Abstract
The Coronavirus disease 2019 (COVID-19) has significantly affected kidney transplantation activities around the world, thus resulting in a substantial decrease in both deceased and living transplants. This study presents a COVID-19 overview from the perspective of the Brazilian kidney transplant program by comparing its differences or similarities with the situations observed in other countries. During the first year of the pandemic, there was a 40% reduction in the number of kidney transplants worldwide. A similar scenario was observed in Brazil, which has the world’s largest public transplantation program. Beyond its effect on transplant activity, COVID-19 has influenced the outcomes of prevalent kidney transplant recipients (KTRs) because the prolonged use of immunosuppressive drugs and comorbidities increase the susceptibility of such patients to severe disease and death. In the pre-vaccination era, almost two-thirds of KTRs required hospitalization, more than 20% required dialysis, and one-third was admitted to the intensive care unit. In the pre-vaccination period in Brazil, 15% and 21% of KTRs died within 28 and 90 days of COVID-19 diagnosis, respectively. Although high vaccination coverage rates have altered the COVID-19 landscape in many populations, persistently low immunogenicity rates following sequential vaccination shots and the absence of targeted treatments for severe cases continue to classify KTRs as highly vulnerable, thus warranting significant concern.
1. Introduction
Since the World Health Organization declared the arrival of the Coronavirus disease 2019 (COVID-19) a pandemic, the disease has significantly affected transplant programs worldwide [1,2]. Such consequences began with the impairment of transplant activity, such as delays in notifying recipients and reductions in the capacity of centers to perform transplants. The pandemic has also compromised regular outpatient follow-up and compelled services to adopt different forms of remote follow-up. Furthermore, COVID-19 is more aggressive in kidney transplant recipients (KTRs), with fatality rates that are seven to eight times higher than in patients who are immunocompetent [3]. Owing to the prolonged use of immunosuppressive drugs, KTRs receive reduced efficacy from vaccines and require additional doses to reach the seroconversion percentages observed in the general population [4,5]. We aimed to present a COVID-19 overview from the perspective of the Brazilian kidney transplant program by comparing its differences or similarities with the situations observed in other countries. We present the impact of the pandemic on transplant activity, several aspects of COVID-19, information on vaccine immunogenicity, and the new scenario after the predominance of the Omicron variant from the perspective of kidney transplantation with a focus on Brazilian data.
2. Effect of the Pandemic on Transplant Activity
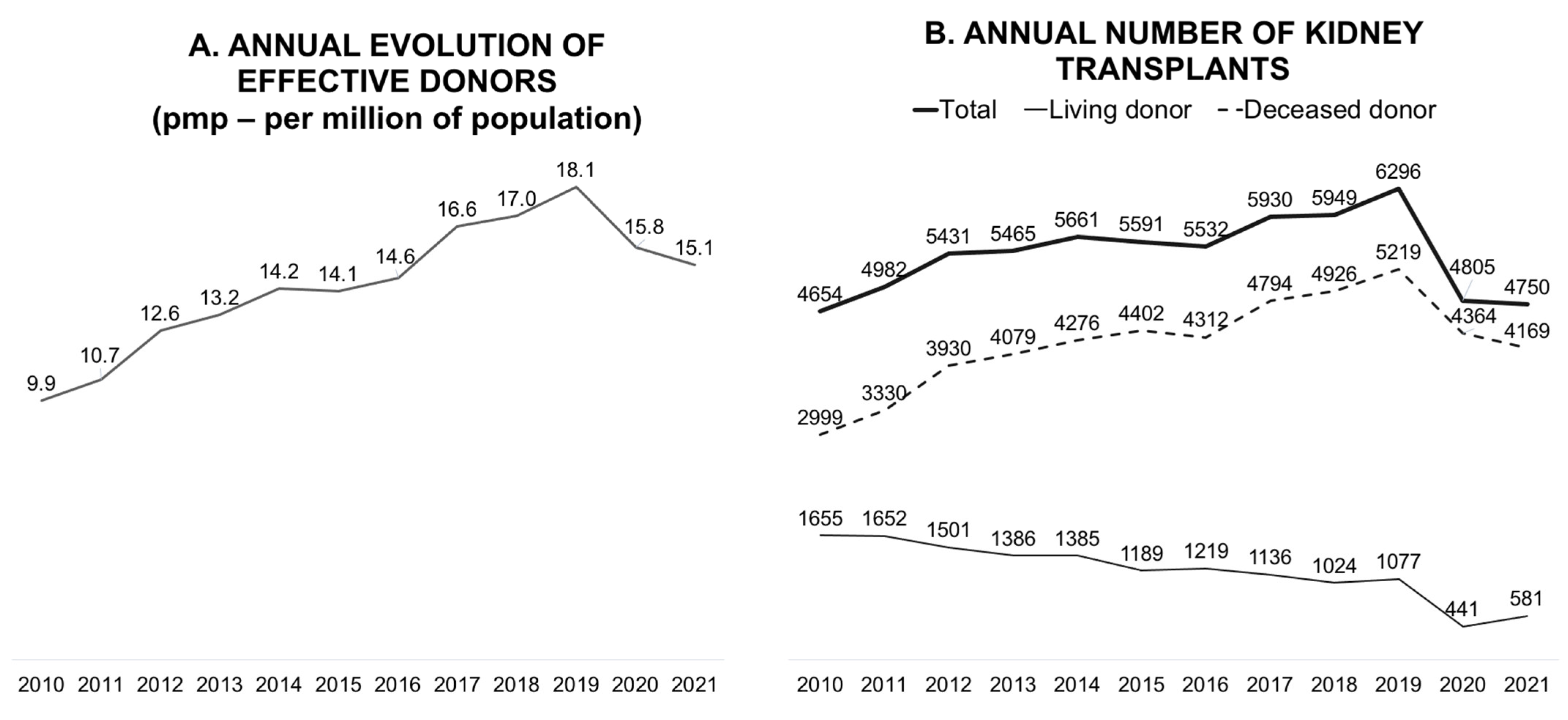
In terms of the absolute number of annual transplants, Brazil has the fourth-largest kidney transplant program and the largest public program in the world [6]. In 2019, the Brazilian transplant program showed continuous and progressive growth, reaching 18.1 donors per million population (pmp) and 6296 kidney transplants annually (Figure 1). The objectives of the program for 2020 were to reach 20 donors pmp and maintain this pace of growth. However, the pandemic has significantly reduced the number of deceased donors; consequently, the number of transplants with this type of donor has also decreased.
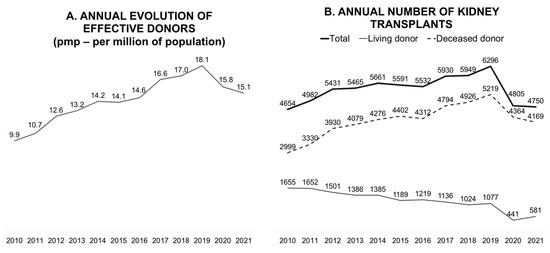
Figure 1.
Annual evolution of effective donors per million population (A) and the annual number of kidney transplants (B).
Globally, all solid organ transplant activities decreased in the first year of the pandemic, although a variation was observed according to the organ type and country [1]. In a population-based, before-and-after observational study that collected the data of consecutive kidney, liver, lung, and heart transplants from nationwide cohorts in 22 countries (January 2020 to December 2020), an overall decrease of 16% was observed in all transplant activities. This decrease was more concentrated in the first three months of 2020 and was higher for kidney transplants (19.1%) than for liver, lung, and heart transplants (10.6%, 15.5%, and 5.4%, respectively) [1]. Additionally, a 40% reduction was observed in living-donor transplantation globally [1,7].
The effect of the pandemic on living-donor transplants was even more significant owing to their elective nature (Figure 1) [8]. Early in the first year of the pandemic, concerns regarding living-donor transplantation activity were evident, as measured in an international survey comprising 33 questions that collected data from 204 centers in 16 countries across 5 continents. For instance, the participant centers in Brazil and the United States represented 39% and 61% of all transplant activities, respectively. Living-donor transplants were held on by 75% of centers, and new donor evaluations were interrupted in 59%. Among the centers that conducted donor evaluations, most centers transferred appointments to virtual platforms, 68% used videos, and 42% used telephones. The safety of donors and recipients was highlighted as the most relevant barrier to transplant activity, and in some countries, it was government restrictions [8].
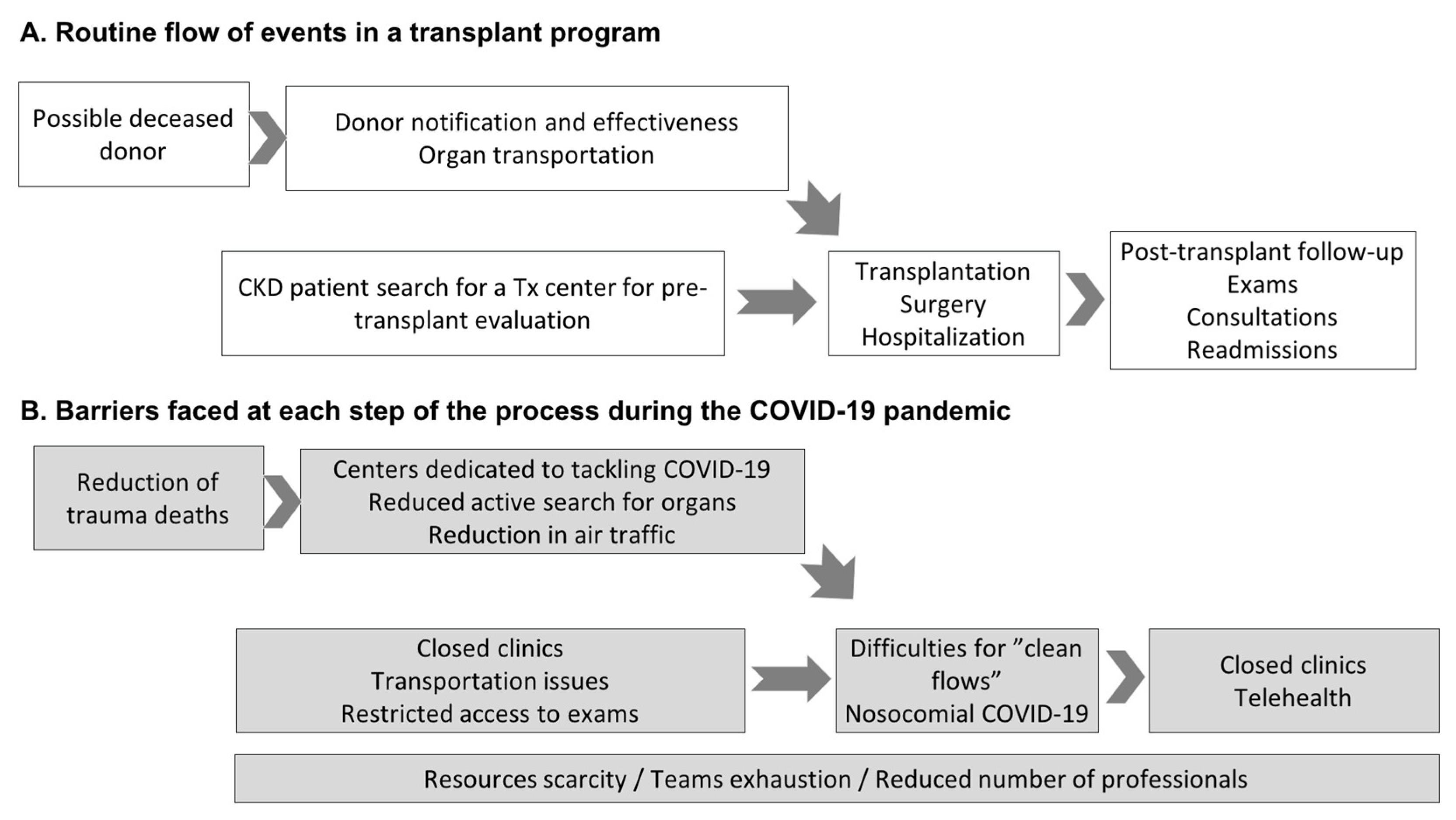
Consequently, the waiting list increased despite a reduction in the entry of new patients owing to the compromised flow of care in pre-transplant outpatient clinics [9]. Figure 2 illustrates the routine flow of events in a transplant program and the events that prevented its full functioning during the pandemic. In a nationwide program with a high number of transplants with deceased donors, one of the most important chains in the whole process is donor identification and notification. As demonstrated in Figure 2A, all other essential chains are required to reach outpatients in the follow-up phase with exams, medical appointments, and eventual hospital readmission. As detailed in Figure 2B, the pandemic affected all processes, from pre-transplant preparation and donor selection to hospitalization for transplant surgery.
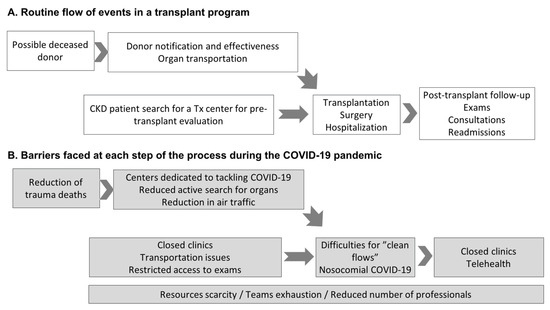
Figure 2.
The flow of events in a transplant program (A) and barriers faced during the COVID-19 pandemic (B). In Figure A, the first box, a possible deceased donor refers to persons with brain death or cardiac death. Of note, in Brazil, donation after cardiac death is not allowed, only after brain death. Abbreviations: Tx: transplant; CKD: chronic kidney disease; CIHDOTT: Intra-Hospital Commission for Donation of Organs and Tissues for Transplants.
In Brazil, the transplantation process was not equally affected in all parts of the country. This can be explained by the migratory nature of COVID-19, particularly in 2020, thus allowing centers in less-affected regions to maintain momentary transplant activity. Hospitals dedicated to transplantation played a fundamental role in this period by ensuring that transplants were performed safely and in a controlled environment [1,10]. For example, although several transplant centers had declared a transplant moratorium using strategic and coordinated decisions, Hospital do Rim continued some transplant activities and served as a national hub for kidney transplantation. Hospital do Rim, which is a 151-bed tertiary hospital located in São Paulo (the most populous city in Brazil), performs almost 1000 kidney transplants annually, which represents 20% of all kidney transplants in Brazil [11].
In addition to performing kidney transplants for recipients on its list, Hospital do Rim took over transplants from other hospitals dedicated to COVID-19 care and accepted kidneys from practically all states of the federation and those that were refused by other transplant centers. By the end of 2022, 1038 kidneys from other states were offered to Hospital do Rim: 73% (n = 762) of the kidneys were transplanted, and 13% were discarded. It is also important to emphasize that over time, owing to a better understanding of the disease and adequate resources, transplant services have adopted measures that allow better control of the disease and the safe resumption of activities [12].
3. COVID-19 in Kidney Transplant Recipients
KTRs have a unique condition characterized by a combination of continuous and prolonged use of immunosuppressive drugs and the sum of morbid conditions. Immunosuppressive drugs modulate the clinical response and outcomes of infectious diseases by inhibiting the action of T and B lymphocytes and antibody production. Approximately two-thirds of KTRs have impaired renal function, and this condition alone is already associated with a worse prognosis after COVID-19. In addition to renal dysfunction, KTRs are often affected by hypertension, dyslipidemia, diabetes, cardiovascular diseases, and frailty syndrome, all of which further exacerbate the likelihood of poor outcomes [13].
3.1. Susceptibility
In a study that compared the pre-vaccination COVID-19 landscape of 11,715 KTRs among 2.63 million people living in São Paulo (March 2020 to March 2021), 11.7% of outpatient KTRs were diagnosed with COVID-19 versus 5.8% of the general population, thus suggesting that KTRs have a higher susceptibility to COVID-19 than other people [14]. KTRs are closely monitored and have easier access to health services and diagnostic tests than the general population. Furthermore, patients with chronic kidney disease on dialysis seem more susceptible to COVID-19 than KTRs, which could be justified by greater exposure resulting from dialysis sessions [15,16]. This pattern was exemplified in initial reports from the Spanish Society of Nephrology Registry (March 2020 to April 2020), where only one-third of patients diagnosed with COVID-19 were KTRs (vs. 63% from hemodialysis centers) [15]. Similar results were observed in an Italian registry, where the frequency of COVID-19 in April 2020 was fourfold higher for patients undergoing hemodialysis (3.55% vs. 0.86%) [16].
Among KTRs, patients who are non-white, are elderly, have pulmonary disease, and are obese, belong to blood group A and are homozygotes at the human leukocyte antigen (HLA) A locus, which is associated with increased susceptibility to the disease [17,18,19]. In our center, we conducted a single-center, observational case–control study that included 720 KTRs with COVID-19 between March 2020 and December 2020 and 1680 KTRs who underwent transplantation in 2018 and 2019. We analyzed 21 HLA-A, 35 HLA-B, and 13 HLA-DRB1 allelic groups and their ABO phenotypic frequencies. No associations were observed between the homozygosities of HLA-A, HLA-B, or HLA-DRB1 and disease severity. However, the probability of COVID-19 was 20% higher for the ABO type A group (odds ratio [OR] = 1.2, 95% CI = 1.0–1.5) and 40% higher for homozygosity at HLA-A (OR = 1.4, 95% confidence interval [CI] = 1.0–1.8) [19].
3.2. Clinical Presentation and Complementary Exams
The first reports from Wuhan provided an overview of the most frequent symptoms of COVID-19 in the general population, including fever (>95%), cough (79%), sputum (23%), fatigue (23%), and myalgia (15%) [20]. A similar pattern was observed in the first report from New York City [21]. The clinical presentation of COVID-19 in KTRs is similar to that of healthy individuals in the pre-vaccination era, with fever, cough, myalgia, anosmia, headache, and fatigue as the most frequently described symptoms. The TANGO International Transplant Consortium described the clinical presentations of the first cases of COVID-19 in KTRs from 12 transplant centers in the United States, Italy, and Spain [22]. Among patients who required hospitalization (March 2020 to May 2020), fever, dyspnea, and myalgia were present in 67%, 68%, and 53%, respectively. The mean time from symptom onset to admission was six days. Interestingly, the frequency of diarrhea was 38%.
The first report from a Brazilian multicenter study that analyzed the data of adult KTRs diagnosed with COVID-19 (March 2020 to November 2020) showed that they were 50 years old on average; 60% and 40% were male and female, respectively, and 89% were non-Afro-Brazilian. Recipients had a long transplantation vintage (5.9 years on average) [3]. The clinical presentation of COVID-19 (Table 1) was consistent with previous reports, with common symptoms including cough (54%), myalgia (40%), dyspnea (37%), and diarrhea (31%). Fever (61%) and hypoxemia (13%) were among the notable clinical signs observed. Attention was drawn to the high occurrence of diarrhea (reported in 30% of patients), which was sometimes the only or predominant symptom [3].

Table 1.
Data from the first 1860 KTRs diagnosed with COVID-19 from the Brazilian multicenter registry.
Regarding the findings of complementary examinations, a high incidence of renal dysfunction has been consistently reported even in patients with mild COVID-19. In the TANGO cohort, the baseline estimated glomerular filtration rate (eGFR) among KTRs requiring hospitalization was 48.9 mL/min/1.73 m2, and the lower graft function differentiated survivors from non-survivors (53.4 vs. 38.0 mL/min/1.73 m2; p = 0.001) [22]. In the Brazilian registry, the baseline eGFR was similar at 48.4 mL/min/1.73 m2, and the frequency of acute kidney injury was 23.2% [3]. In addition, some studies have reported more severe lymphopenia and a higher incidence of anemia in KTRs than in individuals who are immunocompetent [18,23]. Table 2 shows the epidemiologic and demographic data from the Brazilian multicenter registry and data from the databases of other countries.

Table 2.
Summary of main COVID-19 epidemiology and clinical data from Brazilian and international registries.
3.3. Immunosuppression Management and Viral Treatment
To date, there is no conclusive evidence regarding the best strategy for managing immunosuppression in the context of COVID-19 [24]. Immunosuppressive drugs can induce lymphopenia, which is associated with a worse prognosis in COVID-19 cases. These drugs reduce lymphocyte activity and antibody production, thus potentially compromising clinical response and prognosis. However, in vitro studies have shown that drugs such as azathioprine, mycophenolate, calcineurin inhibitors, and mammalian target of rapamycin (mTOR) inhibitors, can inhibit Coronavirus replication, thus suggesting that such drugs have potential benefits in alleviating the clinical presentation of COVID-19. Calcineurin inhibitors have been speculated to modulate the inflammatory response elicited by cytokine release syndrome [25,26,27,28]. However, the high mortality rate observed in KTRs suggests that the potential benefits of these drugs, as demonstrated in vitro or suggested by their mechanisms of action, are outweighed by their immunosuppressive effects.
Despite the lack of robust evidence on immunosuppression management, most transplant centers have chosen to keep the anti-rejection regimen unchanged in outpatients with mild symptoms; withdraw the use of antiproliferative or mTOR inhibitors in patients with moderate symptoms, under hospitalization, or with severe lymphopenia; and completely withdraw the regimen in critically ill patients admitted to intensive care units (ICUs) [3]. In the TANGO cohort, the withdrawal rates of tacrolimus and mycophenolate/everolimus were 23% and 65%, respectively [22]. Similarly, in a Brazilian multicenter study that was conducted before the vaccination era, it was observed that among KTRs diagnosed with COVID-19 and receiving an immunosuppressive regimen based on calcineurin inhibitors, patients treated with mycophenolate had a higher frequency of immunosuppression changes (61.6%, p < 0.001) than those treated with azathioprine (36.0%) or mTOR inhibitors (38.2%) [29]. Similar results were observed when immunosuppression was completely discontinued: mycophenolate (21.5%), azathioprine (19.3%), and mTOR inhibitors (12.9%, p = 0.02). Immunosuppressive changes seem to be associated with survival among patients using a calcineurin inhibitor in combination with mycophenolate. The 30- and 90-day survival rates of patients who continued the drug at their usual dose were 78% and 72%, respectively. For patients in which the dose was reduced or discontinued, the 30- and 90-day survival rates were 83% and 81%, respectively (p < 0.001).
Few studies have investigated specific viral treatments in KTRs. The use of convalescent plasma was considered compassionate therapy; therefore, our group compared the outcomes of 58 KTRs treated with convalescent plasma (January 2021 to March 2021) with a propensity score-matched control group; however, a reduction in disease progression and lethality was not observed [30]. Despite being well-tolerated and safe, no clinical trial investigated the efficacy of remdesivir in KTRs [31], and clinical reports about the combination of nirmatrelvir and ritonavir (Paxlovid) are limited because of the complex management of interactions with calcineurin inhibitors [32]. Finally, results regarding the use of anti-spike monoclonal antibodies for passive immunization are inconclusive [33].
3.4. Outcomes and Predictors
Various studies indicate that KTRs have a high proportion of severe cases and high fatality rates [34,35]. In the first wave in 2020, the crude fatality rate varied from 20% to 25% independent of country. For all solid organ transplant recipients, similar risk factors for mortality were identified early, including age; cumulative number of comorbidities; biochemical changes upon hospital admission including lymphopenia and thrombocytopenia; and high levels of ferritin, C-reactive protein, troponin, or D-dimer [34,36,37].
Data from a Brazilian multicenter study confirmed these findings. In the multicenter registry, we collected data from 35 centers comprising 57% of all transplantation activities in the country. Eligible participants were KTRs diagnosed with COVID-19 in the pre-vaccination era between March 2020 and November 2020. Only patients with at least one COVID-19-attributable symptom associated with a positive test result (polymerase chain reaction [PCR], serology, or viral antigen detection) were considered. Among the first 1680 recipients that were included, the interval time between transplantation and COVID-19 infection was 5.9 years and between symptom onset and the diagnosis was five days. Most patients had hypertension (75.7%), 34% had diabetes, and 23.8% were obese. Previous cardiovascular events were reported in more than 10% of cases, and no comorbidities were registered in 11.4% of cases. Most patients required hospitalization (63%), 23% required dialysis, 35% were admitted to the ICU, 25% required invasive mechanical ventilation, 15% died within 28 days, and 21% died within 90 days [3]. In the multivariable analysis, the risk of hospitalization increased by age (3.3% per each year old); previous diagnoses of hypertension and diabetes increased the risk by 42% and 65%, respectively, and recently, administrations of high doses of steroids for acute rejection increased the risk by 89%. In addition, in the multivariable analysis, the variables associated with death were older age (relative risk [RR] for each year = 1.05, 95% CI = 1.04–1.07), hypertension (RR yes vs. no = 1.57, 95% CI = 1.07–2.29), cardiovascular disease (RR yes vs. no = 1.52, 95% CI = 1.05–2.20), and time after transplantation (RR for each month = 1.02, 95% CI = 1.00–1.05) [3].
In addition to the risk factors for poor COVID-19 outcomes, such as older age, impaired baseline renal function, diabetes mellitus, and cardiovascular disease, the prolonged use of immunosuppressive drugs could also explain the high risk of death. Initial reports questioned the effect of immunosuppression on the poorer outcomes among KTRs because similar outcomes for transplanted patients were observed compared with non-transplanted pairs when age and comorbidities were strictly matched [38,39]. We compared the age-adjusted COVID-19 fatality rate of KTRs with that of people living in the most populous state of Brazil (São Paulo) [14]. Between March 2020 and March 2021, the overall fatality rate was 24.9% for KTRs compared with 3.5% for the general population, and a higher risk was observed across all age groups. In patients aged 20–39 years old, with a lower prevalence of comorbidities, the fatality rate among KTRs was 23 times higher (8.5 vs. 0.37%); this finding strengthens the hypothesis that prolonged immunosuppression plays a determining role [14].
Andersen et al. conducted a retrospective study that included patients with confirmed or suspected COVID-19 in the United States from January 2020 to June 2021 to investigate the association between immunosuppression and the risk of death [40]. More than 12,000 patients with immunosuppression (for different reasons) were paired by propensity score matching with more than 29,000 patients without immunosuppression. An increased risk of death was observed with rituximab for rheumatological disease (RR = 1.72, 95% CI = 1.10–2.69) and for cancer (RR = 2.57, 95% CI = 1.86–3.56) [40]. A Brazilian multicenter study on COVID-19 in KTRs analyzed the data of 1833 patients who were using a regime based on a calcineurin inhibitor; the results showed that a higher hospitalization rate was observed among patients when the associated drug was mycophenolate (45.6% vs. 66.7%, p < 0.001) or mTOR inhibitor (45.6% vs. 61.1%, p = 0.001) than when the drug was azathioprine [29]. Similarly, the requirement for mechanical ventilation was higher for mycophenolate than for azathioprine (26.8% vs. 17.8%, p < 0.001); no difference was found for mTOR inhibitors. In a multivariate analysis, compared with azathioprine, the 90-day risk of death increased by 46% with mycophenolate and decreased by 41% with mTOR inhibitors [29].
The risk of co-infection was high for KTRs requiring hospitalization, particularly those who were under advanced life support in the ICU, thus substantially increasing the risk of death [41]. In addition to healthcare-associated infections, such as central line-associated bloodstream infection, urinary tract infection, ventilator-associated pneumonia, cytomegalovirus infection, herpes zoster infection, mucormycosis infection, and other opportunistic infections have been reported [41].
By using data from the Brazilian multicenter registry and information that were available only in a remote consult, a predictor score was developed to quickly identify patients who are at a higher risk of complications without the need for laboratory or image exams [42]. Thus, for ImAgeS score development, 1635 KTRs with COVID-19 diagnosed by PCR between March and October 2020 were split into a derivation cohort (75%) and an internal validation cohort (25%). Several models were fitted to predict death from any cause within 28 days of COVID-19 diagnosis. An elastic net with few variables achieved better performance in both the derivation and internal validation cohorts and had a low calibration Brier score. Older age, hypertension, diabetes, cardiovascular disease, smoking, impaired kidney function, obesity, and dyspnea were risk factors for death. By contrast, the use of mTOR inhibitors and clinical presentation of anosmia, runny nose, and headache were protective factors. The score was validated in a second validation cohort composed of 374 patients diagnosed with COVID-19 between January and April 2021, and the area under the receiver-operating characteristic curve (AUC) was 0.787 (95% CI = 0.731–0.843).
Domjanović et al. validated the ImAgeS score in an external cohort of KTRs and obtained an AUC of 0.679 (95% CI = 0.519–0.840) and a C concordance index of 0.699 to predict 30-day all-cause mortality. In addition, the ImAgeS score performance was compared with three scores derived from the general population: CHA2DS2-VASC, Wuhan model, and COVID SIEMC [43,44,45]. For these three scores, the AUC to predict mortality varied from 0.62 to 0.69, which was lower than the 0.79 achieved by the ImAgeS score [42].
3.5. Post-Infection and Post-Vaccination Humoral Response
Evidence suggests that transplant patients have a preserved humoral adaptive immune response after natural infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [23,46]. In a single-center observational study by Magicova et al., immunoglobulin G (IgG) seroprevalence in KTRs was compared with that in healthcare workers in the second wave of COVID-19 in Prague, Czech Republic [46]. The study found that among individuals who tested positive for S1 and S2 anti-SARS-CoV-2 IgG, the antibody levels were higher in KTRs than in healthcare workers (31 vs. 15 AU/mL, p < 0.001).
However, the same pattern of immunogenicity was not observed after vaccination. For example, data from two BNT162b2 mRNA vaccine doses showed seroconversion rates ranging from 22% to 37.5% [47]. Grupper et al. compared the humoral response after two doses of BNT162b2 (Pfizer-BioNTech) in 136 KTRs and 25 healthcare workers [47]. Although 100% of the controls showed a positive response, less than 40% of the KTRs were positive (p < 0.001). Among those who seroconverted, the IgG anti-spike antibody was lower in KTRs than in healthcare workers (31.05 vs. 200.5 AU/mL, p < 0.001).
Other studies on mRNA vaccines have explored cellular and humoral immune responses and confirmed that immunogenicity and effectiveness are lower in KTRs than in the general population [48]. Sanders et al. investigated the immune response 6 months after mRNA-1273 vaccination in KTRs (n = 267) compared with that in patients with pre-dialysis chronic kidney disease (n = 152) or those undergoing dialysis (n = 145) and healthy controls (n = 181) [48]. Six months after vaccination, although most non-transplanted patients presented with a positive S1-specific antibody (100% of controls, 98.7% of pre-dialysis patients, and 95.1% of dialysis patients), only 57% of the KTRs were positive. Furthermore, at 28 days, a lower proportion of KTRs showed a detectable T-cell response than the other groups: 18% for KTRs vs. 87.5–69.4% in other groups.
By using four national English registries, Callaghan et al. investigated the effectiveness of two doses of the ChAdOx1-S or BNT162b2 vaccine in solid organ transplant recipients (43,481 patients, 71% KTRs) [49]. Among the transplant recipients, 2.6% received one vaccine dose, 90.3% received two doses, and 7.7% were unvaccinated. The risk of death within 28 days after COVID-19 was only tangentially reduced by 20% (hazard ratio [HR] = 0.80, 95% CI = 0.63–1.00) after vaccination; this was evident with ChAdOx1-S (HR = 0.69, 95% CI = 0.52–0.92) but not with BNT162b2 (HR = 0.97, 95% CI = 0.71–1.31) [49].
After anti-SARS-CoV-2 vaccines were approved in Brazil, we conducted a prospective, single-center, phase 4 interventional study by using two sequential doses of CoronaVac (28 days apart), which is an inactivated whole-virus vaccine [50]. Among the 3371 KTRs enrolled, 942 were randomly selected for immunogenicity assessment. From the first to the second dose, the seroprevalence increased from 15.2% to 43%, which is similar to that observed with other platform vaccines. A reduction in the incidence of COVID-19 was observed during the pandemic (from 6.4% to 4.2%, p < 0.001), but no effect was observed on the 28-day case fatality rate (from 25% to 22%, p = 0.53). We compared the immunogenicity of a third heterologous dose (BNT162b2 mRNA, n = 307) with that of a third homologous dose (n = 777) after two doses of the CoronaVac vaccine [51]. Although the seroconversion rate after the third dose was significantly higher in the heterologous group than in the homologous group (49% vs. 32%, p < 0.001), 42% of the patients remained seronegative. Lastly, among the 279 KTRs who remained seronegative after three doses of CoronaVac and received a fourth sequential homologous dose, the seroconversion rate was only 18.9% [52]. These results underscore the poor vaccinal response in KTRs independent of the vaccine platform investigated.
After the anti-SARS-CoV-2 vaccine was launched, some concerns regarding adverse events were highlighted, even with the safety profile shown in phase III clinical trials of the most common vaccine platforms [53,54]. By considering only clinical trials investigating mRNA vaccines, BNT162b2 and mRNA-1273 were associated with an excess risk of serious adverse events of 10.1 and 15.1 per 10,000 people vaccinated compared with placebo [55]. Some examples of serious events were coagulation disorder, myocarditis, pericarditis, or other forms of acute cardiac injury [55]. However, in a phase IV clinical trial that investigated immunogenicity and reactogenicity after two doses of CoronaVac in COVID-19 convalescent KTRs, we observed that the most common adverse events were local pain or tenderness (38%), headache (12%), and body aches (11%), with no serious adverse reactions [56]. Most recently, a meta-analysis including 727 KTRs from nine studies showed that a fourth dose of the COVID-19 vaccine was well-tolerated with no serious adverse effects [57]. A particular concern has been risk sensitization, although a recent multicenter study demonstrated that no vaccine affected the panel of reactivity of HLA antibodies among wait-listed candidates [58].
3.6. The Scenario after Vaccination Coverage and the Omicron Variant
As of December 2021, a substantial change has been observed in the outcomes related to COVID-19 both in the general population and in KTRs. Two factors seem to have been involved in this change: high vaccination coverage and the predominance of the Omicron variant, which seems to cause less clinically severe conditions despite being more contagious.
Our group conducted a comparison between KTRs in our center (10,497 patients) and residents of Ipaussu, São Paulo, Brazil, a city with over 14,000 inhabitants; results showed that there was a change in COVID-19 behavior after the emergence of the Omicron variant [59]. In addition to having a similar number of persons at risk, both groups had high vaccination coverage rates in December 2021. The number of cases and outcomes associated with COVID-19 were compared in both populations and stratified by two different eras: era 1 (March 2020 to December 2021: before Omicron predominance) and era 2 (December 2021 to March 2022: during Omicron predominance). Although the number of cases increased from 0.3 to 1.0 and from 0.3 to 1.2 per 1000 patient days for KTRs and the inhabitants of Ipaussu, respectively, the need for hospitalization, mechanical ventilation, and case fatality rates decreased in both groups. Among the KTRs, the need for hospitalization and mechanical ventilation was reduced by 50% and 46%, respectively, and the case fatality rate was reduced by 60% [59].
Similar scenarios have been observed in other countries. For instance, in an Australian single-center study that included 41 KTRs diagnosed with the B.1.1.529/BA.1 Omicron variant after at least two doses of the COVID-19 vaccine, the 30-day mortality was 2.4%; also, 4.9% of patients presented with multiorgan failure, and 12.2% of patients presented with respiratory failure [60]. In a multicenter retrospective cohort study that enrolled KTRs with COVID-19 in Spain, those diagnosed in the sixth wave (from December 2021 to February 2022 with Omicron predominance) showed a lower frequency of ventilator support (2.6% vs. 18.5%, p = 0.001), ICU admission (2.6% vs. 22.2%, p < 0.001), and death (3.3% vs. 29.6%, p < 0.001) than those diagnosed in the fifth wave (from June 2021 to November 2021 with Delta predominance) [61].
4. Conclusions
The pandemic has significantly affected kidney transplant activities globally, with a consequent reduction in the number of transplants performed by both deceased and living donors. The evidence has demonstrated that KTRs are significantly vulnerable to severe diseases, thus underscoring the central role of chronic immunosuppression and the cumulative number of comorbidities. Widespread vaccination has reduced the severity and number of cases of COVID-19 in countries with high vaccination coverage rates; this situation motivated the World Health Organization to state that COVID-19 is no longer a public health emergency of international concern. However, KTRs remain at high risk because of persistently low immunogenicity rates following sequential vaccine shots and because of the absence of targeted treatments for severe cases, which warrant significant concern.
5. Future Directions
COVID-19 is considered an established and ongoing health issue as of May 2023. One possible future direction is to move from a pandemic era to an endemic era. One question to be answered in the coming years is whether additional vaccine shots and even annual doses would be required. Another topic is the role of current and new antiviral therapies, particularly for the most vulnerable populations, such as KTRs. In this field, potential drug interactions with calcineurin inhibitors would require additional investigations. Lastly, because the syndrome is not easily defined in clinical practice, the effect of long COVID on personal health; quality of life; long-term graft function; and cellular or humoral immune response, which is associated with the risk of chronic cellular rejection or donor-specific antibody production, must be investigated.
Author Contributions
L.R.-M. wrote and approved the manuscript. R.D.F. reviewed the draft and approved the manuscript. T.V.d.S.-F. wrote and approved the manuscript. J.M.-P. wrote the draft and approved the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study received financial support from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) (process no. 2021/13680-6).
Institutional Review Board Statement
Not Applicable.
Informed Consent Statement
Not Applicable.
Data Availability Statement
Not Applicable.
Conflicts of Interest
The authors have no conflict of interest to declare.
Abbreviations
| AUC | Area under a receiver-operating characteristic curve; |
| CI | Confidence interval; |
| COVID-19 | Coronavirus disease 2019; |
| eGFR | Estimated glomerular filtration rate; |
| HLA | Human leukocyte antigen; |
| HR | Hazard ratio; |
| ICU | Intensive care unit; |
| IGG | Immunoglobulin G; |
| KTR | Kidney transplant recipient; |
| mTOR | Mammalian target of rapamycin; |
| OR | Odds ratio; |
| pmp | Per million population; |
| RR | Relative risk; |
| SARS-CoV-2 | Severe acute respiratory syndrome Coronavirus 2. |
References
- Aubert, O.; Yoo, D.; Zielinski, D.; Cozzi, E.; Cardillo, M.; Durr, M.; Dominguez-Gil, B.; Coll, E.; Da Silva, M.I.; Sallinen, V.; et al. COVID-19 pandemic and worldwide organ transplantation: A population-based study. Lancet Public Health 2021, 6, e709–e719. [Google Scholar] [CrossRef]
- Mahase, E. COVID-19: WHO declares pandemic because of “alarming levels” of spread, severity, and inaction. BMJ 2020, 368, m1036. [Google Scholar] [CrossRef] [PubMed]
- Requiao-Moura, L.R.; Sandes-Freitas, T.V.; Viana, L.A.; Cristelli, M.P.; Andrade, L.G.M.; Garcia, V.D.; Oliveira, C.M.C.; Esmeraldo, R.M.; Abbud Filho, M.; Pacheco-Silva, A.; et al. High mortality among kidney transplant recipients diagnosed with coronavirus disease 2019: Results from the Brazilian multicenter cohort study. PLoS ONE 2021, 16, e0254822. [Google Scholar] [CrossRef] [PubMed]
- Kamar, N.; Abravanel, F.; Marion, O.; Couat, C.; Izopet, J.; Del Bello, A. Three Doses of an mRNA COVID-19 Vaccine in Solid-Organ Transplant Recipients. N. Engl. J. Med. 2021, 385, 661–662. [Google Scholar] [CrossRef] [PubMed]
- Medina-Pestana, J.; Cristelli, M.P.; Viana, L.A.; Foresto, R.D.; Requiao-Moura, L.R.; Tedesco-Silva, H.; Covas, D.T. Clinical Impact, Reactogenicity, and Immunogenicity After the First CoronaVac Dose in Kidney Transplant Recipients. Transplantation 2022, 106, e95–e97. [Google Scholar] [CrossRef] [PubMed]
- Foresto, R.D.; Pestana, J.O.M.; Silva, H.T., Jr. Brasil: The leading public kidney transplant program worldwide. Rev. Assoc. Med. Bras. 2020, 66, 708–709. [Google Scholar] [CrossRef]
- Nimmo, A.; Gardiner, D.; Ushiro-Lumb, I.; Ravanan, R.; Forsythe, J.L.R. The Global Impact of COVID-19 on Solid Organ Transplantation: Two Years Into a Pandemic. Transplantation 2022, 106, 1312–1329. [Google Scholar] [CrossRef]
- Salvalaggio, P.R.; Ferreira, G.F.; Caliskan, Y.; Vest, L.S.; Schnitzler, M.A.; de Sandes-Freitas, T.V.; Moura, L.R.; Lam, N.N.; Maldonado, R.A.; Loupy, A.; et al. An International survey on living kidney donation and transplant practices during the COVID-19 pandemic. Transpl. Infect. Dis. 2021, 23, e13526. [Google Scholar] [CrossRef]
- Registro Brasileiro de Transplantes (RBT). Dimensionamento dos Transplantes no Brasil e em cada Estado (2014–2021). Veículo Oficial da Associação Brasileira de Transplantes de Órgãos. Ano XXVIII No. 4. 2021. Available online: http://abto.org.br (accessed on 9 March 2022).
- Cristelli, M.P.; Sandes-Freitas, T.V.; Viana, L.A.; Requiao-Moura, L.R.; Andrade, L.G.M.; Tedesco-Silva, H.; Medina-Pestana, J. Migratory pattern of the coronavirus disease 2019 and high fatality rates among kidney transplant recipients: Report from the Brazilian Multicenter Cohort Study. J. Bras. Nefrol. 2022, 44, 428–433. [Google Scholar] [CrossRef]
- Medina-Pestana, J.O. Organization of a high-volume kidney transplant program--the “assembly line” approach. Transplantation 2006, 81, 1510–1520. [Google Scholar] [CrossRef]
- de Sandes-Freitas, T.V.; Canito Brasil, I.R.; Oliveira Sales, M.; Studart, E.N.L.M.S.; Pimentel, I.R.S.; Josino da Costa, L.A.T.; Esmeraldo, R.M. Lessons from SARS-CoV-2 screening in a Brazilian organ transplant unit. Transpl. Infect. Dis. 2020, 22, e13376. [Google Scholar] [CrossRef]
- Yilmaz, E.A.; Ozdemir, O. Solid organ transplantations and COVID-19 disease. World J. Transplant. 2021, 11, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Medina-Pestana, J.; Cristelli, M.P.; Foresto, R.D.; Tedesco-Silva, H.; Requiao-Moura, L.R. The Higher COVID-19 Fatality Rate Among Kidney Transplant Recipients Calls for Further Action. Transplantation 2022, 106, 908–910. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Alvarez, J.E.; Perez Fontan, M.; Jimenez Martin, C.; Blasco Pelicano, M.; Cabezas Reina, C.J.; Sevillano Prieto, A.M.; Melilli, E.; Crespo Barrios, M.; Macia Heras, M.; Del Pino, Y.P.M.D. SARS-CoV-2 infection in patients on renal replacement therapy. Report of the COVID-19 Registry of the Spanish Society of Nephrology (SEN). Nefrologia (Eng. Ed.) 2020, 40, 272–278. [Google Scholar] [CrossRef]
- Quintaliani, G.; Reboldi, G.; Di Napoli, A.; Nordio, M.; Limido, A.; Aucella, F.; Messa, P.; Brunori, G.; Italian Society of Nephrology, C.-R.G. Exposure to novel coronavirus in patients on renal replacement therapy during the exponential phase of COVID-19 pandemic: Survey of the Italian Society of Nephrology. J. Nephrol. 2020, 33, 725–736. [Google Scholar] [CrossRef]
- Elias, M.; Pievani, D.; Randoux, C.; Louis, K.; Denis, B.; Delion, A.; Le Goff, O.; Antoine, C.; Greze, C.; Pillebout, E.; et al. COVID-19 Infection in Kidney Transplant Recipients: Disease Incidence and Clinical Outcomes. J. Am. Soc. Nephrol. 2020, 31, 2413–2423. [Google Scholar] [CrossRef]
- Chaudhry, Z.S.; Williams, J.D.; Vahia, A.; Fadel, R.; Parraga Acosta, T.; Prashar, R.; Shrivastava, P.; Khoury, N.; Pinto Corrales, J.; Williams, C.; et al. Clinical characteristics and outcomes of COVID-19 in solid organ transplant recipients: A cohort study. Am. J. Transplant. 2020, 20, 3051–3060. [Google Scholar] [CrossRef]
- De Marco, R.; Faria, T.C.; Mine, K.L.; Cristelli, M.; Medina-Pestana, J.O.; Tedesco-Silva, H.; Gerbase-DeLima, M. HLA-A homozygosis is associated with susceptibility to COVID-19. HLA 2021, 98, 122–131. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W.; the Northwell COVID-19 Research Consortium; Barnaby, D.P.; Becker, L.B.; Chelico, J.D.; et al. Presenting Characteristics, Comorbidities, and Outcomes among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA 2020, 323, 2052–2059. [Google Scholar] [CrossRef]
- Cravedi, P.; Mothi, S.S.; Azzi, Y.; Haverly, M.; Farouk, S.S.; Perez-Saez, M.J.; Redondo-Pachon, M.D.; Murphy, B.; Florman, S.; Cyrino, L.G.; et al. COVID-19 and kidney transplantation: Results from the TANGO International Transplant Consortium. Am. J. Transplant. 2020, 20, 3140–3148. [Google Scholar] [CrossRef] [PubMed]
- Phadke, V.K.; Scanlon, N.; Jordan, S.C.; Rouphael, N.G. Immune Responses to SARS-CoV-2 in Solid Organ Transplant Recipients. Curr. Transplant. Rep. 2021, 8, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Ahmadian, E.; Zununi Vahed, S.; Mammadova, S.; Abediazar, S. Immunosuppressant Management in Renal Transplant Patients with COVID-19. Biomed. Res. Int. 2021, 2021, 9318725. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chou, C.Y.; Chang, G.G. Thiopurine analogue inhibitors of severe acute respiratory syndrome-coronavirus papain-like protease, a deubiquitinating and deISGylating enzyme. Antivir. Chem. Chemother. 2009, 19, 151–156. [Google Scholar] [CrossRef]
- Hart, B.J.; Dyall, J.; Postnikova, E.; Zhou, H.; Kindrachuk, J.; Johnson, R.F.; Olinger, G.G.; Frieman, M.B.; Holbrook, M.R.; Jahrling, P.B.; et al. Interferon-β and mycophenolic acid are potent inhibitors of Middle East respiratory syndrome coronavirus in cell-based assays. J. Gen. Virol. 2014, 95, 571–577. [Google Scholar] [CrossRef]
- Carbajo-Lozoya, J.; Müller, M.A.; Kallies, S.; Thiel, V.; Drosten, C.; von Brunn, A. Replication of human coronaviruses SARS-CoV, HCoV-NL63 and HCoV-229E is inhibited by the drug FK506. Virus Res. 2012, 165, 112–117. [Google Scholar] [CrossRef]
- Appelberg, S.; Gupta, S.; Svensson Akusjärvi, S.; Ambikan, A.T.; Mikaeloff, F.; Saccon, E.; Végvári, Á.; Benfeitas, R.; Sperk, M.; Ståhlberg, M.; et al. Dysregulation in Akt/mTOR/HIF-1 signaling identified by proteo-transcriptomics of SARS-CoV-2 infected cells. Emerg. Microbes Infect. 2020, 9, 1748–1760. [Google Scholar] [CrossRef]
- Requiao-Moura, L.R.; Modelli de Andrade, L.G.; de Sandes-Freitas, T.V.; Cristelli, M.P.; Viana, L.A.; Nakamura, M.R.; Garcia, V.D.; Manfro, R.C.; Simao, D.R.; Almeida, R.; et al. The Mycophenolate-based Immunosuppressive Regimen Is Associated With Increased Mortality in Kidney Transplant Patients With COVID-19. Transplantation 2022, 106, e441–e451. [Google Scholar] [CrossRef]
- Cristelli, M.P.; Langhi Junior, D.M.; Viana, L.A.; de Andrade, L.G.M.; Martins, S.B.S.; Dreige, Y.C.; Bordim, J.O.; Tedesco-Silva, H.; Medina-Pestana, J. Efficacy of Convalescent Plasma to Treat Mild to Moderate COVID-19 in Kidney Transplant Patients: A Propensity Score Matching Analysis. Transplantation 2022, 106, e92–e94. [Google Scholar] [CrossRef]
- Buxeda, A.; Arias-Cabrales, C.; Perez-Saez, M.J.; Cacho, J.; Cabello Pelegrin, S.; Melilli, E.; Aladren, M.J.; Galeano, C.; Lorenzo, I.; Mazuecos, A.; et al. Use and Safety of Remdesivir in Kidney Transplant Recipients With COVID-19. Kidney Int. Rep. 2021, 6, 2305–2315. [Google Scholar] [CrossRef]
- Giguere, P.; Deschenes, M.J.; Loon, M.V.; Hoar, S.; Fairhead, T.; Pazhekattu, R.; Knoll, G.; Karpinski, J.; Parikh, N.; McDougall, J.; et al. Management and Outcome of COVID-19 Infection Using Nirmatrelvir/Ritonavir in Kidney Transplant Patients. Clin. J. Am. Soc. Nephrol. 2023, 18, 913–919. [Google Scholar] [CrossRef] [PubMed]
- Klein, E.J.; Hardesty, A.; Vieira, K.; Farmakiotis, D. Use of anti-spike monoclonal antibodies in kidney transplant recipients with COVID-19: Efficacy, ethnic and racial disparities. Am. J. Transplant. 2022, 22, 640–645. [Google Scholar] [CrossRef] [PubMed]
- Kates, O.S.; Haydel, B.M.; Florman, S.S.; Rana, M.M.; Chaudhry, Z.S.; Ramesh, M.S.; Safa, K.; Kotton, C.N.; Blumberg, E.A.; Besharatian, B.D.; et al. Coronavirus Disease 2019 in Solid Organ Transplant: A Multicenter Cohort Study. Clin. Infect. Dis. 2021, 73, e4090–e4099. [Google Scholar] [CrossRef]
- Caillard, S.; Anglicheau, D.; Matignon, M.; Durrbach, A.; Greze, C.; Frimat, L.; Thaunat, O.; Legris, T.; Moal, V.; Westeel, P.F.; et al. An initial report from the French SOT COVID Registry suggests high mortality due to COVID-19 in recipients of kidney transplants. Kidney Int. 2020, 98, 1549–1558. [Google Scholar] [CrossRef] [PubMed]
- Corbett, R.W.; Blakey, S.; Nitsch, D.; Loucaidou, M.; McLean, A.; Duncan, N.; Ashby, D.R.; West London, R.; Transplant, C. Epidemiology of COVID-19 in an Urban Dialysis Center. J. Am. Soc. Nephrol. 2020, 31, 1815–1823. [Google Scholar] [CrossRef]
- Ravanan, R.; Callaghan, C.J.; Mumford, L.; Ushiro-Lumb, I.; Thorburn, D.; Casey, J.; Friend, P.; Parameshwar, J.; Currie, I.; Burnapp, L.; et al. SARS-CoV-2 infection and early mortality of waitlisted and solid organ transplant recipients in England: A national cohort study. Am. J. Transplant. 2020, 20, 3008–3018. [Google Scholar] [CrossRef]
- Chavarot, N.; Gueguen, J.; Bonnet, G.; Jdidou, M.; Trimaille, A.; Burger, C.; Amrouche, L.; Weizman, O.; Pommier, T.; Aubert, O.; et al. COVID-19 severity in kidney transplant recipients is similar to nontransplant patients with similar comorbidities. Am. J. Transplant. 2021, 21, 1285–1294. [Google Scholar] [CrossRef]
- Molnar, M.Z.; Bhalla, A.; Azhar, A.; Tsujita, M.; Talwar, M.; Balaraman, V.; Sodhi, A.; Kadaria, D.; Eason, J.D.; Hayek, S.S.; et al. Outcomes of critically ill solid organ transplant patients with COVID-19 in the United States. Am. J. Transplant. 2020, 20, 3061–3071. [Google Scholar] [CrossRef]
- Andersen, K.M.; Bates, B.A.; Rashidi, E.S.; Olex, A.L.; Mannon, R.B.; Patel, R.C.; Singh, J.; Sun, J.; Auwaerter, P.G.; Ng, D.K.; et al. Long-term use of immunosuppressive medicines and in-hospital COVID-19 outcomes: A retrospective cohort study using data from the National COVID Cohort Collaborative. Lancet Rheumatol. 2022, 4, e33–e41. [Google Scholar] [CrossRef]
- Choudhary, D.; Kenwar, D.; Sharma, A.; Bhalla, A.; Singh, S.; Singh, M.P.; Kumar, V.; Sharma, A. Risk factors for mortality in kidney transplant recipients with COVID-19: A single centre experience and case-control study. BMC Nephrol. 2022, 23, 241. [Google Scholar] [CrossRef]
- Modelli de Andrade, L.G.; de Sandes-Freitas, T.V.; Requiao-Moura, L.R.; Viana, L.A.; Cristelli, M.P.; Garcia, V.D.; Alcantara, A.L.C.; Esmeraldo, R.M.; Abbud Filho, M.; Pacheco-Silva, A.; et al. Development and validation of a simple web-based tool for early prediction of COVID-19-associated death in kidney transplant recipients. Am. J. Transplant. 2022, 22, 610–625. [Google Scholar] [CrossRef]
- Berenguer, J.; Borobia, A.M.; Ryan, P.; Rodriguez-Bano, J.; Bellon, J.M.; Jarrin, I.; Carratala, J.; Pachon, J.; Carcas, A.J.; Yllescas, M.; et al. Development and validation of a prediction model for 30-day mortality in hospitalised patients with COVID-19: The COVID-19 SEIMC score. Thorax 2021, 76, 920–929. [Google Scholar] [CrossRef] [PubMed]
- Gunduz, R.; Yildiz, B.S.; Ozdemir, I.H.; Cetin, N.; Ozen, M.B.; Bakir, E.O.; Ozgur, S.; Bayturan, O. CHA2DS2-VASc score and modified CHA2DS2-VASc score can predict mortality and intensive care unit hospitalization in COVID-19 patients. J. Thromb. Thrombolysis 2021, 52, 914–924. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zuo, P.; Liu, Y.; Zhang, M.; Zhao, X.; Xie, S.; Zhang, H.; Chen, X.; Liu, C. Clinical and Laboratory Predictors of In-hospital Mortality in Patients With Coronavirus Disease-2019: A Cohort Study in Wuhan, China. Clin. Infect. Dis. 2020, 71, 2079–2088. [Google Scholar] [CrossRef]
- Magicova, M.; Fialova, M.; Zahradka, I.; Rajnochova-Bloudickova, S.; Hackajlo, D.; Raska, P.; Striz, I.; Viklicky, O. Humoral response to SARS-CoV-2 is well preserved and symptom dependent in kidney transplant recipients. Am. J. Transplant. 2021, 21, 3926–3935. [Google Scholar] [CrossRef] [PubMed]
- Grupper, A.; Rabinowich, L.; Schwartz, D.; Schwartz, I.F.; Ben-Yehoyada, M.; Shashar, M.; Katchman, E.; Halperin, T.; Turner, D.; Goykhman, Y.; et al. Reduced humoral response to mRNA SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients without prior exposure to the virus. Am. J. Transplant. 2021, 21, 2719–2726. [Google Scholar] [CrossRef] [PubMed]
- Sanders, J.F.; Messchendorp, A.L.; de Vries, R.D.; Baan, C.C.; van Baarle, D.; van Binnendijk, R.; Diavatopoulos, D.A.; Geers, D.; Schmitz, K.S.; GeurtsvanKessel, C.H.; et al. Antibody and T-Cell Responses 6 Months After Coronavirus Disease 2019 Messenger RNA-1273 Vaccination in Patients with Chronic Kidney Disease, on Dialysis, or Living with a Kidney Transplant. Clin. Infect. Dis. 2023, 76, e188–e199. [Google Scholar] [CrossRef]
- Callaghan, C.J.; Mumford, L.; Curtis, R.M.K.; Williams, S.V.; Whitaker, H.; Andrews, N.; Lopez Bernal, J.; Ushiro-Lumb, I.; Pettigrew, G.J.; Thorburn, D.; et al. Real-world Effectiveness of the Pfizer-BioNTech BNT162b2 and Oxford-AstraZeneca ChAdOx1-S Vaccines Against SARS-CoV-2 in Solid Organ and Islet Transplant Recipients. Transplantation 2022, 106, 436–446. [Google Scholar] [CrossRef]
- Medina-Pestana, J.; Covas, D.T.; Viana, L.A.; Dreige, Y.C.; Nakamura, M.R.; Lucena, E.F.; Requiao-Moura, L.R.; Fortaleza, C.; Foresto, R.D.; Tedesco-Silva, H.; et al. Inactivated Whole-virus Vaccine Triggers Low Response Against SARS-CoV-2 Infection Among Renal Transplant Patients: Prospective Phase 4 Study Results. Transplantation 2022, 106, 853–861. [Google Scholar] [CrossRef]
- Medina-Pestana, J.; Covas, D.T.; Viana, L.A.; Dreige, Y.C.; Requiao-Moura, L.R.; Nakamura, M.R.; Lucena, E.F.; Foresto, R.D.; Tedesco-Silva, H.; Cristelli, M.P. Homologous Third Dose of Inactivated Whole-virion Vaccine Fails to Elicit a Robust Immune Response Among Kidney Seronegative Transplant Recipients. Transplantation 2022, 106, e284–e285. [Google Scholar] [CrossRef]
- Cristelli, M.P.; Nakamura, M.R.; Viana, L.A.; Tedesco-Silva, H.; Medina-Pestana, J. The Fourth Dose of CoronaVac Vaccine Results in a Small Increase of Seroconversion and Antibody Values among Kidney Transplant Recipients. Transplantation 2022, 106, e420–e421. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Perez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Fraiman, J.; Erviti, J.; Jones, M.; Greenland, S.; Whelan, P.; Kaplan, R.M.; Doshi, P. Serious adverse events of special interest following mRNA COVID-19 vaccination in randomized trials in adults. Vaccine 2022, 40, 5798–5805. [Google Scholar] [CrossRef]
- Amorim, L.V.P.; Cristelli, M.P.; Viana, L.A.; Dreige, Y.C.; Requiao-Moura, L.R.; Nakamura, M.R.; Foresto, R.D.; Medina-Pestana, J.; Tedesco-Silva, H. Immunogenicity, Reactogenicity, and Reinfection After 2 Doses of the Inactivated Whole-virion CoronaVac Vaccine in Kidney Transplant Recipients Convalescents From COVID-19. Transplantation 2023, 107, e72–e73. [Google Scholar] [CrossRef] [PubMed]
- Kuniduzi, Y.; Chen, B.; Zeng, J.; Sun, X.; Chen, T.; Qian, X.; Wang, J.; Liang, F.; Abuduxukuer, R.; Yusufu, M.; et al. Efficacy and safety of a fourth dose of the COVID-19 vaccine in kidney transplant recipients: A systematic review and meta-analysis. Transpl. Immunol. 2023, 79, 101864. [Google Scholar] [CrossRef] [PubMed]
- Roll, G.R.; Bray, R.A.; Cooper, M.; Eagar, T.N.; Gebel, H.M.; Vranic, G.M.; Hitchman, K.M.K.; Houp, J.; Kamoun, M.; Killian, J.; et al. COVID-19 infection and vaccination rarely impact HLA antibody profile in waitlisted renal transplant candidates—A multicenter cohort. Hum. Immunol. 2023, 84, 278–285. [Google Scholar] [CrossRef]
- Cristelli, M.P.; Rissoni, R.A.P.; Viana, L.A.; Tedesco-Silva, H.; Medina-Pestana, J. How Did the Omicron Surge Affect Kidney Transplant Recipients Compared With a Cohort From the General Population? Transplantation 2022, 106, e382–e383. [Google Scholar] [CrossRef]
- Wong, G.; Rowlandson, M.; Sabanayagam, D.; Ginn, A.N.; Kable, K.; Sciberras, F.; Au, E.; Draper, J.; Arnott, A.; Sintchenko, V.; et al. COVID-19 Infection With the Omicron SARS-CoV-2 Variant in a Cohort of Kidney and Kidney Pancreas Transplant Recipients: Clinical Features, Risk Factors, and Outcomes. Transplantation 2022, 106, 1860–1866. [Google Scholar] [CrossRef]
- Villanego, F.; Vigara, L.A.; Alonso, M.; Orellana, C.; Gomez, A.M.; Eady, M.; Sanchez, M.G.; Gomez, R.; Garcia, T.; Mazuecos, A. Trends in COVID-19 Outcomes in Kidney Transplant Recipients During the Period of Omicron Variant Predominance. Transplantation 2022, 106, e304–e305. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).