Sugar Extraction from Secondary Agricultural Waste Biomass Using Hydrothermal Carbonization and Direct Contact Membrane Distillation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Hydrothermal Carbonization (HTC)
2.3. High-Performance Liquid Chromatography (HPLC) Analysis
2.4. Direct Contact Membrane Distillation (DCMD)
2.5. Bomb Calorimetry
3. Results and Discussion
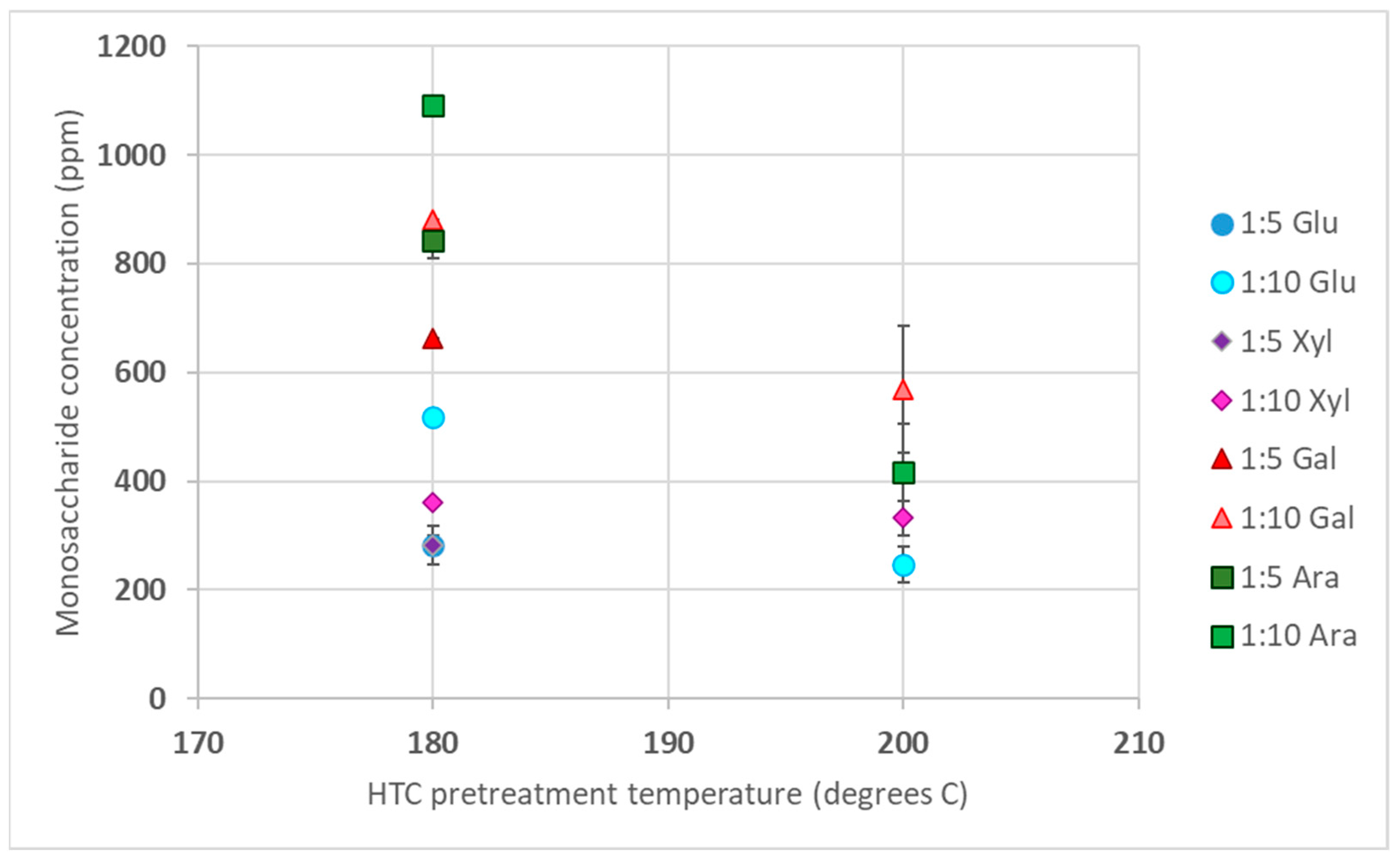
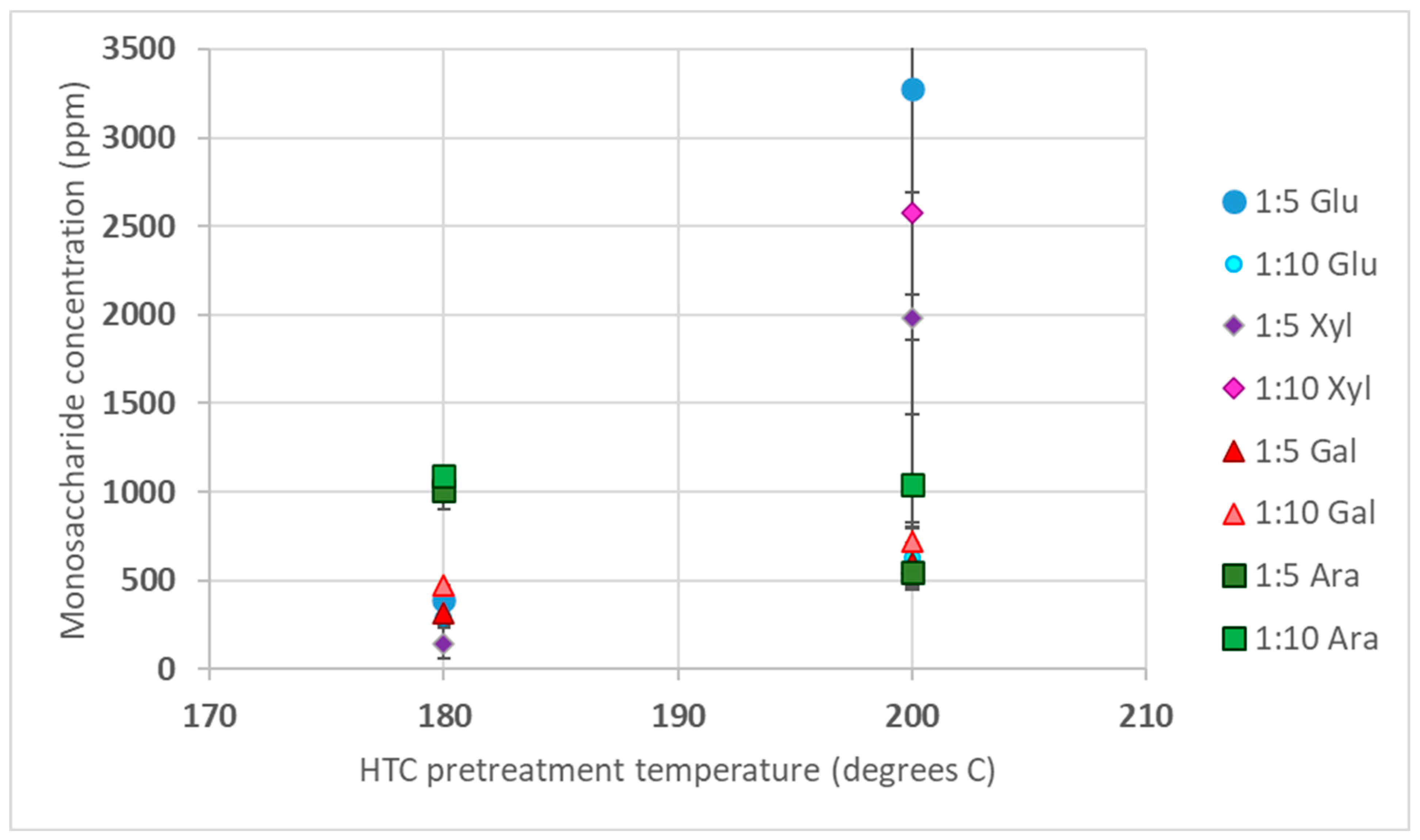
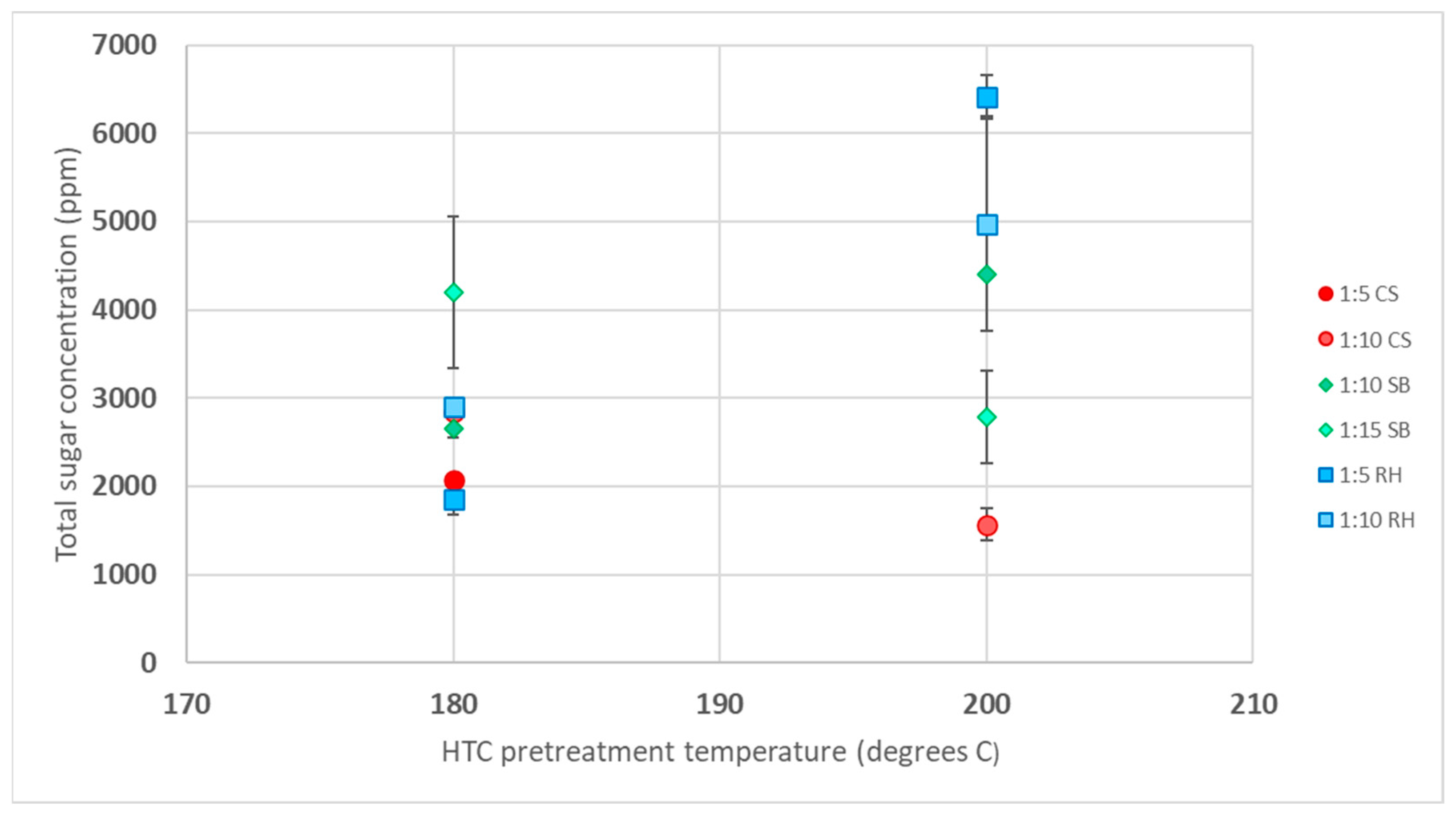
3.1. Sugar Analysis in HTC Liquid Product
3.1.1. CS Sugar Concentration after HTC
3.1.2. SB Sugar Concentration after HTC
3.1.3. RH Sugar Concentration after HTC
3.1.4. Comparison of the Difference in Biomass Responses to HTC
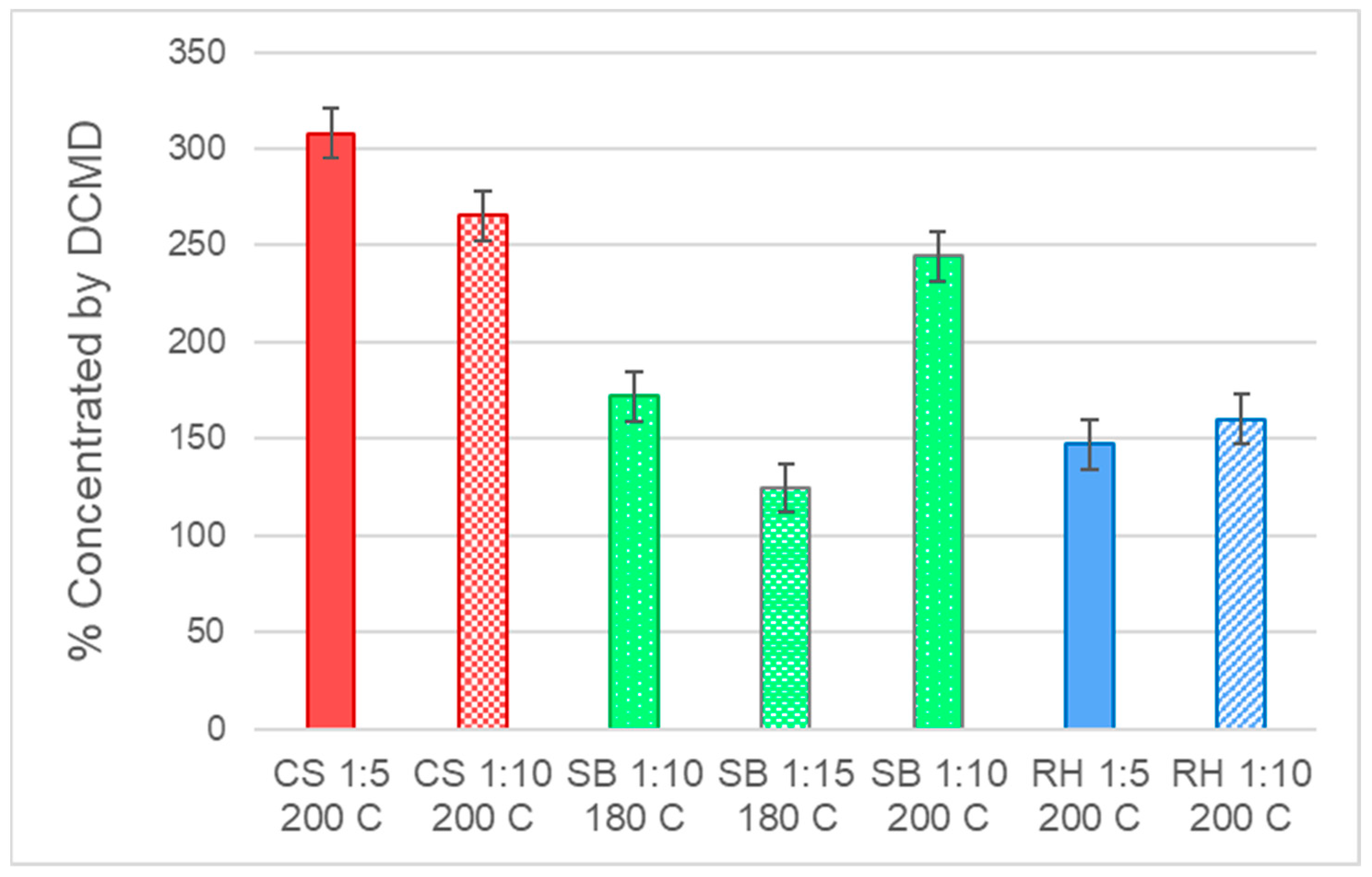
3.2. Direct Contact Membrane Distillation (DCMD)
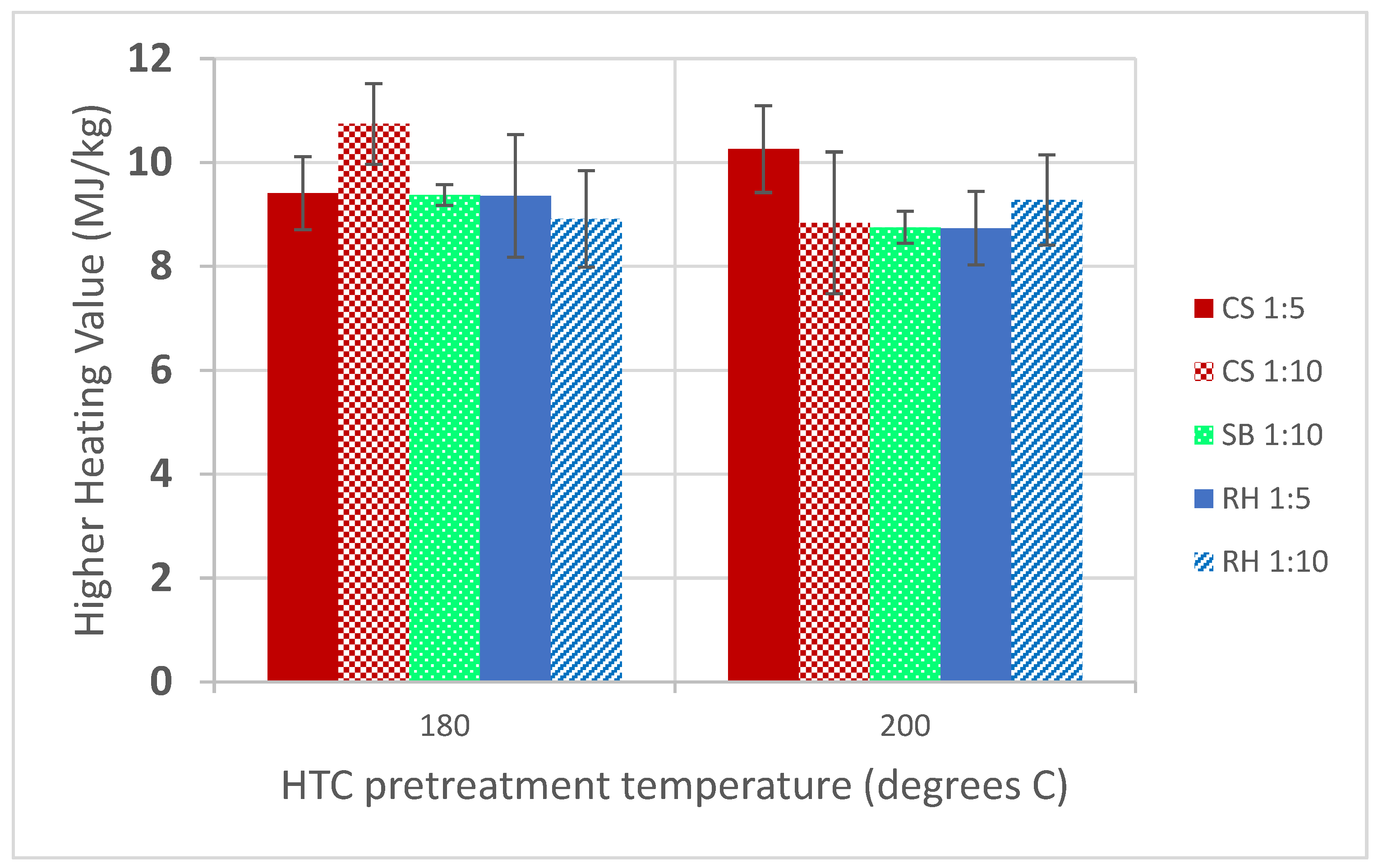
3.3. Higher Heating Values (HHV)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lindsey, R.; Dahlman, L. Climate Change: Global Temperature. NOAA Climate.gov. Available online: https://www.climate.gov/news-features/understanding-climate/climate-change-global-temperature (accessed on 3 February 2023).
- Agrela, F.; Cabrera, M.; Morales, M.M.; Zamorano, M.; Alshaaer, M. 2—Biomass fly ash and biomass bottom ash. In New Trends in Eco-Efficient and Recycled Concrete; de Brito, J., Agrela, F., Eds.; Woodhead Publishing: Sawston, UK, 2019; pp. 23–58. [Google Scholar] [CrossRef]
- Sindhu, R.; Binod, P.; Pandey, A.; Ankaram, S.; Duan, Y.; Awasthi, M.K. Chapter 5—Biofuel Production from Biomass: Toward Sustainable Development. In Current Developments in Biotechnology and Bioengineering; Kumar, S., Kumar, R., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 79–92. [Google Scholar] [CrossRef]
- Wan Azelee, N.I.; Mahdi, H.I.; Cheng, Y.-S.; Nordin, N.; Illias, R.M.; Rahman, R.A.; Shaarani, S.M.; Bhatt, P.; Yadav, S.; Chang, S.W.; et al. Biomass degradation: Challenges and strategies in extraction and fractionation of hemicellulose. Fuel 2023, 339, 126982. [Google Scholar] [CrossRef]
- Dutta, T.; Sun, J.; Wang, E.; Hull, S.; Simmons, B.A.; Singh, S.; Isern, N.G.; Cort, J.R. Survey of Lignin-Structure Changes and Depolymerization during Ionic Liquid Pretreatment. ACS Sustain. Chem. Eng. 2017, 5, 10116–10127. [Google Scholar] [CrossRef]
- Luo, Y.; Li, Z.; Li, X.; Liu, X.; Fan, J.; Clark, J.H.; Hu, C. The production of furfural directly from hemicellulose in lignocellulosic biomass: A review. Catal. Today 2019, 319, 14–24. [Google Scholar] [CrossRef]
- Khan, T.A.; Saud, A.S.; Jamari, S.S.; Rahim, M.H.A.; Park, J.-W.; Kim, H.-J. Hydrothermal carbonization of lignocellulosic biomass for carbon rich material preparation: A review. Biomass Bioenergy 2019, 130, 105384. [Google Scholar] [CrossRef]
- Chacón-Parra, A.; van Eyk, P. Reaction kinetics for the hydrothermal carbonisation of cellulose in a two-phase pathway. Fuel 2022, 309, 122169. [Google Scholar] [CrossRef]
- Volpe, M.; Messineo, A.; Mäkelä, M.; Barr, M.R.; Volpe, R.; Corrado, C.; Fiori, L. Reactivity of cellulose during hydrothermal carbonization of lignocellulosic biomass. Fuel Process. Technol. 2020, 206, 106456. [Google Scholar] [CrossRef]
- Reza, M.T.; Wirth, B.; Lüder, U.; Werner, M. Behavior of selected hydrolyzed and dehydrated products during hydrothermal carbonization of biomass. Bioresour. Technol. 2014, 169, 352–361. [Google Scholar] [CrossRef]
- Kumar, N.; Weldon, R.; Lynam, J.G. Hydrothermal carbonization of coffee silverskins. Biocatal. Agric. Biotechnol. 2021, 36, 102145. [Google Scholar] [CrossRef]
- Coronella, C.J.; Reza, M.T.; Lynam, J.G.; Graves, D.; Uddin, M.H. Engineered Pellets from Biomass Blend. Available online: http://www.aiche.org/resources/chemeondemand/conference-presentations/engineering-pellets-biomass-blends (accessed on 18 September 2023).
- Lynam, J.; Reza, M.T.; Yan, W.; Vásquez, V.; Coronella, C. Hydrothermal carbonization of various lignocellulosic biomass. Biomass Convers. Biorefin. 2015, 5, 173–181. [Google Scholar] [CrossRef]
- Hegde, C.; Ribeiro, R. Preparation and Characterization of Hydrophobic Membranes and Their Seawater Desalination Performance Study by Direct Contact Membrane Distillation. Nat. Environ. Pollut. Technol. 2022, 21, 1599–1608. [Google Scholar] [CrossRef]
- Lynam, J.G.; Chow, G.I.; Coronella, C.J.; Hiibel, S.R. Ionic liquid and water separation by membrane distillation. Chem. Eng. J. 2016, 288, 557–561. [Google Scholar] [CrossRef]
- Sirkar, K.K.; Song, L. Pilot-Scale Studies for Direct Contact Membrane Distillation-Based Desalination Process. In Desalination and Water Purification Research and Development Program Report; No. 134; US Department of the Interior, Bureau of Reclamation: Washington, DC, USA, 2009. [Google Scholar]
- Charisiadis, C. Available online: https://www.lenntech.com/Data-sheets/MD-ZLD-interactive.pdf (accessed on 23 May 2023).
- de Lima, A.E.; Guimarães, R.J.; da Cunha, S.H.B.; Castro, E.M.; Faria, M.M.L.; de Carvalho, A.M. Seedling production of coffea arabica from different cultivars in a modified hydroponic system and nursery using different containers. Cienc. Agrotecnol. 2021, 45, e017821. [Google Scholar] [CrossRef]
- Laviola, B.G.; Martinez, H.E.P.; Mauri, A.L. Influence of the level of fertilization of the matrix plants in the formation of seedlings of coffee plants in hydroponic systems. Cienc. Agrotecnol. 2007, 31, 1043–1047. [Google Scholar] [CrossRef]
- Chapae, C.; Songsri, P.; Kaewpradit, W.; Jongrungklang, N.; Gonkhamdee, S. Suitable Planting Materials and Nutrient Concentrations for Investigating Sugarcanes under Hydroponic System. Int. J. Bot. 2020, 16, 20–33. [Google Scholar] [CrossRef]
- Shandilya, Z.M.; Tanti, B. Hydroponic screening of traditional rice varieties in Assam, India to estimate their potential resistance to Al toxicity under P deficiency. Acta Agrobot. 2019, 72, 1–14. [Google Scholar] [CrossRef]
- Tang, H.; Rising, H.H.; Majji, M.; Brown, R.D. Long-Term Space Nutrition: A Scoping Review. Nutrients 2021, 14, 194. [Google Scholar] [CrossRef]
- Hijosa-Valsero, M.; Garita-Cambronero, J.; Paniagua-García, A.I.; Díez-Antolínez, R. Biobutanol production from coffee silverskin. Microb. Cell Factories 2018, 17, 154. [Google Scholar] [CrossRef]
- Canilha, L.; Santos, V.T.O.; Rocha, G.J.M.; Almeida e Silva, J.B.; Silva, S.S.; Felipe, M.G.A.; Ferraz, A.; Milagres, A.M.F.; Carvalho, W.; Giulietti, M. A study on the pretreatment of a sugarcane bagasse sample with dilute sulfuric acid. J. Ind. Microbiol. Biotechnol. 2011, 38, 1467–1475. [Google Scholar] [CrossRef]
- Cherubini, F. The biorefinery concept: Using biomass instead of oil for producing energy and chemicals. Energy Convers. Manag. 2010, 51, 1412–1421. [Google Scholar] [CrossRef]
- Available online: https://www.theadvocate.com/baton_rouge/louisianas-bagasse-piles-are-bigger-than-ever-could-new-technology-find-other-uses/article_5ea22930-f4e4-11ed-b509-4bcce84c12a8.html (accessed on 23 May 2023).
- Shahbandeh, M. Available online: https://www.statista.com/statistics/249681/total-consumption-of-sugar-worldwide/#:~:text=Global%20sugar%20market,the%20European%20Union%20and%20China (accessed on 24 August 2023).
- Available online: https://www.irri.org/ (accessed on 23 May 2023).
- Available online: https://www.thenewhumanitarian.org/ (accessed on 23 May 2023).
- Kim, H.S.; Yang, H.S.; Kim, H.J.; Park, H.J. Thermogravimetric analysis of rice husk flour filled thermoplastic polymer composites. J. Therm. Anal. Calorim. 2004, 76, 395–404. [Google Scholar]
- Global Markets for Texas Rough Rice. Available online: https://agecoext.tamu.edu/wp-content/uploads/2021/09/GM-for-Rough-Rice.pdf (accessed on 24 August 2023).
- Kumar, N.; Gautam, R.; Stallings, J.D.; Coty, G.G.; Lynam, J.G. Secondary Agriculture Residues Pretreatment Using Deep Eutectic Solvents. Waste Biomass Valorization 2021, 12, 2259–2269. [Google Scholar] [CrossRef]
- Hoekman, S.K.; Broch, A.; Robbins, C. Hydrothermal Carbonization (HTC) of Lignocellulosic Biomass. Energy Fuels 2011, 25, 1802–1810. [Google Scholar] [CrossRef]
- Redgwell, R.J.; Curti, D.; Fischer, M.; Nicolas, P.; Fay, L.B. Coffee bean arabinogalactans: Acidic polymers covalently linked to protein. Carbohydr. Res. 2002, 337, 239–253. [Google Scholar] [CrossRef] [PubMed]
- Hoekman, S.K.; Broch, A.; Felix, L.; Farthing, W. Hydrothermal carbonization (HTC) of loblolly pine using a continuous, reactive twin-screw extruder. Energy Convers. Manag. 2017, 134, 247–259. [Google Scholar] [CrossRef]
- Golon, A.; Kuhnert, N. Characterisation of “caramel-type” thermal decomposition products of selected monosaccharides including fructose, mannose, galactose, arabinose and ribose by advanced electrospray ionization mass spectrometry methods. Food Funct. 2013, 4, 1040–1050. [Google Scholar] [CrossRef]
- Guo, Z.; Ling, Z.; Wang, C.; Zhang, X.; Xu, F. Integration of facile deep eutectic solvents pretreatment for enhanced enzymatic hydrolysis and lignin valorization from industrial xylose residue. Bioresour. Technol. 2018, 265, 334–339. [Google Scholar] [CrossRef]
- da Silva, C.D.M.S.; de Castro, D.A.R.; Santos, M.C.; Almeida, H.D.S.; Schultze, M.; Lüder, U.; Hoffmann, T.; Machado, N.T. Process Analysis of Main Organic Compounds Dissolved in Aqueous Phase by Hydrothermal Processing of Açaí (Euterpe oleraceae, Mart.) Seeds: Influence of Process Temperature, Biomass-to-Water Ratio, and Production Scales. Energies 2021, 14, 5608. [Google Scholar] [CrossRef]
- Ouensanga, A. Variation of Fiber Composition in Sugar Cane Stalks. J. Soc. Wood Sci. Technol. 1989, 21, 105–111. [Google Scholar]
- Yang, H.; Yi, N.; Zhao, S.; Qaseem, M.F.; Zheng, B.; Li, H.; Feng, J.-X.; Wu, A.-M. Characterization of hemicelluloses in sugarcane (Saccharum spp. hybrids) culm during xylogenesis. Int. J. Biol. Macromol. 2020, 165, 1119–1128. [Google Scholar] [CrossRef]
- Mirza Faisal, Q.; Ai-Min, W. Balanced Xylan Acetylation is the Key Regulator of Plant Growth and Development, and Cell Wall Structure and for Industrial Utilization. Int. J. Mol. Sci. 2020, 21, 7875. [Google Scholar] [CrossRef]
- Amornnopparattanakul, N.; Yingkamhaeng, N.; Meesupthong, R.; Pinmanee, P.; Sukyai, P.; Suwanprateep, J.; Nimchua, T. Structure Features of Sugarcane Bagasse Under Ultrasonic with Xylanase and Laccase Treatment. Sugar Tech 2023, 25, 893–905. [Google Scholar] [CrossRef]
- Ahmad, T.; Kenne, L.; Olsson, K.; Theander, O. The formation of 2-furaldehyde and formic acid from pentoses in slightly acidic deuterium oxide studied by 1H NMR spectroscopy. Carbohydr. Res. 1995, 276, 309–320. [Google Scholar] [CrossRef]
- Lynam, J.G.; Reza, M.T.; Vasquez, V.R.; Coronella, C.J. Pretreatment of rice hulls by ionic liquid dissolution. Bioresour. Technol. 2012, 114, 629–636. [Google Scholar] [CrossRef]
- Ouyang, Y.S. Mesomechanical characterization of in situ rice grain hulls. Trans. ASAE 2001, 44, 357–367. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, L.; Wang, Y.; Zhang, C.; Hui, K.S. Microwave-assisted Acid-catalyzed Hydrolysis of Hemicelluloses in Rice Husk into Xylose. In Proceedings of the 2020 International Conference on Petrochemical Engineering and Green Development, Shanghai, China, 17–19 April 2020. [Google Scholar]
- Tae Hoon, K.; Hyun Jin, R.; Kyeong Keun, O. Improvement of Organosolv Fractionation Performance for Rice Husk through a Low Acid-Catalyzation. Energies 2019, 12, 1800. [Google Scholar] [CrossRef]
- Räisänen, U.; Pitkänen, I.; Halttunen, H.; Hurtta, M. Formation of the main degradation compounds from arabinose, xylose, mannose and arabinitol during pyrolysis. J. Therm. Anal. Calorim. 2003, 72, 481–488. [Google Scholar] [CrossRef]
- Niglio, S.; Marzocchella, A.; Procentese, A.; Russo, M.E.; Sannia, G. Combined pretreatments of coffee silverskin to enhance fermentable sugar yield. Biomass Convers. Biorefin. 2020, 10, 1237–1249. [Google Scholar] [CrossRef]
- Hermansyah; Cahyadi, H.; Fatma; Miksusanti; Kasmiarti, G.; Panagan, A.T. Delignification of Lignocellulosic Biomass Sugarcane Bagasse by Using Ozone as Initial Step to Produce Bioethanol. Pol. J. Environ. Stud. 2021, 30, 4405–4411. [Google Scholar] [CrossRef]
- Notzir, N.H.; Masngut, M.I.; Louis, S.R. Physical Structures and Adsorption Efficiencies of Sugarcane Bagasse, Coconut Pulp and Sawdust as Natural Adsorbents in Removal of Heavy Metals from Car Wash Activity. Malays. J. Med. Health Sci. 2022, 18, 108–116. [Google Scholar] [CrossRef]
- Wang, S.; Xu, D.; Guo, Y.; Tang, X.; Wang, Y.; Zhang, J.; Ma, H.; Qian, L.; Li, Y. Introduction. In Supercritical Water Processing Technologies for Environment, Energy and Nanomaterial Applications; Wang, S., Xu, D., Guo, Y., Tang, X., Wang, Y., Zhang, J., Ma, H., Qian, L., Li, Y., Eds.; Springer: Singapore, 2020; pp. 1–24. [Google Scholar]
- Heidari, M.; Salaudeen, S.; Arku, P.; Acharya, B.; Tasnim, S.; Dutta, A. Development of a mathematical model for hydrothermal carbonization of biomass: Comparison of experimental measurements with model predictions. Energy 2021, 214, 119020. [Google Scholar] [CrossRef]
- Rogalinski, T.; Ingram, T.; Brunner, G. Hydrolysis of lignocellulosic biomass in water under elevated temperatures and pressures. J. Supercrit. Fluids 2008, 47, 54–63. [Google Scholar] [CrossRef]
- Zhang, Y.; Nielsen, J.; Liu, Z. Yeast based biorefineries for oleochemical production. Curr. Opin. Biotechnol. 2021, 67, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Khounani, Z.; Nazemi, F.; Shafiei, M.; Aghbashlo, M.; Tabatabaei, M. Techno-economic aspects of a safflower-based biorefinery plant co-producing bioethanol and biodiesel. Energy Convers. Manag. 2019, 201, 112184. [Google Scholar] [CrossRef]
- Siyal, M.I.; Lee, C.-K.; Park, C.; Khan, A.A.; Kim, J.-O. A review of membrane development in membrane distillation for emulsified industrial or shale gas wastewater treatments with feed containing hybrid impurities. J. Environ. Manag. 2019, 243, 45–66. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Park, H. Applicability assessment of subcritical flux operation in crossflow microfiltration with a concentration polarization model. J. Environ. Eng. 2002, 128, 335–340. [Google Scholar] [CrossRef]
- Baghbanbashi, M.; Pazuki, G. Application of SAFT-VR Equation of State for Prediction of Thermophysical Properties of Sugar Solutions. J. Food Process Eng. 2016, 39, 601–609. [Google Scholar] [CrossRef]
- Yu, Y.; Wu, H.W. Understanding the Primary Liquid Products of Cellulose Hydrolysis in Hot-Compressed Water at Various Reaction Temperatures. Energy Fuels 2010, 24, 1963–1971. [Google Scholar] [CrossRef]
- Usman, M.; Chen, H.; Chen, K.; Ren, S.; Luo, G.; Zhang, S.; Clark, J.H.; Fan, J. Characterization and utilization of aqueous products from hydrothermal conversion of biomass for bio-oil and hydro-char production: A review. Green Chem. 2019, 21, 1553–1572. [Google Scholar] [CrossRef]
- Kulikova, M.V.; Krylova, A.Y.; Krysanova, K.O.; Kulikov, A.B.; Maximov, A.L. Mechanisms of Low-Temperature Processes of Biomass Conversion (A Review). Pet. Chem. 2023. [Google Scholar] [CrossRef]
- Sagar, V.; Hardin, M.; Kumar, N.; Lynam, J.G. Characterization and Energy Densification of Mayhaw Jelly Production Wastes Using Hydrothermal Carbonization. Food Technol. Biotechnol. 2023, 61, 118–126. [Google Scholar] [CrossRef]
- Reza, M.T.; Yan, W.; Uddin, M.H.; Lynam, J.G.; Hoekman, S.K.; Coronella, C.J.; Vasquez, V.R. Reaction kinetics of hydrothermal carbonization of loblolly pine. Bioresour. Technol. 2013, 139, 161–169. [Google Scholar] [CrossRef]
- Reza, M.T.; Uddin, M.H.; Lynam, J.G.; Coronella, C.J. Engineered pellets from dry torrefied and HTC biochar blends. Biomass Bioenergy 2014, 63, 229–238. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sagar, V.; Lynam, J.G.; Parrenin, A.G. Sugar Extraction from Secondary Agricultural Waste Biomass Using Hydrothermal Carbonization and Direct Contact Membrane Distillation. Biomass 2023, 3, 323-335. https://doi.org/10.3390/biomass3040020
Sagar V, Lynam JG, Parrenin AG. Sugar Extraction from Secondary Agricultural Waste Biomass Using Hydrothermal Carbonization and Direct Contact Membrane Distillation. Biomass. 2023; 3(4):323-335. https://doi.org/10.3390/biomass3040020
Chicago/Turabian StyleSagar, Viral, Joan G. Lynam, and Amelia G. Parrenin. 2023. "Sugar Extraction from Secondary Agricultural Waste Biomass Using Hydrothermal Carbonization and Direct Contact Membrane Distillation" Biomass 3, no. 4: 323-335. https://doi.org/10.3390/biomass3040020
APA StyleSagar, V., Lynam, J. G., & Parrenin, A. G. (2023). Sugar Extraction from Secondary Agricultural Waste Biomass Using Hydrothermal Carbonization and Direct Contact Membrane Distillation. Biomass, 3(4), 323-335. https://doi.org/10.3390/biomass3040020







