Gibberellin Inhibitors Molecules as a Safeguard against Secondary Growth in Garlic Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. First Experiment: Exploratory Analysis under Controlled Conditions
Plant Biometry and Secondary Growth in Garlic
2.2. Growing Season of 2020: Confirmatory Analysis in the Field
2.2.1. Experimental Environment and Treatments
2.2.2. Plant Biometry
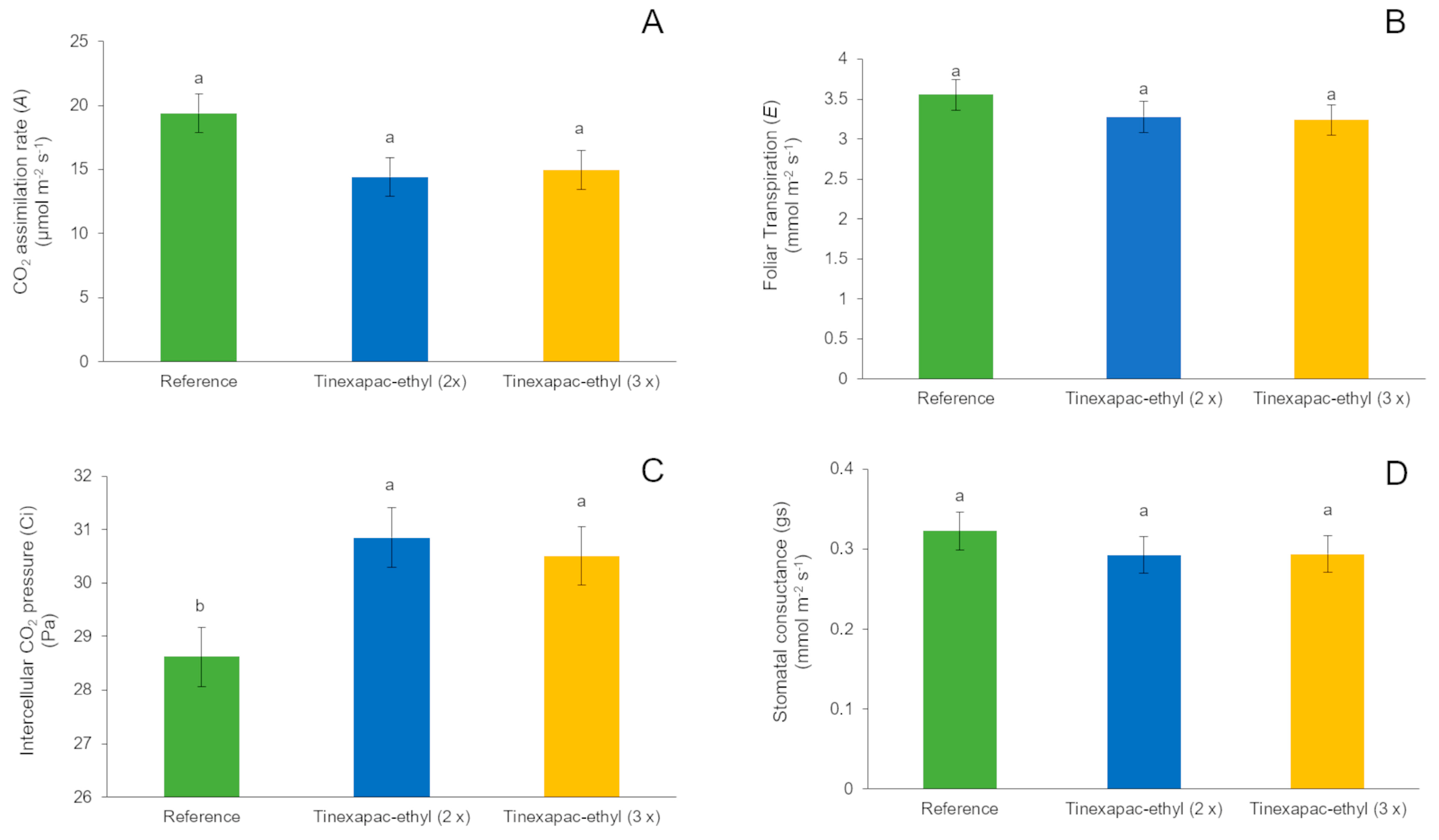
2.2.3. Gas Exchange in Garlic Leaves
2.2.4. Total Soluble Protein Content and the Activities of Antioxidant Enzymes
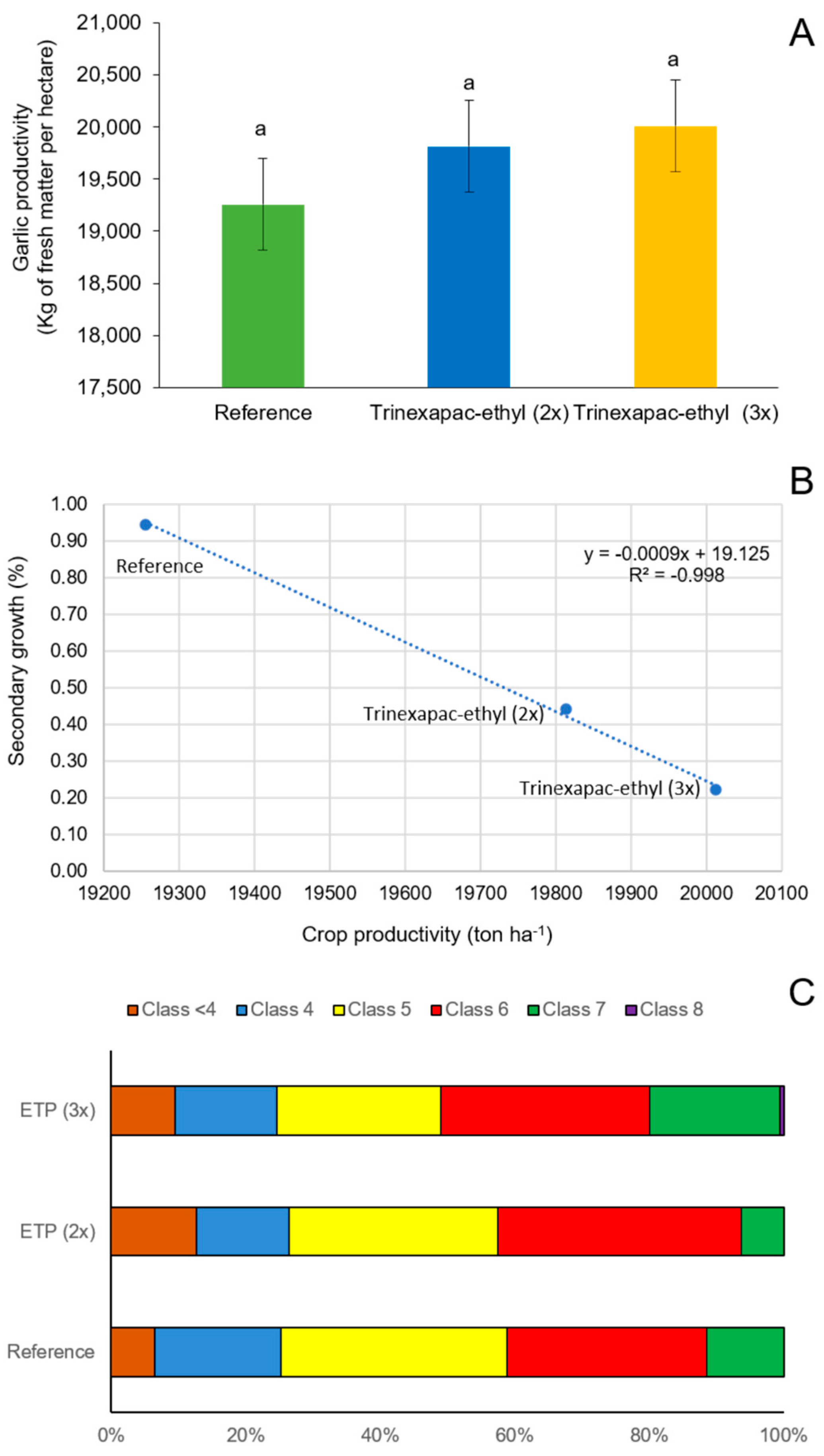
2.2.5. Secondary Growth Index and Garlic Production Parameters
2.3. Growing Season of 2023: Confirmatory Analysis in the Field
2.3.1. Experimental Environment and Treatments
2.3.2. Gas Exchange in Garlic Leaves
2.3.3. Plant Biometry
2.3.4. Total Soluble Protein Content and the Activities of Antioxidant Enzymes
2.3.5. Secondary Growth Index and Garlic Production Parameters
2.4. Statistical Analyses
3. Results
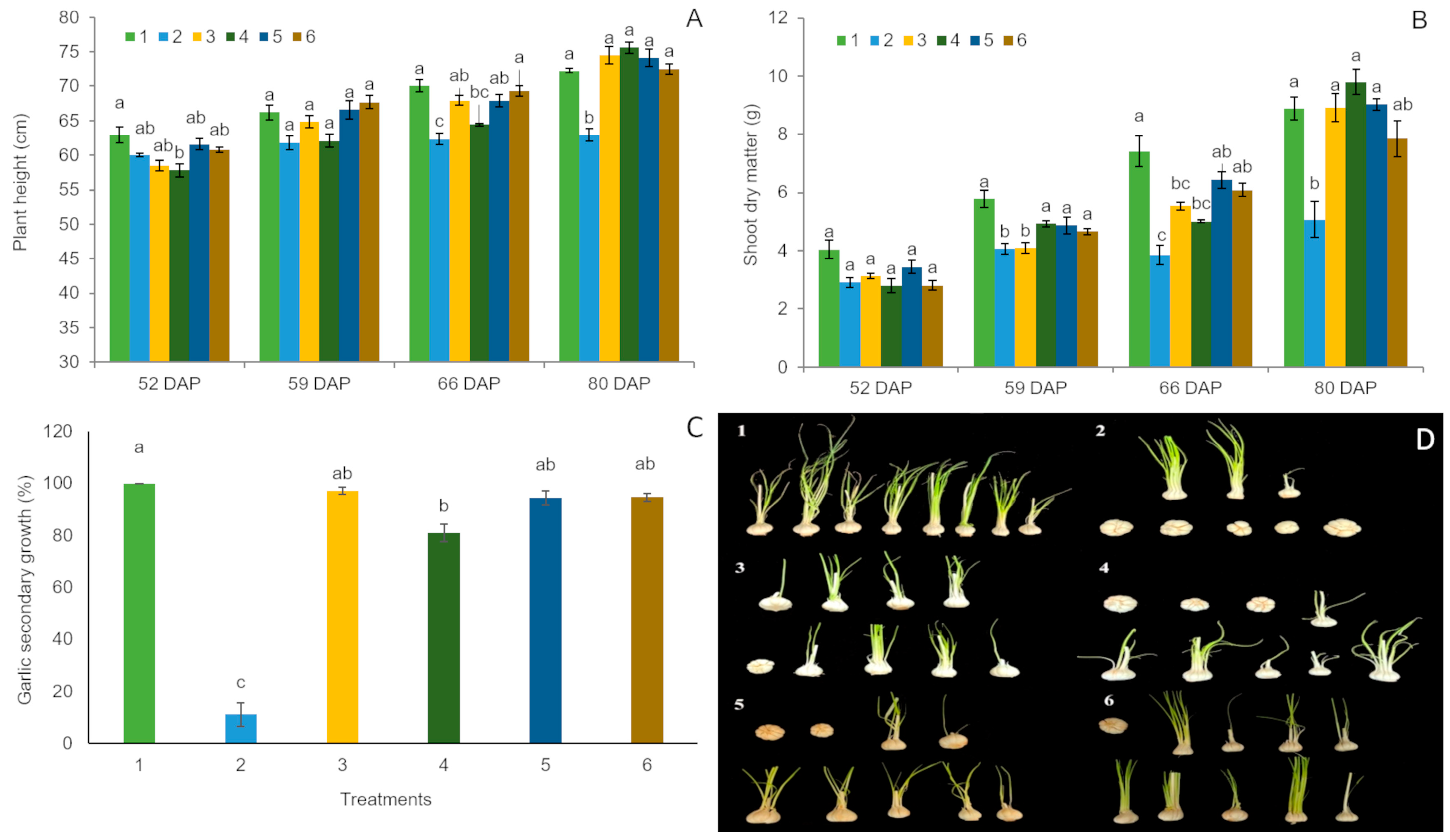
3.1. Exploratory Experiments
3.2. Confirmatory Experiments
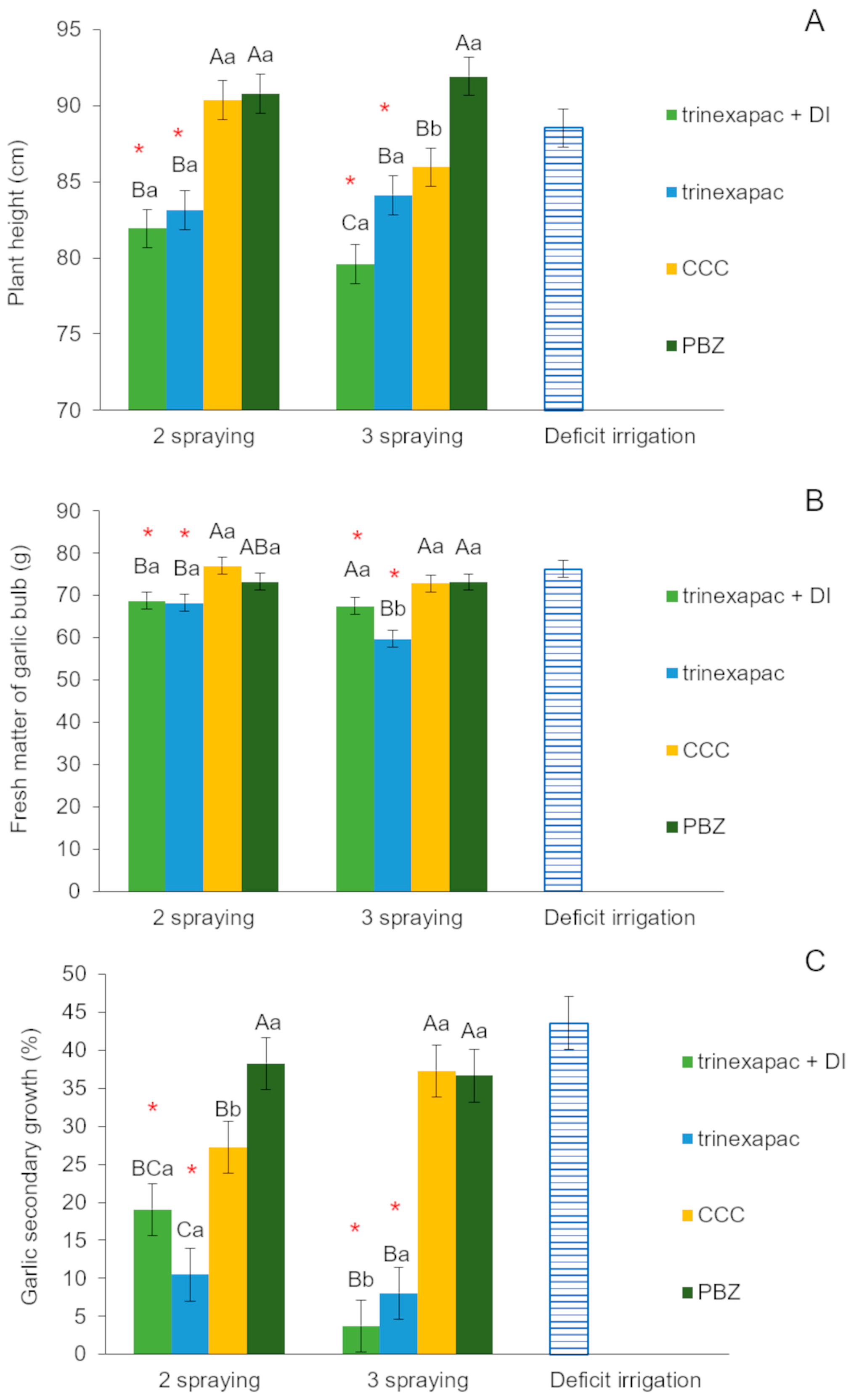
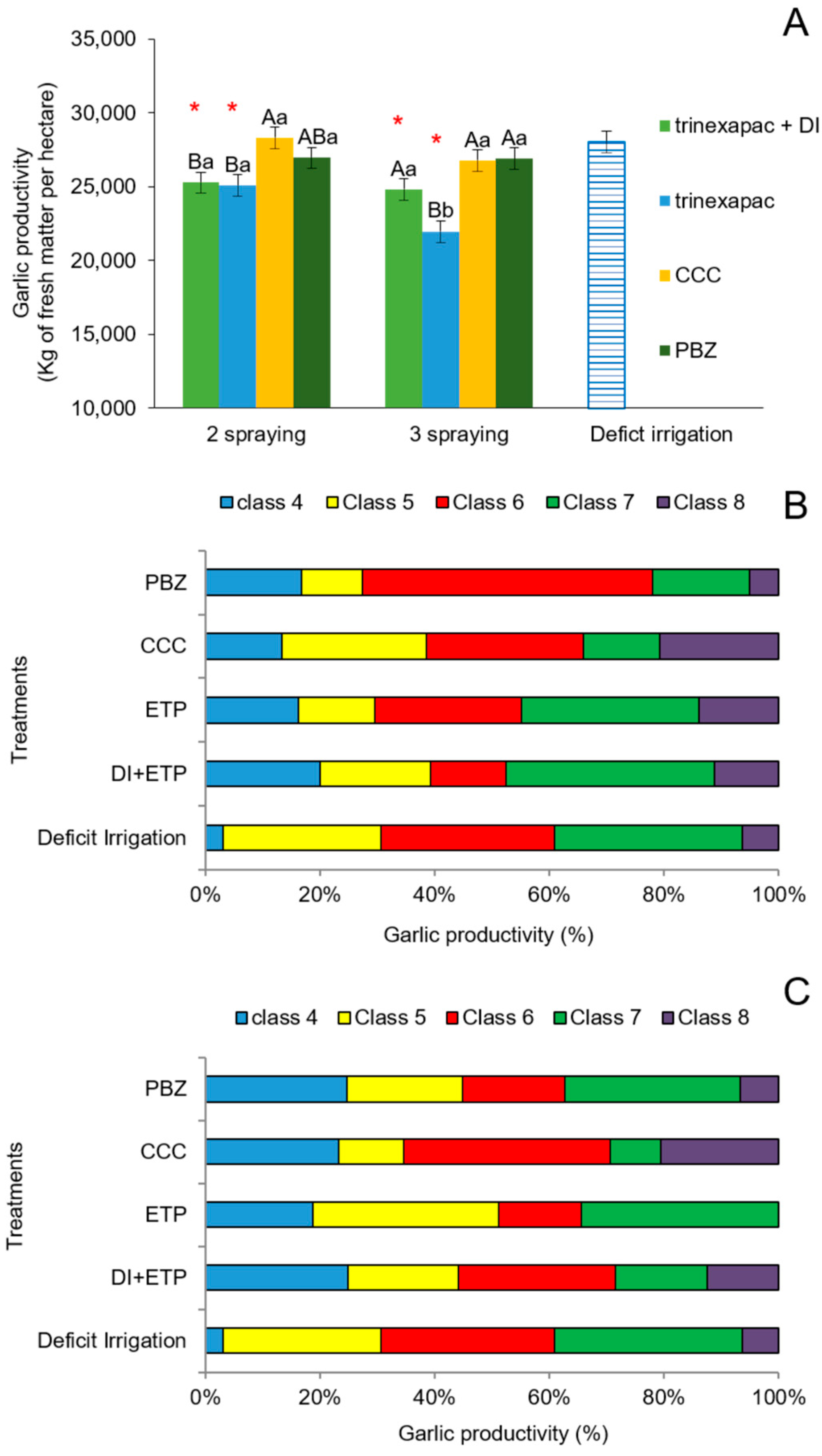
3.2.1. First Field Experiment (2020 Growing Season)
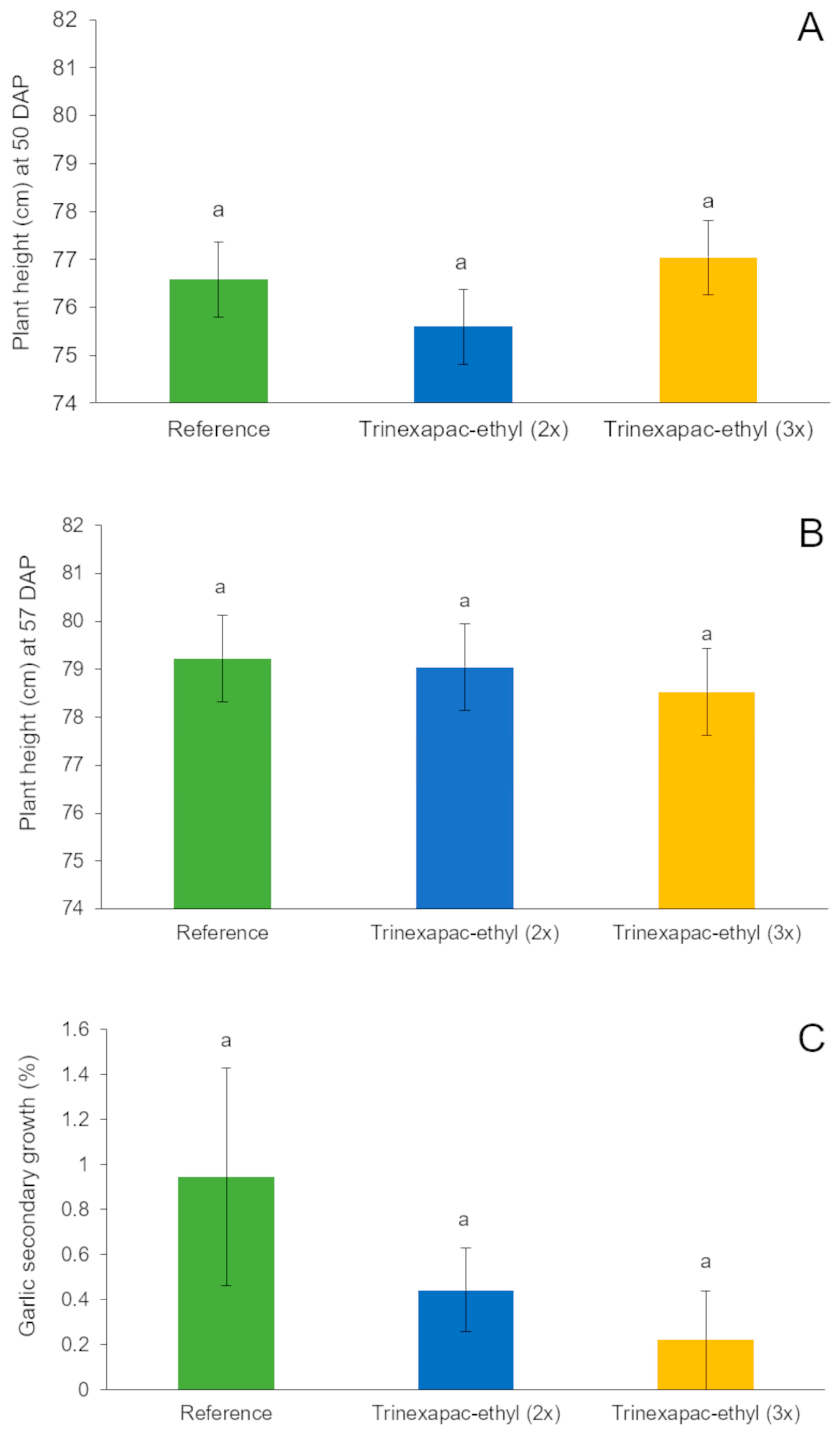
3.2.2. Second Field Experiment (2023 Growing Season)
4. Discussion
4.1. First Experiment
4.2. Field Experiments (2020 and 2023 Growing Seasons)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dufoo-Hurtado, M.D.; Huerta-Ocampo, J.Á.; Barrera-Pacheco, A.; de la Rosa, A.P.; Mercado-Silva, E.M. Low Temperature Conditioning of Garlic (Allium sativum L.) “Seed” Cloves Induces Alterations in Sprouts Proteome. Front. Plant Sci. 2015, 6, 332. [Google Scholar] [CrossRef] [PubMed]
- de Resende, J.T.V.; Morales, R.G.F.; Resende, F.V.; Faria, M.V.; de Souza, R.J.; Marchese, A. Garlic Vernalization and Planting Dates in Guarapuava. Hortic. Bras. 2011, 29, 193–198. [Google Scholar]
- Macêdo, F.S.; Sedoguchi, É.T.; de Souza, R.J.; de Carvalho, J.G. Produtividade de Alho Vernalizado Em Função de Fontes e Doses de Fósforo. Cienc. Rural 2011, 41, 379–383. [Google Scholar] [CrossRef][Green Version]
- Bull, L.T.; Bertani, R.M.A.; Bôas, R.L.; Fernandes, D.M. Produção de Bulbos e Incidência de Pseudoperfilhamento Na Cultura Do Alho Vernalizado Em Função de Adubações Potássicas e Nitrogenadas. Bragantia 2002, 61, 247–255. [Google Scholar] [CrossRef]
- de Souza, R.J.; Casali, V.W.D. Pseudoperfilhamento: Uma Anormalidade Genético-Fisiológica Em Alho. Informe Agropecuário 1986, 12, 36–41. [Google Scholar]
- Resende, G.M.; Souza, R.J. Doses e Épocas de Aplicação de Nitrogênio Sobre a Produtividade e Características Comerciais de Alho. Hortic. Bras. 2001, 19, 126–129. [Google Scholar] [CrossRef]
- de Oliveira, J.T.; de Oliveira, R.A.; Puiatti, M.; Teodoro, P.; Montanari, R. Spatial Analysis and Mapping of the Effect of Irrigation and Nitrogen Application on Lateral Shoot Growing of Garlic. HortScience 2020, 55, 664–665. [Google Scholar] [CrossRef]
- Macêdo, F.S.; de Souza, R.J.; Pereira, G.M. Controle de Superbrotamento e Produtividade de Alho Vernalizado Sob Estresse Hídrico. Pesqui. Agropecu. Bras. 2006, 41, 629–635. [Google Scholar] [CrossRef]
- de Assis, R.P.; de Souza, D.C.; de Andrade Júnior, V.C.; Gonçalves, A.H.; de Souza, R.J. Secondary Growth Control in Garlic With Post Emergency Herbicides. J. Agric. Sci. 2019, 11, 67. [Google Scholar] [CrossRef]
- Wani, S.H.; Kumar, V.; Shriram, V.; Sah, S.K. Phytohormones and Their Metabolic Engineering for Abiotic Stress Tolerance in Crop Plants. Crop J. 2016, 4, 162–176. [Google Scholar] [CrossRef]
- Gabriel, A.; Resende, J.T.V.; Marodin, J.C.; de Matos, R.; Zeist, A.R.; Maciel, C.D.G. Estresse Químico Reduz Pseudoperfilhamento No Alho Vernalizado. Hortic. Bras. 2020, 38, 41–46. [Google Scholar] [CrossRef]
- Tadila, G.; Nigusie, D. Effect of Manure and Nitrogen Rates on Growth and Yield of Garlic (Allium sativum L.) at Haramaya, Eastern Ethiopia. J. Hortic. For. 2018, 10, 135–142. [Google Scholar] [CrossRef]
- Guo, H.Y.; Fan, Z.C. Effect of Soil Water Stress on Photosynthesis of Allium sativum L. Acta Agric. Sin. 1999, 26, 404–405. [Google Scholar]
- Zhou, X.; Condori-Apfata, J.A.; Liu, X.; Condori-Pacsi, S.J.; Valencia, M.V.; Zhang, C. Transcriptomic Changes Induced by Drought Stress in Hardneck Garlic during the Bolting/Bulbing Stage. Agronomy 2021, 11, 246. [Google Scholar] [CrossRef]
- Santos, E.A.; Correia, N.M.; Silva, J.R.M.; Velini, E.D.; Passos, A.B.R.J.; Durigan, J.C. Herbicide Detection in Groundwater in Córrego Rico-SP Watershed. Planta Daninha 2015, 33, 147–155. [Google Scholar] [CrossRef]
- Walperes, K.C.; dos Reis, M.R.; Carneiro, G.D.O.P.; Rocha, B.H.; de Carvalho Dias, R.; Melo, C.A.D.; Gonçalves, C.G. Residual de Metribuzin No Solo Sobre o Crescimento Do Alho, Cebola e Feijão. Rev. Bras. Herbic. 2015, 14, 64–72. [Google Scholar] [CrossRef]
- Rahman, M.H.; Haque, M.S.; Karim, M.A.; Ahmed, M. Effects of Gibberellic Acid (GA 3) on Breaking Dormancy in Garlic (Allium sativum L.). Int. J. Agric. Biol. 2006, 8, 63–65. [Google Scholar]
- Moon, W.; Lee, B.Y. Influences of Short Day Treatment on the Growth and Levels of Endogenous Growth Substances in Garlic Plants (Allium sativum L.). J. Korean Soc. Hortic. Sci. 1980, 21, 109–113. [Google Scholar]
- Liu, H.J.; Huang, C.; Tong, P.; Yang, X.; Cui, M.; Cheng, Z. Response of Axillary Bud Development in Garlic (Allium sativum L.) to Seed Cloves Soaked in Gibberellic Acid (GA3) Solution. J. Integr. Agric. 2020, 19, 1044–1054. [Google Scholar] [CrossRef]
- Rademacher, W. Growth Retardants: Effects on Gibberellin Bioproduction Synthesis and Other Metabolic Pathways. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2000, 51, 501–531. [Google Scholar] [CrossRef]
- Subedi, M.; Karimi, R.; Wang, Z.; Graf, R.J.; Mohr, R.M.; O’donovan, J.T.; Brandt, S.; Beres, B.L.; Canada, A.-F. Winter Cereal Responses to Dose and Application Timing of Trinexapac-Ethyl. Crop Sci. 2021, 61, 2722–2732. [Google Scholar] [CrossRef]
- Ram, M.; Kumar, S.; Chopra, S.; Kumar, M.; Gupta, R.K. Effect of Exogenous Application of Phytohormones and Fungicides on Yield, Quality Storability and Economics of Garlic (Allium sativum L.). Veg. Sci. 2018, 45, 249–253. [Google Scholar] [CrossRef]
- de Resende, G.M.; de Souza, R.J. Efeito de Doses de Paclobutrazol Na Cultura Do Alho. Pesqui. Agropecu. Bras. 2002, 37, 637–641. [Google Scholar] [CrossRef][Green Version]
- Lopez-Bellido, F.J.; Lopez-Bellido, R.J.; Muñoz-Romero, V.; Fernandez-Garcia, P.; Lopez-Bellido, L. New Phenological Growth Stages of Garlic (Allium sativum). Ann. Appl. Biol. 2016, 169, 423–439. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Giannopolitis, C.; Reis, S. Superoxide Dismutases. I. Ocurrence in Higher Plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Havir, E.; McHale, N. Biochemical and Developmental Characterization of Multiple Forms of Catalase in Tobacco Leaves. Plant Physiol. 1987, 84, 450–455. [Google Scholar] [CrossRef]
- MAPA Sistema Integrado de Legislação. Available online: http://sistemasweb.agricultura.gov.br/sislegis/action/detalhaAto.do?method=visualizarAtoPortalMapa&chave=1429352813 (accessed on 3 March 2022).
- Ferreira, D.F. Sisvar: A Computer Statistical Analysis System. Cienc. Agrotecnol. 2011, 35, 1039–1042. [Google Scholar] [CrossRef]
- Carvalho, A.M.; Mendes, F.Q.; Mendes, F.Q.; de Fátima Tavares, L. SPEED Stat: A Free, Intuitive, and Minimalist Spreadsheet Program for Statistical Analyses of Experiments. Crop Breed. Appl. Biotechnol. 2020, 20, e327420312. [Google Scholar] [CrossRef]
- Piepho, H.P.; Edmondson, R.N. A Tutorial on the Statistical Analysis of Factorial Experiments with Qualitative and Quantitative Treatment Factor Levels. J. Agron. Crop Sci. 2018, 204, 429–455. [Google Scholar] [CrossRef]
- Choi, H.-S.; Yang, E.-Y.; Chae, W.-B.; Kwack, Y.-B.; Kim, H.-L. Effect of Soil Temperature, Seedtime, and Fertilization Rate on the Secondary Growth in the Cultivation of the Big Bulbils of Namdo Garlic (Allium sativum L.). J. Bio-Environ. Control 2009, 17, 454–459. [Google Scholar]
- Akbari, S.; Kafi, M.; Beidokhti, S.R. Effect of Drought Stress on Growth and Morphological Characteristics of Two Garlic (Allium sativum L.) Ecotypes in Different Planting Densities. J. Agroecol. 2017, 9, 559–574. [Google Scholar] [CrossRef]
- Ouzounidou, G.; Giannakoula, A.; Asfi, M.; Ilias, I. Differential Responses of Onion and Garlic against Plant Growth Regulators. Pak. J. Bot. 2011, 43, 2051–2057. [Google Scholar]
- Shohat, H.; Cheriker, H.; Kilambi, H.V.; Illouz Eliaz, N.; Blum, S.; Amsellem, Z.; Tarkowská, D.; Aharoni, A.; Eshed, Y.; Weiss, D. Inhibition of Gibberellin Accumulation by Water Deficiency Promotes Fast and Long-Term ‘Drought Avoidance’ Responses in Tomato. New Phytol. 2021, 232, 1985–1998. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.; Chen, Q.; Wu, X.; Chen, F.; Yuan, L.; Mi, G. Gibberellins Synthesis Is Involved in the Reduction of Cell Flux and Elemental Growth Rate in Maize Leaf under Low Nitrogen Supply. Environ. Exp. Bot. 2018, 150, 198–208. [Google Scholar] [CrossRef]
- Linzmeyer Junior, R.; Guimarães, V.F.; dos Santos, D.; Bencke, M.H. Influência de Retardante Vegetal e Densidades de Plantas Sobre o Crescimento, Acamamento e Produtividade Da Soja. Acta Sci. Agron. 2008, 30, 373–379. [Google Scholar] [CrossRef]
- Rademacher, W. Growth Retardants: Biochemical Features and Applications in Horticulture. Acta Hortic. 1995, 394, 57–74. [Google Scholar] [CrossRef]
- Matysiak, K. Influence of Trinexapac-Ethyl on Growth and Development of Winter Wheat. J. Plant Prot. Res. 2006, 46, 133–143. [Google Scholar]
- de Cassia Felix Alvarez, R.; Crusciol, C.A.C.; Nascente, A.S. Produtividade de Arroz de Terras Altas Em Função de Reguladores de Crescimento. Rev. Ceres 2014, 61, 42–49. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A. The Role of Antioxidants in the Chemistry of Oxidative Stress: A Review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef]
- Sánchez-Virosta, Á.; Sadras, V.O.; Sánchez-Gómez, D. Phenotypic Plasticity in Relation to Inter-Cultivar Variation of Garlic (Allium sativum L.) Functional Performance and Yield-Stability in Response to Water Availability. Sci. Hortic. 2021, 285, 110128. [Google Scholar] [CrossRef]
- Tominaga, J.; Shimada, H.; Kawamitsu, Y. Direct Measurement of Intercellular CO2 Concentration in a Gas-Exchange System Resolves Overestimation Using the Standard Method. J. Exp. Bot. 2018, 69, 1981–1991. [Google Scholar] [CrossRef] [PubMed]
- Nasser, V.G.; Macedo, W.R.; Pinto, F.G.; da Silva, J.H.; Sekita, M.C.; Silva, G.H. Plant Growth Regulator from the Essential Oil of Syzygium aromaticum L. for Inhibition of Secondary Growth of Garlic Cultivated under Tropical Conditions. AgriEngineering 2024, 6, 1511–1524. [Google Scholar] [CrossRef]
- Mykhalska, L.M.; Makoveychuk, T.M.; Schwartau, V.V. View of Mode of Physiological Activity of Acylcyclohexadione Retardants. Biosyst. Divers. 2020, 28, 411–418. [Google Scholar] [CrossRef]
- Foda, S.A.; Saleh, H.H.; Shanein, A.H. Effect of Cycocel “chlormequat” on Garlic—World Vegetable Center. Agric. Res. Rev. 1979, 57, 171–177. [Google Scholar]



| First Spraying | Second Spraying | Third Spraying | Treatments | |
|---|---|---|---|---|
| Two sprayings | 8 May 2020 (39 DAP) | 15 May2020 (46 DAP) | --- | T1–T4 |
| Three sprayings | 8 May 2020 (39 DAP) | 13 May 2020 (44 DAP) | 18 May 2020 (49 DAP) | T5–T8 |
| Treatments | First Spraying | Second Spraying | Third Spraying | Treatments |
|---|---|---|---|---|
| Two sprayings | 15 Jun 2023 (40 DAP) | 17 Jun 2023 (42 DAP) | --- | T1–T4 |
| Three sprayings | 15 Jun 2023 (40 DAP) | 17 Jun 2023 (42 DAP) | 19 Jun 2023 (44 DAP) | T5–T8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbosa, I.R.; Cruz, L.d.P.; Costa, R.I.C.d.; Rocha, B.H.; Nasser, V.G.; Silva, G.H.; Macedo, W.R. Gibberellin Inhibitors Molecules as a Safeguard against Secondary Growth in Garlic Plants. Crops 2024, 4, 379-399. https://doi.org/10.3390/crops4030027
Barbosa IR, Cruz LdP, Costa RICd, Rocha BH, Nasser VG, Silva GH, Macedo WR. Gibberellin Inhibitors Molecules as a Safeguard against Secondary Growth in Garlic Plants. Crops. 2024; 4(3):379-399. https://doi.org/10.3390/crops4030027
Chicago/Turabian StyleBarbosa, Iandra Rocha, Luciana de Paula Cruz, Raissa Iêda Cavalcanti da Costa, Bruno Henrique Rocha, Vinícius Guimarães Nasser, Geraldo Humberto Silva, and Willian Rodrigues Macedo. 2024. "Gibberellin Inhibitors Molecules as a Safeguard against Secondary Growth in Garlic Plants" Crops 4, no. 3: 379-399. https://doi.org/10.3390/crops4030027
APA StyleBarbosa, I. R., Cruz, L. d. P., Costa, R. I. C. d., Rocha, B. H., Nasser, V. G., Silva, G. H., & Macedo, W. R. (2024). Gibberellin Inhibitors Molecules as a Safeguard against Secondary Growth in Garlic Plants. Crops, 4(3), 379-399. https://doi.org/10.3390/crops4030027







