Sydnone Imines as a New Class of Promising Plant Growth and Stress Tolerance Modulators—A First Experimental Structure–Activity Overview
Abstract
:1. Introduction
2. NO Signaling in Biological Systems
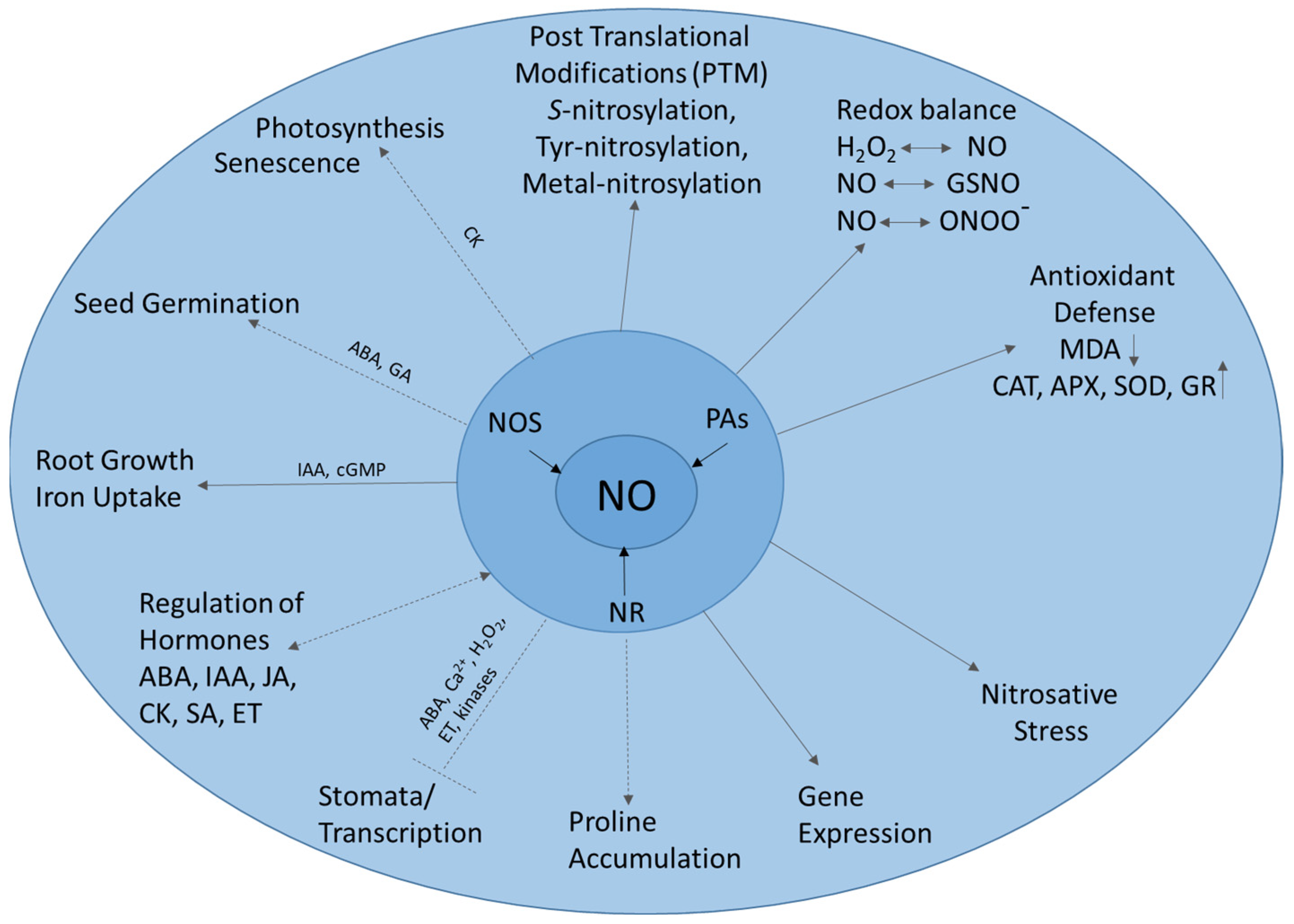
2.1. Sydnone Imines as NO Donors in Pharmaceutical Studies
2.2. Sydnone Imines as Phytoeffectors in Plants
3. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Boyer, J.S. Plant productivity and environment. Science 1982, 218, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Osmolovskaya, N.; Shumilina, J.; Kim, A.; Didio, A.; Grishina, T.; Bilova, T.; Keltsieva, O.A.; Zhukov, V.; Tikhonovich, I.; Tarakhovskaya, E.; et al. Methodology of Drought Stress Research: Experimental Setup and Physiological Characterization. Int. J. Mol. Sci. 2018, 19, 4089. [Google Scholar] [CrossRef]
- Tuteja, N. Abscisic Acid and abiotic stress signaling. Plant Signal. Behav. 2007, 2, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Barnabás, B.; Jäger, K.; Fehér, A. The effect of drought and heat stress on reproductive processes in cereals. Plant Cell Environ. 2008, 31, 11–38. [Google Scholar] [CrossRef] [PubMed]
- Shavrukov, Y.; Kurishbayev, A.; Jatayev, S.; Shvidchenko, V.; Zotova, L.; Koekemoer, F.; de Groot, S.; Soole, K.; Langridge, P. Early Flowering as a Drought Escape Mechanism in Plants: How Can It Aid Wheat Production? Front. Plant Sci. 2017, 8, 1950. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, A.; Sita, K.; Siddique, K.H.M.; Kumar, R.; Bhogireddy, S.; Varshney, R.K.; HanumanthaRao, B.; Nair, R.M.; Prasad, P.V.V.; Nayyar, H. Drought or/and Heat-Stress Effects on Seed Filling in Food Crops: Impacts on Functional Biochemistry, Seed Yields, and Nutritional Quality. Front. Plant Sci. 2018, 9, 1705. [Google Scholar] [CrossRef]
- Ahkami, A.H.; Wang, W.; Wietsma, T.W.; Winkler, T.; Lange, I.; Jansson, C.; Lange, B.M.; McDowell, N.G. Metabolic shifts associated with drought-induced senescence in Brachypodium. Plant Sci. 2019, 289, 110278. [Google Scholar] [CrossRef]
- Shumilina, J.; Gorbach, D.; Popova, V.; Tsarev, A.; Kuznetsova, A.; Grashina, M.; Dorn, M.; Lukasheva, E.; Osmolovskaya, N.; Romanovskaya, E.; et al. Protein glycation and drought response of pea root nodule proteome: A proteomics approach. Biol. Commun. 2021, 66, 210–224. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef]
- Silveira, N.M.; Marcos, F.C.C.; Frungillo, L.; Moura, B.B.; Seabra, A.B.; Salgado, I.; Machado, E.C.; Hancock, J.T.; Ribeiro, R.V. S-nitrosoglutathione spraying improves stomatal conductance, Rubisco activity and antioxidant defense in both leaves and roots of sugarcane plants under water deficit. Physiol. Plant. 2017, 160, 383–395. [Google Scholar] [CrossRef]
- Foyer, C.H. Reactive oxygen species, oxidative signaling and the regulation of photosynthesis. Environ. Exp. Bot. 2018, 154, 134–142. [Google Scholar] [CrossRef]
- Shumilina, J.; Kusnetsova, A.; Tsarev, A.; Janse van Rensburg, H.C.; Medvedev, S.; Demidchik, V.; Van den Ende, W.; Frolov, A. Glycation of Plant Proteins: Regulatory Roles and Interplay with Sugar Signalling? Int. J. Mol. Sci. 2019, 20, 2366. [Google Scholar] [CrossRef] [PubMed]
- Vistoli, G.; De Maddis, D.; Cipak, A.; Zarkovic, N.; Carini, M.; Aldini, G. Advanced glycoxidation and lipoxidation end products (AGEs and ALEs): An overview of their mechanisms of formation. Free Radic. Res. 2013, 47 (Suppl. S1), 3–27. [Google Scholar] [CrossRef]
- Poulsen, M.W.; Hedegaard, R.V.; Andersen, J.M.; de Courten, B.; Bügel, S.; Nielsen, J.; Skibsted, L.H.; Dragsted, L.O. Advanced glycation endproducts in food and their effects on health. Food Chem. Toxicol. 2013, 60, 10–37. [Google Scholar] [CrossRef] [PubMed]
- Soboleva, A.; Frolova, N.; Bureiko, K.; Shumilina, J.; Balcke, G.U.; Zhukov, V.A.; Tikhonovich, I.A.; Frolov, A. Dynamics of Reactive Carbonyl Species in Pea Root Nodules in Response to Polyethylene Glycol (PEG)-Induced Osmotic Stress. Int. J. Mol. Sci. 2022, 23, 2726. [Google Scholar] [CrossRef]
- Leonova, T.; Popova, V.; Tsarev, A.; Henning, C.; Antonova, K.; Rogovskaya, N.; Vikhnina, M.; Baldensperger, T.; Soboleva, A.; Dinastia, E.; et al. Does Protein Glycation Impact on the Drought-Related Changes in Metabolism and Nutritional Properties of Mature Pea (Pisum sativum L.) Seeds? Int. J. Mol. Sci. 2020, 21, 567. [Google Scholar] [CrossRef] [PubMed]
- Lounifi, I.; Arc, E.; Molassiotis, A.; Job, D.; Rajjou, L.; Tanou, G. Interplay between protein carbonylation and nitrosylation in plants. Proteomics 2013, 13, 568–578. [Google Scholar] [CrossRef]
- Mandal, M.; Sarkar, M.; Khan, A.; Biswas, M.; Masi, A.; Rakwal, R.; Agrawal, G.K.; Srivastava, A.; Sarkar, A. Reactive Oxygen Species (ROS) and Reactive Nitrogen Species (RNS) in plants–maintenance of structural individuality and functional blend. Adv. Redox Res. 2022, 5, 100039. [Google Scholar] [CrossRef]
- Poteser, M.; Romanin, C.; Schreibmayer, W.; Mayer, B.; Groschner, K. S-Nitrosation Controls Gating and Conductance of the α1 Subunit of Class C L-type Ca2+ Channels. J. Biol. Chem. 2001, 276, 14797–14803. [Google Scholar] [CrossRef]
- Sanz, L.; Albertos, P.; Mateos, I.; Sánchez-Vicente, I.; Lechón, T.; Fernández-Marcos, M.; Lorenzo, O. Nitric oxide (NO) and phytohormones crosstalk during early plant development. J. Exp. Bot. 2015, 66, 2857–2868. [Google Scholar] [CrossRef]
- Xu, Y.; Burgess, P.; Zhang, X.; Huang, B. Enhancing cytokinin synthesis by overexpressing ipt alleviated drought inhibition of root growth through activating ROS-scavenging systems in Agrostis stolonifera. J. Exp. Bot. 2016, 67, 1979–1992. [Google Scholar] [CrossRef]
- Karky, R.; Perry, M. Disharmonization in the Regulation of Transgenic Plants in Europe. Biotechnol. Law Rep. 2019, 38, 350–375. [Google Scholar] [CrossRef]
- Turnbull, C.; Lillemo, M.; Hvoslef-Eide, T.A.K. Global Regulation of Genetically Modified Crops Amid the Gene Edited Crop Boom—A Review. Front. Plant Sci. 2021, 12, 630396. [Google Scholar] [CrossRef]
- De Block, M.; Verduyn, C.; De Brouwer, D.; Cornelissen, M. Poly(ADP-ribose) polymerase in plants affects energy homeostasis, cell death and stress tolerance. Plant J. 2005, 41, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Geissler, T.; Wessjohann, L.A. A Whole-Plant Microtiter Plate Assay for Drought Stress Tolerance-Inducing Effects. J. Plant Growth Regul. 2011, 30, 504–511. [Google Scholar] [CrossRef]
- Saddhe, A.A.; Malvankar, M.R.; Karle, S.B.; Kumar, K. Reactive nitrogen species: Paradigms of cellular signaling and regulation of salt stress in plants. Environ. Exp. Bot. 2019, 161, 86–97. [Google Scholar] [CrossRef]
- Ederli, L.; Reale, L.; Madeo, L.; Ferranti, F.; Gehring, C.; Fornaciari, M.; Romano, B.; Pasqualini, S. NO release by nitric oxide donors in vitro and in planta. Plant Physiol. Biochem. 2009, 47, 42–48. [Google Scholar] [CrossRef]
- Murgia, I.; Concetta de Pinto, M.; Delledonne, M.; Soave, C.; De Gara, L. Comparative effects of various nitric oxide donors on ferritin regulation, programmed cell death, and cell redox state in plant cells. J. Plant Physiol. 2004, 161, 777–783. [Google Scholar] [CrossRef]
- Ziogas, V.; Tanou, G.; Belghazi, M.; Filippou, P.; Fotopoulos, V.; Grigorios, D.; Molassiotis, A. Roles of sodium hydrosulfide and sodium nitroprusside as priming molecules during drought acclimation in citrus plants. Plant Mol. Biol. 2015, 89, 433–450. [Google Scholar] [CrossRef]
- Fershtat, L.L.; Zhilin, E.S. Recent Advances in the Synthesis and Biomedical Applications of Heterocyclic NO-Donors. Molecules 2021, 26, 5705. [Google Scholar] [CrossRef]
- Cherepanov, I.A.; Moiseev, S.K. Chapter Two—Recent developments in the chemistry of sydnones and sydnone imines. Adv. Heterocycl. Chem. 2020, 131, 49–164. [Google Scholar] [CrossRef]
- Ol’shevskaya, V.; Cherepanov, I.; Spiridonov YuA, S.G.; Makarenkov, A.; Samarskaya, A. Herbicidal activity of carboranes, sydnone imine and ferrocene derivatives. Agrokhimiya 2017, 4, 16–21. [Google Scholar]
- Francisc, P.; Ioana, I. Anthropogenic Air Pollution Sources. In Air Quality; IntechOpen: London, UK, 2010. [Google Scholar] [CrossRef]
- Locey, B.J. Nitrites. In Encyclopedia of Toxicology, 2nd ed.; Wexler, P., Ed.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 232–235. [Google Scholar]
- Hancock, J.T. Nitric Oxide Signaling in Plants. Plants 2020, 9, 1550. [Google Scholar] [CrossRef] [PubMed]
- Baudouin, E.; Hancock, J.T. Nitric oxide signaling in plants. Front. Plant Sci. 2013, 4, 553. [Google Scholar] [CrossRef] [PubMed]
- Lokhande, P.D.; Kuchekar, B.; Chabukswar, A.; Jagdale, S. Nitric Oxide: Role in Biological System. Asian J. Biochem. 2006, 1, 1–17. [Google Scholar] [CrossRef]
- Francis, S.H.; Busch, J.L.; Corbin, J.D. cGMP-Dependent Protein Kinases and cGMP Phosphodiesterases in Nitric Oxide and cGMP Action. Pharmacol. Rev. 2010, 62, 525–563. [Google Scholar] [CrossRef] [PubMed]
- Murad, F.; Mittal, C.K.; Arnold, W.P.; Katsuki, S.; Kimura, H. Guanylate cyclase: Activation by azide, nitro compounds, nitric oxide, and hydroxyl radical and inhibition by hemoglobin and myoglobin. Adv. Cyclic Nucleotide Res. 1978, 9, 145–158. [Google Scholar]
- Bohme, E.; Spies, C.; Grossmann, G.; Herz, J. Stimulation of soluble guanylate cyclase by sydnone imines; relaxants of smooth muscle and inhibitors of platelet aggregation. Naunyn Schmiedebergs Arch. Pharmacol. [Suppl.] 1981, 316, R26. [Google Scholar]
- Furchgott, R.F.; Zawadzki, J.V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 1980, 288, 373–376. [Google Scholar] [CrossRef]
- Palmer, R.M.; Ferrige, A.G.; Moncada, S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature 1987, 327, 524–526. [Google Scholar] [CrossRef]
- Delledonne, M.; Xia, Y.; Dixon, R.A.; Lamb, C. Nitric oxide functions as a signal in plant disease resistance. Nature 1998, 394, 585–588. [Google Scholar] [CrossRef]
- Durner, J.; Wendehenne, D.; Klessig, D.F. Defense gene induction in tobacco by nitric oxide, cyclic GMP, and cyclic ADP-ribose. Proc. Natl. Acad. Sci. USA 1998, 95, 10328–10333. [Google Scholar] [CrossRef]
- Rasheed, R.; Ashraf, M.A.; Ali, S.; Iqbal, M.; Zafar, S.; Akbar, A.; Banik, A. Role of NO in plants: A current update. In Nitric Oxide in Plant Biology; Academic Press: Cambridge, MA, USA, 2022; pp. 139–168. [Google Scholar] [CrossRef]
- Leshem, Y.A.Y.; Haramaty, E. The Characterization and Contrasting Effects of the Nitric Oxide Free Radical in Vegetative Stress and Senescence of Pisum sativum Linn. Foliage. J. Plant Physiol. 1996, 148, 258–263. [Google Scholar] [CrossRef]
- Mur, L.A.J.; Mandon, J.; Persijn, S.; Cristescu, S.M.; Moshkov, I.E.; Novikova, G.V.; Hall, M.A.; Harren, F.J.M.; Hebelstrup, K.H.; Gupta, K.J. Nitric oxide in plants: An assessment of the current state of knowledge. AoB Plants 2013, 5, pls052. [Google Scholar] [CrossRef]
- Hiscock, S.; Bright, J.; McInnis, S.M.; Desikan, R.; Hancock, J.T. Signaling on the Stigma. Plant Signal. Behav. 2007, 2, 23–24. [Google Scholar] [CrossRef]
- Reichler, S.A.; Torres, J.; Rivera, A.L.; Cintolesi, V.A.; Clark, G.; Roux, S.J. Intersection of two signalling pathways: Extracellular nucleotides regulate pollen germination and pollen tube growth via nitric oxide. J. Exp. Bot. 2009, 60, 2129–2138. [Google Scholar] [CrossRef] [PubMed]
- Šírová, J.; Sedlářová, M.; Piterková, J.; Luhová, L.; Petřivalský, M. The role of nitric oxide in the germination of plant seeds and pollen. Plant Sci. 2011, 181, 560–572. [Google Scholar] [CrossRef] [PubMed]
- Arc, E.; Galland, M.; Godin, B.; Cueff, G.; Rajjou, L. Nitric oxide implication in the control of seed dormancy and germination. Front. Plant Sci. 2013, 4, 346. [Google Scholar] [CrossRef]
- Correa-Aragunde, N.; Graziano, M.; Lamattina, L. Nitric oxide plays a central role in determining lateral root development in tomato. Planta 2004, 218, 900–905. [Google Scholar] [CrossRef]
- Lombardo, M.C.; Graziano, M.; Polacco, J.C.; Lamattina, L. Nitric oxide functions as a positive regulator of root hair development. Plant Signal. Behav. 2006, 1, 28–33. [Google Scholar] [CrossRef]
- Neill, S.; Barros, R.; Bright, J.; Desikan, R.; Hancock, J.; Harrison, J.; Morris, P.; Ribeiro, D.; Wilson, I. Nitric oxide, stomatal closure, and abiotic stress. J. Exp. Bot. 2008, 59, 165–176. [Google Scholar] [CrossRef]
- Gayatri, G.; Agurla, S.; Raghavendra, A.S. Nitric oxide in guard cells as an important secondary messenger during stomatal closure. Front. Plant Sci. 2013, 4, 425. [Google Scholar] [CrossRef] [PubMed]
- Fancy, N.N.; Bahlmann, A.K.; Loake, G.J. Nitric oxide function in plant abiotic stress. Plant Cell Environ. 2017, 40, 462–472. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Hasanuzzaman, M.; Nahar, K.; Macovei, A.; Tuteja, N. Importance of nitric oxide in cadmium stress tolerance in crop plants. Plant Physiol. Biochem. 2013, 63, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Tailor, A.; Tandon, R.; Bhatla, S.C. Nitric oxide modulates polyamine homeostasis in sunflower seedling cotyledons under salt stress. Plant Signal. Behav. 2019, 14, 1667730. [Google Scholar] [CrossRef]
- Rai, K.K.; Pandey, N.; Rai, S.P. Salicylic acid and nitric oxide signaling in plant heat stress. Physiol. Plant. 2020, 168, 241–255. [Google Scholar] [CrossRef]
- Lytvyn, D.I.; Raynaud, C.; Yemets, A.I.; Bergounioux, C.; Blume, Y.B. Involvement of Inositol Biosynthesis and Nitric Oxide in the Mediation of UV-B Induced Oxidative Stress. Front. Plant Sci. 2016, 7, 430. [Google Scholar] [CrossRef]
- Mur, L.A.J.; Simpson, C.; Kumari, A.; Gupta, A.K.; Gupta, K.J. Moving nitrogen to the centre of plant defence against pathogens. Ann. Bot. 2017, 119, 703–709. [Google Scholar] [CrossRef]
- Crawford, N.M. Mechanisms for nitric oxide synthesis in plants. J. Exp. Bot. 2006, 57, 471–478. [Google Scholar] [CrossRef]
- Bethke, P.C.; Badger, M.R.; Jones, R.L. Apoplastic synthesis of nitric oxide by plant tissues. Plant Cell 2004, 16, 332–341. [Google Scholar] [CrossRef]
- Campbell, W.H. Structure and function of eukaryotic NAD(P)H:nitrate reductase. Cell. Mol. Life Sci. 2001, 58, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Cueto, M.; Hernández-Perera, O.; Martín, R.; Bentura, M.L.; Rodrigo, J.; Lamas, S.; Golvano, M.P. Presence of nitric oxide synthase activity in roots and nodules of Lupinus albus. FEBS Lett. 1996, 398, 159–164. [Google Scholar] [CrossRef]
- Tun, N.N.; Santa-Catarina, C.; Begum, T.; Silveira, V.; Handro, W.; Floh, E.I.S.; Scherer, G.F.E. Polyamines Induce Rapid Biosynthesis of Nitric Oxide (NO) in Arabidopsis thaliana Seedlings. Plant Cell Physiol. 2006, 47, 346–354. [Google Scholar] [CrossRef]
- García-Mata, C.; Lamattina, L. Nitric oxide induces stomatal closure and enhances the adaptive plant responses against drought stress. Plant Physiol. 2001, 126, 1196–1204. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Ma, W.; Wei, X.; Long, Y.; Zhao, Y.; Su, M.; Luo, Q. Identification of Exogenous Nitric Oxide-Responsive miRNAs from Alfalfa (Medicago sativa L.) under Drought Stress by High-Throughput Sequencing. Genes 2019, 11, 30. [Google Scholar] [CrossRef] [PubMed]
- Santisree, P.; Bhatnagar-Mathur, P.; Sharma, K.K. NO to drought-multifunctional role of nitric oxide in plant drought: Do we have all the answers? Plant Sci. 2015, 239, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Khmelnitskaya, E.Y.; Levina, V.I.; Trukhacheva, L.A.; Grigoriev, N.B.; Kalinin, V.N.; Cherepanov, I.A.; Lebedev, S.N.; Granik, V.G. Sydnonimines as exogenous NO donors. Russ. Chem. Bull. 2004, 53, 2840–2844. [Google Scholar] [CrossRef]
- Schönafinger, K. Heterocyclic NO prodrugs. Il Farm. 1999, 54, 316–320. [Google Scholar] [CrossRef]
- Kankaanranta, H.; Knowles, R.G.; Vuorinen, P.; Kosonen, O.; Holm, P.; Moilanen, E. 3-Morpholino-Sydnonimine-Induced Suppression of Human Neutrophil Degranulation is Not Mediated by Cyclic GMP, Nitric Oxide or Peroxynitrite: Inhibition of the Increase in Intracellular Free Calcium Concentration by N-Morpholino-iminoacetonitrile, a Metabolite of 3-Morpholino-Sydnonimine. Mol. Pharmacol. 1997, 51, 882–888. [Google Scholar] [CrossRef]
- Yashunskii, V.G.; Kholodov, L. The chemistry of sydnone imines. Russ. Chem. Rev. 1980, 49, 28. [Google Scholar] [CrossRef]
- Newton, C.G.; Ramsden, C.A. Meso-ionic heterocycles (1976–1980). Tetrahedron 1982, 38, 2965–3011. [Google Scholar] [CrossRef]
- Rademacher, W. Plant Growth Regulators: Backgrounds and Uses in Plant Production. Russ. Chem. Rev. 2015, 34, 845–872. [Google Scholar] [CrossRef]
- Ingelheim, B.S. Sydnonimine Derivatives. GB 1219254A. 1971. Available online: https://typeset.io/papers/3-morpholino-n6-cyclohexylcarbonyl-sydnonimine-1dge30ms1i (accessed on 3 January 2023).
- Katsutada, M.; Yoshio, I. Sydnonimine Derivatives. US3312690. 1967. Available online: https://worldwide.espacenet.com/patent/search/family/026370876/publication/US3312690A?q=pn%3DUS3312690A (accessed on 3 January 2023).
- Hidaka, H.; Matsumoto, I.; Yoshizawa, J.; Kotani, S. Syndonimine Derivatives, Process for Production Thereof, and Use Thereof. US4421754A. 1983. Available online: https://worldwide.espacenet.com/patent/search/family/015061006/publication/US4421754A?q=pn%3DUS4421754A (accessed on 3 January 2023).
- Provost, P.; Tremblay, J.; Merhi, Y. The antiadhesive and antithrombotic effects of the nitric oxide donor SIN-1 are combined with a decreased vasoconstriction in a porcine model of balloon angioplasty. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 1806–1812. [Google Scholar] [CrossRef] [PubMed]
- Gerzer, R.; Karrenbrock, B.; Siess, W.; Heim, J.M. Direct comparison of the effects of nitroprusside, SIN 1, and various nitrates on platelet aggregation and soluble guanylate cyclase activity. Thromb. Res. 1988, 52, 11–21. [Google Scholar] [CrossRef] [PubMed]
- D’iakonova, T.L.; Samonina, G.E. The naloxone-dependent effects of the psychostimulant sidnofen: A study on identified neurons of the snail. Zhurnal Vyss. Nervn. Deiatelnosti Im. IP Pavlov. 1994, 44, 786–795. [Google Scholar]
- Mashkovsky, M.D.; Yashunsky, V.G.; Altshuller, R.A.; Knolodov, L.E.; Avrutsky, G.Y.; Alexandrovsky, J.A.; Smulevich, A.B. Novel Sydnonimine Derivative. GB1262830A. 1972. Available online: https://worldwide.espacenet.com/patent/search/family/010270292/publication/GB1262830A?q=GB1262830A (accessed on 3 January 2023).
- Rosenkranz, B.; Winkelmann, B.R.; Parnham, M.J. Clinical Pharmacokinetics of Molsidomine. Clin. Pharmacokinet. 1996, 30, 372–384. [Google Scholar] [CrossRef] [PubMed]
- Baker, W.; Ollis, W.D. Meso-ionic compounds. Q. Rev. Chem. Soc. 1957, 11, 15. [Google Scholar] [CrossRef]
- Cherepanov, I.A.; Spiridonov, Y.Y.; Chichvarina, O.A.; Samarskaya, A.S.; Ponomaryov, A.B.; Moiseev, S.K. Growth Stimulating Activity of Sydnonimine Derivatives. Agrochemistry 2018, 9, 50–55. [Google Scholar]
- Cherepanov, I.A.; Shevaldina, E.V.; Lapshin, D.A.; Spiridonov, Y.Y.; Abubikerov, V.A.; Moiseev, S.K. 4-lithiosydnone imines: Generation and stability. Plant growth regulating activity of 4-hydroxymethyl derivatives of sydnone imines. J. Organomet. Chem. 2021, 943, 121841. [Google Scholar] [CrossRef]
- Cherepanov, I.A.; Spiridonov, U.Y.; Abubikerov, V.A.; Spiridonova, I.U.; Kalganova, N.V.; Lapshin, D.A.; Moiseev, S.K. Sydnone Imine Based Herbicide Antidotes. Agrochemistry 2022, 4, 36–45. [Google Scholar]
- Cherepanov, I.A.; Spiridonov, Y.Y.; Moiseev, S.K.; Frolova, N.G. Substituted Sydnone Imines as Herbicides Antidotes, Russia. RU2772224C1. 2022. Available online: https://patents.google.com/patent/RU2772224C1/en (accessed on 3 January 2023).
- Lukatkin, A.S.; Golovatskaya, A.S.; Cherepanov, I.A.; Moiseev, S.K. Evaluation of the potential use of a new class of regulators to stimulate early growth of wheat. In Proceedings of the II Republican Scientific Conference “Adaptation of Living Organisms to Changing Environmental Conditions”, Dushanbe, Tajikistan, 24 September 2021; pp. 21–25. [Google Scholar]
- Guryanova, A.S.; Lukatkin, A.S.; Galkina, A.A.; Kalganova, N.V.; Cherepanov, I.A.; Moiseev, S.K. Protective effect of oxadiazolonium derivatives on growth and temperature stress markers in Triticum aestivum L. and Zea mays L. plants. In Proceedings of the III International Scientific Conference “Cell Biology and Plant Biotechnology”, Minsk, Belarus, 24–27 May 2022; p. 90. [Google Scholar]
- Galkina, A.A.; Ignatieva, O.V.; Lukatkin, A.S.; Kalganova, N.V. The effectiveness of oxadiazolonium derivatives under the effect of temperature stress on corn plants. In Proceedings of the XVII All-Russian Scientific and Practical Conference “Ecology of the native land: Problems and solutions”, Kirov, Russia, 26–27 April 2022; pp. 228–231. [Google Scholar]
- Sokolova, A.S.; Ivanov, B.D. Modification of canola adaptive responses to stress by growth regulators. In Proceedings of the III International Scientific and Practical Conference “Cell Biology and Plant Biotechnology”, Minsk, Belarus, 24–27 May 2022; p. 103. [Google Scholar]
- Lukatkin, A.S.; Sokolova, A.S.; Lukatkin, A.A.; Cherepanov, I.A.; Kalganova, N.V.; Moiseev, S.K. Sydnone Imines: A Novel Class of Plant Growth Regulators. Agrochemicals 2023, 2, 203–219. [Google Scholar] [CrossRef]













Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cherevatskaya, M.; Cherepanov, I.; Kalganova, N.; Erofeeva, N.; Romanovskaya, E.; Frolov, A.; Bilova, T.; Moiseev, S.; Wessjohann, L.A. Sydnone Imines as a New Class of Promising Plant Growth and Stress Tolerance Modulators—A First Experimental Structure–Activity Overview. Stresses 2024, 4, 133-154. https://doi.org/10.3390/stresses4010008
Cherevatskaya M, Cherepanov I, Kalganova N, Erofeeva N, Romanovskaya E, Frolov A, Bilova T, Moiseev S, Wessjohann LA. Sydnone Imines as a New Class of Promising Plant Growth and Stress Tolerance Modulators—A First Experimental Structure–Activity Overview. Stresses. 2024; 4(1):133-154. https://doi.org/10.3390/stresses4010008
Chicago/Turabian StyleCherevatskaya, Maria, Ilia Cherepanov, Natalia Kalganova, Natalia Erofeeva, Ekaterina Romanovskaya, Andrej Frolov, Tatiana Bilova, Sergey Moiseev, and Ludger A. Wessjohann. 2024. "Sydnone Imines as a New Class of Promising Plant Growth and Stress Tolerance Modulators—A First Experimental Structure–Activity Overview" Stresses 4, no. 1: 133-154. https://doi.org/10.3390/stresses4010008
APA StyleCherevatskaya, M., Cherepanov, I., Kalganova, N., Erofeeva, N., Romanovskaya, E., Frolov, A., Bilova, T., Moiseev, S., & Wessjohann, L. A. (2024). Sydnone Imines as a New Class of Promising Plant Growth and Stress Tolerance Modulators—A First Experimental Structure–Activity Overview. Stresses, 4(1), 133-154. https://doi.org/10.3390/stresses4010008






