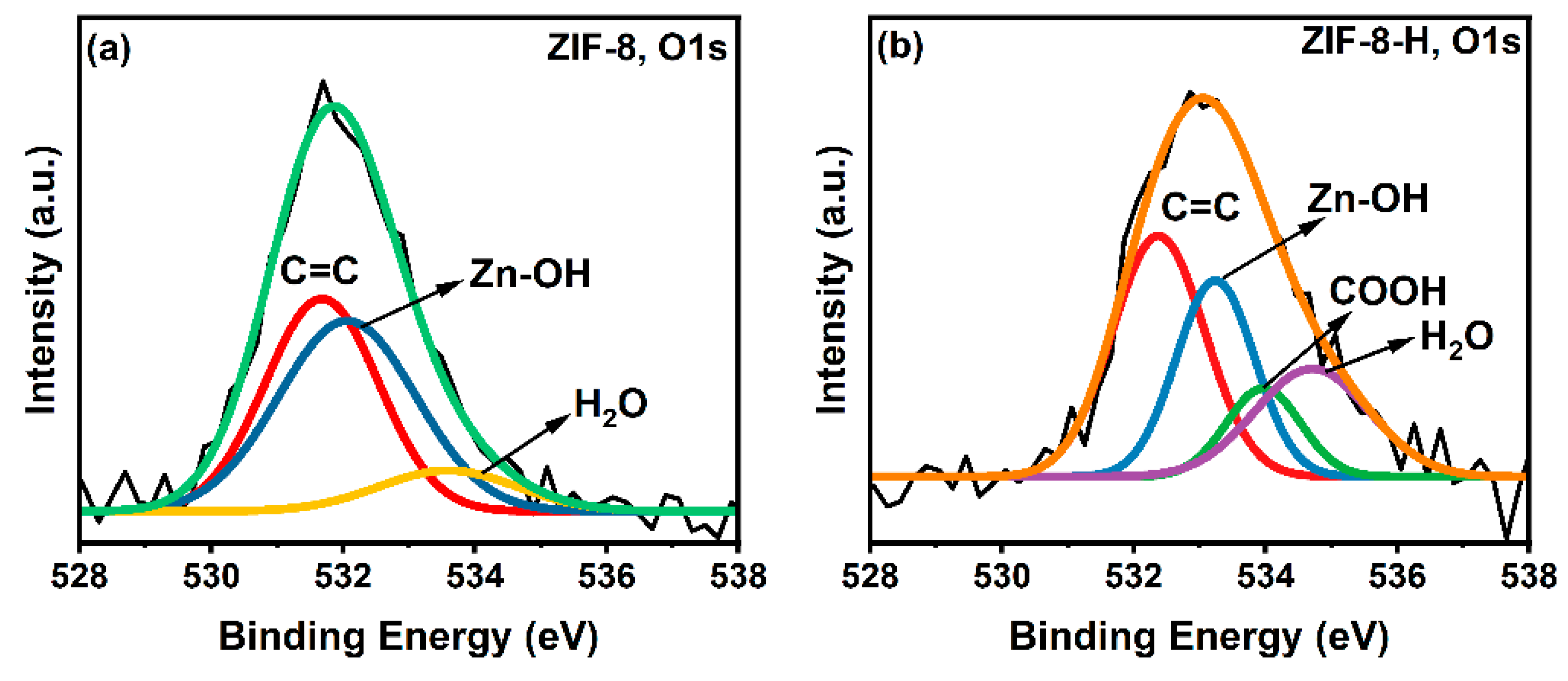
3.2. X-Ray Photoelectron Spectroscopy
3.3. Thermal Stability
3.4. Physisorption Properties
3.4.1. Textural Analysis
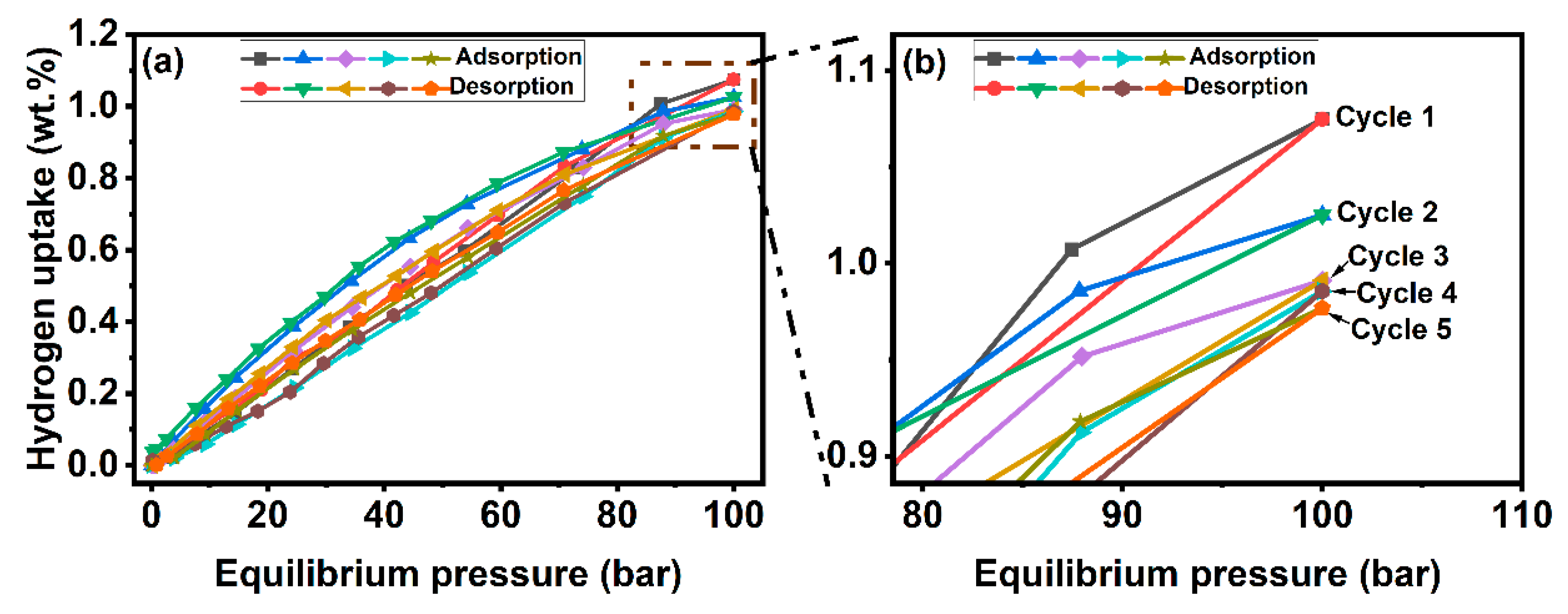
3.4.2. Hydrogen Storage Tests
3.4.3. Hydrogen Adsorption Kinetics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Islam, A.; Teo, S.H.; Ng, C.H.; Taufiq-Yap, Y.H.; Choong, S.Y.T.; Awual, M.R. Progress in Recent Sustainable Materials for Greenhouse Gas (NOx and SOx) Emission Mitigation. Prog. Mater. Sci. 2023, 132, 101033. [Google Scholar] [CrossRef]
- Islam, A.; Malek, A.; Teo, S.H.; Marwani, H.M.; Rahman, M.M.; Asiri, A.M.; Khan, M.A.R.; Taufiq-Yap, Y.H.; Awual, M.R. Smart Materials for CO2 Conversion into Renewable Fuels and Emission Reduction. Sustain. Mater. Technol. 2023, 37, e00636. [Google Scholar] [CrossRef]
- Schlapbach, L.; Züttel, A. Hydrogen-Storage Materials for Mobile Applications. Nature 2001, 414, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Al-Amin, A.Q.; Ambrose, A.F.; Saidur, R. Hydrogen Fuel and Transport System: A Sustainable and Environmental Future. Int. J. Hydrogen Energy 2016, 41, 1369–1380. [Google Scholar] [CrossRef]
- Bourne, S. H2: Flexible Friend? Renew. Energy Focus 2012, 13, 52–54. [Google Scholar] [CrossRef]
- Niaz, S.; Manzoor, T.; Pandith, A.H. Hydrogen Storage: Materials, Methods and Perspectives. Renew. Sustain. Energy Rev. 2015, 50, 457–469. [Google Scholar] [CrossRef]
- Schaefer, S.; Fierro, V.; Szczurek, A.; Izquierdo, M.T.; Celzard, A. Physisorption, Chemisorption and Spill-over Contributions to Hydrogen Storage. Int. J. Hydrogen Energy 2016, 41, 17442–17452. [Google Scholar] [CrossRef]
- Epelle, E.I.; Desongu, K.S.; Obande, W.; Adeleke, A.A.; Ikubanni, P.P.; Okolie, J.A.; Gunes, B. A Comprehensive Review of Hydrogen Production and Storage: A Focus on the Role of Nanomaterials. Int. J. Hydrogen Energy 2022, 47, 20398–20431. [Google Scholar] [CrossRef]
- Zhou, H.-C.; Long, J.R.; Yaghi, O.M. Introduction to Metal–Organic Frameworks. Chem. Rev. 2012, 112, 673–674. [Google Scholar] [CrossRef]
- Wong-Foy, A.G.; Matzger, A.J.; Yaghi, O.M. Exceptional H2 Saturation Uptake in Microporous Metal−Organic Frameworks. J. Am. Chem. Soc. 2006, 128, 3494–3495. [Google Scholar] [CrossRef]
- Shet, S.P.; Shanmuga Priya, S.; Sudhakar, K.; Tahir, M. A Review on Current Trends in Potential Use of Metal-Organic Framework for Hydrogen Storage. Int. J. Hydrogen Energy 2021, 46, 11782–11803. [Google Scholar] [CrossRef]
- Han, S.S.; Choi, S.-H.; Goddard, W.A. Zeolitic Imidazolate Frameworks as H2 Adsorbents: Ab Initio Based Grand Canonical Monte Carlo Simulation. J. Phys. Chem. C 2010, 114, 12039–12047. [Google Scholar] [CrossRef]
- Cravillon, J.; Nayuk, R.; Springer, S.; Feldhoff, A.; Huber, K.; Wiebcke, M. Controlling Zeolitic Imidazolate Framework Nano- and Microcrystal Formation: Insight into Crystal Growth by Time-Resolved In Situ Static Light Scattering. Chem. Mater. 2011, 23, 2130–2141. [Google Scholar] [CrossRef]
- Jin, C.-X. Synthetic Methods, Properties and Controlling Roles of Synthetic Parameters of Zeolite Imidazole Framework-8: A Review. J. Solid State Chem. 2021, 17, 122040. [Google Scholar] [CrossRef]
- Eddaoudi, M.; Sava, D.F.; Eubank, J.F.; Adil, K.; Guillerm, V. Zeolite-like Metal–Organic Frameworks (ZMOFs): Design, Synthesis, and Properties. Chem. Soc. Rev. 2015, 44, 228–249. [Google Scholar] [CrossRef]
- Chen, B.; Yang, Z.; Zhu, Y.; Xia, Y. Zeolitic Imidazolate Framework Materials: Recent Progress in Synthesis and Applications. J. Mater. Chem. A 2014, 2, 16811–16831. [Google Scholar] [CrossRef]
- Wu, H.; Zhou, W.; Yildirim, T. Hydrogen Storage in a Prototypical Zeolitic Imidazolate Framework-8. J. Am. Chem. Soc. 2007, 129, 5314–5315. [Google Scholar] [CrossRef]
- Chen, E.-Y.; Liu, Y.-C.; Zhou, M.; Zhang, L.; Wang, Q. Effects of Structure on Hydrogen Adsorption in Zeolitic Imidazolate Frameworks. Chem. Eng. Sci. 2012, 71, 178–184. [Google Scholar] [CrossRef]
- Assfour, B.; Leoni, S.; Yurchenko, S.; Seifert, G. Hydrogen Storage in Zeolite Imidazolate Frameworks. A Multiscale Theoretical Investigation. Int. J. Hydrogen Energy 2011, 36, 6005–6013. [Google Scholar] [CrossRef]
- Chen, E.-Y.; Liu, Y.-C.; Sun, T.-Y.; Wang, Q.; Liang, L.-J. Effects of Substituent Groups and Central Metal Ion on Hydrogen Adsorption in Zeolitic Imidazolate Frameworks. Chem. Eng. Sci. 2013, 97, 60–66. [Google Scholar] [CrossRef]
- Park, K.S.; Ni, Z.; Côté, A.P.; Choi, J.Y.; Huang, R.; Uribe-Romo, F.J.; Chae, H.K.; O’Keeffe, M.; Yaghi, O.M. Exceptional Chemical and Thermal Stability of Zeolitic Imidazolate Frameworks. Proc. Natl. Acad. Sci. USA 2006, 103, 10186–10191. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Wu, H.; Hartman, M.R.; Yildirim, T. Hydrogen and Methane Adsorption in Metal−Organic Frameworks: A High-Pressure Volumetric Study. J. Phys. Chem. C 2007, 111, 16131–16137. [Google Scholar] [CrossRef]
- Bose, R.; Ethiraj, J.; Sridhar, P.; Varghese, J.J.; Kaisare, N.S.; Selvam, P. Adsorption of Hydrogen and Carbon Dioxide in Zeolitic Imidazolate Framework Structure with SOD Topology: Experimental and Modelling Studies. Adsorption 2020, 26, 1027–1038. [Google Scholar] [CrossRef]
- Hayashi, H.; Côté, A.P.; Furukawa, H.; O’Keeffe, M.; Yaghi, O.M. Zeolite A Imidazolate Frameworks. Nat. Mater. 2007, 6, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Sun, F.; Qin, L. Hydrothermal Synthesis of Zeolitic Imidazolate Framework-67 (ZIF-67) Nanocrystals. Mater. Lett. 2012, 82, 220–223. [Google Scholar] [CrossRef]
- Duan, C.; Yu, Y.; Hu, H. Recent Progress on Synthesis of ZIF-67-Based Materials and Their Application to Heterogeneous Catalysis. Green Energy Environ. 2022, 7, 3–15. [Google Scholar] [CrossRef]
- Kaur, G.; Rai, R.K.; Tyagi, D.; Yao, X.; Li, P.-Z.; Yang, X.-C.; Zhao, Y.; Xu, Q.; Singh, S.K. Room-Temperature Synthesis of Bimetallic Co–Zn Based Zeolitic Imidazolate Frameworks in Water for Enhanced CO2 and H 2 Uptakes. J. Mater. Chem. A 2016, 4, 14932–14938. [Google Scholar] [CrossRef]
- Panchariya, D.K.; Rai, R.K.; Anil Kumar, E.; Singh, S.K. Core–Shell Zeolitic Imidazolate Frameworks for Enhanced Hydrogen Storage. ACS Omega 2018, 3, 167–175. [Google Scholar] [CrossRef]
- Wen, M.; Mori, K.; Futamura, Y.; Kuwahara, Y.; Navlani-García, M.; An, T.; Yamashita, H. PdAg Nanoparticles Within Core-Shell Structured Zeolitic Imidazolate Framework as a Dual Catalyst for Formic Acid-Based Hydrogen Storage/Production. Sci. Rep. 2019, 9, 15675. [Google Scholar] [CrossRef]
- Petit, C.; Bandosz, T.J. Exploring the Coordination Chemistry of MOF–Graphite Oxide Composites and Their Applications as Adsorbents. Dalton Trans. 2012, 41, 4027. [Google Scholar] [CrossRef]
- Kumar, R.; Jayaramulu, K.; Maji, T.K.; Rao, C.N.R. Hybrid Nanocomposites of ZIF-8 with Graphene Oxide Exhibiting Tunable Morphology, Significant CO2 Uptake and Other Novel Properties. Chem. Commun. 2013, 49, 4947. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zhang, J.; Zhang, J.; Yan, X.; Shen, X.; Yuan, A. High-Capacity Room-Temperature Hydrogen Storage of Zeolitic Imidazolate Framework/Graphene Oxide Promoted by Platinum Metal Catalyst. Int. J. Hydrogen Energy 2015, 40, 12275–12285. [Google Scholar] [CrossRef]
- Yang, S.J.; Choi, J.Y.; Chae, H.K.; Cho, J.H.; Nahm, K.S.; Park, C.R. Preparation and Enhanced Hydrostability and Hydrogen Storage Capacity of CNT@MOF-5 Hybrid Composite. Chem. Mater. 2009, 21, 1893–1897. [Google Scholar] [CrossRef]
- Petit, C.; Bandosz, T.J. MOF-Graphite Oxide Composites: Combining the Uniqueness of Graphene Layers and Metal-Organic Frameworks. Adv. Mater. 2009, 21, 4753–4757. [Google Scholar] [CrossRef]
- Jahan, M.; Bao, Q.; Yang, J.-X.; Loh, K.P. Structure-Directing Role of Graphene in the Synthesis of Metal−Organic Framework Nanowire. J. Am. Chem. Soc. 2010, 132, 14487–14495. [Google Scholar] [CrossRef]
- Yang, J.; Li, P.; Wang, L.; Guo, X.; Guo, J.; Liu, S. In-Situ Synthesis of Ni-MOF@CNT on Graphene/Ni Foam Substrate as a Novel Self-Supporting Hybrid Structure for All-Solid-State Supercapacitors with a High Energy Density. J. Electroanal. Chem. 2019, 848, 113301. [Google Scholar] [CrossRef]
- Pinjari, S.; Bera, T.; Kapur, G.S.; Kjeang, E. The Mechanism and Sorption Kinetic Analysis of Hydrogen Storage at Room Temperature Using Acid Functionalized Carbon Nanotubes. Int. J. Hydrogen Energy 2023, 48, 1930–1942. [Google Scholar] [CrossRef]
- Zhang, Y.; Jia, Y.; Li, M.; Hou, L. Influence of the 2-Methylimidazole/Zinc Nitrate Hexahydrate Molar Ratio on the Synthesis of Zeolitic Imidazolate Framework-8 Crystals at Room Temperature. Sci. Rep. 2018, 8, 9597. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, F.; Lu, H.; Hong, X.; Jiang, H.; Wu, Y.; Li, Y. Hollow Zn/Co ZIF Particles Derived from Core-Shell ZIF-67@ZIF-8 as Selective Catalyst for the Semi-Hydrogenation of Acetylene. Angew. Chem. Int. Ed. 2015, 54, 10889–10893. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, T.; Xiao, K.; Cheng, S.; Qian, G.; Wang, Y.; Feng, Y. Novel and Facile Strategy for Controllable Synthesis of Multilayered Core–Shell Zeolitic Imidazolate Frameworks. Cryst. Growth Des. 2016, 16, 6494–6498. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, L.; Chen, S.; Zhu, X.; Deng, Q.; Wang, J.; Zeng, Z.; Deng, S. Facile and Low-Temperature Strategy to Prepare Hollow ZIF-8/CNT Polyhedrons as High-Performance Lithium-Sulfur Cathodes. Chem. Eng. J. 2021, 404, 126579. [Google Scholar] [CrossRef]
- Bahos, F.; Sainz-Vidal, A.; Sánchez-Pérez, C.; Saniger, J.; Gràcia, I.; Saniger-Alba, M.; Matatagui, D. ZIF Nanocrystal-Based Surface Acoustic Wave (SAW) Electronic Nose to Detect Diabetes in Human Breath. Biosensors 2018, 9, 4. [Google Scholar] [CrossRef] [PubMed]
- Zuo, X.; Chang, K.; Zhao, J.; Xie, Z.; Tang, H.; Li, B.; Chang, Z. Bubble-Template-Assisted Synthesis of Hollow Fullerene-like MoS2 Nanocages as a Lithium Ion Battery Anode Material. J. Mater. Chem. A 2016, 4, 51–58. [Google Scholar] [CrossRef]
- Dyer, A. Zeolites. Encyclopedia of Materials: Science and Technology; Buschow, K.H.J., Cahn, R.W., Flemings, M.C., Ilschner, B., Kramer, E.J., Mahajan, S., Veyssière, P., Eds.; Elsevier: Oxford, UK, 2001; pp. 9859–9863. ISBN 978-0-08-043152-9. [Google Scholar]
- Bagai, R.; Christopher, J.; Kapur, G.S. Evaluating Industrial Grade Functionalized Multiwalled Carbon Nanotubes by X-Ray Photoelectron Spectroscopy. Fuller. Nanotub. Carbon Nanostruct. 2019, 27, 240–246. [Google Scholar] [CrossRef]
- Li, Y.; Cai, X.; Chen, S.; Zhang, H.; Zhang, K.H.L.; Hong, J.; Chen, B.; Kuo, D.-H.; Wang, W. Highly Dispersed Metal Carbide on ZIF-Derived Pyridinic-N-Doped Carbon for CO2 Enrichment and Selective Hydrogenation. ChemSusChem 2018, 11, 1040–1047. [Google Scholar] [CrossRef] [PubMed]
- Mallineni, S.S.K.; Bhattacharya, S.; Liu, F.; Puneet, P.; Rao, A.; Srivastava, A.; Podila, R. Defect Engineered 2D Materials for Energy Applications. In Two-Dimensional Materials-Synthesis, Characterization and Potential Applications; Nayak, P.K., Ed.; InTech: London, UK, 2016; ISBN 978-953-51-2554-9. [Google Scholar]
- Muñoz-Gil, D.; Figueiredo, F.M.L. High Surface Proton Conduction in Nanostructured ZIF-8. Nanomaterials 2019, 9, 1369. [Google Scholar] [CrossRef]
- Nqombolo, A.; Munonde, T.S.; Makhetha, T.A.; Moutloali, R.M.; Nomngongo, P.N. Cobalt/Zinc Based Metal Organic Frameworks as an Effective Adsorbent for Improved Removal of As(V) and Cr(VI) in a Wide pH Range. J. Mater. Res. Technol. 2021, 12, 1845–1855. [Google Scholar] [CrossRef]
- Dai, P.; Yao, Y.; Hu, E.; Xu, D.; Li, Z.; Wang, C. Self-Assembled ZIF-67@graphene Oxide as a Cobalt-Based Catalyst Precursor with Enhanced Catalytic Activity toward Methanolysis of Sodium Borohydride. Appl. Surf. Sci. 2021, 546, 149128. [Google Scholar] [CrossRef]
- Song, X.; Yu, J.; Wei, M.; Li, R.; Pan, X.; Yang, G.; Tang, H. Ionic Liquids-Functionalized Zeolitic Imidazolate Framework for Carbon Dioxide Adsorption. Materials 2019, 12, 2361. [Google Scholar] [CrossRef]
- Pinjari, S.; Kumaravelan, M.K.; Peddy, V.C.; Gandham, S.; Patruni, J.; Velluru, S.; Kumar, P. Maximizing the Production of Hydrogen and Carbon Nanotubes: Effect of Ni and Reaction Temperature. Int. J. Hydrogen Energy 2018, 43, 2781–2793. [Google Scholar] [CrossRef]
- Yamamoto, D.; Maki, T.; Watanabe, S.; Tanaka, H.; Miyahara, M.T.; Mae, K. Synthesis and Adsorption Properties of ZIF-8 Nanoparticles Using a Micromixer. Chem. Eng. J. 2013, 227, 145–150. [Google Scholar] [CrossRef]
- Pan, Y.; Liu, Y.; Zeng, G.; Zhao, L.; Lai, Z. Rapid Synthesis of Zeolitic Imidazolate Framework-8 (ZIF-8) Nanocrystals in an Aqueous System. Chem. Commun. 2011, 47, 2071. [Google Scholar] [CrossRef]
- Yue, Y.; Guo, B.; Qiao, Z.-A.; Fulvio, P.F.; Chen, J.; Binder, A.J.; Tian, C.; Dai, S. Multi-Wall Carbon Nanotube@zeolite Imidazolate Framework Composite from a Nanoscale Zinc Oxide Precursor. Microporous Mesoporous Mater. 2014, 198, 139–143. [Google Scholar] [CrossRef]
- Yadav, R.; Baskaran, T.; Kaiprathu, A.; Ahmed, M.; Bhosale, S.V.; Joseph, S.; Al-Muhtaseb, A.H.; Singh, G.; Sakthivel, A.; Vinu, A. Recent Advances in the Preparation and Applications of Organo-Functionalized Porous Materials. Chem. Asian J. 2020, 15, 2588–2621. [Google Scholar] [CrossRef] [PubMed]
- Bosu, S.; Rajamohan, N. Recent Advancements in Hydrogen Storage-Comparative Review on Methods, Operating Conditions and Challenges. Int. J. Hydrogen Energy 2024, 52, 352–370. [Google Scholar] [CrossRef]
- Chen, Z.; Kirlikovali, K.O.; Idrees, K.B.; Wasson, M.C.; Farha, O.K. Porous Materials for Hydrogen Storage. Chem 2022, 8, 693–716. [Google Scholar] [CrossRef]
- Conte, G.; Policicchio, A.; De Luca, O.; Rudolf, P.; Desiderio, G.; Agostino, R.G. Copper-Doped Activated Carbon from Amorphous Cellulose for Hydrogen, Methane and Carbon Dioxide Storage. Int. J. Hydrogen Energy 2022, 47, 18384–18395. [Google Scholar] [CrossRef]
- Zhou, M.; Wang, Q.; Zhang, L.; Liu, Y.-C.; Kang, Y. Adsorption Sites of Hydrogen in Zeolitic Imidazolate Frameworks. J. Phys. Chem. B 2009, 113, 11049–11053. [Google Scholar] [CrossRef]
- Assfour, B.; Leoni, S.; Seifert, G. Hydrogen Adsorption Sites in Zeolite Imidazolate Frameworks ZIF-8 and ZIF-11. J. Phys. Chem. C 2010, 114, 13381–13384. [Google Scholar] [CrossRef]
- Revellame, E.D.; Fortela, D.L.; Sharp, W.; Hernandez, R.; Zappi, M.E. Adsorption Kinetic Modeling Using Pseudo-First Order and Pseudo-Second Order Rate Laws: A Review. Clean. Eng. Technol. 2020, 1, 100032. [Google Scholar] [CrossRef]
- Liu, Y.; Shen, L. From Langmuir Kinetics to First- and Second-Order Rate Equations for Adsorption. Langmuir 2008, 24, 11625–11630. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.L.; Hameed, B.H. Insight into the Adsorption Kinetics Models for the Removal of Contaminants from Aqueous Solutions. J. Taiwan Inst. Chem. Eng. 2017, 74, 25–48. [Google Scholar] [CrossRef]
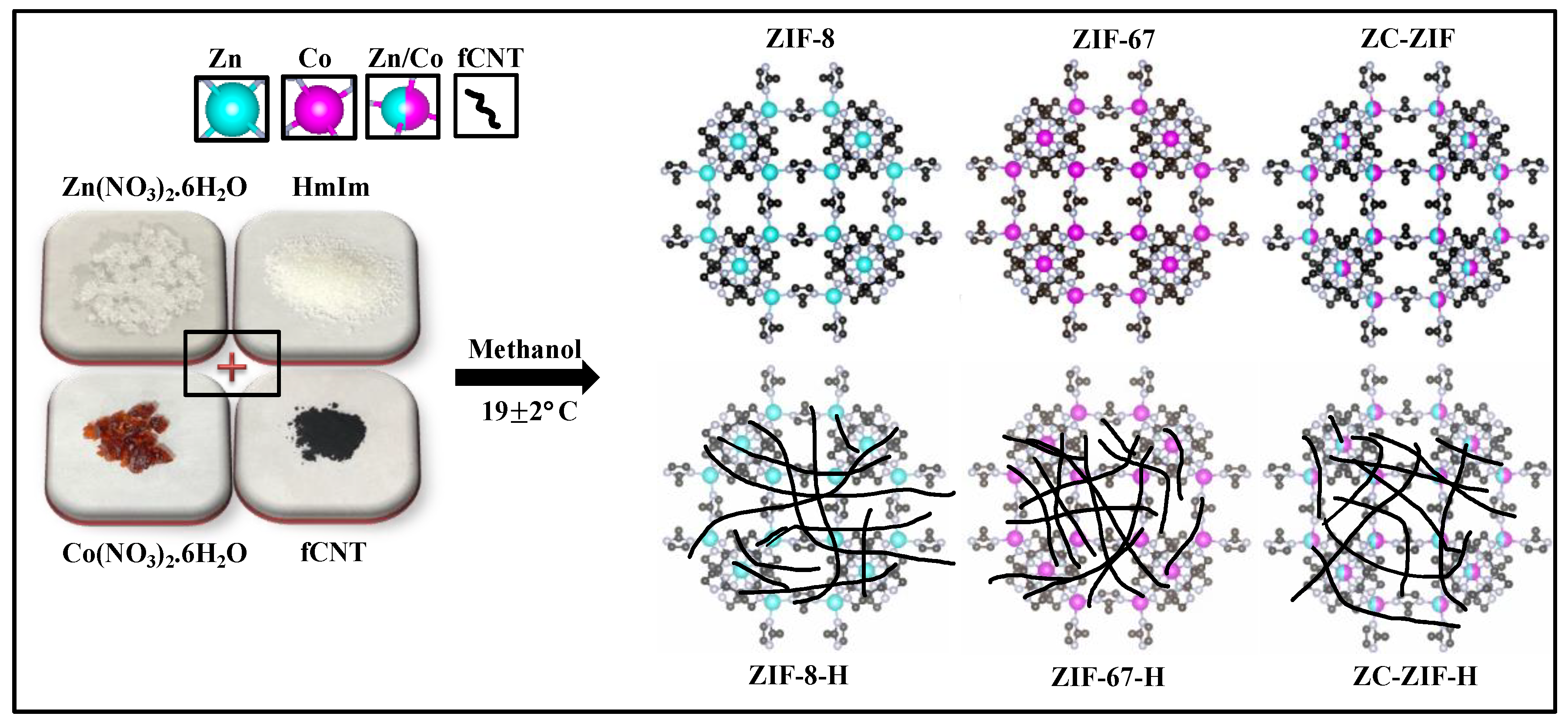

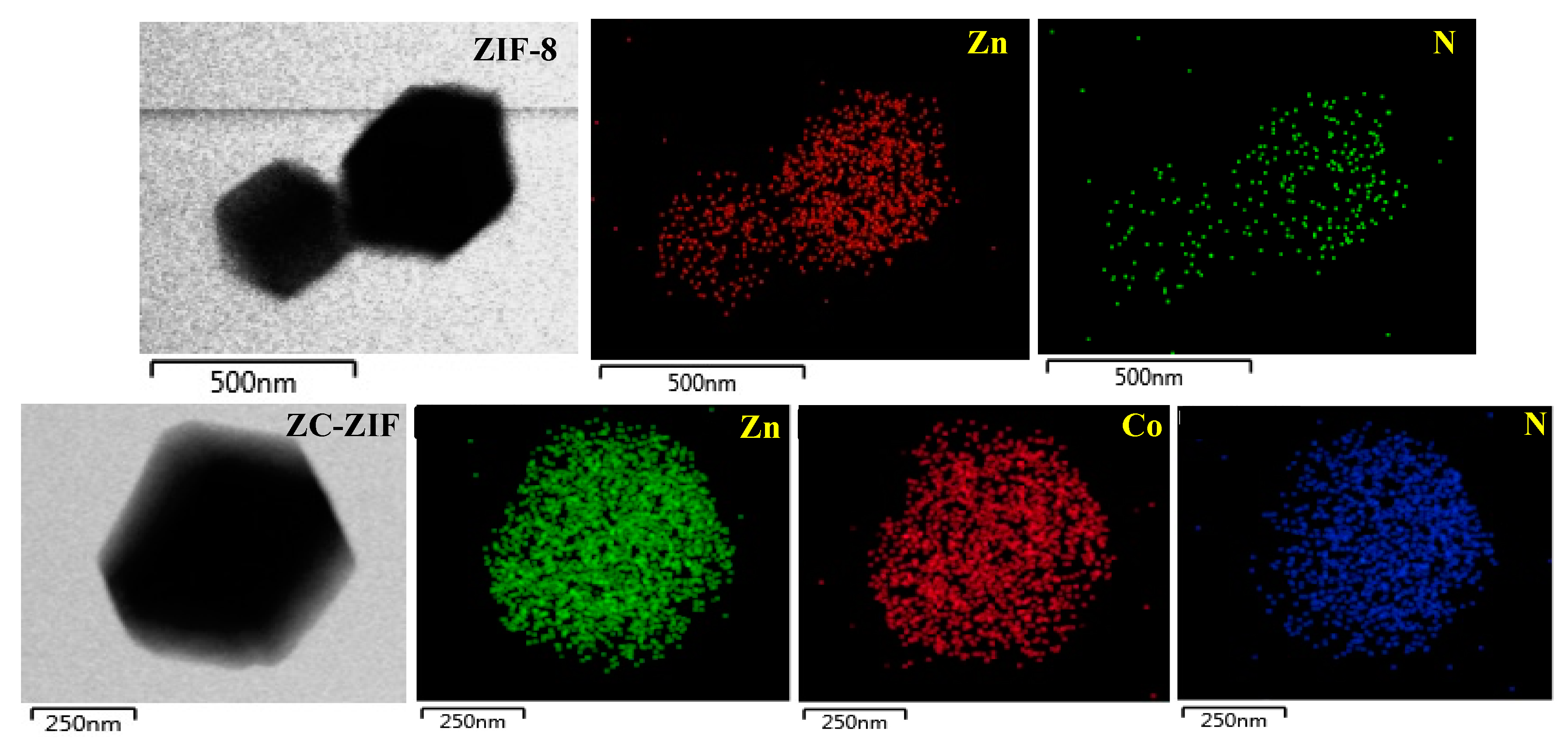

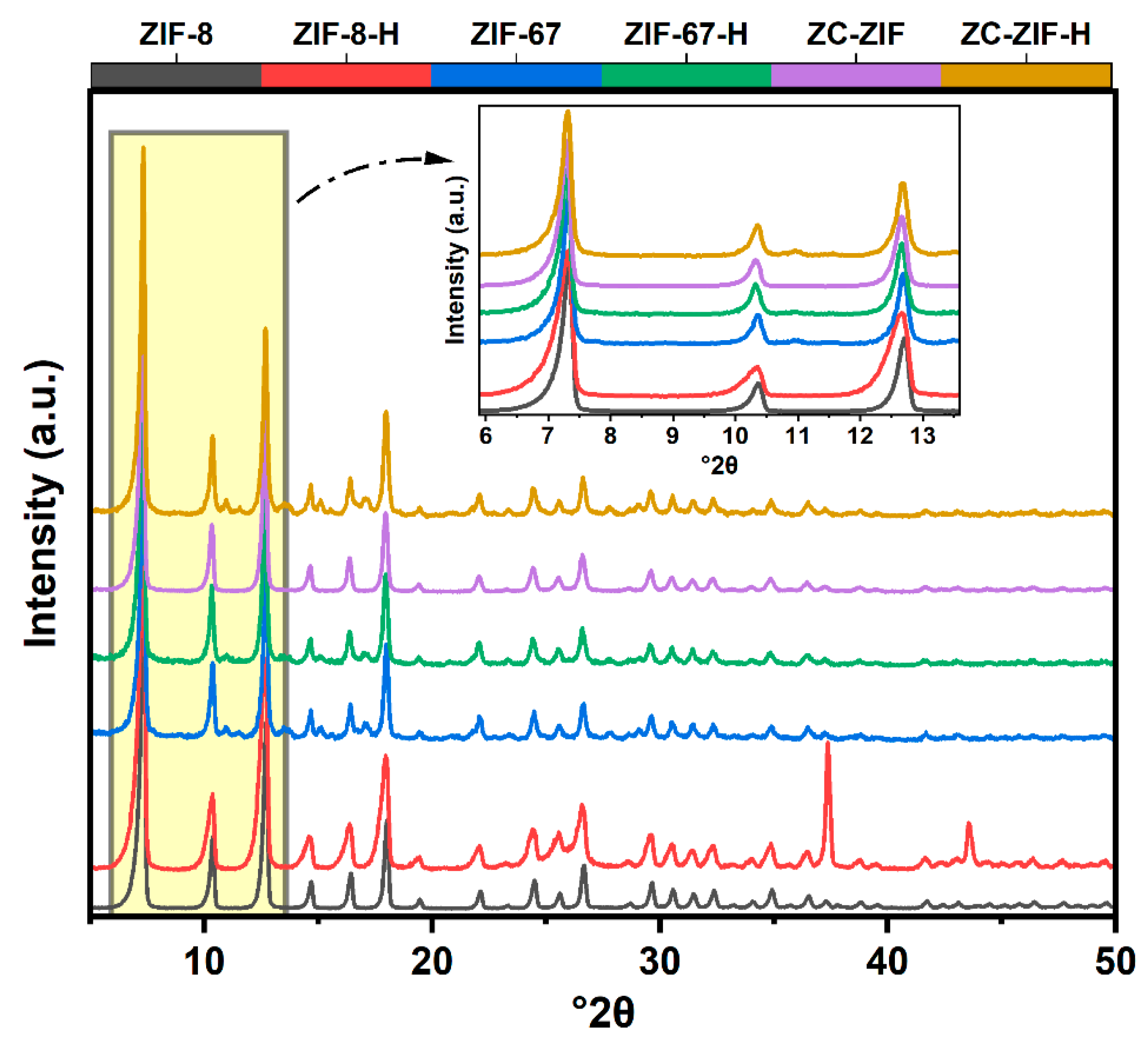


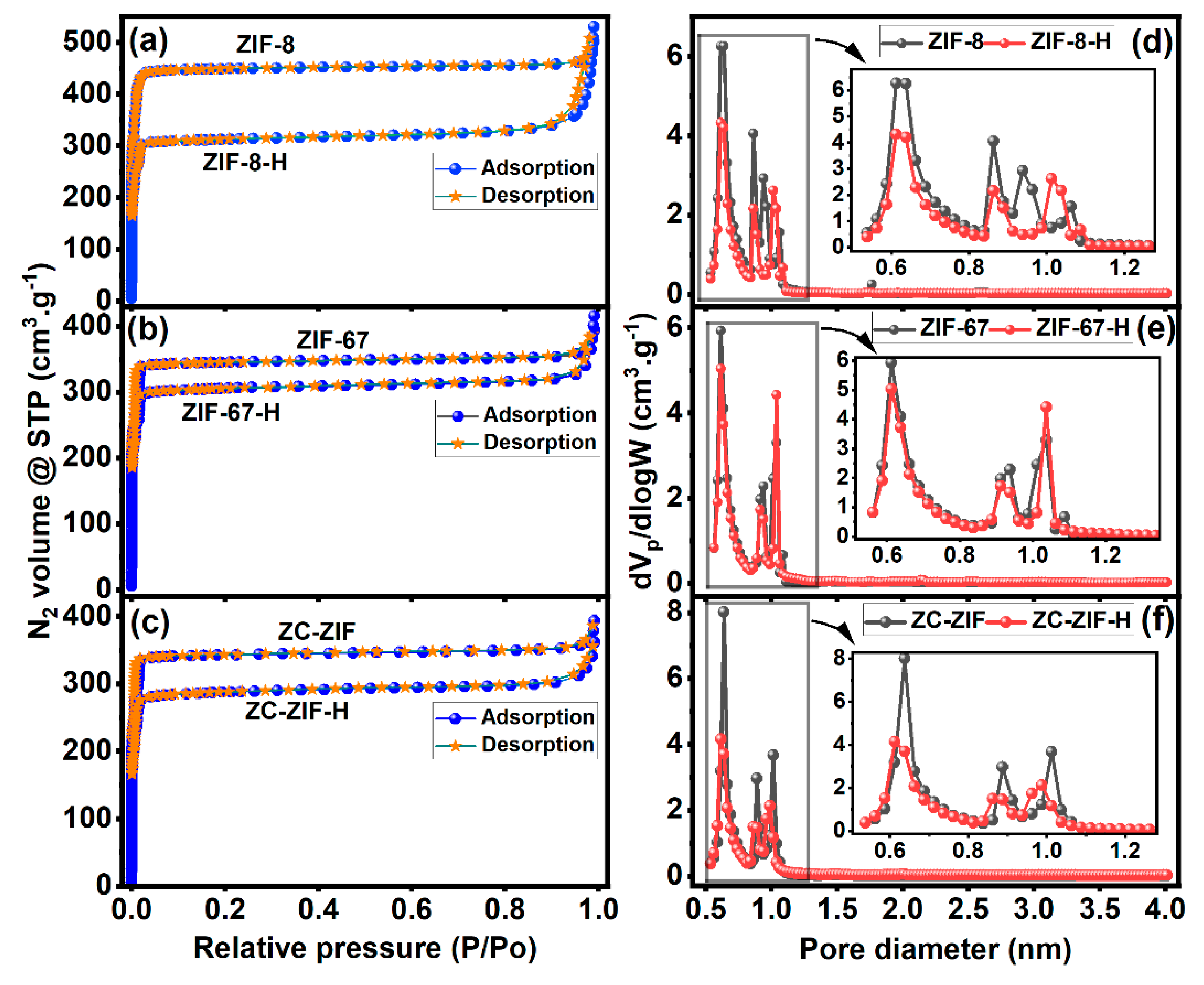
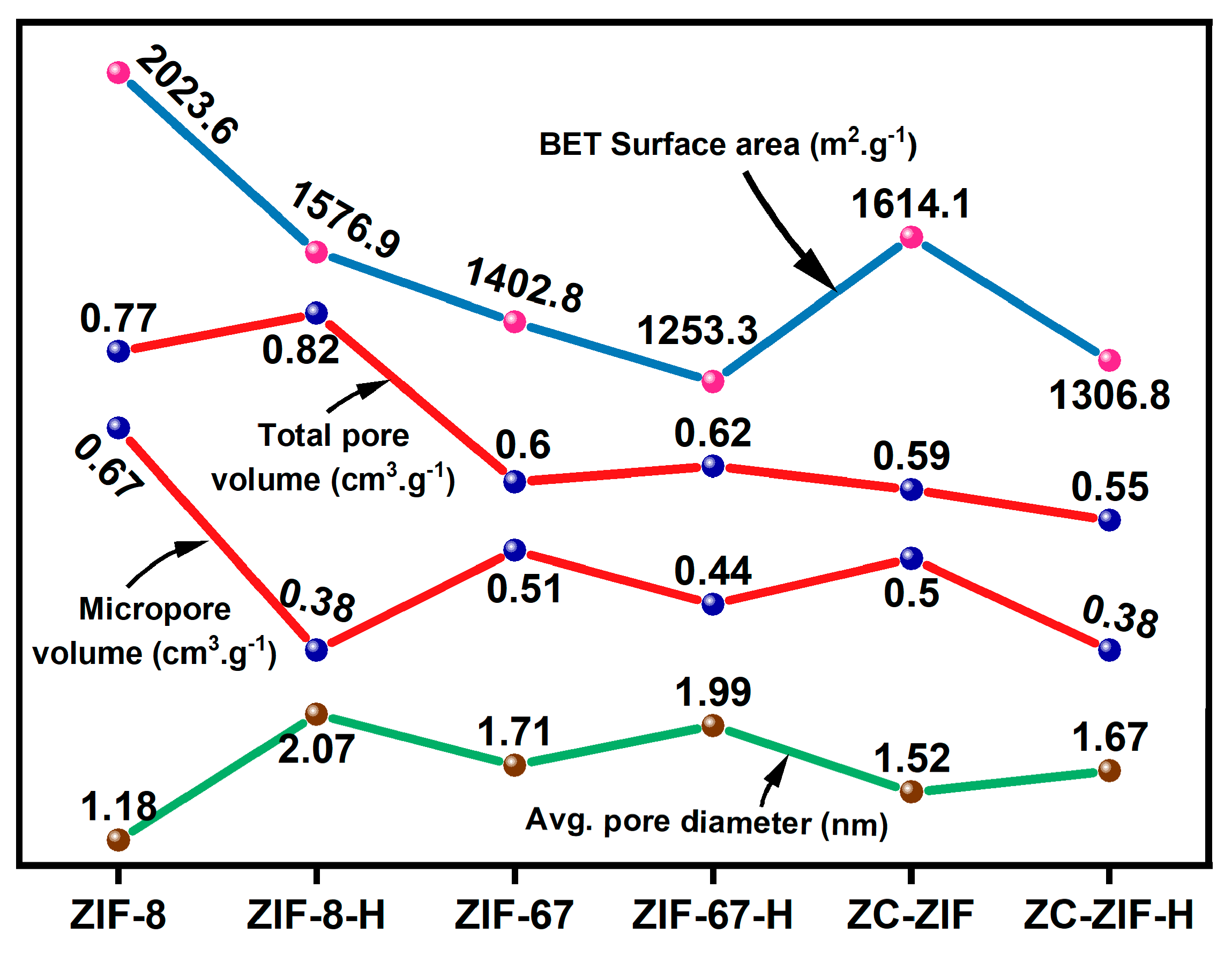
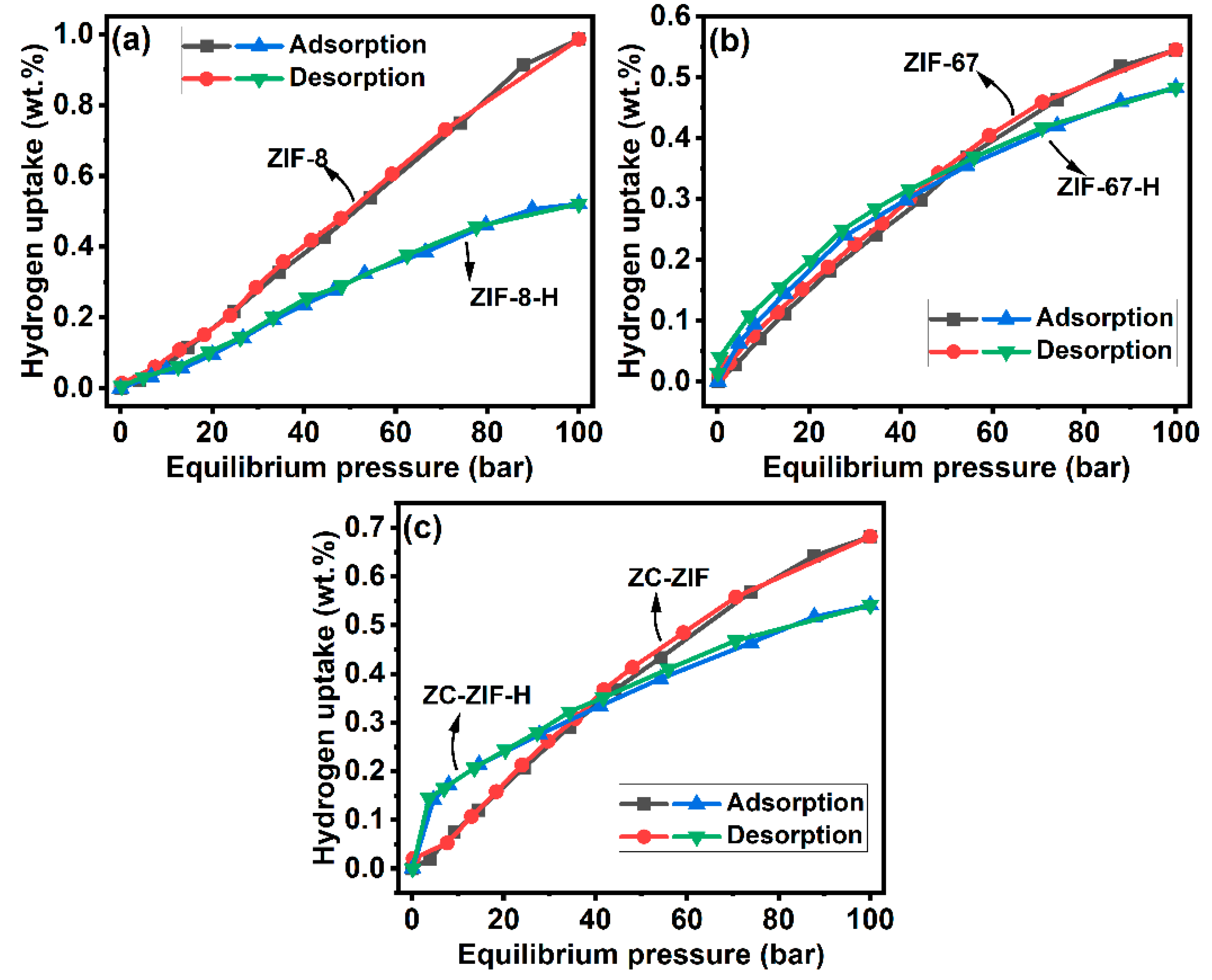
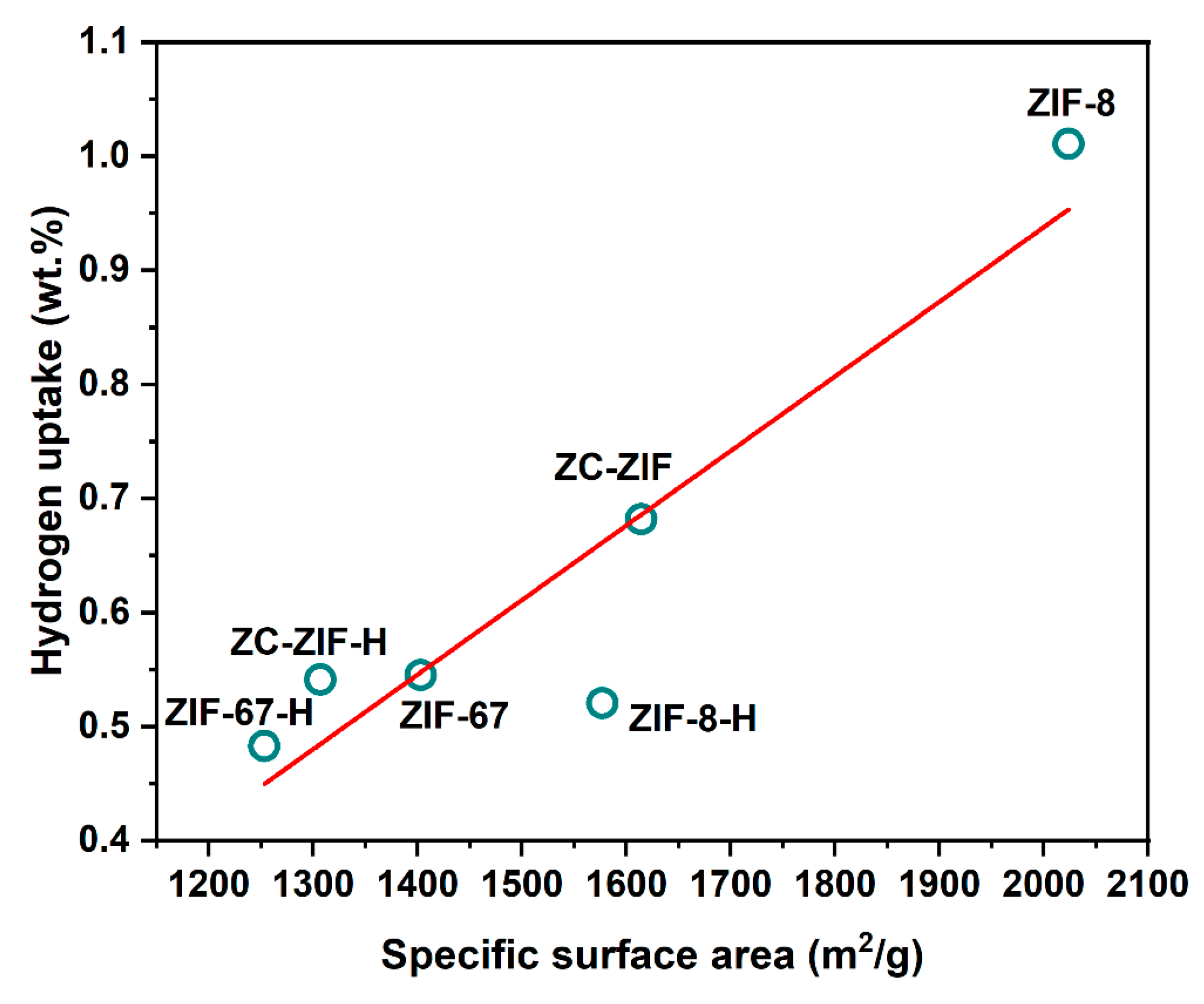

| Samples | °2θ | Relative Intensity (%) | Avg. Crystallite Size (nm) |
|---|---|---|---|
| ZIF-8 | 7.46 | 100.0 | 36.42 |
| ZIF-8-H | 7.90 | 70.7 | 22.48 |
| ZIF-67 | 7.32 | 15.69 | 29.94 |
| ZIF-67-H | 7.30 | 15.54 | 27.92 |
| ZC-ZIF | 7.30 | 49.78 | 30.14 |
| ZC-ZIF-H | 7.32 | 20.51 | 29.64 |
| Samples | Temperature (°C) | Yield (wt. %) | ||
|---|---|---|---|---|
| Onset | Inflection | End Point | ||
| ZIF-8 | 451.4 | 492.0 | 558.6 | 33.8 |
| ZIF-8-H | 446.2 | 485.8 | 637.6 | 25.3 |
| ZIF-67 | 371.5 | 429.8 | 507.2 | 29.7 |
| ZIF-67-H | 380.8 | 433.0 | 452.1 | 29.6 |
| ZC-ZIF | 408.4 | 456.5 | 511.8 | 29.9 |
| ZC-ZIF-H | 428.4 | 464.3 | 540.0 | 31.1 |
| Material | PFO Model | Avrami Model | ||||
|---|---|---|---|---|---|---|
| k × 10−3 (min−1) | Intercept | R2 (%) | kav | n | R2 (%) | |
| ZIF-8 | 4.93 | −0.105252 | 91.32 | 3.03 × 10−4 | 1.43 | 99.42 |
| ZIF-8-H | 3.46 | −0.071934 | 83.61 | 0.0086 | 0.91 | 95.27 |
| ZIF-67 | 3.92 | −0.107561 | 91.38 | 1.14 × 10−4 | 1.55 | 99.23 |
| ZIF-67-H | 3.79 | 0.044047 | 96.06 | 0.0077 | 0.89 | 97.54 |
| ZC-ZIF | 4.18 | −0.12691 | 92.81 | 1.79 × 10−4 | 1.49 | 99.31 |
| ZC-ZIF-H | 5.37 | 0.056535 | 93.87 | 0.0296 | 0.68 | 95.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinjari, S.; Bera, T.; Kjeang, E. Enhanced Room-Temperature Hydrogen Physisorption in Zeolitic Imidazolate Frameworks and Carbon Nanotube Hybrids. Nanoenergy Adv. 2025, 5, 5. https://doi.org/10.3390/nanoenergyadv5020005
Pinjari S, Bera T, Kjeang E. Enhanced Room-Temperature Hydrogen Physisorption in Zeolitic Imidazolate Frameworks and Carbon Nanotube Hybrids. Nanoenergy Advances. 2025; 5(2):5. https://doi.org/10.3390/nanoenergyadv5020005
Chicago/Turabian StylePinjari, Syedvali, Tapan Bera, and Erik Kjeang. 2025. "Enhanced Room-Temperature Hydrogen Physisorption in Zeolitic Imidazolate Frameworks and Carbon Nanotube Hybrids" Nanoenergy Advances 5, no. 2: 5. https://doi.org/10.3390/nanoenergyadv5020005
APA StylePinjari, S., Bera, T., & Kjeang, E. (2025). Enhanced Room-Temperature Hydrogen Physisorption in Zeolitic Imidazolate Frameworks and Carbon Nanotube Hybrids. Nanoenergy Advances, 5(2), 5. https://doi.org/10.3390/nanoenergyadv5020005








