Red Seaweed Pigments from a Biotechnological Perspective
Abstract
1. Introduction
2. Phycobiliproteins
2.1. Distribution, Properties and Structure
2.2. Biotechnological Potential and Applications
2.3. Extraction and Purification Methods
2.4. Production and Commercialization
2.5. Phycoerythrin
2.6. Phycocyanin
2.7. Allophycocyanin
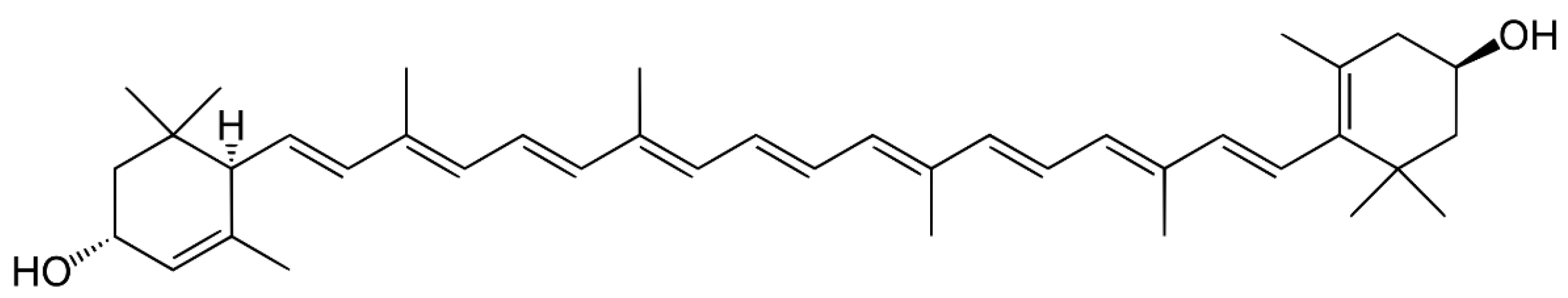
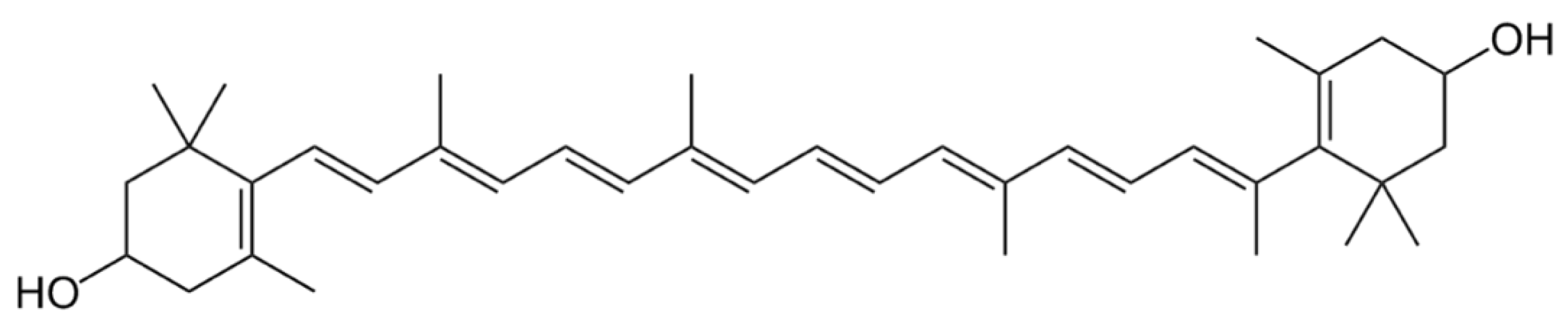
3. Carotenoids
3.1. Distribution, Properties and Structure
3.2. Biotechnological Potential and Applications
3.3. Carotenes
3.4. Xanthophylls
4. Chlorophyll
4.1. Distribution, Properties and Structure
4.2. Biotechnological Potential and Applications
4.3. Extraction and Purification Methods
4.4. Production and Commercialization
5. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, W.; Su, H.N.; Pu, Y.; Chen, J.; Liu, L.N.; Liu, Q.; Qin, S. Phycobiliproteins: Molecular structure, production, applications, and prospects. Biotechnol. Adv. 2019, 37, 340–353. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.S.; Kumari, S.; Singh, K.; Kushwaha, P. Influence of Seasonal Variation on Chemical Composition and Nutritional Profiles of Macro—And Microalgae. In Recent Advances in Micro and Macroalgal Processing; Rajauria, G., Yuan, Y.V., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2021; pp. 14–71. [Google Scholar]
- Cserháti, T. Liquid Chromatography of Natural Pigments and Synthetic Dyes, 1st ed.; Elsevier Science: Amsterdam, The Netherlands, 2006; ISBN 9780080465760. [Google Scholar]
- Kannaujiya, V.K.; Kumar, D.; Singh, V.; Sinha, R.P. Advances in Phycobiliproteins Research: Innovations and Commercialization. In Natural Bioactive Compounds: Technological Advancements; Sinha, R.P., Häder, D.-P., Eds.; Academic Press: London, UK, 2021; pp. 57–81. [Google Scholar]
- Pangestuti, R.; Kim, S.K. Biological activities and health benefit effects of natural pigments derived from marine algae. J. Funct. Foods 2011, 3, 255–266. [Google Scholar] [CrossRef]
- Talarico, L.; Maranzana, G. Light and adaptive responses in red macroalgae: An overview. J. Photochem. Photobiol. B Biol. 2000, 56, 1–11. [Google Scholar] [CrossRef]
- Pereira, L.; Neto, J.M. Marine Algae—Biodiversity, Taxonomy, Environmental Assessment, and Biotechnology, 1st ed.; Pereira, L., Neto, J.M., Eds.; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Bonanno, G.; Orlando-Bonaca, M. Chemical elements in Mediterranean macroalgae. A review. Ecotoxicol. Environ. Saf. 2018, 148, 44–71. [Google Scholar] [CrossRef]
- Begum, H.; Yusoff, F.M.D.; Banerjee, S.; Khatoon, H.; Shariff, M. Availability and Utilization of Pigments from Microalgae. Crit. Rev. Food Sci. Nutr. 2016, 56, 2209–2222. [Google Scholar] [CrossRef]
- Dufossé, L.; Galaup, P.; Yaron, A.; Arad, S.M.; Blanc, P.; Murthy, K.N.C.; Ravishankar, G.A. Microorganisms and microalgae as sources of pigments for food use: A scientific oddity or an industrial reality? Trends Food Sci. Technol. 2005, 16, 389–406. [Google Scholar] [CrossRef]
- Silva, S.C.; Ferreira, I.C.F.R.; Dias, M.M.; Filomena Barreiro, M. Microalgae-derived pigments: A 10-year bibliometric review and industry and market trend analysis. Molecules 2020, 25, 3406. [Google Scholar] [CrossRef] [PubMed]
- Morocho-Jácome, A.L.; Ruscinc, N.; Martinez, R.M.; de Carvalho, J.C.M.; Santos de Almeida, T.; Rosado, C.; Costa, J.G.; Velasco, M.V.R.; Baby, A.R. (Bio)Technological aspects of microalgae pigments for cosmetics. Appl. Microbiol. Biotechnol. 2020, 104, 9513–9522. [Google Scholar] [CrossRef]
- Dufossé, L. Current and Potential Natural Pigments from Microorganisms (Bacteria, Yeasts, Fungi, Microalgae). In Handbook on Natural Pigments in Food and Beverages: Industrial Applications for Improving Food Color; Carle, R., Schweiggert, R.M., Eds.; Woodhead Publishing: Duxford, UK, 2016; pp. 337–354. [Google Scholar]
- Agboyibor, C.; Kong, W.B.; Chen, D.; Zhang, A.M.; Niu, S.Q. Monascus pigments production, composition, bioactivity and its application: A review. Biocatal. Agric. Biotechnol. 2018, 16, 433–447. [Google Scholar] [CrossRef]
- World Intellectual Property Organization (WIPO) Patent Landscape Report: Microalgae-Related Technologies. In Patent Landscape Report on Microalgae-Related Technologies; WIPO Publication No. 947/5E; World Intellectual Property Organization: Geneva, Switzerland, 2016; p. 74.
- World International Property Organization Patentscope. Available online: https://www.wipo.int/patentscope/en/ (accessed on 12 December 2021).
- Hsieh-Lo, M.; Castillo, G.; Ochoa-Becerra, M.A.; Mojica, L. Phycocyanin and phycoerythrin: Strategies to improve production yield and chemical stability. Algal Res. 2019, 42, 101600. [Google Scholar] [CrossRef]
- Beattie, S.W.; Morançais, M.; Déléris, P.; Fleurence, J.; Dumay, J. Extraction of phycocyanin and phycoerythrin pigments. In Protocols for Macroalgae Research; Charrier, B., Wichard, T., Reddy, C.R.K., Eds.; CRC Press: Boca Raton, FL, USA, 2018; pp. 249–265. [Google Scholar]
- Sonani, R.R. Recent advances in production, purification and applications of phycobiliproteins. World J. Biol. Chem. 2016, 7, 100. [Google Scholar] [CrossRef] [PubMed]
- Kuddus, M.; Singh, P.; Thomas, G.; Al-Hazimi, A. Recent developments in production and biotechnological applications of C-phycocyanin. Biomed Res. Int. 2013, 2013, 742859. [Google Scholar] [CrossRef] [PubMed]
- Dumay, J.; Morançais, M.; Munier, M.; Le Guillard, C.; Fleurence, J. Phycoerythrins: Valuable proteinic pigments in red seaweeds. Adv. Bot. Res. 2014, 71, 321–343. [Google Scholar] [CrossRef]
- Cohen-Bazire, G.; Bryant, D.A. Phycobilisomes: Composition and Structure. In The Biology of Cyanobacteria; Carr, N.G., Whitton, B.A., Eds.; Blackwell Publishing: Oxford, UK, 1982; pp. 143–190. [Google Scholar]
- Román, R.B.; Alvárez-Pez, J.M.; Fernández, F.G.A.; Grima, E.M. Recovery of pure B-phycoerythrin from the microalga Porphyridium cruentum. J. Biotechnol. 2002, 93, 73–85. [Google Scholar] [CrossRef]
- Basheva, D.; Moten, D.; Stoyanov, P.; Belkinova, D.; Mladenov, R.; Teneva, I. Content of phycoerythrin, phycocyanin, alophycocyanin and phycoerythrocyanin in some cyanobacterial strains: Applications. Eng. Life Sci. 2018, 18, 861–866. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Sun, L.; Fu, X.; Chen, M. Phycoerythrin-phycocyanin aggregates and phycoerythrin aggregates from phycobilisomes of the marine red alga Polysiphonia urceolata. Int. J. Biol. Macromol. 2019, 126, 685–696. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Jiang, P. Combinational biosynthesis and characterization of fusion proteins with tandem repeats of allophycocyanin holo-α subunits, and their application as bright fluorescent labels for immunofluorescence assay. J. Biosci. Bioeng. 2018, 126, 778–782. [Google Scholar] [CrossRef]
- Adir, N.; Bar-Zvi, S.; Harris, D. The amazing phycobilisome. Biochim. Biophys. Acta Bioenerg. 2020, 1861, 148047. [Google Scholar] [CrossRef] [PubMed]
- Fleurence, J. R-phycoerythrin from red macroalgae: Strategies for extraction and potential application in biotechnology. Appl. Biotechnol. Food Sci. Policy 2003, 1, 1–6. [Google Scholar]
- Wehrmeyer, W. Phycobilisomes: Structure and Function. In Experimental Phycology: Cell Walls and Surfaces, Reproduction, Photosynthesis; Wiesser, W., Robinson, D.G., Starr, R.C., Eds.; Springer: Berlin/Heidelberg, Germany, 1990; pp. 158–172. [Google Scholar]
- López-Figueroa, F. Diurnal Variation in Pigment Content in Porphyra laciniata and Chondrus crispus and its Relation to the Diurnal Changes of Underwater Light Quality and Quantity. Mar. Ecol. 1992, 13, 285–305. [Google Scholar] [CrossRef]
- Niu, J.; Xu, M.; Wang, G.; Zhang, K.; Peng, G. Comprehensive extraction of agar and R-phycoerythrin from Gracilaria lemaneiformis (Bangiales, Rhodophyta). Indian J. Mar. Sci. 2013, 42, 21–28. [Google Scholar]
- Pagels, F.; Guedes, A.C.; Amaro, H.M.; Kijjoa, A.; Vasconcelos, V. Phycobiliproteins from cyanobacteria: Chemistry and biotechnological applications. Biotechnol. Adv. 2019, 37, 422–443. [Google Scholar] [CrossRef] [PubMed]
- Dagnino-Leone, J.; Figueroa, M.; Uribe, E.; Hinrichs, M.V.; Ortiz-López, D.; Martínez-Oyanedel, J.; Bunster, M. Biosynthesis and characterization of a recombinant eukaryotic allophycocyanin using prokaryotic accessory enzymes. Microbiologyopen 2020, 9, e989. [Google Scholar] [CrossRef] [PubMed]
- Leney, A.C.; Tschanz, A.; Heck, A.J.R. Connecting color with assembly in the fluorescent B-phycoerythrin protein complex. FEBS J. 2018, 285, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Rossano, R.; Ungaro, N.; D’Ambrosio, A.; Liuzzi, G.M.; Riccio, P. Extracting and purifying R-phycoerythrin from Mediterranean red algae Corallina elongata Ellis & Solander. J. Biotechnol. 2003, 101, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Bei, H.; Guang-Ce, W.; Chen-Kui, Z.; Zhen-Gang, L. The experimental research of R-phycoerythrin subunits on cancer treatment: A new photosensitizer in PDT. Cancer Biother. Radiopharm. 2002, 17, 35–42. [Google Scholar] [CrossRef]
- Hemlata; Afreen, S.; Fatma, T. Extraction, purification and characterization of phycoerythrin from Michrochaete and its biological activities. Biocatal. Agric. Biotechnol. 2018, 13, 84–89. [Google Scholar] [CrossRef]
- Yabuta, Y.; Fujimura, H.; Kwak, C.S.; Enomoto, T.; Watanabe, F. Antioxidant activity of the phycoerythrobilin compound formed from a dried Korean purple laver (Porphyra sp.) during In Vitro digestion. Food Sci. Technol. Res. 2010, 16, 347–352. [Google Scholar] [CrossRef]
- Nagaraj, S.; Arulmurugan, P.; Rajaram, M.G.; Karuppasamy, K.; Jayappriyan, K.R.; Sundararaj, R.; Vijayanand, N.; Rengasamy, R. Hepatoprotective and antioxidative effects of C-phycocyanin from Arthrospira maxima SAG 25780 in CCl4-induced hepatic damage rats. Biomed. Prev. Nutr. 2012, 2, 81–85. [Google Scholar] [CrossRef]
- Pardhasaradhi, B.V.V.; Mubarak Ali, A.; Leela Kumari, A.; Reddanna, P.; Khar, A. Phycocyanin-mediated apoptosis in AK-5 tumor cells involves down-regulation of Bcl-2 and generation of ROS. Mol. Cancer Ther. 2003, 2, 1165–1170. [Google Scholar]
- Reddy, M.C.; Subhashini, J.; Mahipal, S.V.K.; Bhat, V.B.; Reddy, P.S.; Kiranmai, G.; Madyastha, K.M.; Reddanna, P. C-Phycocyanin, a selective cyclooxygenase-2 inhibitor, induces apoptosis in lipopolysaccharide-stimulated RAW 264.7 macrophages. Biochem. Biophys. Res. Commun. 2003, 304, 385–392. [Google Scholar] [CrossRef]
- Fernández-Rojas, B.; Hernández-Juárez, J.; Pedraza-Chaverri, J. Nutraceutical properties of Phycocyanin. J. Funct. Foods 2014, 11, 375–392. [Google Scholar] [CrossRef]
- Eriksen, N.T. Production of phycocyanin—A pigment with applications in biology, biotechnology, foods and medicine. Appl. Microbiol. Biotechnol. 2008, 80, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Shih, S.R.; Tsai, K.N.; Li, Y.S.; Chueh, C.C.; Chan, E.C. Inhibition of enterovirus 71-induced apoptosis by allophycocyanin isolated from a blue-green alga Spirulina platensis. J. Med. Virol. 2003, 70, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Nishizawa, M.; Shimizu, Y.; Saeki, H. Anti-inflammatory effects of dulse (Palmaria palmata) resulting from the simultaneous water-extraction of phycobiliproteins and chlorophyll a. Food Res. Int. 2017, 100, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.T.; Yeh, H.Y.; Lung, W.Q.C.; Huang, J.; Chen, Y.J.; Chen, B.; Nan, F.H.; Lee, M.C. R-phycoerythrin from Colaconema formosanum (Rhodophyta), an anti-allergic and collagen promoting material for cosmeceuticals. Appl. Sci. 2021, 11, 9425. [Google Scholar] [CrossRef]
- Hao, S.; Li, S.; Wang, J.; Zhao, L.; Zhang, C.; Huang, W.; Wang, C. Phycocyanin Reduces Proliferation of Melanoma Cells through Downregulating GRB2/ERK Signaling. J. Agric. Food Chem. 2018, 66, 10921–10929. [Google Scholar] [CrossRef]
- Pattarayan, D.; Rajarajan, D.; Ayyanar, S.; Palanichamy, R.; Subbiah, R. C-phycocyanin suppresses transforming growth factor-β1-induced epithelial mesenchymal transition in human epithelial cells. Pharmacol. Reports 2017, 69, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Wen, R.; Sui, Z.; Zhang, X.; Zhang, S.; Qin, S. Expression of the phycoerythrin gene of Gracilaria lemaneiformis (Rhodophyta) in E. coli and evaluation of the bioactivity of recombinant PE. J. Ocean Univ. China 2007, 6, 373–377. [Google Scholar] [CrossRef]
- Pan, Q.; Chen, M.; Li, J.; Wu, Y.; Zhen, C.; Liang, B. Antitumor function and mechanism of phycoerythrin from Porphyra haitanensis. Biol. Res. 2013, 46, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Cian, R.E.; López-Posadas, R.; Drago, S.R.; de Medina, F.S.; Martínez-Augustin, O. Immunomodulatory properties of the protein fraction from Phorphyra columbina. J. Agric. Food Chem. 2012, 60, 8146–8154. [Google Scholar] [CrossRef] [PubMed]
- Becker, W.; Richmond, A. Microalgae in human and animal nutrition. In Handbook of Microalgal Culture: Biotechnology and Applied Phycology; Blackwell Publishing Ltd: Hoboken, NJ, USA, 2004. [Google Scholar]
- Munier, M.; Dumay, J.; Morançais, M.; Jaouen, P.; Fleurence, J.; Jaouen, P.; Fleurence, J. Variation in the Biochemical Composition of the Edible Seaweed Grateloupia turuturu Yamada Harvested from Two Sampling Sites on the Brittany Coast (France): The Influence of Storage Method on the Extraction of the Seaweed Pigment R-Phycoerythrin. J. Chem. 2013, 2013, 1–8. [Google Scholar] [CrossRef]
- Moraes, C.C.; Sala, L.; Cerveira, G.P.; Kalil, S.J. C-phycocyanin extraction from Spirulina platensis wet biomass. Braz. J. Chem. Eng. 2011, 28, 45–49. [Google Scholar] [CrossRef]
- Montoya, E.J.O.; Dorion, S.; Atehortua-Garcés, L.; Rivoal, J. Phycobilin heterologous production from the Rhodophyta Porphyridium cruentum. J. Biotechnol. 2021, 341, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Beer, S.; Eshel, A. Determining phycoerythrin and phycocyanin concentrations in aqueous crude extracts of red algae. Mar. Freshw. Res. 1985, 36, 785–792. [Google Scholar] [CrossRef]
- Saluri, M.; Kaldmäe, M.; Tuvikene, R. Reliable quantification of R-phycoerythrin from red algal crude extracts. J. Appl. Phycol. 2020, 32, 1421–1428. [Google Scholar] [CrossRef]
- Pereira, T.; Barroso, S.; Mendes, S.; Amaral, R.A.; Dias, J.R.; Baptista, T.; Saraiva, J.A.; Alves, N.M.; Gil, M.M. Optimization of phycobiliprotein pigments extraction from red algae Gracilaria gracilis for substitution of synthetic food colorants. Food Chem. 2020, 321, 126688. [Google Scholar] [CrossRef] [PubMed]
- Viana Carlos, T.A.; dos Santos Pires Cavalcante, K.M.; de Cássia Evangelista de Oliveira, F.; do Ó. Pessoa, C.; Sant’Ana, H.B.; Feitosa, F.X.; Rocha, M.V.P. Pressurized extraction of phycobiliproteins from Arthrospira platensis and evaluation of its effect on antioxidant and anticancer activities of these biomolecules. J. Appl. Phycol. 2021, 33, 929–938. [Google Scholar] [CrossRef]
- Liu, S.; Chen, Y.; Lu, Y.; Chen, H.; Li, F.; Qin, S. Biosynthesis of fluorescent cyanobacterial allophycocyanin trimer in Escherichia coli. Photosynth. Res. 2010, 105, 135–142. [Google Scholar] [CrossRef]
- Chen, H.; Lin, H.; Li, F.; Jiang, P.; Qin, S. Biosynthesis of a stable allophycocyanin beta subunit in metabolically engineered Escherichia coli. J. Biosci. Bioeng. 2013, 115, 485–489. [Google Scholar] [CrossRef]
- Mysliwa-Kurdziel, B.; Solymosi, K. Phycobilins and Phycobiliproteins Used in Food Industry and Medicine. Mini-Rev. Med. Chem. 2016, 17, 1173–1193. [Google Scholar] [CrossRef] [PubMed]
- Sekar, S.; Chandramohan, M. Phycobiliproteins as a commodity: Trends in applied research, patents and commercialization. J. Appl. Phycol. 2008, 20, 113–136. [Google Scholar] [CrossRef]
- Van Der Weij-De Wit, C.D.; Doust, A.B.; Van Stokkum, I.H.M.; Dekker, J.P.; Wilk, K.E.; Curmi, P.M.G.; Scholes, G.D.; Van Grondelle, R. How energy funnels from the phycoerythrin antenna complex to photosystem i and photosystem II in cryptophyte Rhodomonas CS24 cells. J. Phys. Chem. B 2006, 110, 25066–25073. [Google Scholar] [CrossRef] [PubMed]
- Glazer, A.N. Phycobiliproteins—A family of valuable, widely used fluorophores. J. Appl. Phycol. 1994, 6, 105–112. [Google Scholar] [CrossRef]
- García, A.B.; Longo, E.; Murillo, M.C.; Bermejo, R. Using a B-Phycoerythrin Extract as a Natural Colorant: Application in Milk-Based Products. Molecules 2021, 26, 297. [Google Scholar] [CrossRef] [PubMed]
- Bermejo, R.; Acién, F.G.; Ibáñez, M.J.; Fernández, J.M.; Molina, E.; Alvarez-Pez, J.M. Preparative purification of B-phycoerythrin from the microalga Porphyridium cruentum by expanded-bed adsorption chromatography. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2003, 790, 317–325. [Google Scholar] [CrossRef]
- Munier, M.; Jubeau, S.; Wijaya, A.; Morançais, M.; Dumay, J.; Marchal, L.; Jaouen, P.; Fleurence, J. Physicochemical factors affecting the stability of two pigments: R-phycoerythrin of Grateloupia turuturu and B-phycoerythrin of Porphyridium cruentum. Food Chem. 2014, 150, 400–407. [Google Scholar] [CrossRef]
- Kawsar, S.M.A.; Fujii, Y.; Matsumoto, R.; Yasumitsu, H.; Ozeki, Y. Protein R-phycoerythrin from marine red alga Amphiroa anceps: Extraction, purification and characterization. Phytol. Balc. 2011, 17, 347–354. [Google Scholar]
- Ismail, M.M.; Osman, M.E.H. Seasonal fluctuation of photosynthetic pigments of most common red seaweeds species collected from Abu Qir, Alexandria, Egypt. Rev. Biol. Mar. Oceanogr. 2016, 51, 515–525. [Google Scholar] [CrossRef]
- Kaixian, Q.; Franklin, M.; Borowitzka, M.A. The study for isolation and purification of R-phycoerythrin from a red alga. Appl. Biochem. Biotechnol. 1993, 43, 133–139. [Google Scholar] [CrossRef]
- Hilditch, C.M.; Balding, P.; Jenkins, R.; Smith, A.J.; Rogers, L.J. R-phycoerythrin from the macroalga Corallina officinalis (Rhodophyceae) and application of a derived phycofluor probe for detecting sugar-binding sites on cell membranes. J. Appl. Phycol. 1991, 3, 345–354. [Google Scholar] [CrossRef]
- Sun, L.; Wang, S.; Gong, X.; Zhao, M.; Fu, X.; Wang, L. Isolation, purification and characteristics of R-phycoerythrin from a marine macroalga Heterosiphonia japonica. Protein Expr. Purif. 2009, 64, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Saluri, M.; Kaldmäe, M.; Tuvikene, R. Extraction and quantification of phycobiliproteins from the red alga Furcellaria lumbricalis. Algal Res. 2019, 37, 115–123. [Google Scholar] [CrossRef]
- Mittal, R.; Raghavarao, K.S.M.S. Extraction of R-Phycoerythrin from marine macro-algae, Gelidium pusillum, employing consortia of enzymes. Algal Res. 2018, 34, 1–11. [Google Scholar] [CrossRef]
- Mittal, R.; Sharma, R.; Raghavarao, K. Aqueous two-phase extraction of R-Phycoerythrin from marine macro-algae, Gelidium pusillum. Bioresour. Technol. 2019, 280, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Sudhakar, M.P.; Jagatheesan, A.; Perumal, K.; Arunkumar, K. Methods of phycobiliprotein extraction from Gracilaria crassa and its applications in food colourants. Algal Res. 2015, 8, 115–120. [Google Scholar] [CrossRef]
- Sudhakar, M.P.; Saraswathi, M.; Nair, B.B. Extraction, purification and application study of R-Phycoerythrin from Gracilaria corticata (J. Agardh) J. Agardh var. corticata. Indian J. Nat. Prod. Resour. 2014, 5, 371–374. [Google Scholar]
- Pereira, D.C.; Trigueiro, T.G.; Colepicolo, P.; Marinho-Soriano, E. Seasonal changes in the pigment composition of natural population of Gracilaria domingensis (Gracilariales, Rhodophyta). Braz. J. Pharmacogn. 2012, 22, 874–880. [Google Scholar] [CrossRef]
- Wang, G. Isolation and purification of phycoerythrin from red alga Gracilaria verrucosa by expanded-bed-adsorption and ion-exchange chromatogaphy. Chromatographia 2002, 56, 509–513. [Google Scholar] [CrossRef]
- Francavilla, M.; Franchi, M.; Monteleone, M.; Caroppo, C. The red seaweed Gracilaria gracilis as a multi products source. Mar. Drugs 2013, 11, 3754–3776. [Google Scholar] [CrossRef] [PubMed]
- D’Agnolo, E.; Rizzo, R.; Paoletti, S.; Murano, E. R-phycoerythrin from the red alga Gracilaria longa. Phytochemistry 1994, 35, 693–696. [Google Scholar] [CrossRef]
- Zhao, P.; Wang, X.; Niu, J.; He, L.; Gu, W.; Xie, X.; Wu, M.; Wang, G. Agar extraction and purification of R-phycoerythrin from Gracilaria tenuistipitata, and subsequent wastewater treatment by Ulva prolifera. Algal Res. 2020, 47, 101862. [Google Scholar] [CrossRef]
- Sfriso, A.A.; Gallo, M.; Baldi, F. Phycoerythrin productivity and diversity from five red macroalgae. J. Appl. Phycol. 2018, 30, 2523–2531. [Google Scholar] [CrossRef]
- Gu, D.; Lazo-Portugal, R.; Fang, C.; Wang, Z.; Ma, Y.; Knight, M.; Ito, Y. Purification of R-phycoerythrin from Gracilaria lemaneiformis by centrifugal precipitation chromatography. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2018, 1087–1088, 138–141. [Google Scholar] [CrossRef] [PubMed]
- Denis, C.; Ledorze, C.; Jaouen, P.; Fleurence, J. Comparison of different procedures for the extraction and partial purification of R-phycoerythrin from the red macroalga Grateloupia turuturu. Bot. Mar. 2009, 52, 278–281. [Google Scholar] [CrossRef]
- Le Guillard, C.; Dumay, J.; Donnay-Moreno, C.; Bruzac, S.; Ragon, J.Y.; Fleurence, J.; Bergé, J.P. Ultrasound-assisted extraction of R-phycoerythrin from Grateloupia turuturu with and without enzyme addition. Algal Res. 2015, 12, 522–528. [Google Scholar] [CrossRef]
- Munier, M.; Morançais, M.; Dumay, J.; Jaouen, P.; Fleurence, J. One-step purification of R-phycoerythrin from the red edible seaweed Grateloupia turuturu. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2015, 992, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Malairaj, S.; Muthu, S.; Gopal, V.B.; Perumal, P.; Ramasamy, R. Qualitative and quantitative determination of R-phycoerythrin from Halymenia floresia (Clemente) C. Agardh by polyacrylamide gel using electrophoretic elution technique. J. Chromatogr. A 2016, 1454, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.P.T.; Morançais, M.; Fleurence, J.; Dumay, J. Mastocarpus stellatus as a source of R-phycoerythrin: Optimization of enzyme assisted extraction using response surface methodology. J. Appl. Phycol. 2017, 29, 1563–1570. [Google Scholar] [CrossRef]
- Niu, J.F.; Wang, G.C.; Zhou, B.C.; Lin, X.Z.; Chen, C.S. Purification of R-phycoerythrin from Porphyra haitanensis (Bangiales, Rhodophyta) using expanded-bed absorption. J. Phycol. 2007, 43, 1339–1347. [Google Scholar] [CrossRef]
- Niwa, K.; Iga, H.; Sato, T. Potential of Neoporphyra kitoi (Bangiales, Rhodophyta) as a candidate species for marine crops with high temperature tolerance. Aquaculture 2022, 548, 737650. [Google Scholar] [CrossRef]
- Sano, F.; Murata, K.; Niwa, K. Identification, growth, and pigment content of a spontaneous green mutant of Pyropia kinositae (Bangiales, Rhodophyta). J. Appl. Phycol. 2020, 32, 1983–1994. [Google Scholar] [CrossRef]
- Niu, J.F.; Chen, Z.F.; Wang, G.C.; Zhou, B.C. Purification of phycoerythrin from Porphyra yezoensis Ueda (Bangiales, Rhodophyta) using expanded bed absorption. J. Appl. Phycol. 2010, 22, 25–31. [Google Scholar] [CrossRef]
- Wang, C.; Shen, Z.; Cui, X.; Jiang, Y.; Jiang, X. Response surface optimization of enzyme-assisted extraction of R-phycoerythrin from dry Pyropia yezoensis. J. Appl. Phycol. 2020, 32, 1429–1440. [Google Scholar] [CrossRef]
- Lüder, U.H.; Knoetzel, J.; Wiencke, C. Acclimation of photosynthesis and pigments to seasonally changing light conditions in the endemic antarctic red macroalga Palmaria decipiens. Polar Biol. 2001, 24, 231–236. [Google Scholar] [CrossRef]
- Galland-Irmouli, A.V.; Pons, L.; Luçon, M.; Villaume, C.; Mrabet, N.T.; Guéant, J.L.; Fleurence, J. One-step purification of R-phycoerythrin from the red macroalga Palmaria palmata using preparative polyacrylamide gel electrophoresis. J. Chromatogr. B: Biomed. Sci. Appl. 2000, 739, 117–123. [Google Scholar] [CrossRef]
- Dumay, J.; Clément, N.; Morançais, M.; Fleurence, J. Optimization of hydrolysis conditions of Palmaria palmata to enhance R-phycoerythrin extraction. Bioresour. Technol. 2013, 131, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.H.; Chen, W.C.; Gao, Y.H.; Chen, G.W.; Lin, H.T.V.; Pan, C.L. Enzyme-Assisted Method for Phycobiliproteins Extraction from Porphyra and Evaluation of Their Bioactivity. Processes 2021, 9, 560. [Google Scholar] [CrossRef]
- Niu, J.F.; Wang, G.C.; Tseng, C.K. Method for large-scale isolation and purification of R-phycoerythrin from red alga Polysiphonia urceolata Grev. Protein Expr. Purif. 2006, 49, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Senthilkumar, N.; Suresh, V.; Thangam, R.; Kurinjimalar, C.; Kavitha, G.; Murugan, P.; Rengasamy, R. Isolation and characterization of macromolecular protein R-Phycoerythrin from Portieria hornemannii. Int. J. Biol. Macromol. 2013, 55, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Francavilla, M.; Manara, P.; Kamaterou, P.; Monteleone, M.; Zabaniotou, A. Cascade approach of red macroalgae Gracilaria gracilis sustainable valorization by extraction of phycobiliproteins and pyrolysis of residue. Bioresour. Technol. 2015, 184, 305–313. [Google Scholar] [CrossRef]
- MacColl, R.; Eisele, L.E.; Williams, E.C.; Bowser, S.S. The discovery of a novel R-phycoerythrin from an antarctic red alga. J. Biol. Chem. 1996, 271, 17157–17160. [Google Scholar] [CrossRef] [PubMed]
- MacColl, R.; Eisele, L.E.; Malak, H.; Endres, R.L.; Williams, E.C.; Bowser, S.S. Studies on R-phycoerythrins from two Antarctic marine red algae and a mesophilic red alga. Polar Biol. 1999, 22, 384–388. [Google Scholar] [CrossRef]
- Mittal, R.; Tavanandi, H.A.; Mantri, V.A.; Raghavarao, K.S.M.S. Ultrasound assisted methods for enhanced extraction of phycobiliproteins from marine macro-algae, Gelidium pusillum (Rhodophyta). Ultrason. Sonochem. 2017, 38, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Manirafasha, E.; Guo, L.; Jing, K. Nutraceutical and Pharmaceutical Applications of Phycobiliproteins. In Pigments from Microalgae Handbook; Jacob-Lopes, E., Queiroz, M.I., Zepka, L.Q., Eds.; Springer: Cham, Switzerland, 2020; pp. 577–654. [Google Scholar]
- Dagnino-Leone, J.; Figueroa, M.; Mella, C.; Vorphal, M.A.; Kerff, F.; Vásquez, A.J.; Bunster, M.; Martínez-Oyanedel, J. Structural models of the different trimers present in the core of phycobilisomes from Gracilaria chilensis based on crystal structures and sequences. PLoS ONE 2017, 12, e0177540. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, J.; Liu, D.; Qin, S.; Sun, S.; Zhao, J.; Sui, S.F. Structure of phycobilisome from the red alga Griffithsia pacifica. Nature 2017, 551, 57–63. [Google Scholar] [CrossRef]
- Dumay, J.; Morançais, M. Proteins and Pigments. In Seaweed in Health and Disease Prevention; Fleurence, J., Levine, I.A., Eds.; Elsevier Academic Press: London, UK, 2016; pp. 275–318. [Google Scholar]
- Brauch, J.E. Underutilized Fruits and Vegetables as Potential Novel Pigment Sources. In Handbook on Natural Pigments in Food and Beverages: Industrial Applications for Improving Food Color; Carle, R., Schweiggert, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 305–335. [Google Scholar]
- Jespersen, L.; Strømdahl, L.D.; Olsen, K.; Skibsted, L.H. Heat and light stability of three natural blue colorants for use in confectionery and beverages. Eur. Food Res. Technol. 2005, 220, 261–266. [Google Scholar] [CrossRef]
- Farag, M.R.; Alagawany, M.; El-Hack, M.E.A.; Dhama, K. Nutritional and healthical aspects of Spirulina (Arthrospira) for poultry, animals and human. Int. J. Pharmacol. 2016, 12, 36–51. [Google Scholar] [CrossRef]
- Piniella-Matamoros, B.; Marín-Prida, J.; Pentón-Rol, G. Nutraceutical and therapeutic potential of Phycocyanobilin for treating Alzheimer’s disease. J. Biosci. 2021, 46, 1–16. [Google Scholar] [CrossRef]
- Yu, P.; Wu, Y.; Wang, G.; Jia, T.; Zhang, Y. Purification and bioactivities of phycocyanin. Crit. Rev. Food Sci. Nutr. 2017, 57, 3840–3849. [Google Scholar] [CrossRef] [PubMed]
- Braune, S.; Krüger-Genge, A.; Kammerer, S.; Jung, F.; Küpper, J.H. Phycocyanin from Arthrospira platensis as Potential Anti-Cancer Drug: Review of In Vitro and In Vivo Studies. Life 2021, 11, 91. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, Y.; Yin, Q.; Liu, G.; Liu, H.; Huang, Y.; Li, B. Phycocyanin: A potential drug for cancer treatment. J. Cancer 2017, 8, 3416–3429. [Google Scholar] [CrossRef] [PubMed]
- De Morais, M.G.; Da Fontoura Prates, D.; Moreira, J.B.; Duarte, J.H.; Costa, J.A.V. Phycocyanin from Microalgae: Properties, Extraction and Purification, with Some Recent Applications. Ind. Biotechnol. 2018, 14, 30–37. [Google Scholar] [CrossRef]
- Pez Jaeschke, D.; Rocha Teixeira, I.; Damasceno Ferreira Marczak, L.; Domeneghini Mercali, G. Phycocyanin from Spirulina: A review of extraction methods and stability. Food Res. Int. 2021, 143, 110314. [Google Scholar] [CrossRef] [PubMed]
- Jesús, V.C.; Gutiérrez-Rebolledo, G.A.; Hernández-Ortega, M.; Valadez-Carmona, L.; Mojica-Villegas, A.; Gutiérrez-Salmeán, G.; Chamorro-Cevallos, G. Methods for Extraction, Isolation and Purification of C-phycocyanin: 50 years of research in review. Int. J. Food Nutr. Sci. 2016, 3, 275–284. [Google Scholar] [CrossRef][Green Version]
- Ogashawara, I. Determination of phycocyanin from space-A bibliometric analysis. Remote Sens. 2020, 12, 567. [Google Scholar] [CrossRef]
- Carle, R.; Schweiggert, R.M. 2.6. Phycocyanin. In Handbook on Natural Pigments in Food and Beverages: Industrial Applications for Improving Food Color; Carle, R., Schweiggert, R.M., Eds.; Woodhead Publishing: Duxford, UK, 2016. [Google Scholar]
- Del Rio-Chanona, E.A.; Zhang, D.; Xie, Y.; Manirafasha, E.; Jing, K. Dynamic Simulation and Optimization for Arthrospira platensis Growth and C-Phycocyanin Production. Ind. Eng. Chem. Res. 2015, 54, 10606–10614. [Google Scholar] [CrossRef]
- Sahin, S.C. The potential of Arthrospira platensis extract as a tyrosinase inhibitor for pharmaceutical or cosmetic applications. S. Afr. J. Bot. 2018, 119, 236–243. [Google Scholar] [CrossRef]
- Marková, I.; Koníčková, R.; Vaňková, K.; Leníček, M.; Kolář, M.; Strnad, H.; Hradilová, M.; Šáchová, J.; Rasl, J.; Klímová, Z.; et al. Anti-angiogenic effects of the blue-green alga Arthrospira platensis on pancreatic cancer. J. Cell. Mol. Med. 2020, 24, 2402–2415. [Google Scholar] [CrossRef]
- Ragusa, I.; Nardone, G.N.; Zanatta, S.; Bertin, W.; Amadio, E. Spirulina for Skin Care: A Bright Blue Future. Cosmetics 2021, 8, 7. [Google Scholar] [CrossRef]
- Araujo, G.S.; Santiago, C.S.; Moreira, R.T.; Dantas Neto, M.P.; Fernandes, F.A.N. Nutrient removal by Arthrospira platensis cyanobacteria in cassava processing wastewater. J. Water Process Eng. 2021, 40, 101826. [Google Scholar] [CrossRef]
- Matos, Â.P.; Vadiveloo, A.; Bahri, P.A.; Moheimani, N.R. Anaerobic digestate abattoir effluent (ADAE), a suitable source of nutrients for Arthrospira platensis cultivation. Algal Res. 2021, 54, 102216. [Google Scholar] [CrossRef]
- Matos, J.; Cardoso, C.L.; Falé, P.; Afonso, C.M.; Bandarra, N.M. Investigation of nutraceutical potential of the microalgae Chlorella vulgaris and Arthrospira platensis. Int. J. Food Sci. Technol. 2020, 55, 303–312. [Google Scholar] [CrossRef]
- Wollina, U.; Voicu, C.; Gianfaldoni, S.; Lotti, T.; França, K.; Tchernev, G. Arthrospira platensis—Potential in dermatology and beyond. Open Access Maced. J. Med. Sci. 2018, 6, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Eisele, L.E.; Bakhru, S.H.; Liu, X.; MacColl, R.; Edwards, M.R. Studies on C-phycocyanin from Cyanidium caldarium, a eukaryote at the extremes of habitat. Biochim. Biophys. Acta-Bioenerg. 2000, 1456, 99–107. [Google Scholar] [CrossRef]
- Hirooka, S.; Tomita, R.; Fujiwara, T.; Ohnuma, M.; Kuroiwa, H.; Kuroiwa, T.; Miyagishima, S. Efficient open cultivation of cyanidialean red algae in acidified seawater. Sci. Rep. 2020, 10, 13794. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, Y.; Cao, M.; Pan, T.; Yang, Y.; Mao, H.; Sun, L.; Liu, G. Anti-allergic activity of R-phycocyanin from Porphyra haitanensis in antigen-sensitized mice and mast cells. Int. Immunopharmacol. 2015, 25, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.J.; Yang, Y.H.; Liang, Y.C.; Chiu, C.J.; Chu, K.H.; Chou, H.N.; Chiang, B.L. A novel phycobiliprotein alleviates allergic airway inflammation by modulating immune responses. Am. J. Respir. Crit. Care Med. 2011, 183, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Qu, Y.; Fu, X.; Zhao, M.; Wang, S.; Sun, L. Isolation, Purification and Properties of an R-Phycocyanin from the Phycobilisomes of a Marine Red Macroalga Polysiphonia urceolata. PLoS ONE 2014, 9, e101724. [Google Scholar] [CrossRef] [PubMed]
- Fan-jie, Z.; Zi-xuan, Y.; Li-jin (Li-Chin Chiang), J. Isolation and characterization of R-phycocyanin from Polysiphonia urceolata. Hydrobiologia 1984, 22, 594–596. [Google Scholar] [CrossRef]
- Patil, G.; Raghavarao, K.S.M.S. Aqueous two phase extraction for purification of C-phycocyanin. Biochem. Eng. J. 2007, 34, 156–164. [Google Scholar] [CrossRef]
- Patil, G.; Chethana, S.; Sridevi, A.S.; Raghavarao, K.S.M.S. Method to obtain C-phycocyanin of high purity. J. Chromatogr. A 2006, 1127, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Chaiklahan, R.; Chirasuwan, N.; Bunnag, B. Stability of phycocyanin extracted from Spirulina sp.: Influence of temperature, pH and preservatives. Process Biochem. 2012, 47, 659–664. [Google Scholar] [CrossRef]
- Puzorjov, A.; Dunn, K.E.; McCormick, A.J. Production of thermostable phycocyanin in a mesophilic cyanobacterium. Metab. Eng. Commun. 2021, 13, e00175. [Google Scholar] [CrossRef]
- Phycocyanin Market by Form (Liquid, Powder), by Grade (Food Grade, Cosmetic Grade, Reagent Grade, Analytical Grade) by Application (Food and Beverages, Pharmaceutical and Nutraceutical, Diagnostics and Biomedical), Geography—Global Forecast To 2027. Meticulous Research, Maharashtra, India. 2020. Available online: https://www.marketresearch.com/Meticulous-Research-v4061/Phycocyanin-Form-Liquid-Powder-Grade-13834146/ (accessed on 19 December 2021).
- Liu, J.Y.; Zhang, J.P.; Wan, Z.L.; Liang, D.C.; Zhang, J.P.; Wu, H.J. Crystallization and preliminary X-ray studies of allophycocyanin from red alga Porphyra yezoensis. Acta Crystallogr. Sect. D Biol. Crystallogr. 1998, 54, 662–664. [Google Scholar] [CrossRef]
- Liu, J.Y.; Jiang, T.; Zhang, J.P.; Liang, D.C. Crystal Structure of Allophycocyanin from Red Algae Porphyra yezoensis at 2.2-Å Resolution. J. Biol. Chem. 1999, 274, 16945–16952. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zang, X.; Cao, X.; Zhang, F.; Sun, D.; Shang, M.; Li, R.; Yangzong, Z.; Wei, X.; Zhang, X. Cloning and expression of Allophycocyanin gene from Gracilariopsis lemaneiformis and studying the binding sites of phycocyanobilin on its α and β subunits. J. Appl. Phycol. 2020, 32, 2657–2671. [Google Scholar] [CrossRef]
- Chen, H.; Liu, Q.; Zhao, J.; Jiang, P. Biosynthesis, spectral properties and thermostability of cyanobacterial allophycocyanin holo-α subunits. Int. J. Biol. Macromol. 2016, 88, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Soulier, N.; Bryant, D.A. The structural basis of far-red light absorbance by allophycocyanins. Photosynth. Res. 2021, 147, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, J.; Takatani, N.; Kobayashi, N.; Mikami, K.; Miyashita, K.; Yamano, Y.; Wada, A.; Maoka, T.; Hosokawa, M. Carotenoid profiling of a red seaweed Pyropia yezoensis: Insights into biosynthetic pathways in the order Bangiales. Mar. Drugs 2018, 16, 426. [Google Scholar] [CrossRef] [PubMed]
- Bohn, T.; Bonet, M.L.; Borel, P.; Keijer, J.; Landrier, J.F.; Milisav, I.; Ribot, J.; Riso, P.; Winklhofer-Roob, B.; Sharoni, Y.; et al. Mechanistic Aspects of Carotenoid Health Benefits-Where are we Now? Nutr. Res. Rev. 2021, 34, 276–302. [Google Scholar] [CrossRef] [PubMed]
- Viera, I.; Pérez-Gálvez, A.; Roca, M. Bioaccessibility of marine carotenoids. Mar. Drugs 2018, 16, 397. [Google Scholar] [CrossRef] [PubMed]
- Swapnil, P.; Meena, M.; Singh, S.K.; Dhuldhaj, U.P.; Harish; Marwal, A. Vital roles of carotenoids in plants and humans to deteriorate stress with its structure, biosynthesis, metabolic engineering and functional aspects. Curr. Plant Biol. 2021, 26, 100203. [Google Scholar] [CrossRef]
- Schubert, N.; García-Mendoza, E.; Pacheco-Ruiz, I. Carotenoid composition of marine red algae. J. Phycol. 2006, 42, 1208–1216. [Google Scholar] [CrossRef]
- Kulczyński, B.; Gramza-Michałowska, A.; Kobus-Cisowska, J.; Kmiecik, D. The role of carotenoids in the prevention and treatment of cardiovascular disease—Current state of knowledge. J. Funct. Foods 2017, 38, 45–65. [Google Scholar] [CrossRef]
- Gammone, M.A.; Riccioni, G.; D’Orazio, N. Carotenoids: Potential allies of cardiovascular health? Food Nutr. Res. 2015, 59, 26762. [Google Scholar] [CrossRef] [PubMed]
- Takaichi, S. Carotenoids in Algae: Distributions, Biosyntheses and Functions. Mar. Drugs 2011, 9, 1101–1118. [Google Scholar] [CrossRef] [PubMed]
- Takaichi, S.; Yokoyama, A.; Mochimaru, M.; Uchida, H.; Murakami, A. Carotenogenesis diversification in phylogenetic lineages of Rhodophyta. J. Phycol. 2016, 52, 329–338. [Google Scholar] [CrossRef]
- Takaichi, S. Distributions, biosyntheses and functions of carotenoids in algae. Agro Food Ind. Hi. Tech. 2013, 24, 55–58. [Google Scholar]
- Eggersdorfer, M.; Wyss, A. Carotenoids in human nutrition and health. Arch. Biochem. Biophys. 2018, 652, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Rapoport, A.; Guzhova, I.; Bernetti, L.; Buzzini, P.; Kieliszek, M.; Kot, A.M. Carotenoids and some other pigments from fungi and yeasts. Metabolites 2021, 11, 92. [Google Scholar] [CrossRef]
- Meléndez-Martínez, A.J.; Mandić, A.I.; Bantis, F.; Böhm, V.; Borge, G.I.A.; Brnčić, M.; Bysted, A.; Cano, M.P.; Dias, M.G.; Elgersma, A.; et al. A comprehensive review on carotenoids in foods and feeds: Status quo, applications, patents, and research needs. Crit. Rev. Food Sci. Nutr. 2020, 5, 1–51. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.T.; Matanjun, P.; Yasir, S.M.; Tan, T.S. Antioxidant activities and polyphenolics of various solvent extracts of red seaweed, Gracilaria changii. J. Appl. Phycol. 2015, 27, 2377–2386. [Google Scholar] [CrossRef]
- Dias, M.G.; Borge, G.I.A.; Kljak, K.; Mandić, A.I.; Mapelli-Brahm, P.; Olmedilla-Alonso, B.; Pintea, A.M.; Ravasco, F.; Šaponjac, V.T.; Sereikaitė, J.; et al. European Database of Carotenoid Levels in Foods. Factors Affecting Carotenoid Content. Foods 2021, 10, 912. [Google Scholar] [CrossRef] [PubMed]
- Yabuzaki, J. Carotenoids Database: Structures, chemical fingerprints and distribution among organisms. Database 2017, 2017, bax004. [Google Scholar] [CrossRef] [PubMed]
- Black, H.S.; Boehm, F.; Edge, R.; Truscott, T.G. The Benefits and Risks of Certain Dietary Carotenoids that Exhibit both Anti- and Pro-Oxidative Mechanisms—A Comprehensive Review. Antioxidants 2020, 9, 264. [Google Scholar] [CrossRef]
- Dulińska-Litewka, J.; Hałubiec, P.; Łazarczyk, A.; Szafrański, O.; Sharoni, Y.; McCubrey, J.A.; Gąsiorkiewicz, B.; Bohn, T. Recent Progress in Discovering the Role of Carotenoids and Metabolites in Prostatic Physiology and Pathology-A Review-Part II: Carotenoids in the Human Studies. Antioxidants 2021, 10, 319. [Google Scholar] [CrossRef] [PubMed]
- Meleacutendez-Martiacutenez, A.J.; Bodiehm, V.; Borge, G.I.A.; Cano, M.P.; Fikselovaacute, M.; Gruskiene, R.; Lavelli, V.; Loizzo, M.R.; Mandicacute, A.I.; Brahm, P.M.; et al. Carotenoids: Considerations for Their Use in Functional Foods, Nutraceuticals, Nutricosmetics, Supplements, Botanicals, and Novel Foods in the Context of Sustainability, Circular Economy, and Climate Change. Annu. Rev. Food Sci. Technol. 2021, 12, 433–460. [Google Scholar] [CrossRef] [PubMed]
- Kalra, R.; Gaur, S.; Goel, M. Microalgae bioremediation: A perspective towards wastewater treatment along with industrial carotenoids production. J. Water Process Eng. 2021, 40, 101794. [Google Scholar] [CrossRef]
- Liu, C.; Hu, B.; Cheng, Y.; Guo, Y.; Yao, W.; Qian, H. Carotenoids from fungi and microalgae: A review on their recent production, extraction, and developments. Bioresour. Technol. 2021, 337, 125398. [Google Scholar] [CrossRef] [PubMed]
- Pagels, F.; Vasconcelos, V.; Guedes, A.C. Carotenoids from Cyanobacteria: Biotechnological Potential and Optimization Strategies. Biomolecules 2021, 11, 735. [Google Scholar] [CrossRef] [PubMed]
- Foong, L.C.; Loh, C.W.L.; Ng, H.S.; Lan, J.C.W. Recent development in the production strategies of microbial carotenoids. World J. Microbiol. Biotechnol. 2021, 37, 12. [Google Scholar] [CrossRef] [PubMed]
- Hegazi, M.M.; Pérez-Ruzafa, A.; Almela, L.; Candela, M.E. Separation and identification of chlorophylls and carotenoids from Caulerpa prolifera, Jania rubens and Padina pavonica by reversed-phase high-performance liquid chromatography. J. Chromatogr. A 1998, 829, 153–159. [Google Scholar] [CrossRef]
- Aldred, E.M.; Buck, C.; Vall, K. Terpenes. In Pharmacology: A Handbook for Complementary Healthcare Professionals; Aldred, E.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2009; p. 362. [Google Scholar]
- Asada, K. Production and Scavenging of Reactive Oxygen Species in Chloroplasts and Their Functions. Plant Physiol. 2006, 141, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Murata, N. How do environmental stresses accelerate photoinhibition? Trends Plant Sci. 2008, 13, 178–182. [Google Scholar] [CrossRef]
- Burri, B.J. Carotenoids: Chemistry, Sources and Physiology. In Encyclopedia of Human Nutrition; Caballero, B., Ed.; Academic Press: Waltham, MA, USA, 2012; Volume 1, pp. 283–291. [Google Scholar]
- Murray, M.T.; Capelli, B. β-Carotene and Other Carotenoids. Textb. Nat. Med. 2020, 443–450.e2. [Google Scholar] [CrossRef]
- Latowski, D.; Szymanska, R.; Strzalka, K. Carotenoids Involved in Antioxidant System of Chloroplasts. In Oxidative Damage to Plants: Antioxidant Networks and Signaling; Ahmad, P., Ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 289–319. [Google Scholar]
- De Rosso, V.V.; Mercadante, A.Z. Identification and quantification of carotenoids, by HPLC-PDA-MS/MS, from Amazonian fruits. J. Agric. Food Chem. 2007, 55, 5062–5072. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.; Kimura, M. HarvestPlus Handbook for Carotenoid Analysis; Rodriguez-Amaya, D., Kimura, M., Eds.; HarvestPlus: Washington, DC, USA, 2004. [Google Scholar]
- Poojary, M.M.; Barba, F.J.; Aliakbarian, B.; Donsì, F.; Pataro, G.; Dias, D.A.; Juliano, P. Innovative alternative technologies to extract carotenoids from microalgae and seaweeds. Mar. Drugs 2016, 14, 214. [Google Scholar] [CrossRef]
- Mustafa, A.; Turner, C. Pressurized liquid extraction as a green approach in food and herbal plants extraction: A review. Anal. Chim. Acta 2011, 703, 8–18. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.S. Carotenoid extraction methods: A review of recent developments. Food Chem. 2018, 240, 90–103. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Ahmad, S.; Ahmad, A. Green extraction methods and environmental applications of carotenoids—A review. RSC Adv. 2015, 5. [Google Scholar] [CrossRef]
- Strati, I.F.; Oreopoulou, V. Recovery of carotenoids from tomato processing by-products—A review. Food Res. Int. 2014, 65, 311–321. [Google Scholar] [CrossRef]
- Xu, D.P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.J.; Li, H. Bin Natural Antioxidants in Foods and Medicinal Plants: Extraction, Assessment and Resources. Int. J. Mol. Sci. 2017, 18, 96. [Google Scholar] [CrossRef] [PubMed]
- King, J.W.; Srinivas, K.; Zhang, D. Advances in Critical Fluid Processing. In Alternatives to Conventional Food Processing; Proctor, A., Ed.; The Royal Society of Chemistry: Cambridge, UK, 2010; pp. 93–144. [Google Scholar]
- Billakanti, J.M.; Catchpole, O.J.; Fenton, T.A.; Mitchell, K.A.; Mackenzie, A.D. Enzyme-assisted extraction of fucoxanthin and lipids containing polyunsaturated fatty acids from Undaria pinnatifida using dimethyl ether and ethanol. Process Biochem. 2013, 48, 1999–2008. [Google Scholar] [CrossRef]
- Goto, M.; Kanda, H.; Wahyudiono; Machmudah, S. Extraction of carotenoids and lipids from algae by supercritical CO2 and subcritical dimethyl ether. J. Supercrit. Fluids 2015, 96, 245–251. [Google Scholar] [CrossRef]
- Xie, X.; Lu, X.; Wang, L.; He, L.; Wang, G. High light intensity increases the concentrations of β-carotene and zeaxanthin in marine red macroalgae. Algal Res. 2020, 47, 101852. [Google Scholar] [CrossRef]
- Johnson, E.J. The role of carotenoids in human health. Nutr. Clin. Care 2002, 5, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Di Tomo, P.; Canali, R.; Ciavardelli, D.; Di Silvestre, S.; De Marco, A.; Giardinelli, A.; Pipino, C.; Di Pietro, N.; Virgili, F.; Pandolfi, A. β-Carotene and lycopene affect endothelial response to TNF-α reducing nitro-oxidative stress and interaction with monocytes. Mol. Nutr. Food Res. 2012, 56, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Carpena, M.; Caleja, C.; Pereira, E.; Pereira, C.; Ćirić, A.; Soković, M.; Soria-Lopez, A.; Fraga-Corral, M.; Simal-Gandara, J.; Ferreira, I.C.F.R.; et al. Red Seaweeds as a Source of Nutrients and Bioactive Compounds: Optimization of the Extraction. Chemosensors 2021, 9, 132. [Google Scholar] [CrossRef]
- Mayne, S.T. Beta-carotene, carotenoids, and disease prevention in humans. FASEB J. 1996, 10, 690–701. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Stahl, W. Nutritional protection against skin damage from sunlight. Annu. Rev. Nutr. 2004, 24, 173–200. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, U.; Gärtner, C.; Wiebusch, M.; Eichler, O.; Sies, H.; Tronnier, H.; Stahl, W. Supplementation with β-carotene or a similar amount of mixed carotenoids protects humans from UV-induced erythema. J. Nutr. 2003, 133, 98–101. [Google Scholar] [CrossRef]
- Stahl, W.; Heinrich, U.; Jungmann, H.; Sies, H.; Tronnier, H. Carotenoids and carotenoids plus vitamin E protect against ultraviolet light-induced erythema in humans. Am. J. Clin. Nutr. 2000, 71, 795–798. [Google Scholar] [CrossRef] [PubMed]
- Phan, M.A.T.; Bucknall, M.; Arcot, J. Interactive effects of β-carotene and anthocyanins on cellular uptake, antioxidant activity and anti-inflammatory activity in vitro and ex vivo. J. Funct. Foods 2018, 45, 129–137. [Google Scholar] [CrossRef]
- Jeanette Foss, B.; Nadolski, G.; Lockwood, S. Hydrophilic Carotenoid Amphiphiles: Methods of Synthesis and Biological Applications. Mini-Reviews Med. Chem. 2006, 6, 953–969. [Google Scholar] [CrossRef] [PubMed]
- Gruszecki, W.I.; Strzałka, K. Carotenoids as modulators of lipid membrane physical properties. Biochim. Biophys. Acta—Mol. Basis Dis. 2005, 1740, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Ravikrishnan, R.; Rusia, S.; Ilamurugan, G.; Salunkhe, U.; Deshpande, J.; Shankaranarayanan, J.; Shankaranarayana, M.L.; Soni, M.G. Safety assessment of lutein and zeaxanthin (LutemaxTM 2020): Subchronic toxicity and mutagenicity studies. Food Chem. Toxicol. 2011, 49, 2841–2848. [Google Scholar] [CrossRef] [PubMed]
- Sajilata, M.G.; Singhal, R.S.; Kamat, M.Y. The Carotenoid Pigment Zeaxanthin—A Review. Compr. Rev. Food Sci. Food Saf. 2008, 7, 29–49. [Google Scholar] [CrossRef]
- Koo, S.Y.; Cha, K.H.; Song, D.G.; Chung, D.; Pan, C.H. Optimization of pressurized liquid extraction of zeaxanthin from Chlorella ellipsoidea. J. Appl. Phycol. 2012, 24, 725–730. [Google Scholar] [CrossRef]
- Bhat, I.; Haripriya, G.; Jogi, N.; Mamatha, B.S. Carotenoid composition of locally found seaweeds of Dakshina Kannada district in India. Algal Res. 2021, 53, 102154. [Google Scholar] [CrossRef]
- Ruiz-Domínguez, M.C.; Marticorena, P.; Sepúlveda, C.; Salinas, F.; Cerezal, P.; Riquelme, C. Effect of Drying Methods on Lutein Content and Recovery by Supercritical Extraction from the Microalga Muriellopsis sp. (MCH35) Cultivated in the Arid North of Chile. Mar. Drugs 2020, 18, 528. [Google Scholar] [CrossRef] [PubMed]
- Firdous, A.P.; Kuttan, G.; Kuttan, R. Anti-inflammatory potential of carotenoid meso-zeaxanthin and its mode of action. Pharm. Biol. 2015, 53, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Stahl, W.; Sies, H. Bioactivity and protective effects of natural carotenoids. Biochim. Biophys. Acta Mol. Basis Dis. 2005, 1740, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Lin, X.M. Effects of lutein and zeaxanthin on aspects of eye health. J. Sci. Food Agric. 2010, 90, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.M.; Jiang, Y.; Chen, F. High-yield production of lutein by the green microalga Chlorella protothecoides in heterotrophic fed-batch culture. Biotechnol. Prog. 2002, 18, 723–727. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.K.; Ermis, H.; Murray, P. Marine Microalgae for Potential Lutein Production. Appl. Sci. 2020, 10, 6457. [Google Scholar] [CrossRef]
- Fernández-Sevilla, J.M.; Acién Fernández, F.G.; Molina Grima, E. Biotechnological production of lutein and its applications. Appl. Microbiol. Biotechnol. 2010, 86, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Fábryová, T.; Cheel, J.; Kubáč, D.; Hrouzek, P.; Vu, D.L.; Tůmová, L.; Kopecký, J. Purification of lutein from the green microalgae Chlorella vulgaris by integrated use of a new extraction protocol and a multi-injection high performance counter-current chromatography (HPCCC). Algal Res. 2019, 41, 101574. [Google Scholar] [CrossRef]
- González, S.; Astner, S.; An, W.; Goukassian, D.; Pathak, M.A. Dietary lutein/zeaxanthin decreases ultraviolet B-induced epidermal hyperproliferation and acute inflammation in hairless mice. J. Investig. Dermatol. 2003, 121, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Ranga Rao, A.; Raghunath Reddy, R.L.; Baskaran, V.; Sarada, R.; Ravishankar, G.A. Characterization of Microalgal Carotenoids by Mass Spectrometry and Their Bioavailability and Antioxidant Properties Elucidated in Rat Model. J. Agric. Food Chem. 2010, 58, 8553–8559. [Google Scholar] [CrossRef]
- Hussein, G.; Goto, H.; Oda, S.; Sankawa, U.; Matsumoto, K.; Watanabe, H. Antihypertensive potential and mechanism of action of astaxanthin: III. Antioxidant and histopathological effects in spontaneously hypertensive rats. Biol. Pharm. Bull. 2006, 29, 684–688. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, K.; Ghosh, R.; Homechaudhuri, S.; Mitra, A. Seasonal variation in the biochemical composition of red seaweed (Catenella repens) from Gangetic delta, northeast coast of India. J. Earth Syst. Sci. 2009 1185 2009, 118, 497–505. [Google Scholar] [CrossRef]
- Kang, C.D.; Sim, S.J. Direct extraction of astaxanthin from Haematococcus culture using vegetable oils. Biotechnol. Lett. 2008, 30, 441–444. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.R.; Tang, B.; Row, K.H. Extraction and separation of astaxanthin from marine products. Asian J. Chem. 2014, 26, 4543–4549. [Google Scholar] [CrossRef]
- Yang, M.; Xuan, Z.; Wang, Q.; Yan, S.; Zhou, D.; Naman, C.B.; Zhang, J.; He, S.; Yan, X.; Cui, W. Fucoxanthin has potential for therapeutic efficacy in neurodegenerative disorders by acting on multiple targets. Nutr. Neurosci. 2021, 1–14. [Google Scholar] [CrossRef]
- Wang, C.; Armstrong, D.W.; Chang, C.D. Rapid baseline separation of enantiomers and a mesoform of all-trans-astaxanthin, 13-cis-astaxanthin, adonirubin, and adonixanthin in standards and commercial supplements. J. Chromatogr. A 2008, 1194, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Kurashige, M.; Okimasu, E.; Inoue, M.; Utsumi, K. Inhibition of oxidative injury of biological membranes by astaxanthin—PubMed. Physiol. Chem. Phys. Med. NMR 1990, 22, 27–38. [Google Scholar] [PubMed]
- Hussein, G.; Nakamura, M.; Zhao, Q.; Iguchi, T.; Goto, H.; Sankawa, U.; Watanabe, H. Antihypertensive and neuroprotective effects of astaxanthin in experimental animals. Biol. Pharm. Bull. 2005, 28, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Jenab, M.; Riboli, E.; Ferrari, P.; Friesen, M.; Sabate, J.; Norat, T.; Slimani, N.; Tjønneland, A.; Olsen, A.; Overvad, K.; et al. Plasma and dietary carotenoid, retinol and tocopherol levels and the risk of gastric adenocarcinomas in the European prospective investigation into cancer and nutrition. Br. J. Cancer 2006, 95, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Morishita, Y.; Suzui, M.; Kojima, T.; Okumura, A.; Mori, H. Chemoprevention of mouse urinary bladder carcinogenesis by the naturally occurring carotenoid astaxanthin. Carcinogenesis 1994, 15, 15–19. [Google Scholar] [CrossRef]
- Tanaka, T.; Makita, H.; Ohnishi, M.; Mori, H.; Satoh, K.; Hara, A. Chemoprevention of rat oral carcinogenesis by naturally occurring xanthophylls, astaxanthin and canthaxanthin—PubMed. Cancer Res. 1995, 55, 4059–4064. [Google Scholar] [PubMed]
- Liu, X.; Shibata, T.; Hisaka, S.; Osawa, T. Astaxanthin inhibits reactive oxygen species-mediated cellular toxicity in dopaminergic SH-SY5Y cells via mitochondria-targeted protective mechanism. Brain Res. 2009, 1254, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.C.; Mong, M.C.; Yin, M.C. Antioxidative and anti-inflammatory neuroprotective effects of astaxanthin and canthaxanthin in nerve growth factor differentiated PC12 cells. J. Food Sci. 2009, 74, H225–H231. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Shinoda, T.; Nagao, R.; Akimoto, S.; Suzuki, T.; Dohmae, N.; Chen, M.; Allakhverdiev, S.I.; Shen, J.R.; Akita, F.; et al. Structural basis for the adaptation and function of chlorophyll f in photosystem I. Nat. Commun. 2020, 11, 238. [Google Scholar] [CrossRef] [PubMed]
- Sousa, F.L.; Shavit-Grievink, L.; Allen, J.F.; Martin, W.F. Chlorophyll Biosynthesis Gene Evolution Indicates Photosystem Gene Duplication, Not Photosystem Merger, at the Origin of Oxygenic Photosynthesis. Genome Biol. Evol. 2013, 5, 200–216. [Google Scholar] [CrossRef]
- Mandal, R.; Dutta, G. From photosynthesis to biosensing: Chlorophyll proves to be a versatile molecule. Sensors Int. 2020, 1, 100058. [Google Scholar] [CrossRef]
- Pereira, L. Macroalgae. Encyclopedia 2021, 1, 177–188. [Google Scholar] [CrossRef]
- Lanfer-Marquez, U.M.; Barros, R.M.C.; Sinnecker, P. Antioxidant activity of chlorophylls and their derivatives. Food Res. Int. 2005, 38, 885–891. [Google Scholar] [CrossRef]
- Halim, R.; Hosikian, A.; Lim, S.; Danquah, M.K. Chlorophyll Extraction from Microalgae: A Review on the Process Engineering Aspects. Int. J. Chem. Eng. 2010, 2010, 391632. [Google Scholar] [CrossRef]
- Lee, H.G.; Lu, Y.A.; Je, J.G.; Jayawardena, T.U.; Kang, M.C.; Lee, S.H.; Kim, T.H.; Lee, D.S.; Lee, J.M.; Yim, M.J.; et al. Effects of Ethanol Extracts from Grateloupia elliptica, a Red Seaweed, and Its Chlorophyll Derivative on 3T3-L1 Adipocytes: Suppression of Lipid Accumulation through Downregulation of Adipogenic Protein Expression. Mar. Drugs 2021, 19, 91. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Roca, M. Cooking effects on bioaccessibility of chlorophyll pigments of the main edible seaweeds. Food Chem. 2019, 295, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Castle, S.C.; Morrison, C.D.; Barger, N.N. Extraction of chlorophyll a from biological soil crusts: A comparison of solvents for spectrophotometric determination. Soil Biol. Biochem. 2011, 43, 853–856. [Google Scholar] [CrossRef]
- Samarasinghe, N.; Fernando, S.; Lacey, R.; Faulkner, W.B. Algal cell rupture using high pressure homogenization as a prelude to oil extraction. Renew. Energy 2012, 48, 300–308. [Google Scholar] [CrossRef]
- Martins, M.; Fernandes, A.P.M.; Torres-Acosta, M.A.; Collén, P.N.; Abreu, M.H.; Ventura, S.P.M. Extraction of chlorophyll from wild and farmed Ulva spp. using aqueous solutions of ionic liquids. Sep. Purif. Technol. 2021, 254, 117589. [Google Scholar] [CrossRef]
- Zhu, Z.; Wu, Q.; Di, X.; Li, S.; Barba, F.J.; Koubaa, M.; Roohinejad, S.; Xiong, X.; He, J. Multistage recovery process of seaweed pigments: Investigation of ultrasound assisted extraction and ultra-filtration performances. Food Bioprod. Process. 2017, 104, 40–47. [Google Scholar] [CrossRef]
- Milne, B.F.; Toker, Y.; Rubio, A.; Nielsen, S.B. Unraveling the intrinsic color of chlorophyll. Angew. Chemie—Int. Ed. 2015, 54, 2170–2173. [Google Scholar] [CrossRef] [PubMed]
- Lumbessy, S.Y.; Junaidi, M.; Diniarti, N.; Setyowati, D.N.; Mukhlis, A.; Tambaru, R. Identification of chlorophyll pigment on Gracilaria salicornia seaweed. IOP Conf. Ser. Earth Environ. Sci. 2021, 681, 12017. [Google Scholar] [CrossRef]
- Torres, P.B.; Chow, F.; Furlan, C.M.; Mandelli, F.; Mercadante, A.; dos Santos, D.Y.A.C. Standardization of a protocol to extract and analyze chlorophyll a and carotenoids in Gracilaria tenuistipitata var. liui. Zhang and Xia (Rhodophyta). Braz. J. Oceanogr. 2014, 62, 57–63. [Google Scholar] [CrossRef]
- Chen, K.; Ríos, J.J.; Pérez-Gálvez, A.; Roca, M. Comprehensive chlorophyll composition in the main edible seaweeds. Food Chem. 2017, 228, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Ríos, J.J.; Pérez-Gálvez, A.; Roca, M. Development of an accurate and high-throughput methodology for structural comprehension of chlorophylls derivatives. (I) Phytylated derivatives. J. Chromatogr. A 2015, 1406, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Dias, A.M.; Ferreira, M.L.S. “Supermarket Column Chromatography of Leaf Pigments” Revisited: Simple and Ecofriendly Separation of Plant Carotenoids, Chlorophylls, and Flavonoids from Green and Red Leaves. J. Chem. Educ. 2014, 92, 189–192. [Google Scholar] [CrossRef]
- Chen, K.; Roca, M. In vitro bioavailability of chlorophyll pigments from edible seaweeds. J. Funct. Foods 2018, 41, 25–33. [Google Scholar] [CrossRef]
- Chen, K.; Roca, M. In vitro digestion of chlorophyll pigments from edible seaweeds. J. Funct. Foods 2018, 40, 400–407. [Google Scholar] [CrossRef]
- Jesumani, V.; Du, H.; Aslam, M.; Pei, P.; Huang, N. Potential Use of Seaweed Bioactive Compounds in Skincare—A Review. Mar. Drugs 2019, 17, 688. [Google Scholar] [CrossRef]
- Morais, T.; Cotas, J.; Pacheco, D.; Pereira, L. Seaweeds Compounds: An Ecosustainable Source of Cosmetic Ingredients? Cosmetics 2021, 8, 8. [Google Scholar] [CrossRef]
- Spears, K. Developments in food colourings: The natural alternatives. Trends Biotechnol. 1988, 6, 283–288. [Google Scholar] [CrossRef]
- Solymosi, K.; Mysliwa-Kurdziel, B. Chlorophylls and their Derivatives Used in Food Industry and Medicine. Mini-Rev. Med. Chem. 2016, 17, 1194–1222. [Google Scholar] [CrossRef] [PubMed]
- ChemicalBook:Chlorophyll A. Available online: https://www.chemicalbook.com/ProductChemicalPropertiesCB5471362_EN.htm (accessed on 22 November 2021).
- Janarthanan, M.; Senthil Kumar, M. The properties of bioactive substances obtained from seaweeds and their applications in textile industries. J. Ind. Text. 2017, 48, 361–401. [Google Scholar] [CrossRef]






| Pigment | Number of Patents | ||
|---|---|---|---|
| PBP | PE | 314 | |
| PC | 573 | ||
| APC | 67 | ||
| Carotenoids | Carotenes | α-C | 178 |
| β-C | 2589 | ||
| Xanthophylls | Lutein | 2591 | |
| Zeaxanthin | 1333 | ||
| Astaxanthin | 4236 | ||
| Chlorophylls | Chlorophyll a | 5949 | |
| Species | Extraction Method | Isolation and Purification Steps | PE Concentration (mg g−1 or mg mL−1) or Yield (%) | PE Purity (%) | Reference |
|---|---|---|---|---|---|
| Amphiroa anceps | Grinding with PBS(+) | (NH4)2SO4 salting-out Q-Sepharose IEXC Sepharose CL-6B GFC | 25 mg 35 g−1 dw (PEx) | n/a | Kawsar et al. [69] |
| Carradoriella elongata1 | Extraction with PB | n/a | 3.70 mg g−1 fw | n/a | Ismail and Osman [70] |
| Ceramium isogonum | Washing with KPB Freeze-thaw with Sodium Nitrate French Press | (NH4)2SO4 salting-out Sephadex DEAE IEXC Ultrafiltration | 0.383% dw (PEx) | 2.10 | Kaixian et al. [71] |
| Ceramium tenuicorne | Cryogrinding and suspension in SCB | Phenomenex HPLC-SEC | 2.13% dw 3 (PEx) | n/a | Saluri et al. [57] |
| Coccotylus truncatus | Cryogrinding and suspension in SCB | Phenomenex HPLC-SEC | 0.95% dw 3 (PEx) | n/a | Saluri et al. [57] |
| Colaconema formosanum | Grinding in PBS(+) | (NH4)2SO4 salting-out HiTrap DEAE IEXC | n/a | 5.79 | Lee et al. [46] |
| Constantinea rosa-marina | Cryogrinding and suspension in SCB | Phenomenex HPLC-SEC | ≈0.22% dw 2,3 (PEx) | n/a | Saluri et al. [57] |
| Corallina officinalis | Cryogrinding and homogenization in SPB | (NH4)2SO4 salting-out Sepharose 4B CL-200 SEC DEAE-cellulose IEXC Sephacryl S-200 SEC | n/a | 4.7 | Hilditch et al. [72] |
| Extraction with PB | n/a | >2 mg g−1 fw 2 | n/a | Ismail and Osman [70] | |
| Dasysiphonia japonica1 | Ultrasonication with PB | (NH4)2SO4 salting-out Sepharose CL-4B GFC Sephadex G-200 GFC DEAE-Sepharose IEXC | n/a | 4.89 | Sun et al. [73] |
| Ellisolandia elongata 1 | Grinding with NaPi 4 and NaCl | HAC Superdex 75 GFC | 15 mg 25 g dw (PEx) | 6.67 | Rossano et al. [35] |
| Extraction with PB | n/a | >1 mg g−1 fw 2 | n/a | Ismail and Osman [70] | |
| Furcellaria lumbricalis | Cryogrinding and suspension in CB | Superdex 200 SEC | 0.13% dw (CEx) | 1.41 | Saluri et al. [74] |
| Cryogrinding and suspension in SCB | Phenomenex HPLC-SEC | 0.59% dw 3 (PEx) | n/a | Saluri et al. [57] | |
| Gelidium elegans | Cryogrinding and suspension in SCB | Phenomenex HPLC-SEC | ≈0.17% dw 2,3 (PEx) | n/a | Saluri et al. [57] |
| Gelidium pacificum | Cryogrinding and suspension in SCB | Phenomenex HPLC-SEC | ≈0.18% dw 2,3 (PEx) | n/a | Saluri et al. [57] |
| Gelidium pusillum | Grinding and enzymatic hydrolysis in PB | n/a | 0.29 mg g−1 dw (CEx) | n/a | Mittal and Raghavarao [75] |
| Grinding and ultrasonication with PB | (NH4)2SO4 salting-out ATPE Ultrafiltration | 57% | 1.1 | Mittal et al. [76] | |
| Gracilaria canaliculata1 | Grinding with distilled water | (NH4)2SO4 salting-out DEAE Cellulose AEXC | 0.50 mg g g−1 fw (CEx) | 3.79 | Sudhakar et al. 2015 [77] |
| Gracilaria corticata | Grinding with PB | (NH4)2SO4 salting-out DEAE Cellulose AEXC | 0.024% (PEx) | 1.10 | Sudhakar et al. 2014 [78] |
| Gracilaria domingensis | Cryogrinding and suspension in PB | n/a | 7.69 mg g−1 dw (CEx) | n/a | Pereira et al. 2012 [79] |
| Gracilaria gracilis | Suspension in distilled water | (NH4)2SO4 salting-out Phenyl-Sepharose EBAC Q-Sepharose IEXC | 0.141 mg g−1 fw | 4.4 | Wang et al. [80] |
| Grinding and suspension with acetic-acid-sodium acetate | n/a | 7 mg g−1 dw (CEx) | n/a | Francavilla et al. [81] | |
| Grinding in PB | n/a | 3.6 mg g−1 dw (CEx) | n/a | Pereira et al. [58] | |
| Gracilaria longa | Cryogrinding and suspension in K-Pi and EDTA | (NH4)2SO4 salting-out Superdex 200 GFC | 1 mg 10 g−1 fw (PEx) | 4.5 | D’Agnolo et al. [82] |
| Gracilaria tenuistipitata | Grinding and freeze-thaw with distilled water | (NH4)2SO4 salting-out DEAE-Sepharose AEXC | 30.34 µg g−1 (PEx) | 4.21 | Zhao et al. 2020 [83] |
| Gracilaria vermyculophylla | Grinding and freeze-thaw with EDTA | n/a | ≈1.3 mg g−1 fw 2 | n/a | Sfriso et al. [84] |
| Gracilariopsis lemaneiformis1 | Grinding and freeze-thaw with PB | (NH4)2SO4 salting-out Phenyl-Sepharose EBAC DEAE-Sepharose AEXC | 0.17% (CEx) | 4.2 | Niu et al. [31] |
| Suspension in distilled water | (NH4)2SO4 salting-out CPC | 0.5 mg 100 mg−1 (PEx) | 6.5 | Gu et al. [85] | |
| Gracilariopsis longissima | Grinding and freeze-thaw with EDTA | n/a | ≈1.5 mg g−1 fw 2 | n/a | Sfriso et al. 2018 [84] |
| Grateloupia turuturu | Grinding with distilled water | (NH4)2SO4 salting-out | 2.79 mg g−1 dw (CEx) | 1.14 | Denis et al. [86] |
| Cryogrinding and suspension in SPB | (NH4)2SO4 salting-out | 4.39 mg g−1 dw (CEx) | 0.93 | Munier et al. [53] | |
| Ultrasound-assisted enzymatic hydrolysis | n/a | 3.6 mg g−1 dw (CEx) | n/a | Le Guillard et al. [87] | |
| Cryogrinding and suspension in SPB | Semi-preparative (DEAE-Sepharose) AEXC | 27% (PEx) | 2.89 | Munier et al. [88] | |
| Halymenia floresii | Freeze-thaw with PB | Preparative native-PAGE with electrophoretic elution | 41.1% (PEx) | 5.9 | Sathuvan et al. [89] |
| Jania rubens | Extraction with PB | n/a | 0.91 mg g−1 fw | n/a | Ismail and Osman [70] |
| Mastocarpus stellatus | Cryogrinding, suspension in PB and enzymatic hydrolysis (xylanase) | n/a | 1.63 mg g−1 dw | 0.43 | Nguyen et al. [90] |
| Neodilsea yendoana | Cryogrinding and suspension in SCB | Phenomenex HPLC-SEC | ≈0.24% dw 2,3 (PEx) | n/a | Saluri et al. [57] |
| Neoporphyra haitanensis 1 | Grinding and freeze-thaw with distilled water | Phenyl-Sepharose HIC-EBAC Q-Sepharose IEXC | 1.4 mg g−1 (PEx) | 5.29 | Niu et al. [91] |
| Suspension and freeze-thaw with PBS(+) | (NH4)2SO4 salting-out Sephadex G-100 GFC DEAE-Cellulose IEXC | 1.68 mg g−1 dw | 3.30 | Pan et al. [50] | |
| Neoporphyra kitoi | Grinding in PB | n/a | ≈22 µg cm−2 2 | n/a | Niwa et al. [92] |
| Neopyropia elongata1 | Grinding and freeze-thaw with EDTA | n/a | ≈3 mg g−1 fw 2 | n/a | Sfriso et al. 2018 [84] |
| Neopyropia kinositae 1 | Grinding in PB | n/a | ≈22 µg cm−1 2 | n/a | Sano et al. [93] |
| Neopyropia yezoensis1 | Grinding and freeze-thaw with PB | Phenyl-Sepharose EBAC DEAE-Sepharose IEXC | 0.82% (CEx) | 4.5 | Niu et al. [94] |
| Grinding with SPB | n/a | 6.953% (CEx) | 0.287 | Wang et al. [95] | |
| Palmaria decipiens | Cryogrinding and freeze-thaw with KPB | n/a | 732 µg g−1 fw | n/a | Lüder et al. [96] |
| Palmaria palmata | Cryogrinding and suspension in PB | Preparative PAGE | 1 mg l−1 (CEx) | 3.2 | Galland-Irmouli et al. [97] |
| Cryogrinding, suspension in AB and enzymatic hydrolysis (xylanase) | n/a | 11.27 mg g−1 dw | 0.74 | Dumay et al. [98] | |
| Grinding, suspension in distilled water, (NH4)2SO4 precipitation, and digestion in thermolysin | n/a | 54.3 mg g−1 dw | n/a | Lee et al. [45] | |
| Porphyra sp. | Grinding and suspension in SPB Enzymatic hydrolysis | Ultrafiltration Superdex 200 FPLC | 0.36 mg mL−1 | 3.18 | Huang et al. [99] |
| Polysiphonia morrowii | Grinding and freeze-thaw with EDTA | n/a | ≈0.7 mg g−1 fw 2 | n/a | Sfriso et al. 2018 [84] |
| Polysiphonia stricta1 | Immersion in distilled water | Phenyl-Sepharose EBAC Q-Sepharose AEC HAC | 0.34% (CEx) | 3.90 | Niu et al. [100] |
| Portiera hornemannii | Grinding and freeze-thaw with PB | (NH4)2SO4 salting-out Q-Sepharose AEC | 1.23 mg g−1 fw (CEx) | 5.21 | Senthilkumar et al. [101] |
| Pterocladiella capillacea1 | Extraction with PB | n/a | ≈2 mg g−1 fw 2 | n/a | Ismail and Osman [70] |
| Rhodomela confervoides | Cryogrinding and suspension in SCB | Phenomenex HPLC-SEC | 1.33% dw 3 (PEx) | n/a | Saluri et al. [57] |
| Vertebrata fucoides 1 | Cryogrinding and suspension in SCB | Phenomenex HPLC-SEC | 0.52% dw 3 (PEx) | n/a | Saluri et al. [57] |
| Species | Extraction Enzyme(s) | PE Yield % Improvement | PE Yield | Reference |
|---|---|---|---|---|
| Furcelaria lumbricalis | Xyl + Cel | 130 2 | >0.45% dw (PEx) | Saluri et al. [74] |
| Gelidium pusillum | Xyl + Cel + Aga | 26 2 | 0.29 mg g−1 dw | Mittal and Raghavarao [75] |
| Mastocarpus stellatus | Xyl | 1.8 3 | 1.99 mg g−1 dw | Nguyen et al. [90] |
| Neopyropia yezoensis1 | Cel + Aga | 3.33 2 | 6.953 mg g−1 dw | Wang et al. [95] |
| Palmaria palmata | Xyl | 62 2 | 12.36 mg g−1 dw | Dumay et al. [98] |
| Porphyra sp. | MA103 + MAEF108 4 | n/a | 0.36 mg mL−1 | Huang et al. [99] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freitas, M.V.; Pacheco, D.; Cotas, J.; Mouga, T.; Afonso, C.; Pereira, L. Red Seaweed Pigments from a Biotechnological Perspective. Phycology 2022, 2, 1-29. https://doi.org/10.3390/phycology2010001
Freitas MV, Pacheco D, Cotas J, Mouga T, Afonso C, Pereira L. Red Seaweed Pigments from a Biotechnological Perspective. Phycology. 2022; 2(1):1-29. https://doi.org/10.3390/phycology2010001
Chicago/Turabian StyleFreitas, Marta V., Diana Pacheco, João Cotas, Teresa Mouga, Clélia Afonso, and Leonel Pereira. 2022. "Red Seaweed Pigments from a Biotechnological Perspective" Phycology 2, no. 1: 1-29. https://doi.org/10.3390/phycology2010001
APA StyleFreitas, M. V., Pacheco, D., Cotas, J., Mouga, T., Afonso, C., & Pereira, L. (2022). Red Seaweed Pigments from a Biotechnological Perspective. Phycology, 2(1), 1-29. https://doi.org/10.3390/phycology2010001











