Abstract
The coronavirus disease-2019 (COVID-19) pandemic has had a profound impact on the world, causing loss of life, economic damage, and social disruption. Individuals with chronic kidney disease (CKD) are prone to complications and increased mortality related to COVID-19. Efforts have been made to increase understanding of the effects of COVID-19 in individuals with CKD. This paper aims to gather and discuss the state-of-the-art in the COVID-19 and CKD literature, involving the early history of COVID-19, the immunological aspects of CKD (such as abnormalities in neutrophilicand dendritic cells functions), the mechanisms of kidney injury by SARS-CoV-2 (for example, viral tropism to kidney tissue and direct cytotoxicity), the clinical course of the disease and consequences for CKD individuals (including kidney transplant recipients), and the emerging treatments (such as the use of Remdesivir, nirmatrelvir, and monoclonal neutralizing antibodies) and immunization for the CKD population.
1. Introduction
The World Health Organization (WHO) declared a global pandemic of COVID-19 in March 2020, originating from Wuhan, China. As of February 2023, SARS-CoV-2 has affected over 750 million individuals and caused more than 6.85 million deaths worldwide [1,2,3]. Preliminary studies have describedclinical aspects and potential mechanisms responsible for COVID-19 infection, such as endothelial dysfunction, coagulopathy, and cytokines, and observed increased morbidity and mortality rates among vulnerable populations [4,5,6]. COVID-19 exhibits unique features, such as extrapulmonary damage and rapid progression to acute respiratory syndrome (ARDS) [7], involving organs such as the kidneys, which may be affected by indirect damage due to the involvement of several other systems [8], viral tropism, and the lung-kidney axis in ARDS [9].
Long before the COVID-19 pandemic, chronic kidney disease (CKD) was already a global concern. CKD affects approximately 12% of the global population, with itestimated to increase to more than 20% by 2040 [10,11,12], primarily due to aging and the increased prevalence of associated diseases such as diabetes, obesity, and hypertension. In the pandemic scenario, individuals with CKD are disproportionately affected compared to the general population, mainly due to immunological and clinical aspects of infections. CKD is the most prevalent risk factor for severe COVID-19 worldwide [13], frequently coexisting with other comorbidities, increasing the risk of COVID-19 mortality [14]. Individuals with CKD are at higher risk of worsening conditions due to their chronic immunosuppression, which may result from pharmacological treatments, in the case of transplant patients, or due to the inflammatory state of kidney disease and uremia [15].
This paper aims to provide a narrative review of the scientific literature on COVID-19 and CKD, involving immunological aspects of CKD, the clinical course of the disease, and its consequences for CKD individuals. Mechanisms of kidney injury by SARS-CoV-2 and COVID-19-associated nephropathy are discussed. Kidney transplants and COVID-19 are discussed in a specific section. Finally, emerging treatments and immunization strategies for the CKD population are revised in depth.
2. Effects of CKD on the Immune System and Clinical Consequences Related to COVID-19
2.1. Chronic Impairment of the Immune System in CKD
Individuals with CKD are at an increased risk of death from non-cardiovascular causes, mainly by infection [16]. Of note, kidneys can be among the target organs during infections by SARS-CoV-2 [17,18]. In a systematic review and meta-analysis in a cohort of 17,391 COVID-19 patients, a relation between these two diseases was highlighted: a pooled prevalence of 5.2% of pre-existing CKD before infections were observed [19].
The immune system of those with CKD is chronically impaired, and end-stage kidney disease (ESKD) provides a good model for analyzing the effects of CKD on immunity. The high prevalence of ESKD is concomitant with advanced age and other comorbidities such as hypertension and diabetes [20]. Thus, the mortality rate among individuals in this group of patients is high, with studies pointing to a range of 20 to 50% among hospitalized admitted to intensive care units (ICU) [7,21]. During the pandemic, dialysis patients showed an increased risk of serious complications related to COVID-19, such as arrhythmias, shock, ARDS, and heart failure, in addition to atypical manifestations, such as involvement of the gastrointestinal tract and nervous system [21]. Along with social and specific factors of the treatment routine in dialysis centers, potential mechanisms for these events are discussed soon after.
For these reasons, infections account for 20% of deaths in ESKD patients [22]. Along with infections, the cardiovascular risk of this population is also of concern and contributes even more to mortality, with cardiovascular diseases accounting for 50% of deaths in this group [22]. These two factors together explain the high annual mortality rate ranging from 12% to 20% in this group of patients [22,23], despite advances in handling and care improved over the last few years.
Uremia can lead to a permanent state of immune dysfunction characterized by low-grade inflammation and chronic immunosuppression. One of the particular traits of uremia is hypercytokinemia, which leads to the effects of pro-inflammatory cytokines in different systems [24]. This occurs due to several factors, including a decrease in renal excretion, retention of uremic toxins, oxidative stress, volume overload, and other comorbidities [24].
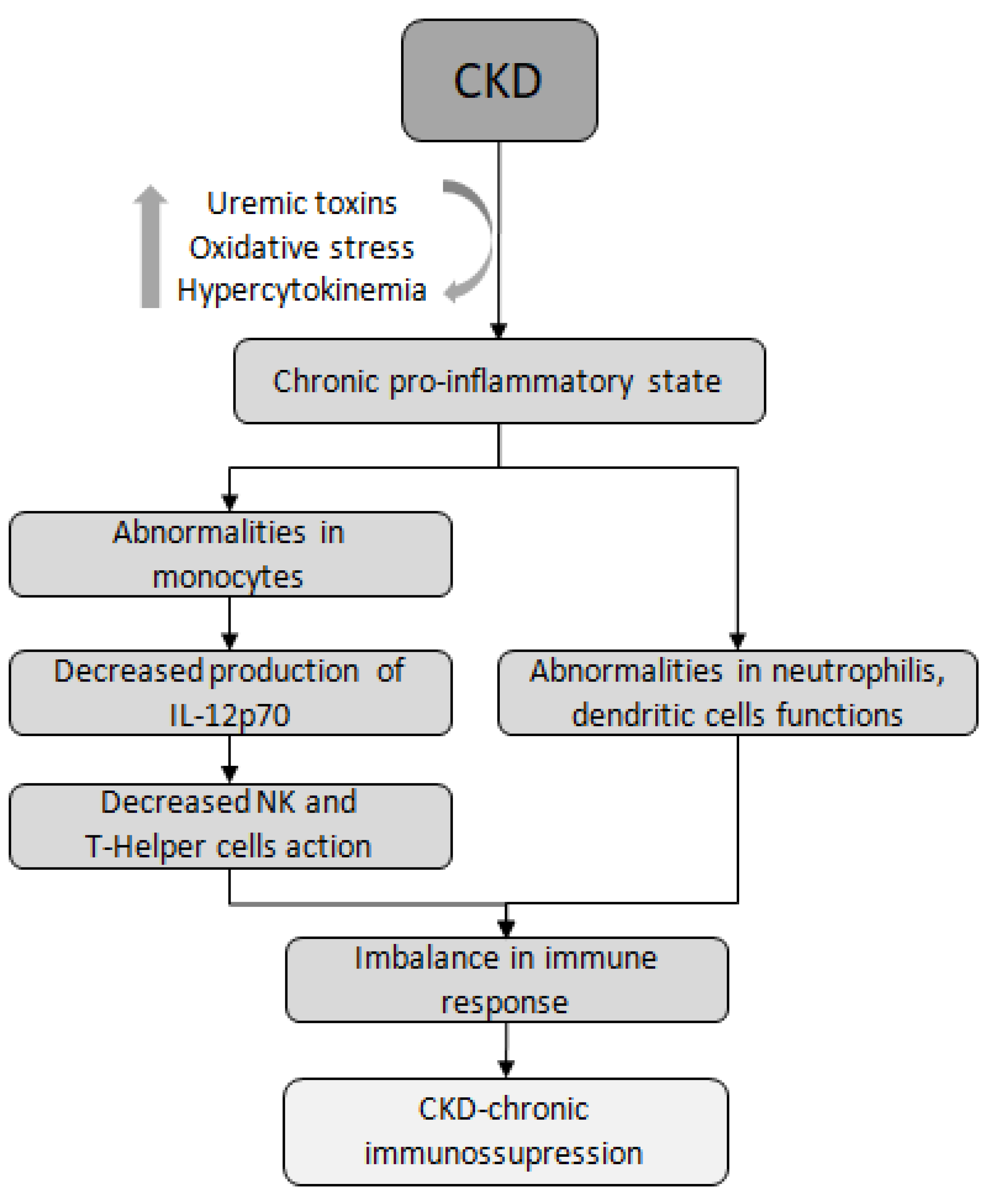
Research suggests that both arms of the immune system, innate and adaptive, contribute to increased rates of infections in CKD. Abnormalities in the function of immune cells, such as monocytes, neutrophils, and dendritic cells (DCs), are directly related to the increased risk and severity of infections in these patients [15,25,26,27,28]. Uremic media, found in individuals with advanced CKD, have been shown to inhibit the function of monocytes and the production of IL-12p70, an interleukin produced by macrophages and DCs. IL-12p70 plays a crucial role in inducing NK cells to engage in cytotoxic action, which is important in viral conditions. Additionally, this interleukin contributes to the production of IFN-γ and facilitates the connection between innate and adaptive immunity [29]. Another mechanism is the impaired maturation of T lymphocytes. For example, in patients undergoing peritoneal dialysis (PD),it was observed a significant reduction in the synthesis of TNF-α and IL-1β, along with the development of a T helper inhibited phenotype, suggesting a potential hyporeaction against infections in these patients [25] (Figure 1).
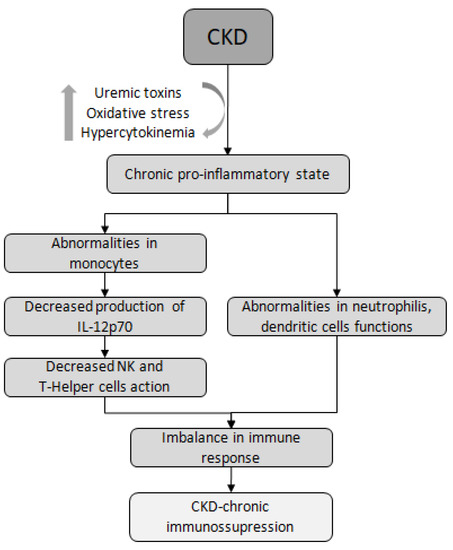
Figure 1.
Immune dysfunction in CKD. CKD, chronic kidney disease; NK, Natural Killer lymphocyte.
Verkade et al. [26] proposed that immunodeficiency in patients with CKD is also attributed to a deficiency in the control and function of cells derived from dendritic cells (moDCs) compared to healthy individuals. Cells from the CKD patient group demonstrated inadequate moDC control, irrespective of the stimulus employed, suggesting compromised terminal differentiation and impaired antigen-presenting capacity. The analysis was based on the membrane marker’s expression of CD14, CD40, CD86, CD83, anti-HLA-ABC, anti-CCR5, and anti-CCR7. The CKD group exhibited lower expression of HLA class II, CD83, and CD86 compared to healthy controls, indicating a higher prevalence of immature cell phenotypes in CKD [26].
Similarly, the maturation and dysfunction of T lymphocytes also affect adaptive immunity in individuals with CKD. While the degree of lymphopenia in these patients alone cannot explain the deficiency of adaptive immunity [30], it is hypothesized that disturbances in cellular behavior and function contribute to the reduced effectiveness of vaccinations against agents such as the hepatitis B virus, influenza virus, Clostridium tetani, or Corynebacterium diphtheriae [31]. In CKD patients, the T lymphocyte proliferation is reduced, and mRNA expression of IL-2 and IFN-γ are reduced [31], suggesting a potentially impaired immune response to COVID-19 vaccination as well.
The chronic immune impairment presented by ESKD patients can be aggravated by immunosuppressive therapies in cases where kidney transplantation (KT) is necessary to reduce the risk of graft rejection and improve survival rates. However, these therapies can increase the risk of infections and, subsequently, the likelihood of developing cardiovascular diseases [32,33], as infections have already been linked to these diseases [34]. Therefore, while KT may initially confer a survival advantage, the increased risk of infections must be considered when making treatment decisions, such as the use of immunosuppressants.
2.2. Increased Infection Rate and Mortality among Patients with CKD
Being a dialysis patient (HD or PD), CKD stages G4–5, or kidney transplant recipient were three of the four top risk categories for death due to COVID-19, according to the OpenSAFELY analysis, which examined data from 17 million patients and 10,926 COVID-19-related deaths [14]. Among patients with CKD, those undergoing hemodialysis (HD) were disproportionately at risk ofSARS-CoV-2 infections in comparison with those with non-dialytic CKD. A meta-analysis by Chung et al. [35], which included 382,407 participants with COVID-19 and CKD, and 1,139,979 total participants with CKD, found that the incidence of COVID-19 was higher in people undergoing HD compared to those with CKD without dialytic therapy or with CKD in all stages (105 per 10,000 person-weeks vs. 16 per 10,000 person-weeks and 66 per 10,000 person-weeks, respectively) [35]. The limitation of this analysis was the low-certainty evidence data available, but its findings were corroborated by another study that concluded that dialysis patients have an increased risk of infections and in-hospital death when compared to pre-dialysis CKD patients, with an incidence ranging from 20% to 31%, underscoring their heightened vulnerability during a pandemic [7,36,37,38,39,40].
Compared to PD patients, those undergoing HD had a fourfold increased incidence rate of SARS-CoV-2 infections, possibly because HD sessions are carried out in dialysis centers, where viral transmission is likely due to prolonged contact between healthcare workers and patients, whereas PD patients may have sessions at home [41]. During the first wave of the pandemic, in countries most affected by infections, the mortality rate reported for HD patients was 30% [42]. Soon after, there was a decrease to 13–16%, partially explained by the finding of a high prevalence of asymptomatic cases among HD patients, as well as in the general population [43,44]; However, this rate still higher for HD patients than that observed in the latter group. Another factor contributing to the increased risk of infection in HD patients is their need to travel to dialysis centers by collective vans or buses [45,46]. Thus, this population was continuously exposed during the pandemic, even in periods when isolation was reinforced for the general population by health authorities [47].
Another great concern with CKD patients during the pandemic was due to their potential higher possibility of increased severity of infections by SARS-CoV-2 with the use of drugs such as angiotensin-converting enzyme inhibitors (ACEis) and angiotensin receptor blockers (ARBs) [48]. It is known that the virus enters cells through ACE2 receptors [49], and, thus, at the beginning of the pandemic, the concern was that this mechanism could further increase the mortality in infected patients with CKD using this medication [48]. However, large studies of systematic reviews and cohorts were unable to prove such an increase in severity or mortality in the group of infected patients using these medications [48,50].
3. Mechanisms of Kidney Injury by SARS-CoV-2
The mechanisms of kidney injury in SARS-CoV-2 infections are diverse and may include an imbalance in the immune response and direct cytotoxicity [51,52]. Evidence suggests that in patients who died from COVID-19, viral proteins and RNA were detected in glomerular, endothelial, and tubular epithelial cells [51,52], indicating the potential for renal tropism of the virus, which may result in direct damage to the organ, in addition to the renal injury classically observed in critically ill patients. The viral action on cells was initially described as the result of the virus’s ability to enter host cells via ACE2 receptors in the kidneys, particularly in the proximal convoluted tubules [53,54].
The high prevalence of COVID-19 globally raised concerns about its impacts on individuals with highly prevalent diseases, such as CKD [55]. CKD is one of the most important risk factors for acute kidney injury (AKI), requiring kidney replacement therapy (KRT) among hospitalized patients with COVID-19 [56,57]. AKI is a common complication of COVID-19, occurring in 9% of hospitalized patients and up to 68% of patients in ICU [58]. This occurrence is associated with a poor prognosis, deterioration of renal function, possible progression to CKD, and potentially the need for KRT [59,60,61]. While most patients who recover from COVID-19-associated AKI regain their kidney function, one-third of them are at risk of remaining on dialysis at discharge [57]. AKI is a common complication independent of baseline levels of kidney function [8] and, in conjunction with hypertension, is associated with a twofold increase in mortality and a threefold increase in the severity of infections [7,62]. The pathogenic mechanisms that could result in AKI include hypoxia, inflammation, sepsis, hemodynamic disturbances, endothelial dysfunction, kidney infarction, use of nephrotoxic drugs, and SARS-CoV-2 infection [8]. In most cases, AKI is mild to moderate, with an increase in serum creatinine, hematuria, proteinuria, and electrolyte disorders [19,63,64].
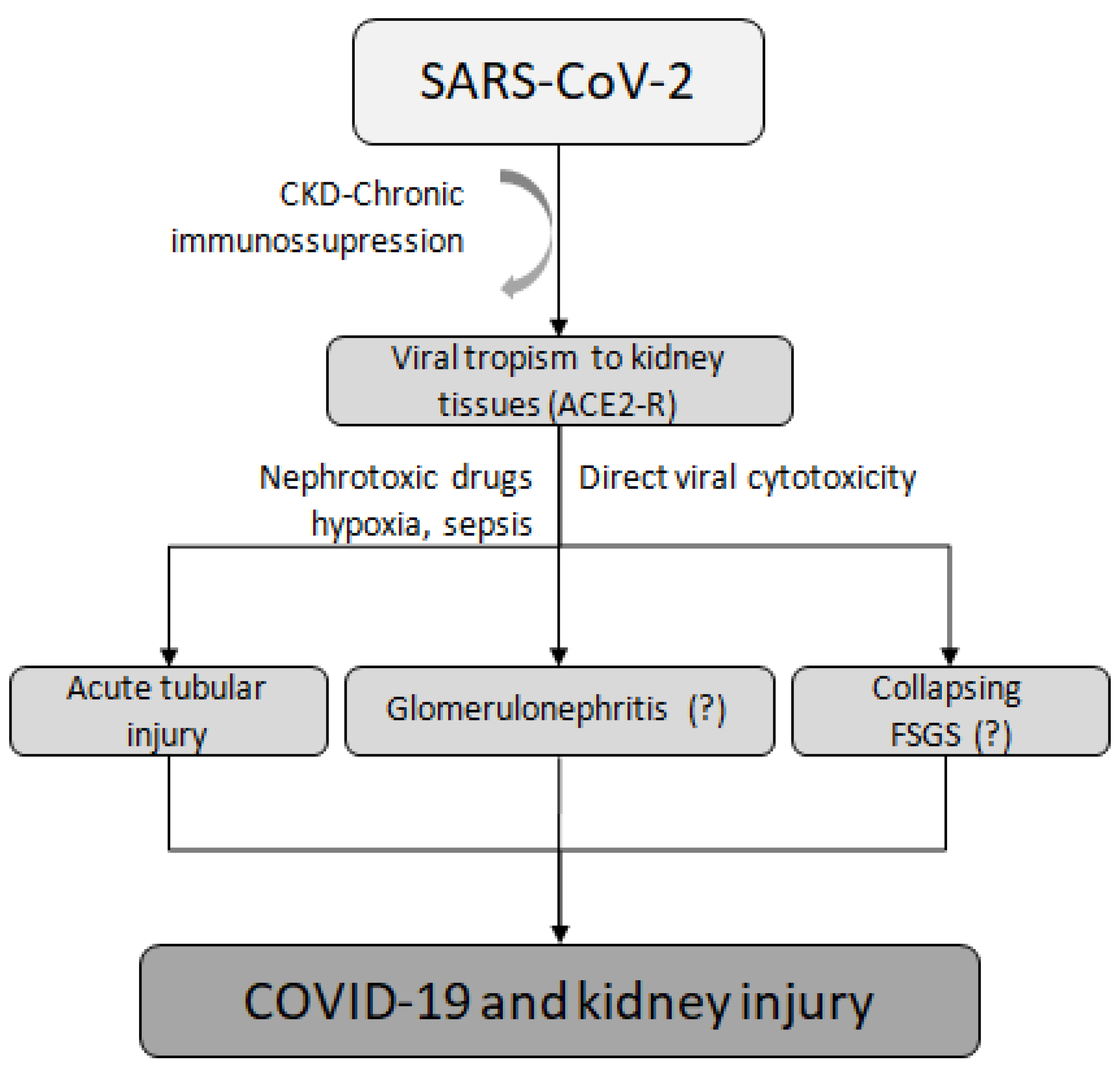
The most common cause of AKI in patients with COVID-19 is acute tubular injury [65,66,67], although other etiologies, such as COVID-19-associated nephropathy (COVAN), have been observed [59]. For instance, a case was reported of an infected man treated with rituximab who initially presented with mild symptoms but then experienced a rapidly progressive decline in renal function caused by COVAN, ultimately leading to ESKD and the need for chronic dialysis [59]. Other forms of kidney damage, such as glomerulonephritis and collapsing focal segmental glomerulosclerosis, are considered rare and are caused by renal virus tropism, direct damage, and nephrotoxicity, as evidenced by the finding of COVID-19 virus-like particles in renal autopsy samples [52,68]. One possible mechanism that could explain this is the ability of SARS-CoV-2 to enter kidney tubule epithelial cells and podocytes via ACE2 receptors, causing direct renal injury [52] (Figure 2).
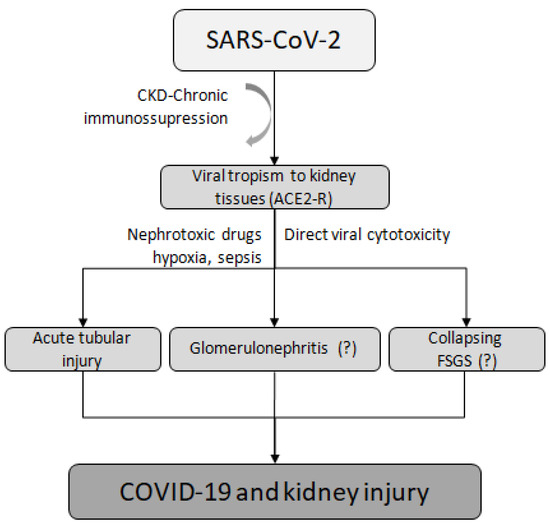
Figure 2.
Mechanisms of kidney injury related to SARS-CoV-2. CKD, chronic kidney disease; ACE2-R, angiotensin-converting enzyme 2 receptor; FSGS, focal segmental glomerulosclerosis.
4. COVID-19 and Kidney Transplant
The COVID-19 pandemic had a significant impact on organ transplantation, particularly on KT from living donors. Some health societies recommended suspending these transplants during periods of increased transmission to reduce the risk of infection and complications for recipients [69,70,71,72]. As a result, there were several restrictions imposed on organ donation and reception to protect potential recipients [68] and a 51% decrease in transplant activity was observed in the United States, while in France, this rate was 91%, both mostly driven by KT [73]. In the first pandemic peak, this decrease was due to scarce hospital resources, insufficient testing capacity and sensitivity, and poor outcomes related to COVID-19 in transplant recipients [74].
Studies have shown the heightened vulnerability of KT recipients to severe COVID-19 infections in comparison to the entire CKD spectrum. For instance, a cohort of 36 KT patients at a Medical Center in New York [75] had a 28% mortality rate within 21 days of diagnosis, with 96% exhibiting radiological signs of pneumonia on X-ray. Among this group, 39% required mechanical ventilation, and 21% required KRT [76]. Therefore, it is crucial to analyze the incidence of COVID-19 in both KT recipients and those on active waiting lists for deceased donors. In a study focused on the transplanted population, Ravanan et al. [77] reported a COVID-19 infection rate of 1.3% in recipient patients (597 cases out of 46,789) and 3.8% in waitlisted patients (197 cases out of 5184). While these rates are not higher than those in the general population, it is essential to give special attention to this group due to their higher mortality rates, as observed in other studies [77].
This increased vulnerability to severe COVID-19 infections in KT patients, as well as for HD patients, is due to chronic immunosuppression and underlying comorbidities. A Spanish prospective cohort study [78] of 1011 COVID-19-positive KT patients found that advanced age, pneumonia, and KT in the previous 6 months were independent predictors of mortality, while the presence of gastrointestinal symptoms was associated with lower death rates. Duivenvoorden et al. [79] analyzed additional variables associated with COVID-19 mortality in this population using ERACODA, a database of KT recipient information from over 130 dialysis centers in 33 countries [79]. Out of the 912 patients included in the study, 487 had available graft function information with a mean eGFR of 40 mL/min/1.73 m2 at admission. The study found that 21.8% of patients experienced a >25% increase in serum creatinine levels compared to pre-infection levels, while biopsy-proven graft rejection was rare, occurring in only fourpatients (0.8%) [79]. The study also reported lower 3-month KRT rates (2.6%), with higher rates in patients admitted to ICU (10.6%). Graft rejection occurred at similar rates in hospitalized (1%) and non-hospitalized (0.7%) patients, with the proportion of rejection increased in ICU patients, 10.7%, with five patients experiencing irreversible rejection by the end of the three-month follow-up [79].
Due to the fragility often found in transplanted patients, immunosuppressive therapy should consider the severity of infections and the potential for aggravation of the conditions presented by individuals because of the possible interaction between antivirals and immunosuppressants. In addition, the use of drugs, dose reduction, or suspension should be considered to prevent rejection and graft loss [80]. Especially in immunosuppressive therapies that involve the induction of T-cell depletion, the effects can be long-lasting, which could lead an individual to perform viral clearance more slowly, making it difficult to assemble humoral responses in cases of COVID-19. This is due to lymphopenia and a low count of T cells in transplanted patients [81]. This situation creates a dilemma between the need for immunosuppression for graft survival and the need not to disarm the immune system so profoundly that immune responses are compromised in a pandemic scenario. Patients with a functioning graft are prone to AKI, a common complication of COVID-19 infections in recipients. AKI is observed in 30–89% of hospitalized transplanted patients and may result from various factors, such as low renal perfusion, multiorgan failure, and cytokine storm [81]. Although AKI associated with SARS-CoV-2 infections usually leads to transient conditions, it may also contribute to graft rejection [81].
Reducing immunosuppressants to prevent severe infections or death from COVID-19 could increase the risk of cardiovascular disease, which is already a major concern for transplant patients due to prolonged inflammatory states associated with CKD. This creates a significant dilemma in managing these patients, particularly in the context of the pandemic. On the one hand, the immune system needs to be competent to fight infections, while on the other hand, chronic inflammatory states can increase cardiovascular risk, leading to damage to vascular and cardiac structures if immune responses are not properly modulated [32,33]. Despite extensive research, there is still no consensus in the literature regarding the reduction or suspension of immunosuppressants, even in the case of acute COVID-19 infections.
5. Emerging Treatments of COVID-19 in CKD Patients
Currently, the effectiveness of strategies used to treat COVID-19 infections in individuals with CKD is not fully validated. While antivirals, monoclonal antibodies, steroids, and anticoagulants are available treatment options for the general population [82], therapies for CKD patients must be individualized to ensure success without causing harm. The decision on therapy and dosage must be made judiciously. One option for individuals with kidney disease is favipiravir, an antiviral drug that was considered one of the most effective treatments for COVID-19 in patients with ESKD. The drug does not require dose adjustment for mild to moderate CKD, but it should be avoided when eGFR < 30 mL/min/1.73m2 [83]. However, its serum concentration in HD patients is similar to that of patients with normal renal function, indicating its potential use for treating individuals with CKD [84].
Clinical studies have also been conducted with Remdesivir, the first drug approved by the FDA for treating COVID-19 infections. Evidence shows that Remdesivir reduces recovery time in hospitalized individuals with pneumonia, from 15 days in the placebo group to 10 days in the treatment group. Moreover, the authors observed a decrease in mortality presented by patients treated vs. placebo group (6.7% vs. 11,9% by day 15, respectively) [85]. A study involving COVID-19 patients with reduced renal function (15 < eGFR < 60 mL/min/1.73 m2) concluded that its use was not associated with an increased risk of adverse renal outcomes [86,87]. However, concerns about its use in individuals with CKD still exist due to the excipient of the drug, sulfobutylether beta-cyclodextrin, which may accumulate in patients with impaired renal function, although there are no reports of significant clinical repercussions [88].
Also, a recent study [78] suggests that nirmatrelvir/ritonavir can be used as a therapeutic option in cases of COVID-19, especially in high-risk populations such as those with advanced CKD. The proposal is to administer adjusted doses of nirmatrelvir/ritonavir when there is infection by the coronavirus [78], but it is contraindicated when eGFR < 15 mL/min/1.73 m2. Ongoing studies are also analyzing the safety and clinical outcomes of these drugs in CKD patients stages 4 and 5 [89] and HD patients [90].
Another treatment with emerging evidence of efficacy is anti-SARS-CoV-2 monoclonal neutralizing antibodies (mAbs). Its advantage lies in its rapid action and the possibility of prophylactic use [91]. However, some antibody lineages are considered obsolete for new strains of the virus due to its high mutation rate. The FDA currently recommends the use of eightmonoclonal antibodies, with cilgavimad and tixagevimad considered pre-exposure prophylactic and treatment options for immunocompromised individuals [91]. Sotrovimab, casirivimab, and imdevimab association do not require a dose adjustment and bypass renal clearance, making them suitable for use in the CKD population [82]. Tocilizumab has been shown to increase the survival of COVID-19 patients and can be used without adjustment in patients with mild to moderate kidney disease, although concerns still exist for patients with ESKD [82].
Significant progress has been made in treating COVID-19 in patients with CKD since the start of the pandemic. However, further studies are needed to develop effective treatment options for different profiles of CKD patients, ensuring that they receive safe and appropriate treatment.
6. COVID-19 Immunization in Individuals with CKD
6.1. CKD Patients Have an Impaired Response to COVID-19 Vaccination
The immunization of individuals with CKD is challenging, despite the great advances in terms of knowledge of kidney disease, its immunological peculiarities, and management. Individuals with kidney disease, including KT patients, exhibit a suboptimal response to vaccination compared to healthy controls [92]. Most studies used mRNA vaccines for immunization, with limited reports on other vaccination mechanisms, such as inactivated viruses or viral vectors. These studies indicated a slight disadvantage in achieving seroconversion in individuals with CKD compared to those who received mRNA vaccines [93,94,95]. Additionally, administering different types of vaccines in a heterologous regimen has been found to increase immune response compared to homologous regimens, as observed in a study of patients undergoing HD [96,97]. Within the CKD spectrum, a significant percentage of patients lost immunity against the virus three months after receiving the last vaccine dose, and the majority exhibited a significant decrease in anti-spike antibody titers [98]. KT patients, who are particularly vulnerable, showed the most prominent decrease in the humoral response, with over 50% of vaccinated patients having undetectable antibodies during this period [98]. This decline was more pronounced in transplant recipients over a six-month period [99]. Consequently, one of the questions raised in the literature pertains to the optimal timing for administering a booster dose of the vaccine to these individuals.
6.2. Immune Response in ESKD, HD, and Kidney Transplant Patients
The impaired immune response and vulnerability of individuals with renal impairment underscore the importance of effective vaccines to protect these patients, despite the aforementioned difficulty in achieving adequate immunization in immunosuppressed patients [100]. Patients with CKD stages G4/5 or on HD exhibit a comparable immune response to vaccines when compared to control groups, while KT recipients have a lower response, necessitating the development of alternative immunization strategies. A Spanish multicenter study [101] evaluated immune responses after vaccination. The trial included individuals with CKD (eGFR < 30 mL/min/1.73 m2), and the responses measured consisted of the production of anti-spike antibodies. KT patients showed a lower response even after completing the entire vaccination schedule. The third dose improved seroconversion in 69% of previously negative patients. Compared to the two-dose vaccination scheme, the application of the third dose was associated with a greater proportion of positive humoral response in transplant recipients (80% vs. 53%), patients on PD (100% vs. 71%), on HD (96% vs. 64%), and non-dialytic CKD (97% vs. 73%) [102]. Differences in the immune response to vaccination were found across the spectrum of CKD. Although the presence of anti-spike antibodies was found in 95% of patients at the end of 28 days after vaccination, a negative humoral response was observed in 21% of KT recipients, highlighting the vulnerability of this population to SARS-CoV-2 infection [101]. Moreover, the type of vaccine used for immunization is an independent predictor of deficient immune response [101].
A Dutch multicenter and prospective study called RECOVACalso found a lower immunization rate in KT patients in comparison to the responses measured for individuals with CKD stages 4, 5, dialysis, and healthy participants [103]. KT patients had significantly lower seroconversion compared to healthy participants (56.9% vs. 100% seroconversion). Factors associated with lower response included mycophenolic acid use, advanced age, lower lymphocyte concentration, lower eGFR, and shorter time after transplantation. Compared to the control group (eGFR > 45 mL/min/1.73 m2), KT patients had lower serum titers of anti-SARS-CoV-2 neutralizing antibodies and lower T-cell response. However, patients with CKD G4/5 had a seroconversion rate of 100%, similar to the control group, and individuals on dialysis had a high rate of seroconversion (99.4%). Nevertheless, the mean serum concentrations of antibodies in patients with CKD G4/5 and on dialysis were lower than those observed in the control group (2.405 and 1.650 vs. 3.186 BAU/mL). Additionally, adverse reactions to vaccination were lower in patients under dialysis treatment or KT recipients [103]. Immunosuppression in KT recipients might influence the development of anti-SARS-CoV-2 antibodies. The exact mechanism is unknown, as well as the impact of different regimens of immunosuppression. Cited studies did not assess cellular immunity. Five ongoing studies are currently analyzing the immune response to COVID-19 vaccination in individuals with CKD, including those on dialysis, or KT, receiving booster doses, and long-term follow-up. These studies reflect the continued need to develop specific immunization strategies for this population [104,105,106,107,108].
7. Conclusions
Since the start of the pandemic, numerous clinical and experimental studies have gathered data about SARS-CoV-2, COVID-19, and CKD. Robust data showed that patients with CKD are more prone to developing severe complications from infection due to several factors, such as an imbalance in immune response, comorbidities, and immunosuppression. Gradually the understanding of the effects of this virus by direct cytotoxicity and kidney tropism effects, as well as the emergence of specific treatments by antiviral drugs and monoclonal antibodies, is advancing. Although much progress has been made in understanding COVID-19 in CKD, many challenges remain.
One crucial step is to conduct further research to develop and test drugs tailored to the needs of individuals with kidney disease at different stages who are infected with SARS-CoV-2. Unique aspects of chronic uremia and immunosuppression make these patients more vulnerable to severe infections and cardiovascular diseases, necessitating distinct management strategies from those for the general population. Moreover, the immune response deficiency in CKD patients underscores the inadequacy of vaccine regimens and agents used for healthy individuals, particularly those with KT.
Author Contributions
Writing—original draft preparation, M.P.M. and R.B.d.O.; writing—review and editing, M.P.M. and R.B.d.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors have no conflictsof interest to declare.
References
- World Health Organization. World Health Organization COVID-19 Dashboard [Internet]. 2023. Available online: https://covid19.who.int (accessed on 1 May 2023).
- Barbosa, E.J.M.; Gefter, W.B.; Ghesu, F.C.; Liu, S.; Mailhe, B.; Mansoor, A.; Grbic, S.; Vogt, S. Automated Detection and Quantification of COVID-19 Airspace Disease on Chest Radiographs: A Novel Approach Achieving Expert Radiologist-Level Performance Using a Deep Convolutional Neural Network Trained on Digital Reconstructed Radiographs From Computed Tomography-Derived Ground Truth. Invest. Radiol. 2021, 56, 471–479. [Google Scholar]
- Das, N.N.; Kumar, N.; Kaur, M.; Kumar, V.; Singh, D. Automated Deep Transfer Learning-Based Approach for Detection of COVID-19 Infection in Chest X-rays. Ing. Rech. Biomed. 2022, 43, 114–119. [Google Scholar] [CrossRef]
- Gupta, N.; Zhao, Y.-Y.; Evans, C.E. The stimulation of thrombosis by hypoxia. Thromb. Res. 2019, 181, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [PubMed]
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Vanstapel, A.; Werlein, C.; Stark, H.; Tzankov, A.; et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in COVID-19. N. Engl. J. Med. 2020, 383, 120–128. [Google Scholar]
- Zhou, S.; Xu, J.; Xue, C.; Yang, B.; Mao, Z.; Ong, A.C.M. Coronavirus-associated kidney outcomes in COVID-19, SARS, and MERS: A meta-analysis and systematic review. Ren. Fail. 2020, 43, 1–15. [Google Scholar] [CrossRef]
- Kellum, J.A.; van Till, J.W.O.; Mulligan, G. Targeting acute kidney injury in COVID-19. Nephrol. Dial. Transplant. 2020, 35, 1652–1662. [Google Scholar] [CrossRef]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. Clinical Characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Koye, D.N.; Magliano, D.J.; Nelson, R.G.; Pavkov, M.E. The Global Epidemiology of Diabetes and Kidney Disease. Adv. Chronic Kidney Dis. 2018, 25, 121–132. [Google Scholar] [CrossRef]
- Glovaci, D.; Fan, W.; Wong, N.D. Epidemiology of Diabetes Mellitus and Cardiovascular Disease. Curr. Cardiol. Rep. 2019, 21, 21. [Google Scholar] [CrossRef]
- Thurlow, J.S.; Joshi, M.; Yan, G.; Norris, K.C.; Agodoa, L.Y.; Yuan, C.M.; Nee, R. Global Epidemiology of End-Stage Kidney Disease and Disparities in Kidney Replacement Therapy. Am. J. Nephrol. 2021, 52, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Council, E.-E.; Ortiz, A.; Cozzolino, M.; Fliser, D.; Fouque, D.; Goumenos, D.; Massy, Z.A.; Rosenkranz, A.R.; Rychlık, I.; Soler, M.J.; et al. Chronic kidney disease is a key risk factor for severe COVID-19: A call to action by the ERA-EDTA. Nephrol. Dial. Transplant. 2021, 36, 87–94. [Google Scholar] [CrossRef]
- Williamson, E.J.; Walker, A.J.; Bhaskaran, K.; Bacon, S.; Bates, C.; Morton, C.E.; Curtis, H.J.; Mehrkar, A.; Evans, D.; Inglesby, P.; et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020, 584, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.; Kireta, S.; Leedham, E.; Russ, G.; Coates, P. Uremia impairs monocyte and monocyte-derived dendritic cell function in hemodialysis patients. Kidney Int. 2007, 72, 1138–1148. [Google Scholar] [CrossRef]
- Ortiz, P.A.; Covic, A.; Fliser, D.; Fouque, D.; Goldsmith, D.; Kanbay, M.; Mallamaci, F.; Massy, Z.A.; Rossignol, P.; Vanholder, R.; et al. Epidemiology, contributors to, and clinical trials of mortality risk in chronic kidney failure. Lancet 2014, 383, 1831–1843. [Google Scholar] [CrossRef]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA J. Am. Med. Assoc. 2020, 323, 1061–1069. [Google Scholar]
- Liakopoulos, V.; Roumeliotis, S.; Papachristou, S.; Papanas, N. COVID-19 and the kidney: Time to take a closer look. Int. Urol. Nephrol. 2022, 54, 1053–1057. [Google Scholar] [CrossRef]
- Kunutsor, S.K.; Laukkanen, J.A. Renal complications in COVID-19: A systematic review and meta-analysis. Ann. Med. 2020, 52, 345–353. [Google Scholar] [CrossRef]
- Valeri, A.M.; Robbins-Juarez, S.Y.; Stevens, J.S.; Ahn, W.; Rao, M.K.; Radhakrishnan, J.; Gharavi, A.G.; Mohan, S.; Husain, S.A. Presentation and Outcomes of Patients with ESKD and COVID-19. J. Am. Soc. Nephrol. 2020, 31, 1409–1415. [Google Scholar] [CrossRef]
- Flythe, J.E.; Assimon, M.M.; Tugman, M.J.; Chang, E.H.; Gupta, S.; Shah, J.; Sosa, M.A.; Renaghan, A.D.; Melamed, M.L.; Wilson, F.P.; et al. Characteristics and Outcomes of Individuals With Pre-existing Kidney Disease and COVID-19 Admitted to Intensive Care Units in the United States. Am. J. Kidney Dis. 2021, 77, 190–203. [Google Scholar] [CrossRef]
- Sarnak, M.J.; Jaber, B.L. Mortality caused by sepsis in patients with end-stage renal disease compared with the general population. Kidney Int. 2000, 58, 1758–1764. [Google Scholar] [PubMed]
- Luxardo, R.; Ceretta, L.; González-Bedat, M.; Ferreiro, A.; Rosa-Diez, G. The Latin American Dialysis and Renal Transplantation Registry: Report 2019. Clin. Kidney J. 2022, 15, 425–431. [Google Scholar] [CrossRef]
- Stenvinkel, P.; Ketteler, M.; Johnson, R.J.; Lindholm, B.; Pecoits-Filho, R.; Riella, M.; Heimbürger, O.; Cederholm, T.; Girndt, M. IL-10, IL-6, and TNF-α: Central factors in the altered cytokine network of uremia—The good, the bad, and the ugly. Kidney Int. 2005, 67, 1216–1233. [Google Scholar] [PubMed]
- Ando, M.; Shibuya, A.; Yasuda, M.; Azuma, N.; Tsuchiya, K.; Akiba, T.; Nitta, K. Impairment of innate cellular response to in vitro stimuli in patients on continuous ambulatory peritoneal dialysis. Nephrol. Dial. Transplant. 2005, 20, 2497–2503. [Google Scholar] [CrossRef] [PubMed]
- Verkade, M.A.; Van Druningen, C.J.; Vaessen, L.M.B.; Hesselink, D.A.; Weimar, W.; Betjes, M.G.H. Functional impairment of monocyte-derived dendritic cells in patients with severe chronic kidney disease. Nephrol. Dial. Transplant. 2007, 22, 128–138. [Google Scholar] [CrossRef]
- Anding, K.; Gross, P.; Rost, J.M.; Allgaier, D.; Jacobs, E. The influence of uraemia and haemodialysis on neutrophil phagocytosis and antimicrobial killing. Nephrol. Dial. Transplant. 2003, 18, 2067–2073. [Google Scholar] [CrossRef]
- Cendoroglo, M.; Jaber, B.L.; Balakrishnan, V.S.; Perianayagam, M.; King, A.J.; Pereira, B.J.G. Neutrophil Apoptosis and Dysfunction in Uremia. J. Am. Soc. Nephrol. 1999, 10, 93–100. [Google Scholar] [CrossRef]
- Benveniste, E.N. Cytokines. Encyclopedia of the Neurological Sciences, 2nd ed.; Daroff, R.B., Aminoff, M.J., Eds.; Academic Press: Cambridge, MA, USA, 2014; pp. 921–925. ISBN 9780123851581. [Google Scholar]
- Deenitchina, S.S.; Ando, T.; Okuda, S.; Kinukawa, N.; Hirakata, H.; Nagashima, A.; Fujishima, M. Cellular Immunity in Hemodialysis Patients: A Quantitative Analysis of Immune Cell Subsets by Flow Cytometry. Am. J. Nephrol. 1995, 15, 57–65. [Google Scholar] [CrossRef]
- Eleftheriadis, T.; Antoniadi, G.; Liakopoulos, V.; Kartsios, C.; Stefanidis, I. Basic Science and Dialysis: Disturbances of Acquired Immunity in Hemodialysis Patients. Semin. Dial. 2007, 20, 440–451. [Google Scholar] [CrossRef]
- Kiechl, S.; Egger, G.; Mayr, M.; Wiedermann, C.J.; Bonora, E.; Oberhollenzer, F.; Muggeo, M.; Xu, Q.; Wick, G.; Poewe, W.; et al. Chronic infections and the risk of carotid atherosclerosis: Prospective results from a large population study. Circulation 2001, 103, 1064–1070. [Google Scholar]
- Smeeth, L.; Thomas, S.L.; Hall, A.J.; Hubbard, R.; Farrington, P.; Vallance, P. Risk of Myocardial Infarction and Stroke after Acute Infection or Vaccination. N. Engl. J. Med. 2004, 351, 2611–2618. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. Inflammation in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2045–2051. [Google Scholar] [PubMed]
- Chung, E.Y.; Palmer, S.C.; Natale, P.; Krishnan, A.; Cooper, T.E.; Saglimbene, V.M.; Ruospo, M.; Au, E.; Jayanti, S.; Liang, A.; et al. Incidence and Outcomes of COVID-19 in People With CKD: A Systematic Review and Meta-analysis. Am. J. Kidney Dis. 2021, 78, 804–815. [Google Scholar] [CrossRef] [PubMed]
- Keller, N.; Chantrel, F.; Krummel, T.; Bazin-Kara, D.; Faller, A.L.; Muller, C.; Nussbaumer, T.; Ismer, M.; Benmoussa, A.; Brahim-Bouna, M.; et al. Impact of first-wave COronaVIrus disease 2019 infection in patients on haemoDIALysis in Alsace: The observational COVIDIAL study. Nephrol. Dial. Transplant. 2020, 35, 1338–1411. [Google Scholar] [CrossRef] [PubMed]
- Tortonese, S.; Scriabine, I.; Anjou, L.; Loens, C.; Michon, A.; Benabdelhak, M.; Ouali, S.; Morin, G.; Laifi, M.; Dobosziewicz, H.; et al. COVID-19 in Patients on Maintenance Dialysis in the Paris Region. Kidney Int. Rep. 2020, 5, 1535–1544. [Google Scholar] [CrossRef] [PubMed]
- Alberici, F.; Delbarba, E.; Manenti, C.; Econimo, L.; Valerio, F.; Pola, A.; Maffei, C.; Possenti, S.; Lucca, B.; Cortinovis, R.; et al. A report from the Brescia Renal COVID Task Force on the clinical characteristics and short-term outcome of hemodialysis patients with SARS-CoV-2 infection. Kidney Int. 2020, 98, 20–26. [Google Scholar]
- Sánchez-Álvarez, J.E.; Fontán, M.P.; Martín, C.J.; Pelícano, M.B.; Reina, C.J.C.; Prieto, Á.M.S.; Melilli, E.; Barrios, M.C.; Heras, M.M.; del Pino, M.D. Situación de la infecciónpor SARS-CoV-2 enpacientesentratamiento renal sustitutivo. Informe del Registro COVID-19 de la Sociedad Española de Nefrología (SEN). Nefrología 2020, 40, 272–278. [Google Scholar]
- Corbett, R.W.; Blakey, S.; Nitsch, D.; Loucaidou, M.; McLean, A.; Duncan, N.; Ashby, D.R. West London Renal and Transplant Centre. Epidemiology of COVID-19 in an Urban Dialysis Center. J. Am. Soc. Nephrol. 2020, 31, 1815–1823. [Google Scholar] [CrossRef]
- Zeng, X.; Huang, X.; Xu, L.; Xiao, J.; Gu, L.; Wang, Y.; Tuo, Y.; Fang, X.; Wang, W.; Li, N.; et al. Clinical outcomes of dialysis patients with COVID-19 in the initial phase of the COVID-19 outbreak in Wuhan, China. Int. Urol. Nephrol. 2021, 53, 353–357. [Google Scholar] [CrossRef]
- Goicoechea, M.; Cámara, L.A.S.; Macías, N.; de Morales, A.M.; Rojas, Á.G.; Bascuñana, A.; Arroyo, D.; Vega, A.; Abad, S.; Verde, E.; et al. COVID-19: Clinical course and outcomes of 36 hemodialysis patients in Spain. Kidney Int. 2020, 98, 27–34. [Google Scholar]
- Da Cunha, T.S.; Gomá-Garcés, E.; Avello, A.; Pereira-García, M.; Mas-Fontao, S.; Ortiz, A.; González-Parra, E. The Spectrum of Clinical and Serological Features of COVID-19 in Urban Hemodialysis Patients. J. Clin. Med. 2020, 9, 2264. [Google Scholar] [CrossRef]
- Albalate, M.; Arribas, P.; Torres, E.; Cintra, M.; Alcázar, R.; Puerta, M.; Ortega, M.; Procaccini, F.; Martin, J.; Jiménez, E.; et al. Alta prevalencia de COVID-19 asintomáticoenhemodiálisis. Aprendiendo día a día el primer mes de pandemia de COVID-19. Nefrología 2020, 40, 279–286. [Google Scholar] [PubMed]
- Fernandez-Prado, R.; Gonzalez-Parra, E.; Ortiz, A. Often forgotten, transport modality to dialysis may be life-saving. Clin. Kidney J. 2020, 13, 510–512. [Google Scholar] [CrossRef] [PubMed]
- Quiroga, B.; Soler, M.J.; Ortiz, A.; Sequera, P.; SENCOVAC Collaborative Network. Lessons from SENCOVAC: A prospective study evaluating the response to SARS-CoV-2 vaccination in the CKD spectrum. Nefrologia, 2022; in press. [Google Scholar] [CrossRef]
- Ortiz, A. Madrid at the center of the Spanish COVID-19 pandemic: The result of ill-advised political decision-making. Port. J. Nephrol. Hypertens. 2021, 35, 205–206. [Google Scholar] [CrossRef]
- de la Cruz, A.; Ashraf, S.; Vittorio, T.J.; Bella, J.N. COVID-19 and renin-angiotensin system modulators: What do we know so far? Expert Rev. Cardiovasc. Ther. 2020, 18, 743–748. [Google Scholar] [CrossRef]
- Zhou, P.; Yang, X.-L.; Wang, X.-G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, C.-L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef]
- European Society of Cardiology. Position Statement of the ESC Council on Hypertension on ACE-Inhibitors and Angiotensin Receptor Blockers. Eur. Soc. Cardiol. 2020. Available online: https://www.escardio.org/Councils/Council-on-Hypertension-(CHT)/News/position-statement-of-the-esc-council-on-hypertension-on-ace-inhibitors-and-ang#:~:text=The%20Council%20on%20Hypertension%20strongly,of%20the%20Covid%2D19%20infection (accessed on 1 May 2023).
- Puelles, V.G.; Lütgehetmann, M.; Lindenmeyer, M.T.; Sperhake, J.P.; Wong, M.N.; Allweiss, L.; Chilla, S.; Heinemann, A.; Wanner, N.; Liu, S.; et al. Multiorgan and Renal Tropism of SARS-CoV-2. N. Engl. J. Med. 2020, 383, 590–592. [Google Scholar] [CrossRef]
- Su, H.; Yang, M.; Wan, C.; Yi, L.-X.; Tang, F.; Zhu, H.-Y.; Yi, F.; Yang, H.-C.; Fogo, A.B.; Nie, X.; et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020, 98, 219–227. [Google Scholar] [CrossRef]
- Fan, C.; Lu, W.; Li, K.; Ding, Y.; Wang, J. ACE2 Expression in Kidney and Testis May Cause Kidney and Testis Infection in COVID-19 Patients. Front. Med. 2021, 7, 563893. [Google Scholar] [CrossRef]
- He, L.; Ding, Y.; Zhang, Q.; Che, X.; He, Y.; Shen, H.; Wang, H.; Li, Z.; Zhao, L.; Geng, J.; et al. Expression of elevated levels of pro-inflammatory cytokines in SARS-CoV-infected ACE2+ cells in SARS patients: Relation to the acute lung injury and pathogenesis of SARS. J. Pathol. A J. Pathol. Soc. Great Br. Irel. 2006, 210, 288–297. [Google Scholar]
- Foreman, K.J.; Marquez, N.; Dolgert, A.; Fukutaki, K.; Fullman, N.; McGaughey, M.; Pletcher, M.A.; Smith, A.E.; Tang, K.; Yuan, C.-W.; et al. Forecasting life expectancy, years of life lost, and all-Cause and cause-Specific mortality for 250 causes of death: Reference and alternative scenarios for 2016-40 for 195 countries and territories. Lancet 2018, 392, 2052–2090. [Google Scholar] [PubMed]
- Hirsch, J.S.; Ng, J.H.; Ross, D.W.; Sharma, P.; Shah, H.H.; Barnett, R.L.; Hazzan, A.D.; Fishbane, S.; Jhaveri, K.D.; on behalf of theNorthwell COVID-19 Research Consortium and theNorthwell Nephrology COVID-19 Research Consortium. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020, 98, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Coca, S.G.; Chan, L.; Melamed, M.L.; Brenner, S.K.; Hayek, S.S.; Sutherland, A.; Puri, S.; Srivastava, A.; Leonberg-Yoo, A.; et al. Faculty Opinions recommendation of AKI Treated with Renal Replacement Therapy in Critically Ill Patients with COVID-19. J. Am. Soc. Nephrol. 2021, 32, 161–176. [Google Scholar] [CrossRef] [PubMed]
- Chan, L.; Chaudhary, K.; Saha, A.; Chauhan, K.; Vaid, A.; Zhao, S.; Paranjpe, I.; Somani, S.; Richter, F.; Miotto, R.; et al. AKI in Hospitalized Patients with COVID-19. J. Am. Soc. Nephrol. 2021, 32, 151–160. [Google Scholar] [CrossRef]
- Maldonado, D.; Ray, J.; Lin, X.; Salem, F.; Brown, M.; Bansal, I. COVAN Leading to ESKD Despite Minimal COVID Symptoms. J. Investig. Med. High Impact Case Rep. 2022, 10, 23247096221093888. [Google Scholar] [CrossRef]
- Kant, S.; Menez, S.P.; Hanouneh, M.; Fine, D.M.; Crews, D.C.; Brennan, D.C.; Sperati, C.J.; Jaar, B.G. The COVID-19 nephrology compendium: AKI, CKD, ESKD and transplantation. BMC Nephrol. 2020, 21, 449. [Google Scholar] [CrossRef]
- Nadim, M.K.; Forni, L.G.; Mehta, R.L.; Connor, M.J., Jr.; Liu, K.D.; Ostermann, M.; Rimmelé, T.; Zarbock, A.; Bell, S.; Bihorac, A.; et al. COVID-19-associated acute kidney injury: Consensus report of the 25th Acute Disease Quality Initiative (ADQI) Workgroup. Nat. Rev. Nephrol. 2020, 16, 747–764. [Google Scholar] [CrossRef]
- Henry, B.M.; Lippi, G. Chronic kidney disease is associated with severe coronavirus disease 2019 (COVID-19) infection. Int. Urol. Nephrol. 2020, 52, 1193–1194. [Google Scholar] [CrossRef]
- Robbins-Juarez, S.Y.; Qian, L.; King, K.L.; Stevens, J.S.; Husain, S.A.; Radhakrishnan, J.; Mohan, S. Outcomes for Patients With COVID-19 and Acute Kidney Injury: A Systematic Review and Meta-Analysis. Kidney Int. Rep. 2020, 5, 1149–1160. [Google Scholar] [CrossRef]
- Kissling, S.; Rotman, S.; Gerber, C.; Halfon, M.; Lamoth, F.; Comte, D.; Lhopitallier, L.; Sadallah, S.; Fakhouri, F. Collapsing glomerulopathy in a COVID-19 patient. Kidney Int. 2020, 98, 228–231. [Google Scholar] [CrossRef]
- Ng, J.H.; Bijol, V.; Sparks, M.A.; Sise, M.E.; Izzedine, H.; Jhaveri, K.D. Pathophysiology and Pathology of Acute Kidney Injury in Patients With COVID-19. Adv. Chronic Kidney Dis. 2020, 27, 365–376. [Google Scholar] [CrossRef]
- Farouk, S.S.; Fiaccadori, E.; Cravedi, P.; Campbell, K.N. COVID-19 and the kidney: What we think we know so far and what we don’t. J. Nephrol. 2020, 33, 1213–1218. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.M.; Lukitsch, I.; Torres-Ortiz, A.E.; Walker, J.B.; Varghese, V.; Hernandez-Arroyo, C.F.; Alqudsi, M.; LeDoux, J.R.; Velez, J.C.Q. Acute Kidney Injury Associated with Coronavirus Disease 2019 in Urban New Orleans. Kidney360 2020, 1, 614–622. [Google Scholar]
- Santoriello, D.; Khairallah, P.; Bomback, A.S.; Xu, K.; Kudose, S.; Batal, I.; Barasch, J.; Radhakrishnan, J.; D’agati, V.; Markowitz, G. Postmortem Kidney Pathology Findings in Patients with COVID-19. J. Am. Soc. Nephrol. 2020, 31, 2158–2167. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, A.B.H.; Nazish, M.; Khan, N.Y.; Manan, F.; Zia, H.H.; Ilyas, A.; Ishtiaq, W.; Khan, N.A. Living Donor Liver Transplantation During the COVID-19 Pandemic: An Evolving Challenge. J. Gastrointest. Surg. 2021, 25, 3092–3098. [Google Scholar] [CrossRef] [PubMed]
- Ahn, C.; Amer, H.; Anglicheau, D.; Ascher, N.; Baan, C.; Battsetset, G.; Bat-Ireedui, B.; Berney, T.; Betjes, M.; Bichu, S.; et al. Global Transplantation COVID Report March 2020. Transplantation 2020, 104, 1974–1983. [Google Scholar] [CrossRef]
- Cravedi, P.; Schold, J.D.; Safa, K.; Kates, O.S.; Elfadawy, N.; Mannon, R.B.; Shah, M.B.; Hammond, S.P.; Avery, R.; Miranda, C.G.; et al. The COVID-19 pandemic: A community approach. Clin. Transplant. 2020, 34, e14059. [Google Scholar] [CrossRef]
- Zidan, A.; Alabbad, S.; Ali, T.; Nizami, I.; Haberal, M.; Tokat, Y.; Kamel, R.; Said, H.; Abdelaal, A.; Elsharkawy, M.; et al. Position Statement of Transplant Activity in the Middle East in Era of COVID-19 Pandemic. Transplantation 2020, 104, 2205–2207. [Google Scholar] [CrossRef]
- Loupy, A.; Aubert, O.; Reese, P.P.; Bastien, O.; Bayer, F.; Jacquelinet, C. Organ procurement and transplantation during the COVID-19 pandemic. Lancet 2020, 395, e95–e96. [Google Scholar] [CrossRef]
- Ajaimy, M.; Liriano-Ward, L.; Graham, J.A.; Akalin, E. Risks and Benefits of Kidney Transplantation during the COVID-19 Pandemic: Transplant or Not Transplant? Kidney360 2021, 2, 1179–1187. [Google Scholar] [CrossRef]
- Akalin, E.; Azzi, Y.; Bartash, R.; Seethamraju, H.; Parides, M.; Hemmige, V.; Ross, M.; Forest, S.; Goldstein, Y.D.; Ajaimy, M.; et al. COVID-19 and Kidney Transplantation. N. Engl. J. Med. 2020, 382, 2475–2477. [Google Scholar] [CrossRef]
- Azzi, Y.; Bartash, R.; Scalea, J.; Loarte-Campos, P.; Akalin, E. COVID-19 and Solid Organ Transplantation: A Review Article. Transplantation 2021, 105, 37–55. [Google Scholar] [CrossRef] [PubMed]
- Ravanan, R.; Callaghan, C.J.; Mumford, L.; Ushiro-Lumb, I.; Thorburn, D.; Casey, J.; Friend, P.; Parameshwar, J.; Currie, I.; Burnapp, L.; et al. SARS-CoV-2 infection and early mortality of waitlisted and solid organ transplant recipients in England: A national cohort study. Am. J. Transplant. 2020, 20, 3008–3018. [Google Scholar] [CrossRef] [PubMed]
- Villanego, F.; Mazuecos, A.; Pérez-Flores, I.M.; Moreso, F.; Andrés, A.; Jiménez-Martín, C.; Molina, M.; Canal, C.; Sánchez-Cámara, L.A.; Zárraga, S.; et al. Predictors of severe COVID-19 in kidney transplant recipients in the different epidemic waves: Analysis of the Spanish Registry. Am. J. Transplant. 2021, 21, 2573–2582. [Google Scholar] [CrossRef] [PubMed]
- Duivenvoorden, R.; Vart, P.; Noordzij, M.; dos Santos, A.C.S.; Zulkarnaev, A.B.; Franssen, C.F.M.; Kuypers, D.; Demir, E.; Rahimzadeh, H.; Kerschbaum, J.; et al. Clinical, Functional, and Mental Health Outcomes in Kidney Transplant Recipients 3 Months After a Diagnosis of COVID-19. Transplantation 2022, 106, 1012–1023. [Google Scholar] [CrossRef] [PubMed]
- Angelico, R.; Blasi, F.; Manzia, T.M.; Toti, L.; Tisone, G.; Cacciola, R. The Management of Immunosuppression in Kidney Transplant Recipients with COVID-19 Disease: An Update and Systematic Review of the Literature. Medicina 2021, 57, 435. [Google Scholar] [CrossRef] [PubMed]
- Lubetzky, M.; Aull, M.J.; Craig-Schapiro, R.; Lee, J.R.; Marku-Podvorica, J.; Salinas, T.; Gingras, L.; Lee, J.B.; Sultan, S.; Kodiyanplakkal, R.P.; et al. Kidney allograft recipients, immunosuppression, and coronavirus disease-2019: A report of consecutive cases from a New York City transplant center. Nephrol. Dial. Transplant. 2020, 35, 1250–1261. [Google Scholar] [CrossRef] [PubMed]
- Violetta, L.; Kartasasmita, A.S.; AmirullahRoesli, R.M.; Rita, C. Pharmacological Treatment Options for Coronavirus Disease-19 in Renal Patients. Int. J. Nephrol. 2021, 2021, 4078713. [Google Scholar]
- Marra, F.; Smolders, E.J.; El-Sherif, O.; Boyle, A.; Davidson, K.; Sommerville, A.J.; Marzolini, C.; Siccardi, M.; Burger, D.; Gibbons, S.; et al. Recommendations for Dosing of Repurposed COVID-19 Medications in Patients with Renal and Hepatic Impairment. Drugs R&D 2020, 21, 9–27. [Google Scholar] [CrossRef]
- Koshi, E.; Saito, S.; Okazaki, M.; Toyama, Y.; Ishimoto, T.; Kosugi, T.; Hiraiwa, H.; Jingushi, N.; Yamamoto, T.; Ozaki, M.; et al. Efficacy of favipiravir for an end stage renal disease patient on maintenance hemodialysis infected with novel coronavirus disease 2019. CEN Case Rep. 2020, 10, 126–131. [Google Scholar] [CrossRef]
- Beigel, J.H.; Tomashek, K.M.; Dodd, L.E.; Mehta, A.K.; Zingman, B.S.; Kalil, A.C.; Hohmann, E.; Chu, H.Y.; Luetkemeyer, A.; Kline, S.; et al. Remdesivir for the Treatment of COVID-19—Final Report. N. Engl. J. Med. 2020, 383, 1813–1826. [Google Scholar] [CrossRef] [PubMed]
- Seethapathy, R.; Wang, Q.; Zhao, S.; Strohbehn, I.A.; Long, J.D.; Dinulos, J.E.; Harden, D.; Kadiyala, V.B.; Moreno, D.; Sise, M.E. Effect of remdesivir on adverse kidney outcomes in hospitalized patients with COVID-19 and impaired kidney function. PLoS ONE 2023, 18, e0279765. [Google Scholar] [CrossRef] [PubMed]
- Aiswarya, D.; Arumugam, V.; Dineshkumar, T.; Gopalakrishnan, N.; Lamech, T.M.; Nithya, G.; Sastry, B.V.; Vathsalyan, P.; Dhanapriya, J.; Sakthirajan, R. Use of Remdesivir in Patients With COVID-19 on Hemodialysis: A Study of Safety and Tolerance. Kidney Int. Rep. 2020, 6, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Thakare, S.; Gandhi, C.; Modi, T.; Bose, S.; Deb, S.; Saxena, N.; Katyal, A.; Patil, A.; Patil, S.; Pajai, A.; et al. Safety of Remdesivir in Patients With Acute Kidney Injury or CKD. Kidney Int. Rep. 2021, 6, 206–210. [Google Scholar] [CrossRef]
- Ming, C.K.; Chinese University of Hong Kong Research Group. Safety and Clinical and Virologic Outcomes in COVID-19 Patients With Chronic Kidney Disease Treated With Nirmatrelvir-Ritonavir. Available online: https://clinicaltrials.gov/ct2/show/NCT05624840?t (accessed on 1 May 2023).
- RenJi Hospital Research Group. The Safety of Paxlovid (Nirmatrelvir/Ritonavir) in Hemodialysis Patients With SARS-CoV-2 Infection. Available online: https://clinicaltrials.gov/ct2/show/NCT05366192?draw=5>.+NCT05366192 (accessed on 1 May 2023).
- Nguyen, Y.; Flahault, A.; Chavarot, N.; Melenotte, C.; Cheminant, M.; Deschamps, P.; Carlier, N.; Lafont, E.; Thomas, M.; Flamarion, E.; et al. Pre-exposure prophylaxis with tixagevimab and cilgavimab (Evusheld) for COVID-19 among 1112 severely immunocompromised patients. Clin. Microbiol. Infect. 2022, 28, 1654.e1–1654.e4. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.M.; Tam, A.R.; Chan, K.W.; Ma, M.K.M.; Hung, I.F.N.; Yap, D.Y.H.; Chan, T.M. Immunogenicity and Safety of COVID-19 Vaccines in Patients Receiving Renal Replacement Therapy: A Systematic Review and Meta-Analysis. Front. Med. 2022, 9, 827859. [Google Scholar] [CrossRef]
- Chen, C.C.; Huang, Y.J.; Lai, M.J.; Lin, M.H.; Lin, W.C.; Lin, H.Y.; Lin, Y.C.; Huang, Y.T.; Lee, Y.F.; Tsai, M.K.; et al. Immunogenicity and safety of two-dose SARS-CoV-2 vaccination via different platforms in kidney transplantation recipients. Front. Immunol. 2022, 13, 951576. [Google Scholar] [CrossRef]
- Asderakis, A.; Khalid, U.; Koimtzis, G.; Ponsford, M.J.; Szabo, L.; Chalklin, C.; Bramhall, K.; Grant, L.; Moat, S.J.; Humphreys, I.R.; et al. An Analysis of Serological Response and Infection Outcomes Following Oxford-AstraZeneca (AZD1222) and Pfizer-BioNTech (mRNA BNT162b2) SARS-CoV-2 Vaccines in Kidney and Kidney-pancreas Transplants. Transplantation 2022, 106, 1421–1429. [Google Scholar] [CrossRef]
- Trakarnvanich, T.; Ngamvichchukorn, T.; Phumisantiphong, U.; Pholtawornkulchai, K.; Phochanasomboon, K.; Manomaipiboon, A. Immune response after COVID-19 vaccination among patients with chronic kidney disease and kidney transplant. Vaccine 2022, 40, 6499–6511. [Google Scholar] [CrossRef]
- Haase, M.; Lesny, P.; Anderson, M.; Cloherty, G.; Stec, M.; Haase-Fielitz, A.; Haarhaus, M.; Santos-Araújo, C.; Veiga, P.M.; Macario, F. Humoral immunogenicity and tolerability of heterologous ChAd/BNT compared with homologous BNT/BNT and ChAd/ChAd SARS-CoV-2 vaccination in hemodialysis patients: A multicenter prospective observational study. J. Nephrol. 2022, 35, 1467–1478. [Google Scholar] [CrossRef]
- Speer, C.; Schaier, M.; Nusshag, C.; Töllner, M.; Buylaert, M.; Kälble, F.; Reichel, P.; Grenz, J.; Süsal, C.; Zeier, M.; et al. Longitudinal Humoral Responses after COVID-19 Vaccination in Peritoneal and Hemodialysis Patients over Twelve Weeks. Vaccines 2021, 9, 1130. [Google Scholar] [CrossRef]
- Quiroga, B.; Soler, M.J.; Ortiz, A.; Bernat, A.; Muñoz Díaz, A.B.; Jarava Mantecón, C.J.; Gómez Pérez, V.O.; Calderón González, C.; Cervienka, M.; Mazuecos, A.; et al. Loss of humoral response 3 months after SARS-CoV-2 vaccination in the CKD spectrum: The multicentric SENCOVAC study. Nephrol. Dial. Transplant. 2022, 37, 994–999. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.M.; Weiner, D.E.; Manley, H.J.; Aweh, G.N.; Ladik, V.; Frament, J.; Miskulin, D.; Argyropoulos, C.; Abreo, K.; Chin, A.; et al. Seroresponse to SARS-CoV-2 Vaccines among Maintenance Dialysis Patients over 6 Months. Clin. J. Am. Soc. Nephrol. 2022, 17, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.; Chitturi, C.; Yee, J. Vaccination in Chronic Kidney Disease. Adv. Chronic Kidney Dis. 2019, 26, 72–78. [Google Scholar] [CrossRef]
- Quiroga, B.; Soler, M.J.; Ortiz, A.; Vaquera, S.M.; Mantecón, C.J.J.; Useche, G.; Márquez, M.G.S.; Carnerero, M.; Rodríguez, M.T.J.; Ramos, P.M.; et al. Safety and immediate humoral response of COVID-19 vaccines in chronic kidney disease patients: The SENCOVAC study. Nephrol. Dial. Transplant. 2022, 37, 1868–1878. [Google Scholar] [CrossRef]
- Quiroga, B.; Soler, M.J.; Ortiz, A.; Orero, E.; Tejedor, S.; Mantecón, C.J.J.; Perez, V.O.G.; Franco, A.J.M.; Sánchez, C.A.; Carretero, M.P.; et al. Humoral Response to Third Dose of SARS-CoV-2 Vaccines in the CKD Spectrum. Clin. J. Am. Soc. Nephrol. 2022, 17, 872–876. [Google Scholar] [CrossRef]
- Sanders, J.-S.F.; Bemelman, F.J.; Messchendorp, A.L.; Baan, C.C.; van Baarle, D.; van Binnendijk, R.; Diavatopoulos, D.A.; Frölke, S.C.; Geers, D.; GeurtsvanKessel, C.H.; et al. The RECOVAC Immune-response Study: The Immunogenicity, Tolerability, and Safety of COVID-19 Vaccination in Patients With Chronic Kidney Disease, on Dialysis, or Living With a Kidney Transplant. Transplantation 2022, 106, 821–834. [Google Scholar] [CrossRef]
- Hemmelder, M.H. The RECOVAC LESS CoV-2 Study—Long Term Efficacy and Safety of SARS-CoV-2 Vaccination in Patients in Patients With Chronic Kidney Disease Stage G4-G5, on Dialysis or After Kidney Transplantation. Available online: https://clinicaltrials.gov/study/NCT04841785 (accessed on 1 May 2023).
- Sunnybrook Health Sciences Centre Research Group. COVID-19 Vaccine Boosters in Patients With CKD (BOOST KIDNEY). Available online: https://clinicaltrials.gov/ct2/show/NCT05022329 (accessed on 1 May 2023).
- Stegbauer, J. Vaccination Against COVID-19 in Chronic Kidney Disease. Available online: https://clinicaltrials.gov/ct2/show/NCT04743947 (accessed on 1 May 2023).
- Matthew, P.M. Phenotyping Seroconversion Following Vaccination Against COVID-19 in Patients on Haemodialysis Study. Available online: https://clinicaltrials.gov/ct2/show/NCT04815850 (accessed on 1 May 2023).
- Ntounousi, E. The Response of the Immune System of Patients With End Stage Kidney Disease on Dialysis and Kidney Transplant Recipients Vaccinated for COVID-19. Available online: https://clinicaltrials.gov/ct2/show/NCT04932876 (accessed on 1 May 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).