A Comprehensive Review of the Strategies to Improve Anaerobic Digestion: Their Mechanism and Digestion Performance
Abstract
:1. Introduction
2. Strategies to Promote the AD Process
2.1. Pre-Treatment
2.1.1. Biological Pre-Treatment
Microbial Pre-Treatment
Enzyme Pre-Treatment
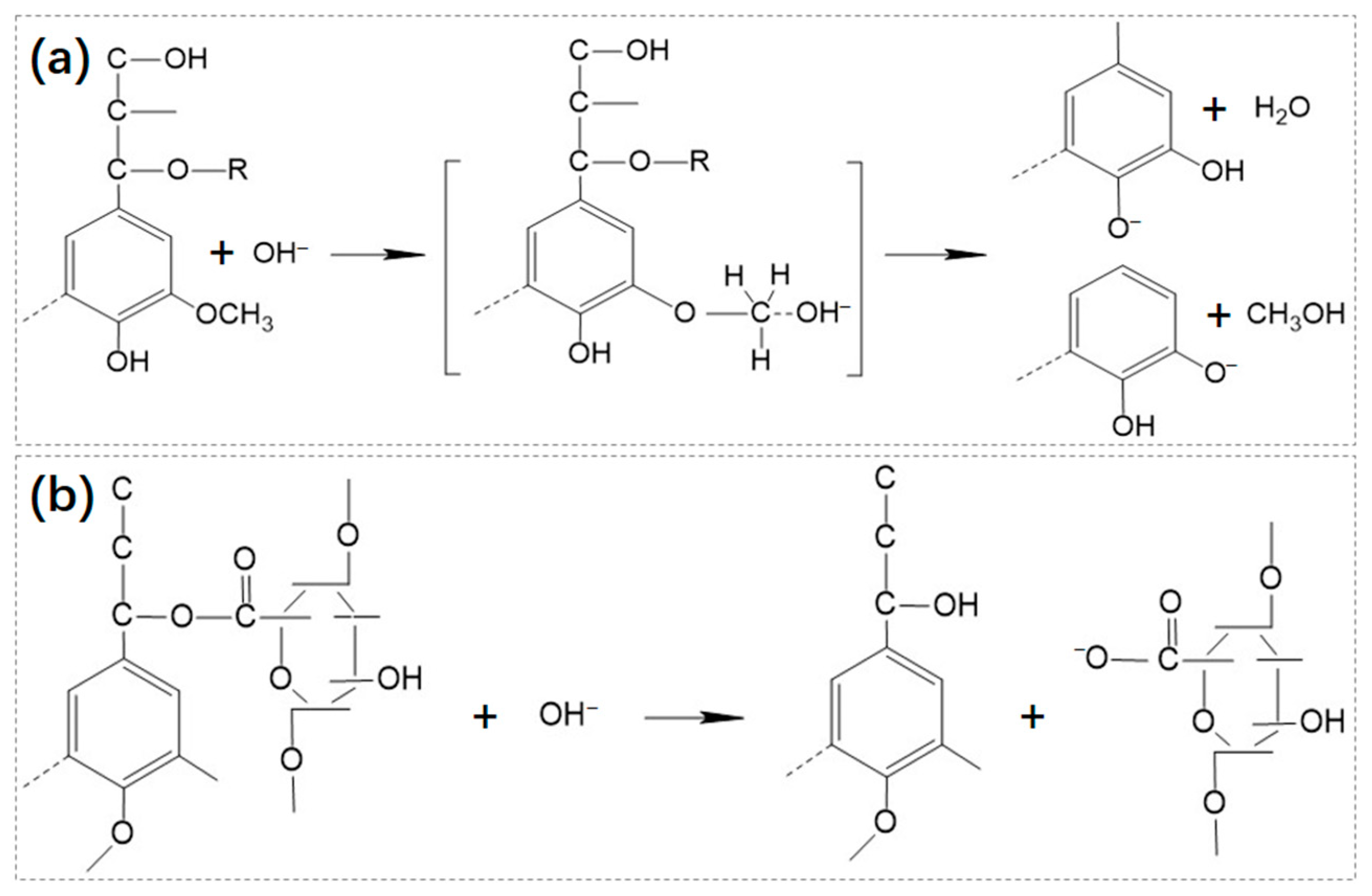
2.1.2. Chemical Pre-Treatment
2.1.3. Physical Pre-Treatment
2.2. Co-Digestion
2.2.1. C/N Ratio: The Most Important Parameter in Co-Digestion
2.2.2. Mechanism by which Co-Digestion Promotes Digestion Efficiency
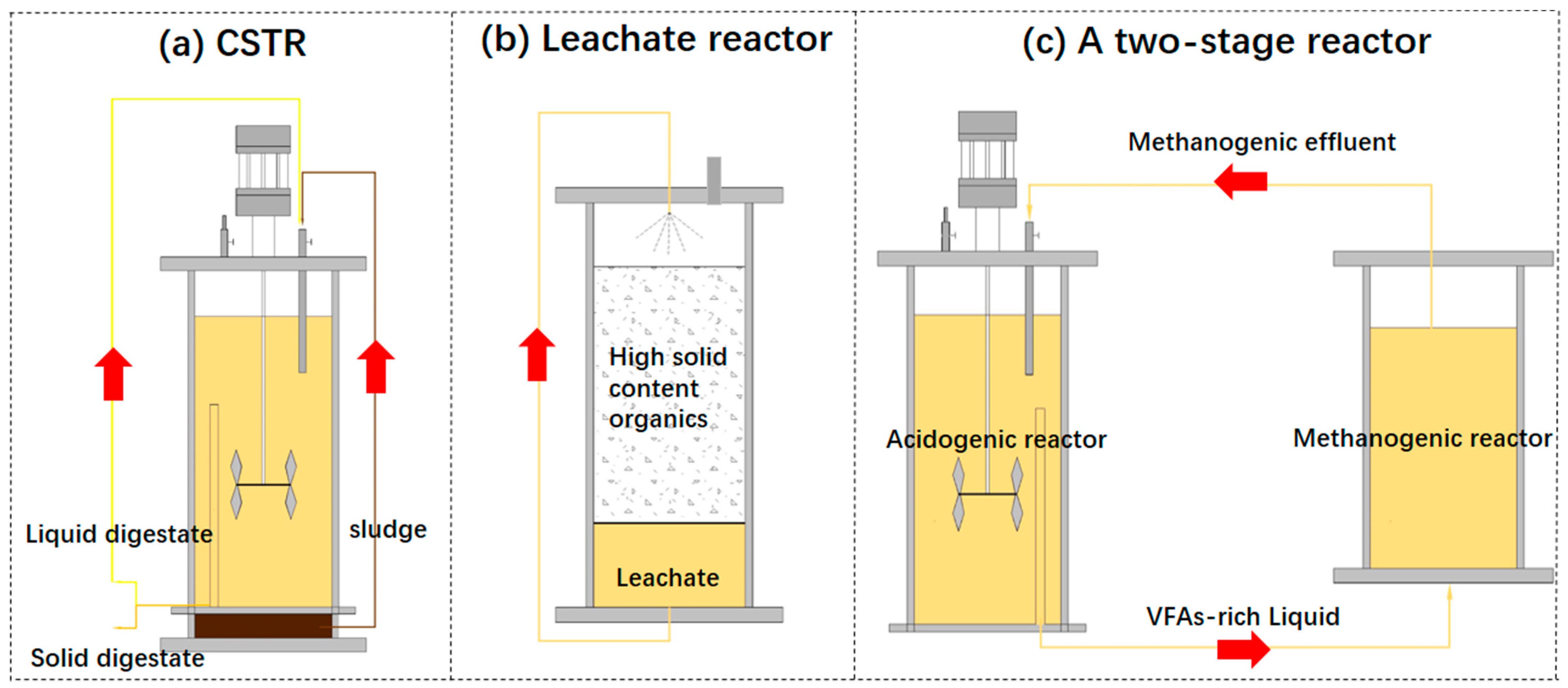
2.3. Recirculation
2.4. Microaeration
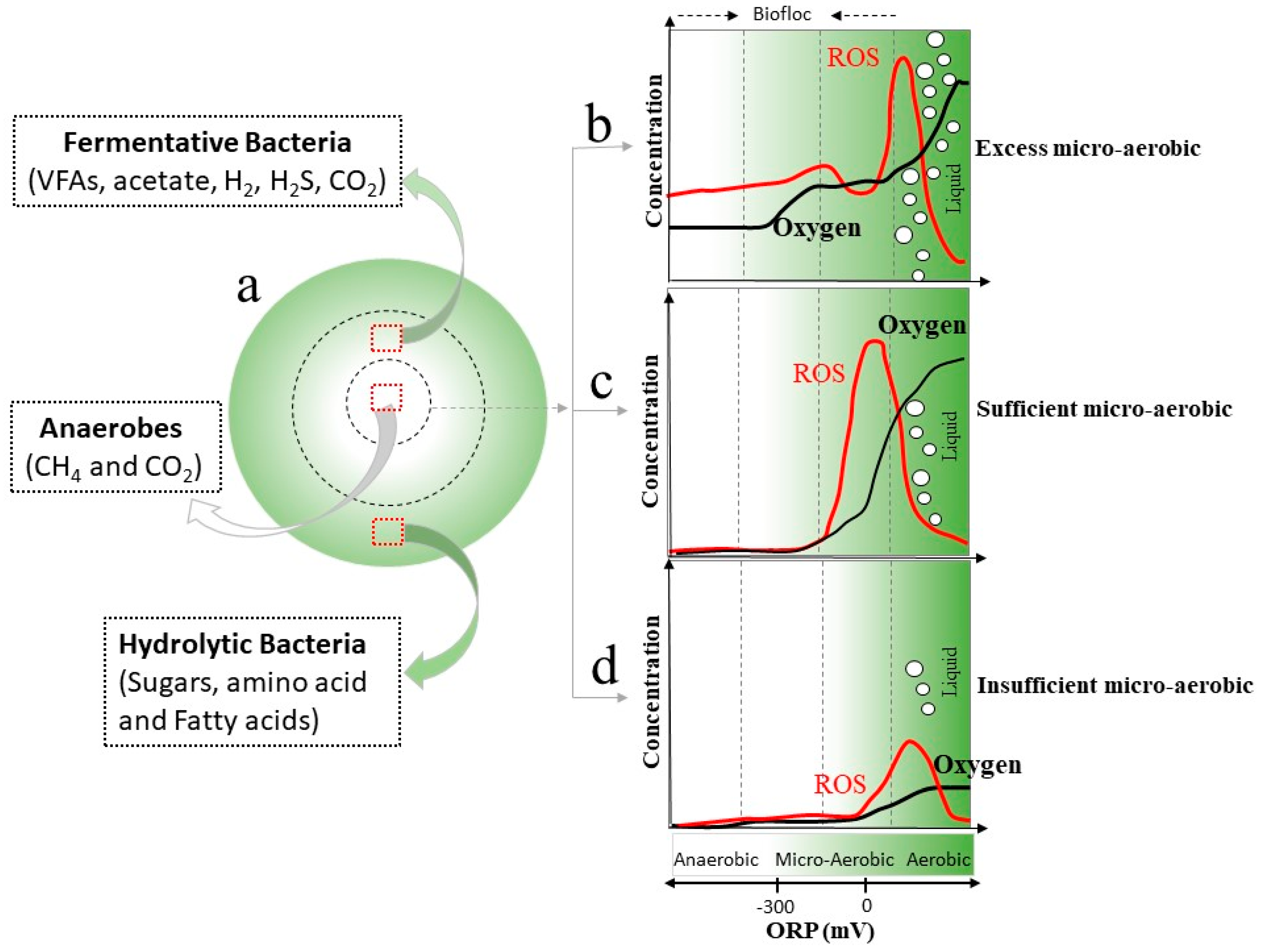
2.4.1. Digestion Performance under Microaerobic Conditions
2.4.2. Co-Existence and Synergistic Interaction Mechanisms between Bacteria and Archaea under Microaerobic Conditions
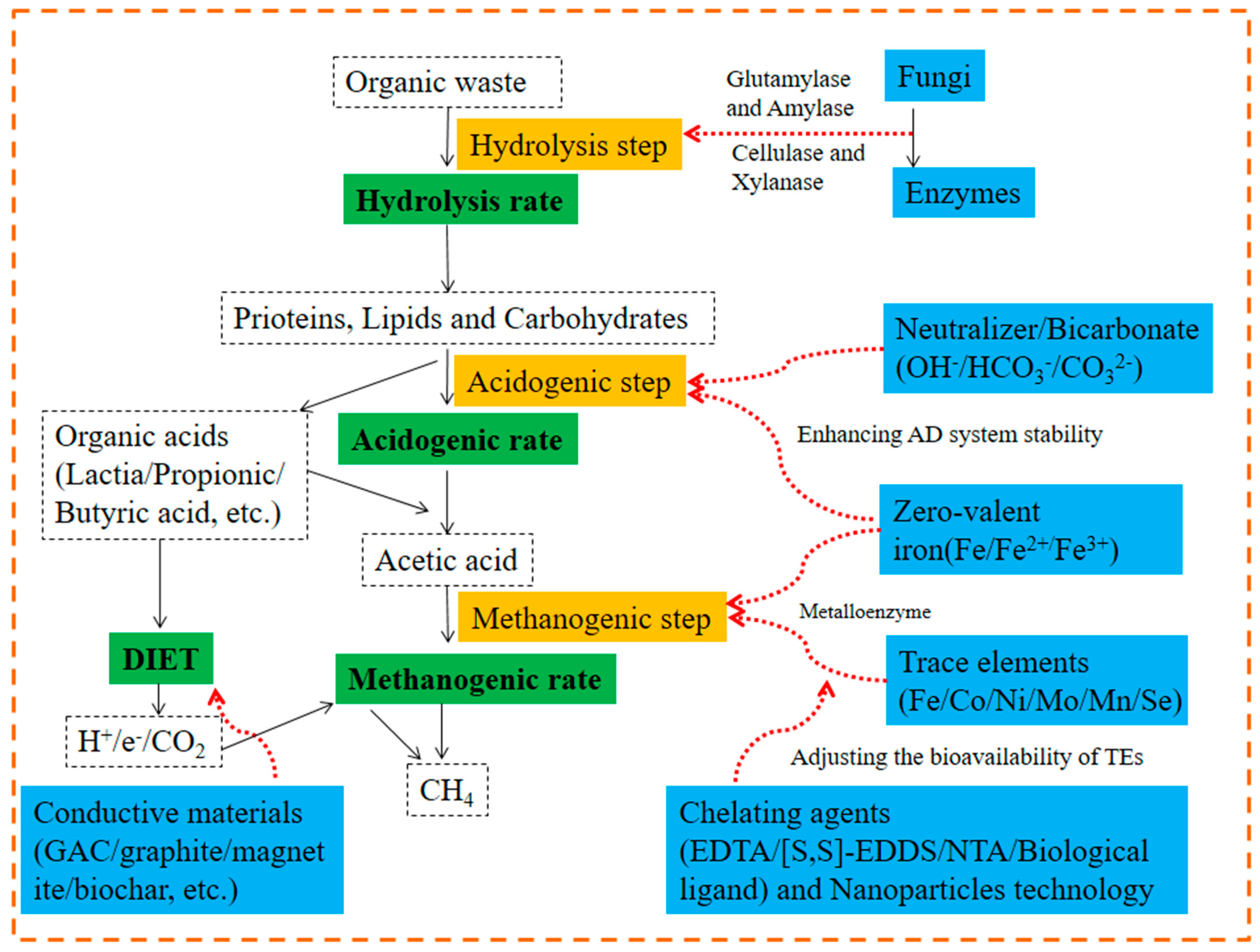
2.5. Additives
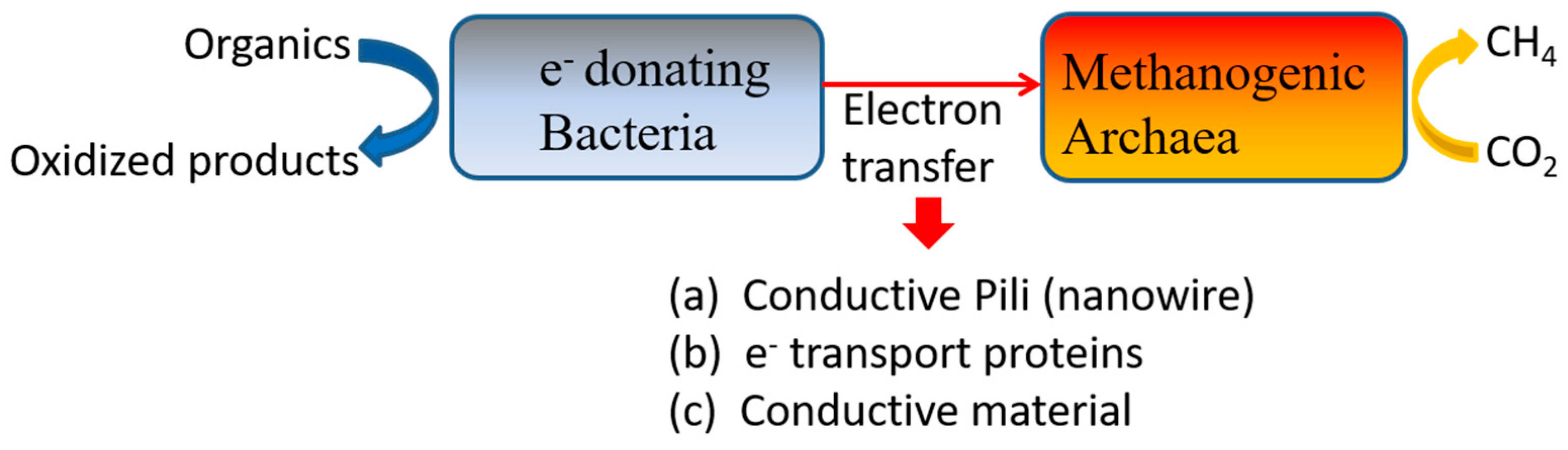
2.5.1. Conductive Materials
Mechanism of AD Promotion by Conductive Materials
2.5.2. Bioaugmentation and Enzymes
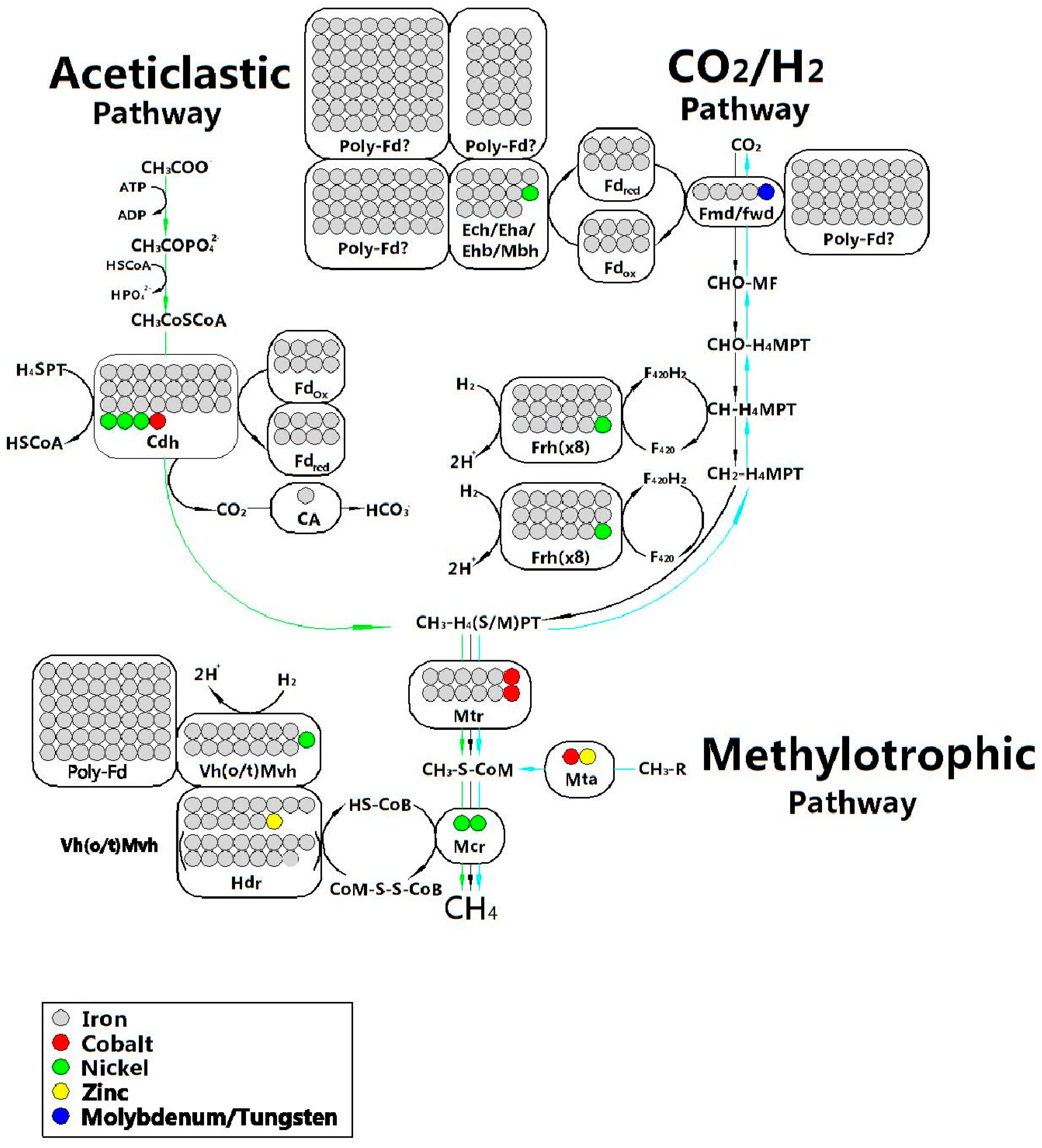
2.5.3. Trace Elements
Relationship between Trace Elements (TEs) and AD
The Requirements for TEs in AD System
The Bioavailability of TE and the Possibility to Regulate
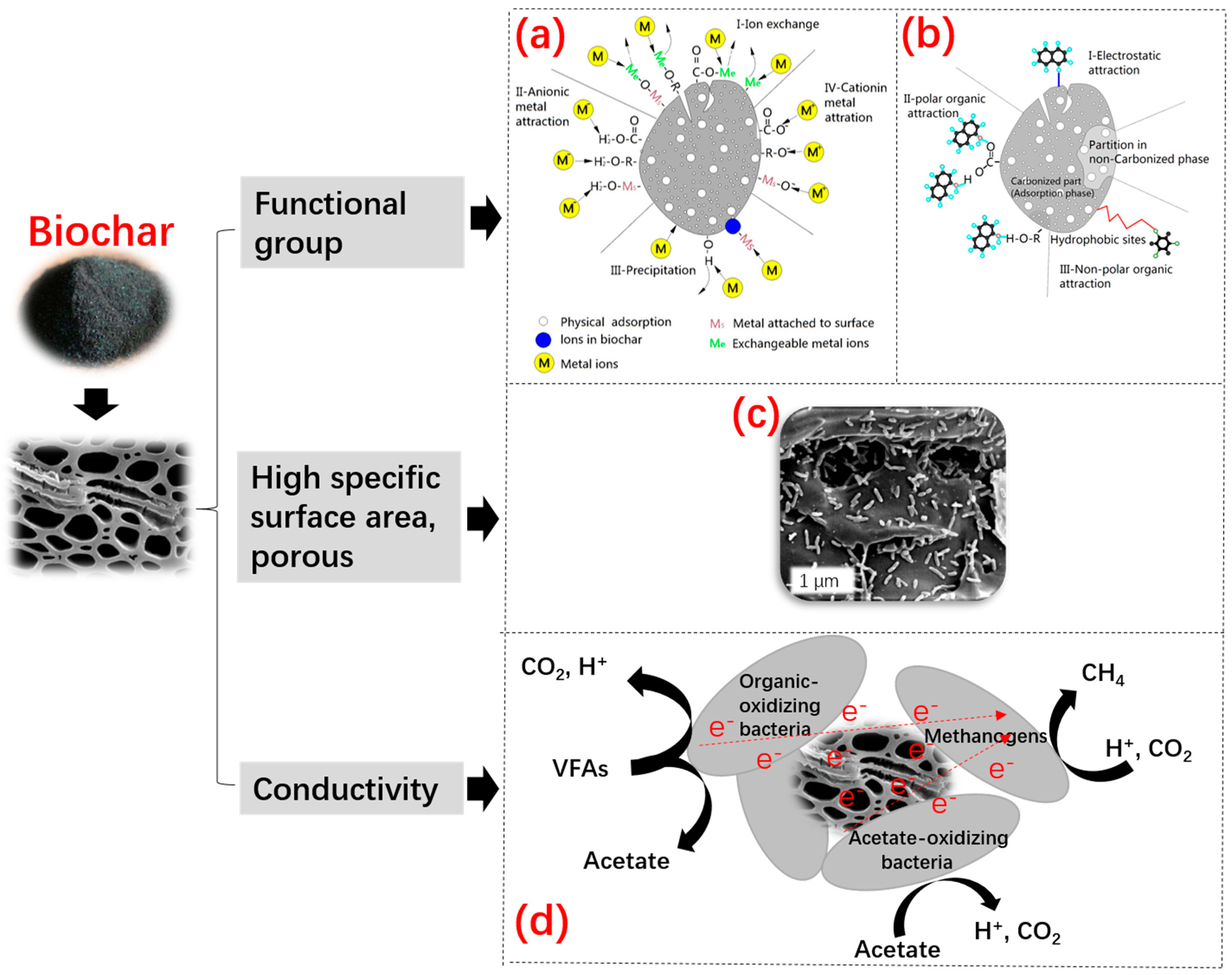
2.5.4. Biochar
3. Conclusions and Recommendations for Future Research
3.1. Conclusions
3.2. Recommendations for Future Research
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Lin, L.; Xu, F.; Ge, X.; Li, Y. Improving the sustainability of organic waste management practices in the food-energy-water nexus: A comparative review of anaerobic digestion and composting. Renew. Sustain. Energy Rev. 2018, 89, 151–167. [Google Scholar] [CrossRef]
- Cai, Y.; Zhao, X.; Zhao, Y.; Wang, H.; Yuan, X.; Zhu, W.; Cui, Z.; Wang, X. Optimization of Fe2+ supplement in anaerobic digestion accounting for the Fe-bioavailability. Bioresour. Technol. 2018, 250, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Y.; Li, X.; Siddiqui, M.A.; Liu, H.; Zhou, T.; Zheng, L.; Huang, S.Y.; Gao, L.; Lin, C.S.K.; Wang, Q.L. Effect of humic substances on the anaerobic digestion of secondary sludge in wastewater treatment plants: A review. Environ. Chem. Lett. 2023, 21, 3023–3040. [Google Scholar] [CrossRef]
- Neshat, S.A.; Mohammadi, M.; Najafpour, G.D.; Lahijani, P. Anaerobic co-digestion of animal manures and lignocellulosic residues as a potent approach for sustainable biogas production. Renew. Sustain. Energy Rev. 2017, 79, 308–322. [Google Scholar] [CrossRef]
- Pan, J.; Ma, J.; Zhai, L.; Liu, H. Enhanced methane production and syntrophic connection between microorganisms during semi-continuous anaerobic digestion of chicken manure by adding biochar. J. Clean. Prod. 2019, 240, 118178. [Google Scholar] [CrossRef]
- Molaey, R.; Bayrakdar, A.; Çalli, B. Long-term influence of trace element deficiency on anaerobic monodigestion of chicken manure. J. Environ. Manag. 2018, 223, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.; Khanal, S.K. A little breath of fresh air into an anaerobic system: How Microaeration facilitates anaerobic digestion process. Biotechnol. Adv. 2018, 36, 1971–1983. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhang, D.; Dai, L.; Dong, B.; Dai, X. Magnetite Triggering Enhanced Direct Interspecies Electron Transfer: A Scavenger for the Blockage of Electron Transfer in Anaerobic digestion of High-Solids Sewage Sludge. Environ. Sci. Technol. 2018, 52, 7160–7169. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Kang, H.J.; Park, K.H.; Park, H.D. Direct interspecies electron transfer via conductive materials: A perspective for anaerobic digestion applications. Bioresour. Technol. 2018, 254, 300–311. [Google Scholar] [CrossRef]
- Hashemi, B.; Sarker, S.; Lamb, J.J.; Lien, K.M. Yield improvements in anaerobic digestion of lignocellulosic feedstocks. J. Clean. Prod. 2021, 288, 125447. [Google Scholar] [CrossRef]
- Sawatdeenarunat, C.; Sung, S.; Khanal, S.K. Enhanced volatile fatty acids production during anaerobic digestion of lignocellulosic biomass via micro-oxygenation. Bioresour. Technol. 2017, 237, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wu, J.; Yuan, X.; Zhu, W.; Wang, X.; Cheng, X.; Cui, Z. The effect of mixing intensity on the performance and microbial dynamics of a single vertical reactor integrating acidogenic and methanogenic phases in lignocellulosic biomass digestion. Bioresour. Technol. 2017, 238, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.C.; Liu, H.B.; Zhang, H.D.; Xu, S.Y.; Lichtfouse, E.; Yun, Y.B. Anaerobic digestion and recycling of kitchen waste: A review. Environ. Chem. Lett. 2022, 20, 1745–1762. [Google Scholar] [CrossRef]
- Tawfik, A.; Eraky, M.; Osman, A.I.; Ai, P.; Zhou, Z.B.; Meng, F.A.; Rooney, D.W. Bioenergy production from chicken manure: A review. Environ. Chem. Lett. 2023, 21, 2707–2727. [Google Scholar] [CrossRef]
- Bayrakdar, A.; Sürmeli, R.O.; Çalli, B. Anaerobic digestion of chicken manure by a leach-bed process coupled with side-stream membrane ammonia separation. Bioresour. Technol. 2018, 258, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Luo, K.; Zhang, Y.; Zheng, Z.; Cai, Y.; Wen, B.; Cui, Z.; Wang, X. Improving the methane yield of maize straw: Focus on the effects of pre-treatment with fungi and their secreted enzymes combined with sodium hydroxide. Bioresour. Technol. 2018, 250, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.W.; Yao, Y.Q.; Deng, J.; Zhang, J.L.; Qiu, Y.J.; Li, G.F.; Liu, J. Hydrogen production via anaerobic digestion of coal modified by white-rot fungi and its application benefits analysis. Renew. Sustain. Energy Rev. 2022, 157, 112091. [Google Scholar] [CrossRef]
- Zhong, W.; Zhang, Z.; Luo, Y.; Sun, S.; Qiao, W.; Xiao, M. Effect of biological pretreatments in enhancing corn straw biogas production. Bioresour Technol. 2011, 102, 11177–11182. [Google Scholar] [CrossRef]
- Yuan, X.; Wen, B.; Ma, X.; Zhu, W.; Wang, X.; Cheng, X.; Cui, Z. Enhancing the anaerobic digestion of lignocellulose of municipal solid waste using a microbial pre-treatment method. Bioresour. Technol. 2014, 154, 1–9. [Google Scholar] [CrossRef]
- Guan, R.; Li, X.; Wachemo, A.C.; Yuan, H.; Liu, Y.; Zou, D. Enhancing anaerobic digestion performance and degradation of lignocellulosic components of rice straw by combined biological and chemical pre-treatment. Sci. Total Environ. 2018, 637–638, 9–17. [Google Scholar] [CrossRef]
- Rouches, E.; Zhou, S.; Sergent, M.; Raouche, S.; Carrere, H. Influence of white-rot fungus Polyporus brumalis BRFM 985 culture conditions on the pre-treatment efficiency for anaerobic digestion of wheat straw. Biomass Bioenergy 2018, 110, 75–79. [Google Scholar] [CrossRef]
- Dong, C.; Chen, J.; Guan, R.; Li, X.; Xin, Y. Dual-frequency ultrasound combined with alkali pre-treatment of corn stalk for enhanced biogas production. Renew. Energy 2018, 127, 444–451. [Google Scholar] [CrossRef]
- Solé-Bundó, M.; Carrère, H.; Garfí, M.; Ferrer, I. Enhancement of microalgae anaerobic digestion by thermo-alkaline pre-treatment with lime (CaO). Algal Res. 2017, 24, 199–206. [Google Scholar] [CrossRef]
- Kang, X.; Sun, Y.; Li, L.; Kong, X.; Yuan, Z. Improving methane production from anaerobic digestion of Pennisetum Hybrid by alkaline pre-treatment. Bioresour. Technol. 2018, 255, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Mancini, G.; Papirio, S.; Lens, P.N.L.; Esposito, G. Increased biogas production from wheat straw by chemical pre-treatments. Renew. Energy 2018, 119, 608–614. [Google Scholar] [CrossRef]
- Wei, W.; Wang, Q.; Zhang, L.; Laloo, A.; Duan, H.; Batstone, D.J.; Yuan, Z. Free nitrous acid pre-treatment of waste activated sludge enhances volatile solids destruction and improves sludge dewaterability in continuous anaerobic digestion. Water Res. 2018, 130, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Bergeron, A.D.; Davaritouchaee, M. Methane recovery from anaerobic digestion of urea-pretreated wheat straw. Renew. Energy 2018, 115, 139–148. [Google Scholar] [CrossRef]
- Passos, F.; Ortega, V.; Donoso-Bravo, A. Thermochemical pre-treatment and anaerobic digestion of dairy cow manure: Experimental and economic evaluation. Bioresour. Technol. 2017, 227, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.L.; Liu, X.F.; Ya, Z.Y.; Yuan, Y.X.; Liao, Y.Z. Comparison of seven chemical pre-treatments of corn straw for improving methane yield by anaerobic digestion. PLoS ONE 2014, 9, e93801. [Google Scholar]
- Dell’Omo, P.P.; Spena, V.A. Mechanical pretreatment of lignocellulosic biomass to improve biogas production: Comparison of results for giant reed and wheat straw. Energy 2020, 203, 07–14. [Google Scholar] [CrossRef]
- Chen, H.; Xia, A.; Zhu, X.; Huang, Y.; Zhu, X.Q.; Liao, Q. Hydrothermal hydrolysis of algal biomass for biofuels production: A review. Bioresour. Technol. 2022, 344, 126213. [Google Scholar] [CrossRef]
- Rajput, A.A.; Sheikh, Z.; Visvanathan, C. Effect of thermal pre-treatment on chemical composition, physical structure and biogas production kinetics of wheat straw. J. Environ. Manag. 2018, 221, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Zhang, L.L.; Fan, Y.M.; Wang, Z.G. The case-dependent lignin role in lignocellulose nanofibers preparation and functional application—A review. Green Energy Environ. 2023, 8, 1553–1566. [Google Scholar] [CrossRef]
- Mönch-Tegeder, M.; Lemmer, A.; Oechsner, H. Enhancement of methane production with horse manure supplement and pre-treatment in a full-scale biogas process. Energy 2014, 73, 523–530. [Google Scholar] [CrossRef]
- Zheng, Z.; Liu, J.; Yuan, X.; Wang, X.; Zhu, W.; Yang, F.; Cui, Z. Effect of dairy manure to switchgrass co-digestion ratio on methane production and the bacterial community in batch anaerobic digestion. Appl. Energy 2015, 151, 249–257. [Google Scholar] [CrossRef]
- Li, Y.; Jin, Y.; Borrion, A.; Li, J. Influence of feed/inoculum ratios and waste cooking oil content on the mesophilic anaerobic digestion of food waste. Waste Manag. 2018, 73, 156–164. [Google Scholar] [CrossRef]
- Rouches, E.; Herpoël-Gimbert, I.; Steyer, J.P.; Carrere, H. Improvement of anaerobic degradation by white-rot fungi pre-treatment of lignocellulosic biomass: A review. Renew. Sustain. Energy Rev. 2016, 59, 179–198. [Google Scholar] [CrossRef]
- Wang, Z.W.; Deuss, P.J. The isolation of lignin with native-like structure. Biotechnol. Adv. 2023, 68, 108230. [Google Scholar] [CrossRef]
- Mustafa, A.M.; Poulsen, T.G.; Xia, Y.; Sheng, K. Combinations of fungal and milling pre-treatments for enhancing rice straw biogas production during solid-state anaerobic digestion. Bioresour. Technol. 2017, 224, 174–182. [Google Scholar] [CrossRef]
- Brémond, U.; Buyer, R.D.; Steyer, J.P.; Bernet, N.; Carrere, H. Biological pre-treatments of biomass for improving biogas production: An overview from lab scale to full-scale. Renew. Sustain. Energy Rev. 2018, 90, 583–604. [Google Scholar] [CrossRef]
- Schroyen, M.; Vervaeren, H.; Van Hulle, S.W.H.; Raes, K. Impact of enzymatic pre-treatment on corn stover degradation and biogas production. Bioresour. Technol. 2014, 173, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Frigon, J.C.; Mehta, P.; Guiot, S.R. Impact of mechanical, chemical and enzymatic pre-treatments on the methane yield from the anaerobic digestion of switchgrass. Biomass Bioenergy 2012, 36, 1–11. [Google Scholar] [CrossRef]
- Schroyen, M.; Vervaeren, H.; Vandepitte, H.; Van Hulle, S.W.H.; Raes, K. Effect of enzymatic pre-treatment of various lignocellulosic substrates on production of phenolic compounds and biomethane potential. Bioresour. Technol. 2015, 192, 696–702. [Google Scholar] [CrossRef] [PubMed]
- Leca, E.; Zennaro, B.; Hamelin, J.; Carrère, H.; Sambusiti, C. Use of additives to improve collective biogas plant performances: A comprehensive review. Biotechnol. Adv. 2023, 65, 108129. [Google Scholar] [CrossRef] [PubMed]
- Wyman, V.; Henríquez, J.; Palma, C.; Carvajal, A. Lignocellulosic waste valorisation strategy through enzyme and biogas production. Bioresour. Technol. 2018, 247, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Zhang, G.; Li, J.; Zhao, Z.; Kang, X. Effect of endogenous hydrolytic enzymes pre-treatment on the anaerobic digestion of sludge. Bioresour. Technol. 2013, 146, 758–761. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Liu, Y.J.; Meng, S.J.; Kiran, E.U.; Liu, Y. Enzymatic pre-treatment of activated sludge, food waste and their mixture for enhanced bioenergy recovery and waste volume reduction via anaerobic digestion. Appl. Energy 2016, 179, 1131–1137. [Google Scholar] [CrossRef]
- Odnell, A.; Recktenwald, M.; Stensén, K.; Jonsson, B.H.; Karlsson, M. Activity, lifetime and effect of hydrolytic enzymes for enhanced biogas production from sludge anaerobic digestion. Water Res. 2016, 103, 462–471. [Google Scholar] [CrossRef]
- Arun, C.; Sivashanmugam, P. Enhanced production of biohydrogen from dairy waste activated sludge pretreated using multi hydrolytic garbage enzyme complex and ultrasound-optimization. Energy Convers. Manag. 2018, 164, 277–287. [Google Scholar] [CrossRef]
- Arun, C.; Sivashanmugam, P. Solubilization of waste activated sludge using a garbage enzyme produced from different pre- consumer organic waste. RSC Adv. 2015, 5, 51421–51427. [Google Scholar] [CrossRef]
- Arun, C.; Sivashanmugam, P. Study on optimization of process parameters for enhancing the multi-hydrolytic enzyme activity in garbage enzyme produced from preconsumer organic waste. Bioresour. Technol. 2017, 226, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Chacana, J.; Alizadeh, S.; Labelle, M.; Laporte, A.; Hawari, J.; Barbeau, B.; Comeau, Y. Effect of ozonation on anaerobic digestion sludge activity and viability. Chemosphere 2017, 176, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Koyama, M.; Yamamoto, S.; Ishikawa, K.; Ban, S.; Toda, T. Inhibition of anaerobic digestion by dissolved lignin derived from alkaline pre-treatment of an aquatic macrophyte. Chem. Eng. J. 2017, 311, 55–62. [Google Scholar] [CrossRef]
- Solé-Bundó, M.; Eskicioglu, C.; Garfí, M.; Carrère, H.; Ferrer, I. Anaerobic co-digestion of microalgal biomass and wheat straw with and without thermo-alkaline pre-treatment. Bioresour. Technol. 2017, 237, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Zan, F.X.; Hao, T.W.; Khanal, S.K.; Chen, G.H. Sewage sludge digestion beyond biogas: Electrochemical pre-treatment for biochemicals. Water Res. 2022, 208, 117839. [Google Scholar] [CrossRef] [PubMed]
- Zhen, G.; Lu, X.; Li, Y.; Zhao, Y. Combined electrical-alkali pre-treatment to increase the anaerobic hydrolysis rate of waste activated sludge during anaerobic digestion. Appl. Energy 2014, 128, 93–102. [Google Scholar] [CrossRef]
- Chen, G.Y.; Cao, J.; Chang, Z.Z.; Ye, X.M.; Du, J. Effect of oganic acids pre-treatment on physico-chemical property and biogas production of wheat straw. Acta Energiae Solaris Sin. 2015, 36, 2559–2564. [Google Scholar]
- Liu, J.; Yang, M.; Zhang, J.; Zheng, J.; Xu, H.; Wang, Y.; Wei, Y. A comprehensive insight into the effects of microwave-H2O2 pre-treatment on concentrated sewage sludge anaerobic digestion based on semi-continuous operation. Bioresour. Technol. 2018, 256, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Gil, A.; Siles, J.A.; Martín, M.A.; Chica, A.F.; Estevez-Pastor, F.S.; Toro-Baptista, E. Effect of microwave pre-treatment on semi-continuous anaerobic digestion of sewage sludge. Renew. Energy 2018, 115, 917–925. [Google Scholar] [CrossRef]
- Bin Kabir, S.; Khalekuzzaman, M.; Hossain, N.; Jamal, M.; Alam, M.A.; Abomohra, A. Progress in biohythane production from microalgae-wastewater sludge co-digestion: An integrated biorefinery approach. Biotechnol. Adv. 2022, 57, 107933. [Google Scholar] [CrossRef]
- Zhang, J.; Lv, C.; Tong, J.; Liu, J.; Liu, J.; Yu, D.; Wang, Y.; Chen, M.; Wei, Y. Optimization and microbial community analysis of anaerobic co-digestion of food waste and sewage sludge based on microwave pre-treatment. Bioresour. Technol. 2016, 200, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Wu, B.; Yao, F.B.; He, L.; Chen, F.; Ma, Y.H.; Shu, X.Y.; Hou, K.J.; Wang, D.B.; Li, X.M. Biogas production from anaerobic co-digestion of waste activated sludge: Co-substrates and influencing parameters. Rev. Environ. Sci. Biotechnol. 2019, 18, 771–793. [Google Scholar] [CrossRef]
- Cesaro, A.; Velten, S.; Belgiorno, V.; Kuchta, K. Enhanced anaerobic digestion by ultrasonic pre-treatment of organic residues for energy production. J. Clean. Prod. 2014, 74, 119–124. [Google Scholar] [CrossRef]
- Passos, F.; Carretero, J.; Ferrer, I. Comparing pre-treatment methods for improving microalgae anaerobic digestion: Thermal, hydrothermal, microwave and ultrasound. Chem. Eng. J. 2015, 279, 667–672. [Google Scholar] [CrossRef]
- Maurya, D.P.; Singla, A.; Negi, S. An overview of key pre-treatment processes for biological conversion of lignocellulosic biomass to bioethanol. Biotech 2015, 5, 597–609. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Chen, Y.; Shi, J.; Zhao, T.; Yu, Q.; Yu, S.; Shi, D.; Chai, H.; Gu, L.; He, Q.; et al. Mesophilic anaerobic co-digestion of residual sludge with different lignocellulosic wastes in the batch digester. Bioresour. Technol. 2018, 268, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Sun, F.; Yu, J.; Cai, Y.; Luo, X.; Cui, Z.; Hu, Y.; Wang, X. Co-digestion of oat straw and cow manure during anaerobic digestion: Stimulative and inhibitory effects on fermentation. Bioresour. Technol. 2018, 269, 143–152. [Google Scholar] [CrossRef]
- Li, Y.; Liu, C.; Wachemo, A.C.; Li, X. Effects of liquid fraction of digestate recirculation on system performance and microbial community structure during serial anaerobic digestion of completely stirred tank reactors for corn stover. Energy 2018, 160, 309–317. [Google Scholar] [CrossRef]
- Jasinska, A.; Grosser, A.; Meers, E. Possibilities and Limitations of Anaerobic Co-Digestion of Animal Manure-A Critical Review. Energies 2023, 120, 3885. [Google Scholar] [CrossRef]
- Wang, X.; Yang, G.; Feng, Y.; Ren, G.; Han, X. Optimizing feeding composition and carbon-nitrogen ratios for improved methane yield during anaerobic co-digestion of dairy, chicken manure and wheat straw. Bioresour. Technol. 2012, 120, 78–83. [Google Scholar] [CrossRef]
- Latha, K.; Velraj, R.; Shanmugam, P.; Sivanesan, S. Mixing strategies of high solids anaerobic co-digestion using food waste with sewage sludge for enhanced biogas production. J. Clean. Prod. 2019, 210, 388–400. [Google Scholar] [CrossRef]
- Sole-Bundo, M.; Passos, F.; Romero-Guiza, M.; Ferrer, I.; Astals, S. Co-digestion strategies to enhance microalgae anaerobic digestion: A review. Renew. Sustain. Energy Rev. 2019, 112, 471–482. [Google Scholar] [CrossRef]
- Zhang, J.; Loh, K.-C.; Lee, J.; Wang, C.-H.; Dai, Y.; Tong, Y.W. Three-stage anaerobic codigestion of food waste and horse manure. Sci. Rep. 2017, 7, 1269. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, B.; Zhang, Q.; Florentino, A.; Xu, R.; Zhang, Y.; Liu, Y. Co-digestion of blackwater with kitchen organic waste: Effects of mixing ratios and insights into microbial community. J. Clean. Prod. 2019, 236, 117703. [Google Scholar] [CrossRef]
- Mu, L.; Zhang, L.; Zhu, K.; Ma, J.; Ifran, M.; Li, A. Anaerobic co-digestion of sewage sludge, food waste and yard waste: Synergistic enhancement on process stability and biogas production. Sci. Total Environ. 2020, 704, 135429. [Google Scholar] [CrossRef] [PubMed]
- Gupte, A.P.; Basaglia, M.; Casella, S.; Favaro, L. Rice waste streams as a promising source of biofuels: Feedstocks, biotechnologies and future perspectives. Renew. Sustain. Energy Rev. 2022, 167, 112673. [Google Scholar] [CrossRef]
- Capson-Tojo, G.; Moscoviz, R.; Astals, S.; Robles, A.; Steyer, J.P. Unraveling the literature chaos around free ammonia inhibition in anaerobic digestion. Renew. Sustain. Energy Rev. 2020, 117, 109487. [Google Scholar] [CrossRef]
- Kurade, M.B.; Saha, S.; Salama, E.S.; Patil, S.M.; Govindwar, S.P.; Jeon, B.H. Acetoclastic methanogenesis led by Methanosarcina in anaerobic codigestion of fats, oil and grease for enhanced production of methane. Bioresour. Technol. 2019, 272, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, K.; Mahapatra, D.; Venkatraman, A.V.; Muthuswamy, S.; Pugazhendhi, A. Advancing anaerobic digestion through two-stage processes: Current developments and future trends. Renew. Sust. Energy Rev. 2020, 123, 109746. [Google Scholar] [CrossRef]
- Gottardo, M.; Micolucci, F.; Bolzonella, D.; Uellendahl, H.; Pavan, P. Pilot scale fermentation coupled with anaerobic digestion of food waste—Effect of dynamic digestate recirculation. Renew. Energy 2017, 114, 455–463. [Google Scholar] [CrossRef]
- Pezzolla, D.; Maria, F.D.; Zadra, C.; Massaccesi, L.; Sordi, A.; Gigliotti, G. Optimization of solid-state anaerobic digestion through the percolate recirculation. Biomass Bioenergy 2017, 96, 112–118. [Google Scholar] [CrossRef]
- Wu, C.; Huang, Q.; Yu, M.; Ren, Y.; Wang, Q.; Sakai, K. Effects of digestate recirculation on a two-stage anaerobic digestion system, particularly focusing on metabolite correlation analysis. Bioresour. Technol. 2018, 251, 40–48. [Google Scholar] [CrossRef]
- Zamanzadeh, M.; Hagen, L.H.; Svensson, K.; Linjordet, R.; Horn, S.J. Anaerobic digestion of food waste-Effect of recirculation and temperature on performance and microbiology. Water Res. 2016, 96, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Ni, P.; Lyu, T.; Sun, H.; Dong, R.; Wu, S. Liquid digestate recycled utilization in anaerobic digestion of pig manure: Effect on methane production, system stability and heavy metal mobilization. Energy 2017, 141, 1695–1704. [Google Scholar] [CrossRef]
- Qian, M.; Zhang, Y.; Li, R.; Nelles, M.; Stinner, W.; Li, Y. Effects of Percolate Recirculation on Dry Anaerobic Co-digestion of Organic Fraction of Municipal Solid Waste and Corn Straw. Energy Fuels 2017, 31, 12183–12191. [Google Scholar] [CrossRef]
- Luo, L.; Wong, J.W.C. Enhanced food waste degradation in integrated two-phase anaerobic digestion: Effect of leachate recirculation ratio. Bioresour. Technol. 2019, 291, 121813. [Google Scholar] [CrossRef] [PubMed]
- Aslam, A.; Khan, S.J.; Shahzad, H.M.A. Impact of sludge recirculation ratios on the performance of anaerobic membrane bioreactor for wastewater treatment. Bioresour. Technol. 2019, 288, 121473. [Google Scholar] [CrossRef]
- Krayzelova, L.; Bartacek, J.; Díaz, I.; Jeison, D.; Volcke, E.I.P.; Jenicek, P. Microaeration for hydrogen sulfide removal during anaerobic treatment: A review. Rev. Environ. Sci. Biotechnol. 2015, 14, 703–725. [Google Scholar] [CrossRef]
- Sasidhar, K.B.; Somasundaram, M.; Ekambaram, P.; Arumugam, S.K.; Nataraj, G.; Murugan, M.A. A critical review on the effects of pneumatic mixing in anaerobic digestion process. J. Clean. Prod. 2022, 378, 134513. [Google Scholar] [CrossRef]
- Krayzelova, L.P.; Mampaey, K.E.; Vannecke, T.P.W.; Bartacek, J.; Jenicek, P.; Volcke, E.I.P. Model-based optimization of Microaeration for biogas desulfurization in UASB reactor. Chem. Eng. J. 2017, 125, 171–179. [Google Scholar]
- Ramos, I.; Fdz-Polanco, M. The potential of oxygen to improve the stability of anaerobic reactors during unbalanced conditions: Results from a pilot-scale digester treating sewage sludge. Bioresour. Technol. 2013, 140, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Wei, Y.; Zhang, Q.; Zhang, J.; Lu, T.; Pei, Y. Enhancement of surfactant biodegradation with an anaerobic membrane bioreactor by introducing microaeration. Chemosphere 2018, 208, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S.; Fonoll, X.; Khanal, S.K.; Raskin, L. Biological strategies for enhanced hydrolysis of lignocellulosic biomass during anaerobic digestion: Current status and future perspectives. Bioresour. Technol. 2017, 245, 1245–1257. [Google Scholar] [CrossRef] [PubMed]
- Diak, J.; Örmeci, B.; Kennedy, K.J. Effect of micro-aeration on anaerobic digestion of primary sludge under septic tank conditions. Bioprocess. Biosyst. Eng. 2013, 36, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Selvam, A.; Wong, J.W.C. Optimization of micro-aeration intensity in acidogenic reactor of a two-phase anaerobic digester treating food waste. Waste Manag. 2014, 34, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Y.; Li, X.; Liu, H.; Zhou, T.; Qin, Z.H.; Mou, J.H.; Sun, J.; Huang, S.Y.; Chaves, A.V.; Gao, L. Bioproduction and applications of short-chain fatty acids from secondary sludge anaerobic fermentation: A critical review. Renew. Sustain. Energy Rev. 2023, 183, 113502. [Google Scholar] [CrossRef]
- Duarte, M.S.; Silva, S.A.; Salvador, A.F.; Cavaleiro, A.J.; Stams, A.J.M.; Alves, M.M.; Pereira, M.A. Insight into the Role of Facultative Bacteria Stimulated by Microaeration in Continuous Bioreactors Converting LCFA to Methane. Environ. Sci. Technol. 2018, 52, 6497–6507. [Google Scholar] [CrossRef] [PubMed]
- Díaz, I.; Donoso-Bravo, A.; Fdz-Polanco, M. Effect of microaerobic conditions on the degradation kinetics of cellulose. Bioresour. Technol. 2011, 102, 10139–10142. [Google Scholar] [CrossRef] [PubMed]
- Krayzelova, L.; Bartacek, J.; Kolesarova, N.; Jenicek, P. Microaeration for hydrogen sulfide removal in UASB reactor. Bioresour. Technol. 2014, 172, 297–302. [Google Scholar] [CrossRef]
- Jenicek, P.; Celis, C.A.; Krayzelova, L.; Anferova, N.; Pokorna, D. Improving products of anaerobic sludge digestion by microaeration. Water Sci. Technol. 2014, 69, 803–809. [Google Scholar] [CrossRef]
- Fu, S.; Wang, F.; Shi, X.; Guo, R. Impacts of microaeration on the anaerobic digestion of corn straw and the microbial community structure. Chem. Eng. J. 2016, 287, 523–528. [Google Scholar] [CrossRef]
- Hanreich, A.; Schimpf, U.; Zakrzewski, M.; Schlüter, A.; Benndorf, D.; Heyer, R.; Rapp, E.; Pühler, A.; Reichl, U.; Klocke, M. Metagenome and metaproteome analyses of microbial communities in mesophilic biogas-producing anaerobic batch fermentations indicate concerted plant carbohydrate degradation. Syst. Appl. Microbiol. 2013, 36, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; May, H.D.; Lu, L.; Liang, P.; Huang, X.; Ren, Z.J. Carbon dioxide and organic waste valorization by microbial electrosynthesis and electro-fermentation. Water Res. 2019, 149, 42–55. [Google Scholar] [CrossRef] [PubMed]
- Zabranska, J.; Pokorna, D. Bioconversion of carbon dioxide to methane using hydrogen and hydrogenotrophic methanogens. Biotechnol. Adv. 2018, 36, 707–720. [Google Scholar] [CrossRef]
- Chen, J.L.; Ortiz, R.; Steele, T.W.J.; Stuckey, D.C. Toxicants inhibiting anaerobic digestion: A review. Biotechnol. Adv. 2014, 32, 1523–1534. [Google Scholar] [CrossRef] [PubMed]
- Horne, A.J.; Lessner, D.J. Assessment of the oxidant tolerance of Methanosarcina acetivorans. FEMS Microbiol. Lett. 2013, 343, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.S.; Franklin, M.J. Physiological heterogeneity in biofilms. Nat. Rev. Microbiol. 2008, 6, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Lü, F.; Hao, L.P.; He, P.J.; Shao, L.M. Regulating the hydrolysis of organic wastes by micro-aeration and effluent recirculation. Waste Manag. 2009, 29, 2042–2050. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Liu, J.; Ma, C.; Li, Y.; Zou, L.; Qian, G.; Xu, Z. Improving the stability and efficiency of anaerobic digestion of food waste using additives: A critical review. J. Clean. Prod. 2018, 192, 316–326. [Google Scholar] [CrossRef]
- Rotaru, A.E.; Shrestha, P.M.; Liu, F.; Shrestha, M.; Shrestha, D.; Embree, M.; Zengler, K.; Wardman, C.; Nevin, K.P.; Lovley, D.R. A new model for electron flow during anaerobic digestion: Direct interspecies electron transfer to Methanosaeta for the reduction of carbon dioxide to methane. Energy Environ. Sci. 2014, 7, 408–415. [Google Scholar] [CrossRef]
- Lin, R.; Cheng, J.; Zhang, J.; Zhou, J.; Cen, K.; Murphy, J.D. Boosting biomethane yield and production rate with graphene: The potential of direct interspecies electron transfer in anaerobic digestion. Bioresour. Technol. 2017, 239, 345–352. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, Y.; Yu, Q.; Zhang, Y. Ferroferric oxide triggered possible direct interspecies electron transfer between Syntrophomonas and Methanosaeta to enhance waste activated sludge anaerobic digestion. Bioresour. Technol. 2018, 250, 79–85. [Google Scholar] [CrossRef]
- Lin, R.; Cheng, J.; Ding, L.; Murphy, J.D. Improved efficiency of anaerobic digestion through direct interspecies electron transfer at mesophilic and thermophilic temperature ranges. Chem. Eng. J. 2018, 350, 681–691. [Google Scholar] [CrossRef]
- Park, J.H.; Seong, H.J.; Sul, W.J.; Jin, H.K.; Park, H.D. Metagenomic insight into methanogenic reactors promoting direct interspecies electron transfer via granular activated carbon. Bioresour. Technol. 2018, 259, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Dang, Y.; Holmes, D.E.; Zhao, Z.; Woodard, T.L.; Zhang, Y.; Sun, D.; Wang, L.; Nevin, K.P.; Lovley, D. Enhancing anaerobic digestion of complex organic waste with carbon-based conductive materials. Bioresour. Technol. 2016, 220, 516–522. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, Y.; Quan, X.; Zhang, Y. Towards engineering application: Potential mechanism for enhancing anaerobic digestion of complex organic waste with different types of conductive materials. Water Res. 2017, 115, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zhang, Y.; Quan, X.; Zhao, H. Evaluation on direct interspecies electron transfer in anaerobic sludge digestion of microbial electrolysis cell. Bioresour. Technol. 2016, 200, 235–244. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, Y.; Chen, S.; Quan, X. Enhanced production of methane from waste activated sludge by the combination of high-solid anaerobic digestion and microbial electrolysis cell with iron–graphite electrode. Chem. Eng. J. 2015, 259, 787–794. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Y.; Gao, X.; Chen, H.; Xu, X.; Zhu, L. Role of biochar in the granulation of anaerobic sludge and improvement of electron transfer characteristics. Bioresour. Technol. 2018, 268, 28–35. [Google Scholar] [CrossRef]
- Romero-Güiza, M.S.; Vila, J.; Mata-Alvarez, J.; Chimenos, J.M.; Astals, S. The role of additives on anaerobic digestion: A review. Renew. Sustain. Energy Rev. 2016, 58, 1486–1499. [Google Scholar] [CrossRef]
- Tuesorn, S.; Wongwilaiwalin, S.; Champreda, V.; Leethochawalit, M.; Nopharatana, A.; Techkarnjanaruk, S.; Chaiprasert, P. Enhancement of biogas production from swine manure by a lignocellulolytic microbial consortium. Bioresour. Technol. 2013, 144, 579–586. [Google Scholar] [CrossRef]
- Weiss, S.; Tauber, M.; Somitsch, W.; Meincke, R.; Müller, H.; Berg, G.; Guebitz, G.M. Enhancement of biogas production by addition of hemicellulolytic bacteria immobilised on activated zeolite. Water Res. 2010, 44, 1970–1980. [Google Scholar] [CrossRef] [PubMed]
- Martin-Ryals, A.; Schideman, L.; Li, P.; Wilkinson, H.; Wagner, R. Improving anaerobic digestion of a cellulosic waste via routine bioaugmentation with cellulolytic microorganisms. Bioresour. Technol. 2015, 189, 62–70. [Google Scholar] [CrossRef]
- Lü, F.; Li, T.; Wang, T.; Shao, L.; He, P. Improvement of sludge digestate biodegradability by thermophilic bioaugmentation. Appl. Microbiol. Biotechnol. 2014, 98, 969–977. [Google Scholar] [CrossRef]
- Li, Y.; Li, L.; Sun, Y.; Yuan, Z. Bioaugmentation strategy for enhancing anaerobic digestion of high C/N ratio feedstock with methanogenic enrichment culture. Bioresour. Technol. 2018, 261, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, G.; Li, L.; Sun, Y. Bioaugmentation for overloaded anaerobic digestion recovery with acid-tolerant methanogenic enrichment. Waste Manag. 2018, 2018, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Fotidis, I.A.; Karakashev, D.; Angelidaki, I. Bioaugmentation with an acetate-oxidising consortium as a tool to tackle ammonia inhibition of anaerobic digestion. Bioresour. Technol. 2013, 146, 57–62. [Google Scholar] [CrossRef]
- Tian, H.; Mancini, E.; Treu, L.; Angelidaki, I.; Fotidis, I.A. Bioaugmentation strategy for overcoming ammonia inhibition during biomethanation of a protein-rich substrate. Chemosphere 2019, 231, 415–422. [Google Scholar] [CrossRef]
- Fotidis, I.A.; Wang, H.; Fiedel, N.R.; Luo, G.; Karakashev, D.B.; Angelidaki, I. Bioaugmentation as a solution to increase methane production from an ammoniarich substrate. Environ. Sci. Technol. 2014, 48, 7669–7676. [Google Scholar] [CrossRef]
- Tian, H.; Yan, M.; Treu, L.; Angelidaki, I.; Fotidis, I.A. Hydrogenotrophic methanogens are the key for a successful bioaugmentation to alleviate ammonia inhibition in thermophilic anaerobic digesters. Bioresour. Technol. 2019, 293, 122070. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, W.; Liu, C.; Zhang, R.; Liu, G. Mitigation of ammonia inhibition through bioaugmentation with different microorganisms during anaerobic digestion: Selection of strains and reactor performance evaluation. Water Res. 2019, 155, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Donoso-Bravo, A.; Fdz-Polanco, M. Anaerobic co-digestion of sewage sludge and grease trap: Assessment of enzyme addition. Process Biochem. 2013, 48, 936–940. [Google Scholar] [CrossRef]
- Thanh, P.M.; Ketheesan, B.; Zhou, Y.; Stuckey, D. Trace metal speciation and bioavailability in anaerobic digestion: A review. Biotechnol. Adv. 2016, 34, 122–136. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.J.P.; Fraústo da Silva, J.J.R. The distribution of elements in cells. Coord. Chem. Rev. 2000, 200–202, 247–248. [Google Scholar] [CrossRef]
- Cai, Y.; Wang, X.; Cui, Z. A critical review on trace elements and bioavailability in anaerobic digestion system. J. China Agric. Univ. 2017, 22, 1–11. (In Chinese) [Google Scholar]
- Hochheimer, A.; Hedderich, R.; Thauer, R.K. The formylmethanofuran dehydrogenase isoenzymes in Methanobacterium wolfei and Methanobacterium thermoautotrophicum: Induction of the molybdenum isoenzyme by molybdate and constitutive synthesis of the tungsten isoenzyme. Arch. Microbiol. 1998, 170, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Harrop, T.C.; Mascharak, P.K. Structural and spectroscopic models of the Acluster of acetyl coenzyme a synthase/carbon monoxide dehydrogenase: Nature’s Monsanto acetic acid catalyst. Coord. Chem. Rev. 2005, 249, 3007–3024. [Google Scholar] [CrossRef]
- Ferry, J.G. The chemical biology of methanogenesis. Planet. Space Sci. 2010, 58, 1775–1783. [Google Scholar] [CrossRef]
- Glass, J.B.; Orphan, V.J. Trace metal requirements for microbial enzymes involved in the production and consumption of methane and nitrous oxide. Front. Microbiol. 2012, 3, 20413. [Google Scholar] [CrossRef]
- Cai, Y.; Hua, B.; Gao, L.; Hu, Y.; Yuan, X.; Cui, Z.; Zhu, W.; Wang, X. Effects of adding trace elements on rice straw anaerobic mono-digestion: Focus on changes in microbial communities using high-throughput sequencing. Bioresour. Technol. 2017, 239, 454–463. [Google Scholar] [CrossRef]
- Cai, Y.; Zheng, Z.; Zhao, Y.; Zhang, Y.; Guo, S.; Cui, Z.; Wang, X. Effects of molybdenum, selenium and manganese supplementation on the performance of anaerobic digestion and the characteristics of bacterial community in acidogenic stage. Bioresour. Technol. 2018, 266, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Hu, K.; Zheng, Z.; Zhang, Y.; Guo, S.; Zhao, X.; Cui, Z.; Wang, X. Effects of adding EDTA and Fe2+ on the performance of reactor and microbial community structure in two simulated phases of anaerobic digestion. Bioresour. Technol. 2019, 275, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Bi, S.; Westerholm, M.; Qiao, W.; Mahdy, A.; Xiong, L.; Yin, D.; Fan, R.; Dach, J.; Dong, R. Enhanced methanogenic performance and metabolic pathway of high solid anaerobic digestion of chicken manure by Fe2+ and Ni2+ supplementation. Waste Manag. 2019, 94, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Choong, Y.Y.; Norli, I.; Abdullah, A.Z.; Yhaya, M.F. Impacts of trace element supplementation on the performance of AD process: A critical review. Bioresour. Technol. 2016, 209, 369–379. [Google Scholar] [CrossRef]
- Kong, X.; Wei, Y.; Xu, S.; Liu, J.; Li, H.; Liu, Y.; Yu, S. Inhibiting excessive acidification using zero-valent iron in anaerobic digestion of food waste at high organic load rates. Bioresour. Technol. 2016, 211, 65–71. [Google Scholar] [CrossRef]
- Takashima, M.; Shimada, K.; Speece, R.E. Minimum Requirements for trace metals (iron, nickel, cobalt, and zinc) in thermophilic and mesophilic methane fermentation from glucose. Water Environ. Res. 2011, 83, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Marcato, C.; Pinelli, E.; Cecchi, M.; Winterton, P.; Guiresse, M. Bioavailability of Cu and Zn in raw and anaerobically digested pig slurry. Ecotoxicol. Environ. Saf. 2009, 72, 1538–1544. [Google Scholar] [CrossRef]
- Cai, Y.; Wang, J.; Zhao, Y.; Zhao, X.; Zheng, Z.; Wen, B.; Cui, Z.; Wang, X. A new perspective of using sequential extraction: To predict the deficiency of trace elements during anaerobic digestion. Water Res. 2018, 140, 335–343. [Google Scholar] [CrossRef]
- Zandvoort, M.H.; van Hullebusch, E.D.; Fermoso, F.G.; Lens, P.N.L. Trace metals in anaerobic granular sludge reactors: Bioavailability and dosing strategies. Eng. Life Sci. 2006, 6, 293–301. [Google Scholar] [CrossRef]
- Frunzo, L.; Fermoso, F.G.; Luongo, V.; Mattei, M.R.; Esposito, G. ADM1-based mechanistic model for the role of trace elements in anaerobic digestion processes. J. Environ. Manag. 2019, 241, 587–602. [Google Scholar] [CrossRef]
- Gustavsson, J.; Yekta, S.S.; Sundberg, C.; Karlsson, A.; Ejlertsson, J.; Skyllberg, U.; Svensson, B.H. Bioavailability of cobalt and nickel during anaerobic digestion of sulfur-rich stillage for biogas formation. Appl. Energy 2013, 112, 473–477. [Google Scholar] [CrossRef]
- Thanh, P.M.; Ketheesan, B.; Stuckey, D.C.; Zhou, Y. Dosing of Ethylenediamine-N,N′-disuccinic acid (EDDS) to improve the bioavailability of Fe2+ in the presence of sulfide in a submerged anaerobic membrane bioreactor. Chem. Eng. J. 2017, 330, 175–182. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, L.; Li, A. Enhanced anaerobic digestion of food waste by trace metal elements supplementation and reduced metals dosage by green chelating agent [S, S]-EDDS via improving metals bioavailability. Water Res. 2015, 84, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Vintiloiu, A.; Boxriker, M.; Lemmer, A.; Oechsner, H.; Jungbluth, T.; Mathies, E.; Ramhold, D. Effect of ethylenediaminetetraacetic acid (EDTA) on the bioavailability of trace elements during anaerobic digestion. Chem. Eng. J. 2013, 223, 436–441. [Google Scholar] [CrossRef]
- Chen, J.; Steele, T.W.J.; Stuckey, D.C. Stimulation and inhibition of anaerobic digestion by nickel and cobalt: A rapid assessment using the resazurin reduction assay. Environ. Sci. Technol. 2016, 50, 11154–11163. [Google Scholar] [CrossRef] [PubMed]
- Serrano, A.; Pinto-Ibieta, F.; Braga, A.; Jeison, D.; Borja, R.; Fermoso, F. Risks of using EDTA as an agent for trace metals dosing in anaerobic digestion of olive mill solid waste. Environ. Technol. 2017, 38, 3137–3144. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Gu, J.; Wang, X.; Qian, X.; Tuo, X. Impacts of biochar on the environmental risk of antibiotic resistance genes and mobile genetic elements during AD of cattle farm wastewater. Bioresour. Technol. 2018, 256, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Masebinu, S.O.; Akinlabi, E.T.; Muzenda, E.; Aboyade, A.O. A review of biochar properties and their roles in mitigating challenges with anaerobic digestion. Renew. Sustain. Energy Rev. 2019, 103, 291–307. [Google Scholar] [CrossRef]
- Qambrani, N.A.; Rahman, M.M.; Won, S.; Shim, S.; Ra, C. Biochar properties and ecofriendly applications for climate change mitigation, waste management, and wastewater treatment: A review. Renew. Sustain. Energy Rev. 2017, 79, 255–273. [Google Scholar] [CrossRef]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.S.; Ok, Y.S. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef]
- Baek, G.; Kim, J.; Kim, J.; Lee, C. Role and potential of direct interspecies electron transfer in anaerobic digestion. Energies 2018, 11, 107. [Google Scholar] [CrossRef]
- Wang, D.; Ai, J.; Shen, F.; Yang, G.; Zhang, Y.; Deng, S.; Zhang, J.; Zeng, Y.; Song, C. Improving anaerobic digestion of easy-acidification substrates by promoting buffering capacity using biochar derived from vermicompost. Bioresour. Technol. 2017, 227, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Mumme, J.; Srocke, F.; Heeg, K.; Werner, M. Use of biochars in anaerobic digestion. Bioresour. Technol. 2014, 164, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Fagbohungbe, M.; Herbert, B.; Hurst, L.; Li, H.; Usmani, S.; Semple, K. Impact of biochar on the anaerobic digestion of citrus peel waste. Bioresour. Technol. 2016, 216, 142–149. [Google Scholar] [CrossRef]
- Shen, Y.; Linville, J.L.; Ignacio-de Leon, P.A.A.; Schoene, R.P.; Urgun-Demirtas, M. Towards a sustainable paradigm of waste-to-energy process: Enhanced anaerobic digestion of sludge with woody biochar. J. Clean. Prod. 2016, 135, 1054–1064. [Google Scholar] [CrossRef]


| Time (Year) | 2015 | 2016 | 2017 | 2018 | 2019 | |
|---|---|---|---|---|---|---|
| Published paper number of pre-treatment | Microbial pre-treatment | 64 | 99 | 94 | 142 | 182 |
| Physical pre-treatment | 19 | 27 | 15 | 27 | 25 | |
| Chemical pre-treatment | 132 | 148 | 154 | 179 | 206 | |
| Published paper number of Co-digestion | Co-digestion | 403 | 475 | 575 | 720 | 778 |
| Published paper number of recirculation | Recirculation | 34 | 55 | 48 | 57 | 55 |
| Published paper number of microaeration | Microaeration | 2 | 6 | 7 | 7 | 7 |
| Published paper number of additives | Biochar | 13 | 31 | 48 | 67 | 103 |
| Bioaugmentation and enzymes | 20 | 19 | 17 | 39 | 38 | |
| Trace element | 30 | 28 | 28 | 53 | 63 | |
| Conductive material | 2 | 9 | 17 | 33 | 14 | |
| Published paper number of anaerobic digestion | Anaerobic digestion | 1863 | 2149 | 2311 | 2633 | 2573 |
| The ratio of strategies/ anaerobic digestion | 38.6% | 41.7% | 43.4% | 50.3% | 57.2% |
| Pre-Treatment Methods | Mechanism | Cost | Advantage | Disadvantage |
|---|---|---|---|---|
| Physical pre-treatment | Break complex structures and increase specific surface area | +++ | Simple principle and operation, no inhibitors generate | High energy consumption |
| Chemical Pre-treatment | Destroy molecular structure, reduce the crystallinity of lignocellulosic, dissolve lignin | ++ | High efficiency | Potential secondary pollution |
| Biological pre-treatment | Production of enzymes capable of decomposing complex organic matter | + | No environment pollution, mild reaction, less energy consumption | Long pre-treatment cycle, complex culture conditions, loss of organic matter, low efficiency |
| Pre-Treatment | Condition | T (°C) | Substrate Type | Methane Yield a | Methane Yield b | Ref. |
|---|---|---|---|---|---|---|
| Biological Pre-treatment | Secreted enzymes | 37 ± 2 °C | Maize straw | 250.2 c | 277.0 c | [16] |
| Biological Pre-treatment | Fungi | 37 ± 1 °C | Yard trimmings | 8.5 d | 40.0 d | [17] |
| Biological Pre-treatment | Fungi | Not given | Corn straw | 131.0 d | 239.0 d | [18] |
| Biological Pre-treatment | Bacterium | 35 °C | MSW | 97.8 d | 221.0 d | [19] |
| Biological Pre-treatment | Biogas slurry | 35 ± 1 °C | Rice straw | 174.3 d | 233.3 d | [20] |
| Biological Pre-treatment | Fungi | 36 °C | Wheat straw | 118.0 c | 182.0 c | [21] |
| Chemical Pre-treatment | 2% NaOH | 35 ± 1 °C | Corn stalk | 187.0 d | 196.0 d | [22] |
| Chemical Pre-treatment | 10% CaO | 35 °C | Microalgae | 257.0 d | 292.0 d | [23] |
| Chemical Pre-treatment | 4% NaOH | 37 ± 0.5 °C | Pennisetum Hybrid | 249.3 d | 281.4 d | [24] |
| Chemical Pre-treatment | 1.6% NaOH | 37 ± 2 °C | Wheat straw | 263.0 d | 314.0 d | [25] |
| Chemical Pre-treatment | 20 g N/L NaNO2 | 35 °C | Waste activated sludge | 132.0 d | 153.0 d | [26] |
| Chemical Pre-treatment | 1% urea | 35 °C | Wheat straw | 210.4 d | 305.5 d | [27] |
| Chemical Pre-treatment | 10.0% NaOH | 37 °C | Dairy cow manure | 292.1 d | 361.0 d | [28] |
| Chemical Pre-treatment | 3%H2O2 | 25 ± 2 °C | Corn straw | 100.6 d | 216.7 d | [29] |
| Physical Pre-treatment | milling | 38 °C | Wheat straw | 127.4 d | 250.3 d | [30] |
| Physical Pre-treatment | Microwave | 35 °C | Microalgae | 170.0 d | 270.0 d | [31] |
| Physical Pre-treatment | Microwave | 37 ± 0.5 °C | FW and Sewage sludge | 285.0 d | 310.0 d | [32] |
| Physical Pre-treatment | Thermal | 37 °C | Algae | 279.0 e | 391.0 e | [33] |
| Physical Pre-treatment | Thermal | 35 °C | Wheat straw | 404.0 e | 615.0 e | [34] |
| Physical Pre-treatment | Thermal | 35 °C | Microalgae | 181.0 d | 106.0 d | [35] |
| Substrate | Reactor Type | Recirculation Type | Conclusions | Ref. |
|---|---|---|---|---|
| Vegetable waste | Two-stage reactor | Recirculation rates from 0 to 1.4 a | pH was significantly increased in acidogenic reactor. Biogas production rates increased more than 3 times. | [83] |
| Corn stover | CSTR | Liquid fraction of the digestate total recirculation | Methane and biogas production were increased significantly by 2.3% and 10.8% due to increased process stability. | [68] |
| FW | Integrated two-phase reactor | Leachate recirculation rates b at 0%, 25%, 50%, or 75% of collected leachate | Enhance the hydrolysis efficiency and methanogenic reaction, 50% recirculation obtained optimal effect. | [86] |
| Wastewater | CSTR and AnMBR | Sludge recirculation | COD removal rate reaches its highest, at 96.7%, when sludge recirculation rate is 2. | [87] |
| FW | CSTR | Recirculation liquid fraction of the digestate, recirculation rate is 2 c | The methane yield of recirculation and no-recirculation was similar. | [83] |
| Pig slurry and straw (3:1, w/w) | Leachate reactor | Recirculation of all leachate | A better system stability was obtained because recirculation avoided the accumulation of VFAs. | [81] |
| Pig manure | CSTR | Liquid digestate | Recirculation operation could improve the bioenergy production under OLRs below 5 g VS L−1 d−1. However, OLRs more than 6 g VS L−1 d−1 recirculation decreased mass transfer characteristics and increased heavy metal accumulation. | [84] |
| OFMSW and Corn Straw | Leachate reactor | Leachate recirculation rates are 0.3, 0.6, 1.2, 2.4, and 4.8 d | High recirculation rate positively contributed to the hydrolysis and acidogenesis rate due to its inoculation effect and mass transfer enhancement. Highest methane yield was obtained when recirculation rate was 0.3. | [85] |
| Objective | Reactor Type | Substrate | Oxygen Dosing Rate Equivalent (L O2/Lreactor/d) * | Results | Ref. |
|---|---|---|---|---|---|
| Enhance hydrolysis | CSTR | FW and brown water | 0.005 and 0.007 | Bacterial diversity and concentration of VFAs increased. | [61] |
| Enhance hydrolysis | CSTR | Primary sludge | 0.21 | Hydrolysis rate increased by 50–60%. However, methane yield, VFAs, and sCOD decreased due to aerobic substrate consumption. | [91] |
| Enhance hydrolysis | CSTR | Primary sludge | 0.5 | Hydrolysis of carbohydrates and protein was enhanced accompanied by increased solubilization of COD. | [94] |
| Enhance hydrolysis | Leach bed reactor | Synthetic FW | 2.1, 4.4 and 6.5 | Middle aeration rate was best: increased hydrolysis. | [95] |
| Enhance methane yield | Batch reactor | Corn straw | 0.003–0.021 | At lower micro-aeration intensity, enhanced methane yield, diversity of phylum Firmicutes, and VS removal were obtained. | [96] |
| Enhance methane yield | Batch reactor | Long-chain fatty acids | Not given | A significant increase in methane yield. | [97] |
| Remove H2S | Sludge reactor | Waste-activated sludge | 0.01 | 98% H2S removal from biogas. | [98] |
| Remove H2S | UASB | Synthetic brewery | 0.08 | 73% H2S removal. | [99] |
| Remove H2S | UASB | Wastewater | 0.03 mol O2 m−3 | The highest H2S removal efficiency was 91.2% and obtained for an O2:S ratio of 0.5. | [92] |
| Control VFA accumulation and improve effluent quality | CSTR | Waste-activated sludge | 0.03 | 3.5 times lower VFAs and 33% lower sCOD were obtained. Compared with anaerobic conditions, microaerobic conditions have lower foaming and better dewaterability. | [100] |
| Overcome overloading and improve reactor stability | CSTR | Waste-activated sludge | 0.01 | Overcame hydraulic overloading, promoted growth of hydrogenotrophic bacteria. | [93] |
| Produce VFAs | Batch | Batch reactor | 0.09 and 1.9 | Highest VFA production was obtained with 15 mL O2/g VS and 3 days’ incubation time using cattle manure as inoculum. | [11] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Wang, Z.; He, Y.; Wang, Y.; Wang, S.; Zheng, Z.; Wang, S.; Xu, J.; Cai, Y.; Ying, H. A Comprehensive Review of the Strategies to Improve Anaerobic Digestion: Their Mechanism and Digestion Performance. Methane 2024, 3, 227-256. https://doi.org/10.3390/methane3020014
Li X, Wang Z, He Y, Wang Y, Wang S, Zheng Z, Wang S, Xu J, Cai Y, Ying H. A Comprehensive Review of the Strategies to Improve Anaerobic Digestion: Their Mechanism and Digestion Performance. Methane. 2024; 3(2):227-256. https://doi.org/10.3390/methane3020014
Chicago/Turabian StyleLi, Xiaoyong, Zhi Wang, Yun He, Yuzhong Wang, Shilei Wang, Zehui Zheng, Songtao Wang, Jingliang Xu, Yafan Cai, and Hanjie Ying. 2024. "A Comprehensive Review of the Strategies to Improve Anaerobic Digestion: Their Mechanism and Digestion Performance" Methane 3, no. 2: 227-256. https://doi.org/10.3390/methane3020014
APA StyleLi, X., Wang, Z., He, Y., Wang, Y., Wang, S., Zheng, Z., Wang, S., Xu, J., Cai, Y., & Ying, H. (2024). A Comprehensive Review of the Strategies to Improve Anaerobic Digestion: Their Mechanism and Digestion Performance. Methane, 3(2), 227-256. https://doi.org/10.3390/methane3020014







