Phosphate Solubilization and Plant Growth Promotion by Enterobacter sp. Isolate
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strain
2.2. Morphological and Biochemical Characterization
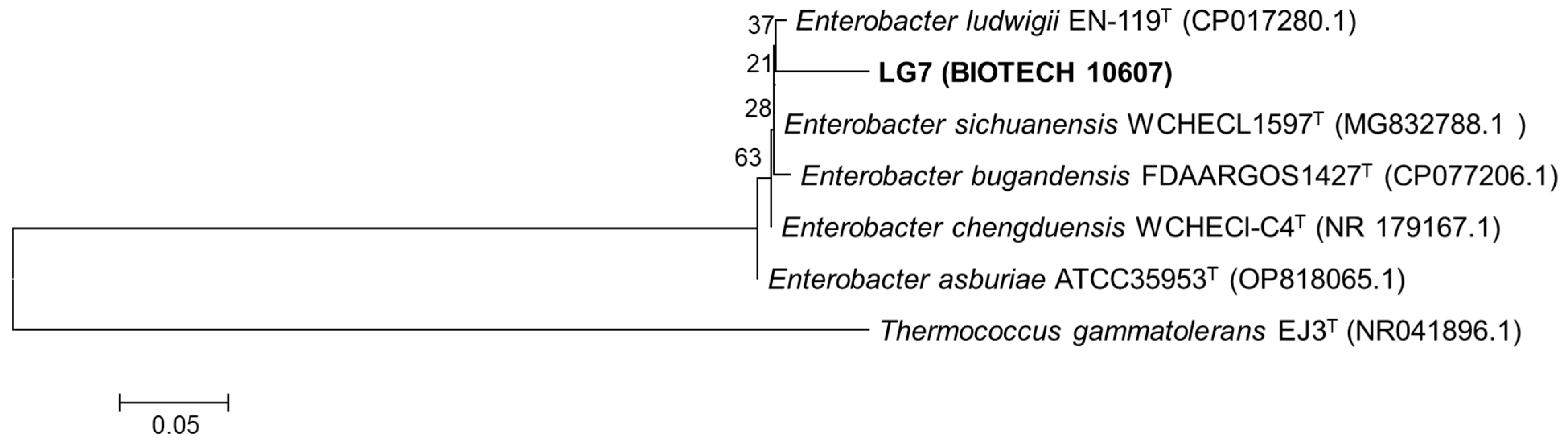
2.3. DNA Extraction, Amplification, and Sequencing
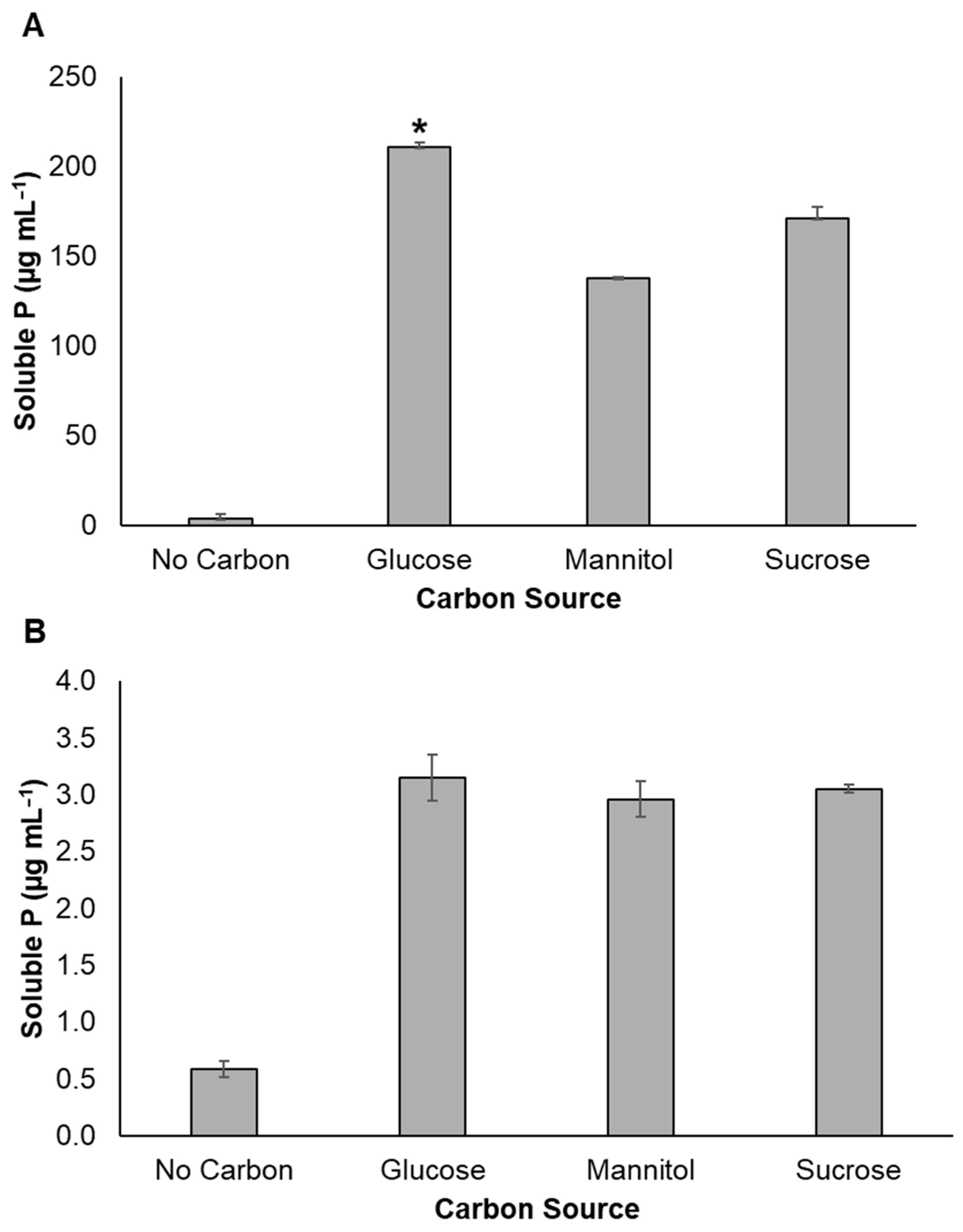
2.4. Effect of Carbon Source on Mineral Phosphate Solubilization
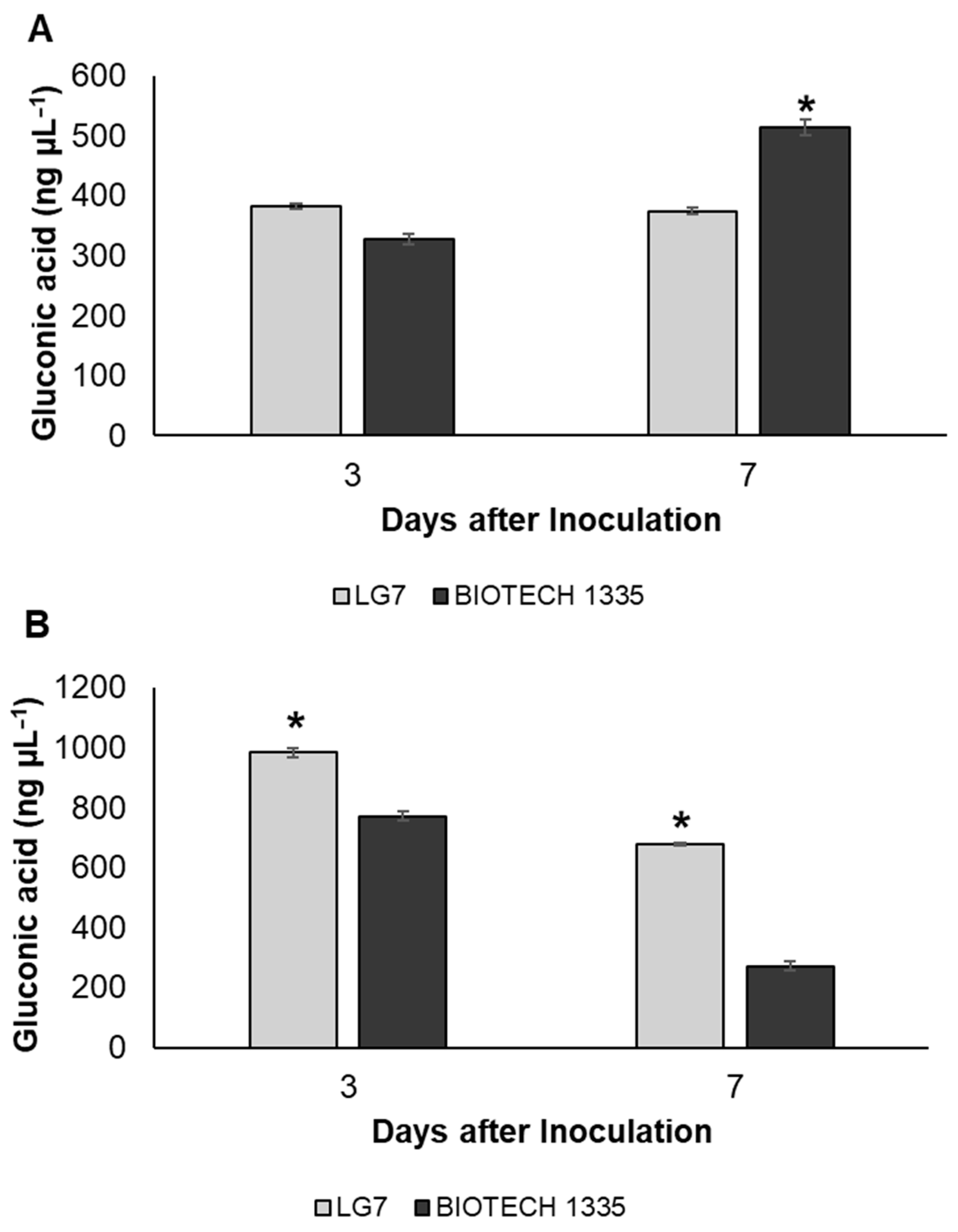
2.5. Estimation of Phosphate-Solubilizing Ability and Gluconic Acid
2.6. Plant Growth Assay as Single Inoculant
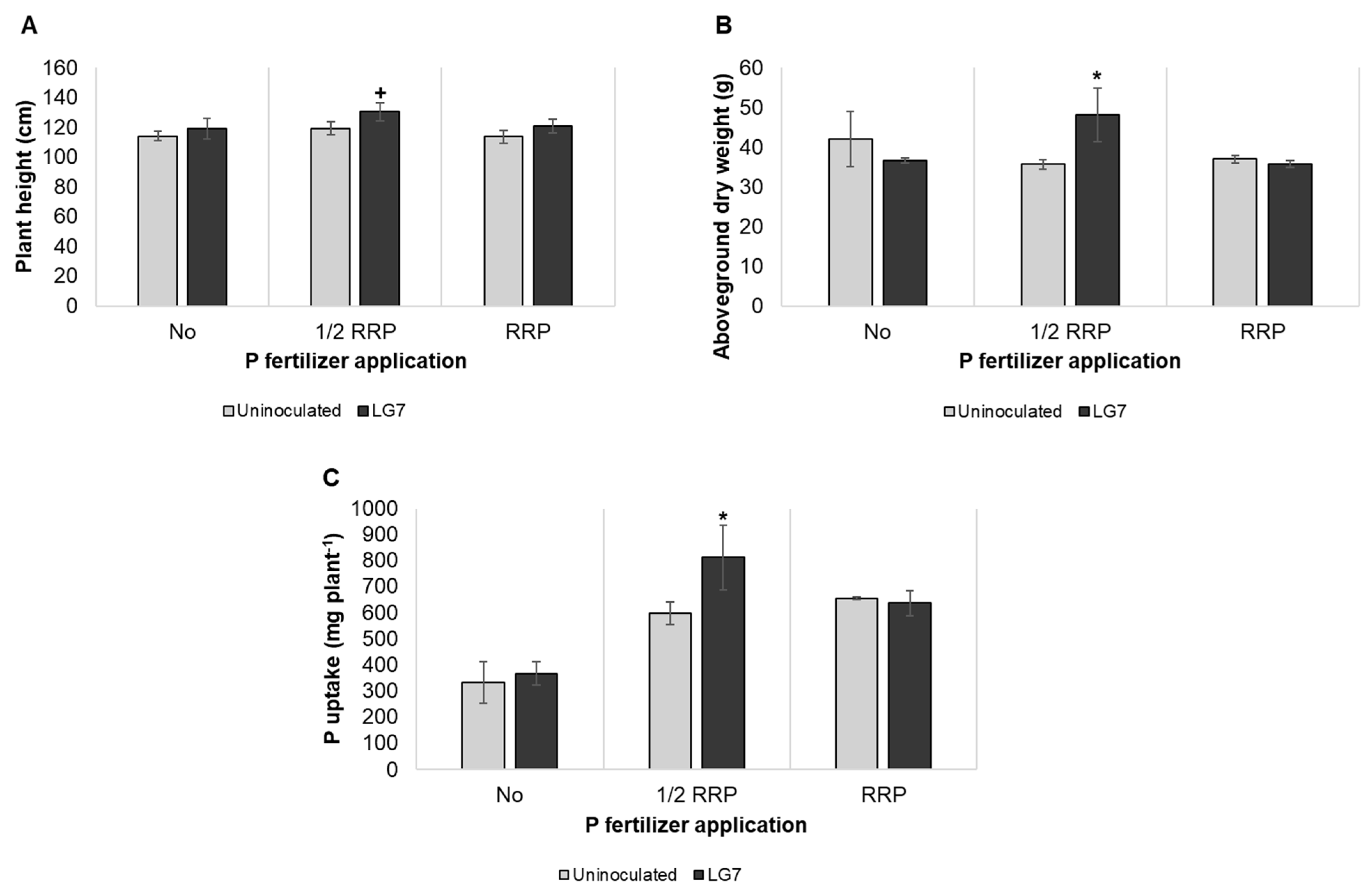
2.7. Plant Growth Assay as Co-Inoculant with Rhizobium
2.7.1. Inoculant Production
2.7.2. Viability and Competence of LG7-Rhizobia Inoculant
2.7.3. Screenhouse Experiment of LG7-Rhizobia Inoculant
2.8. Statistical Analyses
3. Results
3.1. Morphological, Biochemical, and Genetic Characterization of LG7
3.2. Effect of Varying Carbon Sources on Mineral Phosphate Solubilization
3.3. Estimation of Phosphate-Solubilizing Ability and Gluconic Acid
3.4. Effect of Single Inoculation of LG7 on Rice Growth Promotion
3.5. Effect of Co-Inoculation of LG7 and Rhizobia on Peanut Growth Promotion
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bulgarelli, D.; Schlaeppi, K.; Spaepen, S.; van Themaat, E.V.L.; Schulze-Lefert, P. Structure and Functions of the Bacterial Microbiota of Plants. Annu. Rev. Plant Biol. 2013, 64, 807–838. [Google Scholar] [CrossRef] [PubMed]
- Garbeva, P.; van Veen, J.; van Elsas, J. MICROBIAL DIVERSITY IN SOIL: Selection of Microbial Populations by Plant and Soil Type and Implications for Disease Suppressiveness. Annu. Rev. Phytopathol. 2004, 42, 243–270. [Google Scholar] [CrossRef] [PubMed]
- Boraste, A.; Vamsi, K.K.; Jhadav, A.; Khairnar, Y.; Gupta, N.; Trivedi, S.; Patil, P.; Gupta, G.; Gupta, M.; Mujapara, A.K.; et al. Biofertilizers: A novel tool for agriculture. Int. J. Microbiol. Res. 2009, 1, 23–31. [Google Scholar] [CrossRef]
- Lugtenberg, B.; Kamilova, F. Plant-growth-promoting rhizobacteria. Annu. Rev. Microbiol. 2009, 63, 541–556. [Google Scholar] [CrossRef] [PubMed]
- Berg, G.; Rybakova, D.; Grube, M.; Köberl, M. The plant microbiome explored: Implications for experimental botany. J. Exp. Bot. 2016, 67, 995–1002. [Google Scholar] [CrossRef] [PubMed]
- Terrazas, R.A.; Giles, C.; Paterson, E.; Robertson-Albertyn, S.; Cesco, S.; Mimmo, T.; Pii, Y.; Bulgarelli, D. Plant-microbiota interactions as a driver of the mineral Turnover in the Rhizosphere. Adv. Appl. Microbiol. 2016, 95, 1–67. [Google Scholar] [CrossRef]
- Pikovskaya, R. Mobilization of phosphorus in soil in connection with vital capacity of source microbial species. Microbiologia 1948, 17, 362–370. [Google Scholar]
- Zaidi, A.; Khan, M.; Ahemad, M.; Oves, M. Plant growth promotion by phosphate solubilizing bacteria. Acta Microbiol. Immunol. Hung. 2009, 56, 263–284. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.Y.; McDonald, G.A.; Jordan, D. Solubilization of hydroxyapatite by Enterobacter agglomerans and cloned Escherichia coli in culture medium. Biol. Fertil. Soils 1997, 24, 347–352. [Google Scholar] [CrossRef]
- Lin, T.-F.; Huang, H.-I.; Shen, F.-T.; Young, C.-C. The protons of gluconic acid are the major factor responsible for the dissolution of tricalcium phosphate by Burkholderia cepacia CC-Al74. Bioresour. Technol. 2006, 97, 957–960. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.K.; Archana, G.; Kumar, G.N. Variation in the Nature of Organic Acid Secretion and Mineral Phosphate Solubilization by Citrobacter sp. DHRSS in the Presence of Different Sugars. Curr. Microbiol. 2008, 56, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Ben Farhat, M.; Farhat, A.; Bejar, W.; Kammoun, R.; Bouchaala, K.; Fourati, A.; Antoun, H.; Bejar, S.; Chouayekh, H. Characterization of the mineral phosphate solubilizing activity of Serratia marcescens CTM 50650 isolated from the phosphate mine of Gafsa. Arch. Microbiol. 2009, 191, 815–824. [Google Scholar] [CrossRef] [PubMed]
- Richardson, A.E.; Barea, J.-M.; McNeill, A.M.; Prigent-Combaret, C. Acquisition of phosphorus and nitrogen in the rhizosphere and plant growth promotion by microorganisms. Plant Soil 2009, 321, 305–339. [Google Scholar] [CrossRef]
- Jones, D.; Oburger, E. Solubilization of phosphorus by soil microorganism. In Phosphorus in Action; Buenemann, E., Oberson, A., Frossard, E., Eds.; Springer: New York, NY, USA, 2011; pp. 169–198. [Google Scholar]
- Bieleski, R.L. Phosphate Pools, Phosphate Transport, and Phosphate Availability. Annu. Rev. Plant Physiol. 1973, 24, 225–252. [Google Scholar] [CrossRef]
- Raghothama, K.G. Phosphate acquisition. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 665–693. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, H.; Fraga, R. Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol. Adv. 1999, 17, 4–5. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, P.; Logan, T. Myths and science about the chemistry and fertility of soils in the tropics. In Myths and Science of Soils of the Tropics; Lal, R., Sanchez, P., Eds.; Soil Science Society of America: Madison, WI, USA, 1992; pp. 35–46. [Google Scholar]
- Richardson, A.E. Prospects for using soil microorganisms to improve the acquisition of phosphorus by plants. Funct. Plant Biol. 2001, 28, 897–906. [Google Scholar] [CrossRef]
- Goldstein, A.H.; Braverman, K.; Osorio, N. Evidence for mutualism between a plant growing in a phosphate-limited desert environment and a mineral phosphate solubilizing (MPS) rhizobacterium. FEMS Microbiol. Ecol. 2006, 30, 295–300. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bennett, E.M.; Carpenter, S.R.; Caraco, N.F. Human Impact on Erodable Phosphorus and Eutrophication: A Global Perspective: Increasing accumulation of phosphorus in soil threatens rivers, lakes, and coastal oceans with eutrophication. Bioscience 2001, 51, 227–234. [Google Scholar] [CrossRef]
- Brownlie, W.J.; Sutton, M.A.; Reay, D.S.; Heal, K.V.; Hermann, L.; Kabbe, C.; Spears, B.M. Global actions for a sustainable phosphorus future. Nat. Food 2021, 2, 71–74. [Google Scholar] [CrossRef]
- Mogollón, J.M.; Bouwman, A.F.; Beusen, A.H.W.; Lassaletta, L.; van Grinsven, H.J.M.; Westhoek, H. More efficient phosphorus use can avoid cropland expansion. Nat. Food 2021, 2, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Nedelciu, C.E.; Ragnarsdottir, K.V.; Schlyter, P.; Stjernquist, I. Global phosphorus supply chain dynamics: Assessing regional impact to 2050. Glob. Food Secur. 2020, 26, 100426. [Google Scholar] [CrossRef] [PubMed]
- Brownlie, W.J.; Sutton, M.A.; Cordell, D.; Reay, D.S.; Heal, K.V.; Withers, P.J.A.; Vanderbeck, I.; Spears, B.M. Phosphorus price spikes: A wake-up call for phosphorus resilience. Front. Sustain. Food Syst. 2023, 7, 1088776. [Google Scholar] [CrossRef]
- IAASTD. Global Report International Assessment of Agriculture at a Crossroads; IAASTD: Washington, DC, USA, 2009. [Google Scholar]
- Barbieri, P.; MacDonald, G.K.; Bernard de Raymond, A.; Nesme, T. Food system resilience to phosphorus shortages on a telecoupled planet. Nat. Sustain. 2021, 5, 114–122. [Google Scholar] [CrossRef]
- Verma, S.C.; Ladha, J.K.; Tripathi, A.K. Evaluation of plant growth promoting and colonization ability of endophytic diazotrophs from deep water rice. J. Biotechnol. 2001, 91, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Sherpa, M.T.; Sharma, L.; Bag, N.; Das, S. Isolation, Characterization, and Evaluation of Native Rhizobacterial Consortia Developed From the Rhizosphere of Rice Grown in Organic State Sikkim, India, and Their Effect on Plant Growth. Front. Microbiol. 2021, 12, 713660. [Google Scholar] [CrossRef] [PubMed]
- Alagawadi, A.R.; Gaur, A.C. Associative effect of Rhizobium and phosphate-solubilizing bacteria on the yield and nutrient uptake of chickpea. Plant Soil 1988, 105, 241–246. [Google Scholar] [CrossRef]
- Cheng, H.-R.; Jiang, N. Extremely Rapid Extraction of DNA from Bacteria and Yeasts. Biotechnol. Lett. 2006, 28, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Weisburg, W.G.; Barns, S.M.; Pelletier, D.A.; Lane, D.J. 16S Ribosomal DNA Amplification for Phylogenetic Study. J. Bacteriol. 1991, 173, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.; Nautiyal, C.S. An Efficient Method for Qualitative Screening of Phosphate-Solubilizing Bacteria. Curr. Microbiol. 2001, 43, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Philippine Council for Agriculture and Resources Research. Standard Methods of Analysis for Soil, Plant Tissue, Water and Fertilizer, 22nd ed.; Philippine Council for Agriculture and Resources Research, Farm Resources and Systems Research Division: Los Baños, Philippines, 1991; Volume 120. [Google Scholar]
- Ahemad, M.; Khan, M.S. Phosphate-Solubilizing and Plant-Growth-Promoting Pseudomonas aeruginosa PS1 Improves Greengram Performance in Quizalafop-p-ethyl and Clodinafop Amended Soil. Arch. Environ. Contam. Toxicol. 2010, 58, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Edi Premono, M.; Moawad, A.M.; Vlek, P.L.G. Effect of phosphate solubilizing Pseudomonas putida on the growth of maize and its survival in the rhizosphere. Indones. J. Crop Sci. 1996, 11, 13–23. [Google Scholar]
- Philippine Rice Research Institute. Rc 222 Boosts Yield by 20 Cavans. Available online: https://www.philrice.gov.ph (accessed on 17 March 2018).
- Tejima, K.; Arima, Y.; Yokoyama, T.; Sekimoto, H. Composition of amino acids, organic acids, and sugars in the peribacteroid space of soybean root nodules. Soil Sci. Plant Nutr. 2003, 49, 239–247. [Google Scholar] [CrossRef]
- Rhoades, R.; Nazarea, V. Peanut in Local and Global Food Systems Series Report No. 1, 1998.
- Zarate, J.T.; Aquino, G.M.B.; Cruz, J.M.C.; Villa, N.O.; Rosana, A.R.R. Draft Genome Sequence of Enterobacter sp. Strain AD2-3, Isolated from a Postmining Site in Benguet, Philippines. Microbiol. Resour. Announc. 2019, 8, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Kirui, C.K.; Njeru, E.M.; Runo, S. Diversity and Phosphate Solubilization Efficiency of Phosphate Solubilizing Bacteria Isolated from Semi-Arid Agroecosystems of Eastern Kenya. Microbiol. Insights 2022, 15, 117863612210889. [Google Scholar] [CrossRef] [PubMed]
- Muindi, M.M.; Muthini, M.; Njeru, E.M.; Maingi, J. Symbiotic efficiency and genetic characterization of rhizobia and non rhizobial endophytes associated with cowpea grown in semi-arid tropics of Kenya. Heliyon 2021, 7, e06867. [Google Scholar] [CrossRef] [PubMed]
- Ajibade, O.A.; Oladipo, E.K.; Kwenda, S.; Khumalo, Z.; Ismail, A.; Oloke, J.K.; Oyawoye, O.M.; Onyeaka, H. Whole genomic sequence of Enterobacter sichuanensis AJI 2411—A plant growth promoting rhizobacteria. Gene 2023, 887, 147725. [Google Scholar] [CrossRef] [PubMed]
- Chinachanta, K.; Shutsrirung, A.; Herrmann, L.; Lesueur, D. Isolation and characterization of KDML105 aromatic rice rhizobacteria producing indole-3-acetic acid: Impact of organic and conventional paddy rice practices. Lett. Appl. Microbiol. 2022, 74, 354–366. [Google Scholar] [CrossRef] [PubMed]
- Bashan, Y.; Kamnev, A.A.; de-Bashan, L.E. Tricalcium phosphate is inappropriate as a universal selection factor for isolating and testing phosphate-solubilizing bacteria that enhance plant growth: A proposal for an alternative procedure. Biol. Fertil. Soils 2013, 49, 465–479. [Google Scholar] [CrossRef]
- Monroy Miguel, R.; Carrillo González, R.; Rios Leal, E.; González-Chávez, M.d.C.A. Screening bacterial phosphate solubilization with bulk-tricalcium phosphate and hydroxyapatite nanoparticles. Antonie Leeuwenhoek 2020, 113, 1033–1047. [Google Scholar] [CrossRef] [PubMed]
- Zuluaga, M.Y.A.; de Oliveira, A.L.M.; Valentinuzzi, F.; Jayme, N.S.; Monterisi, S.; Fattorini, R.; Cesco, S.; Pii, Y. An insight into the role of the organic acids produced by Enterobacter sp. strain 15S in solubilizing tricalcium phosphate: In situ study on cucumber. BMC Microbiol. 2023, 23, 184. [Google Scholar] [CrossRef]
- Chen, X.; Yang, C.; Palta, J.A.; Li, Y.; Fan, X. An Enterobacter cloacae strain NG-33 that can solubilize phosphate and promote maize growth. Front. Microbiol. 2022, 13, 1047313. [Google Scholar] [CrossRef] [PubMed]
- Damo, J.L.C.; Ramirez, M.D.A.; Agake, S.-I.; Pedro, M.; Brown, M.; Sekimoto, H.; Yokoyama, T.; Sugihara, S.; Okazaki, S.; Ohkama-Ohtsu, N. Isolation and Characterization of Phosphate Solubilizing Bacteria from Paddy Field Soils in Japan. Microbes Environ. 2022, 37, ME21085. [Google Scholar] [CrossRef]
- Li, X.L.; Zhao, X.Q.; Dong, X.Y.; Ma, J.F.; Shen, R.F. Secretion of Gluconic Acid from Nguyenibacter sp. L1 Is Responsible for Solubilization of Aluminum Phosphate. Front. Microbiol. 2021, 12, 784025. [Google Scholar] [CrossRef] [PubMed]
- Buch, A.; Archana, G.; Naresh, K. Metabolic chanelling of glucose towards gluconate in phosphate solubilizing Pseudomonas aeruginosa P4 under phosphorus deficiency. Res. Microbiol. 2008, 159, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Stella, M.; Halimi, M.S. Gluconic acid production by bacteria to liberate phosphorus from insoluble phosphate complexes. J. Trop. Agric. Food Sci. 2015, 43, 41–53. [Google Scholar]
- Goldstein, A.H. Recent Progress in Understanding the Molecular Genetics and Biochemistry of Calcium Phosphate Solubilization by Gram Negative Bacteria. Biol. Agric. Hortic. 1995, 12, 185–193. [Google Scholar] [CrossRef]
- Wang, D.; Zhan, J.; Sun, Q.-Y. Phosphate solubilization of Aureobasidium pullulan F4 and its mechanism. Ying Yong Sheng Tai Xue Bao 2014, 25, 2079–2084. [Google Scholar] [PubMed]
- Ranawat, B.; Bachani, P.; Singh, A.; Mishra, S. Enterobacter hormaechei as Plant Growth-Promoting Bacteria for Improvement in Lycopersicum esculentum. Curr. Microbiol. 2021, 78, 1208–1217. [Google Scholar] [CrossRef] [PubMed]
- Anzuay, M.S.; Prenollio, A.; Ludueña, L.M.; Morla, F.D.; Cerliani, C.; Lucero, C.; Angelini, J.G.; Taurian, T. Enterobacter sp. J49: A Native Plant Growth-Promoting Bacteria as Alternative to the Application of Chemical Fertilizers on Peanut and Maize Crops. Curr. Microbiol. 2023, 80, 85. [Google Scholar] [CrossRef] [PubMed]
- Madhaiyan, M.; Peng, N.; Te, N.S.; Hsin I, C.; Lin, C.; Lin, F.; Reddy, C.; Yan, H.; Ji, L. Improvement of plant growth and seed yield in Jatropha curcas by a novel nitrogen-fixing root associated Enterobacter species. Biotechnol. Biofuels 2013, 6, 140. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Reddy, M.S. Phosphate solubilizing rhizobacteria from an organic farm and their influence on the growth and yield of maize (Zea mays L.). J. Gen. Appl. Microbiol. 2013, 59, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Sulieman, S.; Tran, L.-S.P. Phosphorus homeostasis in legume nodules as an adaptive strategy to phosphorus deficiency. Plant Sci. 2015, 239, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.; Ryan, M.H.; Lambers, H.; Siddique, K.H. Phosphorus acquisition and utilisation in crop legumes under global change. Curr. Opin. Plant Biol. 2018, 45, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Ljones, T. Nitrogen fixation and bioenergetics: The role of ATP in nitrogenase catalysis. FEBS Lett. 1979, 98, 981–988. [Google Scholar] [CrossRef] [PubMed]


| Substrates for Assimilation Tests | Result * |
|---|---|
| Ala-Phe-Pro-Arylamidase | − |
| Adonitol | − |
| L-Pyrrolydonyl-Arylamidase | − |
| L-Arabitol | − |
| D-Cellobiose | + |
| Beta-Galactosidase | + |
| H2S Production | − |
| Beta-N-Acetyl-Glucosaminidase | + |
| Glutamyl Arylamidase pNA | − |
| D-Glucose | + |
| Gamma-Glutamyl-Transferase | + |
| Fermentation/Glucose | + |
| Beta-Glucosidase | − |
| D-Maltose | + |
| D-Mannitol | + |
| D-Mannose | + |
| Beta-Xylosidase | + |
| Beta-Alanine arylamidase pNA | − |
| L-Proline Arylamidase | + |
| Lipase | − |
| Palatinose | + |
| Tyrosine Arylamidase | + |
| Urease | − |
| D-Sorbitol | + |
| Saccharose/Sucrose | + |
| D-Tagatose | − |
| D-Trehalose | + |
| Citrate (Sodium) | + |
| Malonate | − |
| 5-Keto-D-Gluconate | − |
| L-Lactate alkalinization | + |
| Alpha-Glucosidase | − |
| Succinate alkalization | + |
| Beta-N-Acetyl-Galactosaminidase | + |
| Alpha-Galactosidase | − |
| Phosphatase | − |
| Glycine Arylamidase | + |
| Ornithine Decarboxylase | + |
| Decarboxylase Base | − |
| L-Histidine Assimilation | − |
| Coumarate | − |
| Beta-Glucuronidase | − |
| 0/129 Resistance (comp. Vibrio) | + |
| Glu-Gly-Arg-Arylamidase | − |
| L-Malate Assimilation | − |
| Ellman | − |
| L-Lactate assimilation | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Damo, J.L.C.; Pedro, M.; Sison, M.L. Phosphate Solubilization and Plant Growth Promotion by Enterobacter sp. Isolate. Appl. Microbiol. 2024, 4, 1177-1192. https://doi.org/10.3390/applmicrobiol4030080
Damo JLC, Pedro M, Sison ML. Phosphate Solubilization and Plant Growth Promotion by Enterobacter sp. Isolate. Applied Microbiology. 2024; 4(3):1177-1192. https://doi.org/10.3390/applmicrobiol4030080
Chicago/Turabian StyleDamo, Jean Louise Cocson, Mannix Pedro, and Maria Lourdes Sison. 2024. "Phosphate Solubilization and Plant Growth Promotion by Enterobacter sp. Isolate" Applied Microbiology 4, no. 3: 1177-1192. https://doi.org/10.3390/applmicrobiol4030080
APA StyleDamo, J. L. C., Pedro, M., & Sison, M. L. (2024). Phosphate Solubilization and Plant Growth Promotion by Enterobacter sp. Isolate. Applied Microbiology, 4(3), 1177-1192. https://doi.org/10.3390/applmicrobiol4030080






