Abstract
Improving maize yield is an objective of utmost importance for food security in Uganda. In the evaluation of soil microorganisms in crop production, it is important to assess the composition and diversity of the arbuscular mycorrhizal fungi (AMF) species in different agroecosystems. AMF play an important role in improving crop growth and yield. We present a study of the morphological diversity of native AMF species associated with the rhizosphere of maize in two locations in eastern Uganda (the Amuria and Serere districts). The effects of soil chemical properties on this diversity are also assessed. AMF diversity is assessed by the morphological identification of the spores extracted from soils samples by the wet sieving method. Spores abundance, species richness, and diversity are determined. A total of 19 AMF morphotypes distributed in 7 genera (Gigaspora, Scutellospora, Glomus, Acaulospora, Archaeospora, Entrophospora, and Paraglomus) are observed. Glomus species are abundant in all sites. Spore densities are higher in Amuria than in Serere. Soil pH, CEC, and phosphorus content influence AMF distribution. Finding the species in various agroecological environments indicates that they are adapted to the environments. Maize grown in eastern Uganda is associated with a diversity of AMF that could be selected as bio-fertilizers to improve crop production.
1. Introduction
Maize (Zea mays L.), a tropical cereal crop, is one of the most economic and nutritional cereals grown in the African continent. It is used as a staple food and as livestock feed, and also in industrial production (biofuel) [1]. In sub-Saharan Africa (SSA), particularly in the eastern region, maize is the main source of carbohydrates and protein that contribute to populations’ food security [2]. Its dry grains contain a high level of carbohydrate (73%), protein (9.1–14%), and oligo-elements such as iron and zinc [3,4]. In addition, another important beneficial attribute of this cereal is that it is particularly adapted to regions of limited rainfall (under 600 mm per year), and those that are sub-humid to semi-arid (1000–1500 mm per year). In Uganda, it is the most important cereal crop; it accounts for 16% of caloric intake and, per capita, maize consumption ranges from 28 to 125 kg per annum [5]. Maize is planted in all agroecological zones in Uganda, but more intensely in the eastern region (Mbale, Jinja, Iganga, and Teso sub-regions). More than 90% of Uganda’s maize is produced by small-hold farmers, of which about 60% of the annual production is consumed on the farm, and it is generally managed with low- or zero-input technology, due to the capacity of farmers in buying chemical fertilizers [6]. It is reported that 2.9 million tons of maize grain were produced in 2018, but this level of production is largely below the national demand [7]. Constraints limiting maize production in Uganda include pests and diseases [8], as well as low soil fertility and erratic rainfall pattern [9]. The challenge of the National Agricultural Research Organization (NARO) in Uganda is the improvement of maize production using new, affordable, and sustainable technologies, contributing to maintaining biodiversity and preserving the environment. It is proven that the soil microbiota has an important role in the development of sustainable agriculture [10]. Among the key microbial communities developing in the rhizosphere are arbuscular mycorrhizal fungi (AMF). This ancient group of fungi is composed of obligate symbionts forming associations with a large majority of plant species (about 72% of terrestrial plant species) [11]. Several studies report on their roles in mineral nutrition and water supply of plants, as well as the effects on improving plant resistance to abiotic and biotic stresses [12,13]. Thus, AMF is used in agriculture to increase crop yields while reducing the application of chemical fertilizer inputs [14]. Numerous studies in Senegal and Kenya show substantial yield increases through long-term mycorrhizal inoculation [15,16]. The selection of efficient production of biofertilizers in quality and quantity are critical issues for the application of AMF technology in agricultural production in Africa. The use of indigenous mycorrhiza from soil has many benefits in restoration schemes, such as their high level of adaptation to local conditions and low ecological risk. However, little is known about native AMF associated with annual crops in fields in Uganda. The first study on mycorrhizal symbiosis in Uganda was conducted on bananas in 2001 by Msiska [17]. One recent study helped to identify the diversity of AMF in Soroti (Uganda) based on spore morphology [18]. Spores are one of the most important and convenient characteristic features, and they can help research in the rapid identification of mycorrhizae, faster than sequence techniques. Many studies reveal that spore morphology is necessary when working on AMF. About 150 AMF species were described employing morphological features of spores. Though several studies were carried out on the use of AMF in crop yield, studies on the morphological diversity of AMF are almost missing in Uganda. To the best of our knowledge, no information is available on the morphological diversity of AMF associated with maize crops grown in East African fields. Hence, the present work was conducted to find out the morpho-types of arbuscular mycorrhizal fungi associated with maize (Zea mays) in eastern Uganda. This study is probably one of the first to assess the diversity of AMF associated with the rhizosphere of maize in East Africa. The results obtained provide background information on the indigenous AMF associated with maize, and are a necessary step towards forecasting the integration of AMF biofertilizer in improving grain yields.
2. Materials and Methods
2.1. Study Area
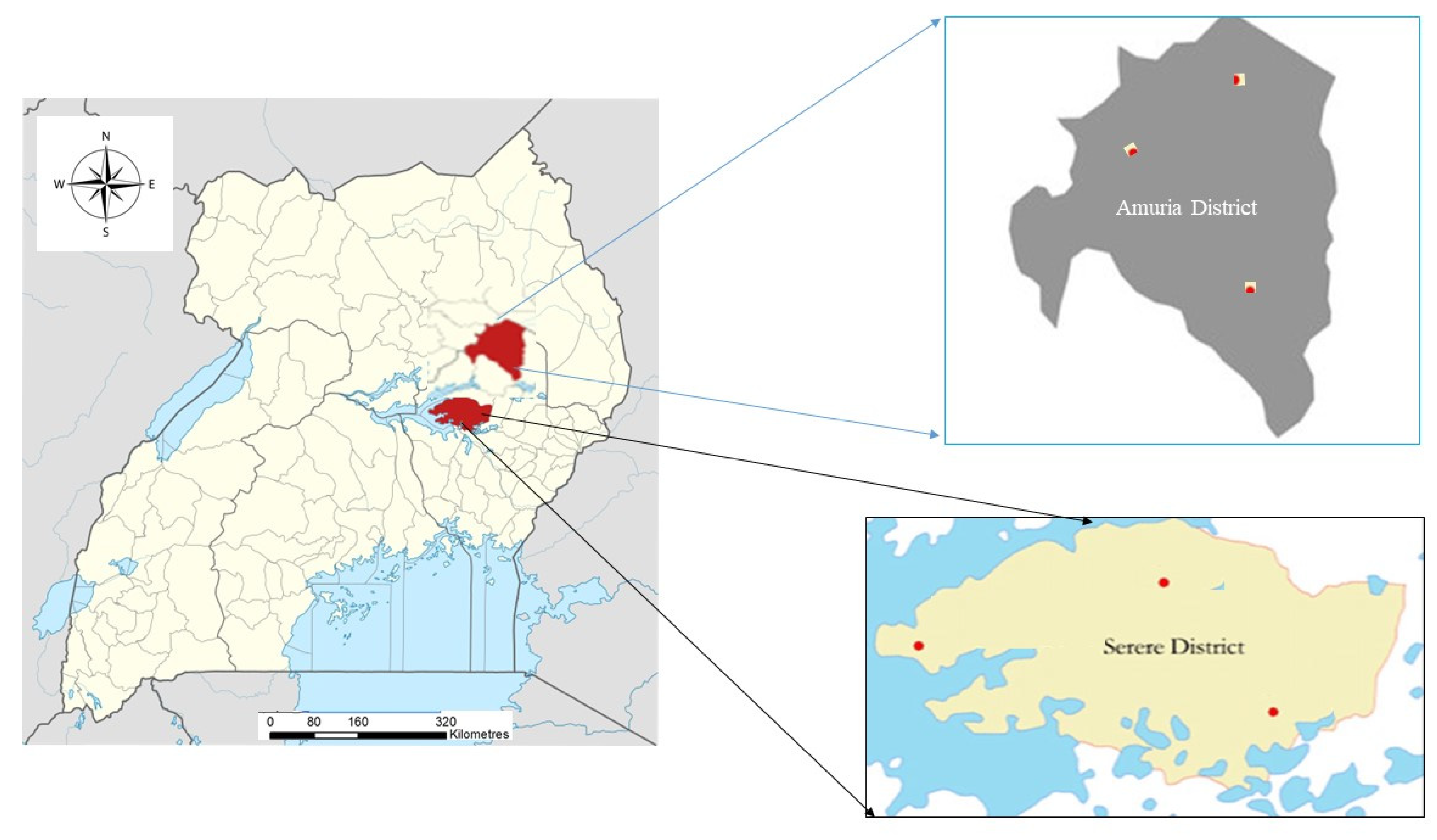
This study arose from the need to achieve a long-term objective: to improve maize production in Uganda by using the diversity of native arbuscular mycorrhizal fungi associated with maize rhizosphere. The study areas were the Amuria and Serere districts, as depicted in Figure 1. The Serere district is located in eastern Uganda, in the Teso sub-region, and covers approximately 1965 km2 [19], with an elevation about 1095 m above sea level [20]. The Amuria district is also situated in the Teso sub-region, and covers a surface of 2588 km2, with an elevation of 1096 m above sea level. The area experiences a humid and hot climate, receiving bimodal rainfall, with an annual average of 1350 mm, between March and May. There are decreasing light showers between June and August, and heavier rains again between September and November. The dry season begins in December and lasts until February. The climate of the sub-region is modified by the large swamp wetland area that surrounds it. The minimum temperature is 18 °C and the maximum temperature is 31.3 °C. However, extremes usually occur in February, when the temperature can exceed 35 °C. Teso land slopes from east to west, and lies at a lower altitude than the Karamoja highlands and Sebei uplands sub-regions of Uganda, thereby receiving water discharges [21]. The economic activities in the sub-region are based on subsistence agriculture and livestock rearing. Farmers grow a diversity of crops, especially legumes and cereals including millet, sorghum, rice, and maize.
Figure 1.
Study area and soil sampling sites.
2.2. Soils Sampling and Analysis
Soils were sampled from maize fields, at the end of the rainy season in December 2021. Cores were collected from 20 random points, and the soils were pooled to obtain representative bulk samples. Samples were collected from between 2–30 cm in depth by removing the top 2 cm of soil and excavating maize rhizosphere soil. The fields selected were active, experimental, conventionally managed fields in maize cultivation, and had been used for multiple seasons. The soils were then placed in a zip-lock freezer bag and kept at 4 °C until their utilization. Soil samples from the fields were analyzed in Makerere University lab (Uganda), according to the procedure described by [22]. The soil samples were air-dried and used for the determination of physical and chemical properties including soil texture, pH, organic carbon (C), total nitrogen (N), total phosphorus (P), available phosphorus (P), and the CEC.
2.3. Extraction, Enumeration, and Morphological Identification of AMF Spores
Maize rhizosphere soils collected were wet-sieved and sucrose-centrifuged to extract AMF spores, following the method described by [23]. In summary, 100 g of rhizosphere soil was suspended in 1000 mL tap water, stirred for 1 min, and the solutions were sieved sequentially through 400, 200, 100, and 50 µm sieves under flowing tap water, to separate the spores according to their size. The soil fraction in each sieve was collected into the beaker. Then, spore suspensions were transferred to 50 mL centrifugation tubes and centrifuged with a water–sucrose solution (20% and 60% w/v) for 5 min at 4000 rpm [24]. The supernatant was decanted into a 32 µm sieve, washed, and transferred to Petri dishes for quantification under binoculars, and grouped according to their morphological characteristics (spore size, color, and hyphal attachment). The spore density was expressed as the total number of spores per 100 g of soil [25]. Some spores morphotypes were mounted on slides in polyvinyl–lactic acid–glycerine (PVLG) and a mixture of PVLG with Melzer’s reagent (1:1; v/v) (Brundrett et al., 1996), to observe wall structures and other specific attributes using a compound microscope at 400× magnification (Motic, MIPLUS20). Then, the spores were identified based on descriptions and identification criteria presented on the International Culture Collection of Vesicular–Arbuscular Mycorrhizal Fungi website (http://fungi.invam.wvu.edu/the-fungi/species-descriptions.html (accessed on 20 January 2022), and on the descriptions in the literature. Morphotypes were classified to the genus level and, when possible, to the species level, according to the current valid taxonomy [26].
2.4. AMF Roots Colonization Measurement
Capillary root segments were cleaned in 10% KOH solution for 20 min at 90 °C in a bain-marie, then rinsed with water several times using mesh and forceps, and acidified with 1% HCI solution for 30 min. Finally, the root pieces were stained with 0.05% trypan blue in lactoglycerol (1:1:1 lactic acid, glycerol) for 10 min at 90 °C in a bain-marie. The stained root segments were placed in a 50% (v/v) glycerol solution for distaining. The distained root samples were then placed on slides and observed under a compound microscope. Root segments were randomly chosen from the distained root samples. Ten slides were prepared by mounting 10 root fragments on each; therefore, a total of 100 root fragments were prepared per treatment. Mycorrhizal colonization was estimated according to the method proposed by [27].
2.5. Statistical Analysis
The significance of differences between treatments concerning spore abundance, species numbers, and AMF diversity (Shannon–Weiner index) was tested using Fisher’s least significant difference at p < 0.05 after one-way ANOVA. Statistical analyses for spore abundance, species numbers, and AMF diversity (Shannon–Weiner index) were performed with Minitab software version 17. The data were subjected to an analysis of variance (ANOVA) test. The Fisher least significant difference (LSD) was used to separate the means at the 5% level of significance (p < 0.05). Linear regression clarified the influence of soil chemical parameters on communities of AMF. Principal components analysis (PCA) was performed using XlStat Excel extension 2020 to determine the relationship between soil chemical properties and spore’s abundance. The principal component analysis (PCA) analyzed species composition about soil chemical properties using XlStat.
The diversity was calculated from the Shannon–Weiner diversity index (H’) with the formula:
where ni = the spore abundance for an individual species (S) and N = the total spore abundance of the population of all the species in a sample.
A low H′ value generally suggests a site with few species and a few dominant species, while a high H′ value suggests considerably more species. The more species we observe, the more diverse the area.
3. Results
3.1. Soils Chemical and Physical Characterization
The characteristics of the soils from the study sites are given in Table 1. The soil pH from Amuria is neutral (6.0 < pH < 7.5), while the soils from Serere are strongly acidic (pH < 5.5). The P content also differs, with significant variation between sites, as the levels of P contents are 9.52 mg/kg and 4.62 mg/kg for Amuria and Serere, respectively. The organic matter is moderate in Amuria (2.09%) and Serere (1.952%). The CEC is low (8.6 cmol/kg) in the sandy loam soil from Amuria, and moderate (19.9 cmol/kg) in the clay loam from Serere. Total nitrogen (TN) differs significantly between sites; it is 0.047% and 0.093% for Amuria and Serere, respectively.

Table 1.
Physical and chemical properties of soils in the study area.
3.2. Intensity of Maize Mycorrhization
The microscopic observation of maize root samples shows the presence of hyphal, vesicular, and arbuscular features of AMF, and the presence of spores within the roots. Statistical analysis of the frequency and intensity of mycorrhization shows that the site has no significant effect on the mycorrhizal colonization of roots. We notice that the roots isolated from the Amuria fields show almost a similar value for mycorrhization intensity (56%) when compared to the Serere field roots that present intensity of (55%) (Table 2).

Table 2.
AMF spore abundance, species richness, and intensity of mycorrhization found in the field samples of the different sites.
3.3. Diversity and Abundance of AMF Spores
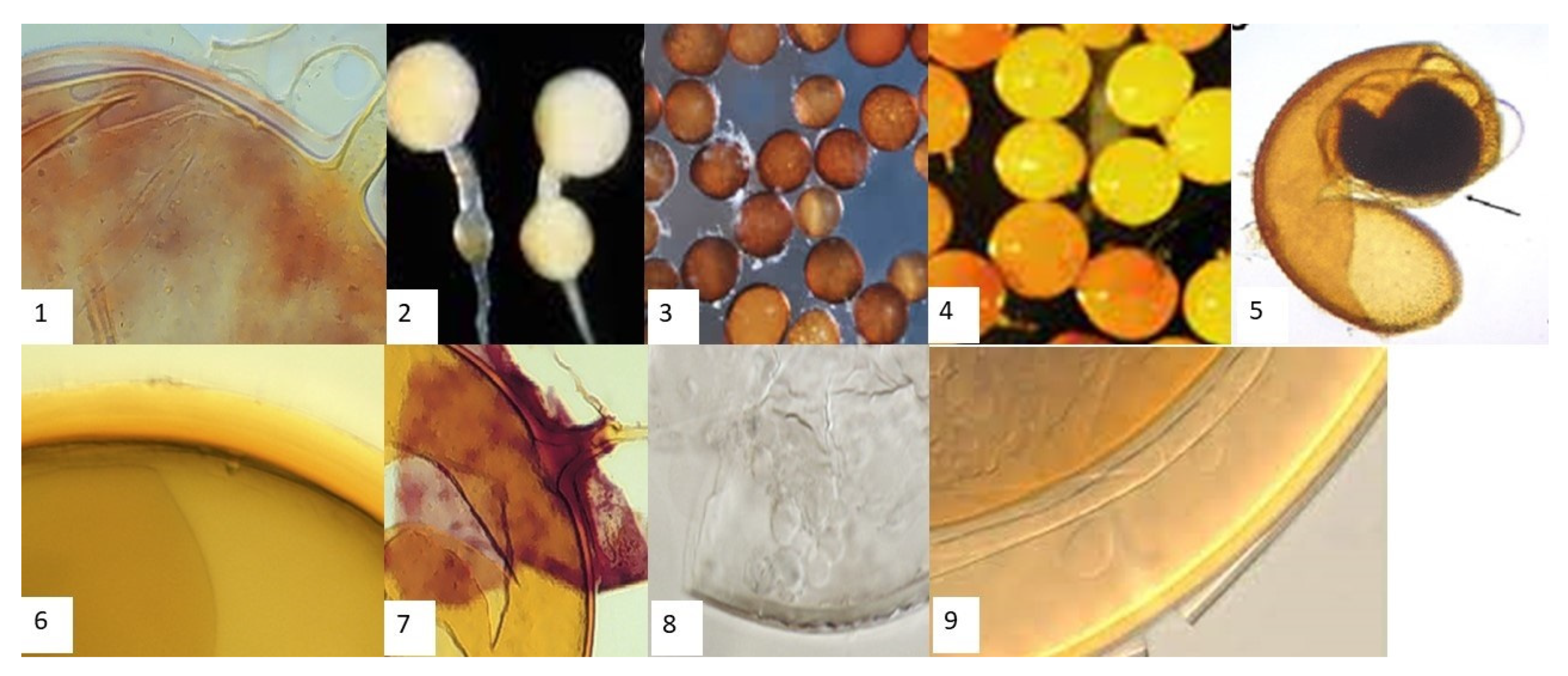
Seven genera of AMF are associated with maize in both sites. The genera are Gigaspora, Scutellospora, Glomus, Acaulospora, Archaeospora, Entrophospora, and Paraglomus, as described by [28]. In total, 19 AMF morphotypes are recovered. Eight species are unequivocally assigned to known species of the Glomales, namely, three species of the genus Glomus, two of the genus Acaulospora, one of the genus Gigaspora, one of the genus Scutellospora, and one of the genus Entrophospora. Eleven spore types are not distinguishable at the species level, because they lack enough distinct features. Figure 1 presents some AMF spores observed in the study area. As shown in Table 3, all of the 19 AMF species found in the present study are present in the samples from Amuria. Only 17 species are observed in the samples from Serere. Regarding relative spore abundance, most of the Glomus species show no significant differences between Amuria and Serere. Glomus and Acaulospora spores are relatively more numerous than other AMF spore types in the Amuria fields, whereas Glomus and Entrophospora spores are relatively more abundant than other AMF spore types in the Serere fields. Interestingly, spores of Paraglomus are not found in Serere. Spores of the genera Archaeospora, Gigaspora, and Scutellospora are found to be ubiquitous in all the sites, and they are: Archaeospora schenckii, Archaeospora sp., Gigaspora gigantean, Gigaspora sp., Scutellospora. Pellucida, and Scutellospora sp.

Table 3.
Relative spore abundance (%) (mean of three field replicates) from each AMF species distinguished in the soil samples from the different sites.
The number of AMF spores per gram of soil and the number of AMF species found per site are shown in Table 3. The highest value is observed in Amuria, with 5.1 spores g−1 of soil (p < 0.05) compared to Serere (3.7 spores g−1 of soil) (Figure 2). Likewise, the species numbers are higher in Amuria compared to Serere. The AMF species diversity, as expressed by the H′, does not show much difference between sites, with higher diversity obtained in the soils from Amuria (H’ = 1.84) compared to Serere (H′ = 1.81) (Table 3). The Shannon–Weiner diversity index (H’) shows similar values (3.35–3.38) for all soil microbial communities, and these differences between soils are not statistically significant (Table 2).
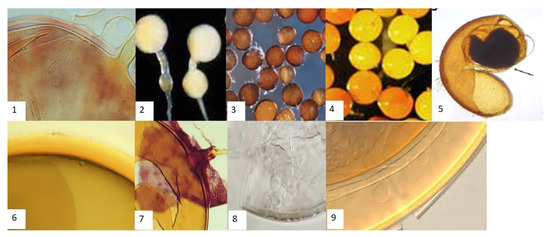
Figure 2.
AMF Spores isolated from the rhizosphere of maize in eastern Uganda. 1—Glomus intraradices. 2—Entrophospora colombiana. 3—Acaulospora foveate. 4—Gigaspora gigantean. 5—Acaulospora spinose. 6—Glomus geosporum. 7—Glomus mossae. 8—Archaeospora schenckii. 9—Scutellospora pellucida.
3.4. Relationship between Soil Chemical Properties and Species Diversity
A strong positive and statistically significant correlation is observed between the soil chemical parameters (pH and P) and the species (b, c, e, g, j m, o, q, s, u, and v) (Table 4). The same species are negatively correlated with the CEC. Chemical parameters (such as O.M and TN) are not correlated with the species abundance.

Table 4.
Pearson’s correlation coefficients between soil chemical properties and AMF community.
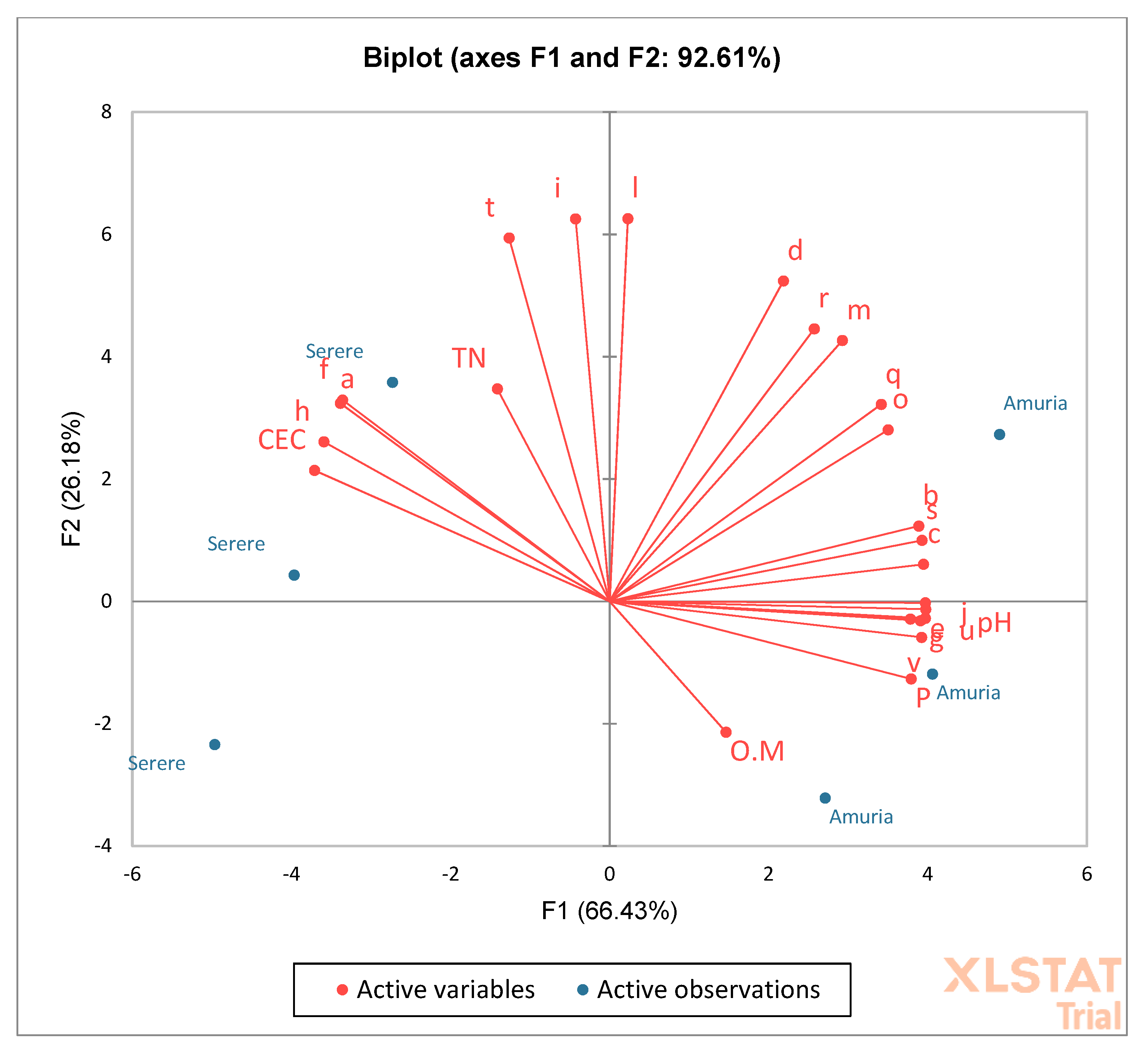
To understand the correlation between the AMF community structure and soil chemical parameters, principal component analysis (PCA) was undertaken (Figure 3). A large number of species occur in soils with lower CEC and sufficient available P, and tend to prefer neutral soils. The distribution of the species is mostly influenced by the pH, CEC, and P. The abundance of the majority of spores is positively correlated with P and pH, and negatively correlated with CEC. This implies that the higher the pH and available P, and the lower the CEC, the higher the species abundance, and vice versa.
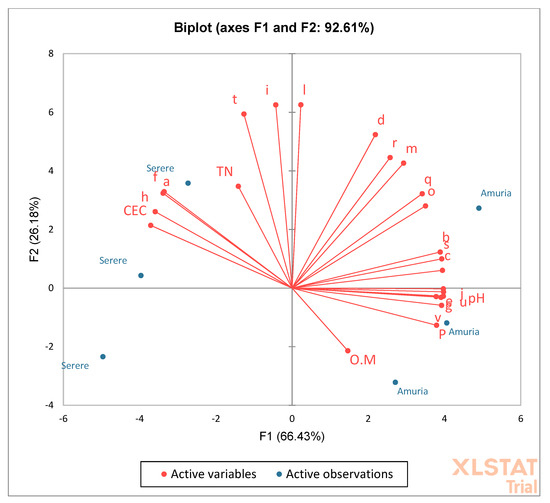
Figure 3.
Principal component analysis of the species occurrence related to soil chemical properties.
4. Discussion
4.1. Intensity of Maize Mycorrhization
The intensities of the mycorrhization of maize roots observed in this study are high (about 55%), and show no significant differences between the sites. These results confirm those obtained by [29,30], who observed a mycorrhization rate of between 50% and 70%. This can be explained by the fact that maize roots are less abundant, stocky, and devoid of absorbent hairs, and are, therefore, particularly dependent on AMF [31]. The root system of maize is characterized by the presence of adventitious roots that only absorb nutrients from the superficial soil layer. Thus, AMF helps root development, and improves water and nutrient uptake from the soil.
4.2. Diversity and Abundance of AMF Spores
Taking into account all the AMF species identified directly in the field samples, 19 species are divided into 7 genera: Gigaspora, Scutellospora, Glomus, Acaulospora, Archaeospora, Entrophospora, and Paraglomus, which are observed under maize cultivation in all sites. This specific richness is higher than that obtained for the sorghum crops in Uganda by [18] and by [32] for pearl millet in Senegal, as well as by [33] for maize crop in Benin. On the other hand, this specific richness is lower than that obtained from the maize field (39 species) by [34] in South Kivu (DR Congo). This difference could be due to the period of collection of soil samples and previous crops, which are important parameters to consider in the evaluation of the density of spores in the soil. The seven genera of AMF are described by [35,36,37,38]. Acaulospora, Entrophospora, and Glomus genera constitute the dominant genera in this area. This finding is in line with the results of [39] in India. These authors demonstrate that Glomus, Gigaspora, Scutellospora, Acaulospora, Paraglomus Archaeospora, and Funneliformis are the most dominant genera associated with maize crops. The results reported in this study indicate the dominance of spores of the Glomus genus. Glomus appears to be the most dominant in croplands [32], and they are the principal maize colonizers [40]. The predominance of species of the genus Glomus in the study area suggests a better adaptation of this genus to the environmental conditions and a wide range of ecological niches [41]. In addition, ref [42,43] demonstrate that maize monoculture also reduces AMF diversity. Maize monoculture is practiced in the fields where the soils are sampled. The result reveals that the area is rich in spores of the Archaeospora and Paraglomus genera. This result is in opposition to those of [44] who observe that Archaeospora and Paraglomus genera are not colonizers of maize grown in monoculture. The results of the diversity index indicate that the AMF community is very diverse, regardless of the site. In this study, the high diversity of AMF species found associated with maize might mean that the mycorrhizal fungi host specificity is low in maize crops. Host specificity of AMF is a longtime debate among researchers. Many authors argue that AMF has no host-plant specificity [45], but several studies demonstrate the preference of some AMF genera for some plant species [46]. Thus, the maize crop has a relatively high mycorrhizal dependency [47]. This mycorrhizal dependency is characterized by the type of crop, the soil properties, and the effect of the cropping system [48]. The variation in diversity index in the area, which varies between 1.81 and 1.84, might result from the variability in soil properties and management practices observed in the study area, where most of the fields are managed in a conventional setting with a medium level of nutrient input. This is in line with the findings of [49], who observe that a high diversity of AMF species results from management practices that affect the nutrient status in the soil.
The number of AMF morphotypes identified is high, which means that eastern Uganda has a high diversity of AMF species. Some AMF species could not be identified in the field samples; the morphological features of the spores were not distinct enough and, thus, these spores could only be grouped into a species group (for example Acaulospora sp.). Using bimolecular tools, this area could reveal greater AMF diversity. Therefore, to ensure the species composition, molecular methods of analysis should be used to confirm the identification of these species.
The results from this study reveal that spore density varies between 3.7 to 5.1 spores per g of soil for all sites. This density is significantly lower than that obtained by [33] (about 1340 spores per g of soil) for maize in Benin but is higher than that observed in DR Congo (0.7 to 1.5 spores per g of soil) by [34]. This difference could be due to the period of collection of soil samples and previous crops, which are important parameters to consider in the evaluation of the density of spores in the soil. According to [50,51], the number of spores is higher in the soil after long-term stress conditions. The number of undiscovered morphotypes may be even higher, affirming that many species are yet to be discovered. Spores abundance is moderate in the field soils. Similar results of moderate abundance are reported in the Willamette Valley of western Oregon (USA) in a field evaluation of AMF fungal diversity by Cheeke et al. 2013, who recorded 15.42 and 16.05 spores g-1. Low spore abundance was discovered in South Kivu (0.6 to 1.5 spores g-1) [34].
4.3. Relationship between Soil Chemical Properties and Species Diversity
In this study, differences in the composition of AMF communities associated with maize were recorded and characterized. The difference in the composition of AMF diversity indicates that soil properties are the main factors of AMF communities associated with maize. Indeed, previous studies demonstrate the influence of soil properties on the composition of AMF communities [52,53]. The results from this study show the existence of a strong relationship between some chemical parameters and the total species diversity and density in the study area. These results are consistent with those of [33]. There is a positive and negative correlation between available P, pH, and CEC, and spore density and diversity, depending on the genus. The low number of morphotypes recovered in Serere compared to Amuria could be explained by the chemical properties of the sandy loam soils, which are neutral with a high level of available P. According to [54], AMF proliferation depends on pH, with a preference for slightly acidic conditions. This is in contradiction with the results of this study, which reveals that AMF is more abundant in neutral soil than in acidic soil. The genera Acaulospora and Glomus show a positive correlation with soil pH. This finding is consistent with the observation of [55]. In addition, AMF plays a major role in P mobilization for the host plant. Depending on the pH of the soil, this element is strongly adsorbed by iron, aluminum, or calcium, in forms that are not available for plants [56]. Phosphorus is a limiting factor for the abundance of AMF spores, whether at very high or very low concentrations [57,58]. Mycorrhizal associations play a significant role in the mineralization of organic phosphorus and mobilize nutrients for the benefit of the host plant [59]. In addition, the diversity and abundance of AMF species concerning soil chemical properties vary among species, as some species are associated with some specific soil conditions. For instance, in this study, AMF species of the genus Entrophospora prefer acidic soil, low P content, and high soil CEC, while the genus Paraglomus are only found in neutral soils with a high level of P content. Many species are found to be generalists in all the sites, especially the species from the Archaeospora, Gigaspora, Scutellospora, and Glomus genera, which are not dependent on soil chemical properties. This study reveals that maize can associate with a range of AMF; thus, a mycorrhizal-based fertilizer produced from the associated species could have a higher chance of thriving in the region.
5. Conclusions
Our study is the first report on arbuscular mycorrhizal fungi (AMF) diversity associated with maize in Uganda. The overall objective of this study was to highlight the diversity of AMF associated with maize in the eastern region of Uganda. Little is currently known about the AMF composition in the roots of other cereal or other plants in the semi-arid regions of Uganda. The results show a higher spore density and a dominance of the Glomus genus in the rhizosphere soil of maize. A total of 19 species divided into 7 genera are identified, with an abundance of the Glomus genus. However, further research is needed to identify and explore more diversity of AMF species associated with this cereal. The molecular identification of the species is recommended for accurate identification. There is a strong relationship between the chemical parameters and the total spore density in the area, as well as with the diversity. The soil pH and P contribute to the distribution of AMF species. Some of the species from the genera Gigaspora, Acaulospora, Glomus, Archaeospora, Gigaspora, and Scutellospora are found in all sites. These genera can be recommended for further research aimed at improving the agricultural benefits of indigenous AMF for enhanced root functions, and the subsequent productivity in farming systems in the region.
Author Contributions
A.F.F. contributed to the inception of the paper, research, and writing. G.N. contributed to the inception and reviews of the paper. J.S. contributed to reviews. H.F.-M. contributed to the inception of the work. A.B. contributed to the data analysis. I.B. and M.N. contributed to the write-up and review. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Regional Academic Exchange for Enhanced Skills in Fragile Ecosystem Management in Africa (REFORM), grant number: 2017–2861.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors are thankful to Keryose, Katende, Jude Ssebuwufu, and J.B. of the College of Agriculture Sciences and Biotechnology department, Makerere University for providing laboratory facilities.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Ekpa, O.; Palacios-Rojas, N.; Kruseman, G.; Fogliano, V.; Linnemann, A.R. Sub-Saharan African maize-based foods-processing practices, challe, needs, and opportunities. Food Rev. Int. 2019, 35, 609–639. [Google Scholar] [CrossRef] [Green Version]
- Mumo, L.; Yu, J.; Ojara, M.; Lukorito, C.; Kerandi, N. Assessing changes in climate suitability and yields of maize and sorghum crops over Kenya in the twenty-first century. Theor. Appl. Climatol. 2021, 146, 381–394. [Google Scholar] [CrossRef]
- Ochieng, I.O.; Gitari, H.I.; Mochoge, B.; Rezaei-Chiyaneh, E.; Gweyi-Onyango, J.P. Optimizing maize yield, nitrogen efficacy, and grain protein content under different N forms and rates. J. Soil Sci. Plant Nutr. 2021, 21, 1867–1880. [Google Scholar] [CrossRef]
- Wu, Y.; Messing, J. Proteome balancing of the maize seed for higher nutritional value. Front. Plant Sci. 2016, 5, 240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharon, B.; Michael, M.; Bwayo, M.F. Severity and prevalence of the destructive fall armyworm on maize in Uganda: A case of Bulambuli District. Afr. J. Agric. Res. 2020, 16, 777–784. [Google Scholar]
- UBOS. Uganda National Panel Survey 2015/2016, Technical Report 1224; Uganda Bureau of Statistics: Kampala, Uganda, 2016. [Google Scholar]
- FAO. World Food and Agriculture—Statistical Yearbook; FAO: Rome, Italy, 2020. [Google Scholar]
- Oloo, A. Constraints to Maize Production in Kamuli District, Eastern Uganda. Ph.D. Thesis, Makerere University, Kampala, Uganda, 2021. [Google Scholar]
- Okoboi, G.; Muwanga, J.; Mwebaze, T. Use of improved inputs and its effect on maize yield and profit in Uganda. Afr. J. Food Agric. Nutr. Dev. 2012, 12, 7. [Google Scholar] [CrossRef]
- Yadav, A.N. Plant Microbiomes for Sustainable Agriculture: Current Research and Future Challenges. In Sustainable Development and Biodiversity; Springer Nature: Cham, Switzerland, 2020; Volume 25, pp. 475–482. [Google Scholar]
- Brundrett, M.C.; Tedersoo, L. Evolutionary history of mycorrhizal symbioses and global host plant diversity. New Phytol. 2018, 220, 1108–1115. [Google Scholar] [CrossRef]
- Wu, Q.S. Arbuscular Mycorrhizas and Stress Tolerance of Plants; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Dowarah, B.; Gill, S.S.; Agarwala, N. Arbuscular Mycorrhizal Fungi in Conferring Tolerance to Biotic Stresses in Plants. J. Plant Growth Regul. 2022, 41, 1429–1444. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, Y.; Jiang, S.; Deng, Y.; Christie, P.; Murray, P.J.; Li, X.; Zhang, J. Arbuscular mycorrhizal fungi in soil and roots respond differently to phosphorus inputs in an intensively managed calcareous agricultural soil. Sci. Rep. 2016, 6, 24902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leye, E.H.M.; Ndiaye, M.; Diouf, M.; Diop, T. Etude comparative de l’effet de souches de champignons mycorhiziens arbusculaires sur la croissance et la nutrition minérale du sésame cultivé au Sénégal. Afr. Crop Sci. J. 2015, 23, 211–219. [Google Scholar]
- Nyaga, J.; Jefwa, J.M.; Muthuri, C.W.; Matiru, V.N.; Wachira, P.M.; Okoth, S.A. Arbuscular mycorrhizal fungi with different soil fertility amendment practices in agricultural landscapes of Kenyan Highlands. Nutr. Cycl. Agroecosyst. 2015, 103, 229–240. [Google Scholar] [CrossRef]
- Msiska, Z. Arbuscular Mycorrhizal Fungi of Ugandan Banana Plantation Soils. Ph.D. Thesis, University of Pretoria, Pretoria, South Africa, 2007. [Google Scholar]
- Sebuliba, E.; Nyeko, P.; Majaliwa, J.G.M.; Kizza, L.C.; Eilu, G.; Adipala, E. Effect of selected arbuscular mycorrhiza fungi on the growth of Calliandra calothyrsus and Sorghum bicolor in eastern Uganda. In Proceedings of the Second RUFORUM Biennial Meeting, Entebbe, Uganda, 20–24 September 2010; pp. 20–24. [Google Scholar]
- Agwot, R.K. Contract Management and Service Delivery in Local Governments in Uganda: A case of Serere District Local Government. Ph.D. Thesis, Uganda Management Institute, Kampala, Uganda, 2018. [Google Scholar]
- Nsabagwa, M.; Mwije, A.; Nimusiima, A.; Odongo, R.I.; Ogwang, B.A.; Wasswa, P.; Mugume, I.; Basalirwa, C.; Nalwanga, F.; Kakuru, R.; et al. Examining the Ability of Communities to Cope with Food Insecurity due to Climate Change. Sustainability 2021, 13, 11047. [Google Scholar] [CrossRef]
- Egeru, A. Role of indigenous knowledge in climate change adaptation: A case study of the Teso Sub-Region, Eastern Uganda. Indian J. Tradit. Knowl. 2012, 11, 217–224. [Google Scholar]
- Okalebo, J.R.; Gathua, K.W.; Woomer, P.L. Laboratory Methods of Soil and Plant Analysis: A Working Manual, 2nd ed.; Sacred Africa: Nairobi, Kenya, 2002; Volume 21, pp. 25–26. [Google Scholar]
- Gerdemann, J.W.; Nicolson, T.H. Spores of mycorrhizal Endogone species extracted from soil by wet sieving and decanting. Trans. Br. Mycol. Soc. 1963, 46, 235–244. [Google Scholar] [CrossRef]
- Oehl, F.; Sieverding, E.; Ineichen, K.; Mäder, P.; Boller, T.; Wiemken, A. Impact of land-use intensity on the species diversity of arbuscular mycorrhizal fungi in agroecosystems of Central Europe. Appl. Environ. Microbiol. 2003, 69, 2816–2824. [Google Scholar] [CrossRef] [Green Version]
- Sasvári, Z.; Magurno, F.; Galanics, D.; Hang, T.T.N.; Ha, T.T.H.; Luyen, N.D.; Posta, K. Isolation and identification of arbuscular mycorrhizal fungi from agricultural fields of Vietnam. Am. J. Plant Sci. 2012, 3, 1796–1801. [Google Scholar] [CrossRef] [Green Version]
- Redecker, D.; Schüßler, A.; Stockinger, H.; Stürmer, S.L.; Morton, J.B.; Walker, C. An evidence-based consensus for the classification of arbuscular mycorrhizal fungi (Glomeromycota). Mycorrhiza 2013, 23, 515–531. [Google Scholar] [CrossRef]
- Trouvelot, A. Mesure du taux de mycorhization VA d’un système radiculaire. Recherche de méthodes d’estimation ayant une signification fonctionnelle. In Physiological and genetical aspects of mycorrhizae, Proceedings of the 1st European Symposium on Mycorrhizae, Dijon, France 1–5 July 1985; INRA: Paris, France, 1986; pp. 217–221. ISBN 2-85340-774-8. [Google Scholar]
- Morton, J.B.; Benny, G.L. Revised classification of arbuscular mycorrhizal fungi (Zygomycetes): A new order, Glomales, two new suborders, Glomineae and Gigasporineae, and two new families, Acaulosporaceae and Gigasporaceae, with an emendation of Glomaceae. Mycotaxon 1990, 37, 471–491. [Google Scholar]
- Houngnandan, P.; Yemadje, R.G.H.; Kane, A.; Boeckx, P.; Van Cleemput, O. Les glomales indigènes de la forêt claire à Isoberlinia doka (Craib et Stapf) à Wari-Maro au centre du Bénin. Tropicultura 2009, 27, 83–87. [Google Scholar]
- Tawaraya, K. Arbuscular mycorrhizal dependency of different plant species and cultivars. Soil Sci. Plant Nutr. 2003, 49, 655–668. [Google Scholar] [CrossRef]
- Garbaye, J. La Symbiose Mycorhizienne: Une Association Entre Les Plantes et Les Champignons. La Symbiose Mycorhizienne; Editions Quae: Versailles, France, 2013; pp. 1–280. ISBN 9782759219636. [Google Scholar]
- Fall, A.F.; Founoune-Mboup, H.; Diatta, S.; Diakhaté, S.; Ndoye, I. Arbuscular mycorrhizal fungi associated with the rhizosphere of Piliostigma reticulatum and Guiera senegalensis shrubs in Senegal. Afr. Crop. Sci. J. 2021, 29, 433. [Google Scholar] [CrossRef]
- Borriello, R.; Lumini, E.; Girlanda, M.; Bonfante, P.; Bianciotto, V. Effects of different management practices on arbuscular mycorrhizal fungal diversity in maize fields by a molecular approach. Biol. Fertil. Soils 2012, 48, 911–922. [Google Scholar] [CrossRef]
- Blaszkowski, J.; Czerniawska, B. Arbuscular mycorrhizal fungi (Glomeromycota) are associated with roots of Ammophila Arenaria growing in maritime dunes of Bornholm (Denmark). Acta Soc. Bot. Pol. 2011, 80, 63–76. [Google Scholar] [CrossRef] [Green Version]
- Hijri, I.; Sýkorová, Z.; Oehl, F.; Ineichen, K.; Maeder, P.; Wiemken, A.; Redecker, D. Communities of arbuscular mycorrhizal fungi in arable soils are not necessarily low in diversity. Mol. Ecol. 2006, 15, 2277–2289. [Google Scholar] [CrossRef] [PubMed]
- Alguacil, M.M.; Lumini, E.; Roldan, A.; Salinas-Garcia, J.R.; Bonfante, P.; Bianciotto, V. The impact of tillage practices on arbuscular mycorrhizal fungal diversity in subtropical crops. Ecol. Appl. 2008, 18, 527–536. [Google Scholar] [CrossRef]
- Sasvári, Z.; Hornok, L.; Posta, K. The community structure of arbuscular mycorrhizal fungi in roots of maize grown in a 50-year monoculture. Biol. Fertil. Soils 2011, 47, 167–176. [Google Scholar] [CrossRef]
- Sanders, I.R. Specificity in the arbuscular mycorrhizal symbiosis. In Mycorrhizal Ecology; Springer: Berlin/Heidelberg, Germany, 2002; pp. 415–437. [Google Scholar]
- Jacquemyn, H.; Merckx, V.; Brys, R.; Tyteca, D.; Cammue, B.P.; Honnay, O.; Lievens, B. Analysis of network architecture reveals phylogenetic constraints on mycorrhizal specificity in the genus Orchis (Orchidaceae). New Phytol. 2011, 192, 518–528. [Google Scholar] [CrossRef]
- Hao, L.; Zhang, Z.; Hao, B.; Diao, F.; Zhang, J.; Bao, Z.; Guo, W. Arbuscular mycorrhizal fungi alter the microbiome structure of rhizosphere soil to enhance maize tolerance to La. Ecotoxicol. Environ. Saf. 2021, 212, 111996. [Google Scholar] [CrossRef]
- Chifflot, V.; Rivest, D.; Olivier, A.; Cogliastro, A.; Khasa, D. Molecular analysis of arbuscular mycorrhizal community structure and spores distribution in tree-based intercropping and forest systems. Agric. Ecosyst. Environ. 2009, 131, 32–39. [Google Scholar] [CrossRef]
- Vieira, C.K.; Marascalchi, M.N.; Rodrigues, A.V.; de Armas, R.D.; Stürmer, S.L. Morphological and molecular diversity of arbuscular mycorrhizal fungi in the revegetated iron-mining sites has the same magnitude as adjacent pristine ecosystems. J. Environ. Sci. 2018, 67, 330–343. [Google Scholar] [CrossRef] [PubMed]
- Bossou, L.D.R.; Houngnandan, H.B.; Adandonon, A.; Zoundji, C.; Houngnandan, P. Diversité des champignons mycorhiziens arbusculaires associés à la culture du maïs (Zea mays L.) au Bénin. Int. J. Biol. Chem. Sci. 2019, 13, 597–609. [Google Scholar] [CrossRef] [Green Version]
- Malembaka, R.E.B.; Onwonga, R.; Jefwa, J.; Ayuke, F.; Nabahungu, L. Diversity and distribution of arbuscular mycorrhizal fungi in maize (Zea mays) cropping fields in South Kivu, Democratic Republic of Congo. Afr. J. Agric. Res. 2021, 17, 604–617. [Google Scholar]
- Johnson, J.M.; Houngnandan, P.; Kane, A.; Sanon, K.B.; Neyra, M. Diversity patterns of indigenous arbuscular mycorrhizal fungi associated with the rhizosphere of cowpea (Vigna unguiculata (L.) Walp.) in Benin, West Africa. Pedobiologia 2013, 56, 121–128. [Google Scholar] [CrossRef]
- Mbogne, J.T.; Temegne, C.N.; Hougnandan, P.; Youmbi, E.; Tonfack, L.B.; Ntsomboh-Ntsefong, G. Biodiversity of arbuscular mycorrhizal fungi of pumpkins (Cucurbita spp.) under the influence of fertilizers in ferralitic soils of Cameroon and Benin. J. Appl. Biol. Biotechnol. 2015, 3, 1–10. [Google Scholar]
- Moreira, H.; Pereira, S.I.; Marques, A.P.; Rangel, A.O.; Castro, P.M. Mine land valorization through energy maize production enhanced by the application of plant growth-promoting rhizobacteria and arbuscular mycorrhizal fungi. Environ. Sci. Pollut. Res. 2016, 23, 6940–6950. [Google Scholar] [CrossRef] [PubMed]
- Crossay, T.; Cilia, A.; Cavaloc, Y.; Amir, H.; Redecker, D. Four new species of arbuscular mycorrhizal fungi (Glomeromycota) associated with endemic plants from ultramafic soils of New Caledonia. Mycol. Prog. 2018, 17, 729–744. [Google Scholar] [CrossRef]
- Songachan, L.S.; Kayang, H. Diversity and distribution of arbuscular mycorrhizal fungi in Solanum species growing in natural conditions. Agric. Res. 2012, 1, 258–264. [Google Scholar] [CrossRef]
- Bohrer, G.; Kagan-Zur, V.; Roth-Bejerano, N.; Ward, D.; Beck, G.; Bonifacio, E. Effects of different Kalahari-desert VA mycorrhizal communities on mineral acquisition and depletion from the soil by host plants. J. Arid Environ. 2003, 55, 193–208. [Google Scholar] [CrossRef]
- Rosendahl, S. Communities, Populations, and individuals of arbuscular mycorrhizal fungi. New Phytol. 2008, 178, 253–266. [Google Scholar] [CrossRef]
- Jansa, J.; Erb, A.; Oberholzer, H.R.; Šmilauer, P.; Egli, S. Soil and geography are more important determinants of indigenous arbuscular mycorrhizal communities than management practices in Swiss agricultural soils. Mol. Ecol. 2014, 23, 2118–2135. [Google Scholar] [CrossRef]
- Xiang, X.; Gibbons, S.M.; He, J.S.; Wang, C.; He, D.; Li, Q.; Ni, Y.; Chu, H. Rapid response of arbuscular mycorrhizal fungal communities to short-term fertilization in an alpine grassland on the Qinghai-Tibet Plateau. PeerJ 2016, 4, e2226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, X.; Yang, W.; Song, F.; Li, X. Diversity and composition of arbuscular mycorrhizal fungal communities in the cropland black soils of China. Glob. Ecol. Conserv. 2020, 22, e00964. [Google Scholar] [CrossRef]
- Séry, D.; Kouadjo, Z.G.; Voko, B.R.; Zeze, A. Selecting native arbuscular mycorrhizal fungi to promote cassava growth and increase yield under field conditions. Front. Microbiol. 2016, 7, 2063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hinsinger, P. Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: A review. Plant Soil 2001, 237, 173–195. [Google Scholar] [CrossRef]
- Amijee, F.; Tinker, P.B.; Stribley, D.P. The development of endomycorrhizal root systems: VII. A detailed study of effects of soil phosphorus on colonization. New Phytol. 1989, 111, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Lagrange, A.; L’Huillier, L.; Amir, H. Mycorrhizal status of Cyperaceae from New Caledonian ultramafic soils: Effects of phosphorus availability on arbuscular mycorrhizal colonization of Costularia comosa under field conditions. Mycorrhiza 2013, 23, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.; Xiao, L.; Liu, R. Response of arbuscular mycorrhizal fungi and phosphorus solubilizing bacteria to remediation abandoned solid waste of coal mine. Int. J. Coal Sci. Technol. 2019, 6, 603–610. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).