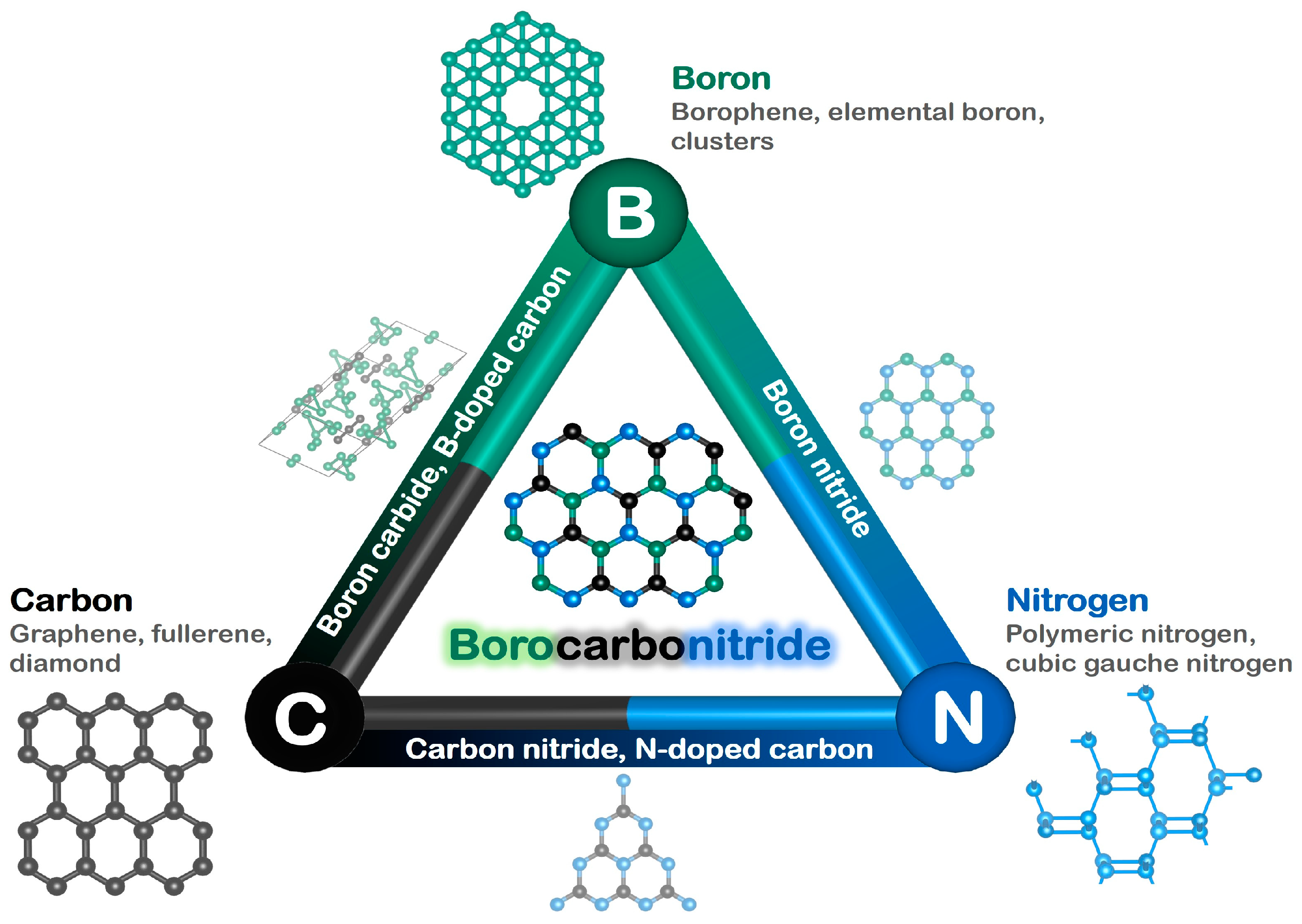
Borocarbonitrides for Decarbonization: From CO2 Utilization to Renewable Fuel Synthesis
Abstract
:1. Introduction
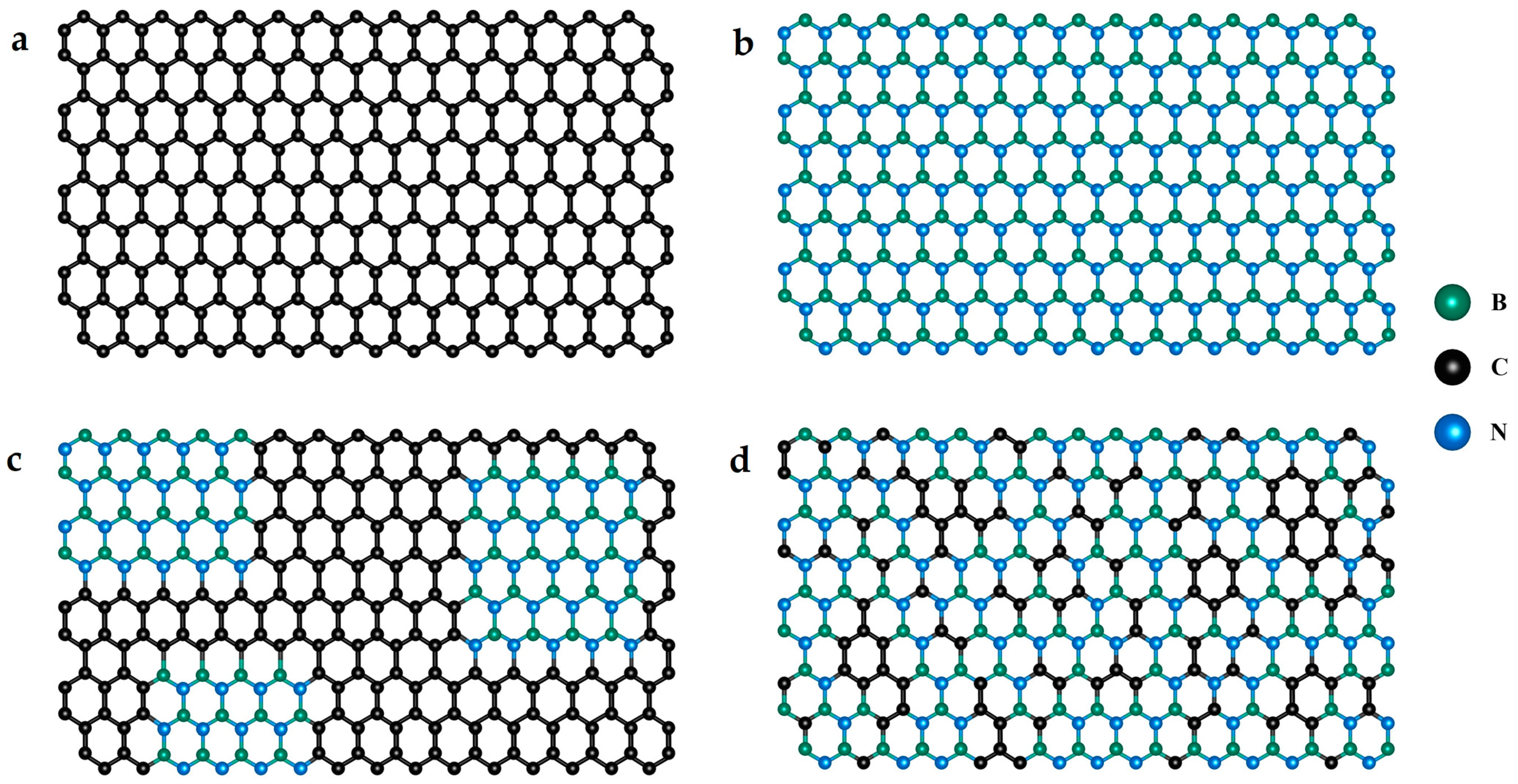
2. Synthesis and Characterization of Borocarbonitrides
2.1. Synthesis

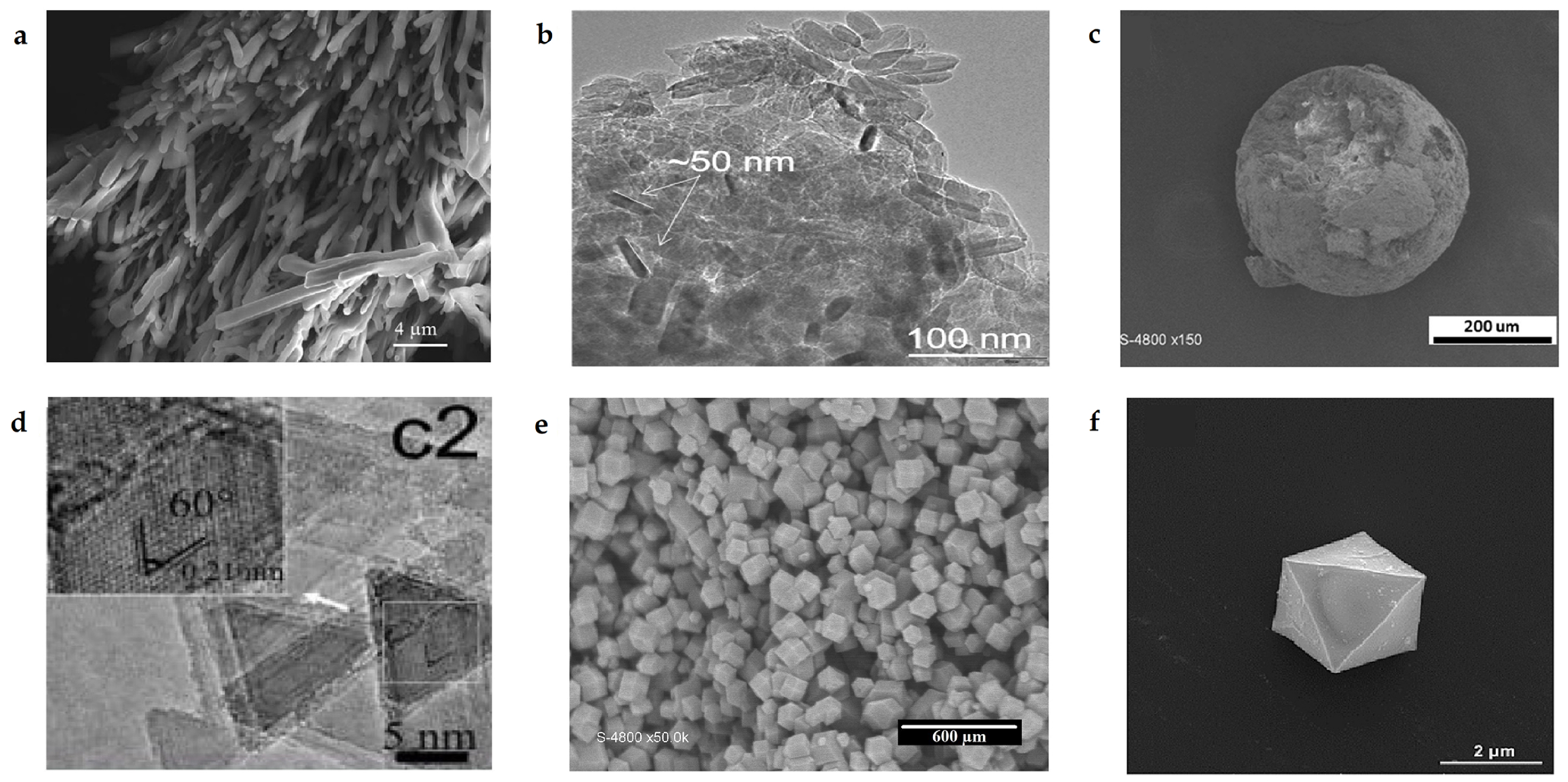
2.2. Characterization
2.2.1. Elemental Analysis
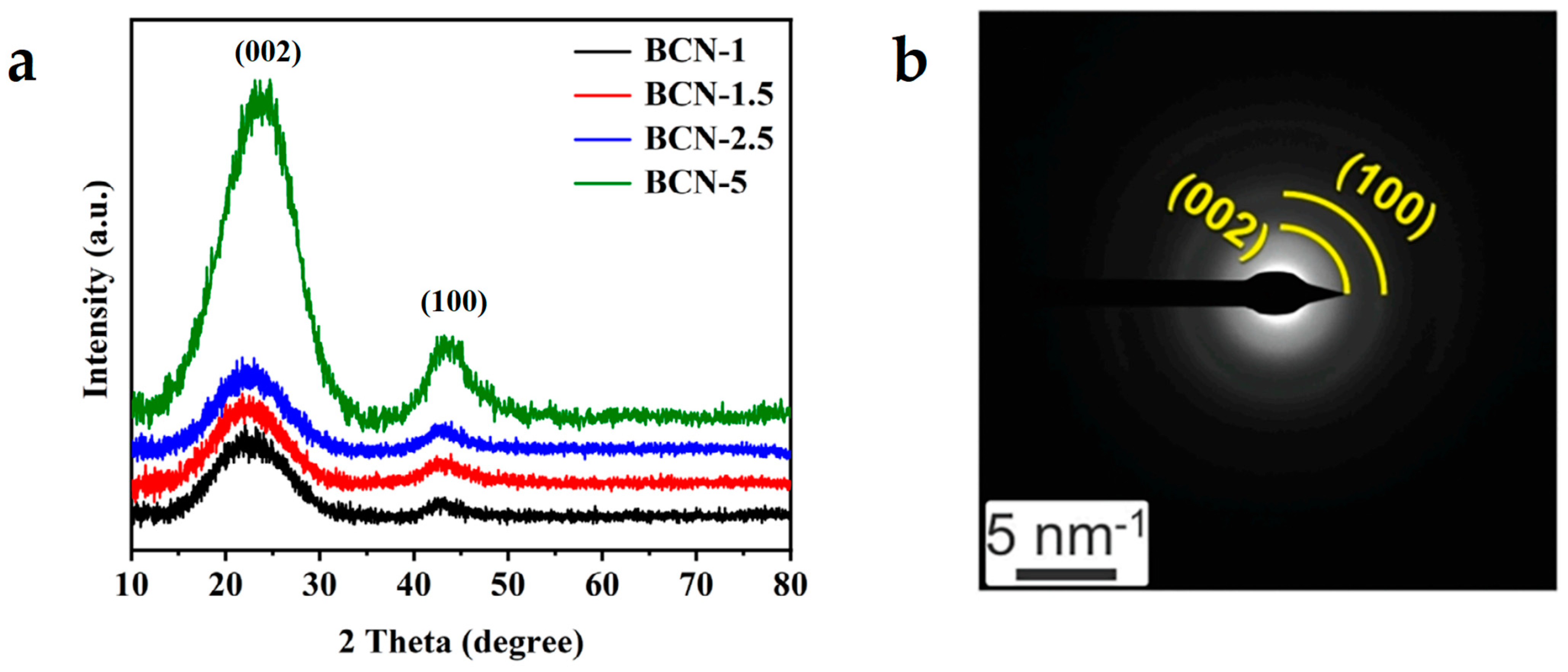
2.2.2. Structural Analyses
2.2.3. Spectroscopic Analyses
2.2.4. Optic Analyses
2.2.5. Quantification of Acidic and Basic Sites
3. Application of Borocarbonitrides in Decarbonization Technologies
3.1. Gas Adsorption
| Material | nads (mmol g−1) | wt% | SSA (m2 g−1) | Ref. |
|---|---|---|---|---|
| Nanoporous coral-like BCN | 16.3 | 3.26 | 988 | [103] |
| Two-dimensional porous BCN | 14.5 | 2.91 | 3310 | [106] |
| Graphene-like BCN | 13 | 2.60 | 2911 | [139] |
| Nanoporous BCN | 5.35 | 1.07 | 1560 | [125] |
| Material | nads (mmol g−1) | wt% | −ΔH (kJ mol−1) | SSA (m2 g−1) | Ref. |
|---|---|---|---|---|---|
| Porous BCN | 3.74–3.91 | 16.5–17.2 | 33.5 ± 1.5 | 727 | [127] |
| Microporous BCN | 3.23 | 14.2 | 33 ± 2 | 511 | [96] |
| Two-dimensional porous BCN | 2.39 | 10.5 | - | 3310 | [106] |
| BCN with oxygenated surface | 2.38 | 10.4 | 23.7 ± 1.4 | 2991 | [32] |
| C-doped BN–UV irradiation | 2.38 | 10.4 | - | - | [132] |
| Nanostructured BN/C spheres | 1.97 | 8.6 | 27.5 ± 2.5 | 767 | [111] |
| C/BN composite | 1.94 a | 8.5a | 12 ± 4 | 56 | [113] |
| Mesoporous BCN | 1.64 | 7.2 | 14.1 ± 7.6 | 1166 | [107] |
| Nanoporous coral-like BCN | 1.36 | 6 | - | 988 | [103] |
| Graphene-like BCN | 1.16 | 5.1 | - | 1991 | [140] |
| Carbon-doped BN | 0.73 | 3.2 | 72 | 368 b | [138] |
| Graphene-like BCN | 0.67 | 2.9 | - | 2911 | [139] |

3.2. Metal-Free Catalysis
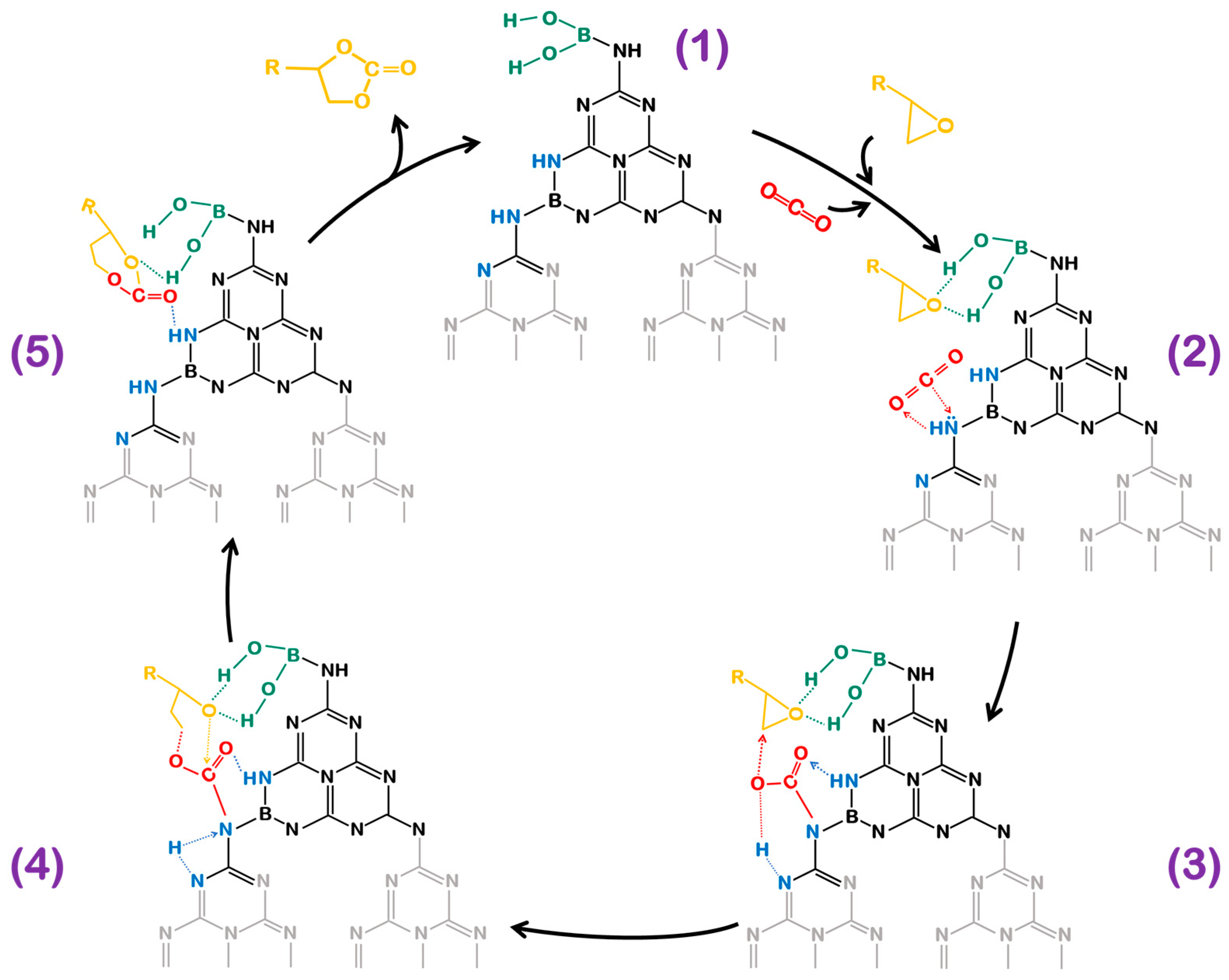
3.2.1. CO2 Cycloaddition
3.2.2. Desulfurization of Fuels
3.2.3. Oxidative Dehydrogenation
3.3. Photocatalysis
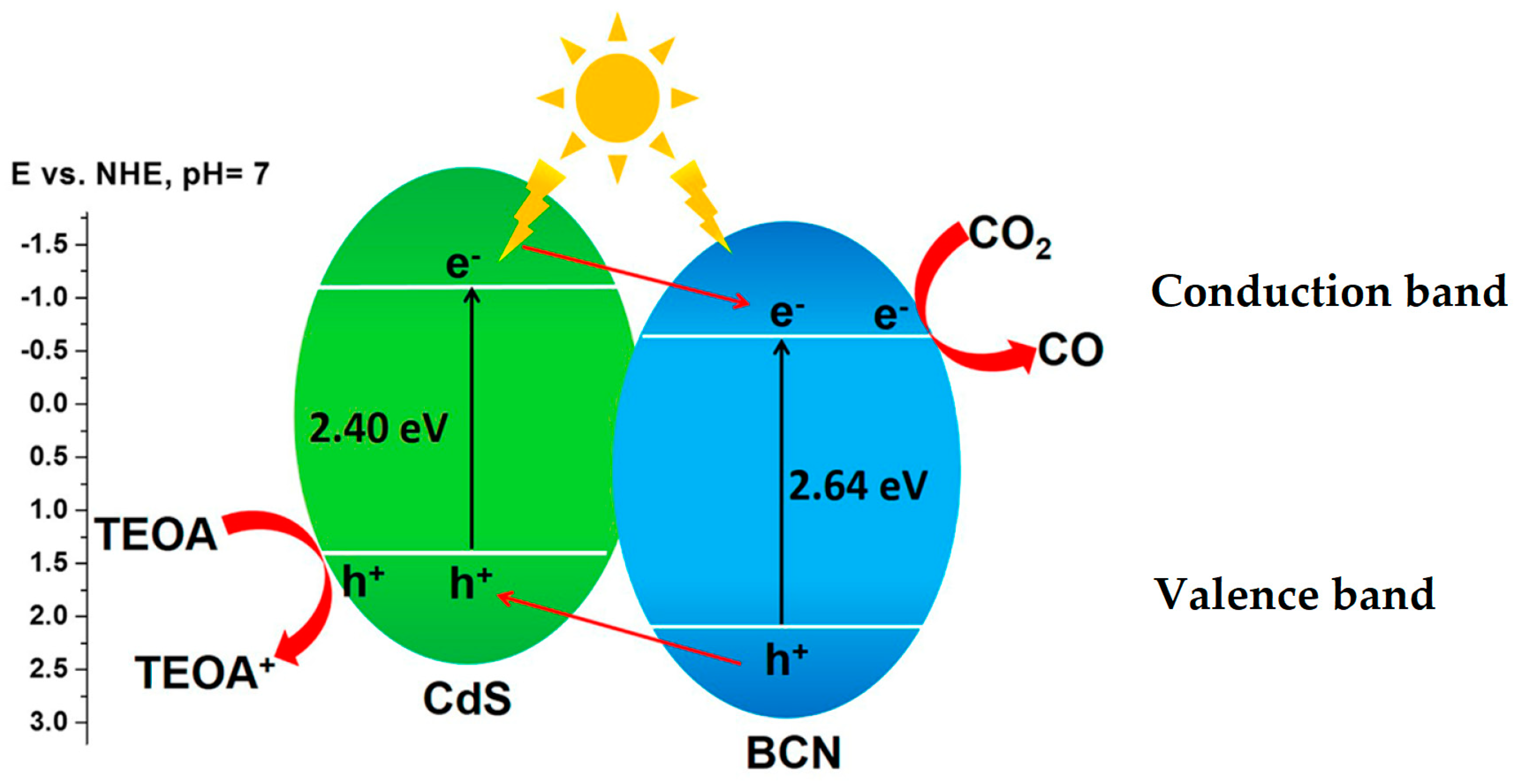
3.3.1. CO2 Photoreduction
3.3.2. Water Splitting
3.4. Electrocatalysis
3.4.1. CO2 Reduction
3.4.2. Oxygen Reduction Reaction
3.4.3. Hydrogen Evolution Reaction
3.4.4. Electrochemical Ammonia Production
4. Concluding Remarks and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| Ammonia | NH3 |
| Ammonia borane | AB |
| Boric acid | B(OH)3 |
| Borocarbonitride(s) | BCN(s) |
| Boron nitride | BN |
| Carbon dioxide | CO2 |
| Chemical vapor deposition | CVD |
| Density functional theory | DFT |
| Electron Energy Loss Spectroscopy | EELS |
| Energy-Dispersive X-ray | EDX |
| Faraday efficiency(ies) | FE(s) |
| Graphitic carbon nitride | g-C3N4 |
| Hexagonal boron nitride | h-BN |
| High Resolution | HR |
| Hydrogen evolution reaction | HER |
| Hydrogen peroxide | H2O2 |
| Hydrogen | H2 |
| Inductively Coupled Plasma | ICP |
| Infrared | IR |
| Isosteric heat of adsorption | −ΔH |
| Magic-Angle Spinning Nuclear Magnetic Resonance | MAS NMR |
| Mass Spectrometry | MS |
| Melamine | C3H6N6 |
| Metal-organic framework(s) | MOF(s) |
| Methane | CH4 |
| Nitrogen | N2 |
| Nitrogen reduction reaction | NRR |
| Normal Hydrogen Electrode | NHE |
| Optical Emission Spectroscopy | OES |
| Oxidative dehydrogenation | ODH |
| Oxidative desulfurization | ODS |
| Oxygen evolution reaction | OER |
| Oxygen reduction reaction | ORR |
| Physical vapor deposition | PVD |
| Reversible Hydrogen Electrode | RHE |
| Scanning Electron Microscopy | SEM |
| Selected-Area Electron Diffraction | SAED |
| Specific surface area | SSA |
| Temperature-Programmed Desorption | TPD |
| Thermogravimetric Analysis | TGA |
| Transmission Electron Microscopy | TEM |
| Triethanolamine | TEOA |
| Two-dimensional | 2D |
| Ultraviolet–Visible | UV-Vis |
| Urea | (NH2)2CO |
| Water | H2O |
| Wavelength | λ |
| X-ray Absorption Near-Edge Structure | XANES |
| X-ray Diffraction | XRD |
| X-ray Photoelectron Spectroscopy | XPS |
References
- EPA.gov Sources of Greenhouse Gas Emissions. Available online: https://www.epa.gov/ghgemissions/sources-greenhouse-gas-emissions (accessed on 4 November 2024).
- Holmes, K.J.; Zeitler, E.; Kerxhalli-Kleinfield, M.; DeBoer, R. Scaling Deep Decarbonization Technologies. Earth’s Future 2021, 9, e2021EF002399. [Google Scholar] [CrossRef]
- Borup, R.; Krause, T.; Brouwer, J. Hydrogen Is Essential for Industry and Transportation Decarbonization. Electrochem. Soc. Interface 2021, 30, 79–84. [Google Scholar] [CrossRef]
- Kojima, Y.; Yamaguchi, M. Ammonia as a Hydrogen Energy Carrier. Int. J. Hydrogen Energy 2022, 47, 22832–22839. [Google Scholar] [CrossRef]
- Le, T.-H.; Tran, N.; Lee, H.-J. Development of Liquid Organic Hydrogen Carriers for Hydrogen Storage and Transport. Int. J. Mol. Sci. 2024, 25, 1359. [Google Scholar] [CrossRef] [PubMed]
- Zainal, B.S.; Ker, P.J.; Mohamed, H.; Ong, H.C.; Fattah, I.M.R.; Rahman, S.M.A.; Nghiem, L.D.; Mahlia, T.M.I. Recent Advancement and Assessment of Green Hydrogen Production Technologies. Renew. Sustain. Energy Rev. 2024, 189, 113941. [Google Scholar] [CrossRef]
- Alamiery, A. Advancements in Materials for Hydrogen Production: A Review of Cutting-Edge Technologies. ChemPhysMater 2023, 3, 64–79. [Google Scholar] [CrossRef]
- Singh, G.; Lee, J.; Karakoti, A.; Bahadur, R.; Yi, J.; Zhao, D.; AlBahily, K.; Vinu, A. Emerging Trends in Porous Materials for CO2 Capture and Conversion. Chem. Soc. Rev. 2020, 49, 4360–4404. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.J.; Sun, J.; Ling, H.; Xu, N.; Ying, Z.F.; Wu, J.D. Preparation of Thin Films of Carbon-Based Compounds. Appl. Surf. Sci. 2003, 218, 298–305. [Google Scholar] [CrossRef]
- Eremets, M.I.; Gavriliuk, A.G.; Trojan, I.A.; Dzivenko, D.A.; Boehler, R. Single-Bonded Cubic Form of Nitrogen. Nat. Mater. 2004, 3, 558–563. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Roy, S.; Zhang, X.; Puthirath, A.B.; Meiyazhagan, A.; Bhattacharyya, S.; Rahman, M.M.; Babu, G.; Susarla, S.; Saju, S.K.; Tran, M.K.; et al. Structure, Properties and Applications of Two-dimensional Hexagonal Boron Nitride. Adv. Mater. 2021, 33, 2101589. [Google Scholar] [CrossRef] [PubMed]
- Marbaniang, P.; Ingavale, S.; Catherin, D.; Ramgir, N.; Swami, A.; Kakade, B. Forming a B–B Bond in Boron Carbon Nitride Composite: A Way for Metal Free Electrocatalyst for Oxygen Reduction Reaction in Alkaline Medium. J. Catal. 2019, 378, 104–112. [Google Scholar] [CrossRef]
- Wang, J.; Ma, F.; Liang, W.; Wang, R.; Sun, M. Optical, Photonic and Optoelectronic Properties of Graphene, h-BN and Their Hybrid Materials. Nanophotonics 2017, 6, 943–976. [Google Scholar] [CrossRef]
- Lu, Y.; Yu, Y.; Zhu, X.; Wang, M. Two Predicted Two-Dimensional BCN Structures: A First-Principles Study. Phys. E Low-Dimens. Syst. Nanostruct. 2021, 125, 114413. [Google Scholar] [CrossRef]
- Yuge, K. Prediction of Superhard Cubic Boron–Carbon Nitride through First Principles. J. Phys. Condens. Matter 2009, 21, 415403. [Google Scholar] [CrossRef]
- Qu, N.-R.; Wang, H.-C.; Li, Q.; Li, Y.-D.; Li, Z.-P.; Gou, H.-Y.; Gao, F.-M. Surperhard Monoclinic BC6N Allotropes: First-Principles Investigations*. Chin. Phys. B 2019, 28, 096201. [Google Scholar] [CrossRef]
- Wang, H.; Li, Z.; Gou, H.; Gao, F. Superhard Orthorhombic BCN Allotropes: OIM12-BCN and oPM12-BCN. Diam. Relat. Mater. 2023, 132, 109689. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Lv, J.; Zhu, C.; Li, Q.; Zhang, M.; Li, Q.; Ma, Y. First-Principles Structural Design of Superhard Materials. J. Chem. Phys. 2013, 138, 114101. [Google Scholar] [CrossRef]
- Wang, H.; Qu, N.; Li, Q.; Li, Y.; Li, Z.; Gou, H.; Gao, F. First-Principles Calculations on Two Superhard BCN Allotropes: P3m1-BCN and I41Md-BCN. Comput. Mater. Sci. 2020, 184, 109869. [Google Scholar] [CrossRef]
- Nakano, S.; Akaishi, M.; Sasaki, T.; Yamaoka, S. Segregative Crystallization of Several Diamond-like Phases from the Graphitic BC2N without an Additive at 7.7 Gpa. Chem. Mater. 1994, 6, 2246–2251. [Google Scholar] [CrossRef]
- Liu, F.; Zhao, X.; Shi, P.; Li, L.; Dong, Q.; Tian, M.; Wu, Y.; Sun, X. A Review on Recent Progress Achieved in Boron Carbon Nitride Nanomaterials for Supercapacitor Applications. Batteries 2023, 9, 396. [Google Scholar] [CrossRef]
- Radhakrishnan, S.; Patra, A.; Manasa, G.; Belgami, M.A.; Mun Jeong, S.; Rout, C.S. Borocarbonitride-based Emerging Materials for Supercapacitor Applications: Recent Advances, Challenges, and Future Perspectives. Adv. Sci. 2024, 11, 2305325. [Google Scholar] [CrossRef]
- Thomas, S.A.; Cherusseri, J.; Pallavolu, M.R.; Rajendran, D.N.; Kumar, D. Boron Carbon Nitride (BCN): An Emerging Two-Dimensional Material for Rechargeable Batteries. Energy Fuels 2024, 38, 13704–13721. [Google Scholar] [CrossRef]
- Sohrabi, H.; Arbabzadeh, O.; Falaki, M.; Vatanpour, V.; Majidi, M.R.; Kudaibergenov, N.; Joo, S.W.; Khataee, A. Advances in Fabrication, Physio-Chemical Properties, and Sensing Applications of Non-Metal Boron Nitride and Boron Carbon Nitride-Based Nanomaterials. Surf. Interfaces 2023, 41, 103152. [Google Scholar] [CrossRef]
- Aghaei, S.M.; Aasi, A.; Farhangdoust, S.; Panchapakesan, B. Graphene-like BC6N Nanosheets Are Potential Candidates for Detection of Volatile Organic Compounds (VOCs) in Human Breath: A DFT Study. Appl. Surf. Sci. 2021, 536, 147756. [Google Scholar] [CrossRef]
- Ahmed, M.T.; Islam, S.; Ahmed, F. Density Functional Theory Study of Mobius Boron-Carbon-Nitride as Potential CH4, H2S, NH3, COCl2 and CH3OH Gas Sensor. R. Soc. Open Sci. 2022, 9, 220778. [Google Scholar] [CrossRef]
- Dindorkar, S.S.; Sinha, N.; Yadav, A. Comparative Study on Adsorption of Volatile Organic Compounds on Graphene, Boron Nitride and Boron Carbon Nitride Nanosheets. Solid State Commun. 2023, 359, 115021. [Google Scholar] [CrossRef]
- Rocky, M.H.; Khatun, M.; Al Roman, A.; Roy, D.; Ahmed, M.T. A DFT Study on Boron Carbon Nitride and In-Plane Graphene-Boron Nitride Nanosheets for O3 and F2 Gas Sensing. Comput. Theor. Chem. 2024, 1237, 114639. [Google Scholar] [CrossRef]
- Yadav, A.; Yadav, R.; Sinha, N. A Comparative Study of NH3 and H2S Sensing Performance on Monolayer Nanosheets through First-Principle Studies. Colloids Surfaces A Physicochem. Eng. Asp. 2024, 693, 133997. [Google Scholar] [CrossRef]
- Chalase, P.; Deshpande, S.; Kumavat, S.; Deshpande, M. Adsorption Mechanism of Different Toxic Gases onto Pristine BNC2 and Al-Doped BNC2 Monolayers. Phys. Chem. Chem. Phys. 2023, 25, 17337–17351. [Google Scholar] [CrossRef] [PubMed]
- Bahadur, R.; Singh, G.; Bando, Y.; Vinu, A. Advanced Porous Borocarbonitride Nanoarchitectonics: Their Structural Designs and Applications. Carbon 2022, 190, 142–169. [Google Scholar] [CrossRef]
- Rao, C.N.R.; Chhetri, M. Borocarbonitrides as Metal-free Catalysts for the Hydrogen Evolution Reaction. Adv. Mater. 2019, 31, 1803668. [Google Scholar] [CrossRef] [PubMed]
- Garg, H.; Patial, S.; Raizada, P.; Nguyen, V.-H.; Kim, S.Y.; Van Le, Q.; Ahamad, T.; Alshehri, S.M.; Hussain, C.M.; Nguyen, T.T.H.; et al. Hexagonal-Borocarbonitride (h-BCN) Based Heterostructure Photocatalyst for Energy and Environmental Applications: A Review. Chemosphere 2023, 313, 137610. [Google Scholar] [CrossRef]
- Wan, Y.; Fang, C.; Yang, X.; Liu, J.; Lin, Y. Borocarbonitride Materials as Metal-Free Catalysts for Advanced Catalysis. J. Mater. Chem. A 2024, 12, 33392–33426. [Google Scholar] [CrossRef]
- Thomas, S.; Asle Zaeem, M. A New Planar BCN Lateral Heterostructure with Outstanding Strength and Defect-Mediated Superior Semiconducting to Metallic Properties. Phys. Chem. Chem. Phys. 2020, 22, 22066–22077. [Google Scholar] [CrossRef]
- Tazekritt, S.; Gallouze, M.; Kellou, A. DFT Study of Energetics and Optoelectronics Properties of B, C, and N Binary and Ternary Honeycomb Structures. J. Appl. Phys. 2024, 135, 094302. [Google Scholar] [CrossRef]
- Wan, J.; Wang, H.; Shu, H. Electro-Optical Properties of a Strain-Induced Borocarbonitride Monolayer from Many-Body Perturbation Theory. J. Mater. Chem. C 2024, 12, 14642–14649. [Google Scholar] [CrossRef]
- Kumawat, M.K.; Yadav, V.; Tiwari, S.; Mahanta, T.; Mohanty, T. Temperature Controlled Synthesis of Boron Carbon Nitride Nanosheets and Study of Their Bandgap Modulation and Nonlinear Optical Properties. Carbon 2023, 214, 118363. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, Y.; Li, X.; Zhuang, Q.; Zhang, Z.; Zhou, H.; Ding, Q.; Wang, Y.; Dang, Y.; Duan, L.; et al. Charge State Modulation on Boron Site by Carbon and Nitrogen Localized Bonding Microenvironment for Two-Electron Electrocatalytic H2O2 Production. Chinese Chem. Lett. 2023, 34, 107596. [Google Scholar] [CrossRef]
- Chen, F.; Lv, X.; Wang, H.; Wen, F.; Qu, L.; Zheng, G.; Han, Q. Weak-Field Electro-Flash Induced Asymmetric Catalytic Sites toward Efficient Solar Hydrogen Peroxide Production. JACS Au 2024, 4, 1219–1228. [Google Scholar] [CrossRef]
- Yang, D.; Li, Y.; Chen, R.; Wang, X.; Li, Z.; Xing, T.; Wei, L.; Xu, S.; Dai, P.; Wu, M. Flower-like Superstructure of Boron Carbon Nitride Nanosheets with Adjustable Band Gaps for Photocatalytic Hydrogen Peroxide Production. J. Mater. Sci. Technol. 2024, 183, 23–31. [Google Scholar] [CrossRef]
- Lu, L.; He, J.; Wu, P.; Wu, Y.; Chao, Y.; Li, H.H.; Tao, D.; Fan, L.; Li, H.H.; Zhu, W. Taming Electronic Properties of Boron Nitride Nanosheets as Metal-Free Catalysts for Aerobic Oxidative Desulfurization of Fuels. Green Chem. 2018, 20, 4453–4460. [Google Scholar] [CrossRef]
- Li, X.; Lin, B.; Li, H.; Yu, Q.; Ge, Y.; Jin, X.; Liu, X.; Zhou, Y.; Xiao, J. Carbon Doped Hexagonal BN as a Highly Efficient Metal-Free Base Catalyst for Knoevenagel Condensation Reaction. Appl. Catal. B Environ. 2018, 239, 254–259. [Google Scholar] [CrossRef]
- Horimoto, T.; Kannari, N.; Sato, K. Boron-Carbon-Nitrogen-Containing Heterogeneous Catalysts for Base-Catalyzed Reaction. Appl. Surf. Sci. 2021, 558, 149841. [Google Scholar] [CrossRef]
- Xu, G.; Zheng, H.; Zhang, X.; Li, W.; Zhou, L.; Qin, L. BCN Materials with Adjustable Active Sites as Heterogeneous Acid–Base Catalysts for Knoevenagel Condensation Reaction. Mol. Catal. 2023, 549, 113447. [Google Scholar] [CrossRef]
- Leng, J.; Ali, M.S.; Al-Lohedan, H.A.; Rout, C.S.; Pramoda, K.; Sharath Kumar, K.S. Frustrated Lewis Pairs in Two-Dimensional Borocarbonitride for the Facile Synthesis of 3-Aminoimidazo[1,2-α]Pyridines Using TMSCN as an Isonitrile Substitute. New J. Chem. 2024, 48, 5971–5980. [Google Scholar] [CrossRef]
- Wang, J.; Hao, J.; Liu, D.; Qin, S.; Chen, C.; Yang, C.; Liu, Y.; Yang, T.; Fan, Y.; Chen, Y.; et al. Flower Stamen-like Porous Boron Carbon Nitride Nanoscrolls for Water Cleaning. Nanoscale 2017, 9, 9787–9791. [Google Scholar] [CrossRef]
- Wang, H.; Tian, L.; Huang, Z.; Liang, F.; Guan, K.; Jia, Q.; Zhang, H.; Zhang, S. Molten Salt Synthesis of Carbon-Doped Boron Nitride Nanosheets with Enhanced Adsorption Performance. Nanotechnology 2020, 31, 505606. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, G.; Weng, H.; Wang, L.; Chen, J.; Cheng, S.; Zhang, P.; Wang, M.; Ge, X.; Chen, H.; et al. Carbon-Doped Boron Nitride Nanosheets with Adjustable Band Structure for Efficient Photocatalytic U(VI) Reduction under Visible Light. Chem. Eng. J. 2021, 410, 128280. [Google Scholar] [CrossRef]
- Yang, C.; Bu, D.; Huang, S. Solvent-Free Synthesis of Highly Porous Boron Carbon Nitride for Effective Water Cleaning. Ceram. Int. 2022, 48, 27658–27663. [Google Scholar] [CrossRef]
- Yadav, A.; Dindorkar, S.S.; Ramisetti, S.B.; Sinha, N. Simultaneous Adsorption of Methylene Blue and Arsenic on Graphene, Boron Nitride and Boron Carbon Nitride Nanosheets: Insights from Molecular Simulations. J. Water Process Eng. 2022, 46, 102653. [Google Scholar] [CrossRef]
- Jokar, Z.; Khademiyan, A.; Fallah, M.-A.; Smida, K.; Sajadi, S.M.; Inc, M. Molecular Dynamics Simulation of Urea Adsorption on Various Nanoparticles in a Spiral Microfluidic System. Eng. Anal. Bound. Elem. 2022, 145, 271–285. [Google Scholar] [CrossRef]
- Recepoglu, Y.K.; Goren, A.Y.; Vatanpour, V.; Yoon, Y.; Khataee, A. Boron Carbon Nitride Nanosheets in Water and Wastewater Treatment: A Critical Review. Desalination 2022, 533, 115782. [Google Scholar] [CrossRef]
- Hao, Q.; Song, Y.; Mo, Z.; Mishra, S.; Pang, J.; Liu, Y.; Lian, J.; Wu, J.; Yuan, S.; Xu, H.; et al. Highly Efficient Adsorption of Oils and Pollutants by Porous Ultrathin Oxygen-Modified BCN Nanosheets. ACS Sustain. Chem. Eng. 2019, 7, 3234–3242. [Google Scholar] [CrossRef]
- Peng, D.; Jiang, W.; Li, F.-F.; Zhang, L.; Liang, R.-P.; Qiu, J.-D. One-Pot Synthesis of Boron Carbon Nitride Nanosheets for Facile and Efficient Heavy Metal Ions Removal. ACS Sustain. Chem. Eng. 2018, 6, 11685–11694. [Google Scholar] [CrossRef]
- Patel, M.R.; Park, T.J.; Kailasa, S.K. Synthesis of Fluorescent Boron Carbon Nitride Nanosheets for the Detection of Cu2+ Ions and Epinephrine. New J. Chem. 2023, 47, 9279–9287. [Google Scholar] [CrossRef]
- Chen, T.; Liu, T.; Zhou, L.; Li, M.; Meng, Q.; Yu, K.; Lian, J.; Zhu, W. Ternary Boron Carbon Nitrides Hollow Nanotubes with Tunable P-n Homojunction for Photo-Assisted Uranium Extraction: A Combined Batch, EXAFS and DFT Calculations. Appl. Catal. B Environ. 2022, 318, 121815. [Google Scholar] [CrossRef]
- Hirata, Y.; Takeuchi, R.; Taniguchi, H.; Kawagoe, M.; Iwamoto, Y.; Yoshizato, M.; Akasaka, H.; Ohtake, N. Structural and Mechanical Properties of A-BCN Films Prepared by an Arc-Sputtering Hybrid Process. Materials 2021, 14, 719. [Google Scholar] [CrossRef]
- Chakraborty, H.; Mogurampelly, S.; Yadav, V.K.; Waghmare, U.V.; Klein, M.L. Phonons and Thermal Conducting Properties of Borocarbonitride (BCN) Nanosheets. Nanoscale 2018, 10, 22148–22154. [Google Scholar] [CrossRef] [PubMed]
- Yadav, V.K.; Chakraborty, H.; Klein, M.L.; Waghmare, U.V.; Rao, C.N.R. Defect-Enriched Tunability of Electronic and Charge-Carrier Transport Characteristics of 2D Borocarbonitride (BCN) Monolayers from Ab Initio Calculations. Nanoscale 2019, 11, 19398–19407. [Google Scholar] [CrossRef]
- Bafekry, A.; Stampfl, C. Band-Gap Control of Graphenelike Borocarbonitride g−BC6N Bilayers by Electrical Gating. Phys. Rev. B 2020, 102, 195411. [Google Scholar] [CrossRef]
- Li, S.; Shi, L.; Zhu, H.; Xia, W. Elastic and Bandgap Modulations of Hexagonal BC2N from First-principles Calculations. Phys. Status Solidi 2019, 256, 1900281. [Google Scholar] [CrossRef]
- Hussain, K.; Younis, U.; Muhammad, I.; Qie, Y.; Guo, Y.; Li, T.; Xie, H.; Sun, Q. Three-Dimensional Porous Borocarbonitride BC2N with Negative Poisson’s Ratio. J. Mater. Chem. C 2020, 8, 15771–15777. [Google Scholar] [CrossRef]
- Jongwannasiri, C.; Yoshida, S.; Watanabe, S. Tribological Behavior under High Temperature of BCN Films Deposited by Sputtering-PBII Hybrid System. Surf. Interfaces 2020, 18, 100434. [Google Scholar] [CrossRef]
- Yadav, A.; Dindorkar, S.S. Vacancy Defects in Monolayer Boron Carbon Nitride for Enhanced Adsorption of Paraben Compounds from Aqueous Stream: A Quantum Chemical Study. Surf. Sci. 2022, 723, 122131. [Google Scholar] [CrossRef]
- Zhou, Z.; Bello, I.; Lei, M..; Li, K..; Lee, C..; Lee, S. Synthesis and Characterization of Boron Carbon Nitride Films by Radio Frequency Magnetron Sputtering. Surf. Coatings Technol. 2000, 128–129, 334–340. [Google Scholar] [CrossRef]
- Prakash, A.; Sundaram, K.B. Optical and XPS Studies of BCN Thin Films by Co-Sputtering of B4C and BN Targets. Appl. Surf. Sci. 2017, 396, 484–491. [Google Scholar] [CrossRef]
- Attri, R.; Roychowdhury, S.; Biswas, K.; Rao, C.N.R. Low Thermal Conductivity of 2D Borocarbonitride Nanosheets. J. Solid State Chem. 2020, 282, 121105. [Google Scholar] [CrossRef]
- Bhattacharyya, P.; Sahoo, S.; Seikh, A.H.; Mohammed, S.M.A.K.; Sarkar, A.; Alharthi, N. Synthesis, Characterization and Optical Property Study of BCNO and BCN Related Nanopowder. Diam. Relat. Mater. 2019, 92, 235–241. [Google Scholar] [CrossRef]
- Mirzaee, M.; Rashidi, A.; Zolriasatein, A.; Rezaei Abadchi, M. Solid-State Synthesis and Characterization of Two-Dimensional Hexagonal BCN Nanosheet Using a Free Template Method. Diam. Relat. Mater. 2021, 115, 108350. [Google Scholar] [CrossRef]
- Zhou, M.; Li, S.; Wang, S.; Jiang, Z.; Yang, C.; Guo, F.; Wang, X.; Ho, W.-k. Anchoring ZnIn2S4 Nanosheets on Ultrathin Boron Carbon Nitride Layers for Improved Photo-Redox Catalysis. Appl. Surf. Sci. 2022, 599, 153985. [Google Scholar] [CrossRef]
- Mou, P.; Zhao, J.; Wang, G.; Shi, S.; Wan, G.; Zhou, M.; Deng, Z.; Teng, S.; Wang, G. BCN Nanosheets Derived from Coconut Shells with Outstanding Microwave Absorption and Thermal Conductive Properties. Chem. Eng. J. 2022, 437, 135285. [Google Scholar] [CrossRef]
- Raghu, M.S.; Parashuram, L.; Kumar, K.Y.; Prasanna, B.P.; Rao, S.; Krishnaiah, P.; Prashanth, K.N.; Kumar, C.B.P.; Alrobei, H. Facile Green Synthesis of Boroncarbonitride Using Orange Peel; Its Application in High-Performance Supercapacitors and Detection of Levodopa in Real Samples. Mater. Today Commun. 2020, 24, 101033. [Google Scholar] [CrossRef]
- Ma, F.; Wang, M.; Shao, Y.; Wang, L.; Wu, Y.; Wang, Z.; Hao, X. ‘Thermal Substitution’ for Preparing Ternary BCN Nanosheets with Enhanced and Controllable Nonlinear Optical Performance. J. Mater. Chem. C 2017, 5, 2559–2565. [Google Scholar] [CrossRef]
- Barua, M.; Sreedhara, M.B.; Pramoda, K.; Rao, C.N.R. Quantification of Surface Functionalities on Graphene, Boron Nitride and Borocarbonitrides by Fluorescence Labeling. Chem. Phys. Lett. 2017, 683, 459–466. [Google Scholar] [CrossRef]
- Attri, R.; Sreedhara, M.B.; Rao, C.N.R. Compositional Tuning of Electrical and Optical Properties of PLD-Generated Thin Films of 2D Borocarbonitrides (BN)1–x(C)x. ACS Appl. Electron. Mater. 2019, 1, 569–576. [Google Scholar] [CrossRef]
- Guo, F.; Li, S.; Yang, C.; Zhang, J.; Hou, Y.; Wang, X. A Highly Crystallized Hexagonal BCN Photocatalyst with Superior Anticorrosion Properties. Adv. Opt. Mater. 2022, 10, 2200282. [Google Scholar] [CrossRef]
- Giusto, P.; Arazoe, H.; Cruz, D.; Lova, P.; Heil, T.; Aida, T.; Antonietti, M. Boron Carbon Nitride Thin Films: From Disordered to Ordered Conjugated Ternary Materials. J. Am. Chem. Soc. 2020, 142, 20883–20891. [Google Scholar] [CrossRef]
- Zhu, J.; Diao, T.; Wang, W.; Xu, X.; Sun, X.; Carabineiro, S.A.C.; Zhao, Z. Boron Doped Graphitic Carbon Nitride with Acid-Base Duality for Cycloaddition of Carbon Dioxide to Epoxide under Solvent-Free Condition. Appl. Catal. B Environ. 2017, 219, 92–100. [Google Scholar] [CrossRef]
- Wang, X.; Yang, L.; Fu, G.; Chen, Y.; Yang, C.; Sun, J. Experimental and Theoretical Investigation for the Cycloaddition of Carbon Dioxide to Epoxides Catalyzed by Potassium and Boron Co-Doped Carbon Nitride. J. Colloid Interface Sci. 2022, 609, 523–534. [Google Scholar] [CrossRef]
- Yang, C.; Zhao, X.; Yang, T. Boron and Phosphorus Co-Doped Graphitic Carbon Nitride Cooperate with Bu4NBr as Binary Heterogeneous Catalysts for the Cycloaddition of CO2 to Epoxides. Catalysts 2022, 12, 1196. [Google Scholar] [CrossRef]
- Giusto, P.; Cruz, D.; Heil, T.; Tarakina, N.; Patrini, M.; Antonietti, M. Chemical Vapor Deposition of Highly Conjugated, Transparent Boron Carbon Nitride Thin Films. Adv. Sci. 2021, 8, 2101602. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhou, M.; Luo, Z.; Wakeel, M.; Asiri, A.M.; Wang, X. Template-Free Synthesis of Carbon-Doped Boron Nitride Nanosheets for Enhanced Photocatalytic Hydrogen Evolution. Appl. Catal. B Environ. 2019, 241, 246–255. [Google Scholar] [CrossRef]
- Chithaiah, P.; Pramoda, K.; Kulkarni, G.U.; Rao, C.N.R. A Simple Chemical Route to Borocarbonitride Nanotubes. Eur. J. Inorg. Chem. 2020, 2020, 1230–1232. [Google Scholar] [CrossRef]
- Thaweesak, S.; Wang, S.; Lyu, M.; Xiao, M.; Peerakiatkhajohn, P.; Wang, L. Boron-Doped Graphitic Carbon Nitride Nanosheets for Enhanced Visible Light Photocatalytic Water Splitting. Dalt. Trans. 2017, 46, 10714–10720. [Google Scholar] [CrossRef]
- Bahadur, R.; Singh, G.; Li, M.; Chu, D.; Yi, J.; Karakoti, A.; Vinu, A. BCN Nanostructures Conjugated Nanoporous Carbon with Oxygenated Surface and High Specific Surface Area for Enhanced CO2 Capture and Supercapacitance. Chem. Eng. J. 2023, 460, 141793. [Google Scholar] [CrossRef]
- Kosaka, M.; Urakami, N.; Hashimoto, Y. Formation of Graphitic Carbon Nitride and Boron Carbon Nitride Film on Sapphire Substrate. Jpn. J. Appl. Phys. 2018, 57, 02CB09. [Google Scholar] [CrossRef]
- Alrebh, A.; Meunier, J.-L. Boron Carbon Nitride Nanosheets via Induction Plasma: Insights on Synthesis, Characterization, and Control of Band Gap. Carbon 2023, 215, 118472. [Google Scholar] [CrossRef]
- Sutorius, A.; Weißing, R.; Rindtorff Pèrez, C.; Fischer, T.; Hartl, F.; Basu, N.; Shin, H.S.; Mathur, S. Understanding Vapor Phase Growth of Hexagonal Boron Nitride. Nanoscale 2024, 16, 15782–15792. [Google Scholar] [CrossRef]
- Alrebh, A.; Ruth, D.; Plunkett, M.; Gaburici, L.; Couillard, M.; Lacelle, T.; Kingston, C.T.; Kim, K.S. Boron Nitride Nanotubes Synthesis from Ammonia Borane by an Inductively Coupled Plasma. Chem. Eng. J. 2023, 472, 144891. [Google Scholar] [CrossRef]
- Leardini, F.; Flores, E.; Galvis E, A.R.; Ferrer, I.J.; Ares, J.R.; Sánchez, C.; Molina, P.; van der Meulen, H.P.; Navarro, C.G.; Polin, G.L.; et al. Chemical Vapor Deposition Growth of Boron–Carbon–Nitrogen Layers from Methylamine Borane Thermolysis Products. Nanotechnology 2018, 29, 025603. [Google Scholar] [CrossRef] [PubMed]
- Leardini, F.; Jiménez-Arévalo, N.; Ferrer, I.J.; Ares, J.R.; Molina, P.; Navarro, C.G.; Manzanares, Y.; Granados, D.; Urbanos, F.J.; García-García, F.J.; et al. A Fast Synthesis Route of Boron–Carbon–Nitrogen Ultrathin Layers towards Highly Mixed Ternary B–C–N Phases. 2D Mater. 2019, 6, 035015. [Google Scholar] [CrossRef]
- Leardini, F.; Massimi, L.; Flores-Cuevas, E.; Fernández, J.; Ares, J.; Betti, M.; Mariani, C. Synthesis of Ternary Borocarbonitrides by High Temperature Pyrolysis of Ethane 1,2-Diamineborane. Materials 2015, 8, 5974–5985. [Google Scholar] [CrossRef] [PubMed]
- Massimi, L.; Betti, M.G.; Caramazza, S.; Postorino, P.; Mariani, C.; Latini, A.; Leardini, F. In-Vacuum Thermolysis of Ethane 1,2-Diamineborane for the Synthesis of Ternary Borocarbonitrides. Nanotechnology 2016, 27, 435601. [Google Scholar] [CrossRef]
- Mighri, R.; Demirci, U.B.; Alauzun, J.G. Microporous Borocarbonitrides BxCyNz: Synthesis, Characterization, and Promises for CO2 Capture. Nanomaterials 2023, 13, 734. [Google Scholar] [CrossRef]
- Tay, R.Y.; Li, H.; Tsang, S.H.; Zhu, M.; Loeblein, M.; Jing, L.; Leong, F.N.; Teo, E.H.T. Trimethylamine Borane: A New Single-Source Precursor for Monolayer h-BN Single Crystals and h-BCN Thin Films. Chem. Mater. 2016, 28, 2180–2190. [Google Scholar] [CrossRef]
- Galligan, P.R.; Xu, Y.; Tang, T.W.; Liu, H.; Tamtaji, M.; Zhou, Y.; Luo, Z. Aligned Carbon-Doping to Modulate Thermal and Electrical Conductivity of Boron Carbon Nitride Grown from Chemical Vapor Deposition. Carbon 2023, 215, 118397. [Google Scholar] [CrossRef]
- Beniwal, S.; Hooper, J.; Miller, D.P.; Costa, P.S.; Chen, G.; Liu, S.-Y.; Dowben, P.A.; Sykes, E.C.H.; Zurek, E.; Enders, A. Graphene-like Boron–Carbon–Nitrogen Monolayers. ACS Nano 2017, 11, 2486–2493. [Google Scholar] [CrossRef]
- Zeng, X.; Chen, H.; He, X.; Zhang, H.; Fang, W.; Du, X.; Li, W.; Huang, Z.; Zhao, L. In-Situ Synthesis of Non-Phase-Separated Boron Carbon Nitride for Photocatalytic Reduction of CO2. Environ. Res. 2022, 207, 112178. [Google Scholar] [CrossRef]
- Fellinger, T.P.; Su, D.S.; Engenhorst, M.; Gautam, D.; Schlögl, R.; Antonietti, M. Thermolytic Synthesis of Graphitic Boron Carbon Nitride from an Ionic Liquid Precursor: Mechanism, Structure Analysis and Electronic Properties. J. Mater. Chem. 2012, 22, 23996–24005. [Google Scholar] [CrossRef]
- Su, Q.; Yao, X.; Cheng, W.; Zhang, S. Boron-Doped Melamine-Derived Carbon Nitrides Tailored by Ionic Liquids for Catalytic Conversion of CO2 into Cyclic Carbonates. Green Chem. 2017, 19, 2957–2965. [Google Scholar] [CrossRef]
- Jayaramulu, K.; Kumar, N.; Hazra, A.; Maji, T.K.; Rao, C.N.R. A Nanoporous Borocarbonitride (BC4N) with Novel Properties Derived from a Boron-Imidazolate-Based Metal-Organic Framework. Chem.-A Eur. J. 2013, 19, 6966–6970. [Google Scholar] [CrossRef] [PubMed]
- Bhaduri, B. High Temperature Synthesis, Surface Characterization, and Photoluminescence Property of Boron Carbon Nitride. Mater. Lett. 2024, 362, 136231. [Google Scholar] [CrossRef]
- Zhang, M.; Zhou, M.; Luo, Z.; Zhang, J.; Wang, S.; Wang, X. Molten Salt Assisted Assembly Growth of Atomically Thin Boron Carbon Nitride Nanosheets for Photocatalytic H2 Evolution. Chem. Commun. 2020, 56, 2558–2561. [Google Scholar] [CrossRef] [PubMed]
- Panda, P.; Samanta, R.; Barman, S. Facile Synthesis of Two-Dimensional (2D) Boron Carbonitride and 2D Porous Boron Carbonitride for Excellent Energy Storage and Gas Adsorption Applications. Energy Fuels 2023, 37, 5540–5555. [Google Scholar] [CrossRef]
- Sathish, C.; Kothandam, G.; Selvarajan, P.; Lei, Z.; Lee, J.; Qu, J.; Al-Muhtaseb, A.H.; Yu, X.; Breese, M.B.H.; Zheng, R.; et al. Ordered Mesoporous Boron Carbon Nitrides with Tunable Mesopore Nanoarchitectonics for Energy Storage and CO2 Adsorption Properties. Adv. Sci. 2022, 9, 2105603. [Google Scholar] [CrossRef]
- Luo, J.; Wang, C.; Liu, J.; Wei, Y.; Chao, Y.; Zou, Y.; Mu, L.; Huang, Y.; Li, H.; Zhu, W. High-performance Adsorptive Desulfurization by Ternary Hybrid Boron Carbon Nitride Aerogel. AIChE J. 2021, 67, e17280. [Google Scholar] [CrossRef]
- Ryan Galligan, P.; Liu, H.; Wang, G.; Tamtaji, M.; Li, Y.; Wing Tang, T.; Zhou, Y.; Luo, Z. Lightweight, Freestanding Hybrids of Graphene and Hexagonal Boron Nitride Foams. Compos. Part A Appl. Sci. Manuf. 2024, 182, 108176. [Google Scholar] [CrossRef]
- Marbaniang, P.; Patil, I.; Lokanathan, M.; Parse, H.; Catherin Sesu, D.; Ingavale, S.; Kakade, B. Nanorice-like Structure of Carbon-Doped Hexagonal Boron Nitride as an Efficient Metal-Free Catalyst for Oxygen Electroreduction. ACS Sustain. Chem. Eng. 2018, 6, 11115–11122. [Google Scholar] [CrossRef]
- Mighri, R.; Turani-I-Belloto, K.; Demirci, U.B.; Alauzun, J.G. Nanostructured Carbon-Doped BN for CO2 Capture Applications. Nanomaterials 2023, 13, 2389. [Google Scholar] [CrossRef]
- Wang, L.; Wang, C.; Zhang, Z.; Wu, J.; Ding, R.; Lv, B. Thermal Induced BCN Nanosheets Evolution and Its Usage as Metal-Free Catalyst in Ethylbenzene Dehydrogenation. Appl. Surf. Sci. 2017, 422, 574–581. [Google Scholar] [CrossRef]
- Castilla-Martinez, C.A.; Charmette, C.; Cartier, J.; Demirci, U.B. A Boron Nitride-Carbon Composite Derived from Ammonia Borane and ZIF-8 with Promises for the Adsorption of Carbon Dioxide. New J. Chem. 2024, 48, 8534–8544. [Google Scholar] [CrossRef]
- Shi, L.; Bi, S.; Qi, Y.; Ning, G.; Ye, J. Highly Efficient Metal-Free Borocarbonitride Catalysts for Electrochemical Reduction of N2 to NH3. J. Colloid Interface Sci. 2023, 641, 577–584. [Google Scholar] [CrossRef]
- Chen, X.; Ma, N.; Liu, X.; Wei, C.; Cui, C.; Cao, B.; Guo, Y.; Wang, L.; Gu, Q.; Chen, X. Facile Synthesis of Unsolvated Alkali Metal Octahydrotriborate Salts MB3H8 (M = K, Rb, and Cs), Mechanisms of Formation, and the Crystal Structure of KB3H8. Angew. Chem. 2019, 131, 2746–2750. [Google Scholar] [CrossRef]
- Barnakov, C.N.; Khokhlova, G.P.; Malysheva, V.Y.; Popova, A.N.; Ismagilov, Z.R. X-Ray Diffraction Analysis of the Crystal Structures of Different Graphites. Solid Fuel Chem. 2015, 49, 25–29. [Google Scholar] [CrossRef]
- Qiu, T.; Yang, J.-G.; Bai, X.-J.; Wang, Y.-L. The Preparation of Synthetic Graphite Materials with Hierarchical Pores from Lignite by One-Step Impregnation and Their Characterization as Dye Absorbents. RSC Adv. 2019, 9, 12737–12746. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Dai, X.; Xie, Z.; Qi, W. Borocarbonitride Catalyzed Ethylbenzene Oxidative Dehydrogenation: Activity Enhancement via Encapsulation of Mn Clusters inside the Tube. Small 2024, 20, 2401532. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, X.; Yan, Y.; Huang, X.; Xie, Z. New Insight into Structural Transformations of Borocarbonitride in Oxidative Dehydrogenation of Propane. Appl. Catal. A Gen. 2021, 628, 118402. [Google Scholar] [CrossRef]
- Shi, Y.; Hamsen, C.; Jia, X.; Kim, K.K.; Reina, A.; Hofmann, M.; Hsu, A.L.; Zhang, K.; Li, H.; Juang, Z.-Y.; et al. Synthesis of Few-Layer Hexagonal Boron Nitride Thin Film by Chemical Vapor Deposition. Nano Lett. 2010, 10, 4134–4139. [Google Scholar] [CrossRef]
- Wu, J.-B.; Lin, M.-L.; Cong, X.; Liu, H.-N.; Tan, P.-H. Raman Spectroscopy of Graphene-Based Materials and Its Applications in Related Devices. Chem. Soc. Rev. 2018, 47, 1822–1873. [Google Scholar] [CrossRef]
- Huang, C.; Chen, C.; Zhang, M.; Lin, L.; Ye, X.; Lin, S.; Antonietti, M.; Wang, X. Carbon-Doped BN Nanosheets for Metal-Free Photoredox Catalysis. Nat. Commun. 2015, 6, 7698. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zeng, X.; Wang, D.; Zhang, H.; Du, X.; He, X.; Li, W.; Fang, W.; Huang, Z.; Chen, H. In-Plane Graphene Incorporated Borocarbonitride: Directional Utilization of Disorder Charge via Micro π-Conjugated Heterointerface for Photocatalytic CO2 Reduction. Carbon 2023, 203, 847–855. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, C.; Liu, L.; Xu, Z.; Wei, J.; Wang, W.; Bai, X.; Wang, E. Towards the Controlled CVD Growth of Graphitic B–C–N Atomic Layer Films: The Key Role of B–C Delivery Molecular Precursor. Nano Res. 2016, 9, 1221–1235. [Google Scholar] [CrossRef]
- Portehault, D.; Giordano, C.; Gervais, C.; Senkovska, I.; Kaskel, S.; Sanchez, C.; Antonietti, M. High-Surface-Area Nanoporous Boron Carbon Nitrides for Hydrogen Storage. Adv. Funct. Mater. 2010, 20, 1827–1833. [Google Scholar] [CrossRef]
- Bai, X.; Zhang, X.; Sun, Y.; Huang, M.; Fan, J.; Xu, S.; Li, H. Low Ruthenium Content Confined on Boron Carbon Nitride as an Efficient and Stable Electrocatalyst for Acidic Oxygen Evolution Reaction. Angew. Chemie-Int. Ed. 2023, 62, e202308704. [Google Scholar] [CrossRef]
- Chen, S.; Li, P.; Xu, S.; Pan, X.; Fu, Q.; Bao, X. Carbon Doping of Hexagonal Boron Nitride Porous Materials toward CO2 Capture. J. Mater. Chem. A 2018, 6, 1832–1839. [Google Scholar] [CrossRef]
- Lim, W.H.; Hamzah, A.; Ahmadi, M.T.; Ismail, R. Band Gap Engineering of BC2N for Nanoelectronic Applications. Superlattices Microstruct. 2017, 112, 328–338. [Google Scholar] [CrossRef]
- Chand, H.; Choudhary, P.; Kumar, A.; Kumar, A.; Krishnan, V. Atmospheric Pressure Conversion of Carbon Dioxide to Cyclic Carbonates Using a Metal-Free Lewis Acid-Base Bifunctional Heterogeneous Catalyst. J. CO2 Util. 2021, 51, 101646. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Q.; Chen, Q.; Li, X.; Li, Y.; Kang, M.; Li, Q.; Wang, J. A New Boron Modified Carbon Nitride Metal-Free Catalyst for the Cycloaddition of CO2 and Bisepoxides. Appl. Catal. A Gen. 2024, 675, 119615. [Google Scholar] [CrossRef]
- Lei, G.; Qi, S.; Li, H.; Xue, Y.; Shen, L.; Zheng, X.; Wang, S.; Cao, Y.; Zhan, Y. Carbon-Doped Boron Nitride Nanosheets as an Efficient Metal-Free Catalyst for the Selective Oxidation of H2S. Phys. Chem. Chem. Phys. 2023, 25, 32317–32322. [Google Scholar] [CrossRef]
- Duan, X.; Song, G.; Song, C.; Lu, G.; Wang, Y.; Sun, J.; Chen, A.; Xie, X. Photoinduced Enhanced CO2 Capture Performance on Carbon-Doped Boron Nitride Adsorbent. Sep. Purif. Technol. 2024, 339, 126685. [Google Scholar] [CrossRef]
- Mondal, K.; Malode, S.J.; Shetti, N.P.; Alqarni, S.A.; Pandiaraj, S.; Alodhayb, A. Porous Nanostructures for Hydrogen Generation and Storage. J. Energy Storage 2024, 76, 109719. [Google Scholar] [CrossRef]
- Hirscher, M.; Zhang, L.; Oh, H. Nanoporous Adsorbents for Hydrogen Storage. Appl. Phys. A 2023, 129, 112. [Google Scholar] [CrossRef]
- Chen, Z.; Kirlikovali, K.O.; Idrees, K.B.; Wasson, M.C.; Farha, O.K. Porous Materials for Hydrogen Storage. Chem 2022, 8, 693–716. [Google Scholar] [CrossRef]
- Villajos, J.A.; Balderas-Xicohténcatl, R.; Al Shakhs, A.N.; Berenguer-Murcia, Á.; Buckley, C.E.; Cazorla-Amorós, D.; Charalambopoulou, G.; Couturas, F.; Cuevas, F.; Fairen-Jimenez, D.; et al. Establishing ZIF-8 as a Reference Material for Hydrogen Cryoadsorption: An Interlaboratory Study. ChemPhysChem 2024, 25, e202300794. [Google Scholar] [CrossRef] [PubMed]
- Broom, D.P.; Hirscher, M. Irreproducibility in Hydrogen Storage Material Research. Energy Environ. Sci. 2016, 9, 3368–3380. [Google Scholar] [CrossRef]
- Li, Y.; Liu, L.; Yu, H.; Zhao, Y.; Dai, J.; Zhong, Y.; Pan, Z.; Yu, H. Synergy of Developed Micropores and Electronic Structure Defects in Carbon-Doped Boron Nitride for CO2 Capture. Sci. Total Environ. 2022, 811, 151384. [Google Scholar] [CrossRef]
- Raidongia, K.; Nag, A.; Hembram, K.P.S.S.P.S.S.; Waghmare, U.V.; Datta, R.; Rao, C.N.R.N.R. BCN: A Graphene Analogue with Remarkable Adsorptive Properties. Chem.-A Eur. J. 2010, 16, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Subrahmanyam, K.S.; Chaturbedy, P.; Raidongia, K.; Govindaraj, A.; Hembram, K.P.S.S.; Mishra, A.K.; Waghmare, U.V.; Rao, C.N.R. Remarkable Uptake of CO2 and CH4 by Graphene-like Borocarbonitrides, BxCyNZ. ChemSusChem 2011, 4, 1662–1670. [Google Scholar] [CrossRef]
- Mishra, A.K.; Mishra, S. Tuning of Adsorption Energies of CO2 and CH4 in Borocarbonitrides BxCyNz: A First-Principles Study. J. Mol. Graph. Model. 2019, 93, 107446. [Google Scholar] [CrossRef]
- Ibarra-Rodríguez, M.; Sánchez, M. Graphitic Carbon Nitride Functionalized with Four Boron Atoms for Adsorption and Separation of CO2/CH4: DFT Calculations. Adsorption 2020, 26, 597–605. [Google Scholar] [CrossRef]
- Moghaddam, F.E.; Shayeganfar, F.; Ramazani, A. Boron-Rich Enhanced Ambient CO2 Capture and Storage of Boron-Carbon-Nitride Hybrid Nanotubes. J. Mater. Chem. A 2023, 11, 17594–17608. [Google Scholar] [CrossRef]
- Wang, J.; Luo, X. Theoretical Investigation of the BCN Monolayer and Their Derivatives for Metal-Free CO2 Photocatalysis, Capture, and Utilization. ACS Omega 2024, 9, 3772–3780. [Google Scholar] [CrossRef]
- Ali Khan, A.; Ahmad, A.; Al-Swaidan, H.M.; Haider, S.; Saeed Akhtar, M. CO2 Capture and Separation from H2/CH4/N2 Gas Mixtures by a Novel Ternary Pentagonal Monolayer “Penta-BCN”: First Principles Investigation. J. Mol. Liq. 2022, 348, 118474. [Google Scholar] [CrossRef]
- Talapaneni, S.N.; Singh, G.; Kim, I.Y.; AlBahily, K.; Al-Muhtaseb, A.H.; Karakoti, A.S.; Tavakkoli, E.; Vinu, A. Nanostructured Carbon Nitrides for CO2 Capture and Conversion. Adv. Mater. 2020, 32, 1904635. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Zhang, H.; Wei, J.; Zhang, H.-X.; Wu, X.; Li, Y.; Li, C.; Zhang, J.; Ye, J. Integrating the G-C3N4 Nanosheet with B–H Bonding Decorated Metal–Organic Framework for CO2 Activation and Photoreduction. ACS Nano 2018, 12, 5333–5340. [Google Scholar] [CrossRef]
- Peng, H.-L.; Zhong, F.-Y.; Zhang, J.-B.; Zhang, J.-Y.; Wu, P.-K.; Huang, K.; Fan, J.-P.; Jiang, L.-L. Graphitic Carbon Nitride Functionalized with Polyethylenimine for Highly Effective Capture of Carbon Dioxide. Ind. Eng. Chem. Res. 2018, 57, 11031–11038. [Google Scholar] [CrossRef]
- Castilla-Martinez, C.A.; Mighri, R.; Charmette, C.; Cartier, J.; Demirci, U.B. Boron Nitride from Ammonia Borane and Alkali Amidoboranes and Its Features for Carbon Dioxide Capture. Energy Technol. 2023, 11, 2201521. [Google Scholar] [CrossRef]
- Yang, C.; Wang, J.; Chen, Y.; Liu, D.; Huang, S.; Lei, W. One-Step Template-Free Synthesis of 3D Functionalized Flower-like Boron Nitride Nanosheets for NH3 and CO2 Adsorption. Nanoscale 2018, 10, 10979–10985. [Google Scholar] [CrossRef]
- Gou, J.; Liu, C.; Lin, J.; Yu, C.; Fang, Y.; Liu, Z.; Guo, Z.; Tang, C.; Huang, Y. Densification and Pelletization of Porous Boron Nitride Fibers for Effective CO2 Adsorption. Ceram. Int. 2022, 48, 11636–11643. [Google Scholar] [CrossRef]
- Ghanbari, T.; Abnisa, F.; Wan Daud, W.M.A. A Review on Production of Metal Organic Frameworks (MOF) for CO2 Adsorption. Sci. Total Environ. 2020, 707, 135090. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, S.; Lahtinen, M. Recent Progress in Metal-Organic Frameworks (MOFs) for CO2 Capture at Different Pressures. J. Environ. Chem. Eng. 2022, 10, 108930. [Google Scholar] [CrossRef]
- Pescarmona, P.P. Cyclic Carbonates Synthesised from CO2: Applications, Challenges and Recent Research Trends. Curr. Opin. Green Sustain. Chem. 2021, 29, 100457. [Google Scholar] [CrossRef]
- Huang, Z.; Li, F.; Chen, B.; Yuan, G. Cycloaddition of CO2 and Epoxide Catalyzed by Amino- and Hydroxyl-Rich Graphitic Carbon Nitride. Catal. Sci. Technol. 2016, 6, 2942–2948. [Google Scholar] [CrossRef]
- Rajendran, A.; Cui, T.; Fan, H.; Yang, Z.; Feng, J.; Li, W. A Comprehensive Review on Oxidative Desulfurization Catalysts Targeting Clean Energy and Environment. J. Mater. Chem. A 2020, 8, 2246–2285. [Google Scholar] [CrossRef]
- Lin, A.; Bagnato, G. Revolutionising Energy Storage: The Latest Breakthrough in Liquid Organic Hydrogen Carriers. Int. J. Hydrogen Energy 2024, 63, 315–329. [Google Scholar] [CrossRef]
- Wei, Y.; Wu, P.; Luo, J.; Dai, L.; Li, H.H.; Zhang, M.; Chen, L.; Wang, L.; Zhu, W.; Li, H.H. Synthesis of Hierarchical Porous BCN Using Ternary Deep Eutectic Solvent as Precursor and Template for Aerobic Oxidative Desulfurization. Microporous Mesoporous Mater. 2020, 293, 109788. [Google Scholar] [CrossRef]
- Sheng, J.; Yan, B.; Lu, W.-D.; Qiu, B.; Gao, X.-Q.; Wang, D.; Lu, A.-H. Oxidative Dehydrogenation of Light Alkanes to Olefins on Metal-Free Catalysts. Chem. Soc. Rev. 2021, 50, 1438–1468. [Google Scholar] [CrossRef]
- Guo, F.; Yang, P.; Pan, Z.; Cao, X.X.-N.; Xie, Z.; Wang, X. Carbon-doped BN Nanosheets for the Oxidative Dehydrogenation of Ethylbenzene. Angew. Chem. 2017, 129, 8343–8347. [Google Scholar] [CrossRef]
- Zhang, X.; Dai, X.; Wu, K.-H.; Su, B.; Chen, J.; Qi, W.; Xie, Z. A Generalized Approach to Adjust the Catalytic Activity of Borocarbonitride for Alkane Oxidative Dehydrogenation Reactions. J. Catal. 2022, 405, 105–115. [Google Scholar] [CrossRef]
- Wang, G.; Chen, S.; Duan, Q.; Wei, F.; Lin, S.; Xie, Z. Surface Chemistry and Catalytic Reactivity of Borocarbonitride in Oxidative Dehydrogenation of Propane. Angew. Chemie Int. Ed. 2023, 62, e202307470. [Google Scholar] [CrossRef]
- Yang, D.; Liu, D.; Li, Y.; Gan, H.; Xu, P.; Tian, Y.; Li, Z.; Xing, T.; Gu, X.; Li, L.; et al. Photo-Thermal Synergistic Catalytic Oxidative Dehydrogenation of Propane over a Spherical Superstructure of Boron Carbon Nitride Nanosheets. Appl. Surf. Sci. 2023, 639, 158258. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, Y.; Han, Y.; Feng, R.; Xie, Z. Unravelling the Role of Boron Dopant in Borocarbonitirde Catalytic Dehydrogenation Reaction. J. Energy Chem. 2023, 85, 137–143. [Google Scholar] [CrossRef]
- Wang, G.; Hu, A.; Duan, Q.; Cui, L.; Chen, Z.; Huang, Z.; Zhang, X.; Huang, S.; Xie, Z. Hierarchical Boroncarbonitride Nanosheets as Metal-Free Catalysts for Enhanced Oxidative Dehydrogenation of Propane. Chem. Eng. Sci. 2024, 288, 119848. [Google Scholar] [CrossRef]
- Mukherjee, D.; Park, S.-E.; Reddy, B.M. CO2 as a Soft Oxidant for Oxidative Dehydrogenation Reaction: An Eco Benign Process for Industry. J. CO2 Util. 2016, 16, 301–312. [Google Scholar] [CrossRef]
- Zhang, X.; Yan, P.; Xu, J.; Li, F.; Herold, F.; Etzold, B.J.M.; Wang, P.; Su, D.S.; Lin, S.; Qi, W.; et al. Methanol Conversion on Borocarbonitride Catalysts: Identification and Quantification of Active Sites. Sci. Adv. 2020, 6, eaba5778. [Google Scholar] [CrossRef]
- Villa, K.; Galán-Mascarós, J.R.; López, N.; Palomares, E. Photocatalytic Water Splitting: Advantages and Challenges. Sustain. Energy Fuels 2021, 5, 4560–4569. [Google Scholar] [CrossRef]
- Nasir, M.S.; Yang, G.; Ayub, I.; Wang, S.; Wang, L.; Wang, X.; Yan, W.; Peng, S.; Ramakarishna, S. Recent Development in Graphitic Carbon Nitride Based Photocatalysis for Hydrogen Generation. Appl. Catal. B Environ. 2019, 257, 117855. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.; Zhang, S.; Xu, S.; Wu, X.; Chang, J.; He, Z. Hexagonal Boron Nitride Composite Photocatalysts for Hydrogen Production. J. Alloys Compd. 2021, 864, 158153. [Google Scholar] [CrossRef]
- Wan, Q.; Wei, F.; Ma, Z.; Anpo, M.; Lin, S. Novel Porous Boron Nitride Nanosheet with Carbon Doping: Potential Metal-free Photocatalyst for Visible-light-driven Overall Water Splitting. Adv. Theory Simul. 2019, 2, 1800174. [Google Scholar] [CrossRef]
- Zheng, M.; Ghosh, I.; König, B.; Wang, X. Metal-free Semiconductor Photocatalysis for Sp2 C−H Functionalization with Molecular Oxygen. ChemCatChem 2019, 11, 703–706. [Google Scholar] [CrossRef]
- Shi, J.; Yuan, T.; Zheng, M.; Wang, X. Metal-Free Heterogeneous Semiconductor for Visible-Light Photocatalytic Decarboxylation of Carboxylic Acids. ACS Catal. 2021, 11, 3040–3047. [Google Scholar] [CrossRef]
- Zhou, M.; Chen, Z.; Yang, P.; Wang, S.; Huang, C.; Wang, X. Hydrogen Reduction Treatment of Boron Carbon Nitrides for Photocatalytic Selective Oxidation of Alcohols. Appl. Catal. B Environ. 2020, 276, 118916. [Google Scholar] [CrossRef]
- Zhou, M.; Yang, P.; Wang, S.; Luo, Z.; Huang, C.; Wang, X. Structure-mediated Charge Separation in Boron Carbon Nitride for Enhanced Photocatalytic Oxidation of Alcohol. ChemSusChem 2018, 11, 3949–3955. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Yuan, T.; Wang, R.; Zheng, M.; Wang, X. Boron Carbonitride Photocatalysts for Direct Decarboxylation: The Construction of C(Sp3)–N or C(Sp3)–C(Sp2) Bonds with Visible Light. Green Chem. 2021, 23, 3945–3949. [Google Scholar] [CrossRef]
- Sivaprakash, K.; Induja, M.; Gomathi Priya, P. Facile Synthesis of Metal Free Non-Toxic Boron Carbon Nitride Nanosheets with Strong Photocatalytic Behavior for Degradation of Industrial Dyes. Mater. Res. Bull. 2018, 100, 313–321. [Google Scholar] [CrossRef]
- Wei, B.; Sun, J.; Mei, Q.; An, Z.; Wang, X.; Cao, H.; Han, D.; He, M. Feasibility of Carbon-Doped BN Nanosheets as Photocatalyst for Degradation of 4-Chloroguaiacol and Ecotoxicity Fate during Indirect Photochemical Transformation. J. Catal. 2019, 379, 10–17. [Google Scholar] [CrossRef]
- Ebadi, S.; Ghasemipanah, K.; Alaie, E.; Rashidi, A.; Khataee, A. Using BCN Nanostructure as Anode Electrode for Photoelectrocatalytic Degradation of Organics: A Statistical Approach. J. Water Supply Res. Technol. 2021, 70, 856–867. [Google Scholar] [CrossRef]
- Sivaprakash, K.; Induja, M.; Gomathipriya, P.; Karthikeyan, S.; Umabharathi, S.T. Single-Step Synthesis of Efficient Nanometric Boron Carbon Nitride Semiconductor for Photocatalysis. Mater. Res. Bull. 2021, 134, 111106. [Google Scholar] [CrossRef]
- Shakunthala, R.; Sivaa Vignesh, C.; Viswanathan, R.; Matheswaran, M. Solar Photocatalytic Process Using Biomass-Derived Boron Carbon Nitride (BM-BCN) for the Treatment of Synthetic Textile Effluent. Catal. Today 2024, 432, 114583. [Google Scholar] [CrossRef]
- Wang, B.; Anpo, M.; Lin, J.; Yang, C.; Zhang, Y.; Wang, X. Direct Hydroxylation of Benzene to Phenol on H-BCN Nanosheets in the Presence of FeCl3 and H2O2 under Visible Light. Catal. Today 2019, 324, 73–82. [Google Scholar] [CrossRef]
- Jiang, H.; Zang, C.; Cheng, H.; Sun, B.; Gao, X. Photocatalytic Green Synthesis of Benzazoles from Alcohol Oxidation/Toluene Sp3 C–H Activation over Metal-Free BCN: Effect of Crystallinity and N–B Pair Exposure. Catal. Sci. Technol. 2021, 11, 7955–7962. [Google Scholar] [CrossRef]
- Luo, Z.; Fang, Y.; Zhou, M.; Wang, X. A Borocarbonitride Ceramic Aerogel for Photoredox Catalysis. Angew. Chemie Int. Ed. 2019, 58, 6033–6037. [Google Scholar] [CrossRef]
- Zhou, M.; Wang, S.; Yang, P.; Huang, C.; Wang, X. Boron Carbon Nitride Semiconductors Decorated with CdS Nanoparticles for Photocatalytic Reduction of CO2. ACS Catal. 2018, 8, 4928–4936. [Google Scholar] [CrossRef]
- Yuan, T.; Wu, Z.; Zhai, S.; Wang, R.; Wu, S.; Cheng, J.; Zheng, M.; Wang, X. Photosynthetic Fixation of CO2 in Alkenes by Heterogeneous Photoredox Catalysis with Visible Light. Angew. Chemie-Int. Ed. 2023, 62, e202304861. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liang, F.; Gu, H.; Wu, S.; Cao, Y.; Lv, G.; Zhang, H.; Jia, Q.; Zhang, S. In Situ Synthesized α-Fe2O3/BCN Heterojunction for Promoting Photocatalytic CO2 Reduction Performance. J. Colloid Interface Sci. 2022, 621, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Ri, M.; Choe, K.; Kim, K.; Gao, Y.; Tang, Z. C-Doping into h-BN at Low Annealing Temperature by Alkaline Earth Metal Borate for Photoredox Activity. RSC Adv. 2018, 8, 42109–42115. [Google Scholar] [CrossRef]
- Zeng, X.; Bu, X.; Chen, H.; Huang, Z.; Fang, W.; Wang, D.; He, X.; Du, X.; Li, W.; Zhang, H.; et al. Regulation of Charge Transfer Direction and Key Steps via Y Modification of Redox-Active Sites on Borocarbonitride for Photocatalytic CO2 Reduction. Compos. Part B Eng. 2024, 287, 111838. [Google Scholar] [CrossRef]
- Zeng, X.; Chen, H.; Fang, W.; Huang, Z.; Wang, D.; He, X.; Du, X.; Li, W.; Zhang, H.; Zhao, L. Local Spatial Polarization Induced Efficient Electron Transfer in Fluorinated Borocarbonitride for Boosting CO2 Photoreduction. Chem. Eng. J. 2024, 488, 151042. [Google Scholar] [CrossRef]
- Ishaq, T.; Yousaf, M.; Bhatti, I.A.; Batool, A.; Asghar, M.A.; Mohsin, M.; Ahmad, M. A Perspective on Possible Amendments in Semiconductors for Enhanced Photocatalytic Hydrogen Generation by Water Splitting. Int. J. Hydrogen Energy 2021, 46, 39036–39057. [Google Scholar] [CrossRef]
- Babu, P.; Mohanty, S.; Naik, B.; Parida, K. Synergistic Effects of Boron and Sulfur Co-Doping into Graphitic Carbon Nitride Framework for Enhanced Photocatalytic Activity in Visible Light Driven Hydrogen Generation. ACS Appl. Energy Mater. 2018, 1, 5936–5947. [Google Scholar] [CrossRef]
- Lin, S.; Huang, H.; Ma, T.; Zhang, Y. Photocatalytic Oxygen Evolution from Water Splitting. Adv. Sci. 2021, 8, 2002458. [Google Scholar] [CrossRef]
- Luo, Z.; Chen, J.; Fang, Y.; Xie, L.; Liu, Q.; Huang, J.; Liu, M. Synthesis of Borocarbonitride Nanosheets from Biomass for Enhanced Charge Separation and Hydrogen Production. Sci. Rep. 2024, 14, 14443. [Google Scholar] [CrossRef]
- Chen, Q.; Gao, G.; Guo, H.; Wang, S.A.; Wang, Q.; Fang, Y.; Hu, X.; Duan, R. Boron-Doped Polymeric Carbon Nitride Co-Modified with Carbon-Ring and Carboxyl for Efficient Photocatalytic Overall Water-Splitting. Chem. Eng. J. 2023, 470, 144199. [Google Scholar] [CrossRef]
- Chen, L.; Wang, X. Bio-Templated Fabrication of Metal-Free Boron Carbonitride Tubes for Visible Light Photocatalysis. Chem. Commun. 2017, 53, 11988–11991. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Dong, C.L.; Wang, B.; Chen, C.; Huang, Y.C.; Diao, Z.; Li, S.; Guo, L.; Shen, S. Synergy of Dopants and Defects in Graphitic Carbon Nitride with Exceptionally Modulated Band Structures for Efficient Photocatalytic Oxygen Evolution. Adv. Mater. 2019, 31, 1903545. [Google Scholar] [CrossRef]
- Zhao, D.; Wang, Y.; Dong, C.L.; Huang, Y.C.; Chen, J.; Xue, F.; Shen, S.; Guo, L. Boron-Doped Nitrogen-Deficient Carbon Nitride-Based Z-Scheme Heterostructures for Photocatalytic Overall Water Splitting. Nat. Energy 2021, 6, 388–397. [Google Scholar] [CrossRef]
- Nehate, S.D.; Saikumar, A.K.; Prakash, A.; Sundaram, K.B. A Review of Boron Carbon Nitride Thin Films and Progress in Nanomaterials. Mater. Today Adv. 2020, 8, 100106. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Cheng, K.; Quan, X.; Fan, X.; Su, Y.; Chen, S.; Zhao, H.; Zhang, Y.; Yu, H.; et al. Selective Electrochemical Reduction of Carbon Dioxide to Ethanol on a Boron- and Nitrogen-Co-Doped Nanodiamond. Angew. Chemie-Int. Ed. 2017, 56, 15607–15611. [Google Scholar] [CrossRef]
- Cheng, C.; Shao, J.; Wei, P.; Song, Y.; Li, H.; Gao, D.; Wang, G. Nitrogen and Boron Co-doped Carbon Spheres for Carbon Dioxide Electroreduction. ChemNanoMat 2021, 7, 635–640. [Google Scholar] [CrossRef]
- Jia, C.; Ren, W.; Chen, X.; Yang, W.; Zhao, C. (N, B) Dual Heteroatom-Doped Hierarchical Porous Carbon Framework for Efficient Electroreduction of Carbon Dioxide. ACS Sustain. Chem. Eng. 2020, 8, 6003–6010. [Google Scholar] [CrossRef]
- Ma, X.; Du, J.; Sun, H.; Ye, F.; Wang, X.; Xu, P.; Hu, C.; Zhang, L.; Liu, D. Boron, Nitrogen Co-Doped Carbon with Abundant Mesopores for Efficient CO2 Electroreduction. Appl. Catal. B Environ. 2021, 298, 120543. [Google Scholar] [CrossRef]
- Ayyub, M.M.; Rao, C.N.R. Borocarbonitrides as Metal-Free Electrocatalysts for the Electrochemical Reduction of CO2. Chem. Mater. 2022, 34, 6626–6635. [Google Scholar] [CrossRef]
- Khan, A.F.; Ferrari, A.G.-M.; Hughes, J.P.; Smith, G.C.; Banks, C.E.; Rowley-Neale, S.J. 2D-Hexagonal Boron Nitride Screen-Printed Bulk-Modified Electrochemical Platforms Explored towards Oxygen Reduction Reactions. Sensors 2022, 22, 3330. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hao, J.; Liu, D.; Qin, S.; Portehault, D.; Li, Y.; Chen, Y.; Lei, W. Porous Boron Carbon Nitride Nanosheets as Efficient Metal-Free Catalysts for the Oxygen Reduction Reaction in Both Alkaline and Acidic Solutions. ACS Energy Lett. 2017, 2, 306–312. [Google Scholar] [CrossRef]
- Moses, K.; Kiran, V.; Sampath, S.; Rao, C.N.R. Few-layer Borocarbonitride Nanosheets: Platinum-free Catalyst for the Oxygen Reduction Reaction. Chem.–An Asian J. 2014, 9, 838–843. [Google Scholar] [CrossRef]
- Liu, F.; Gao, D.; Wang, F.; Shen, P.; Liu, Y.; Zhang, S.; Li, Y.; Zhang, J.; Xue, Y.; Tang, C. Boron Carbon Nitride as Efficient Oxygen Reduction Reaction Support. J. Colloid Interface Sci. 2024, 673, 901–908. [Google Scholar] [CrossRef]
- Patil, I.M.; Lokanathan, M.; Ganesan, B.; Swami, A.; Kakade, B. Carbon Nanotube/Boron Nitride Nanocomposite as a Significant Bifunctional Electrocatalyst for Oxygen Reduction and Oxygen Evolution Reactions. Chem.–A Eur. J. 2017, 23, 676–683. [Google Scholar] [CrossRef]
- Weber, M.; Tuleushova, N.; Zgheib, J.; Lamboux, C.; Iatsunskyi, I.; Coy, E.; Flaud, V.; Tingry, S.; Cornu, D.; Miele, P.; et al. Enhanced Electrocatalytic Performance Triggered by Atomically Bridged Boron Nitride between Palladium Nanoparticles and Carbon Fibers in Gas-Diffusion Electrodes. Appl. Catal. B Environ. 2019, 257, 117917. [Google Scholar] [CrossRef]
- Gu, S.; Chandra Mallick, B.; Hsieh, C.-T.; Gandomi, Y.A.; Zhang, R.-S. Hexagonal Boron Nitride Nanosheets as Metal-Free Electrochemical Catalysts for Oxygen Reduction Reactions. Ceram. Int. 2022, 48, 9506–9517. [Google Scholar] [CrossRef]
- Xu, X.; Zhao, Y.; Yuan, Q.; Wu, Y.; He, J.; Fan, M. Porous Heterostructure of H-BN/Carbon as an Efficient Electrocatalyst for Hydrogen Peroxide Generation. Carbon Lett. 2024, 34, 1629–1637. [Google Scholar] [CrossRef]
- Nazer, E.A.A.; Muthukrishnan, A. Synergistic Effect on BCN Nanomaterials for the Oxygen Reduction Reaction—A Kinetic and Mechanistic Analysis to Explore the Active Sites. Catal. Sci. Technol. 2020, 10, 6659–6668. [Google Scholar] [CrossRef]
- Wu, W.; He, Z.; Xiao, Y.; Zhang, X.; Chen, K.; Fan, J.; Li, X.; Zhao, Y.; Qu, L. A Versatile, Heat-Resisting, Electrocatalytic Active Graphene Framework by in-Situ Formation of Boron Nitride Quantum Dots. Carbon 2022, 192, 123–132. [Google Scholar] [CrossRef]
- Yu, J.; Tang, C.; Zhang, W.; Jiang, Z.; Qi, L.; Mu, Y.; Huang, T.; Zhu, X. Embedded Ag Nanoparticles on Boron Nitride by Introduced Carbon Nanotubes to Optimize ORR and OER Performance. Int. J. Hydrogen Energy 2024, 73, 294–304. [Google Scholar] [CrossRef]
- Ďurovič, M.; Hnát, J.; Bouzek, K. Electrocatalysts for the Hydrogen Evolution Reaction in Alkaline and Neutral Media. A Comparative Review. J. Power Sources 2021, 493, 229708. [Google Scholar] [CrossRef]
- Zaman, N.; Noor, T.; Iqbal, N. Recent Advances in the Metal–Organic Framework-Based Electrocatalysts for the Hydrogen Evolution Reaction in Water Splitting: A Review. RSC Adv. 2021, 11, 21904–21925. [Google Scholar] [CrossRef]
- Chhetri, M.; Maitra, S.; Chakraborty, H.; Waghmare, U.V.; Rao, C.N.R. Superior Performance of Borocarbonitrides, BxCyNz, as Stable, Low-Cost Metal-Free Electrocatalysts for the Hydrogen Evolution Reaction. Energy Environ. Sci. 2016, 9, 95–101. [Google Scholar] [CrossRef]
- Kaur, M.; Ahmad Mir, R.; Chauhan, I.; Singh, K.; Krishnan, U.; Kumar, M.; Devi, P.; Pandey, O.P.; Kumar, A. Defect States Induced Luminescence and Electrochemical Studies of Boron Carbon Nitride Nanosheets. Appl. Surf. Sci. 2021, 559, 149982. [Google Scholar] [CrossRef]
- Liu, Y.; Ali, R.; Ma, J.; Jiao, W.; Yin, L.; Mu, C.; Jian, X. Graphene-Decorated Boron–Carbon–Nitride-Based Metal-Free Catalysts for an Enhanced Hydrogen Evolution Reaction. ACS Appl. Energy Mater. 2021, 4, 3861–3868. [Google Scholar] [CrossRef]
- Kumar, P.S.; Prakash, P. Metal Free Nanocomposite of Graphitic Carbon Nitride, Boron Nitride and Chitosan for Efficient Evolution of Hydrogen: A Strategic Approach to Achieving Sustainable and Effective Electrocatalysis. J. Environ. Chem. Eng. 2023, 11, 109045. [Google Scholar] [CrossRef]
- Yang, L.; Wang, X.; Wang, J.; Cui, G.; Liu, D. Graphite Carbon Nitride/Boron-Doped Graphene Hybrid for Efficient Hydrogen Generation Reaction. Nanotechnology 2018, 29, 345705. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.; Zhang, Z.; Zhang, H.; Zhang, H.; Dong, S. Transformation from Graphitic C3N4 to Nitrogen-Boron-Carbon Ternary Nanosheets as Efficient Metal-Free Bifunctional Electrocatalyst for Oxygen Reduction Reaction and Hydrogen Evolution Reaction. Appl. Surf. Sci. 2018, 448, 618–627. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, Y.; Wang, B.; Zou, R.; Huang, Y.; Sun, W. Dynamic Analysis of Equivalent Circuit Model Value of CoP/Boron Nitride Doped Carbon for Hydrogen Evolution Reaction. Electrochim. Acta 2022, 406, 139846. [Google Scholar] [CrossRef]
- Salah, A.; Ren, H.-D.; Al-Ansi, N.; Tan, H.; Yu, F.; Yanchun, L.; Thamer, B.M.; Al-Salihy, A.; Zhao, L.; Li, Y. Dispersing Small Ru Nanoparticles into Boron Nitride Remodified by Reduced Graphene Oxide for High-Efficient Electrocatalytic Hydrogen Evolution Reaction. J. Colloid Interface Sci. 2023, 644, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Chandrashekharan, B.; Sampatkumar, H.G.; George, D.; Antony, A.M.; Doddamani, S.V.; Sasidhar, B.S.; Balakrishna, R.G.; Patil, S.A. Palladium Nanoparticle Immobilized on Coconut Coir Extract Coated Boron Carbon Nitride: A Green and Sustainable Nanocatalyst for Cross-Coupling Reactions and HER Studies. Diam. Relat. Mater. 2024, 147, 111261. [Google Scholar] [CrossRef]
- Pramoda, K.; Ayyub, M.M.; Singh, N.K.; Chhetri, M.; Gupta, U.; Soni, A.; Rao, C.N.R. Covalently Bonded MoS2–Borocarbonitride Nanocomposites Generated by Using Surface Functionalities on the Nanosheets and Their Remarkable HER Activity. J. Phys. Chem. C 2018, 122, 13376–13384. [Google Scholar] [CrossRef]
- Pramoda, K.; Binwal, D.C.; Rao, C.N.R. Nanocomposites of MoS2 Nanoparticles with Carboxyl-Functionalized Carbon Nanotubes and Borocarbonitrides Nanosheets, and Their Electrocatalytic Hydrogen Evolution Reaction Activity. Mater. Res. Bull. 2022, 149, 111697. [Google Scholar] [CrossRef]
- Pramoda, K.; Servottam, S.; Kaur, M.; Rao, C.N.R. Layered Nanocomposites of Polymer-Functionalized Reduced Graphene Oxide and Borocarbonitride with MoS2 and MoSe2 and Their Hydrogen Evolution Reaction Activity. ACS Appl. Nano Mater. 2020, 3, 1792–1799. [Google Scholar] [CrossRef]
- Beere, H.K.; Shantharaja; Yatish, K.V.; Aravind, K.; Ghosh, D.; Balakrishna, R.G.; Pramoda, K. Unveiling Favorable Synergy of Tubules-like NiMoSe2 with Defect-Rich Borocarbonitride over Graphene or MXene for Efficient Hydrogen Evolution Reaction Electrocatalysis. Int. J. Hydrogen Energy 2024, 54, 1582–1592. [Google Scholar] [CrossRef]
- Binwal, D.C.; Pramoda, K.; Zak, A.; Kaur, M.; Chithaiah, P.; Rao, C.N.R. Nanocomposites of 1D MoS2 with Polymer-Functionalized Nanotubes of Carbon and Borocarbonitride, and Their HER Activity. ACS Appl. Energy Mater. 2021, 4, 2339–2347. [Google Scholar] [CrossRef]
- Xu, W.; Chen, C.; Tang, C.; Li, Y.; Xu, L. Design of Boron Doped C2N-C3N Coplanar Conjugated Heterostructure for Efficient HER Electrocatalysis. Sci. Rep. 2018, 8, 5661. [Google Scholar] [CrossRef]
- Ng, S.-F.; Chen, X.; Foo, J.J.; Xiong, M.; Ong, W.-J. 2D Carbon Nitrides: Regulating Non-Metal Boron-Doped C3N5 for Elucidating the Mechanism of Wide PH Range Photocatalytic Hydrogen Evolution Reaction. Chin. J. Catal. 2023, 47, 150–160. [Google Scholar] [CrossRef]
- Erfani, N.; Baharudin, L.; Watson, M. Recent Advances and Intensifications in Haber-Bosch Ammonia Synthesis Process. Chem. Eng. Process.-Process Intensif. 2024, 204, 109962. [Google Scholar] [CrossRef]
- Soloveichik, G. Electrochemical Synthesis of Ammonia as a Potential Alternative to the Haber–Bosch Process. Nat. Catal. 2019, 2, 377–380. [Google Scholar] [CrossRef]
- Chang, B.; Li, L.; Shi, D.; Jiang, H.; Ai, Z.; Wang, S.; Shao, Y.; Shen, J.; Wu, Y.; Li, Y.; et al. Metal-Free Boron Carbonitride with Tunable Boron Lewis Acid Sites for Enhanced Nitrogen Electroreduction to Ammonia. Appl. Catal. B Environ. 2021, 283, 119622. [Google Scholar] [CrossRef]
- Ma, C.; Zhang, Y.; Yan, S.; Liu, B. Carbon-Doped Boron Nitride Nanosheets: A High-Efficient Electrocatalyst for Ambient Nitrogen Reduction. Appl. Catal. B Environ. 2022, 315, 121574. [Google Scholar] [CrossRef]
- Lin, W.; Chen, H.; Lin, G.; Yao, S.; Zhang, Z.; Qi, J.; Jing, M.; Song, W.; Li, J.; Liu, X.; et al. Creating Frustrated Lewis Pairs in Defective Boron Carbon Nitride for Electrocatalytic Nitrogen Reduction to Ammonia. Angew. Chemie Int. Ed. 2022, 61, e202207807. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Lin, W.; Guo, T.; Wang, J.; Li, B.; Fu, J. Highly Defective Boron Carbon Nitride Nanosheets: A Robust Electro-Catalyst for Efficient Nitrogen Fixation. Ind. Eng. Chem. Res. 2023, 62, 10391–10398. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Y.; Wang, X.; Chen, S.; Duan, L.; Zhang, W.; Li, W.; Liu, J. Tailoring Electron-Riched Boron Sites in BCN for Nitrogen Fixation via Alternate Mechanism. Adv. Mater. Interfaces 2022, 9, 2101842. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, R.; Cui, Y.; Shang, J.; Wei, X.; Wang, S.; Jia, S.; Qin, K.; Zhang, H.; Wang, H.; et al. Tuning Electronic Environment of B Sites in Boron Carbonitride Nanoribbon Boosts Catalytic Activity of Reducing N2 to NH3. Fuel 2024, 369, 131750. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, N.; Zhang, J.; Deng, B.; Cao, Z.; Wang, Z.; Wei, G.; Zhang, Q.; Jia, R.; Xiang, P.; et al. Critical Review in Electrocatalytic Nitrate Reduction to Ammonia towards a Sustainable Nitrogen Utilization. Chem. Eng. J. 2024, 483, 148952. [Google Scholar] [CrossRef]
- Zhao, X.; Hu, G.; Tan, F.; Zhang, S.; Wang, X.; Hu, X.; Kuklin, A.V.; Baryshnikov, G.V.; Ågren, H.; Zhou, X.; et al. Copper Confined in Vesicle-like BCN Cavities Promotes Electrochemical Reduction of Nitrate to Ammonia in Water. J. Mater. Chem. A 2021, 9, 23675–23686. [Google Scholar] [CrossRef]
- Zhao, X.; Jia, X.; He, Y.; Zhang, H.; Zhou, X.; Zhang, H.; Zhang, S.; Dong, Y.; Hu, X.; Kuklin, A.V.; et al. Two-Dimensional BCN Matrix Inlaid with Single-Atom-Cu Driven Electrochemical Nitrate Reduction Reaction to Achieve Sustainable Industrial-Grade Production of Ammonia. Appl. Mater. Today 2021, 25, 101206. [Google Scholar] [CrossRef]
- Zhao, X.; Li, X.; Zhang, H.; Chen, X.; Xu, J.; Yang, J.; Zhang, H.; Hu, G. Atomic-Dispersed Copper Simultaneously Achieve High-Efficiency Removal and High-Value-Added Conversion to Ammonia of Nitrate in Sewage. J. Hazard. Mater. 2022, 424, 127319. [Google Scholar] [CrossRef]
- Li, X.; Zhao, X.; Zhou, Y.; Hu, J.; Zhang, H.; Hu, X.; Hu, G. Pd Nanocrystals Embedded in BC2N for Efficient Electrochemical Conversion of Nitrate to Ammonia. Appl. Surf. Sci. 2022, 584, 152556. [Google Scholar] [CrossRef]
- Zhao, X.; Zhu, Z.; He, Y.; Zhang, H.; Zhou, X.; Hu, W.; Li, M.; Zhang, S.; Dong, Y.; Hu, X.; et al. Simultaneous Anchoring of Ni Nanoparticles and Single-Atom Ni on BCN Matrix Promotes Efficient Conversion of Nitrate in Water into High-Value-Added Ammonia. Chem. Eng. J. 2022, 433, 133190. [Google Scholar] [CrossRef]
- Wang, J.; Fan, Z.; Zhao, H.; Liu, X.; Zheng, M.; Zhang, L.; Zhou, Y.; Sun, L.; Liu, J.; Zhang, H. High Faraday Efficiency of Cu1Co1-BCN Based on a Dodecahydro-Closo-Dodecaborate Hybrid for Electrocatalytic Reduction of Nitrate to Ammonia. J. Mater. Chem. A 2023, 11, 20234–20241. [Google Scholar] [CrossRef]
- Wang, Z.; Xia, S.; Deng, X.; Baryshnikov, G.; Kuklin, A.; Ågren, H.; Zhang, H. Platinum Group Nanoparticles Doped BCN Matrix: Efficient Catalysts for the Electrocatalytic Reduction of Nitrate to Ammonia. J. Colloid Interface Sci. 2024, 664, 84–95. [Google Scholar] [CrossRef]
- Lu, X.; Wei, J.; Lin, H.; Li, Y.; Li, Y.Y. Boron Regulated Fe Single-Atom Structures for Electrocatalytic Nitrate Reduction to Ammonia. ACS Appl. Nano Mater. 2024, 7, 14654–14664. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castilla-Martinez, C.A.; Meléndez-González, P.C.; Demirci, U.B. Borocarbonitrides for Decarbonization: From CO2 Utilization to Renewable Fuel Synthesis. Nanoenergy Adv. 2025, 5, 6. https://doi.org/10.3390/nanoenergyadv5020006
Castilla-Martinez CA, Meléndez-González PC, Demirci UB. Borocarbonitrides for Decarbonization: From CO2 Utilization to Renewable Fuel Synthesis. Nanoenergy Advances. 2025; 5(2):6. https://doi.org/10.3390/nanoenergyadv5020006
Chicago/Turabian StyleCastilla-Martinez, Carlos A., Perla C. Meléndez-González, and Umit B. Demirci. 2025. "Borocarbonitrides for Decarbonization: From CO2 Utilization to Renewable Fuel Synthesis" Nanoenergy Advances 5, no. 2: 6. https://doi.org/10.3390/nanoenergyadv5020006
APA StyleCastilla-Martinez, C. A., Meléndez-González, P. C., & Demirci, U. B. (2025). Borocarbonitrides for Decarbonization: From CO2 Utilization to Renewable Fuel Synthesis. Nanoenergy Advances, 5(2), 6. https://doi.org/10.3390/nanoenergyadv5020006










