Valorisation of Spent Yeast Fermentation Media through Compositional-Analysis-Directed Supplementation
Abstract
1. Introduction
2. Materials and Methods
2.1. Generation of Spent BMMY for Use in Secondary Fermentations
2.2. Inductively Coupled Plasma Mass Spectrometry (ICP-MS) Analysis of Media Samples
2.3. Metabolomic Analysis of Amino Acid Content via LC-MS/MS
2.4. Preparation and Supplementation of Spent BMMY for Secondary E. coli Fermentations
2.5. Expression and Purification of mCherry-EF2
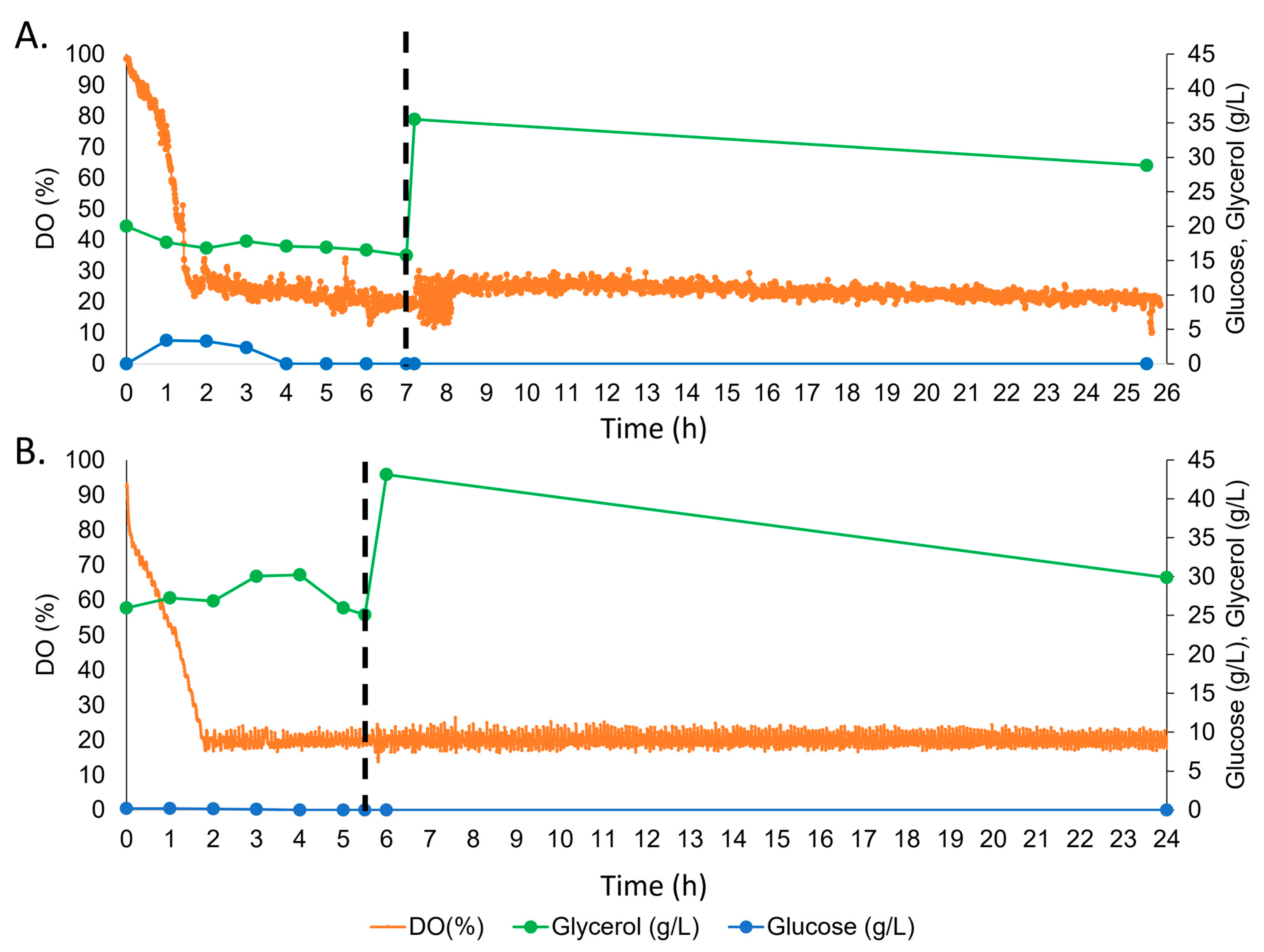
2.6. Bioreactor Fermentation of YSM-fed E. coli
3. Results
3.1. Elemental and Amino Acid Composition Analysis of Fresh and Spent Media
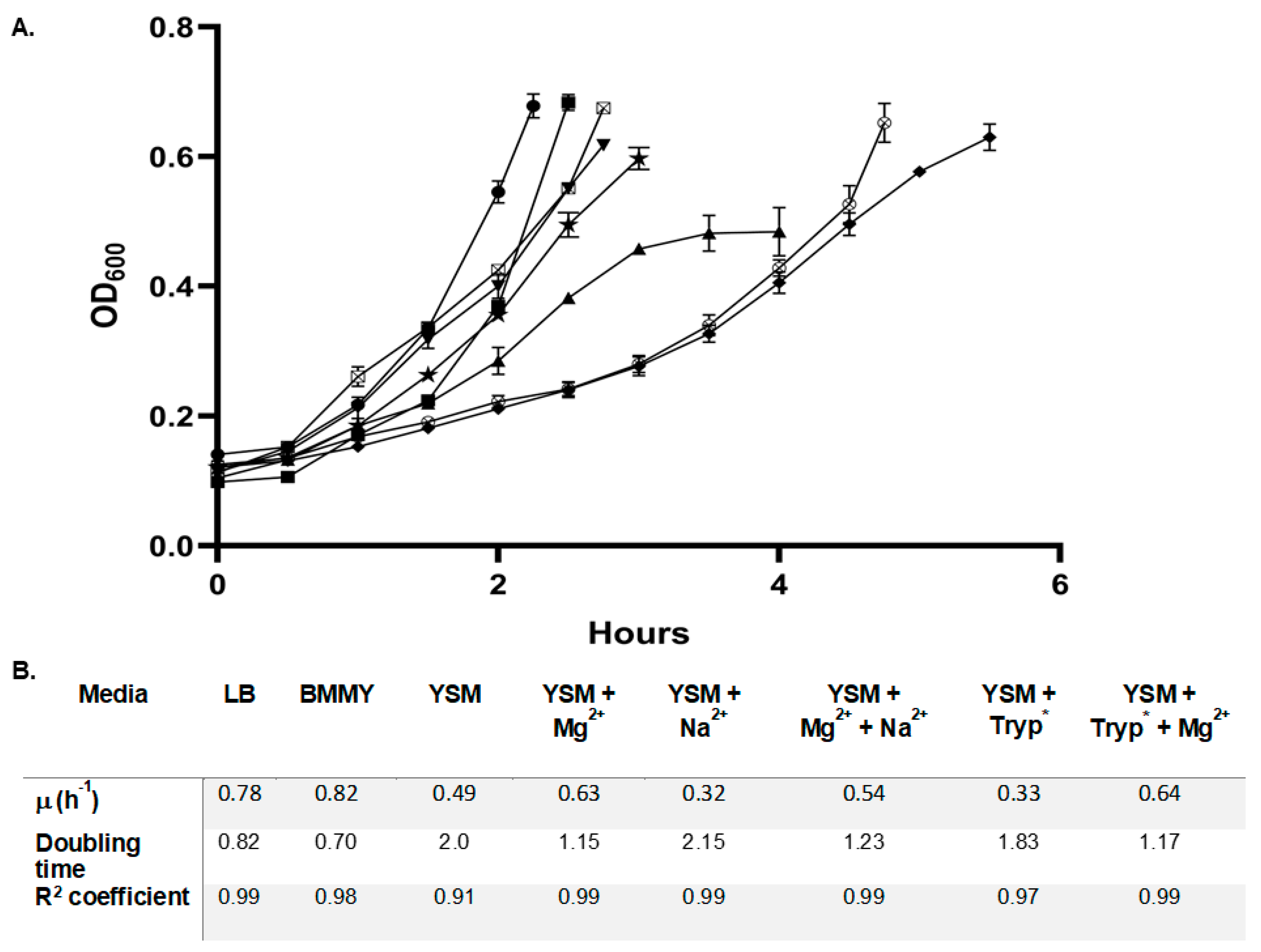
3.2. Supplementation in Shake Flask Experiments
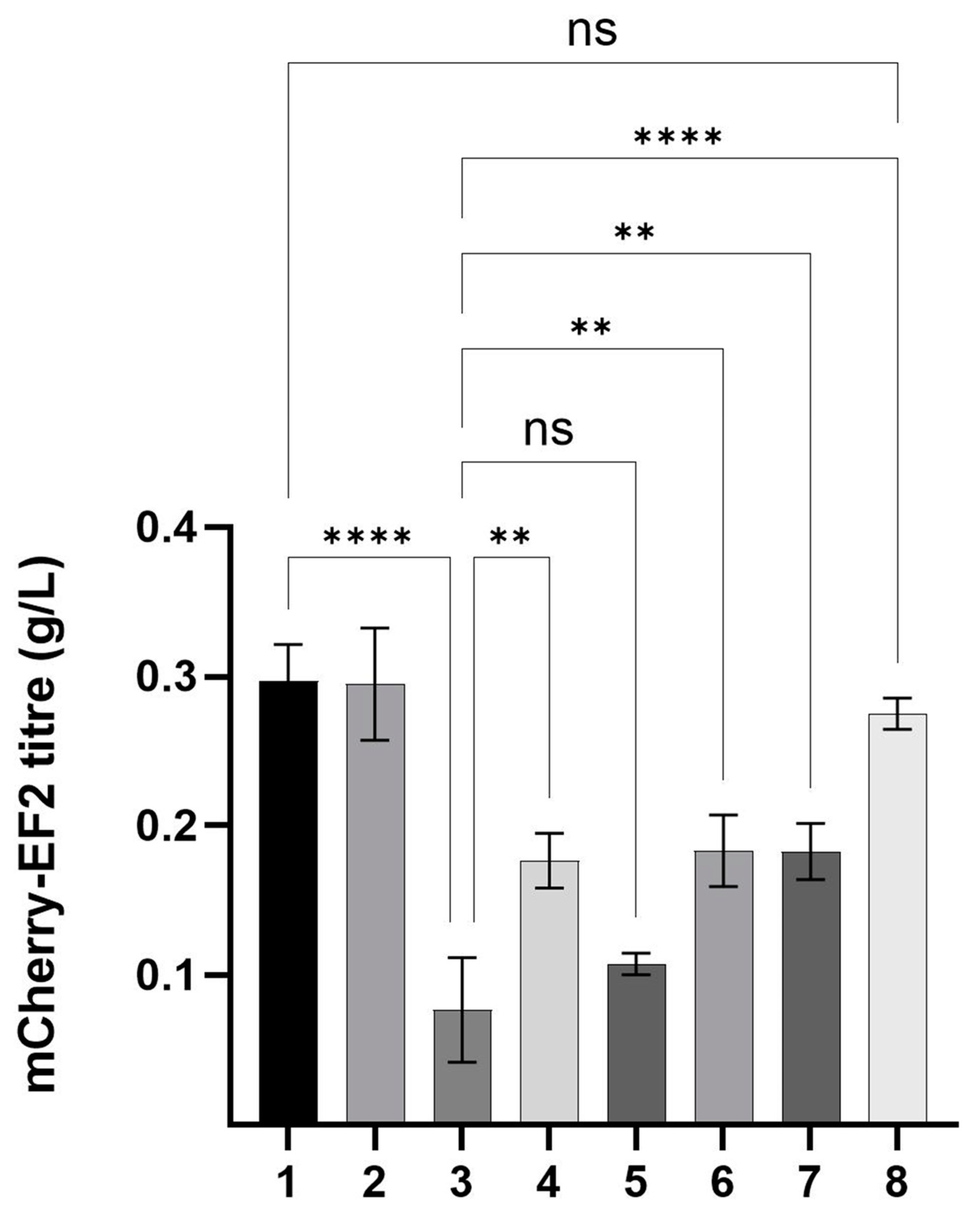
3.3. Determination of Recombinant Protein Expression Titres in Shake Flask Cultures
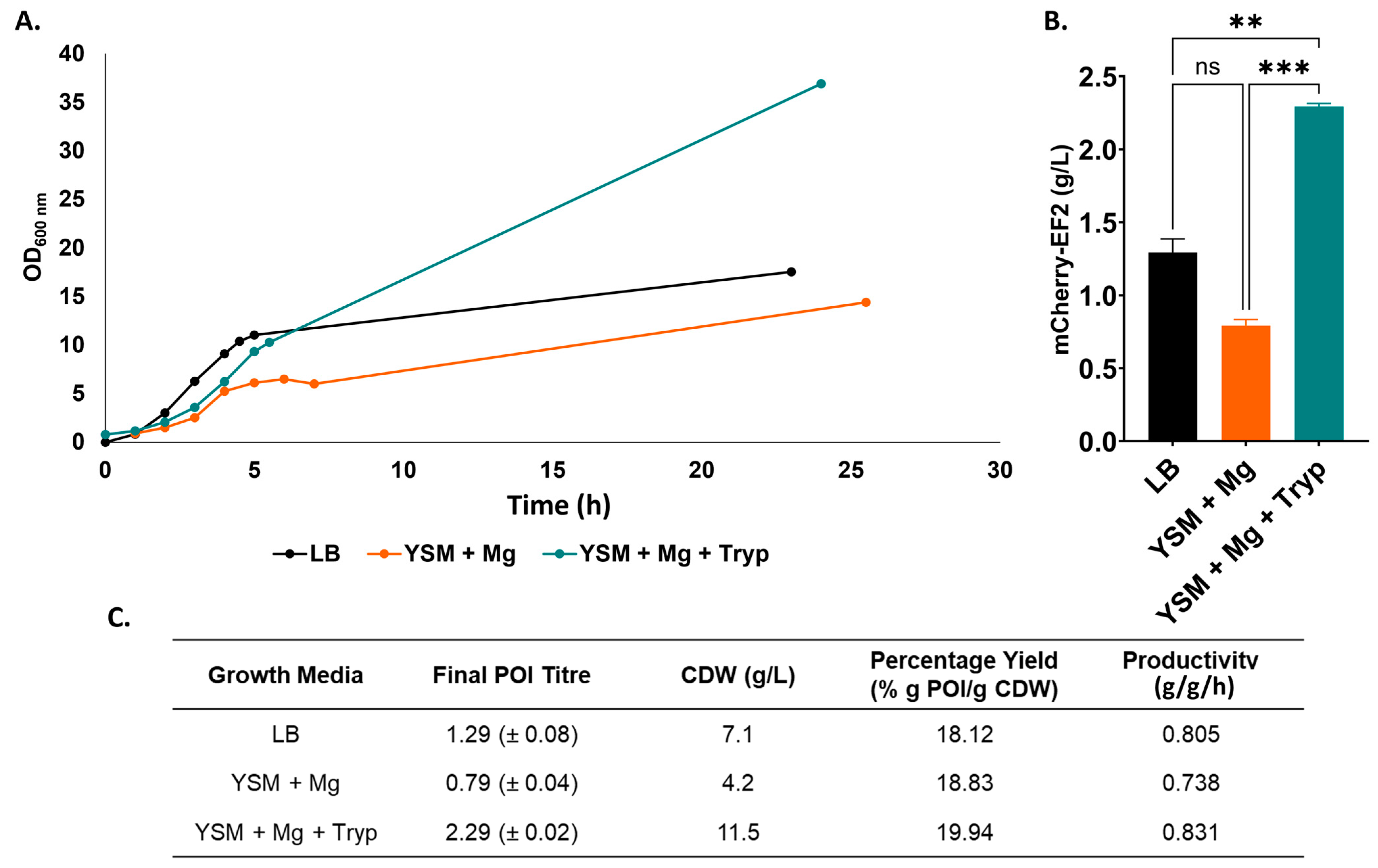
3.4. Recombinant Protein Expression Titres in Bioreactor Fermentation
4. Discussion
4.1. Spent Media Analysis of YSM Enabled Efficient Reuse of This Waste Resource
4.2. Titre of Recombinant Protein Is Increased with Optimised Supplementation of YSM in Bioreactor Fermentation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brandão, A.S.; Gonçalves, A.; Santos, J.M.R.C.A. Circular bioeconomy strategies: From scientific research to commercially viable products. J. Clean. Prod. 2021, 295, 126407. [Google Scholar] [CrossRef]
- Stegmann, P.; Londo, M.; Junginger, M. The circular bioeconomy: Its elements and role in European bioeconomy clusters. Resour. Conserv. Recycl. X 2020, 6, 100029. [Google Scholar] [CrossRef]
- Lynch, C.; Jordan, L.; O’ Connell, D.J. Redesigning Spent Media from Cell Culture Bioprocess to Feed New Bacterial Fermentations. In Cell Culture Engineering and Technology: In Appreciation to Professor Mohamed Al-Rubeai; Pörtner, R., Ed.; Springer International Publishing: Cham, Switzerland, 2021; pp. 129–146. [Google Scholar] [CrossRef]
- Amasawa, E.; Kuroda, H.; Okamura, K.; Badr, S.; Sugiyama, H. Cost–Benefit Analysis of Monoclonal Antibody Cultivation Scenarios in Terms of Life Cycle Environmental Impact and Operating Cost. ACS Sustain. Chem. Eng. 2021, 9, 14012–14021. [Google Scholar] [CrossRef]
- Budzinski, K.; Blewis, M.; Dahlin, P.; D’Aquila, D.; Esparza, J.; Gavin, J.; Ho, S.V.; Hutchens, C.; Kahn, D.; Koenig, S.G.; et al. Introduction of a process mass intensity metric for biologics. New Biotechnol. 2019, 49, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Gonzalez, C.; Ponder, C.S.; Broxterman, Q.B.; Manley, J.B. Using the Right Green Yardstick: Why Process Mass Intensity Is Used in the Pharmaceutical Industry to Drive More Sustainable Processes. Org. Process Res. Dev. 2011, 15, 912–917. [Google Scholar] [CrossRef]
- Cataldo, A.L.; Sissolak, B.; Metzger, K.; Budzinski, K.; Shirokizawa, O.; Luchner, M.; Jungbauer, A.; Satzer, P. Water related impact of energy: Cost and carbon footprint analysis of water for biopharmaceuticals from tap to waste. Chem. Eng. Sci. X 2020, 8, 100083. [Google Scholar] [CrossRef]
- Lynch, C.D.; Cerrone, F.; Connor, K.E.O.; O’Connell, D.J. Feeding secondary fermentations with mammalian and fungal culture waste streams increases productivity and resource efficiency. bioRxiv 2023. [Google Scholar] [CrossRef]
- Lynch, C.D.; O’Connell, D.J. Conversion of mammalian cell culture media waste to microbial fermentation feed efficiently supports production of recombinant protein by Escherichia coli. PLoS ONE 2022, 17, e0266921. [Google Scholar] [CrossRef] [PubMed]
- Lahue, C.; Madden, A.A.; Dunn, R.R.; Smukowski Heil, C. History and Domestication of Saccharomyces cerevisiae in Bread Baking. Front. Genet. 2020, 11, 584718. [Google Scholar] [CrossRef]
- Raihofer, L.; Zarnow, M.; Gastl, M.; Hutzler, M. A short history of beer brewing: Alcoholic fermentation and yeast technology over time: Alcoholic fermentation and yeast technology over time. EMBO Rep. 2022, 23, e56355. [Google Scholar] [CrossRef]
- Chance, R.E.; Frank, B.H. Research, development, production, and safety of biosynthetic human insulin. Diabetes Care 1993, 16 (Suppl. 3), 133–142. [Google Scholar] [CrossRef]
- Nielsen, J. Production of biopharmaceutical proteins by yeast: Advances through metabolic engineering. Bioengineered 2013, 4, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Baeshen, N.A.; Baeshen, M.N.; Sheikh, A.; Bora, R.S.; Ahmed, M.M.M.; Ramadan, H.A.; Saini, K.S.; Redwan, E.M. Cell factories for insulin production. Microb. Cell Fact. 2014, 13, 141. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, S. Eptinezumab: First Approval. Drugs 2020, 80, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Walsh, G.; Walsh, E. Biopharmaceutical benchmarks 2022. Nat. Biotechnol. 2022, 40, 1722–1760. [Google Scholar] [CrossRef]
- Walsh, G. Biopharmaceutical benchmarks 2018. Nat. Biotechnol. 2018, 36, 1136–1145. [Google Scholar] [CrossRef]
- Pais-Chanfrau, J.M.; Trujillo-Toledo, L.E. Optimization of Culture Medium for Large-Scale Production of Heterologous Proteins in Pichia pastoris to be used in Nanoscience and other Biotechnological Fields. Biol. Med. 2016, 8, 279. [Google Scholar] [CrossRef]
- Joseph, J.A.; Akkermans, S.; Cornillie, E.; Deberlanger, J.; Van Impe, J.F.M. Optimal culture medium selection and supplementation for recombinant thaumatin II production by Komagataella phaffii. Food Bioprod. Process 2023, 139, 190–203. [Google Scholar] [CrossRef]
- Bustos, C.; Quezada, J.; Veas, R.; Altamirano, C.; Braun-Galleani, S.; Fickers, P.; Berrios, J. Advances in Cell Engineering of the Komagataella phaffii Platform for Recombinant Protein Production. Metabolites 2022, 12, 346. [Google Scholar] [CrossRef]
- Lanser, D.M.; Gelli, A. Optimized Expression and Isolation of Recombinant Active Secreted Proteases Using Pichia pastoris. Bio Protoc. 2023, 13, e4628. [Google Scholar] [CrossRef]
- Pinheiro, F.C.; Babos, D.V.; Barros, A.I.; Pereira-Filho, E.R.; Nóbrega, J.A. Microwave-assisted digestion using dilute nitric acid solution and investigation of calibration strategies for determination of As, Cd, Hg and Pb in dietary supplements using ICP-MS. J. Pharm. Biomed. Anal. 2019, 174, 471–478. Available online: https://www.sciencedirect.com/science/article/pii/S0731708519310921?casa_token=76ujxFeBWbcAAAAA:spal-DNjD3m2H7uwph-y339LBeWZOQXZx5nJ6UnMSccWlqLnFdD-CCwLLVFb7pgSjef3xuMjgQ (accessed on 30 May 2024). [CrossRef]
- Lindman, S.; Johansson, I.; Thulin, E.; Linse, S. Green fluorescence induced by EF-hand assembly in a split GFP system. Protein Sci. 2009, 18, 1221–1229. [Google Scholar] [CrossRef]
- Yeliseev, A.A.; O’Connell, D.J. Application of a Small EF Hand Affinity Tag for Expression, Purification and Biophysical Studies of G Protein-Coupled Membrane Receptors. Available online: https://researchrepository.ucd.ie/rest/bitstreams/40073/retrieve (accessed on 20 December 2023).
- Gao, J.; Opiteck, G.J.; Friedrichs, M.S.; Dongre, A.R.; Hefta, S.A. Changes in the protein expression of yeast as a function of carbon source. J. Proteome Res. 2003, 2, 643–649. [Google Scholar] [CrossRef]
- Dodia, H.; Sunder, A.V.; Borkar, Y.; Wangikar, P.P. Precision fermentation with mass spectrometry-based spent media analysis. Biotechnol. Bioeng. 2023, 120, 2809–2826. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Wang, X.; Zhan, Y. Effect of precipitation pH and coexisting magnesium ion on phosphate adsorption onto hydrous zirconium oxide. J. Environ. Sci. 2019, 76, 167–187. [Google Scholar] [CrossRef]
- Innovating for Sustainable Growth: A Bioeconomy for Europe, EC, 2012. European Environment Agency. 21 April 2013. Available online: https://www.eea.europa.eu/policy-documents/innovating-for-sustainable-growth-a (accessed on 17 August 2023).
- Yano, T.; Mori, H.; Kobayashi, T.; Shimizu, S. Reusability of Broth Supernatant as Medium. J. Ferment. Technol. 1980, 58, 259–266. Available online: https://ci.nii.ac.jp/naid/110002691431/ (accessed on 30 May 2024).
- Nierhaus, K.H. Mg2+, K+, and the ribosome. J. Bacteriol. 2014, 196, 3817–3819. [Google Scholar] [CrossRef] [PubMed]
- Froschauer, E.M.; Kolisek, M.; Dieterich, F.; Schweigel, M.; Schweyen, R.J. Fluorescence measurements of free [Mg2+] by use of mag-fura 2 in Salmonella enterica. FEMS Microbiol. Lett. 2004, 237, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Richey, B.; Cayley, D.S.; Mossing, M.C.; Kolka, C.; Anderson, C.F.; Farrar, T.C.; Record, M.T., Jr. Variability of the intracellular ionic environment of Escherichia coli. Differences between in vitro and in vivo effects of ion concentrations on protein-DNA interactions and gene expression. J. Biol. Chem. 1987, 262, 7157–7164. Available online: https://www.ncbi.nlm.nih.gov/pubmed/3108249 (accessed on 30 May 2024). [CrossRef]
- Zhu, Y.; Rinzema, A.; Tramper, J.; Bol, J. Medium design based on stoichiometric analysis of microbial transglutaminase production by Streptoverticillium mobaraense. Biotechnol. Bioeng. 1996, 50, 291–298. [Google Scholar] [CrossRef]
- Yegane-Sarkandy, S.; Farnoud, A.M.; Shojaosadati, S.A.; Khalilzadeh, R.; Sadeghyzadeh, M.; Ranjbar, B.; Babaeipour, V. Overproduction of human interleukin-2 in recombinant Escherichia coli BL21 high-cell-density culture by the determination and optimization of essential amino acids using a simple stoichiometric model. Biotechnol. Appl. Biochem. 2009, 54, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Stadtman, E.R.; Shapiro, B.M.; Kingdon, H.S.; Woolfolk, C.A.; Hubbard, J.S. Cellular regulation of glutamine synthetase activity in Escherichia coli. Adv. Enzym. Regul. 1968, 6, 257–289. [Google Scholar] [CrossRef] [PubMed]
- Pizer, L.I. Glycine synthesis and metabolism in Escherichia coli. J. Bacteriol. 1965, 89, 1145–1150. [Google Scholar] [CrossRef] [PubMed]
- Helling, R.B. Pathway choice in glutamate synthesis in Escherichia coli. J. Bacteriol. 1998, 180, 4571–4575. [Google Scholar] [CrossRef] [PubMed]
- Abdulkarim, S.M.; Fatimah, A.B.; Anderson, J.G. Effect of salt concentrations on the growth of heat-stressed and unstressed Escherichia coli. Int. J. Food Agric. Environ. 2009, 7, 3. Available online: https://strathprints.strath.ac.uk/18874/ (accessed on 30 May 2024).
- Kaleta, C.; Schäuble, S.; Rinas, U.; Schuster, S. Metabolic costs of amino acid and protein production in Escherichia coli. Biotechnol. J. 2013, 8, 1105–1114. [Google Scholar] [CrossRef]
| Element | LB (mg/L) | YSM (mg/L) |
| Sodium | 362.25 | 34.36 |
| Magnesium | 0.51 | 0.27 |
| Phosphorus | 18.07 | 408.62 |
| Sulfur | 8.77 | 311.66 |
| Potassium | 32.96 | 616.72 |
| Calcium | 0.49 | 1.38 |
| Amino acid | LB (mM) | YSM (mM) |
| Alanine | 2682.33 | 50.07 |
| Arginine | 523.33 | 4.91 |
| Asparagine | 607.00 | 35.23 |
| Aspartate | 1232.67 | 17.03 |
| Cysteine | 38.50 | 38.73 |
| Glutamate | 27.70 | 19.23 |
| Glutamine | 2848.00 | 84.10 |
| Glycine | 1258.33 | 17.97 |
| Histidine | 425.00 | 3.78 |
| Isoleucine | 1784.67 | 30.77 |
| Leucine | 4179.33 | 60.83 |
| Lysine | 2397.00 | 1482.33 |
| Methionine | 717.33 | 300.33 |
| Phenylalanine | 1518.33 | 765.33 |
| Proline | 569.00 | 22.97 |
| Serine | 1489.33 | 13.37 |
| Threonine | 1204.67 | 10.63 |
| Tryptophan | 526.00 | 3.13 |
| Tyrosine | 413.00 | 161.67 |
| Valine | 1250.67 | 35.80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murphy, L.; Lynch, C.D.; O’Connell, D.J. Valorisation of Spent Yeast Fermentation Media through Compositional-Analysis-Directed Supplementation. Appl. Microbiol. 2024, 4, 959-971. https://doi.org/10.3390/applmicrobiol4020065
Murphy L, Lynch CD, O’Connell DJ. Valorisation of Spent Yeast Fermentation Media through Compositional-Analysis-Directed Supplementation. Applied Microbiology. 2024; 4(2):959-971. https://doi.org/10.3390/applmicrobiol4020065
Chicago/Turabian StyleMurphy, Laura, Ciara D. Lynch, and David J. O’Connell. 2024. "Valorisation of Spent Yeast Fermentation Media through Compositional-Analysis-Directed Supplementation" Applied Microbiology 4, no. 2: 959-971. https://doi.org/10.3390/applmicrobiol4020065
APA StyleMurphy, L., Lynch, C. D., & O’Connell, D. J. (2024). Valorisation of Spent Yeast Fermentation Media through Compositional-Analysis-Directed Supplementation. Applied Microbiology, 4(2), 959-971. https://doi.org/10.3390/applmicrobiol4020065







