Biologics, Small Molecules and More in Inflammatory Bowel Disease: The Present and the Future
Abstract
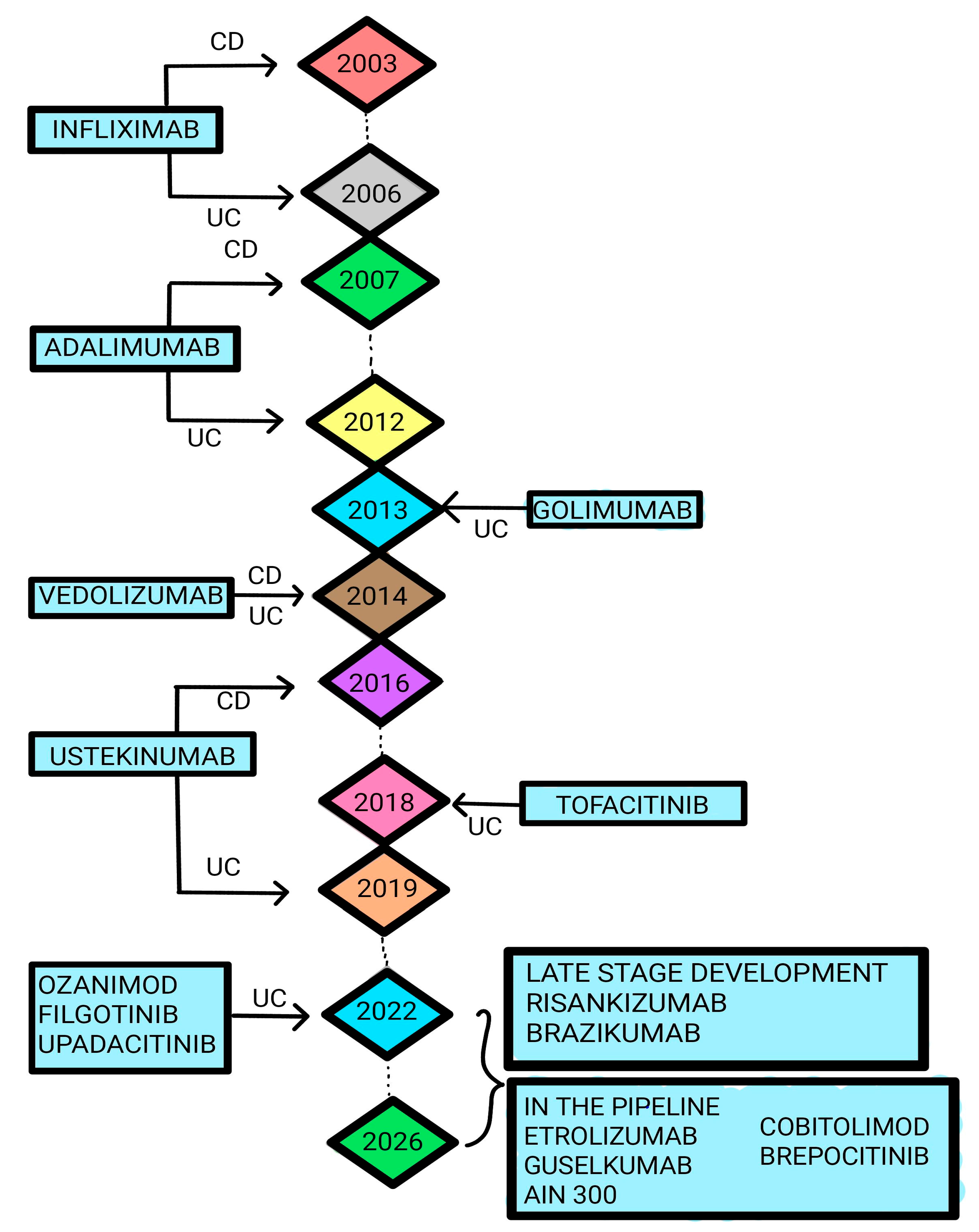
:1. Introduction
2. Materials and Methods
3. Anti-TNF Therapy in IBD: The Evidence
3.1. Infliximab
3.1.1. Infliximab in Crohn’s Disease
3.1.2. Infliximab in the Postoperative Recurrence of CD
3.1.3. Pediatric CD
3.1.4. Infliximab in Ulcerative Colitis
3.1.5. Infliximab in Pouchitis
3.2. Adalimumab
3.2.1. Adalimumab in CD
3.2.2. Adalimumab in UC
3.3. Golimumab
3.3.1. Golimumab in UC
3.3.2. Golimumab in CD
3.4. Anti-TNF in Pregnancy
3.5. Effect on Extraintestinal Manifestations (EIMs)
3.6. Adverse Effects of Anti-TNF Drugs
4. Anti-Interleukin-12/23 p 40 Biologics
4.1. Introduction
4.2. Ustekinumab
4.2.1. Ustekinumab in CD
4.2.2. Ustekinumab in UC
4.2.3. Long-Term Efficacy of Ustekinumab in UC and CD
4.2.4. Safety Profile of Ustekinumab
4.2.5. Ustekinumab and Pregnancy
4.2.6. Ustekinumab in the Pediatric Age Group
4.2.7. Ustekinumab in Postoperative IBD
4.2.8. Ustekinumab in Stricturing CD
4.2.9. Ustekinumab in Perianal Disease
4.2.10. Ustekinumab in Extraintestinal Manifestations (EIMs)
5. Anti-Integrins
5.1. Leukocyte Trafficking and Role of ‘Integrins’ in Propagating Inflammation in IBD
5.2. Vedolizumab in UC and CD
5.3. Safety Profile of Vedolizumab
5.4. Vedolizumab and Pregnancy
5.5. Vedolizumab in the Pediatric Age Group
5.6. Vedolizumab in Fistulizing Crohn’s Disease
5.7. Vedolizumab in Extraintestinal Manifestations
6. Small Molecules in IBD
6.1. Introduction
6.2. Targets of Small molecules and Mechanism of Action
6.2.1. JAK Inhibitors
6.2.2. S1P Receptor Modulators
6.2.3. PDE 4 Inhibitors
6.2.4. TLR 9 Agonist
6.2.5. TYK 2 Inhibitors
6.3. Use in Special Situations
6.3.1. ASUC/Acute Flare of CD
6.3.2. Pregnancy
6.3.3. Pediatric Population
6.3.4. EIM
6.3.5. Post-Surgery
6.4. Adverse Effects
6.4.1. Jak Inhibitors (Tofacitinib/Upadacitinib/Filgotinib)
Hyperlipidemia
Venous Thromboembolism
Cytopenias
6.4.2. Ozanimod
Hypertension
Infections
Increased Transaminases
6.5. Summary
7. Role of the Combination of Biologics and/or Small Molecules
7.1. Indications
7.2. Evidence
7.3. Pros and Cons of Combination Therapy
7.4. Expert Opinion
8. Newer and Emerging Modalities
8.1. Gut Microbiota Modulation
8.2. Stem Cell Therapy
8.3. Regulation of Fibrosis
8.4. Regulation of Innate Lymphoid Cells
8.5. Regulation of B Cells
8.6. Regulation of the Gut-Brain Axis
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Murch, S.H.; Lamkin, V.A.; Savage, M.O.; Walker-Smith, J.A.; MacDonald, T.T. Serum concentrations of tumour necrosis factor alpha in childhood chronic inflammatory bowel disease. Gut 1991, 32, 913–917. [Google Scholar] [CrossRef]
- Braegger, C.P.; Nicholls, S.; Murch, S.H.; MacDonald, T.T.; Stephens, S. Tumour necrosis factor alpha in stool as a marker of intestinal inflammation. Lancet 1992, 339, 89–91. [Google Scholar] [CrossRef] [PubMed]
- Breese, E.J.; Michie, C.A.; Nicholls, S.W.; Murch, S.H.; Williams, C.B.; Domizio, P.; Walker-Smith, J.A.; MacDonald, T.T. Tumor necrosis factor alpha-producing cells in the intestinal mucosa of children with inflammatory bowel disease. Gastroenterology 1994, 106, 1455–1466. [Google Scholar] [CrossRef] [PubMed]
- Derkx, B.; Taminiau, J.; Radema, S.; Stronkhorst, A.; Wortel, C.; Tytgat, G.; van Deventer, S. Tumour-necrosis-factor antibody treatment in Crohn’s disease. Lancet 1993, 342, 173–174. [Google Scholar] [CrossRef]
- Papamichael, K.; Gils, A.; Rutgeerts, P.; Levesque, B.G.; Vermeire, S.; Sandborn, W.J.; Vande Casteele, N. Role for therapeutic drug monitoring during induction therapy with TNF antagonists in IBD: Evolution in the definition and management of primary nonresponse. Inflamm. Bowel Dis. 2015, 21, 182–197. [Google Scholar] [CrossRef] [PubMed]
- Papamichael, K.; Chachu, K.A.; Vajravelu, R.K.; Vaughn, B.P.; Ni, J.; Osterman, M.T.; Cheifetz, A.S. Improved Long-term Outcomes of Patients With Inflammatory Bowel Disease Receiving Proactive Compared With Reactive Monitoring of Serum Concentrations of Infliximab. Clin. Gastroenterol. Hepatol. 2017, 15, 1580–1588.e3. [Google Scholar] [CrossRef] [PubMed]
- Zurba, Y.; Gros, B.; Shehab, M. Exploring the Pipeline of Novel Therapies for Inflammatory Bowel Disease; State of the Art Review. Biomedicines 2023, 11, 747. [Google Scholar] [CrossRef] [PubMed]
- Targan, S.R.; Hanauer, S.B.; van Deventer, S.J.; Mayer, L.; Present, D.H.; Braakman, T.; DeWoody, K.L.; Schaible, T.F.; Rutgeerts, P.J. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn’s disease. Crohn’s Disease cA2 Study Group. N. Engl. J. Med. 1997, 337, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
- Hanauer, S.B.; Feagan, B.G.; Lichtenstein, G.R.; Mayer, L.F.; Schreiber, S.; Colombel, J.F.; Rachmilewitz, D.; Wolf, D.C.; Olson, A.; Bao, W.; et al. Maintenance infliximab for Crohn’s disease: The ACCENT I randomised trial. Lancet 2002, 359, 1541–1549. [Google Scholar] [CrossRef]
- Present, D.H.; Rutgeerts, P.; Targan, S.; Hanauer, S.B.; Mayer, L.; van Hogezand, R.A.; Podolsky, D.K.; Sands, B.E.; Braakman, T.; DeWoody, K.L.; et al. Infliximab for the treatment of fistulas in patients with Crohn’s disease. N. Engl. J. Med. 1999, 340, 1398–1405. [Google Scholar] [CrossRef]
- Sands, B.E.; Anderson, F.H.; Bernstein, C.N.; Chey, W.Y.; Feagan, B.G.; Fedorak, R.N.; Kamm, M.A.; Korzenik, J.R.; Lashner, B.A.; Onken, J.E.; et al. Infliximab maintenance therapy for fistulizing Crohn’s disease. N. Engl. J. Med. 2004, 350, 876–885. [Google Scholar] [CrossRef]
- Colombel, J.F.; Sandborn, W.J.; Reinisch, W.; Mantzaris, G.J.; Kornbluth, A.; Rachmilewitz, D.; Lichtiger, S.; D’Haens, G.; Diamond, R.H.; Broussard, D.L.; et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N. Engl. J. Med. 2010, 362, 1383–1395. [Google Scholar] [CrossRef]
- Schnitzler, F.; Fidder, H.; Ferrante, M.; Noman, M.; Arijs, I.; Van Assche, G.; Hoffman, I.; Van Steen, K.; Vermeire, S.; Rutgeerts, P. Long-term outcome of treatment with infliximab in 614 patients with Crohn’s disease: Results from a single-centre cohort. Gut 2009, 58, 492–500. [Google Scholar] [CrossRef]
- Fidder, H.; Schnitzler, F.; Ferrante, M.; Noman, M.; Katsanos, K.; Segaert, S.; Henckaerts, L.; Van Assche, G.; Vermeire, S.; Rutgeerts, P. Long-term safety of infliximab for the treatment of inflammatory bowel disease: A single-centre cohort study. Gut 2009, 58, 501–508. [Google Scholar] [CrossRef]
- Billiet, T.; Cleynen, I.; Ballet, V.; Ferrante, M.; Van Assche, G.; Gils, A.; Vermeire, S. Prognostic factors for long-term infliximab treatment in Crohn’s disease patients: A 20-year single centre experience. Aliment. Pharmacol. Ther. 2016, 44, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Olivera, P.; Thiriet, L.; Luc, A.; Baumann, C.; Danese, S.; Peyrin-Biroulet, L. Treatment Persistence for Infliximab Versus Adalimumab in Crohn’s Disease: A 14-Year Single-Center Experience. Inflamm. Bowel Dis. 2017, 23, 976–985. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, G.R.; Feagan, B.G.; Cohen, R.D.; Salzberg, B.A.; Safdi, M.; Popp, J.W.; Langholff, W.; Sandborn, W.J. Infliximab for Crohn’s Disease: More Than 13 Years of Real-world Experience. Inflamm. Bowel Dis. 2018, 24, 490–501. [Google Scholar] [CrossRef]
- Lichtenstein, G.R.; Rutgeerts, P.; Sandborn, W.J.; Sands, B.E.; Diamond, R.H.; Blank, M.; Montello, J.; Tang, L.; Cornillie, F.; Colombel, J.-F. A pooled analysis of infections, malignancy, and mortality in infliximab- and immunomodulator-treated adult patients with inflammatory bowel disease. Am. J. Gastroenterol. 2012, 107, 1051–1063. [Google Scholar] [CrossRef] [PubMed]
- Mao, E.J.; Hazlewood, G.S.; Kaplan, G.G.; Peyrin-Biroulet, L.; Ananthakrishnan, A.N. Systematic review with meta-analysis: Comparative efficacy of immunosuppressants and biologics for reducing hospitalisation and surgery in Crohn’s disease and ulcerative colitis. Aliment. Pharmacol. Ther. 2017, 45, 3–13. [Google Scholar] [CrossRef]
- Singh, S.; Fumery, M.; Sandborn, W.J.; Murad, M.H. Systematic review and network meta-analysis: First- and second-line biologic therapies for moderate-severe Crohn’s disease. Aliment. Pharmacol. Ther. 2018, 48, 394–409. [Google Scholar] [CrossRef]
- Barreiro-de Acosta, M.; García-Bosch, O.; Souto, R.; Mañosa, M.; Miranda, J.; García-Sanchez, V.; Gordillo, J.; Chacon, S.; Loras, C.; Carpio, D.; et al. Efficacy of infliximab rescue therapy in patients with chronic refractory pouchitis: A multicenter study. Inflamm. Bowel Dis. 2012, 18, 812–817. [Google Scholar] [CrossRef]
- Regueiro, M.; Feagan, B.G.; Zou, B.; Johanns, J.; Blank, M.A.; Chevrier, M.; Plevy, S.; Popp, J.; Cornillie, F.J.; Lukas, M.; et al. Infliximab Reduces Endoscopic, but Not Clinical, Recurrence of Crohn’s Disease After Ileocolonic Resection. Gastroenterology. 2016, 150, 1568–1578. [Google Scholar] [CrossRef]
- Huguet, M.; Pereira, B.; Goutte, M.; Goutorbe, F.; Dubois, A.; Bommelaer, G.; Buisson, A. Systematic Review With Meta-Analysis: Anti-TNF Therapy in Refractory Pouchitis and Crohn’s Disease-Like Complications of the Pouch After Ileal Pouch-Anal Anastomosis Following Colectomy for Ulcerative Colitis. Inflamm. Bowel Dis. 2018, 24, 261–268. [Google Scholar] [CrossRef]
- Cezard, J.P.; Nouaili, N.; Talbotec, C.; Hugot, J.P.; Gobert, J.G.; Schmitz, J.; Mougenot, J.F.; Alberti, C.; Goulet, O. A prospective study of the efficacy and tolerance of a chimeric antibody to tumor necrosis factors in severe pediatric Crohn’s disease. J. Pediatr. Gastroenterol. Nutr. 2003, 36, 632–636. [Google Scholar]
- Armuzzi, A.; Felice, C.; Papa, A.; Marzo, M.; Pugliese, D.; Andrisani, G.; Federico, F.; De Vitis, I.; Rapaccini, G.L.; Guidi, L. Prevention of postoperative recurrence with azathioprine or infliximab in patients with Crohn’s disease: An open-label pilot study. J. Crohn’s Colitis 2013, 7, e623–e629. [Google Scholar] [CrossRef]
- Rutgeerts, P.; Sandborn, W.J.; Feagan, B.G.; Reinisch, W.; Olson, A.; Johanns, J.; Travers, S.; Rachmilewitz, D.; Hanauer, S.B.; Lichtenstein, G.R.; et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 2005, 353, 2462–2476. [Google Scholar] [CrossRef]
- Järnerot, G.; Hertervig, E.; Friis-Liby, I.; Blomquist, L.; Karlén, P.; Grännö, C.; Vilien, M.; Ström, M.; Danielsson, A.; Verbaan, H.; et al. Infliximab as rescue therapy in severe to moderately severe ulcerative colitis: A randomized, placebo-controlled study. Gastroenterology 2005, 128, 1805–1811. [Google Scholar] [CrossRef]
- Laharie, D.; Bourreille, A.; Branche, J.; Allez, M.; Bouhnik, Y.; Filippi, J.; Zerbib, F.; Savoye, G.; Nachury, M.; Moreau, J.; et al. Ciclosporin versus infliximab in patients with severe ulcerative colitis refractory to intravenous steroids: A parallel, open-label randomised controlled trial. Lancet 2012, 380, 1909–1915. [Google Scholar] [CrossRef] [PubMed]
- Laharie, D.; Bourreille, A.; Branche, J.; Allez, M.; Bouhnik, Y.; Filippi, J.; Zerbib, F.; Savoye, G.; Vuitton, L.; Moreau, J.; et al. Long-term outcome of patients with steroid-refractory acute severe UC treated with ciclosporin or infliximab. Gut 2018, 67, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.E.; McGrail, K.M.; Peterson, S.; Raval, M.J.; Karimuddin, A.A.; Phang, P.T.; Bressler, B.; Brown, C.J. Infliximab in ulcerative colitis: The impact of preoperative treatment on rates of colectomy and prescribing practices in the province of British Columbia, Canada. Dis. Colon. Rectum 2014, 57, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Arias, M.T.; Vande Casteele, N.; Vermeire, S.; de Buck van Overstraeten, A.; Billiet, T.; Baert, F.; Wolthuis, A.; Van Assche, G.; Noman, M.; Hoffman, I.; et al. A panel to predict long-term outcome of infliximab therapy for patients with ulcerative colitis. Clin. Gastroenterol. Hepatol. 2015, 13, 531–538. [Google Scholar] [CrossRef]
- Kelly, O.B.; Rosenberg, M.; Tyler, A.D.; Stempak, J.M.; Steinhart, A.H.; Cohen, Z.; Greenberg, G.R.; Silverberg, M.S. Infliximab to Treat Refractory Inflammation After Pelvic Pouch Surgery for Ulcerative Colitis. J. Crohn’s Colitis 2016, 10, 410–417. [Google Scholar] [CrossRef]
- Oussalah, A.; Evesque, L.; Laharie, D.; Roblin, X.; Boschetti, G.; Nancey, S.; Filippi, J.; Flourié, B.; Hebuterne, X.; Bigard, M.-A.; et al. A multicenter experience with infliximab for ulcerative colitis: Outcomes and predictors of response, optimization, colectomy, and hospitalization. Am. J. Gastroenterol. 2010, 105, 2617–2625. [Google Scholar] [CrossRef]
- Ferrante, M.; D’Haens, G.; Dewit, O.; Baert, F.; Holvoet, J.; Geboes, K.; De Hertogh, G.; Van Assche, G.; Vermeire, S.; Rutgeerts, P.; et al. Efficacy of infliximab in refractory pouchitis and Crohn’s disease-related complications of the pouch: A Belgian case series. Inflamm. Bowel Dis. 2010, 16, 243–249. [Google Scholar] [CrossRef]
- Baldassano, R.; Braegger, C.P.; Escher, J.C.; DeWoody, K.; Hendricks, D.F.; Keenan, G.F.; Winter, H.S. Infliximab (REMICADE) therapy in the treatment of pediatric Crohn’s disease. Am. J. Gastroenterol. 2003, 98, 833–838. [Google Scholar] [CrossRef]
- Hanauer, S.B.; Sandborn, W.J.; Rutgeerts, P.; Fedorak, R.N.; Lukas, M.; MacIntosh, D.; Panaccione, R.; Wolf, D.; Pollack, P. Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn’s disease: The CLASSIC-I trial. Gastroenterology 2006, 130, 323–333, quiz 591. [Google Scholar] [CrossRef] [PubMed]
- Hyams, J.; Crandall, W.; Kugathasan, S.; Griffiths, A.; Olson, A.; Johanns, J.; Liu, G.; Travers, S.; Heuschkel, R.; Markowitz, J.; et al. Induction and maintenance infliximab therapy for the treatment of moderate-to-severe Crohn’s disease in children. Gastroenterology 2007, 132, 863–873, q. [Google Scholar] [CrossRef] [PubMed]
- Baert, F.; Noman, M.; Vermeire, S.; Van Assche, G.; D’ Haens, G.; Carbonez, A.; Rutgeerts, P. Influence of immunogenicity on the long-term efficacy of infliximab in Crohn’s disease. N. Engl. J. Med. 2003, 348, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Nanda, K.S.; Cheifetz, A.S.; Moss, A.C. Impact of antibodies to infliximab on clinical outcomes and serum infliximab levels in patients with inflammatory bowel disease (IBD): A meta-analysis. Am. J. Gastroenterol. 2013, 108, 40–47, quiz 48. [Google Scholar] [CrossRef] [PubMed]
- Bálint, A.; Farkas, K.; Palatka, K.; Lakner, L.; Miheller, P.; Rácz, I.; Hegede, G.; Vincze, Á.; Horváth, G.; Szabó, A.; et al. Efficacy and Safety of Adalimumab in Ulcerative Colitis Refractory to Conventional Therapy in Routine Clinical Practice. J. Crohn’s Colitis 2016, 10, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Sandborn, W.J.; Hanauer, S.B.; Rutgeerts, P.; Fedorak, R.N.; Lukas, M.; MacIntosh, D.G.; Panaccione, R.; Wolf, D.; Kent, J.D.; Bittle, B.; et al. Adalimumab for maintenance treatment of Crohn’s disease: Results of the CLASSIC II trial. Gut 2007, 56, 1232–1239. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Feagan, B.G.; Marano, C.; Zhang, H.; Strauss, R.; Johanns, J.; Adedokun, O.J.; Guzzo, C.; Colombel, J.-F.; Reinisch, W.; et al. Subcutaneous golimumab induces clinical response and remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology 2014, 146, 85–95, quiz e14–15. [Google Scholar] [CrossRef]
- Hibi, T.; Imai, Y.; Senoo, A.; Ohta, K.; Ukyo, Y. Efficacy and safety of golimumab 52-week maintenance therapy in Japanese patients with moderate to severely active ulcerative colitis: A phase 3, double-blind, randomized, placebo-controlled study-(PURSUIT-J study). J. Gastroenterol. 2017, 52, 1101–1111. [Google Scholar] [CrossRef] [PubMed]
- Probert, C.S.; Sebastian, S.; Gaya, D.R.; Hamlin, P.J.; Gillespie, G.; Rose, A.; Tate, H.; Wheeler, C.; Irving, P.M. Golimumab induction and maintenance for moderate to severe ulcerative colitis: Results from GO-COLITIS (Golimumab: A Phase 4, UK, open label, single arm study on its utilization and impact in ulcerative Colitis). BMJ Open Gastroenterol. 2018, 5, e000212. [Google Scholar] [CrossRef] [PubMed]
- Greener, T.; Boland, K.; Steinhart, A.H.; Silverberg, M.S. The Unfinished Symphony: Golimumab Therapy for Anti-Tumour Necrosis Factor Refractory Crohn’s Disease. J. Crohn’s Colitis 2018, 12, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Pichler, J.; Memaran, N.; Huber, W.D.; Aufricht, C.; Bidmon-Fliegenschnee, B. Golimumab in adolescents with Crohn’s disease refractory to previous tumour necrosis factor antibody. Acta Paediatr. 2021, 110, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Simister, N.E. Placental transport of immunoglobulin G. Vaccine 2003, 21, 3365–3369. [Google Scholar] [CrossRef] [PubMed]
- Mahadevan, U.; Long, M.D.; Kane, S.V.; Roy, A.; Dubinsky, M.C.; Sands, B.E.; Cohen, R.D.; Chambers, C.D.; Sandborn, W.J. Crohn’s Colitis Foundation Clinical Research Alliance Pregnancy and Neonatal Outcomes After Fetal Exposure to Biologics and Thiopurines Among Women With Inflammatory Bowel Disease. Gastroenterology 2021, 160, 1131–1139. [Google Scholar] [CrossRef] [PubMed]
- Narula, N.; Wong, E.C.L.; Dulai, P.S.; Marshall, J.K.; Colombel, J.-F.; Reinisch, W. Outcomes of Passable and Non-passable Strictures in Clinical Trials of Crohn’s Disease: A Post-hoc Analysis. J. Crohn’s Colitis 2021, 15, 1649–1657. [Google Scholar] [CrossRef]
- Mahadevan, U.; Robinson, C.; Bernasko, N.; Boland, B.; Chambers, C.; Dubinsky, M.; Friedman, S.; Kane, S.; Manthey, J.; Sauberan, J.; et al. Inflammatory Bowel Disease in Pregnancy Clinical Care Pathway: A Report From the American Gastroenterological Association IBD Parenthood Project Working Group. Gastroenterology 2019, 156, 1508–1524. [Google Scholar] [CrossRef]
- Reinisch, W.; Sandborn, W.J.; Hommes, D.W.; D’Haens, G.; Hanauer, S.; Schreiber, S.; Panaccione, R.; Fedorak, R.N.; Tighe, M.B.; Huang, B.; et al. Adalimumab for induction of clinical remission in moderately to severely active ulcerative colitis: Results of a randomised controlled trial. Gut 2011, 60, 780–787. [Google Scholar] [CrossRef] [PubMed]
- Adalimumab in the Treatment of Moderate-to-Severe Ulcerative Colitis: ULTRA 2 Trial Results. Gastroenterol. Hepatol. 2013, 9, 317–320.
- Colombel, J.-F.; Sandborn, W.J.; Ghosh, S.; Wolf, D.C.; Panaccione, R.; Feagan, B.; Reinisch, W.; Robinson, A.M.; Lazar, A.; Kron, M.; et al. Four-year maintenance treatment with adalimumab in patients with moderately to severely active ulcerative colitis: Data from ULTRA 1, 2, and 3. Am. J. Gastroenterol. 2014, 109, 1771–1780. [Google Scholar] [CrossRef]
- Levine, J.S.; Burakoff, R. Extraintestinal manifestations of inflammatory bowel disease. Gastroenterol. Hepatol. 2011, 7, 235–241. [Google Scholar]
- Vavricka, S.R.; Gubler, M.; Gantenbein, C.; Spoerri, M.; Froehlich, F.; Seibold, F.; Protic, M.; Michetti, P.; Straumann, A.; Fournier, N.; et al. Anti-TNF Treatment for Extraintestinal Manifestations of Inflammatory Bowel Disease in the Swiss IBD Cohort Study. Inflamm. Bowel Dis. 2017, 23, 1174–1181. [Google Scholar] [CrossRef]
- Thiebault, H.; Boyard-Lasselin, P.; Guignant, C.; Guillaume, N.; Wacrenier, A.; Sabbagh, C.; Rebibo, L.; Brazier, F.; Meynier, J.; Nguyen-Khac, E.; et al. Paradoxical articular manifestations in patients with inflammatory bowel diseases treated with infliximab. Eur. J. Gastroenterol. Hepatol. 2016, 28, 876–881. [Google Scholar] [CrossRef]
- Kugathasan, S.; Miranda, A.; Nocton, J.; Drolet, B.A.; Raasch, C.; Binion, D.G. Dermatologic manifestations of Crohn disease in children: Response to infliximab. J. Pediatr. Gastroenterol. Nutr. 2003, 37, 150–154. [Google Scholar]
- Fries, W.; Giofré, M.R.; Catanoso, M.; Lo Gullo, R. Treatment of acute uveitis associated with Crohn’s disease and sacroileitis with infliximab. Am. J. Gastroenterol. 2002, 97, 499–500. [Google Scholar] [CrossRef] [PubMed]
- Moreland, L.W.; Baumgartner, S.W.; Schiff, M.H.; Tindall, E.A.; Fleischmann, R.M.; Weaver, A.L.; Ettlinger, R.E.; Cohen, S.; Koopman, W.J.; Mohler, K.; et al. Treatment of rheumatoid arthritis with a recombinant human tumor necrosis factor receptor (p75)-Fc fusion protein. N. Engl. J. Med. 1997, 337, 141–147. [Google Scholar] [CrossRef]
- Benson, J.M.; Peritt, D.; Scallon, B.J.; Heavner, G.A.; Shealy, D.J.; Giles-Komar, J.M.; Mascelli, M.A. Discovery and mechanism of ustekinumab. MAbs 2011, 3, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Ahluwalia, B.; Magnusson, M.K.; Öhman, L. Mucosal immune system of the gastrointestinal tract: Maintaining balance between the good and the bad. Scand. J. Gastroenterol. 2017, 52, 1185–1193. [Google Scholar] [CrossRef]
- Ahluwalia, B.; Moraes, L.; Magnusson, M.K.; Öhman, L. Immunopathogenesis of inflammatory bowel disease and mechanisms of biological therapies. Scand. J. Gastroenterol. 2018, 53, 379–389. [Google Scholar] [CrossRef]
- Monteleone, G.; Biancone, L.; Marasco, R.; Morrone, G.; Marasco, O.; Luzza, F.; Pallone, F. Interleukin 12 is expressed and actively released by Crohn’s disease intestinal lamina propria mononuclear cells. Gastroenterology 1997, 112, 1169–1178. [Google Scholar] [CrossRef] [PubMed]
- McGovern, D.; Powrie, F. The IL23 axis plays a key role in the pathogenesis of IBD. Gut 2007, 56, 1333–1336. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, W.; Kolls, J.K.; Zheng, Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity 2008, 28, 454–467. [Google Scholar] [CrossRef] [PubMed]
- Gubler, U.; Chua, A.O.; Schoenhaut, D.S.; Dwyer, C.M.; McComas, W.; Motyka, R.; Nabavi, N.; Wolitzky, A.G.; Quinn, P.M.; Familletti, P.C. Coexpression of two distinct genes is required to generate secreted bioactive cytotoxic lymphocyte maturation factor. Proc. Natl. Acad. Sci. USA 1991, 88, 4143–4147. [Google Scholar] [CrossRef] [PubMed]
- Oppmann, B.; Lesley, R.; Blom, B.; Timans, J.C.; Xu, Y.; Hunte, B.; Vega, F.; Yu, N.; Wang, J.; Singh, K.; et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity 2000, 13, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Moschen, A.R.; Tilg, H.; Raine, T. IL-12, IL-23 and IL-17 in IBD: Immunobiology and therapeutic targeting. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Adedokun, O.J.; Xu, Z.; Gasink, C.; Kowalski, K.; Sandborn, W.J.; Feagan, B. Population Pharmacokinetics and Exposure-Response Analyses of Ustekinumab in Patients With Moderately to Severely Active Crohn’s Disease. Clin. Ther. 2022, 44, 1336–1355. [Google Scholar] [CrossRef] [PubMed]
- Feagan, B.G.; Sandborn, W.J.; Gasink, C.; Jacobstein, D.; Lang, Y.; Friedman, J.R.; Blank, M.A.; Johanns, J.; Gao, L.-L.; Miao, Y.; et al. Ustekinumab as Induction and Maintenance Therapy for Crohn’s Disease. N. Engl. J. Med. 2016, 375, 1946–1960. [Google Scholar] [CrossRef]
- Hanauer, S.B.; Sandborn, W.J.; Feagan, B.G.; Gasink, C.; Jacobstein, D.; Zou, B.; Johanns, J.; Adedokun, O.J.; Sands, B.E.; Rutgeerts, P.; et al. IM-UNITI: Three-year Efficacy, Safety, and Immunogenicity of Ustekinumab Treatment of Crohn’s Disease. J. Crohn’s Colitis 2020, 14, 23–32. [Google Scholar] [CrossRef]
- Abreu, M.T.; Rowbotham, D.S.; Danese, S.; Sandborn, W.J.; Miao, Y.; Zhang, H.; Tikhonov, I.; Panaccione, R.; Hisamatsu, T.; Scherl, E.J.; et al. Efficacy and Safety of Maintenance Ustekinumab for Ulcerative Colitis Through 3 Years: UNIFI Long-term Extension. J. Crohn’s Colitis 2022, 16, 1222–1234. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Feagan, B.G.; Danese, S.; O’Brien, C.D.; Ott, E.; Marano, C.; Baker, T.; Zhou, Y.; Volger, S.; Tikhonov, I.; et al. Safety of Ustekinumab in Inflammatory Bowel Disease: Pooled Safety Analysis of Results from Phase 2/3 Studies. Inflamm. Bowel Dis. 2021, 27, 994–1007. [Google Scholar] [CrossRef] [PubMed]
- Chebli, J.M.F.; Parra, R.S.; Flores, C.; Moraes, A.C.; Nones, R.B.; Gomes, T.N.F.; Perdomo, A.M.B.; Scapini, G.; Zaltman, C. Effectiveness and Safety of Ustekinumab for Moderate to Severely Active Crohn’s Disease: Results from an Early Access Program in Brazil. J. Clin. Med. 2022, 11, 6481. [Google Scholar] [CrossRef]
- Avni-Biron, I.; Mishael, T.; Zittan, E.; Livne-Margolin, M.; Zinger, A.; Tzadok, R.; Goldenberg, R.; Kopylov, U.; Ron, Y.; Hadar, E.; et al. Ustekinumab during pregnancy in patients with inflammatory bowel disease: A prospective multicentre cohort study. Aliment. Pharmacol. Ther. 2022, 56, 1361–1369. [Google Scholar] [CrossRef] [PubMed]
- Mitrova, K.; Pipek, B.; Bortlik, M.; Bouchner, L.; Brezina, J.; Douda, T.; Drasar, T.; Klvana, P.; Kohout, P.; Leksa, V.; et al. Safety of Ustekinumab and Vedolizumab During Pregnancy-Pregnancy, Neonatal, and Infant Outcome: A Prospective Multicentre Study. J. Crohn’s Colitis 2022, 16, 1808–1815. [Google Scholar] [CrossRef] [PubMed]
- Admin, S. European Crohn’s and Colitis Organisation—ECCO—DOP52 Safety of Inflammatory Bowel Disease Drugs during Pregnancy and Breastfeeding: Mothers and babies’ Outcomes (DUMBO Registry). Available online: https://www.ecco-ibd.eu/publications/congress-abstracts/item/dop52-safety-of-inflammatory-bowel-disease-drugs-during-pregnancy-and-breastfeeding-mothers-and-babies-outcomes-dumbo-registry-2.html (accessed on 16 November 2023).
- Rosh, J.R.; Turner, D.; Griffiths, A.; Cohen, S.A.; Jacobstein, D.; Adedokun, O.J.; Padgett, L.; Terry, N.A.; O’Brien, C.; Hyams, J.S. Ustekinumab in Paediatric Patients with Moderately to Severely Active Crohn’s Disease: Pharmacokinetics, Safety, and Efficacy Results from UniStar, a Phase 1 Study. J. Crohn’s Colitis 2021, 15, 1931–1942. [Google Scholar] [CrossRef] [PubMed]
- Buisson, A.; Seigne, A.-L.; D’huart, M.-C.; Bigard, M.-A.; Peyrin-Biroulet, L. The extra burden of infliximab infusions in inflammatory bowel disease. Inflamm. Bowel Dis. 2013, 19, 2464–2467. [Google Scholar] [CrossRef]
- Tursi, A.; Mocci, G.; Picchio, M.; Elisei, W.; Maconi, G. Letter: Ustekinumab for the treatment of post-surgical and refractory Crohn’s disease. Aliment. Pharmacol. Ther. 2021, 53, 859–860. [Google Scholar] [CrossRef] [PubMed]
- Inc, M.G. Vedolizumab and Ustekinumab for the Treatment of Symptomatic Small… by Dr. Sara El Ouali. Available online: https://eposters.ddw.org/ddw/2021/ddw-2021-virtual/320964/sara.el.ouali.vedolizumab.and.ustekinumab.for.the.treatment.of.symptomatic.html?f=listing%3D5%2Abrowseby%3D8%2Asortby%3D2%2Ace_id%3D2020%2Alabel%3D21916%2Amarker%3D1277 (accessed on 16 November 2023).
- Singh, S.; Proctor, D.; Scott, F.I.; Falck-Ytter, Y.; Feuerstein, J.D. AGA Technical Review on the Medical Management of Moderate to Severe Luminal and Perianal Fistulizing Crohn’s Disease. Gastroenterology 2021, 160, 2512–2556.e9. [Google Scholar] [CrossRef]
- Chapuis-Biron, C.; Kirchgesner, J.; Pariente, B.; Bouhnik, Y.; Amiot, A.; Viennot, S.; Serrero, M.; Fumery, M.; Allez, M.; Siproudhis, L.; et al. Ustekinumab for Perianal Crohn’s Disease: The BioLAP Multicenter Study From the GETAID. Am. J. Gastroenterol. 2020, 115, 1812–1820. [Google Scholar] [CrossRef]
- Attauabi, M.; Burisch, J.; Seidelin, J.B. Efficacy of ustekinumab for active perianal fistulizing Crohn’s disease: A systematic review and meta-analysis of the current literature. Scand. J. Gastroenterol. 2021, 56, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Guillo, L.; D’Amico, F.; Danese, S.; Peyrin-Biroulet, L. Ustekinumab for Extra-intestinal Manifestations of Inflammatory Bowel Disease: A Systematic Literature Review. J. Crohn’s Colitis 2021, 15, 1236–1243. [Google Scholar] [CrossRef] [PubMed]
- Sands, B.E.; Sandborn, W.J.; Panaccione, R.; O’Brien, C.D.; Zhang, H.; Johanns, J.; Adedokun, O.J.; Li, K.; Peyrin-Biroulet, L.; Van Assche, G.; et al. Ustekinumab as Induction and Maintenance Therapy for Ulcerative Colitis. N. Engl. J. Med. 2019, 381, 1201–1214. [Google Scholar] [CrossRef] [PubMed]
- Sandborn, W.J.; Rebuck, R.; Wang, Y.; Zou, B.; Adedokun, O.J.; Gasink, C.; Sands, B.E.; Hanauer, S.B.; Targan, S.; Ghosh, S.; et al. Five-Year Efficacy and Safety of Ustekinumab Treatment in Crohn’s Disease: The IM-UNITI Trial. Clin. Gastroenterol. Hepatol. 2022, 20, 578–590.e4. [Google Scholar] [CrossRef] [PubMed]
- Wright, R.D.; Cooper, D. Glycobiology of leukocyte trafficking in inflammation. Glycobiology 2014, 24, 1242–1251. [Google Scholar] [CrossRef] [PubMed]
- Arseneau, K.O.; Cominelli, F. Targeting leukocyte trafficking for the treatment of inflammatory bowel disease. Clin. Pharmacol. Ther. 2015, 97, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Schippers, A.; Muschaweck, M.; Clahsen, T.; Tautorat, S.; Grieb, L.; Tenbrock, K.; Gaßler, N.; Wagner, N. β7-Integrin exacerbates experimental DSS-induced colitis in mice by directing inflammatory monocytes into the colon. Mucosal. Immunol. 2016, 9, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Rosario, M.; Dirks, N.L.; Milch, C.; Parikh, A.; Bargfrede, M.; Wyant, T.; Fedyk, E.; Fox, I. A Review of the Clinical Pharmacokinetics, Pharmacodynamics, and Immunogenicity of Vedolizumab. Clin. Pharmacokinet. 2017, 56, 1287–1301. [Google Scholar] [CrossRef]
- Milch, C.; Wyant, T.; Xu, J.; Parikh, A.; Kent, W.; Fox, I.; Berger, J. Vedolizumab, a monoclonal antibody to the gut homing α4β7 integrin, does not affect cerebrospinal fluid T-lymphocyte immunophenotype. J. Neuroimmunol. 2013, 264, 123–126. [Google Scholar] [CrossRef]
- Zeissig, S.; Rosati, E.; Dowds, C.M.; Aden, K.; Bethge, J.; Schulte, B.; Pan, W.H.; Mishra, N.; Zuhayra, M.; Marx, M.; et al. Vedolizumab is associated with changes in innate rather than adaptive immunity in patients with inflammatory bowel disease. Gut 2019, 68, 25–39. [Google Scholar] [CrossRef]
- Battat, R.; Dulai, P.S.; Jairath, V.; Vande Casteele, N. A product review of vedolizumab in inflammatory bowel disease. Hum. Vaccin. Immunother. 2019, 15, 2482–2490. [Google Scholar] [CrossRef]
- Feagan, B.G.; McDonald, J.; Greenberg, G.; Wild, G.; Pare, P.; Fedorak, R.N.; Landau, S.B.; Brettrnan, L.R. An ascending dose trial of a humanized A4B7 antibody in ulcerative colitis (UC). Gastroenterology 2000, 118, a874. [Google Scholar] [CrossRef]
- Feagan, B.G.; Greenberg, G.R.; Wild, G.; Fedorak, R.N.; Paré, P.; McDonald, J.W.D.; Dubé, R.; Cohen, A.; Steinhart, A.H.; Landau, S.; et al. Treatment of ulcerative colitis with a humanized antibody to the alpha4beta7 integrin. N. Engl. J. Med. 2005, 352, 2499–2507. [Google Scholar] [CrossRef]
- Feagan, B.G.; Rutgeerts, P.; Sands, B.E.; Hanauer, S.; Colombel, J.-F.; Sandborn, W.J.; Van Assche, G.; Axler, J.; Kim, H.-J.; Danese, S.; et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 2013, 369, 699–710. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Feagan, B.G.; Rutgeerts, P.; Hanauer, S.; Colombel, J.-F.; Sands, B.E.; Lukas, M.; Fedorak, R.N.; Lee, S.; Bressler, B.; et al. Vedolizumab as induction and maintenance therapy for Crohn’s disease. N. Engl. J. Med. 2013, 369, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Sands, B.E.; Feagan, B.G.; Rutgeerts, P.; Colombel, J.-F.; Sandborn, W.J.; Sy, R.; D’Haens, G.; Ben-Horin, S.; Xu, J.; Rosario, M.; et al. Effects of vedolizumab induction therapy for patients with Crohn’s disease in whom tumor necrosis factor antagonist treatment failed. Gastroenterology 2014, 147, 618–627.e3. [Google Scholar] [CrossRef] [PubMed]
- Bickston, S.J.; Behm, B.W.; Tsoulis, D.J.; Cheng, J.; MacDonald, J.K.; Khanna, R.; Feagan, B.G. Vedolizumab for induction and maintenance of remission in ulcerative colitis. Cochrane Database Syst. Rev. 2014, 8, CD007571. [Google Scholar] [CrossRef] [PubMed]
- Mosli, M.H.; MacDonald, J.K.; Bickston, S.J.; Behm, B.W.; Tsoulis, D.J.; Cheng, J.; Khanna, R.; Feagan, B.G. Vedolizumab for induction and maintenance of remission in ulcerative colitis: A Cochrane systematic review and meta-analysis. Inflamm. Bowel Dis. 2015, 21, 1151–1159. [Google Scholar] [CrossRef] [PubMed]
- Sands, B.E.; Peyrin-Biroulet, L.; Loftus, E.V.; Danese, S.; Colombel, J.-F.; Törüner, M.; Jonaitis, L.; Abhyankar, B.; Chen, J.; Rogers, R.; et al. Vedolizumab versus Adalimumab for Moderate-to-Severe Ulcerative Colitis. N. Engl. J. Med. 2019, 381, 1215. [Google Scholar] [CrossRef] [PubMed]
- Travis, S.; Silverberg, M.S.; Danese, S.; Gionchetti, P.; Löwenberg, M.; Jairath, V.; Feagan, B.G.; Bressler, B.; Ferrante, M.; Hart, A.; et al. Vedolizumab for the Treatment of Chronic Pouchitis. N. Engl. J. Med. 2023, 388, 1191–1200. [Google Scholar] [CrossRef] [PubMed]
- Loftus, E.V., Jr.; Feagan, B.G.; Panaccione, R.; Colombel, J.F.; Sandborn, W.J.; Sands, B.E.; Danese, S.; D’Haens, G.; Rubin, D.T.; Shafran, I.; et al. Long-term safety of vedolizumab for inflammatory bowel disease. Aliment Pharmacol Ther. 2020, 52, 1353–1365. [Google Scholar] [CrossRef] [PubMed]
- Cohen, R.D.; Bhayat, F.; Blake, A.; Travis, S. The Safety Profile of Vedolizumab in Ulcerative Colitis and Crohn’s Disease: 4 Years of Global Post-marketing Data. J. Crohn’s Colitis 2020, 14, 192–204. [Google Scholar] [CrossRef] [PubMed]
- Mahadevan, U.; Vermeire, S.; Lasch, K.; Abhyankar, B.; Bhayat, F.; Blake, A.; Dubinsky, M. Vedolizumab exposure in pregnancy: Outcomes from clinical studies in inflammatory bowel disease. Aliment. Pharmacol. Ther. 2017, 45, 941–950. [Google Scholar] [CrossRef] [PubMed]
- Wils, P.; Seksik, P.; Stefanescu, C.; Nancey, S.; Allez, M.; Pineton de Chambrun, G.; Altwegg, R.; Gilletta, C.; Vuitton, L.; Viennot, S.; et al. Safety of ustekinumab or vedolizumab in pregnant inflammatory bowel disease patients: A multicentre cohort study. Aliment. Pharmacol. Ther. 2021, 53, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Bell, C.; Tandon, P.; Lentz, E.; Marshall, J.K.; Narula, N. Systematic review and meta-analysis: Safety of vedolizumab during pregnancy in patients with inflammatory bowel disease. J. Gastroenterol. Hepatol. 2021, 36, 2640–2648. [Google Scholar] [CrossRef] [PubMed]
- Ledder, O.; Assa, A.; Levine, A.; Escher, J.C.; de Ridder, L.; Ruemmele, F.; Shah, N.; Shaoul, R.; Wolters, V.M.; Rodrigues, A.; et al. Vedolizumab in Paediatric Inflammatory Bowel Disease: A Retrospective Multi-Centre Experience From the Paediatric IBD Porto Group of ESPGHAN. J. Crohn’s Colitis 2017, 11, 1230–1237. [Google Scholar] [CrossRef]
- Fabiszewska, S.; Derda, E.; Szymanska, E.; Osiecki, M.; Kierkus, J. Safety and Effectiveness of Vedolizumab for the Treatment of Pediatric Patients with Very Early Onset Inflammatory Bowel Diseases. J. Clin. Med. 2021, 10, 2997. [Google Scholar] [CrossRef]
- Fang, S.; Song, Y.; Zhang, C.; Wang, L. Efficacy and safety of vedolizumab for pediatrics with inflammatory bowel disease: A systematic review. BMC Pediatr. 2022, 22, 175. [Google Scholar] [CrossRef]
- Chapuis-Biron, C.; Bourrier, A.; Nachury, M.; Nancey, S.; Bouhnik, Y.; Serrero, M.; Armengol-Debeir, L.; Buisson, A.; Tran-Minh, M.-L.; Zallot, C.; et al. Vedolizumab for perianal Crohn’s disease: A multicentre cohort study in 151 patients. Aliment. Pharmacol. Ther. 2020, 51, 719–727. [Google Scholar] [CrossRef]
- Schwartz, D.A.; Peyrin-Biroulet, L.; Lasch, K.; Adsul, S.; Danese, S. Efficacy and Safety of 2 Vedolizumab Intravenous Regimens for Perianal Fistulizing Crohn’s Disease: ENTERPRISE Study. Clin. Gastroenterol. Hepatol. 2022, 20, 1059–1067.e9. [Google Scholar] [CrossRef]
- Ayoub, F.; Odenwald, M.; Micic, D.; Dalal, S.R.; Pekow, J.; Cohen, R.D.; Rubin, D.T.; Sakuraba, A. Vedolizumab for perianal fistulizing Crohn’s disease: Systematic review and meta-analysis. Intest. Res. 2022, 20, 240–250. [Google Scholar] [CrossRef]
- Hanzel, J.; Ma, C.; Casteele, N.V.; Khanna, R.; Jairath, V.; Feagan, B.G. Vedolizumab and Extraintestinal Manifestations in Inflammatory Bowel Disease. Drugs 2021, 81, 333–347. [Google Scholar] [CrossRef]
- Vedolizumab as Induction and Maintenance Therapy for Ulcerative Colitis | NEJM. Available online: https://www.nejm.org/doi/10.1056/NEJMoa1215734?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200www.ncbi.nlm.nih.gov (accessed on 8 October 2023).
- Ma, C.; Huang, V.; Fedorak, D.K.; Kroeker, K.I.; Dieleman, L.A.; Halloran, B.P.; Fedorak, R.N. Outpatient Ulcerative Colitis Primary Anti-TNF Responders Receiving Adalimumab or Infliximab Maintenance Therapy Have Similar Rates of Secondary Loss of Response. J. Clin. Gastroenterol. 2015, 49, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Roblin, X.; Williet, N.; Boschetti, G.; Phelip, J.-M.; Del Tedesco, E.; Berger, A.-E.; Vedrines, P.; Duru, G.; Peyrin-Biroulet, L.; Nancey, S.; et al. Addition of azathioprine to the switch of anti-TNF in patients with IBD in clinical relapse with undetectable anti-TNF trough levels and antidrug antibodies: A prospective randomised trial. Gut 2020, 69, 1206–1212. [Google Scholar] [CrossRef]
- Ungar, B.; Chowers, Y.; Yavzori, M.; Picard, O.; Fudim, E.; Har-Noy, O.; Kopylov, U.; Eliakim, R.; Ben-Horin, S. ABIRISK consortium The temporal evolution of antidrug antibodies in patients with inflammatory bowel disease treated with infliximab. Gut 2014, 63, 1258–1264. [Google Scholar] [CrossRef]
- Eriksson, C.; Rundquist, S.; Lykiardopoulos, V.; Udumyan, R.; Karlén, P.; Grip, O.; Söderman, C.; Almer, S.; Hertervig, E.; Marsal, J.; et al. Real-world effectiveness of vedolizumab in inflammatory bowel disease: Week 52 results from the Swedish prospective multicentre SVEAH study. Therap. Adv. Gastroenterol. 2021, 14, 17562848211023386. [Google Scholar] [CrossRef] [PubMed]
- Shim, H.H.; Chan, P.W.; Chuah, S.W.; Schwender, B.J.; Kong, S.C.; Ling, K.L. A review of vedolizumab and ustekinumab for the treatment of inflammatory bowel diseases. JGH Open 2018, 2, 223–234. [Google Scholar] [CrossRef]
- Fiorino, G.; Allocca, M.; Correale, C.; Roda, G.; Furfaro, F.; Loy, L.; Zilli, A.; Peyrin-Biroulet, L.; Danese, S. Positioning ustekinumab in moderate-to-severe ulcerative colitis: New kid on the block. Expert Opin. Biol. Ther. 2020, 20, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Kedia, S.; Jain, S.; Gupta, V.; Bopanna, S.; Yadav, D.P.; Goyal, S.; Mouli, V.P.; Dhingra, R.; Makharia, G.; et al. High risk of tuberculosis during infliximab therapy despite tuberculosis screening in inflammatory bowel disease patients in India. Intest. Res. 2018, 16, 588–598. [Google Scholar] [CrossRef]
- Fehily, S.R.; Al-Ani, A.H.; Abdelmalak, J.; Rentch, C.; Zhang, E.; Denholm, J.T.; Johnson, D.; Ng, S.C.; Sharma, V.; Rubin, D.T.; et al. Review article: Latent tuberculosis in patients with inflammatory bowel diseases receiving immunosuppression-risks, screening, diagnosis and management. Aliment. Pharmacol. Ther. 2022, 56, 6–27. [Google Scholar] [CrossRef]
- Higashiyama, M.; Hokari, R. New and Emerging Treatments for Inflammatory Bowel Disease. Digestion 2022, 104, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Al-Bawardy, B.; Shivashankar, R.; Proctor, D.D. Novel and Emerging Therapies for Inflammatory Bowel Disease. Front. Pharmacol. 2021, 12. [Google Scholar] [CrossRef]
- Anderson, C.A.; Boucher, G.; Lees, C.W.; Franke, A.; D’Amato, M.; Taylor, K.D.; Lee, J.C.; Goyette, P.; Imielinski, M.; Latiano, A.; et al. Meta-analysis identifies 29 additional ulcerative colitis risk loci, increasing the number of confirmed associations to 47. Nat. Genet. 2011, 43, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Barrett, J.C.; Hansoul, S.; Nicolae, D.L.; Cho, J.H.; Duerr, R.H.; Rioux, J.D.; Brant, S.R.; Silverberg, M.S.; Taylor, K.D.; Barmada, M.M.; et al. Genome-wide association defines more than 30 distinct susceptibility loci for Crohn’s disease. Nat. Genet. 2008, 40, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Kang, G.; Wang, J.; Gao, X.; Wang, X.; Ye, Y.; Liu, L.; Zhao, J.; Liu, X.; Huang, H.; et al. Breaking through the therapeutic ceiling of inflammatory bowel disease: Dual-targeted therapies. Biomed. Pharmacother. 2023, 158, 114174. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Feagan, B.G.; D’Haens, G.; Wolf, D.C.; Jovanovic, I.; Hanauer, S.B.; Ghosh, S.; Petersen, A.; Hua, S.Y.; Lee, J.H.; et al. Ozanimod as Induction and Maintenance Therapy for Ulcerative Colitis. N. Engl. J. Med. 2021, 385, 1280–1291. [Google Scholar] [CrossRef]
- Danese, S.; Neurath, M.F.; Kopoń, A.; Zakko, S.F.; Simmons, T.C.; Fogel, R.; Siegel, C.A.; Panaccione, R.; Zhan, X.; Usiskin, K.; et al. Effects of Apremilast, an Oral Inhibitor of Phosphodiesterase 4, in a Randomized Trial of Patients With Active Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2020, 18, 2526–2534.e9. [Google Scholar] [CrossRef]
- Atreya, R.; Bloom, S.; Scaldaferri, F.; Gerardi, V.; Admyre, C.; Karlsson, Å.; Knittel, T.; Kowalski, J.; Lukas, M.; Löfberg, R.; et al. Clinical Effects of a Topically Applied Toll-like Receptor 9 Agonist in Active Moderate-to-Severe Ulcerative Colitis. J. Crohn’s Colitis 2016, 10, 1294–1302. [Google Scholar] [CrossRef]
- Nogueira, M.; Puig, L.; Torres, T. JAK Inhibitors for Treatment of Psoriasis: Focus on Selective TYK2 Inhibitors. Drugs 2020, 80, 341–352. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Su, C.; Sands, B.E.; D’Haens, G.R.; Vermeire, S.; Schreiber, S.; Danese, S.; Feagan, B.G.; Reinisch, W.; Niezychowski, W.; et al. Tofacitinib as Induction and Maintenance Therapy for Ulcerative Colitis. N. Engl. J. Med. 2017, 376, 1723–1736. [Google Scholar] [CrossRef]
- Sands, B.E.; Armuzzi, A.; Marshall, J.K.; Lindsay, J.O.; Sandborn, W.J.; Danese, S.; Panés, J.; Bressler, B.; Colombel, J.-F.; Lawendy, N.; et al. Efficacy and safety of tofacitinib dose de-escalation and dose escalation for patients with ulcerative colitis: Results from OCTAVE Open. Aliment. Pharmacol. Ther. 2020, 51, 271–280. [Google Scholar] [CrossRef]
- Vermeire, S.; Su, C.; Lawendy, N.; Kobayashi, T.; Sandborn, W.J.; Rubin, D.T.; Modesto, I.; Gardiner, S.; Kulisek, N.; Zhang, H.; et al. Outcomes of Tofacitinib Dose Reduction in Patients with Ulcerative Colitis in Stable Remission from the Randomised RIVETING Trial. J. Crohn’s Colitis 2020, 15, 1130–1141. [Google Scholar] [CrossRef] [PubMed]
- Panés, J.; Sandborn, W.J.; Schreiber, S.; Sands, B.E.; Vermeire, S.; D’Haens, G.; Panaccione, R.; Higgins, P.D.R.; Colombel, J.-F.; Feagan, B.G.; et al. Tofacitinib for induction and maintenance therapy of Crohn’s disease: Results of two phase IIb randomised placebo-controlled trials. Gut 2017, 66, 1049–1059. [Google Scholar] [CrossRef]
- Loftus, E.V.; Panés, J.; Lacerda, A.P.; Peyrin-Biroulet, L.; D’Haens, G.; Panaccione, R.; Reinisch, W.; Louis, E.; Chen, M.; Nakase, H.; et al. Upadacitinib Induction and Maintenance Therapy for Crohn’s Disease. N. Engl. J. Med. 2023, 388, 1966–1980. [Google Scholar] [CrossRef]
- Danese, S.; Vermeire, S.; Zhou, W.; Pangan, A.L.; Siffledeen, J.; Greenbloom, S.; Hébuterne, X.; D’Haens, G.; Nakase, H.; Panés, J.; et al. Upadacitinib as induction and maintenance therapy for moderately to severely active ulcerative colitis: Results from three phase 3, multicentre, double-blind, randomised trials. Lancet 2022, 399, 2113–2128. [Google Scholar] [CrossRef]
- Schreiber, S.; Feagan, B.G.; Peyrin-Biroulet, L.; Vermeire, S.; Faes, M.; Harris, K.; Oortwijn, A.; Daniele, P.; Patel, H.; Danese, S. Filgotinib Improved Health-Related Quality of Life and Led to Comprehensive Disease Control in Individuals with Ulcerative Colitis: Data from the SELECTION Trial. J. Crohn’s Colitis 2023, 17, 863–875. [Google Scholar] [CrossRef] [PubMed]
- Vermeire, S.; Schreiber, S.; Petryka, R.; Kuehbacher, T.; Hebuterne, X.; Roblin, X.; Klopocka, M.; Goldis, A.; Wisniewska-Jarosinska, M.; Baranovsky, A.; et al. Clinical remission in patients with moderate-to-severe Crohn’s disease treated with filgotinib (the FITZROY study): Results from a phase 2, double-blind, randomised, placebo-controlled trial. Lancet 2017, 389, 266–275. [Google Scholar] [CrossRef]
- D’Haens, G.R.; Lee, S.; Taylor, S.A.; Serone, A.; Rimola, J.; Colombel, J.-F.; Besuyen, R.; Gecse, K.; McKevitt, M.; Reinisch, W.; et al. Filgotinib for the Treatment of Small Bowel Crohn’s Disease: The DIVERGENCE 1 Trial. Gastroenterology 2023, 165, 289–292.e3. [Google Scholar] [CrossRef] [PubMed]
- Sandborn, W.J.; Feagan, B.G.; Hanauer, S.; Vermeire, S.; Ghosh, S.; Liu, W.J.; Petersen, A.; Charles, L.; Huang, V.; Usiskin, K.; et al. Long-Term Efficacy and Safety of Ozanimod in Moderately to Severely Active Ulcerative Colitis: Results From the Open-Label Extension of the Randomized, Phase 2 TOUCHSTONE Study. J. Crohn’s Colitis 2021, 15, 1120–1129. [Google Scholar] [CrossRef]
- Feagan, B.G.; Sandborn, W.J.; Danese, S.; Wolf, D.C.; Liu, W.J.; Hua, S.Y.; Minton, N.; Olson, A.; D’Haens, G. Ozanimod induction therapy for patients with moderate to severe Crohn’s disease: A single-arm, phase 2, prospective observer-blinded endpoint study. Lancet Gastroenterol. Hepatol. 2020, 5, 819–828. [Google Scholar] [CrossRef]
- Feagan, B.G.; Schreiber, S.; Afzali, A.; Rieder, F.; Hyams, J.; Kollengode, K.; Pearlman, J.; Son, V.; Marta, C.; Wolf, D.C.; et al. Ozanimod as a novel oral small molecule therapy for the treatment of Crohn’s disease: The YELLOWSTONE clinical trial program. Contemp. Clin. Trials 2022, 122, 106958. [Google Scholar] [CrossRef] [PubMed]
- Hanauer, S.; Panaccione, R.; Danese, S.; Cheifetz, A.; Reinisch, W.; Higgins, P.D.R.; Woodworth, D.A.; Zhang, H.; Friedman, G.S.; Lawendy, N.; et al. Tofacitinib Induction Therapy Reduces Symptoms Within 3 Days for Patients With Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2019, 17, 139–147. [Google Scholar] [CrossRef]
- Berinstein, J.A.; Sheehan, J.L.; Dias, M.; Berinstein, E.M.; Steiner, C.A.; Johnson, L.A.; Regal, R.E.; Allen, J.I.; Cushing, K.C.; Stidham, R.W.; et al. Tofacitinib for Biologic-Experienced Hospitalized Patients With Acute Severe Ulcerative Colitis: A Retrospective Case-Control Study. Clin. Gastroenterol. Hepatol. 2021, 19, 2112–2120.e1. [Google Scholar] [CrossRef]
- Gilmore, R.; Hilley, P.; Srinivasan, A.; Choy, M.; De Cruz, P. Sequential Use of High-Dose Tofacitinib After Infliximab Salvage Theapy in Acute Severe Ulcerative Colitis. J. Crohn’s Colitis 2022, 16, 166–168. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Benoit, N.; Sedano, R.; Jairath, V.; Narula, N.; McCurdy, J.D.; Rosenfeld, G.; Afif, W.; Lakatos, P.L.; Bessissow, T. Effectiveness of Tofacitinib for Hospitalized Patients with Acute Severe Ulcerative Colitis: Case Series. Dig. Dis. Sci. 2022, 67, 5213–5219. [Google Scholar] [CrossRef] [PubMed]
- Jena, A.; Mishra, S.; Sachan, A.; Singh, H.; Singh, A.K.; Sharma, V. Tofacitinib in Acute Severe Ulcerative Colitis: Case Series and a Systematic Review. Inflamm. Bowel Dis. 2021, 27, e101–e103. [Google Scholar] [CrossRef]
- Uzzan, M.; Bresteau, C.; Laharie, D.; Stefanescu, C.; Bellanger, C.; Carbonnel, F.; Serrero, M.; Viennot, S.; Nachury, M.; Amiot, A.; et al. Tofacitinib as salvage therapy for 55 patients hospitalised with refractory severe ulcerative colitis: A GETAID cohort. Aliment. Pharmacol. Ther. 2021, 54, 312–319. [Google Scholar] [CrossRef]
- Gilmore, R.; Tan, W.L.; Fernandes, R.; An, Y.K.; Begun, J. Upadacitinib Salvage Therapy for Infliximab-Experienced Patients with Acute Severe Ulcerative Colitis. J. Crohn’s Colitis 2023, 17, 2033–2036. [Google Scholar] [CrossRef]
- Cohen, N.A.; Dalal, S.R.; Choi, D.; Rubin, D.T. Ozanimod Maintenance Therapy After Cyclosporine Induction in Acute Severe Ulcerative Colitis. ACG Case Rep. J. 2022, 9, e00832. [Google Scholar] [CrossRef]
- Agrawal, M.; Kim, E.S.; Colombel, J.F. JAK Inhibitors Safety in Ulcerative Colitis: Practical Implications. J. Crohn’s Colitis 2020, 14 (Suppl. S2), S755–S760. [Google Scholar] [CrossRef]
- Mahadevan, U.; Dubinsky, M.C.; Su, C.; Lawendy, N.; Jones, T.V.; Marren, A.; Zhang, H.; Graham, D.; Clowse, M.E.B.; Feldman, S.R.; et al. Outcomes of Pregnancies With Maternal/Paternal Exposure in the Tofacitinib Safety Databases for Ulcerative Colitis. Inflamm. Bowel Dis. 2018, 24, 2494–2500. [Google Scholar] [CrossRef]
- Clowse, M.E.B.; Feldman, S.R.; Isaacs, J.D.; Kimball, A.B.; Strand, V.; Warren, R.B.; Xibillé, D.; Chen, Y.; Frazier, D.; Geier, J.; et al. Pregnancy Outcomes in the Tofacitinib Safety Databases for Rheumatoid Arthritis and Psoriasis. Drug Saf. 2016, 39, 755–762. [Google Scholar] [CrossRef]
- Padda, I.S.; Bhatt, R.; Parmar, M. Upadacitinib. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: http://www.ncbi.nlm.nih.gov/books/NBK572088/ (accessed on 8 November 2023).
- Dubinsky, M.C.; Mahadevan, U.; Charles, L.; Afsari, S.; Henry, A.; Comi, G.; Selmaj, K.; van der Woude, C.J. DOP53 Pregnancy outcomes in the ozanimod clinical development program in relapsing multiple sclerosis, Ulcerative Colitis, and Crohn’s Disease. J. Crohn’s Colitis 2021, 15 (Suppl. S1), S088–S089. [Google Scholar] [CrossRef]
- Dolinger, M.T.; Rolfes, P.; Phan, B.L.; Dubinsky, M.C. Letter: Tofacitinib use for bio- logic-refractory paediatric inflammatory bowel disease. Aliment Pharmacol. Ther. 2019, 50, 966–967. [Google Scholar] [CrossRef]
- Collen, L.V. Rapid Clinical Remission With Upadacitinib in a Pediatric Patient With Refractory Crohn’s Disease. Inflamm. Bowel. Dis. 2023, 29, 1175–1176. [Google Scholar] [CrossRef]
- A Study Investigating Oral Ozanimod (RPC1063) in Pediatric Participants with Moderate to Severe Active Ulcerative Colitis [Internet]. Crohn’s & Colitis Foundation. Available online: https://www.crohnscolitisfoundation.org/a-study-investigating-oral-ozanimod-rpc1063-pediatric-participants-with-moderate-to-severe-active-0 (accessed on 18 October 2023).
- Rubin, D.T.; Reinisch, W.; Greuter, T.; Kotze, P.G.; Pinheiro, M.; Mundayat, R.; Maller, E.; Fellmann, M.; Lawendy, N.; Modesto, I.; et al. Extraintestinal manifestations at baseline, and the effect of tofacitinib, in patients with moderate to severe ulcerative colitis. Ther. Adv. Gastroenterol. 2021, 14, 17562848211005708. [Google Scholar] [CrossRef]
- Colombel, J.; Cao, Q.; Ghosh, S.; Reinisch, W.; Zhou, W.; Ilo, D.; Shu, L.; Yao, X.; Rubin, D. OP33 Effect of upadacitinib (UPA) treatment on extraintestinal manifestations (EIMs) in patients with moderate-to-severe Ulcerative Colitis (UC): Results from the UPA Phase 3 programme. J. Crohn’s Colitis 2022, 16, i036–i037. [Google Scholar] [CrossRef]
- Peyrin-Biroulet, L.; Danese, S.; Louis, E.; Higgins, P.D.R.; Dubinsky, M.; Cataldi, F.; Zhou, Q.; Lee, W.-J.; Kligys, K.; Lacerda, A.P. DOP50 Effect of upadacitinib on extra-intestinal manifestations in patients with moderate to severe Crohn’s disease: Data from the CELEST study. J. Crohn’s Colitis 2019, 13, S057. [Google Scholar] [CrossRef]
- Lightner, A.L.; Vaidya, P.; Holubar, S.; Warusavitarne, J.; Sahnan, K.; Carrano, F.M.; Spinelli, A.; Zaghiyan, K.; Fleshner, P.R. Perioperative safety of tofacitinib in surgical ulcerative colitis patients. Colorectal. Dis. 2021, 23, 2085–2090. [Google Scholar] [CrossRef] [PubMed]
- Deepak, P.; Alayo, Q.A.; Khatiwada, A.; Lin, B.; Fenster, M.; Dimopoulos, C.; Bader, G.; Weisshof, R.; Jacobs, M.; Gutierrez, A.; et al. Safety of Tofacitinib in a Real-World Cohort of Patients With Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2021, 19, 1592–1601.e3. [Google Scholar] [CrossRef]
- Winthrop, K.L.; Melmed, G.Y.; Vermeire, S.; Long, M.D.; Chan, G.; Pedersen, R.D.; Lawendy, N.; Thorpe, A.J.; Nduaka, C.I.; Su, C. Herpes Zoster Infection in Patients With Ulcerative Colitis Receiving Tofacitinib. Inflamm. Bowel. Dis. 2018, 24, 2258–2265. [Google Scholar] [CrossRef] [PubMed]
- Colombel, J.-F. Herpes Zoster in Patients Receiving JAK Inhibitors for Ulcerative Colitis: Mechanism, Epidemiology, Management, and Prevention. Inflamm. Bowel Dis. 2018, 24, 2173–2182. [Google Scholar] [CrossRef]
- Kim, E.S.; Keam, S.J. Filgotinib in Rheumatoid Arthritis: A Profile of Its Use. Clin. Drug Investig. 2021, 41, 741–749. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Feagan, B.G.; Loftus, E.V.; Peyrin-Biroulet, L.; Van Assche, G.; D’Haens, G.; Schreiber, S.; Colombel, J.F.; Lewis, J.D.; Ghosh, S.; et al. Efficacy and Safety of Upadacitinib in a Randomized Trial of Patients With Crohn’s Disease. Gastroenterology 2020, 158, 2123–2138.e8. [Google Scholar] [CrossRef] [PubMed]
- Spiewak, T.A.; Patel, A. User’s guide to JAK inhibitors in inflammatory bowel disease. Curr. Res. Pharmacol. Drug Discov. 2022, 3, 100096. [Google Scholar] [CrossRef] [PubMed]
- López-Sanromán, A.; Esplugues, J.V.; Domènech, E. Pharmacology and safety of tofacitinib in ulcerative colitis. Gastroenterol. Hepatol. 2021, 44, 39–48. [Google Scholar] [CrossRef]
- Feagan, B.G.; Danese, S.; Loftus, E.V.; Vermeire, S.; Schreiber, S.; Ritter, T.; Fogel, R.; Mehta, R.; Nijhawan, S.; Kempiński, R.; et al. Filgotinib as induction and maintenance therapy for ulcerative colitis (SELECTION): A phase 2b/3 double-blind, randomised, placebo-controlled trial. Lancet 2021, 397, 2372–2384. [Google Scholar] [CrossRef]
- Charles-Schoeman, C.; Fleischmann, R.M.; Mysler, E.; Greenwald, M.; Wang, C.; Chen, A.S.; Connell, C.A.; Woolcott, J.; Menon, S.; Chen, Y.; et al. Pos0239 risk of venous thromboembolic events in patients with rheumatoid arthritis aged ≥50 years with ≥1 cardiovascular risk factor: Results from a phase 3b/4 randomised study of tofacitinib vs tumour necrosis factor inhibitors. Ann. Rheum. Dis. 2022, 81, 358–359. [Google Scholar] [CrossRef]
- Mannucci, A.; D’Amico, F.; El Saadi, A.; Peyrin-Biroulet, L.; Danese, S. Filgotinib for moderately to severely active ulcerative colitis. Expert Rev. Gastroenterol. Hepatol. 2022, 16, 927–940. [Google Scholar] [CrossRef]
- Colombel, J.F.; Panaccione, R.; Nakase, H.; Burmester, G.; Cohen, S.B.; Mease, P.; Guttman-Yassky, E.; Liu, J.; Zhou, W.; Ilo, D.; et al. P573 The safety profile of upadacitinib maintenance therapy in ulcerative colitis in the Phase 3 U-ACHIEVE study is consistent with that in approved indications. J. Crohn’s Colitis 2022, 16, i514. [Google Scholar] [CrossRef]
- Wollenhaupt, J.; Lee, E.-B.; Curtis, J.R.; Silverfield, J.; Terry, K.; Soma, K.; Mojcik, C.; DeMasi, R.; Strengholt, S.; Kwok, K.; et al. Safety and efficacy of tofacitinib for up to 9.5 years in the treatment of rheumatoid arthritis: Final results of a global, open-label, long-term extension study. Arthritis Res. Ther. 2019, 21, 89. [Google Scholar] [CrossRef]
- Bristol Myers Squibb Presents New Zeposia (ozanimod) Data on Long-Term Disease Progression and Cognition in Patients with Relapsing Forms of Multiple Sclerosis. Available online: https://news.bms.com/news/corporate-financial/2023/Bristol-Myers-Squibb-Presents-New-Zeposia-ozanimod-Data-on-Long-Term-Disease-Progression-and-Cognition-in-Patients-with-Relapsing-Forms-of-Multiple-Sclerosis/default.aspx (accessed on 21 November 2023).
- Long-Term Use of Ozanimod in Patients With Moderately to Severely Active Ulcerative Colitis. Gastroenterol. Hepatol. 2022, 18 (Suppl. S1), 6.
- Cree, B.; Danese, S.; Wolf, D.; Alekseeva, O.; Charles, L.; Petersen, A.; Sheffield, J.; Cheng, C.Y.; Riolo, J.; Silva, D.; et al. Long-term Safety of Ozanimod in Relapsing Multiple Sclerosis and Moderately to Severely Active Ulcerative Colitis (P7-3.007). Neurol. J. 2023, 100. [Google Scholar] [CrossRef]
- Graham, D.B.; Xavier, R.J. Pathway paradigms revealed from the genetics of inflammatory bowel disease. Nature 2020, 578, 527–539. [Google Scholar] [CrossRef]
- Gold, S.L.; Steinlauf, A.F. Efficacy and Safety of Dual Biologic Therapy in Patients with Inflammatory Bowel Disease: A Review of the Literature. Gastroenterol. Hepatol. 2021, 17, 406–414. [Google Scholar]
- Ahmed, W.; Galati, J.; Kumar, A.; Christos, P.J.; Longman, R.; Lukin, D.J.; Scherl, E.; Battat, R. Dual Biologic or Small Molecule Therapy for Treatment of Inflammatory Bowel Disease: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2022, 20, e361–e379. [Google Scholar] [CrossRef] [PubMed]
- Alayo, Q.A.; Fenster, M.; Altayar, O.; Glassner, K.L.; Llano, E.; Clark-Snustad, K.; Patel, A.; Kwapisz, L.; Yarur, A.J.; Cohen, B.L.; et al. Systematic Review With Meta-analysis: Safety and Effectiveness of Combining Biologics and Small Molecules in Inflammatory Bowel Disease. Crohns Colitis 360 2022, 4, otac002. [Google Scholar] [CrossRef] [PubMed]
- Bonovas, S.; Fiorino, G.; Allocca, M.; Lytras, T.; Nikolopoulos, G.K.; Peyrin-Biroulet, L.; Danese, S. Biologic Therapies and Risk of Infection and Malignancy in Patients with Inflammatory Bowel Disease: A Systematic Review and Network Meta-analysis. Clin. Gastroenterol. Hepatol. 2016, 14, 1385–1397.e10. [Google Scholar] [CrossRef] [PubMed]
- Amoroso, C.; Perillo, F.; Strati, F.; Fantini, M.C.; Caprioli, F.; Facciotti, F. The Role of Gut Microbiota Biomodulators on Mucosal Immunity and Intestinal Inflammation. Cells 2020, 9, 1234. [Google Scholar] [CrossRef]
- Tan, X.-Y.; Xie, Y.-J.; Liu, X.-L.; Li, X.-Y.; Jia, B. A Systematic Review and Meta-Analysis of Randomized Controlled Trials of Fecal Microbiota Transplantation for the Treatment of Inflammatory Bowel Disease. Evid. Based Complement. Alternat. Med. 2022, 2022, 8266793. [Google Scholar] [CrossRef]
- Bernardo, M.E.; Fibbe, W.E. Mesenchymal stromal cells: Sensors and switchers of inflammation. Cell Stem Cell 2013, 13, 392–402. [Google Scholar] [PubMed]
- Iwata, K.; Mikami, Y.; Kato, M.; Yahagi, N.; Kanai, T. Pathogenesis and management of gastrointestinal inflammation and fibrosis: From inflammatory bowel diseases to endoscopic surgery. Inflamm. Regen. 2021, 41, 21. [Google Scholar] [CrossRef] [PubMed]
- Saez, A.; Gomez-Bris, R.; Herrero-Fernandez, B.; Mingorance, C.; Rius, C.; Gonzalez-Granado, J.M. Innate Lymphoid Cells in Intestinal Homeostasis and Inflammatory Bowel Disease. Int. J. Mol. Sci. 2021, 22, 7618. [Google Scholar] [CrossRef] [PubMed]
- Castro-Dopico, T.; Colombel, J.F.; Mehandru, S. Targeting B cells for inflammatory bowel disease treatment: Back to the future. Curr. Opin. Pharmacol. 2020, 55, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Günther, C.; Rothhammer, V.; Karow, M.; Neurath, M.; Winner, B. The Gut-Brain Axis in Inflammatory Bowel Disease-Current and Future Perspectives. Int. J. Mol. Sci. 2021, 22, 8870. [Google Scholar] [CrossRef]
- Bonaz, B. Is-there a place for vagus nerve stimulation in inflammatory bowel diseases? Bioelectron. Med. 2018, 4, 4. [Google Scholar] [CrossRef]

| N | FU (Wks.) | Design | Medication | Cres (%) | SAE (%) | Peads (n) | Fistula Closure (%) | Infection (%) | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| IFX | CD | 108 | 12 | DB, PC RCT | Placebo (n = 25) | 12 | - | - | - | - | Tagran et al. [8] |
| IFX 5 mg/kg (n = 27) | 48 | ||||||||||
| IFX 10 mg/kg (n = 28) | 29 | ||||||||||
| IFX 20 mg/kg (n = 28) | 46 | ||||||||||
| 335 | 54 | DB, PC RCT | Placebo (n = 110) | 21 | 29 | - | - | 4 | Hanau et al. [9] (ACCENT I) | ||
| IFX 5 mg/kg (n = 113) | 39 | 28 | 4 | ||||||||
| IFX 5 mg/kg (n = 112) | 45 | 22 | 3 | ||||||||
| 94 | 18 | DB, PC RCT | Placebo (n = 31) | 26 | - | - | 26 | - | Present et al. [10] | ||
| IFX 5 mg/kg (n = 31) | 68 | 68 | |||||||||
| IFX 10 mg/kg (n = 32) | 56 | 56 | |||||||||
| 195 | 54 | DB, PC RCT | Placebo (n = 31) | 23 | 23 | - | 36 | 6 | Sands et al. [11] (ACCENT II) | ||
| IFX 5 mg/kg (n = 31) | 46 | 14 | 19 | 3 | |||||||
| 508 | 30 | DB RCT | AZA (n = 170) | 30 | 27 | - | - | 6 | Colombel et al. [12] (SONIC) | ||
| IFX 5 mg/kg (n = 169) | 44 | 18 | 5 | ||||||||
| IFX + AZA (n = 169) | 57 | 15 | 4 | ||||||||
| 614 | 200 | OB | IFX 5 mg/kg | 64 | - | - | Schnitzler et al. [13] | ||||
| 1400 | OB | IFX 5 mg/kg (n = 734) | 13 | - | - | 1.6 # | Fidder et al. [14] | ||||
| Control (n = 666) | 19 | 1.1 # | |||||||||
| 6233 | 650 | REG | IFX (n = 3400) | - | - | - | - | 2.15 # | Lichtenstein et al. [17] | ||
| Others (n = 2833) | 0.86 # | ||||||||||
| 21 | 4 | OB | IFX 5 mg/kg | 100 | 21 | Baldassano et al. [35] | |||||
| 21 | 8 | OB | IFX 5 mg/kg | 91 | 21 | 100 | Cezzard et al. [36] | ||||
| 112 | 30 | OB | IFX 5 mg/kg | 88 | 112 | 59 | Hyams et al. [37] (REACH) | ||||
| UC | 364 | 54 | DB, PC RCT | Placebo (n = 121) | 20 | 26 | - | NA | 4 | Rutgeerts et al. [26] (ACT I) | |
| IFX 5 mg/kg (n = 121) | 55 | 22 | 3 | ||||||||
| IFX 10 mg/kg (n = 122) | 54 | 24 | 7 | ||||||||
| 364 | 30 | DB, PC RCT | Placebo (n = 123) | 26 | 20 | - | - | 1 | Rutgeerts et al. [26] (ACT II) | ||
| IFX 5 mg/kg (n = 121) | 47 | 12 | 2 | ||||||||
| IFX 10 mg/kg (n = 120) | 60 | 9 | 3 | ||||||||
| 45 | 12 | DB, RCT | Placebo | 67 $ | - | - | - | - | Järnerot et al. [22] | ||
| IFX 5 mg/kg | 30 $ | ||||||||||
| 115 | 14 | RCT | Cyclosporine (n = 58) | 40 | 16 | - | 4 | Laharie et al. [28] | |||
| IFX 5 mg/kg (n = 57) | 46 | 25 | 5 | ||||||||
| 115 | 250 | RCT | Cyclosporine (n = 58) | 39 $ | - | - | - | - | Laharie et al. [29] | ||
| IFX 5 mg/kg (n = 57) | 35 $ | ||||||||||
| 7227 | - | REG | Control | 10 $ | - | - | - | - | Moore et al. [30] | ||
| IFX | 8.8 $ |
| N | FU (Wks.) | Design | Medication | Cres (%) | SAE (%) | Peads (n) | Fistula Closure (%) | Infection (%) | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ADA | CD | 299 | 4 | DB, PC RCT | Placebo | 12 | 3 | - | 17 | 16 | Hanauer et al. [36] (CLASSIC I) |
| ADA 40 mg/20 mg | 18 | 1 | 75 | 10 | |||||||
| ADA 80 mg/40 mg | 24 | 1 | 17 | ||||||||
| ADA 160 mg/80 mg | 36 | 0 | 21 | ||||||||
| 276 | 56 | DB, PC RCT | Placebo | 44 | 11 | - | - | 0 | Sandborn et al. [41] (CLASSIC II) | ||
| ADA 40 mg alt week | 79 | 5 | 0 | ||||||||
| ADA 40 mg weekly | 83 | 6 | 0 | ||||||||
| ADA 40 mg weekly * | 46 | 18 | 4 | ||||||||
| UC | 8 | DB, PC RCT | ADA 160/80/40 mg | 19 | 8 | - | - | 0 | Reinisch et al. [51] (ULTRA I) | ||
| ADA 80/40 mg | 10 | 4 | 0 | ||||||||
| Placebo | 9.2 | 4 | 1 | ||||||||
| 494 | 52 | DB, PC RCT | Placebo | 9 | 12 | - | - | 2 | Sandborn et al. [52] (ULTRA II) | ||
| ADA 160/80/40 mg | 17 | 12 | 2 | ||||||||
| 71 | - | OB | ADA 160/80/40 mg | 49 | - | - | - | 0 | Balint et al. [40] | ||
| 588 | 200 | OB | ADA 160/80/40 mg | 62 | 30 | - | - | 2 | Colombel et al. [53] (ULTRA III) | ||
| GOL | CD | 45 | 12 | OB | GOL 200/100 mg | 78 | 4.4 | - | - | 11 | Greener et al. [45] |
| 7 | 28 | OB | GOL 200/100 mg | 72 | 0 | 7 | - | - | Pichler et al. [46] | ||
| UC | 774 | 6 | DB, PC RCT | Placebo | 31 | 6.1 | - | - | 1.8 | Sandborn et al. [42] (PURSUIT SC) | |
| GOL 200/100 mg | 51 | 3.0 | - - | 0.5 | |||||||
| GOL 400/200 mg | 55 | ||||||||||
| 464 | 54 | DB, PC RCT | Placebo | 32 | 7.7 | - | - | 1.9 | Sandborn et al. [42] (PURSUIT M) | ||
| GOL 50 mg 4 weekly | 50 | 8.4 | 3.2 | ||||||||
| GOL 100 mg 4 weekly | 47 | 14.3 | 3.2 | ||||||||
| 144 | 52 | PC RCT | Placebo | 20 | 12.9 | - | - | - | Hibi et al. [43] (PURSUIT J) | ||
| GOL 100 mg 4 weekly | 57 | 3.1 | |||||||||
| 205 | 54 | OB | GOL 200/100 mg | 25 | - | - | - | - | Probert et al. [43] |
| Trial | Inclusion Criteria | Study Cohorts | Treatment Cohort | Response |
|---|---|---|---|---|
| Ustekinumab as induction and maintenance therapy in UC, double-blind, placebo-controlled RCT [86] | Moderate-to-Severe UC (51.1%) with prior treatment failure | Induction | Divided into 3 groups | |
|
| |||
|
| |||
|
| |||
| Maintenance until 44 weeks | Divided into 3 groups | |||
|
| |||
|
| |||
|
| |||
| IM-UNITI trial ustekinumab as induction and maintenance therapy for CD, double-blind, placebo-controlled RCT [68] | Pts with moderate-to-severe CD who had completed induction and had a clinical response | Maintenance until 52 weeks | Divided into 3 groups | |
|
| |||
|
| |||
|
|
| Trial | Inclusion Criteria | Study Cohorts | Remission (%) |
|---|---|---|---|
| UNIFI Long-Term Extension study [72] | Patients completing 44 weeks of maintenance on ustekinumab for UC in the previous RCT | Divided into 2 groups (n = 399) | |
|
| ||
|
| ||
| IM-UNITI 3-year safety, efficacy, and immunogenicity of ustekinumab [71] | Patients completing 44 weeks of maintenance on ustekinumab for CD in the previous RCT | Divided into 2 groups (n = 567) | |
|
| ||
|
| ||
| IM-UNITI 5-year safety and efficacy of ustekinumab in CD [87] | Patients completing 44 weeks of maintenance on ustekinumab for CD in the previous RCT | Divided into two groups (n = 124) | |
|
| ||
|
|
| Trial | Inclusion Criteria | Study Cohorts | Treatment Cohort | Response |
|---|---|---|---|---|
| Phase Ib/II placebo-controlled RCT [95] | Moderate-to-severe UC | Induction (n = 29) | Divided into 2 groups | |
| 40% achieved endoscopic remission | |||
| None achieved endoscopic remission | |||
| Phase II, placebo-controlled, double-blind RCT [96] | Moderate-to-severe UC | Induction (n = 181) | Divided into 3 groups | |
|
| |||
|
| |||
|
| |||
| GEMINI I phase III double-blind RCT [97] | Treatment-naïve patients with UC | Induction (n = 374) | Divided into 2 groups | |
|
| |||
|
| |||
| Maintenance | Divided into 3 groups | |||
|
| |||
|
| |||
|
| |||
| GEMINI 2 Placebo controlled Phase III double blind RCT[98] | Adult patients with active CD | Induction (n = 368) | Divided into 2 groups | |
|
| |||
|
| |||
| Maintenance (n = 747) | Divided into 3 groups | |||
|
| |||
|
| |||
|
| |||
| GEMINI-3 placebo-controlled, double-blind RCT [99] | Adult patients with active CD with a loss of response, inadequate response, or intolerance to TNFα antagonists | Induction (n = 315) | Divided into 2 groups | |
|
| |||
|
|
| No | Class | Drug | Target | Clinical Trial | |
|---|---|---|---|---|---|
| UC | CD | ||||
| 1. | JAK | Tofacitinib | JAK1/JAK3 | FDA approved | Phase IIb |
| Filgotinib | JAK1 | Phase III | Phase III | ||
| Upadacitinib | JAK1 | FDA approved | FDA approved | ||
| Izencitinib | JAK1 | Phase IIb | - | ||
| Peficitinib | JAK1 | Phase IIb | - | ||
| Ritlecitinib | JAK1 | Umbrella study | - | ||
| 2. | JAK/TYK2 | Brepocitinib | TYK2/JAK1 | Umbrella study | |
| 3. | S1P receptor modulators | Ozanimod | S1P1, S1P5 | FDA approved | Phase III |
| Etrasimod | S1P1, S1P4, S1P5 | Phase II | Phase II/III | ||
| Amiselimod | S1P, S1PR1 | Phase II | Phase II/III | ||
| 4. | TLR9 agonist | Cobitolimod | TLR9 | Phase III | - |
| 5. | PDE4 inhibitors | Apremilast | PDE4 | Phase II | |
| Types of SMDs | Phase, Indication | Design and Intervention | Results | |
|---|---|---|---|---|
| JAK inhibitor | Tofacitinib (pankinase, JAK1, JAK3) | III, UC OCTAVE1&2 [134] | An 8-week induction trial conducted in 598 and 541 patients with moderate-to-severe disease, respectively, who were either biologic- naïve or biologic-experienced. Patients were given either 10 mg of the drug or placebo (4:1). | Higher clinical remission in the active group than in the placebo group (OCTAVE-1: 18.5% vs. 8.2%, p = 0.007; OCTAVE-2: 16.6% vs. 3.6%, p < 0.001). |
| III, UC OCTAVE Sustain [134] | A 52-week sustained trial for 593 responders from OCTAVE induction trials was conducted with three arms consisting of 10 mg, 5 mg, and the placebo (1:1:1). | Higher clinical remission in the 10 mg group vs. the placebo group (40.6% vs. 11.1%, p < 0.001), but not in the 5 mg group (34.3% vs. 11.1%). | ||
| III, UC OCTAVE Open [135] | A total of 944 patients included OCTAVE Induction 1&2 non-responders and OCTAVE Sustain completers/treatment failures. Patients in remission at OCTAVE Open baseline received tofacitinib 5 mg b.d.; all others received 10 mg b.d. | At month 36, 66.9% and 40.3% showed a clinical response, 64.6% and 37.1% had endoscopic improvement, and 58.9% and 33.7% maintained or achieved remission with tofacitinib 5 and 10 mg b.d., respectively. Demonstrated safety up to 7 yrs. | ||
| III, UC RIVETING [136] | A total of 140 patients were randomised [1:1] to tofacitinib 5 or 10 mg BID. Patients had received tofacitinib 10 mg BID for ≥2 consecutive years and had been in stable remission for ≥6 months before enrollment. | A total of 77.1% and 90.0% of patients in the 5 and 10 mg BID groups, respectively, were in remission at month 6. | ||
| IIb, CD [137] | An 8-week induction trial in 180 patients with moderate-to-severe disease, naïve or biologic-experienced 10 mg/5 mg/placebo (1:1:1). | No significant improvement in remission (43.0%/43.5%/36.7%, all tests vs. placebo NS). | ||
| A total of 180 patients from the induction study were re-randomised to maintenance treatment with placebo or tofacitinib 5 or 10 mg twice daily for 26 weeks. | Clinical response-100 or remission was 55.8% with tofacitinib 10 mg twice daily compared with 39.5% with tofacitinib 5 mg twice daily and 38.1% with placebo (p = 0.130 for 10 mg twice daily vs. placebo). No significant difference between the intervention and placebo. | |||
| Upadacitinib | III, CD [138] U EXCEL U EXCEED | Induction: 526 and 495 patients with moderate-to-severe Crohn’s disease to receive 45 mg of upadacitinib or placebo (2:1 ratio) once daily for 12 weeks, respectively. | Clinical remission in U-EXCEL, 49.5% vs. 29.1%; in U-EXCEED, 38.9% vs. 21.1%; and an endoscopic response in U-EXCEL, 45.5% vs. 13.1%; in U-EXCEED, 34.6% vs. 3.5% (p < 0.001 for all comparisons). | |
| U ENDURE [138] | Maintenance: 502 of those who had a clinical response in induction were assigned in the maintenance trial to receive 15 or 30 mg of upadacitinib or placebo (1:1:1 ratio) once daily for 52 weeks. | At week 52, a higher percentage of patients had clinical remission with 15 mg of upadacitinib (37.3%) or 30 mg of upadacitinib (47.6%) than with the placebo (15.1%), and a higher percentage had an endoscopic response with 15 mg of upadacitinib (27.6%) or 30 mg of upadacitinib (40.1%) than with the placebo (7.3%) (p < 0.001 for all comparisons). | ||
| III, UC [139] U-ACHIEVE (UC1) (induction) U-ACCOMPLISH (UC2) | Induction: 474 and 522 patients with moderate-to-severe active UC were randomly assigned (2:1) to oral upadacitinib 45 mg once daily or the placebo for 8 weeks. | Statistically significantly more patients achieved clinical remission with upadacitinib 45 mg, 26% vs. 5% in UC1 and 34% vs. 4% in UC2; p < 0·0001. | ||
| U-ACHIEVE [139] (Maintenance) | Maintenance: 451 patients who achieved remission in induction study were re-randomly assigned (1:1:1) to upadacitinib 15 mg, upadacitinib 30 mg, or the placebo for 52 weeks. | Statistically significant more clinical remission in patients receiving upadacitinib 15 mg [42%] and 30 mg [52%] than in those receiving the placebo [12%]; p < 0.0001. | ||
| Filgotinib | III, UC SELECTION-I [140] | A 10-week induction trial in 659 patients with moderate-to-severe biologic- naïve disease and in 689 biologic-experienced patients receiving 200 mg/100 mg/placebo (2:2:1). | Efficacy (clinical remission) of 200 mg filgotinib vs. placebo in both biologic- naïve (26.1% vs. 15.3%) biologic-experienced patients (11.5% vs. 4.2%) | |
| II, CD FITZROY [141] | A 10-week induction in 172 patients with moderate-to-severe CD, naïve or biologic-experienced 200 mg vs. placebo (3:1) | A higher remission rate for 200 mg of filgotinib (47% vs. 23%, p < 0.001) | ||
| II, CD DIVERGENCE 1 [142] | Seventy-eight patients were randomly assigned (2:2:1) to receive filgotinib 200 mg, filgotinib 100 mg, or the placebo orally once daily for up to 24 weeks. | Clinical remission at week 24 for 200 mg, 100 mg, placebo was 25% vs. 25% vs. 16.7% (the difference was not statistically significant) | ||
| S1P receptor modulator | Ozanimod | II, UC [143] (TOUCHSTONE) | An 8-week induction trial in 197 patients with moderate-to-severe disease, naïve or biologic-experienced receiving 1 mg/0.5 mg/placebo (1:1:1). | A higher clinical remission rate in the 1 mg group vs. the placebo group (16% vs. 6%, p = 0.048) |
| III, UC [130] (TRUE NORTH STUDY) | Induction: 10 weeks, cohort 1 received oral ozanimod 1 mg or the placebo once daily in a double-blind manner, and cohort 2 received open-label ozanimod at the same daily dose. Maintenance: Patients with a clinical response to ozanimod in either cohort underwent randomization again to receive double-blind ozanimod or the placebo for the maintenance period (through week 52). | Clinical remission was significantly higher among patients who received ozanimod than among those who received the placebo during both induction (18.4% vs. 6.0%, p < 0.001) and maintenance (37.0% vs. 18.5% [among patients with a response at week 10], p < 0.001). | ||
| II, CD [144] (STEPSTONE) | An uncontrolled trial in 69 patients with moderate-to-severe disease, naïve or biologic-experienced received 1 mg ozanimod for 11 weeks after a 7-day dose escalation. | A total of 39.1% (95% CI 27.6–51.6) achieved clinical remission (CDAI < 150) and 56.5% (95% CI 44.0–68.4) exhibited a clinical response (CDAI decrease from baseline ≥ 100). | ||
| III, CD [145] (YELLOWSTONE) | Patients with an inadequate response to treatment are randomized to ozanimod 0.92 mg or the placebo for 12 weeks during induction. Those who respond to ozanimod are rerandomized to continue ozanimod or placebo maintenance therapy for 52 weeks. | Expected 2023 (induction studies), 2024 (maintenance study), and 2026 (OLE) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manrai, M.; Jha, A.A.; Dawra, S.; Pachisia, A.V. Biologics, Small Molecules and More in Inflammatory Bowel Disease: The Present and the Future. Future Pharmacol. 2024, 4, 279-316. https://doi.org/10.3390/futurepharmacol4010017
Manrai M, Jha AA, Dawra S, Pachisia AV. Biologics, Small Molecules and More in Inflammatory Bowel Disease: The Present and the Future. Future Pharmacology. 2024; 4(1):279-316. https://doi.org/10.3390/futurepharmacol4010017
Chicago/Turabian StyleManrai, Manish, Atul Abhishek Jha, Saurabh Dawra, and Aditya Vikram Pachisia. 2024. "Biologics, Small Molecules and More in Inflammatory Bowel Disease: The Present and the Future" Future Pharmacology 4, no. 1: 279-316. https://doi.org/10.3390/futurepharmacol4010017
APA StyleManrai, M., Jha, A. A., Dawra, S., & Pachisia, A. V. (2024). Biologics, Small Molecules and More in Inflammatory Bowel Disease: The Present and the Future. Future Pharmacology, 4(1), 279-316. https://doi.org/10.3390/futurepharmacol4010017







