Biogeochemical Markers to Identify Spatiotemporal Gradients of Phytoplankton across Estuaries
Abstract
:1. Introduction
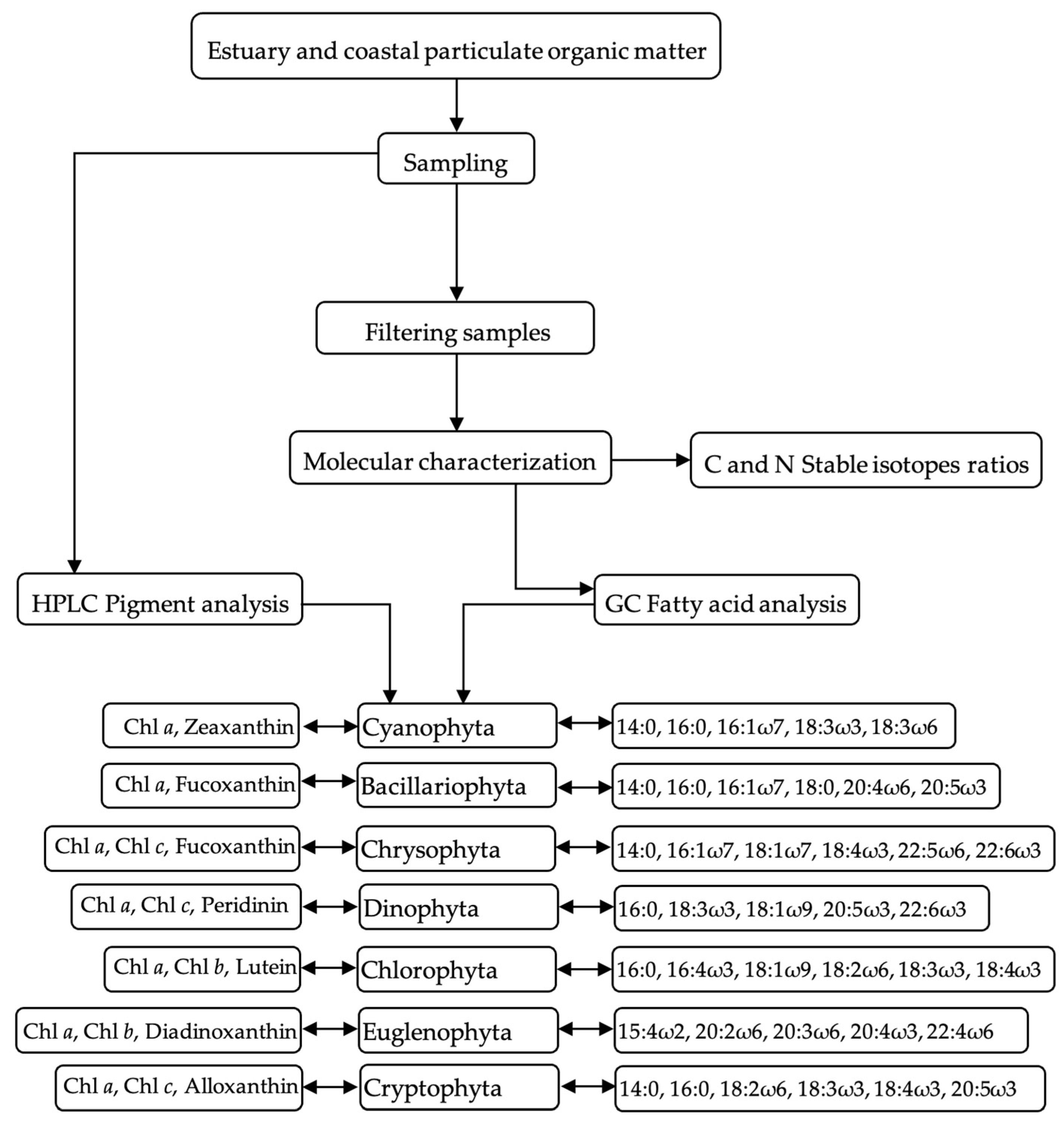
2. Methods
3. In Situ Measurements and Phytoplankton Composition
3.1. Fatty Acids to Identify Phytoplankton Functional Groups
3.2. Pigments to Identify Phytoplankton Groups
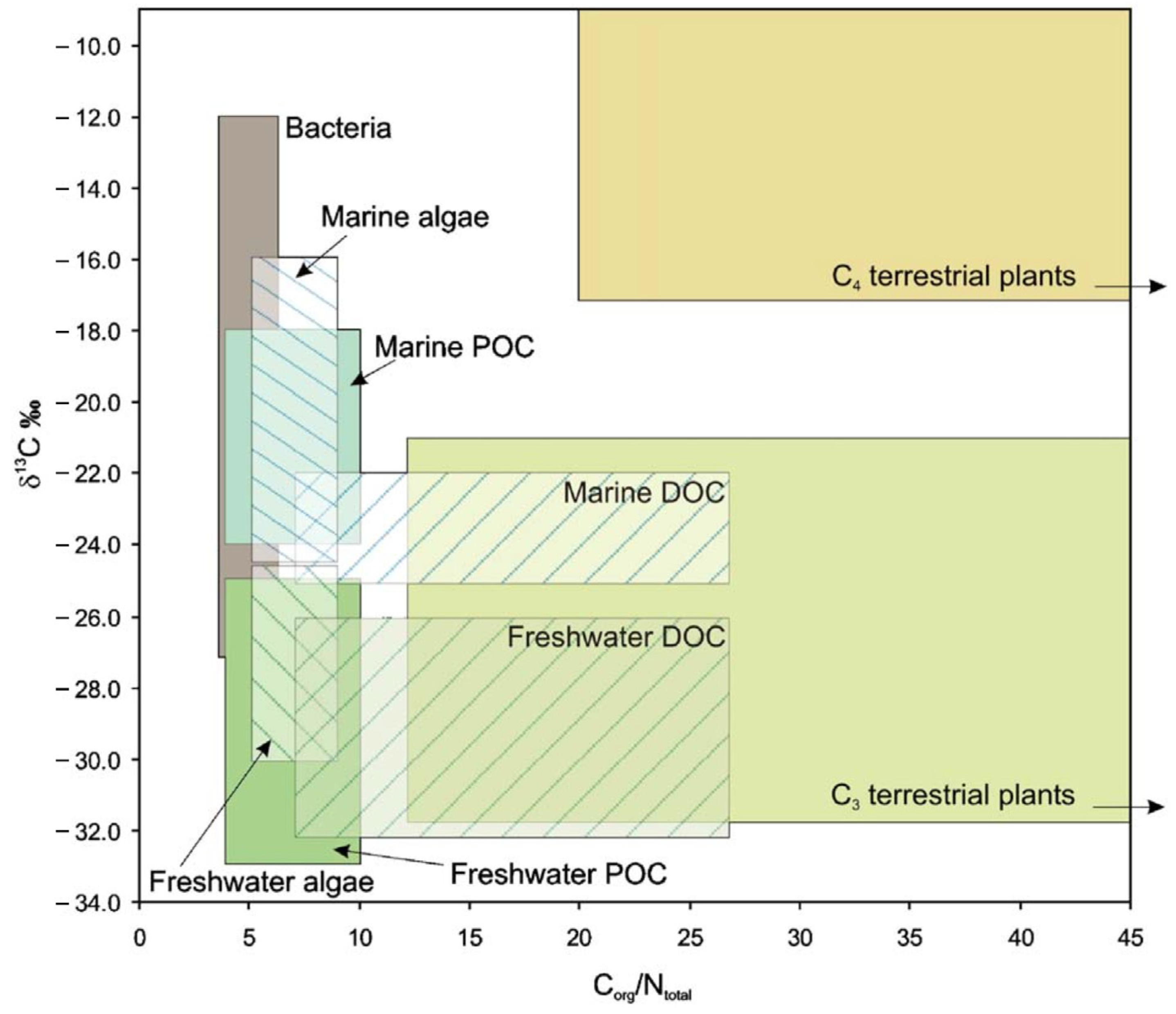
3.3. Stable Isotopes Assess Contribution of Terrestrial Inputs
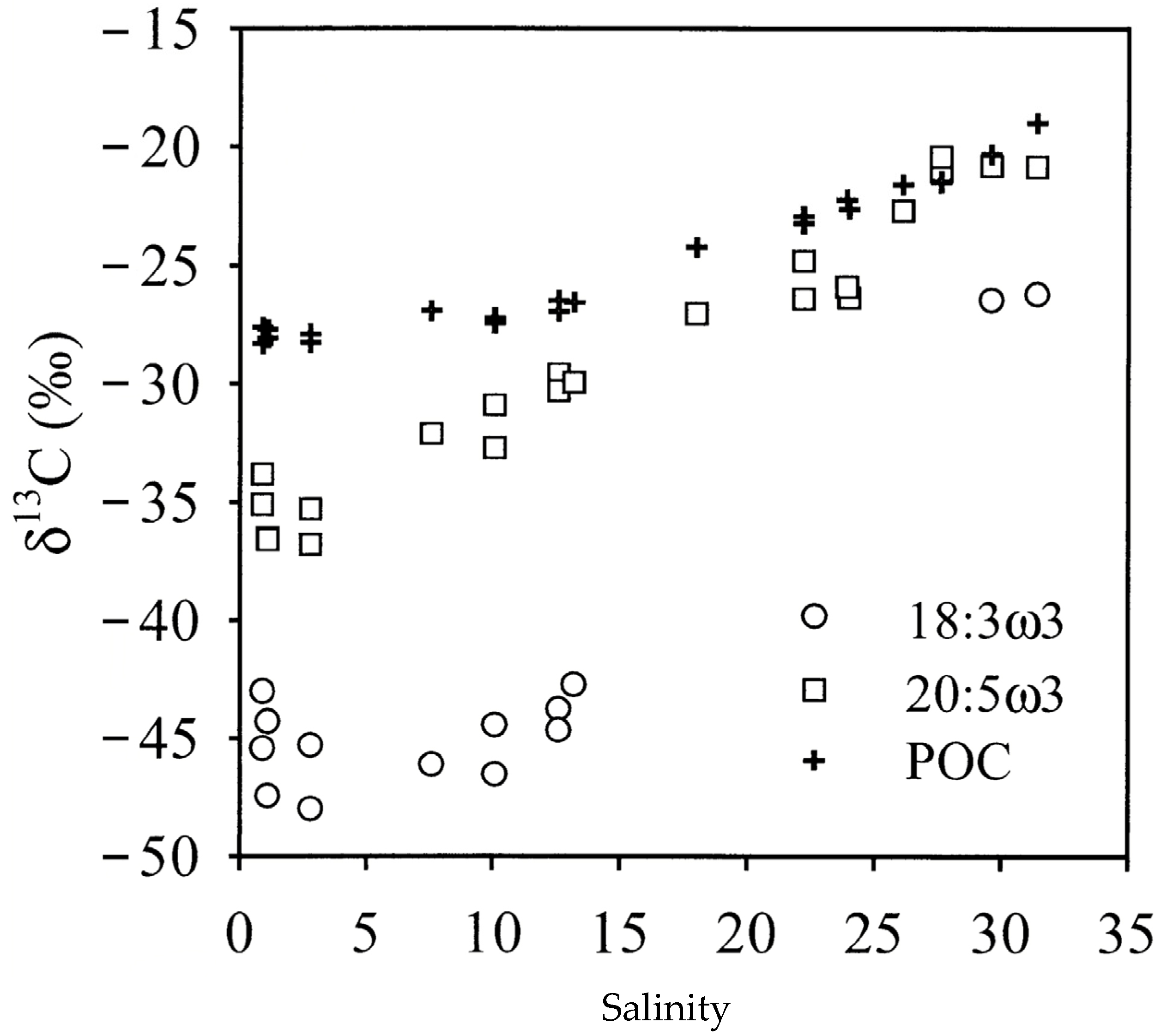
3.4. Combining Pigments, Fatty Acids Biomarkers and Stable Isotopes
3.5. Factors Influencing Phytoplankton Distribution
3.5.1. Seasonal Variability of Phytoplankton Groups
3.5.2. Salinity
3.5.3. Turbidity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cloern, J.E.; Foster, S.Q.; Kleckner, A.E. Phytoplankton primary production in the world’s estuarine-coastal ecosystems. Biogeosciences 2014, 11, 2477–2501. [Google Scholar] [CrossRef]
- Geyer, W.R.; Hill, P.S.; Kineke, G.C. The transport, transformation and dispersal of sediment by buoyant coastal flows. Cont. Shelf Res. 2004, 24, 927–949. [Google Scholar] [CrossRef]
- Conroy, T.; Sutherland, D.A.; Ralston, D.K. Estuarine Exchange Flow Variability in a Seasonal, Segmented Estuary. J. Phys. Oceanogr. 2020, 50, 595–613. [Google Scholar] [CrossRef]
- Humborg, C.; Danielsson, Å.; Sjöberg, B.; Green, M. Nutrient land–sea fluxes in oligothrophic and pristine estuaries of the Gulf of Bothnia, Baltic Sea. Estuar. Coast. Shelf Sci. 2003, 56, 781–793. [Google Scholar] [CrossRef]
- Inoue, M.; Wiseman, W.J. Transport, Mixing and Stirring Processes in a Louisiana Estuary: A Model Study. Estuar. Coast. Shelf Sci. 2000, 50, 449–466. [Google Scholar] [CrossRef]
- Kitheka, J.U.; Obiero, M.; Nthenge, P. River discharge, sediment transport and exchange in the Tana Estuary, Kenya. Estuar. Coast. Shelf Sci. 2005, 63, 455–468. [Google Scholar] [CrossRef]
- Osadchiev, A.; Medvedev, I.; Shchuka, S.; Kulikov, M.; Spivak, E.; Pisareva, M.; Semiletov, I. Influence of estuarine tidal mixing on structure and spatial scales of large river plumes. Ocean Sci. 2020, 16, 781–798. [Google Scholar] [CrossRef]
- Wei, X.; Kumar, M.; Schuttelaars, H.M. Three-Dimensional Salt Dynamics in Well-Mixed Estuaries: Influence of Estuarine Convergence, Coriolis, and Bathymetry. J. Phys. Oceanogr. 2017, 47, 1843–1871. [Google Scholar] [CrossRef]
- Fisher, T.R.; Harding, L.W., Jr.; Stanley, D.W.; Ward, L.G. Phytoplankton, nutrients, and turbidity in the Chesapeake, Delaware, and Hudson estuaries. Estuar. Coast. Shelf Sci. 1988, 27, 61–93. [Google Scholar] [CrossRef]
- Chen, C.Y.; Buckman, K.L.; Shaw, A.; Curtis, A.; Taylor, M.; Montesdeoca, M.; Driscoll, C. The influence of nutrient loading on methylmercury availability in Long Island estuaries. Environ. Pollut. 2021, 268. [Google Scholar] [CrossRef]
- Sanders, T.; Schöl, A.; Dähnke, K. Hot Spots of Nitrification in the Elbe Estuary and Their Impact on Nitrate Regeneration. Estuaries Coasts 2017, 41, 128–138. [Google Scholar] [CrossRef]
- McGovern, M.; Pavlov, A.K.; Deininger, A.; Granskog, M.A.; Leu, E.; Søreide, J.E.; Poste, A.E. Terrestrial Inputs Drive Seasonality in Organic Matter and Nutrient Biogeochemistry in a High Arctic Fjord System (Isfjorden, Svalbard). Front. Mar. Sci. 2020, 7. [Google Scholar] [CrossRef]
- Reese, L.; Gräwe, U.; Klingbeil, K.; Li, X.; Lorenz, M.; Burchard, H. Local Mixing Determines Spatial Structure of Diahaline Exchange Flow in a Mesotidal Estuary: A Study of Extreme Runoff Conditions. J. Phys. Oceanogr. 2024, 54, 3–27. [Google Scholar] [CrossRef]
- Stefánsson, U.; Richards, F.A. Processes contributing to the nutrient distribution off the columbia river and strait of juan de fuca 1. Limnol. Oceanogr. 1963, 8, 394–410. [Google Scholar] [CrossRef]
- Ouyang, W.; Wang, R.; Ji, K.; Liu, X.; Geng, F.; Hao, X.; Lin, C. Phytoplankton biomass dynamics with diffuse terrestrial nutrients pollution discharge into bay. Chemosphere 2023, 313, 137674. [Google Scholar] [CrossRef]
- Adyasari, D.; Oehler, T.; Afiati, N.; Moosdorf, N. Groundwater nutrient inputs into an urbanized tropical estuary system in Indonesia. Sci. Total Environ. 2018, 627, 1066–1079. [Google Scholar] [CrossRef]
- Kaul, L.W.; Froelich, P.N. Modeling estuarine nutrient geochemistry in a simple system. Geochim. Cosmochim. Acta 1984, 48, 1417–1433. [Google Scholar] [CrossRef]
- Seitzinger, S.P.; Sanders, R.W. Atmospheric inputs of dissolved organic nitrogen stimulate estuarine bacteria and phytoplankton. Limnol. Oceanogr. 1999, 44, 721–730. [Google Scholar] [CrossRef]
- Vendramini, J.; Silveira, M.; Dubeux, J., Jr.; Sollenberger, L. Environmental impacts and nutrient recycling on pastures grazed by cattle. Rev. Bras. Zootec. 2007, 36, 139–149. [Google Scholar] [CrossRef]
- Bull, E.; Cunha, C.d.L.; Scudelari, A. Water quality impact from shrimp farming effluents in a tropical estuary. Water Sci. Technol. 2021, 83, 123–136. [Google Scholar] [CrossRef]
- Conrad, S.R.; Santos, I.R.; White, S.; Sanders, C.J. Nutrient and Trace Metal Fluxes into Estuarine Sediments Linked to Historical and Expanding Agricultural Activity (Hearnes Lake, Australia). Estuaries Coasts 2019, 42, 944–957. [Google Scholar] [CrossRef]
- Bukaveckas, P.A.; Isenberg, W. Loading, transformation, and retention of nitrogen and phosphorus in the tidal freshwater James River (Virginia). Estuaries Coasts 2013, 36, 1219–1236. [Google Scholar] [CrossRef]
- Paczkowska, J.; Brugel, S.; Rowe, O.; Lefébure, R.; Brutemark, A.; Andersson, A. Response of Coastal Phytoplankton to High Inflows of Terrestrial Matter. Front. Mar. Sci. 2020, 7. [Google Scholar] [CrossRef]
- Geider, R.J.; Delucia, E.H.; Falkowski, P.G.; Finzi, A.C.; Grime, J.P.; Grace, J.; Kana, T.M.; La Roche, J.; Long, S.P.; Osborne, B.A.; et al. Primary productivity of planet earth: Biological determinants and physical constraints in terrestrial and aquatic habitats. Glob. Chang. Biol. 2001, 7, 849–882. [Google Scholar] [CrossRef]
- Dzwonkowski, B.; Greer, A.T.; Briseño-Avena, C.; Krause, J.W.; Soto, I.M.; Hernandez, F.J.; Deary, A.L.; Wiggert, J.D.; Joung, D.; Fitzpatrick, P.J.; et al. Estuarine influence on biogeochemical properties of the Alabama shelf during the fall season. Cont. Shelf Res. 2017, 140, 96–109. [Google Scholar] [CrossRef]
- Davis, K.A.; Banas, N.S.; Giddings, S.N.; Siedlecki, S.A.; MacCready, P.; Lessard, E.J.; Kudela, R.M.; Hickey, B.M. Estuary-enhanced upwelling of marine nutrients fuels coastal productivity in the U.S. Pacific Northwest. J. Geophys. Res. Ocean. 2014, 119, 8778–8799. [Google Scholar] [CrossRef]
- Maier, G.O.; Toft, J.D.; Simenstad, C.A. Variability in Isotopic (δ13C, δ15N, δ34S) Composition of Organic Matter Contributing to Detritus-Based Food Webs of the Columbia River Estuary. Northwest Sci. 2011, 85, 41–54. [Google Scholar] [CrossRef]
- Gardade, L.; Khandeparker, L.; Desai, D.V.; Atchuthan, P.; Anil, A.C. Fatty acids as indicators of sediment organic matter dynamics in a monsoon-influenced tropical estuary. Ecol. Indic. 2021, 130, 108014. [Google Scholar] [CrossRef]
- Napolitano, G.E.; Pollero, R.J.; Gayoso, A.M.; Macdonald, B.A.; Thompson, R.J. Fatty acids as trophic markers of phytoplankton blooms in the Bahia Blanca estuary (Buenos Aires, Argentina) and in Trinity Bay (Newfoundland, Canada). Biochem. Syst. Ecol. 1997, 25, 739–755. [Google Scholar] [CrossRef]
- Cañavate, J.-P.; van Bergeijk, S.; González-Ortegón, E.; Vílas, C. Contrasting fatty acids with other indicators to assess nutritional status of suspended particulate organic matter in a turbid estuary. Estuar. Coast. Shelf Sci. 2021, 254, 107329. [Google Scholar] [CrossRef]
- Antonio, E.S.; Richoux, N.B. Tide-Induced Variations in the Fatty Acid Composition of Estuarine Particulate Organic Matter. Estuaries Coasts 2015, 39, 1072–1083. [Google Scholar] [CrossRef]
- Cañavate, J.-P.; van Bergeijk, S.; Giráldez, I.; González-Ortegón, E.; Vílas, C. Fatty Acids to Quantify Phytoplankton Functional Groups and Their Spatiotemporal Dynamics in a Highly Turbid Estuary. Estuaries Coasts 2019, 42, 1971–1990. [Google Scholar] [CrossRef]
- Fischer, A.M.; Ryan, J.P.; Levesque, C.; Welschmeyer, N. Characterizing estuarine plume discharge into the coastal ocean using fatty acid biomarkers and pigment analysis. Mar. Environ. Res. 2014, 99, 106–116. [Google Scholar] [CrossRef]
- Kopprio, G.A.; Dutto, M.S.; Garzón Cardona, J.E.; Gärdes, A.; Lara, R.J.; Graeve, M. Biogeochemical markers across a pollution gradient in a Patagonian estuary: A multidimensional approach of fatty acids and stable isotopes. Mar. Pollut. Bull. 2018, 137, 617–626. [Google Scholar] [CrossRef]
- Ruiz-Ruiz, P.A.; Contreras, S.; Urzúa, Á.; Quiroga, E.; Rebolledo, L. Fatty acid biomarkers in three species inhabiting a high latitude Patagonian fjord (Yendegaia Fjord, Chile). Polar Biol. 2021, 44, 147–162. [Google Scholar] [CrossRef]
- Pang, S.Y.; Suratman, S.; Fadzil, M.F.; Tan, H.S.; Le, D.Q.; Mostapa, R.; Simoneit, B.R.T.; Tahir, N.M. Lipid biomarkers, stable C and N isotope ratios coupled with multivariate statistics as sources indicators in coastal sediments of Brunei Bay, Southern South China Sea. Reg. Stud. Mar. Sci. 2021, 48, 11. [Google Scholar] [CrossRef]
- Chuecas, L.; Riley, J.P. Component Fatty Acids of the Total Lipids of Some Marine Phytoplankton. J. Mar. Biol. Assoc. UK 1969, 49, 97–116. [Google Scholar] [CrossRef]
- DeMort, C.L.; Lowry, R.; Tinsley, I.; Phinney, H. The biochemical analysis of some estuarine phytoplankton species. i. fatty acid composition 1 2. J. Phycol. 1972, 8, 211–216. [Google Scholar] [CrossRef]
- Bosley, K.M.; Copeman, L.A.; Dumbauld, B.R.; Bosley, K.L. Identification of burrowing shrimp food sources along an estuarine gradient using fatty acid analysis and stable isotope ratios. Estuaries Coasts 2017, 40, 1113–1130. [Google Scholar] [CrossRef]
- Richoux, N.B.; Froneman, P.W. Trophic ecology of dominant zooplankton and macrofauna in a temperate, oligotrophic South African estuary: A fatty acid approach. Mar. Ecol. Prog. Ser. 2008, 357, 121–137. [Google Scholar] [CrossRef]
- Sofía Dutto, M.; Kopprio, G.A.; Hoffmeyer, M.S.; Alonso, T.S.; Graeve, M.; Kattner, G. Planktonic trophic interactions in a human-impacted estuary of Argentina: A fatty acid marker approach. J. Plankton Res. 2014, 36, 776–787. [Google Scholar] [CrossRef]
- Kelly, J.R.; Scheibling, R.E. Fatty acids as dietary tracers in benthic food webs. Mar. Ecol. Prog. Ser. 2012, 446, 1–22. [Google Scholar] [CrossRef]
- Dijkman, N.A.; Kromkamp, J.C. Phospholipid-derived fatty acids as chemotaxonomic markers for phytoplankton: Application for inferring phytoplankton composition. Mar. Ecol. Prog. Ser. 2006, 324, 113–125. [Google Scholar] [CrossRef]
- Jónasdóttir, S.H. Fatty acid profiles and production in marine phytoplankton. Mar. Drugs 2019, 17, 151. [Google Scholar] [CrossRef]
- Shilla, D.; Routh, J. Using biochemical and isotopic tracers to characterise organic matter sources and their incorporation into estuarine food webs (Rufiji delta, Tanzania). Chem. Ecol. 2017, 33, 893–917. [Google Scholar] [CrossRef]
- Taipale, S.J.; Peltomaa, E.; Hiltunen, M.; Jones, R.I.; Hahn, M.W.; Biasi, C.; Brett, M.T. Inferring phytoplankton, terrestrial plant and bacteria bulk δ¹³C values from compound specific analyses of lipids and fatty acids. PLoS ONE 2015, 10, e0133974. [Google Scholar] [CrossRef]
- Špoljarić, I.V.; Novak, T.; Gašparović, B.; Kazazić, S.P.; Čanković, M.; Ljubešić, Z.; Hrustić, E.; Mlakar, M.; Du, J.; Zhang, R. Impact of environmental conditions on phospholipid fatty acid composition: Implications from two contrasting estuaries. Aquat. Ecol. 2021, 55, 1–20. [Google Scholar] [CrossRef]
- Schnurr, P.J.; Drever, M.C.; Kling, H.J.; Elner, R.W.; Arts, M.T. Seasonal changes in fatty acid composition of estuarine intertidal biofilm: Implications for western sandpiper migration. Estuar. Coast. Shelf Sci. 2019, 224, 94–107. [Google Scholar] [CrossRef]
- Lee, M.; Won, N.-I.; Baek, S.H. Comparison of HPLC Pigment Analysis and Microscopy in Phytoplankton Assessment in the Seomjin River Estuary, Korea. Sustainability 2020, 12, 1675. [Google Scholar] [CrossRef]
- Strandberg, U.; Taipale, S.J.; Hiltunen, M.; Galloway, A.W.E.; Brett, M.T.; Kankaala, P. Inferring phytoplankton community composition with a fatty acid mixing model. Ecosphere 2015, 6, art16. [Google Scholar] [CrossRef]
- Boschker, H.T.S.; Kromkamp, J.C.; Middelburg, J.J. Biomarker and carbon isotopic constraints on bacterial and algal community structure and functioning in a turbid, tidal estuary. Limnol. Oceanogr. 2005, 50, 70–80. [Google Scholar] [CrossRef]
- Wright, S.W.; Jeffrey, S. Pigment Markers for Phytoplankton Production. In Marine Organic Matter: Biomarkers, Isotopes and DNA; Springer: Berlin/Heidelberg, Germany, 2006; pp. 71–104. [Google Scholar]
- Dursun, F.; Tas, S.; Ediger, D. Assessment of phytoplankton group composition in the Golden Horn Estuary (Sea of Marmara, Turkey) determined with pigments measured by HPLC-CHEMTAX analyses and microscopy. J. Mar. Biol. Assoc. UK 2021, 101, 649–665. [Google Scholar] [CrossRef]
- Damar, A.; Colijn, F.; Hesse, K.-J.; Kurniawan, F. Coastal Phytoplankton Pigments Composition in Three Tropical Estuaries of Indonesia. J. Mar. Sci. Eng. 2020, 8, 311. [Google Scholar] [CrossRef]
- Chai, C.; Jiang, T.; Cen, J.; Ge, W.; Lu, S. Phytoplankton pigments and functional community structure in relation to environmental factors in the Pearl River Estuary. Oceanologia 2016, 58, 201–211. [Google Scholar] [CrossRef]
- Laza-Martinez, A.; Seoane, S.; Zapata, M.; Orive, E. Phytoplankton pigment patterns in a temperate estuary: From unialgal cultures to natural assemblages. J. Plankton Res. 2007, 29, 913–929. [Google Scholar] [CrossRef]
- Wright, S.; Jeffrey, S.; Mantoura, R. Phytoplankton Pigments in Oceanography: Guidelines to Modern Methods; Unesco Pub.: Paris, France, 2005. [Google Scholar]
- Gibb, S.W.; Cummings, D.G.; Irigoien, X.; Barlow, R.G.; Fauzi, R.; Mantoura, C. Phytoplankton pigment chemotaxonomy of the northeastern Atlantic. Deep Sea Res. Part II Top. Stud. Oceanogr. 2001, 48, 795–823. [Google Scholar] [CrossRef]
- Vijayan, A.K.; Reddy, B.B.; Sudheesh, V.; Marathe, P.H.; Nampoothiri, V.N.; Harikrishnachari, N.V.; Kavya, P.; Gupta, G.V.M.; Ramanamurthy, M.V. Phytoplankton community structure in a contrasting physico-chemical regime along the eastern Arabian Sea during the winter monsoon. J. Mar. Syst. 2021, 215. [Google Scholar] [CrossRef]
- Schmidt, H.-L.; Robins, R.J.; Werner, R.A. Multi-factorial in vivo stable isotope fractionation: Causes, correlations, consequences and applications. Isot. Environ. Health Stud. 2015, 51, 155–199. [Google Scholar] [CrossRef] [PubMed]
- Benner, R. What happens to terrestrial organic matter in the ocean? Mar. Chem. 2004, 92, 307–310. [Google Scholar] [CrossRef]
- Botto, F.; Gaitán, E.; Mianzan, H.; Acha, M.; Giberto, D.; Schiariti, A.; Iribarne, O. Origin of resources and trophic pathways in a large SW Atlantic estuary: An evaluation using stable isotopes. Estuar. Coast. Shelf Sci. 2011, 92, 70–77. [Google Scholar] [CrossRef]
- Hoefs, J. Stable Isotope Geochemistry; Springer: Berlin, Germany, 1997. [Google Scholar]
- De Laeter, J.R.; Böhlke, J.K.; De Bievre, P.; Hidaka, H.; Peiser, H.; Rosman, K.; Taylor, P. Atomic weights of the elements. Review 2000 (IUPAC Technical Report). Pure Appl. Chem. 2003, 75, 683–800. [Google Scholar] [CrossRef]
- Berg, T.; Strand, D.H. 13C labelled internal standards—A solution to minimize ion suppression effects in liquid chromatography–tandem mass spectrometry analyses of drugs in biological samples? J. Chromatogr. A 2011, 1218, 9366–9374. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D. δ15N as an integrator of the nitrogen cycle. Trends Ecol. Evol. 2001, 16, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Boschker, H.; Middelburg, J. Stable isotopes and biomarkers in microbial ecology. FEMS Microbiol. Ecol. 2002, 40, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Guo, W.; Wei, G.; Jia, G. The Sources and Transformations of Dissolved Organic Matter in the Pearl River Estuary, China, as Revealed by Stable Isotopes. J. Geophys. Res. Ocean. 2018, 123, 6893–6908. [Google Scholar] [CrossRef]
- Alfaro, A.C.; Thomas, F.; Sergent, L.; Duxbury, M. Identification of trophic interactions within an estuarine food web (northern New Zealand) using fatty acid biomarkers and stable isotopes. Estuar. Coast. Shelf Sci. 2006, 70, 271–286. [Google Scholar] [CrossRef]
- Cloern, J.E.; Canuel, E.A.; Harris, D. Stable carbon and nitrogen isotope composition of aquatic and terrestrial plants of the San Francisco Bay estuarine system. Limnol. Oceanogr. 2002, 47, 713–729. [Google Scholar] [CrossRef]
- Lamb, A.L.; Wilson, G.P.; Leng, M.J. A review of coastal palaeoclimate and relative sea-level reconstructions using δ13C and C/N ratios in organic material. Earth-Sci. Rev. 2006, 75, 29–57. [Google Scholar] [CrossRef]
- Bergamino, L.; Dalu, T.; Richoux, N.B. Evidence of spatial and temporal changes in sources of organic matter in estuarine sediments: Stable isotope and fatty acid analyses. Hydrobiologia 2014, 732, 133–145. [Google Scholar] [CrossRef]
- Hu, J.; Peng, P.A.; Jia, G.; Mai, B.; Zhang, G. Distribution and sources of organic carbon, nitrogen and their isotopes in sediments of the subtropical Pearl River estuary and adjacent shelf, Southern China. Mar. Chem. 2006, 98, 274–285. [Google Scholar] [CrossRef]
- McIntosh, H.A.; McNichol, A.P.; Xu, L.; Canuel, E.A. Source—Age dynamics of estuarine particulate organic matter using fatty acid δ13C and Δ14C composition. Limnol. Oceanogr. 2015, 60, 611–628. [Google Scholar] [CrossRef]
- Wissel, B.; Gaçe, A.; Fry, B. Tracing river influences on phytoplankton dynamics in two louisiana estuaries. Ecology 2005, 86, 2751–2762. [Google Scholar] [CrossRef]
- Bargu, S.; Justic, D.; White, J.R.; Lane, R.; Day, J.; Paerl, H.; Raynie, R. Mississippi River diversions and phytoplankton dynamics in deltaic Gulf of Mexico estuaries: A review. Estuar. Coast. Shelf Sci. 2019, 221, 39–52. [Google Scholar] [CrossRef]
- Pulina, S.; Satta, C.T.; Padedda, B.M.; Sechi, N.; Lugliè, A. Seasonal variations of phytoplankton size structure in relation to environmental variables in three Mediterranean shallow coastal lagoons. Estuar. Coast. Shelf Sci. 2018, 212, 95–104. [Google Scholar] [CrossRef]
- Dijkstra, Y.M.; Chant, R.J.; Reinfelder, J.R. Factors controlling seasonal phytoplankton dynamics in the Delaware River Estuary: An idealized model study. Estuaries Coasts 2019, 42, 1839–1857. [Google Scholar] [CrossRef]
- Liu, B.; D’Sa, E.J.; Maiti, K.; Rivera-Monroy, V.H.; Xue, Z. Biogeographical trends in phytoplankton community size structure using adaptive sentinel 3-OLCI chlorophyll a and spectral empirical orthogonal functions in the estuarine-shelf waters of the northern Gulf of Mexico. Remote Sens. Environ. 2021, 252, 112154. [Google Scholar] [CrossRef]
- Carstensen, J.; Klais, R.; Cloern, J.E. Phytoplankton blooms in estuarine and coastal waters: Seasonal patterns and key species. Estuar. Coast. Shelf Sci. 2015, 162, 98–109. [Google Scholar] [CrossRef]
- Ge, J.; Torres, R.; Chen, C.; Liu, J.; Xu, Y.; Bellerby, R.; Shen, F.; Bruggeman, J.; Ding, P. Influence of suspended sediment front on nutrients and phytoplankton dynamics off the Changjiang Estuary: A FVCOM-ERSEM coupled model experiment. J. Mar. Syst. 2020, 204, 103292. [Google Scholar] [CrossRef]
- Labry, C.; Herbland, A.; Delmas, D.; Laborde, P.; Lazure, P.; Froidefond, J.; Jegou, A.-M.; Sautour, B. Initiation of winter phytoplankton blooms within the Gironde plume waters in the Bay of Biscay. Mar. Ecol. Prog. Ser. 2001, 212, 117–130. [Google Scholar] [CrossRef]
- Moschonas, G.; Gowen, R.J.; Paterson, R.F.; Mitchell, E.; Stewart, B.M.; McNeill, S.; Glibert, P.M.; Davidson, K. Nitrogen dynamics and phytoplankton community structure: The role of organic nutrients. Biogeochemistry 2017, 134, 125–145. [Google Scholar] [CrossRef]
- Soria-Píriz, S.; García-Robledo, E.; Papaspyrou, S.; Aguilar, V.; Seguro, I.; Acuña, J.; Morales, Á.; Corzo, A. Size fractionated phytoplankton biomass and net metabolism along a tropical estuarine gradient. Limnol. Oceanogr. 2017, 62, S309–S326. [Google Scholar] [CrossRef]
- Jiang, Z.; Chen, J.; Zhou, F.; Shou, L.; Chen, Q.; Tao, B.; Yan, X.; Wang, K. Controlling factors of summer phytoplankton community in the Changjiang (Yangtze River) Estuary and adjacent East China Sea shelf. Cont. Shelf Res. 2015, 101, 71–84. [Google Scholar] [CrossRef]
- Cañavate, J.-P.; Fernández-Díaz, C. Salinity induces unique changes in lipid classes and fatty acids of the estuarine haptophyte Diacronema vlkianum. Eur. J. Phycol. 2021, 57, 297–317. [Google Scholar] [CrossRef]
- Viličić, D.; Terzić, S.; Ahel, M.; Burić, Z.; Jasprica, N.; Carić, M.; Mihalić, K.C.; Olujić, G. Phytoplankton abundance and pigment biomarkers in the oligotrophic, eastern Adriatic estuary. Environ. Monit. Assess. 2008, 142, 199–218. [Google Scholar] [CrossRef] [PubMed]
- Lovelock, C.E.; Feller, I.C.; Ellis, J.; Schwarz, A.M.; Hancock, N.; Nichols, P.; Sorrell, B. Mangrove growth in New Zealand estuaries: The role of nutrient enrichment at sites with contrasting rates of sedimentation. Oecologia 2007, 153, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Lancelot, C.; Muylaert, K. 7.02 Trends in estuarine phytoplankton ecology. In Treatise on Estuarine and Coastal Science; Academic Press: Waltham, MA, USA, 2011; pp. 5–15. [Google Scholar]
- O’Meara, T.A.; Hillman, J.R.; Thrush, S.F. Rising tides, cumulative impacts and cascading changes to estuarine ecosystem functions. Sci. Rep. 2017, 7, 10218. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, H.; Lin, J.; Zhu, J.; Zhang, W.; Li, C. Phytoplankton Blooms off a High Turbidity Estuary: A Case Study in the Changjiang River Estuary. J. Geophys. Res. Ocean. 2019, 124, 8036–8059. [Google Scholar] [CrossRef]
- Moisan, T.A.; Rufty, K.M.; Moisan, J.R.; Linkswiler, M.A. Satellite Observations of Phytoplankton Functional Type Spatial Distributions, Phenology, Diversity, and Ecotones. Front. Mar. Sci. 2017, 4. [Google Scholar] [CrossRef]
| Phytoplankton Group | Fatty Acid Biomarker | Reference |
|---|---|---|
| Diatoms | 16:1ω7, 20:5ω3 | [34,43,44] |
| Chlorophytes | 16:4ω3, 18:1ω9, 18:2ω6, 18:3ω3 | [38,45] |
| Cryptophytes | 18:3ω3, 18:4ω4, 20:5ω6 | [42,43,46] |
| Cyanobacteria | 16:1ω7, 18:3ω6, 20:5ω6 | [46] |
| Dinoflagellates | 18:5ω3, 22:6ω3 | [34,43] |
| Vascular plants | 20:0, 22:0, 23:0, 24:0 | [46] |
| Pigment | Phytoplankton Groups | Reference |
|---|---|---|
| Chlorophyll a | All—except Prochlorophytes | [49,53,54] |
| Fucoxanthin, Chlorophyll c | Diatoms | [54] |
| Peridinin, Chlorophyll c | Dinoflagellates | [54] |
| Lutein | Chlorophytes | [54,55,59] |
| Alloxanthin | Cryptophytes | [53] |
| Prasinoxanthin | Prasinophytes | [55,56] |
| Zeaxanthin | Cyanobacteria |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Egoda Gamage, A.; Fischer, A.M.; Nichols, D.S.; Lee Chang, K.J. Biogeochemical Markers to Identify Spatiotemporal Gradients of Phytoplankton across Estuaries. Coasts 2024, 4, 469-481. https://doi.org/10.3390/coasts4030024
Egoda Gamage A, Fischer AM, Nichols DS, Lee Chang KJ. Biogeochemical Markers to Identify Spatiotemporal Gradients of Phytoplankton across Estuaries. Coasts. 2024; 4(3):469-481. https://doi.org/10.3390/coasts4030024
Chicago/Turabian StyleEgoda Gamage, Anushka, Andrew M. Fischer, David S. Nichols, and Kim Jye Lee Chang. 2024. "Biogeochemical Markers to Identify Spatiotemporal Gradients of Phytoplankton across Estuaries" Coasts 4, no. 3: 469-481. https://doi.org/10.3390/coasts4030024
APA StyleEgoda Gamage, A., Fischer, A. M., Nichols, D. S., & Lee Chang, K. J. (2024). Biogeochemical Markers to Identify Spatiotemporal Gradients of Phytoplankton across Estuaries. Coasts, 4(3), 469-481. https://doi.org/10.3390/coasts4030024








