Advancements and Prospects in Algal Biofuel Production: A Comprehensive Review
Abstract
:1. Introduction
2. Cultivation and Harvesting of Microalgae for Production of Algae Biofuel
2.1. Cultivation Systems for Microalgae-Based Biofuel Production
2.2. Cultivation Modes for Enhanced Microalgae Biomass and Biodiesel Production
2.3. Wastewater-Based Microalgae Cultivation: Achieving Dual Benefits
2.4. Harvesting
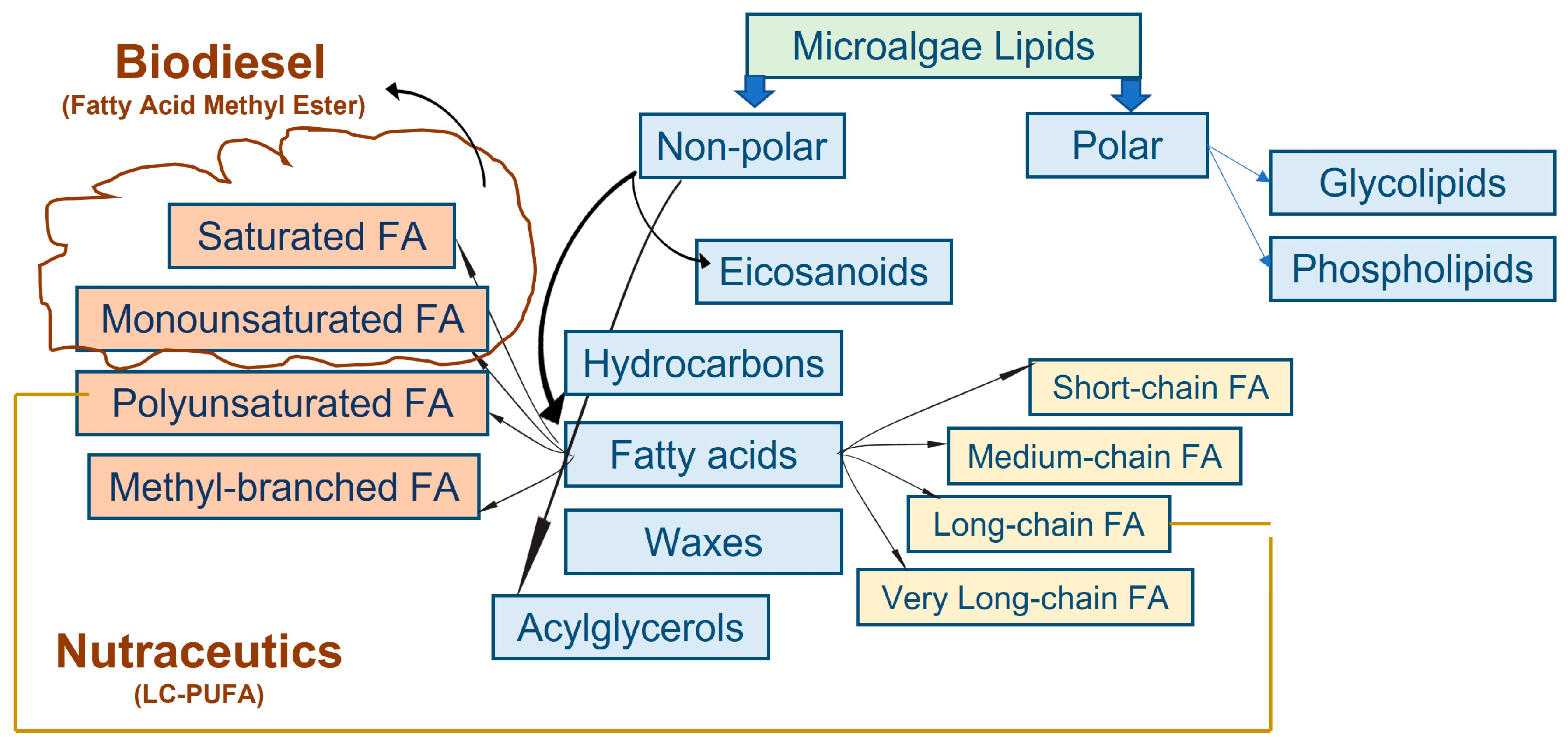
3. Lipid Productivity and Fatty Acid Composition
3.1. Fatty Acid Composition of Microalgae as the Important Property of Biodiesel Feedstock
3.2. Enhancing Lipid Productivity in Microalgae through Various Manipulation Techniques
3.2.1. Nutrient Stress as an Approach to Enhance Lipid Content in Microalgae
3.2.2. Salinity Stress as an Approach to Enhance Lipid Content in Microalgae
3.2.3. CO2 Manipulation as an Approach to Enhance Lipid Content in Microalgae
3.2.4. Genome-Editing Techniques as an Approach to Improve Enhance Lipid Content and Biomass in Microalgae
4. Physicochemical Properties of Algae Biodiesel
4.1. Kinematic Viscosity
4.2. Surface Tension
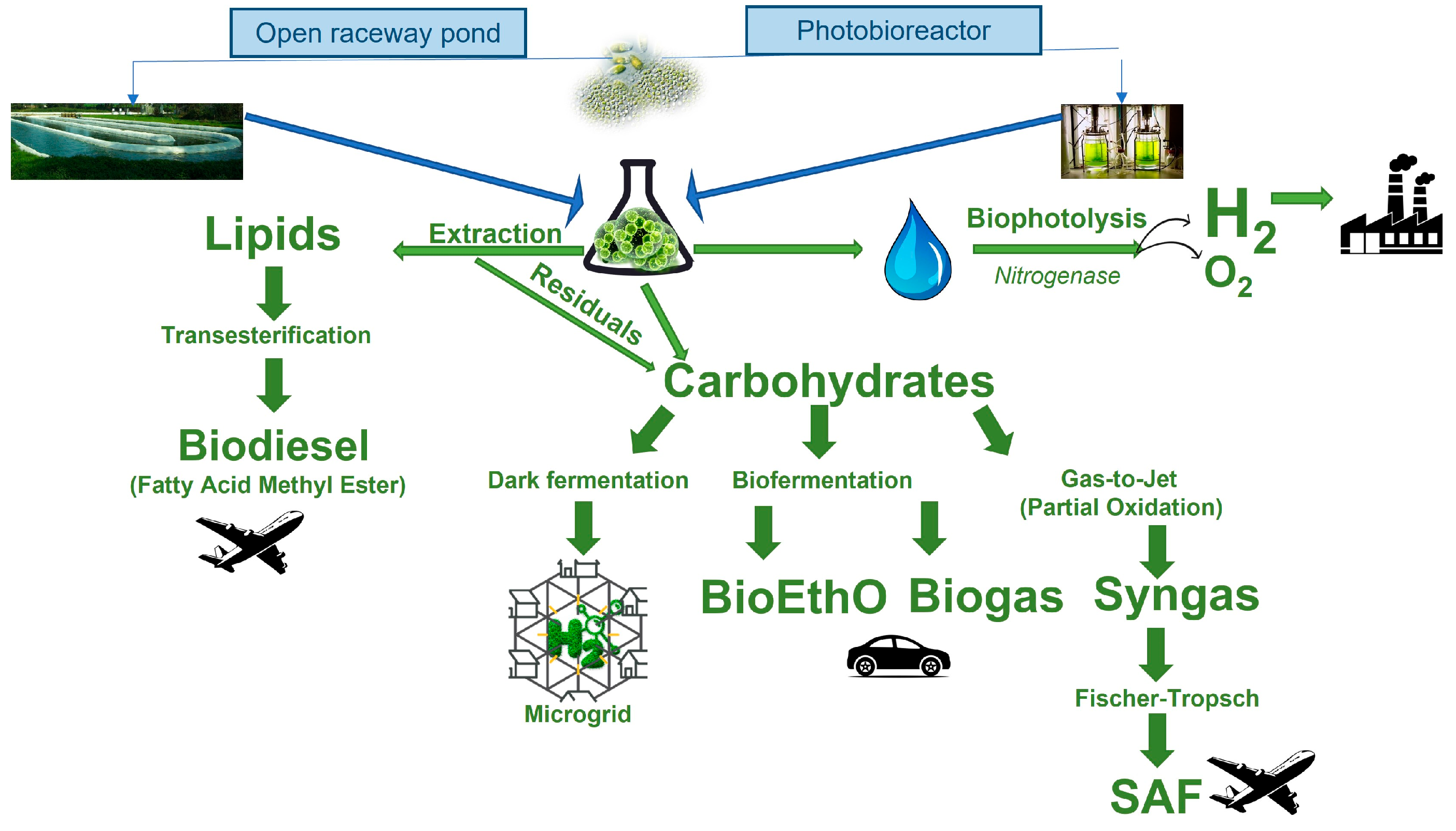
5. Microalgae as the Sustainable Feedstock for Sustainable Aviation Fuel and Biohydrogen
5.1. Biodiesel and Sustainable Aviation Fuel
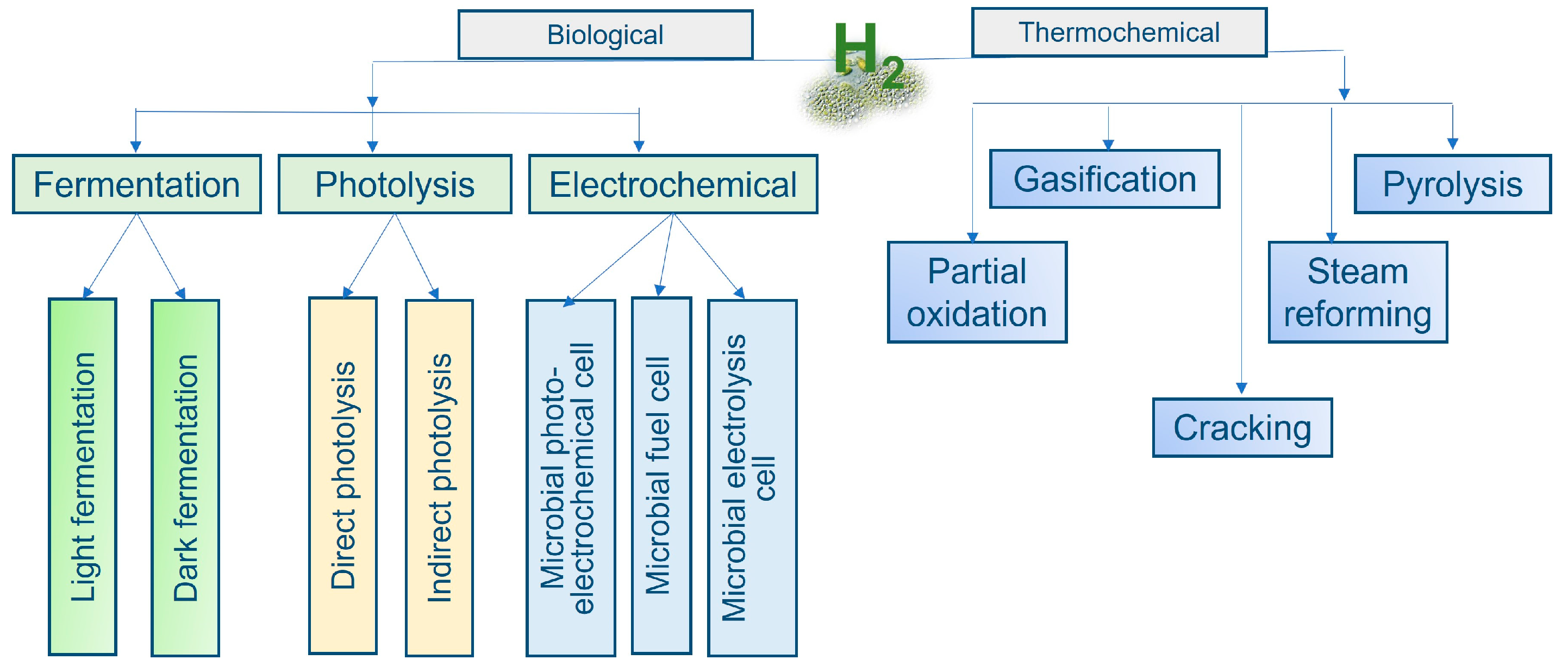
5.2. Biohydrogen
6. Conclusions and Perspectives
- Cultivation Optimization: Tailoring microalgal cultivation conditions to optimize the production of desirable fatty acids can enhance fuel properties, while genetic engineering technologies such as CRISPR/Cas9 offer precision in modifying lipid biosynthetic pathways for higher yields and better fuel characteristics. Employing hybrid cultivation systems to balance cost and productivity can also optimize resource use.
- Advanced Harvesting Techniques: Developing energy-efficient harvesting methods, such as enhanced flocculation, co-cultivation with other microorganisms, and the use of biofilm reactors to reduce costs.
- Resource Efficiency: Integrating microalgae cultivation with wastewater treatment facilities to maximize the use of nutrient-dense waste streams, hence decreasing operational expenses and overall environmental footprint.
- Policy Support and Incentives: Government subsidies, tax incentives, and other financial support are essential to making SAF from microalgae commercially viable. Most bio-jet fuel production technologies incur costs that are at least 120% higher than conventional fossil-based jet fuel, while achieving emissions reductions of at least 27%. Despite these high costs, only 38% of existing policies provide monetary incentives to SAF producers, resulting in SAF production operating at only 3.5% of its total potential capacity [160].
- Genetic Modifications: Enhancing microalgal strains through genetic engineering to increase hydrogenase activity and overall hydrogen yield.
- Bioreactor Design: Developing advanced bioreactors that maximize sunlight capture and gas exchange and optimize growth conditions.
- Integration with Waste Treatment: Using wastewater as a nutrient source for microalgae cultivation can provide a low-cost substrate while treating the wastewater, thus achieving dual benefits.
- Integration of Biohydrogen Production Into Microgrids: Implementing biohydrogen production facilities within microgrids can significantly benefit rural and remote areas. By utilizing local biomass waste residues and cultivating microalgae biomass in wastewater treatment facilities, these regions can enhance their energy independence and sustainability. In an ideal scenario, microgrids powered by green hydrogen derived from biohydrogen may offer advantages over those using electrolytically produced hydrogen.
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jabłońska-Trypuć, A.; Wołejko, E.; Ernazarovna, M.D.; Głowacka, A.; Sokołowska, G.; Wydro, U. Using Algae for Biofuel Production: A Review. Energies 2023, 16, 1758. [Google Scholar] [CrossRef]
- Häder, D. On the Way to Mars—Flagellated Algae in Bioregenerative Life Support Systems Under Microgravity Conditions. Front. Plant Sci. 2020, 10, 1621. [Google Scholar] [CrossRef] [PubMed]
- Ślusarczyk, J.; Adamska, E.; Czerwik-Marcinkowska, J. Fungi and Algae as Sources of Medicinal and Other Biologically Active Compounds: A Review. Nutrients 2021, 13, 3178. [Google Scholar] [CrossRef] [PubMed]
- Henao, E.; Murphy, P.J.; Falfushynska, H.; Horyn, O.; Evans, D.M.; Klimaszyk, P.; Rzymski, P. Polymethoxy-1-Alkenes Screening of Chlorella and Spirulina Food Supplements Coupled with In Vivo Toxicity Studies. Toxins 2020, 12, 111. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.; Shin, J.H.; Kim, J.D. The Promising Future of Microalgae: Current Status, Challenges, and Optimization of a Sustainable and Renewable Industry for Biofuels, Feed, and Other Products. Microb. Cell Fact. 2018, 17, 36. [Google Scholar] [CrossRef]
- Chen, W.-H.; Lin, B.-J.; Huang, M.-Y.; Chang, J.-S. Thermochemical Conversion of Microalgal Biomass into Biofuels: A Review. Bioresour. Technol. 2015, 184, 314–327. [Google Scholar] [CrossRef]
- Mahmood, T.; Hussain, N.; Shahbaz, A.; Mulla, S.I.; Iqbal, H.M.N.; Bilal, M. Sustainable Production of Biofuels from the Algae-Derived Biomass. Bioprocess. Biosyst. Eng. 2023, 46, 1077–1097. [Google Scholar] [CrossRef]
- Kim, S.; Im, H.; Yu, J.; Kim, K.; Kim, M.; Lee, T. Biofuel Production from Euglena: Current Status and Techno-Economic Perspectives. Bioresour. Technol. 2023, 371, 128582. [Google Scholar] [CrossRef]
- Mofijur, M.; Ashrafur Rahman, S.M.; Nguyen, L.N.; Mahlia, T.M.I.; Nghiem, L.D. Selection of Microalgae Strains for Sustainable Production of Aviation Biofuel. Bioresour. Technol. 2022, 345, 126408. [Google Scholar] [CrossRef]
- Net Electricity Consumption Worldwide in Select Years from 1980 to 2022. Available online: https://www.statista.com/statistics/280704/world-power-consumption (accessed on 4 October 2024).
- Shafiee, S.; Topal, E. When Will Fossil Fuel Reserves Be Diminished? Energy Policy 2009, 37, 181–189. [Google Scholar] [CrossRef]
- Renewable Energy Targets. Available online: https://energy.ec.europa.eu/topics/renewable-energy/renewable-energy-directive-targets-and-rules/renewable-energy-targets_en (accessed on 4 October 2024).
- Electricity 2030: Longterm Trends–Tasks for the Coming Years. Available online: https://www.bmwk.de/Redaktion/EN/Publikationen/electricity-2030-long-term-trends.pdf?__blob=publicationFile&v=1 (accessed on 4 October 2024).
- Moioli, E.; Schildhauer, T. Negative CO2 Emissions from Flexible Biofuel Synthesis: Concepts, Potentials, Technologies. Renew. Sustain. Energy Rev. 2022, 158, 112120. [Google Scholar] [CrossRef]
- Falfushynska, H. Navigating Environmental Concerns: Assessing the Ecological Footprint of Photovoltaic-Produced Energy. Environments 2024, 11, 140. [Google Scholar] [CrossRef]
- Lee, R.A.; Lavoie, J.-M. From First- to Third-Generation Biofuels: Challenges of Producing a Commodity from a Biomass of Increasing Complexity. Anim. Front. 2013, 3, 6–11. [Google Scholar] [CrossRef]
- Yin, Z.; Zhu, L.; Li, S.; Hu, T.; Chu, R.; Mo, F.; Hu, D.; Liu, C.; Li, B. A Comprehensive Review on Cultivation and Harvesting of Microalgae for Biodiesel Production: Environmental Pollution Control and Future Directions. Bioresour. Technol. 2020, 301, 122804. [Google Scholar] [CrossRef]
- Doliente, S.S.; Narayan, A.; Tapia, J.F.D.; Samsatli, N.J.; Zhao, Y.; Samsatli, S. Bio-Aviation Fuel: A Comprehensive Review and Analysis of the Supply Chain Components. Front. Energy Res. 2020, 8, 110. [Google Scholar] [CrossRef]
- Islam, M.A.; Heimann, K.; Brown, R.J. Microalgae Biodiesel: Current Status and Future Needs for Engine Performance and Emissions. Renew. Sustain. Energy Rev. 2017, 79, 1160–1170. [Google Scholar] [CrossRef]
- Wijffels, R.H.; Barbosa, M.J. An Outlook on Microalgal Biofuels. Science 2010, 329, 796–799. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.S.; Lee, S.Y.; Chew, K.W.; Lam, M.K.; Lim, J.W.; Ho, S.-H.; Show, P.L. A Review on Microalgae Cultivation and Harvesting, and Their Biomass Extraction Processing Using Ionic Liquids. Bioengineered 2020, 11, 116–129. [Google Scholar] [CrossRef]
- The Future of the EU Algae Sector. Available online: https://www.europarl.europa.eu/RegData/etudes/STUD/2023/733114/IPOL_STU(2023)733114_EN.pdf (accessed on 4 October 2024).
- Araújo, R.; Vázquez Calderón, F.; Sánchez López, J.; Azevedo, I.C.; Bruhn, A.; Fluch, S.; Garcia Tasende, M.; Ghaderiardakani, F.; Ilmjärv, T.; Laurans, M.; et al. Current Status of the Algae Production Industry in Europe: An Emerging Sector of the Blue Bioeconomy. Front. Mar. Sci. 2021, 7, 626389. [Google Scholar] [CrossRef]
- Abdur Razzak, S.; Bahar, K.; Islam, K.M.O.; Haniffa, A.K.; Faruque, M.O.; Hossain, S.M.Z.; Hossain, M.M. Microalgae Cultivation in Photobioreactors: Sustainable Solutions for a Greener Future. Green. Chem. Eng. 2024, 5, 418–439. [Google Scholar] [CrossRef]
- Cui, X.; Yang, J.; Cui, M.; Zhang, W.; Zhao, J. Comparative Experiments of Two Novel Tubular Photobioreactors with an Inner Aerated Tube for Microalgal Cultivation: Enhanced Mass Transfer and Improved Biomass Yield. Algal Res. 2021, 58, 102364. [Google Scholar] [CrossRef]
- Huang, Q.; Jiang, F.; Wang, L.; Yang, C. Design of Photobioreactors for Mass Cultivation of Photosynthetic Organisms. Engineering 2017, 3, 318–329. [Google Scholar] [CrossRef]
- Standard Specification for Biodiesel Fuel Blend Stock (B100) for Middle Distillate Fuels. Available online: https://www.astm.org/d6751-20a.html (accessed on 4 October 2024).
- EN 14214. Available online: https://www.chemeurope.com/en/encyclopedia/EN_14214.html (accessed on 4 October 2024).
- Sharma, A.K.; Sharma, A.; Singh, Y.; Chen, W.-H. Production of a Sustainable Fuel from Microalgae Chlorella minutissima Grown in a 1500 L Open Raceway Ponds. Biomass Bioenergy 2021, 149, 106073. [Google Scholar] [CrossRef]
- Schneider, R.D.C.D.S.; De Moura Lima, M.; Hoeltz, M.; De Farias Neves, F.; John, D.K.; De Azevedo, A. Life Cycle Assessment of Microalgae Production in a Raceway Pond with Alternative Culture Media. Algal Res. 2018, 32, 280–292. [Google Scholar] [CrossRef]
- Rayen, F.; Behnam, T.; Dominique, P. Optimization of a Raceway Pond System for Wastewater Treatment: A Review. Crit. Rev. Biotechnol. 2019, 39, 422–435. [Google Scholar] [CrossRef]
- Adesanya, V.O.; Cadena, E.; Scott, S.A.; Smith, A.G. Life Cycle Assessment on Microalgal Biodiesel Production Using a Hybrid Cultivation System. Bioresour. Technol. 2014, 163, 343–355. [Google Scholar] [CrossRef]
- Yun, J.-H.; Cho, D.-H.; Lee, S.; Heo, J.; Tran, Q.-G.; Chang, Y.K.; Kim, H.-S. Hybrid Operation of Photobioreactor and Wastewater-Fed Open Raceway Ponds Enhances the Dominance of Target Algal Species and Algal Biomass Production. Algal Res. 2018, 29, 319–329. [Google Scholar] [CrossRef]
- Kumar, M.S.; Hwang, J.-H.; Abou-Shanab, R.A.I.; Kabra, A.N.; Ji, M.-K.; Jeon, B.-H. Influence of CO2 and Light Spectra on the Enhancement of Microalgal Growth and Lipid Content. J. Renew. Sustain. Energy 2014, 6, 063107. [Google Scholar] [CrossRef]
- Assis, L.R.D.; Ferreira, J.; Assemany, P.P.; Teixeira, J.S.; Castro, J.D.S.; Pereira, H.A.; Calijuri, M.L. Environmental Benefits of a Hybrid System for Algal Biomass Production, Harvesting and Nutrient Recovery under a Life-Cycle Assessment. Algal Res. 2023, 73, 103163. [Google Scholar] [CrossRef]
- Abreu, A.P.; Morais, R.C.; Teixeira, J.A.; Nunes, J. A Comparison between Microalgal Autotrophic Growth and Metabolite Accumulation with Heterotrophic, Mixotrophic and Photoheterotrophic Cultivation Modes. Renew. Sustain. Energy Rev. 2022, 159, 112247. [Google Scholar] [CrossRef]
- Yun, H.-S.; Kim, Y.-S.; Yoon, H.-S. Effect of Different Cultivation Modes (Photoautotrophic, Mixotrophic, and Heterotrophic) on the Growth of Chlorella sp. and Biocompositions. Front. Bioeng. Biotechnol. 2021, 9, 774143. [Google Scholar] [CrossRef] [PubMed]
- Proietti Tocca, G.; Agostino, V.; Menin, B.; Tommasi, T.; Fino, D.; Di Caprio, F. Mixotrophic and Heterotrophic Growth of Microalgae Using Acetate from Different Production Processes. Rev. Environ. Sci. Biotechnol. 2024, 23, 93–132. [Google Scholar] [CrossRef]
- Grubišić, M.; Peremin, I.; Djedović, E.; Šantek, B.; Ivančić Šantek, M. Cultivation of a Novel Strain of Chlorella vulgaris S2 under Phototrophic, Mixotrophic, and Heterotrophic Conditions, and Effects on Biomass Growth and Composition. Fermentation 2024, 10, 270. [Google Scholar] [CrossRef]
- Verma, R.; Kumari, K.V.L.K.; Srivastava, A.; Kumar, A. Photoautotrophic, Mixotrophic, and Heterotrophic Culture Media Optimization for Enhanced Microalgae Production. J. Environ. Chem. Eng. 2020, 8, 104149. [Google Scholar] [CrossRef]
- Youssef, A.M.; Gomaa, M.; Mohamed, A.K.S.H.; El-Shanawany, A.-R.A. Enhancement of Biomass Productivity and Biochemical Composition of Alkaliphilic Microalgae by Mixotrophic Cultivation Using Cheese Whey for Biofuel Production. Environ. Sci. Pollut. Res. 2024, 31, 42875–42888. [Google Scholar] [CrossRef]
- Zuliani, L.; Frison, N.; Jelic, A.; Fatone, F.; Bolzonella, D.; Ballottari, M. Microalgae Cultivation on Anaerobic Digestate of Municipal Wastewater, Sewage Sludge and Agro-Waste. Int. J. Mol. Sci. 2016, 17, 1692. [Google Scholar] [CrossRef]
- Kong, W.; Kong, J.; Feng, S.; Yang, T.; Xu, L.; Shen, B.; Bi, Y.; Lyu, H. Cultivation of Microalgae–Bacteria Consortium by Waste Gas–Waste Water to Achieve CO2 Fixation, Wastewater Purification and Bioproducts Production. Biotechnol. Biofuels 2024, 17, 26. [Google Scholar] [CrossRef]
- Velásquez-Orta, S.B.; Yáñez-Noguez, I.; Ramírez, I.M.; Ledesma, M.T.O. Pilot-Scale Microalgae Cultivation and Wastewater Treatment Using High-Rate Ponds: A Meta-Analysis. Environ. Sci. Pollut. Res. 2024, 31, 46994–47021. [Google Scholar] [CrossRef]
- Zewdie, D.T.; Ali, A.Y. Cultivation of Microalgae for Biofuel Production: Coupling with Sugarcane-Processing Factories. Energy Sustain. Soc. 2020, 10, 27. [Google Scholar] [CrossRef]
- Osman, M.E.H.; Abo-Shady, A.M.; Gheda, S.F.; Desoki, S.M.; Elshobary, M.E. Unlocking the Potential of Microalgae Cultivated on Wastewater Combined with Salinity Stress to Improve Biodiesel Production. Environ. Sci. Pollut. Res. 2023, 30, 114610–114624. [Google Scholar] [CrossRef]
- Koley, S.; Prasad, S.; Bagchi, S.K.; Mallick, N. Development of a Harvesting Technique for Large-Scale Microalgal Harvesting for Biodiesel Production. RSC Adv. 2017, 7, 7227–7237. [Google Scholar] [CrossRef]
- Wrede, D.; Taha, M.; Miranda, A.F.; Kadali, K.; Stevenson, T.; Ball, A.S.; Mouradov, A. Co-Cultivation of Fungal and Microalgal Cells as an Efficient System for Harvesting Microalgal Cells, Lipid Production and Wastewater Treatment. PLoS ONE 2014, 9, e113497. [Google Scholar] [CrossRef] [PubMed]
- Najjar, Y.S.H.; Abu-Shamleh, A. Harvesting of Microalgae by Centrifugation for Biodiesel Production: A Review. Algal Res. 2020, 51, 102046. [Google Scholar] [CrossRef]
- Bwapwa, J.K.; Anandraj, A.; Trois, C. Possibilities for Conversion of Microalgae Oil into Aviation Fuel: A Review. Renew. Sustain. Energy Rev. 2017, 80, 1345–1354. [Google Scholar] [CrossRef]
- Luo, S.; Wu, X.; Jiang, H.; Yu, M.; Liu, Y.; Min, A.; Li, W.; Ruan, R. Edible Fungi-Assisted Harvesting System for Efficient Microalgae Bio-Flocculation. Bioresour. Technol. 2019, 282, 325–330. [Google Scholar] [CrossRef]
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Microalgae for Biodiesel Production and Other Applications: A Review. Renew. Sustain. Energy Rev. 2010, 14, 217–232. [Google Scholar] [CrossRef]
- De Morais, E.G.; Sampaio, I.C.F.; Gonzalez-Flo, E.; Ferrer, I.; Uggetti, E.; García, J. Microalgae Harvesting for Wastewater Treatment and Resources Recovery: A Review. New Biotechnol. 2023, 78, 84–94. [Google Scholar] [CrossRef]
- Zhu, L.; Li, Z.; Hiltunen, E. Microalgae Chlorella vulgaris Biomass Harvesting by Natural Flocculant: Effects on Biomass Sedimentation, Spent Medium Recycling and Lipid Extraction. Biotechnol. Biofuels 2018, 11, 183. [Google Scholar] [CrossRef]
- Milledge, J.J.; Heaven, S. A Review of the Harvesting of Micro-Algae for Biofuel Production. Rev. Environ. Sci. Biotechnol. 2013, 12, 165–178. [Google Scholar] [CrossRef]
- Khoo, K.S.; Ahmad, I.; Chew, K.W.; Iwamoto, K.; Bhatnagar, A.; Show, P.L. Enhanced Microalgal Lipid Production for Biofuel Using Different Strategies Including Genetic Modification of Microalgae: A Review. Prog. Energy Combust. Sci. 2023, 96, 101071. [Google Scholar] [CrossRef]
- Sun, X.-M.; Ren, L.-J.; Zhao, Q.-Y.; Ji, X.-J.; Huang, H. Microalgae for the Production of Lipid and Carotenoids: A Review with Focus on Stress Regulation and Adaptation. Biotechnol. Biofuels 2018, 11, 272. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, S.; Bala, K.; Kumar, R. Selection of Microalgae Species Based on Their Lipid Content, Fatty Acid Profile and Apparent Fuel Properties for Biodiesel Production. Environ. Sci. Pollut. Res. 2019, 26, 24462–24473. [Google Scholar] [CrossRef]
- Morales, M.; Aflalo, C.; Bernard, O. Microalgal Lipids: A Review of Lipids Potential and Quantification for 95 Phytoplankton Species. Biomass Bioenergy 2021, 150, 106108. [Google Scholar] [CrossRef]
- Demirbas, A.; Fatih Demirbas, M. Importance of Algae Oil as a Source of Biodiesel. Energy Convers. Manag. 2011, 52, 163–170. [Google Scholar] [CrossRef]
- Mostafa, S.S.M.; El-Gendy, N.S. Evaluation of Fuel Properties for Microalgae Spirulina Platensis Bio-Diesel and Its Blends with Egyptian Petro-Diesel. Arab. J. Chem. 2017, 10, S2040–S2050. [Google Scholar] [CrossRef]
- Lee, S.-J.; Go, S.; Jeong, G.-T.; Kim, S.-K. Oil Production from Five Marine Microalgae for the Production of Biodiesel. Biotechnol. Bioprocess Eng. 2011, 16, 561–566. [Google Scholar] [CrossRef]
- Pugliese, A.; Biondi, L.; Bartocci, P.; Fantozzi, F. Selenastrum Capricornutum a New Strain of Algae for Biodiesel Production. Fermentation 2020, 6, 46. [Google Scholar] [CrossRef]
- Hawrot-Paw, M.; Ratomski, P.; Koniuszy, A.; Golimowski, W.; Teleszko, M.; Grygier, A. Fatty Acid Profile of Microalgal Oils as a Criterion for Selection of the Best Feedstock for Biodiesel Production. Energies 2021, 14, 7334. [Google Scholar] [CrossRef]
- Andrew, A.R.; Yong, W.T.L.; Misson, M.; Anton, A.; Chin, G.J.W.L. Selection of Tropical Microalgae Species for Mass Production Based on Lipid and Fatty Acid Profiles. Front. Energy Res. 2022, 10, 912904. [Google Scholar] [CrossRef]
- Yang, Y.; Du, L.; Hosokawa, M.; Miyashita, K. Total Lipids Content, Lipid Class and Fatty Acid Composition of Ten Species of Microalgae. J. Oleo Sci. 2020, 69, 1181–1189. [Google Scholar] [CrossRef]
- Enwereuzoh, U.; Harding, K.; Low, M. Characterization of Biodiesel Produced from Microalgae Grown on Fish Farm Wastewater. SN Appl. Sci. 2020, 2, 970. [Google Scholar] [CrossRef]
- Rodríguez-Palacio, M.C.; Cabrera-Cruz, R.B.E.; Rolón-Aguilar, J.C.; Tobías-Jaramillo, R.; Martínez-Hernández, M.; Lozano-Ramírez, C. The Cultivation of Five Microalgae Species and Their Potential for Biodiesel Production. Energy Sustain. Soc. 2022, 12, 10. [Google Scholar] [CrossRef]
- Anandraj, A.; White, S.; Bwapwa, J.K. Temperature and Nutrient Coupled Stress on Microalgal Neutral Lipids for Low-carbon Fuels Production. Biofuels Bioprod. Bioref. 2021, 15, 1073–1086. [Google Scholar] [CrossRef]
- Gui, J.; Chen, S.; Luo, G.; Wu, Z.; Fan, Y.; Yao, L.; Xu, H. Nutrient Deficiency and an Algicidal Bacterium Improved the Lipid Profiles of a Novel Promising Oleaginous Dinoflagellate, Prorocentrum donghaiense, for Biodiesel Production. Appl. Environ. Microbiol. 2021, 87, e01159-21. [Google Scholar] [CrossRef] [PubMed]
- El-Sheekh, M.M.; Mansour, H.M.; Bedaiwy, M.Y.; El-shenody, R.A. Influence of Nutrient Supplementation and Stress Conditions on the Biomass and Lipid Production of Microchloropsis Salina for Biodiesel Production. Biomass Conv. Bioref. 2022. [Google Scholar] [CrossRef]
- Udayan, A.; Pandey, A.K.; Sirohi, R.; Sreekumar, N.; Sang, B.-I.; Sim, S.J.; Kim, S.H.; Pandey, A. Production of Microalgae with High Lipid Content and Their Potential as Sources of Nutraceuticals. Phytochem. Rev. 2023, 22, 833–860. [Google Scholar] [CrossRef]
- Kim, S.; Kim, M.; Chang, Y.K.; Kim, D. Lipid Production under a Nutrient-Sufficient Condition Outperforms Starvation Conditions Due to a Natural Polarization of Lipid Content in Algal Biofilm. Fuel 2023, 339, 126902. [Google Scholar] [CrossRef]
- Ratomski, P.; Hawrot-Paw, M. Influence of Nutrient-Stress Conditions on Chlorella vulgaris Biomass Production and Lipid Content. Catalysts 2021, 11, 573. [Google Scholar] [CrossRef]
- Abd El Baky, H.; El Baroty, G. Cultivation of Pseudochlorella Pringsheimii for Biodiesel Production in a Scalable Indoor Photobioreactor: Case Studies from Egypt. J. Genet. Eng. Biotechnol. 2023, 21, 25. [Google Scholar] [CrossRef]
- El-Sheekh, M.M.; Galal, H.R.; Mousa, A.S.H.; Farghl, A.A.M. Impact of Macronutrients and Salinity Stress on Biomass and Biochemical Constituents in Monoraphidium Braunii to Enhance Biodiesel Production. Sci. Rep. 2024, 14, 2725. [Google Scholar] [CrossRef]
- Yun, C.-J.; Hwang, K.-O.; Han, S.-S.; Ri, H.-G. The Effect of Salinity Stress on the Biofuel Production Potential of Freshwater Microalgae Chlorella vulgaris YH703. Biomass Bioenergy 2019, 127, 105277. [Google Scholar] [CrossRef]
- Vrana, I.; Bakija Alempijević, S.; Novosel, N.; Ivošević DeNardis, N.; Žigon, D.; Ogrinc, N.; Gašparović, B. Hyposalinity Induces Significant Polar Lipid Remodeling in the Marine Microalga Dunaliella tertiolecta (Chlorophyceae). J. Appl. Phycol. 2022, 34, 1457–1470. [Google Scholar] [CrossRef]
- Khatoon, H.; Haris, N.; Banerjee, S.; Rahman, N.A.; Begum, H.; Mian, S.; Abol-Munafi, A.B.; Endut, A. Effects of Different Salinities on the Growth and Proximate Composition of Dunaliella sp. Isolated from South China Sea at Different Growth Phases. Process Saf. Environ. Prot. 2017, 112, 280–287. [Google Scholar] [CrossRef]
- Conde, T.; Aveiro, S.; Melo, T.; Santos, T.; Neves, B.; Domingues, P.; Varela, J.; Pereira, H.; Domingues, M.R. Cross-Stress Lipid Response of Tetraselmis striata CTP4 to Temperature and Salinity Variation. Algal Res. 2023, 74, 103218. [Google Scholar] [CrossRef]
- Singh, R.P.; Yadav, P.; Kumar, A.; Hashem, A.; Avila-Quezada, G.D.; Abd_Allah, E.F.; Gupta, R.K. Salinity-Induced Physiochemical Alterations to Enhance Lipid Content in Oleaginous Microalgae Scenedesmus sp. BHU1 via Two-Stage Cultivation for Biodiesel Feedstock. Microorganisms 2023, 11, 2064. [Google Scholar] [CrossRef]
- Yang, Z.-Y.; Huang, K.-X.; Zhang, Y.-R.; Yang, L.; Zhou, J.-L.; Yang, Q.; Gao, F. Efficient Microalgal Lipid Production Driven by Salt Stress and Phytohormones Synergistically. Bioresour. Technol. 2023, 367, 128270. [Google Scholar] [CrossRef]
- Li, H.; Sun, X.; Sun, Y.; Ye, L.; Xue, H.; Gao, F.; Yang, Y. Enhancing Lipid Production in Chlorella under Successive Stresses of Periodic Micro-Current and Salinity: Performance and Contribution. Chem. Eng. J. 2024, 486, 150409. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, C.; Vaidyanathan, S. Influence of Gas Management on Biochemical Conversion of CO2 by Microalgae for Biofuel Production. Appl. Energy 2020, 261, 114420. [Google Scholar] [CrossRef]
- Wu, S.; Gu, W.; Huang, A.; Li, Y.; Kumar, M.; Lim, P.E.; Huan, L.; Gao, S.; Wang, G. Elevated CO2 Improves Both Lipid Accumulation and Growth Rate in the Glucose-6-Phosphate Dehydrogenase Engineered Phaeodactylum Tricornutum. Microb. Cell Fact. 2019, 18, 161. [Google Scholar] [CrossRef]
- Widjaja, A.; Chien, C.-C.; Ju, Y.-H. Study of Increasing Lipid Production from Fresh Water Microalgae Chlorella vulgaris. J. Taiwan Inst. Chem. Eng. 2009, 40, 13–20. [Google Scholar] [CrossRef]
- Levitan, O.; Dinamarca, J.; Zelzion, E.; Gorbunov, M.Y.; Falkowski, P.G. An RNA Interference Knock-down of Nitrate Reductase Enhances Lipid Biosynthesis in the Diatom Phaeodactylum tricornutum. Plant J. 2015, 84, 963–973. [Google Scholar] [CrossRef]
- Takahashi, K.; Ide, Y.; Hayakawa, J.; Yoshimitsu, Y.; Fukuhara, I.; Abe, J.; Kasai, Y.; Harayama, S. Lipid Productivity in TALEN-Induced Starchless Mutants of the Unicellular Green Alga Coccomyxa sp. Strain Obi. Algal Res. 2018, 32, 300–307. [Google Scholar] [CrossRef]
- Kurita, T.; Moroi, K.; Iwai, M.; Okazaki, K.; Shimizu, S.; Nomura, S.; Saito, F.; Maeda, S.; Takami, A.; Sakamoto, A.; et al. Efficient and Multiplexable Genome Editing Using Platinum TALENs in Oleaginous Microalga, Nannochloropsis oceanica NIES-2145. Genes Cells 2020, 25, 695–702. [Google Scholar] [CrossRef]
- Dhokane, D.; Shaikh, A.; Yadav, A.; Giri, N.; Bandyopadhyay, A.; Dasgupta, S.; Bhadra, B. CRISPR-Based Bioengineering in Microalgae for Production of Industrially Important Biomolecules. Front. Bioeng. Biotechnol. 2023, 11, 1267826. [Google Scholar] [CrossRef]
- Lin, W.-R.; Ng, I.-S. Development of CRISPR/Cas9 System in Chlorella vulgaris FSP-E to Enhance Lipid Accumulation. Enzym. Microb. Technol. 2020, 133, 109458. [Google Scholar] [CrossRef]
- Kasai, Y.; Takagi, S.; Ota, S.; Ishii, K.; Takeshita, T.; Kawano, S.; Harayama, S. Development of a CRISPR/Cas9-Mediated Gene-Editing Method to Isolate a Mutant of the Unicellular Green Alga Parachlorella Kessleri Strain NIES-2152 with Improved Lipid Productivity. Biotechnol. Biofuels 2024, 17, 36. [Google Scholar] [CrossRef]
- Chang, K.S.; Kim, J.; Park, H.; Hong, S.-J.; Lee, C.-G.; Jin, E. Enhanced Lipid Productivity in AGP Knockout Marine Microalga Tetraselmis sp. Using a DNA-Free CRISPR-Cas9 RNP Method. Bioresour. Technol. 2020, 303, 122932. [Google Scholar] [CrossRef]
- ASTM Biodiesel Specifications. Available online: https://afdc.energy.gov/fuels/biodiesel-specifications (accessed on 4 October 2024).
- ASTM D975-20a; Specification for Diesel Fuel. ASTM International: West Conshohocken, PA, USA, 2019.
- Das, S.; Chowdhury, A. An Exploration of Biodiesel for Application in Aviation and Automobile Sector. Energy Nexus 2023, 10, 100204. [Google Scholar] [CrossRef]
- Alptekin, E.; Canakci, M. Determination of the Density and the Viscosities of Biodiesel–Diesel Fuel Blends. Renew. Energy 2008, 33, 2623–2630. [Google Scholar] [CrossRef]
- Bharti, R.K.; Kaushal, C.; Singh, A.; Dhar, D.W.; Babu, R.; Kaushik, A. Evaluation of Fuel Properties for Possible Biodiesel Output Based on the Fatty Acid Composition of Oleaginous Plants and Microalgae. Sci. Total Environ. 2024, 918, 170448. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Bukkarapu, K.R.; Krishnasamy, A. A Composition Based Approach to Predict Density, Viscosity and Surface Tension of Biodiesel Fuels. Fuel 2021, 285, 119056. [Google Scholar] [CrossRef]
- Park, S.H.; Khan, N.; Lee, S.; Zimmermann, K.; DeRosa, M.; Hamilton, L.; Hudson, W.; Hyder, S.; Serratos, M.; Sheffield, E.; et al. Biodiesel Production from Locally Sourced Restaurant Waste Cooking Oil and Grease: Synthesis, Characterization, and Performance Evaluation. ACS Omega 2019, 4, 7775–7784. [Google Scholar] [CrossRef]
- Bessières, D.; Bazile, J.-P.; Tanh, X.N.T.; García-Cuadra, F.; Acien, F.G. Thermophysical Behavior of Three Algal Biodiesels over Wide Ranges of Pressure and Temperature. Fuel 2018, 233, 497–503. [Google Scholar] [CrossRef]
- Bucy, H.B.; Baumgardner, M.E.; Marchese, A.J. Chemical and Physical Properties of Algal Methyl Ester Biodiesel Containing Varying Levels of Methyl Eicosapentaenoate and Methyl Docosahexaenoate. Algal Res. 2012, 1, 57–69. [Google Scholar] [CrossRef]
- Al-Ansari, M.M.; Al-Humaid, L.; Al-Dahmash, N.D.; Aldawsari, M. Assessing the Benefits of Chlorella vulgaris Microalgal Biodiesel for Internal Combustion Engines: Energy and Exergy Analyses. Fuel 2023, 344, 128055. [Google Scholar] [CrossRef]
- Sharma, A.K.; Sahoo, P.K.; Singhal, S. Feasibility of Biodiesel Production from Chlorella vulgaris Grown in a Flat Plate Photobioreactor under Outdoor Conditions. Int. J. ChemTech Res. 2015, 8, 671–678. [Google Scholar]
- Karmakar, R.; Kundu, K.; Rajor, A. Fuel Properties and Emission Characteristics of Biodiesel Produced from Unused Algae Grown in India. Pet. Sci. 2018, 15, 385–395. [Google Scholar] [CrossRef]
- Ejim, C.E.; Fleck, B.A.; Amirfazli, A. Analytical Study for Atomization of Biodiesels and Their Blends in a Typical Injector: Surface Tension and Viscosity Effects. Fuel 2007, 86, 1534–1544. [Google Scholar] [CrossRef]
- Das, M.; Sarkar, M.; Datta, A.; Santra, A.K. Study on Viscosity and Surface Tension Properties of Biodiesel-Diesel Blends and Their Effects on Spray Parameters for CI Engines. Fuel 2018, 220, 769–779. [Google Scholar] [CrossRef]
- Al-lwayzy, S.; Yusaf, T.; Al-Juboori, R. Biofuels from the Fresh Water Microalgae Chlorella vulgaris (FWM-CV) for Diesel Engines. Energies 2014, 7, 1829–1851. [Google Scholar] [CrossRef]
- Pillet, F.; Dague, E.; Pečar Ilić, J.; Ružić, I.; Rols, M.-P.; Ivošević DeNardis, N. Changes in Nanomechanical Properties and Adhesion Dynamics of Algal Cells during Their Growth. Bioelectrochemistry 2019, 127, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.S.; Francesco, F.D.; Aguinaga, A.; Parameswaran, P.; Rittmann, B.E. Improving Lipid Recovery from Scenedesmus Wet Biomass by Surfactant-Assisted Disruption. Green Chem. 2016, 18, 1319–1326. [Google Scholar] [CrossRef]
- Shimi, E.; Sheltawy, E.M.; Diwani, E. Reactive Extraction of Microalgae for Biodiesel Production; an Optimization Study. Environ. Sci. Eng. Chem. 2014. Available online: https://api.semanticscholar.org/CorpusID:52467259 (accessed on 4 October 2024).
- Salam, K.A. Reactive Extraction of Microalgae for Biodiesel Production. Ph.D. Thesis, Newcastle University, Newcastle upon Tyne, UK, 2015. Available online: https://core.ac.uk/download/pdf/153779776.pdf (accessed on 4 October 2024).
- Salam, K.A.; Velasquez-Orta, S.B.; Harvey, A.P. Surfactant-Assisted Direct Biodiesel Production from Wet Nannochloropsis occulata by in Situ Transesterification/Reactive Extraction. Biofuel Res. J. 2016, 3, 366–371. [Google Scholar] [CrossRef]
- Racheva, R.; Tietgens, N.; Kerner, M.; Smirnova, I. In Situ Continuous Countercurrent Cloud Point Extraction of Microalgae Cultures. Sep. Purif. Technol. 2018, 190, 268–277. [Google Scholar] [CrossRef]
- Lim, J.H.K.; Gan, Y.Y.; Ong, H.C.; Lau, B.F.; Chen, W.-H.; Chong, C.T.; Ling, T.C.; Klemeš, J.J. Utilization of Microalgae for Bio-Jet Fuel Production in the Aviation Sector: Challenges and Perspective. Renew. Sustain. Energy Rev. 2021, 149, 111396. [Google Scholar] [CrossRef]
- Atnoorkar, S.; Wiatrowski, M.; Newes, E.; Davis, R.; Peterson, S. Algae to HEFA: Economics and Potential Deployment in the United States. Biofuels Bioprod. Bioref. 2024, 18, 1121–1136. [Google Scholar] [CrossRef]
- Algal Biofuels: Long-Term Energy Benefits Drive U.S. Research. Available online: https://www.energy.gov/eere/bioenergy/articles/algal-biofuels-long-term-energy-benefits-drive-us-research (accessed on 4 October 2024).
- Gasoline and Diesel Fuel Update. Available online: https://www.eia.gov/petroleum/gasdiesel (accessed on 4 October 2024).
- Sun, J.; Xiong, X.; Wang, M.; Du, H.; Li, J.; Zhou, D.; Zuo, J. Microalgae Biodiesel Production in China: A Preliminary Economic Analysis. Renew. Sustain. Energy Rev. 2019, 104, 296–306. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, B.; Cheng, J.; Pu, S. Socio-Economic Impacts of Algae-Derived Biodiesel Industrial Development in China: An Input–Output Analysis. Algal Res. 2015, 9, 74–81. [Google Scholar] [CrossRef]
- Germans and French Bring Algal Biofuel to Indian Ocean. Available online: https://advancedbiofuelsusa.info/germans-and-french-bring-algal-biofuel-to-indian-ocean (accessed on 4 October 2024).
- Bavarian Facility Can Now Grow Global Strains of Algae for Aerospace Biofuel Production. Available online: https://www.labiotech.eu/trends-news/aviation-biofuels-bavarian-algae-plant (accessed on 4 October 2024).
- New EU-Funded FUELGAE Project Aims to Resurrect Algae as a Promising Source for Aviation Biofuels. 2024. Available online: https://www.greenairnews.com/?p=5193 (accessed on 4 October 2024).
- D-FACTORY: The Micro-Algae Biorefinery. Available online: https://www.ifeu.de/en/project/d-factory-the-micro-algae-biorefinery (accessed on 4 October 2024).
- Raheem, A.; Abbasi, S.A.; Mangi, F.H.; Ahmed, S.; He, Q.; Ding, L.; Memon, A.A.; Zhao, M.; Yu, G. Gasification of Algal Residue for Synthesis Gas Production. Algal Res. 2021, 58, 102411. [Google Scholar] [CrossRef]
- Al-Rabiah, A.A.; Al-Dawsari, J.N.; Ajbar, A.M.; Al Darwish, R.K.; Abdelaziz, O.Y. Development of a Biomass Gasification Process for the Coproduction of Methanol and Power from Red Sea Microalgae. Energies 2022, 15, 7890. [Google Scholar] [CrossRef]
- Ebadi, A.G.; Hisoriev, H.; Zarnegar, M.; Ahmadi, H. Hydrogen and Syngas Production by Catalytic Gasification of Algal Biomass (Cladophora glomerata L.) Using Alkali and Alkaline-Earth Metals Compounds. Environ. Technol. 2019, 40, 1178–1184. [Google Scholar] [CrossRef] [PubMed]
- Raheem, A.; Dupont, V.; Channa, A.Q.; Zhao, X.; Vuppaladadiyam, A.K.; Taufiq-Yap, Y.-H.; Zhao, M.; Harun, R. Parametric Characterization of Air Gasification of Chlorella vulgaris Biomass. Energy Fuels 2017, 31, 2959–2969. [Google Scholar] [CrossRef]
- Marangon, B.B.; Castro, J.D.S.; Assemany, P.P.; Machado, N.A.; Calijuri, M.L. Wastewater-Grown Microalgae Biomass as a Source of Sustainable Aviation Fuel: Life Cycle Assessment Comparing Hydrothermal Routes. J. Environ. Manag. 2024, 360, 121164. [Google Scholar] [CrossRef]
- Kenkel, P.; Wassermann, T.; Zondervan, E. Renewable Fuels from Integrated Power- and Biomass-to-X Processes: A Superstructure Optimization Study. Processes 2022, 10, 1298. [Google Scholar] [CrossRef]
- Yousefian, F.; Babatabar, M.A.; Eshaghi, M.; Poor, S.M.; Tavasoli, A. Pyrolysis of Rice Husk, Coconut Shell, and Cladophora Glomerata Algae and Application of the Produced Biochars as Support for Cobalt Catalyst in Fischer–Tropsch Synthesis. Fuel Process. Technol. 2023, 247, 107818. [Google Scholar] [CrossRef]
- Bayat, F.; Pirbazari, S.M.; Shojaei, N.; Kiani, S.; Tavasoli, A. Green Catalyst Innovation: Enhanced Fischer-Tropsch Synthesis Using Potassium-Promoted Cobalt Catalysts Supported on Pyrolyzed Peanut Shells and Cladophora Glomerata Modified Biochars. Fuel Process. Technol. 2024, 258, 108094. [Google Scholar] [CrossRef]
- Chiaramonti, D.; Talluri, G.; Scarlat, N.; Prussi, M. The Challenge of Forecasting the Role of Biofuel in EU Transport Decarbonisation at 2050: A Meta-Analysis Review of Published Scenarios. Renew. Sustain. Energy Rev. 2021, 139, 110715. [Google Scholar] [CrossRef]
- Sharma, V.; Hossain, A.K.; Duraisamy, G.; Griffiths, G. Microalgal Biodiesel: A Challenging Route toward a Sustainable Aviation Fuel. Fermentation 2023, 9, 907. [Google Scholar] [CrossRef]
- Velmozhina, K.; Shinkevich, P.; Zhazhkov, V.; Politaeva, N.; Korablev, V.; Vladimirov, I.; Morales, T.C. Production of Biohydrogen from Microalgae Biomass after Wastewater Treatment and Air Purification from CO2. Processes 2023, 11, 2978. [Google Scholar] [CrossRef]
- Falfushynska, H.; Zhadan, V.; Holz, M. Advancement and Assessment of Power-to-X Strategies as a Significant Contribution for the De-Fossilization of Economies. Universitäts- und Landesbibliothek Sachsen-Anhalt. Proc. Int. Conf. Appl. Innov. IT 2024, 12, 233–241. [Google Scholar] [CrossRef]
- Zhang, J.; Xue, D.; Wang, C.; Fang, D.; Cao, L.; Gong, C. Genetic Engineering for Biohydrogen Production from Microalgae. iScience 2023, 26, 107255. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Zhang, J.; Pan, M.; Hao, Y.; Hu, R.; Xiao, W.; Li, G.; Lyu, T. Valorisation of Microalgae Residues after Lipid Extraction: Pyrolysis Characteristics for Biofuel Production. Biochem. Eng. J. 2022, 179, 108330. [Google Scholar] [CrossRef]
- Wang, K.; Khoo, K.S.; Chew, K.W.; Selvarajoo, A.; Chen, W.-H.; Chang, J.-S.; Show, P.L. Microalgae: The Future Supply House of Biohydrogen and Biogas. Front. Energy Res. 2021, 9, 660399. [Google Scholar] [CrossRef]
- Falfushynska, H.; Holz, M. Power-to-X Strategies: A Key Driver for Decarbonization and Renewable Energy Integration in Economies. Lecture Notes in Networks and Systems. Lect. Notes Netw. Syst. 2024, in press. [Google Scholar]
- Chen, S.; Qu, D.; Xiao, X.; Miao, X. Biohydrogen Production with Lipid-Extracted Dunaliella Biomass and a New Strain of Hyper-Thermophilic Archaeon Thermococcus eurythermalis A501. Int. J. Hydrogen Energy 2020, 45, 12721–12730. [Google Scholar] [CrossRef]
- Wang, J.; Yin, Y. Fermentative Hydrogen Production Using Pretreated Microalgal Biomass as Feedstock. Microb. Cell Fact. 2018, 17, 22. [Google Scholar] [CrossRef]
- Mustafi, N.N.; Hossain, M.I.; Ahammad, M.F.; Naz, S. Biohydrogen Production from Euglena acus Microalgae Available in Bangladesh. MethodsX 2023, 10, 101976. [Google Scholar] [CrossRef]
- Biohydrogen: Affordable, Green and yet Overlooked. 2023. Available online: https://www.europeanbiogas.eu/biohydrogen-affordable-green-and-yet-overlooked (accessed on 4 October 2024).
- Jiao, H.; Tsigkou, K.; Elsamahy, T.; Pispas, K.; Sun, J.; Manthos, G.; Schagerl, M.; Sventzouri, E.; Al-Tohamy, R.; Kornaros, M.; et al. Recent Advances in Sustainable Hydrogen Production from Microalgae: Mechanisms, Challenges, and Future Perspectives. Ecotoxicol. Environ. Saf. 2024, 270, 115908. [Google Scholar] [CrossRef]
- Genç, Ş.; Koku, H. A Preliminary Techno-Economic Analysis of Photofermentative Hydrogen Production. Int. J. Hydrogen Energy 2024, 52, 212–222. [Google Scholar] [CrossRef]
- Somers, M.D.; Chen, P.; Clippinger, J.; Cruce, J.R.; Davis, R.; Lammers, P.J.; Quinn, J.C. Techno-Economic and Life-Cycle Assessment of Fuel Production from Mixotrophic Galdieria Sulphuraria Microalgae on Hydrolysate. Algal Res. 2021, 59, 102419. [Google Scholar] [CrossRef]
- Yang, D.-W.; Syn, J.-W.; Hsieh, C.-H.; Huang, C.-C.; Chien, L.-F. Genetically Engineered Hydrogenases Promote Biophotocatalysis-Mediated H2 Production in the Green Alga Chlorella sp. DT. Int. J. Hydrogen Energy 2019, 44, 2533–2545. [Google Scholar] [CrossRef]
- Min Woon, J.; Shiong Khoo, K.; Akermi, M.; Alanazi, M.M.; Wei Lim, J.; Jing Chan, Y.; Sean Goh, P.; Silas Chidi, B.; Kee Lam, M.; Zaini, J.; et al. Reviewing Biohydrogen Production from Microalgal Cells through Fundamental Mechanisms, Enzymes and Factors That Engendering New Challenges and Prospects. Fuel 2023, 346, 128312. [Google Scholar] [CrossRef]
- Babu Pallam, R.; Ramamoorthy, N.K.; Rakshit, S.; Jaiswal, K.K.; Sarma, V.V. Microalgae for the Sustainable Production of Biohydrogen. In Clean Energy Transition-via-Biomass Resource Utilization; Kumar, S., Sundaramurthy, S., Kumar, D., Chandel, A.K., Eds.; Green Energy and Technology; Springer Nature: Singapore, 2024; pp. 151–175. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Jagtap, S.S.; Bedekar, A.A.; Bhatia, R.K.; Rajendran, K.; Pugazhendhi, A.; Rao, C.V.; Atabani, A.E.; Kumar, G.; Yang, Y.-H. Renewable Biohydrogen Production from Lignocellulosic Biomass Using Fermentation and Integration of Systems with Other Energy Generation Technologies. Sci. Total Environ. 2021, 765, 144429. [Google Scholar] [CrossRef] [PubMed]
- Xia, A.; Cheng, J.; Song, W.; Su, H.; Ding, L.; Lin, R.; Lu, H.; Liu, J.; Zhou, J.; Cen, K. Fermentative Hydrogen Production Using Algal Biomass as Feedstock. Renew. Sustain. Energy Rev. 2015, 51, 209–230. [Google Scholar] [CrossRef]
- Kiehbadroudinezhad, M.; Merabet, A.; Ghenai, C.; Abo-Khalil, A.G.; Salameh, T. The Role of Biofuels for Sustainable MicrogridsF: A Path towards Carbon Neutrality and the Green Economy. Heliyon 2023, 9, e13407. [Google Scholar] [CrossRef]
- Navas, S.J.; Cabello González, G.M.; Pino, A.; Pino, F.J. Integration of Microgrids in Chemical Industries with Hydrogen as a Byproduct: Styrene Production Case Study. Int. J. Hydrogen Energy 2024, 59, 947–957. [Google Scholar] [CrossRef]
- Eloffy, M.G.; Elgarahy, A.M.; Saber, A.N.; Hammad, A.; El-Sherif, D.M.; Shehata, M.; Mohsen, A.; Elwakeel, K.Z. Biomass-to-Sustainable Biohydrogen: Insights into the Production Routes, and Technical Challenges. Chem. Eng. J. Adv. 2022, 12, 100410. [Google Scholar] [CrossRef]
- Mandalika, A.S.; Chou, K.J.; Decker, S.R. Biohydrogen: Prospects for Industrial Utilization and Energy Resiliency in Rural Communities. Front. Ind. Microbiol. 2024, 2, 1428686. [Google Scholar] [CrossRef]
- Biofuels Saved 9.7 Million Tonnes of CO2 Equivalent in 2019. Available online: https://www.ble.de/SharedDocs/Meldungen/EN/2021/210226_biofuels.html (accessed on 4 October 2024).
- Final Report Summary-BIOALGAESORB. Available online: https://cordis.europa.eu/project/id/243752/reporting/it (accessed on 4 October 2024).
- Biofuels Dashboard 2023. Available online: https://www.ifpenergiesnouvelles.com/article/biofuels-dashboard-2023 (accessed on 4 October 2024).
- Watson, M.J.; Machado, P.G.; Da Silva, A.V.; Saltar, Y.; Ribeiro, C.O.; Nascimento, C.A.O.; Dowling, A.W. Sustainable Aviation Fuel Technologies, Costs, Emissions, Policies, and Markets: A Critical Review. J. Clean. Prod. 2024, 449, 141472. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Falfushynska, H. Advancements and Prospects in Algal Biofuel Production: A Comprehensive Review. Phycology 2024, 4, 548-575. https://doi.org/10.3390/phycology4040030
Falfushynska H. Advancements and Prospects in Algal Biofuel Production: A Comprehensive Review. Phycology. 2024; 4(4):548-575. https://doi.org/10.3390/phycology4040030
Chicago/Turabian StyleFalfushynska, Halina. 2024. "Advancements and Prospects in Algal Biofuel Production: A Comprehensive Review" Phycology 4, no. 4: 548-575. https://doi.org/10.3390/phycology4040030
APA StyleFalfushynska, H. (2024). Advancements and Prospects in Algal Biofuel Production: A Comprehensive Review. Phycology, 4(4), 548-575. https://doi.org/10.3390/phycology4040030





