Abstract
Salinity is a dominant obstacle to the proper germination of seeds, growth of seedlings, and, consequently, the production of crops. The priming of seeds with different treating agents can efficiently impart salinity tolerance. Kidney bean is a nutritious and popular vegetable crop in the world. Literature shows that salt stress negatively disturbs the germination and growth of kidney beans. In the present research, we investigated the potentiality of salicylic acid (SA) and hydrogen peroxide (H2O2) as priming and exogenous agents to alleviate the salinity-inhibited germination and growth of kidney beans. The seeds were pretreated with SA (1 mM and 2 mM) and H2O2 (0.1 mM and 0.15 mM) and soaked in normal tap water (hydro-priming) for 60 min. In addition, for the control experiment, untreated seeds were used. Finally, primed seeds were subjected to salt stress (150 mM NaCl). Our results exhibited that salt stress considerably lowered the percentage of germination (GP), germination index (GI), seed vigor index (SVI), shoot length (SL), root length (RL), shoot–root fresh and dry biomass, and plant growth. The results also exhibited that salt stress significantly decreased the relative water content (RWC) and photosynthetic pigments such as chlorophyll, carotenoids, lycopene, and beta-carotene contents. The SA- and H2O2- and hydro-priming stimulated the GP, GI, SL, RL, SVI, and seedling growth. Data also revealed that the supplementation of SA and H2O2 enhanced RWC and photosynthetic pigments. When compared to other treatments, pretreatment with 1 mM SA was determined to be comparatively more effective at imparting the salt tolerance of kidney beans. Overall, these results, via a heatmap and principal component analysis, uncovered that priming and exogenous applications of SA and H2O2 can improve salt tolerance and enhance germination and seedling characteristics of kidney beans.
1. Introduction
Successful seed germination and strong seedling vigor are critical determinants for crop growth in their success, as these parameters lead to consistent plant growth and, consequently, high production [1]. As a result, increasing seed vigor and obtaining proper germination is the main goal of the seed grower in order to improve the vital and yield-defining stage of crop establishment. However, different biotic and abiotic stresses hamper seed germination, vigor, seedling characteristics, and plant growth [2]. Drought, salt, and severe temperatures are examples of abiotic stresses that create osmotic stress in crop plants, resulting in an imbalance at the cellular, molecular, and physiological levels, ultimately leading to plant mortality [3]. Salinity is one of the injurious abiotic stresses that extremely affect the germination, growth, and output of crops [4]. Excessive salt levels in cultivated fields are a big worldwide issue at the moment. According to recent statistics, salt disrupts approximately 0.80 billion hectares of terrestrial regions, severely restraining the usage of land for agricultural purposes [5]. From an agricultural standpoint, salinity levels influence approximately 20% of the crop-cultivable area and approximately 33% of irrigated cropland to variable degrees, and by 2050, this figure will surpass 50% [6]. To face salt stress, plants have evolved diverse mechanisms and plant growers are applying different tactics such as seed priming, the supplementation of plant growth regulators, organic fertilizers, and the screening of the right varieties [7,8,9,10].
Priming is a simple technology that hydrates seeds to a point where metabolic activity for germination is initiated, but the emergence of a radicle does not arise [11,12]. This method can help a variety of crops whose germination and emergence are hampered by unfavorable soil conditions. Priming stimulates germination by inducing a variety of metabolic changes in the seed and increases seed vitality, resulting in quick and homogenous emergence as well as strong stand establishment [13]. Moreover, seed priming mitigates abiotic stresses in plants by regulating antioxidant enzyme activities, ionic homeostasis, and photosynthetic attributes [14]. Furthermore, priming leads to a faster and stronger induction of basal resistance mechanisms in response to subsequent pathogen attacks, as well as increased tolerance to abiotic stimuli [15,16]. Different signaling molecules and plant hormones used in priming have been well-reported, as have agents that enhance the germination rate and seedling emergence in crops [17,18]. Salicylic acid (SA) is a phenolic endogenous growth regulator that influences a variety of plant progressions, including germination, growth, photosynthesis, and stress tolerance [19,20]. On the other hand, hydrogen peroxide (H2O2) performs a vital role in signal transduction, activating a chain of physiological mechanisms that increase plant tolerance to salt stress [21,22]. Literature shows that rice seedlings boosted salt resistance due to seed priming, and supplemented H2O2 application also boosted salinity resistance in rice seedlings [23]. Hemalatha et al. [24] reported that H2O2-primed rice seeds had better germination and growth when compared with hydro-primed and unprimed seeds. In addition, hydro-priming has the potential for seed germination and crop growth enhancement. Damalas et al. [25] reported that hydro-priming enhances the germination and field performance of faba bean. Recently, Tania et al. [26] reported that SA, H2O2, and hydro-primed wheat seeds enhance germination and seedling growth.
Beans are an important legume crop because of their notable health benefits and soil-friendly properties. The kidney bean is one of the most popular and common varieties of bean and is also called the French bean, snap bean, haricot bean, or navy bean [27]. The seeds of these beans are excellent plant-based sources of protein (23%), flavonoids, and carotenoids [28]. According to the National Nutrient Database of USDA, one standard cup of canned Kidney beans (approximately 150 g) contains 0.55 g of fat, 5.66 g of carbohydrate, 2.6 g of fiber, 1.94 g of sugar, 1.42 g of protein, 17 mg of calcium, 1.2 mg of iron, 18 mg magnesium, 30 mg of phosphorus, and 130 mg potassium [29]. Furthermore, the cultivation of this bean is becoming more popular due to its dual purpose as a pulse while also being consumed as immature tender fruits. A number of studies found that seed priming and the supplementation of various chemicals increased faba bean, broad bean, and common bean performance under stress conditions [17,30,31]. However, research is scant on the germination and seedling growth of kidney beans under stress. To the best of our knowledge, there is no published research available on the role of SA and H2O2 priming on the germination and seedling traits of kidney beans under salt stress. Therefore, in this research, we explored the potentiality of SA and H2O2 priming on the germination and seedling growth of kidney beans under salt stress.
2. Materials and Methods
2.1. Site of Experiment, Treating Conditions, and Germination Indices Measurement
A Petri-dish and pot experiment was conducted in the laboratory and net house, respectively, at the Seed Science and Technology department, Bangladesh Agricultural University, Mymensingh. The local, high-yielding, popular kidney bean (Phaseolus vulgaris L.) variety “Lal Rajma” was collected from the local market (Mymensingh, Bangladesh) and used in the experiment. To prevent the growth of microbial contaminants, present on the seed surface, sodium-hypochlorite (1%) was used for 5 min to sterilize the seeds. The seeds were soaked for 60 min for priming in 1 mM and 2 mM concentrations of SA, and 0.1 mM and 0.15 mM concentrations of H2O2, each in individual screw-plugged pots, and in double-distilled water for hydro-priming. Moreover, for control experiments, untreated seeds were used. The concentrations of SA and H2O2 were selected based on previous experiments as well as the literature [8,23,26]. Thirty bean seeds were soaked for each concentration of treatment. The primed seeds were positioned on Petri dishes (150 × 25 mm diameter) prepared with 3 layers of Whatman filter papers and kept at room temperature 25 ± 1 °C and relative humidity of 96%. Along with priming, seeds were exposed to salt stress. For the salt treatment, 10 mL of a 150 mM NaCl solution was placed into every Petri dish, while the non-saline condition was controlled by a Petri dish holding 15 mL of pure water. With 3 independent repetitions, the experiment was operated in a completely randomized block design, and the treatment conditions shown in Table 1 were followed. All the chemicals used in this experiment were purchased from Sigma-Aldrich (St. Louis, MO, USA).

Table 1.
Treatment conditions and their denotation.
Seedling emergence was recorded daily, and radicles 1 mm or more in length were considered germinated. The number of seeds that germinated was documented at 12 h intervals from the first emergence up to the 7th day. After that GP, MGT, and GI were calculated. At 22 days after sowing (DAS), SL (cm) and RL (cm) were measured, and SVI was calculated. According to Rhaman et al. [7], the respective formulas were used to calculate the GP, MGT, GI, and SVI.
“n” is the seed number on day D and D is the number of days calculated from the beginning of germination.
The GI was computed using the following formula:
where seedling length = shoot length + root length.
Seed vigor index (SVI) = GP × seedling length (cm)
2.2. Pot Experiment
Uniform-size seedlings were transplanted from the Petri dish experiments for each treatment and positioned on plastic pots (22.5 × 25.5 cm) packed with soil (6 seedlings/pot; seedling size was approximately 6–7 cm. Though seedlings were collected from Petri dish priming experiments, seedlings (17 days old) were again pretreated with SA and H2O2, except hydro-primed and control plants (concentrations are similar to those during the germination period) for 3 days. After that, the seedlings were subjected to 150 mM NaCl stress and grown for 5 days. After 5 days of salt exposure, the plant height, relative water content, and photosynthetic pigments were analyzed. For the fertilizer application, the soil was thoroughly mixed with Hyponex liquid fertilizer (Osaka, Japan), which contains nitrogen (6%), phosphorus (10%), potassium (5%), and other micronutrients. In the pots, 3 mL of the fertilizer solution was applied twice a week in each pot. The treatment conditions were as follows: C, control; SS, 150 mM NaCl; Hp, seedling from hydro-primed seed; HpSS, seedling from hydro-primed seed + 150 mM NaCl; SA1, 1 mM SA; SA1SS, 1 mM SA + 150 mM NaCl; SA2, 2 mM SA; SA2SS, 2 mM SA + 150 mM NaCl; HP1, 0.1 mM H2O2; HP1SS, 0.1 mM H2O2 + 150 mM NaCl; HP2, 0.15 mM H2O2; HP2SS, 0.15 mM H2O2 + 150 mM NaCl.
2.3. Relative Water Content Determination
The relative water content (RWC) was finalized using the standard techniques of Mostofa and Fujita [32]. To determine RWC, after 25 days of planting, leaf samples were gathered, and leaves fresh weights (FW) were taken. The leaves were then immersed in dH2O and remained for 1 h and 2 h, and the turgid weights of leaves were taken. Afterward, surplus water was wiped away from the turgid leaves and the turgid weight (TW) was immediately noted. The leaves were then oven-dried at 70 degrees Celsius for 48 h to determine their dry weight (DW). The RWC was analyzed according to the equation below:
RWC (%) = (FW − DW)/(TW − DW) × 100
2.4. Estimation of Photosynthetic Pigments
The contents of photosynthetic leaf pigments Chl a and b, carotenoid, lycopene, and beta carotene were quantified using a spectro-photometric approach based on Lichtenthaler’s method [33]. Fresh leaves weighing 0.5 g were placed in a tiny vial holding 10 mL of 80% ethanol. For pigment extraction, the bowls were wrapped in aluminum foil and kept in the dark for seven days. Using a spectrophotometer, the absorbance of Chl a and b, lycopene, beta carotene, and carotenoids was determined at 663, 645, 645, 505, and 453 nm wavelengths for chlorophyll, lycopene, beta carotene, and carotenoid concentrations (Shimadzu UV-2550, Kyoto, Japan). The following formulae were used to determine the photosynthetic pigments:
Total Chlorophyll = Chlorophyll a + Chlorophyll b
Chlorophyll a = (0.999 × A663 − 0.0989 × A645)
Chlorophyll b = (−0.328 × A663 + 1.77 × A645)
Lycopene = (0.0458 × A663 + 0.204 × A645 + 0.372 × A505 − 0.0806 × A453)
Beta-carotene = (0.216 × A663 − 1.22 × A645 − 0.304 × A505 + 0.452 × A453)
Carotenoids = (A480 + (0.114 × A663 − 0.638 × A645)
2.5. Statistical Analysis
Minitab 17.0 statistical software was employed to accomplish a one-way analysis of variance (ANOVA) on the collected data, and Tukey’s pairwise comparisons were used to compare statistical variances between the mean values of treatment conditions and salt stress at the 5% significance level. In R 3.6.1, the “pheatmap” was used to create a heatmap and perform hierarchical clustering analysis using Euclidean distances, while the “ggplot2”, “factoextra”, and FactoMineR” packages were used for principal component analysis (PCA).
3. Results
3.1. Priming Boosts Germination Indices and Traits of Seedlings under Salt Stress
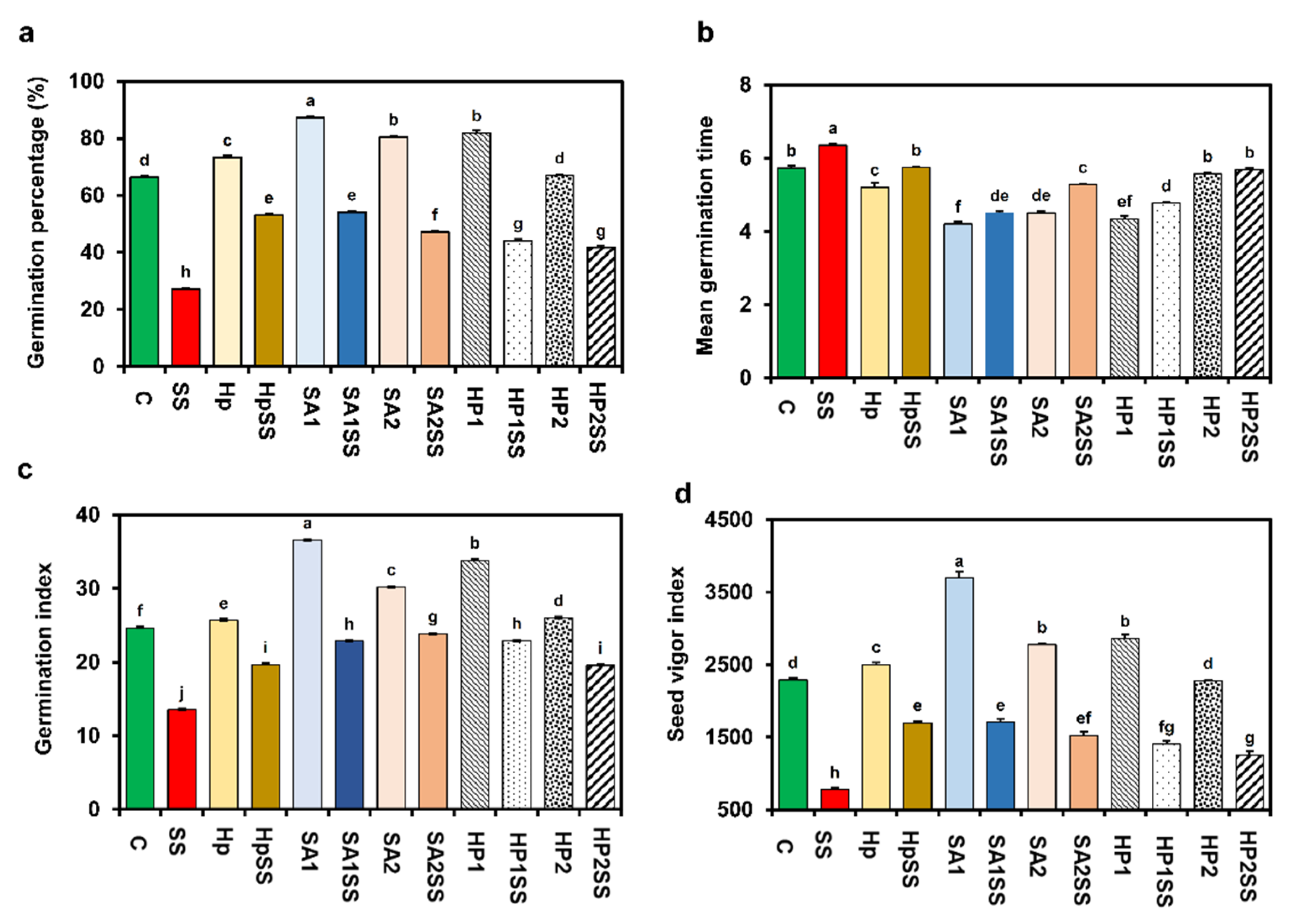
The impacts of SA and H2O2 priming on the germination indices of kidney beans under salt stress are displayed in Figure 1. The findings show that salt stress significantly reduced GP by 58.99% compared to the control condition. In both stress and without-stress situations, Hp, SA1, SA2, HP1, and HP2 priming showed a significant effect on GP (Figure 1a). While the highest GP (87.2%) was recorded for SA1, the lowest GP (41.6%) was recorded for HP2SS (Figure 1a). In the case of MGT, significant results were found for different priming treatments compared to salt stress. MGT decreased by 9.6, 29.1, 17.0, 24.8 and 10.5%, respectively, for HpSS, SA1SS, SA2SS, HP1SS, and HP2SS compared to salt stress (Figure 1b). Salt stress significantly reduced GI and priming with SA and H2O2 increased GI under salt settings, and the application of priming agents without stress also increased GI (Figure 1c). Similarly, different priming conditions increased SVI but salt stress significantly reduced SVI (66.1%) compared with the control. The results indicated that SVI increased by 119.2, 119.8, 96.9, 81.1 and 62.1%, respectively, for HpSS, SA1SS, SA2SS, HP1SS, and HP2SS priming compared to salt stress (Figure 1d).
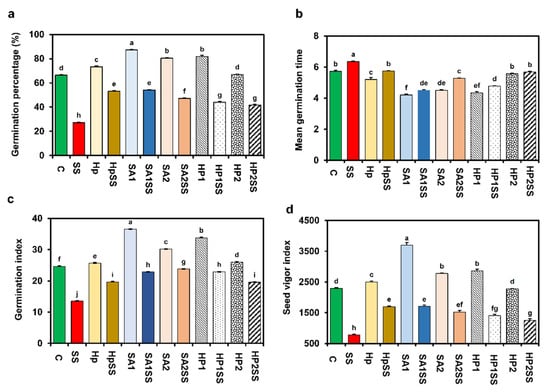
Figure 1.
Effects of SA-, H2O2-, and hydro-priming on the germination indices of kidney bean under salt stress. (a) Germination percentage; (b) mean germination time; (c) germination index; (d) seed vigor index. The error bar represents standard error. Different letters among treatments were analyzed by Tukey’s test: p < 0.05.
To assess the effects of salt stress and stress-decreasing acts of SA and H2O2 on the growth of bean seedlings, we recorded the SL and RL. The results indicated that salt stress significantly diminished SL and RL by 14.8% and 26.6%, respectively, compared with the native plants (Table 2). All the priming conditions significantly increased shoot and root length under salt stress. The highest shoot (30.3 cm) and root (12.0 cm) lengths were observed in the case of SA1 priming. Similarly, shoot fresh weight (SFW), shoot dry weight (SDW), and root fresh and dry weight (RFW, RDW) were decreased by salt stress (Table 2). SA and H2O2 priming significantly increased SFW, SDW, RFW, and RDW under salt stress. These results clearly indicate that SA and H2O2 priming improved germination indices and seedling traits of kidney beans under salt stress.

Table 2.
Effects of SA-, H2O2-, and hydro-priming on the seedling traits of kidney bean under salt stress. Shoot length (SL), root length (RL), shoot fresh weight (SFW), shoot dry weight (SDW), root fresh weight (RFW), root dry weight (DRW). Values are means of 3 replicates and means with different letters in a column indicating significant differences (p ≤ 0.05).
3.2. Exogenous SA and H2O2 Enhance Growth and RWC of Plants under Salt Stress
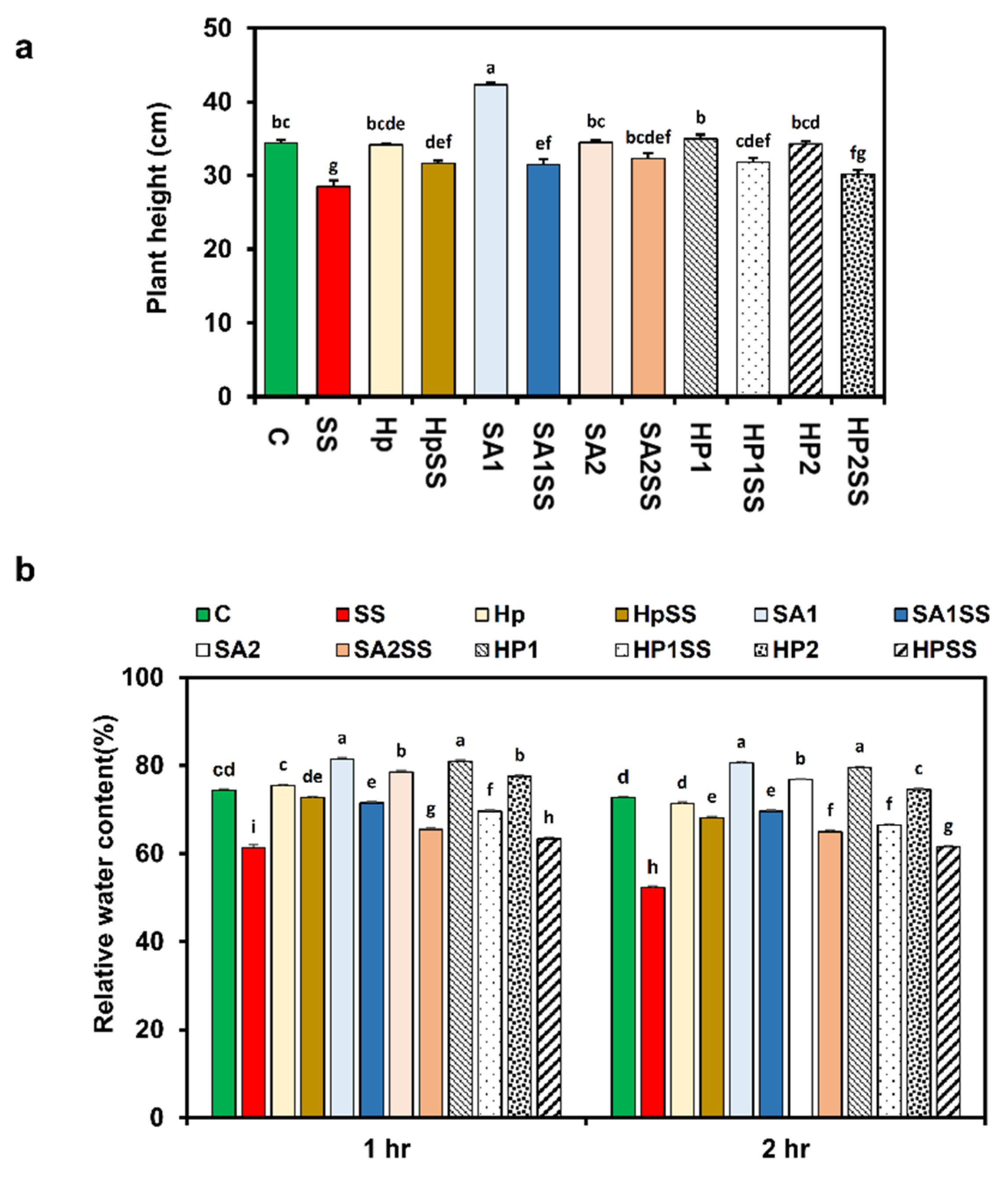
The influences of exogenous SA and H2O2 on the growth of kidney beans were investigated in this study. When compared to the control, salt stress dramatically reduced plant height (17%) (Figure 2a). Under salt stress, the supplementation of kidney beans with SA (1 mM and 2 mM) and H2O2 (0.1 mM and 0.15 mM) improved plant height. Plant height was also increased by a single exposure to SA, H2O2, and Hp (Figure 2a).
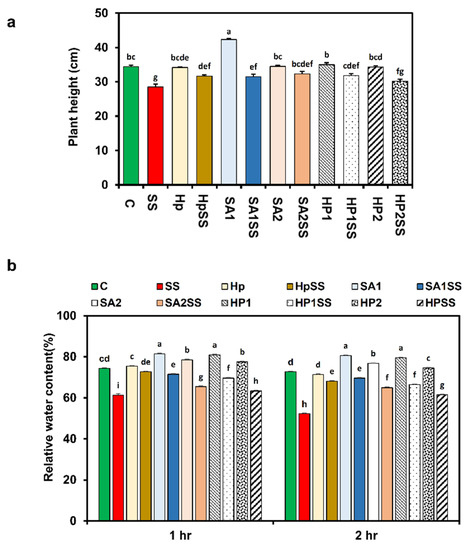
Figure 2.
Effects of SA-, H2O2-, and hydro-priming on the plant height and relative water content of kidney beans under salt stress. (a) Plant height; (b) relative water content. The error bar represents the standard error. Different letters among treatments were analyzed by Tukey’s test: p < 0.05.
The water status of kidney bean plants was studied in this work by measuring RWC with and without salt stress using priming agents. The results showed that salt stress significantly reduced RWC by 17.5% at 1 h and 28.2% at 2 h (Figure 2b). The application of priming agents responded strongly to RWC at both times in comparison to salt conditions. The highest RWC increased by HpSS 15.5% at 1 h, and at 2 h, the maximum RWC displayed by SA1SS was 24.9% higher compared to SS.
3.3. Pretreatment of SA and H2O2 Regulate Photosynthetic Pigment of Kidney under Salt Stress
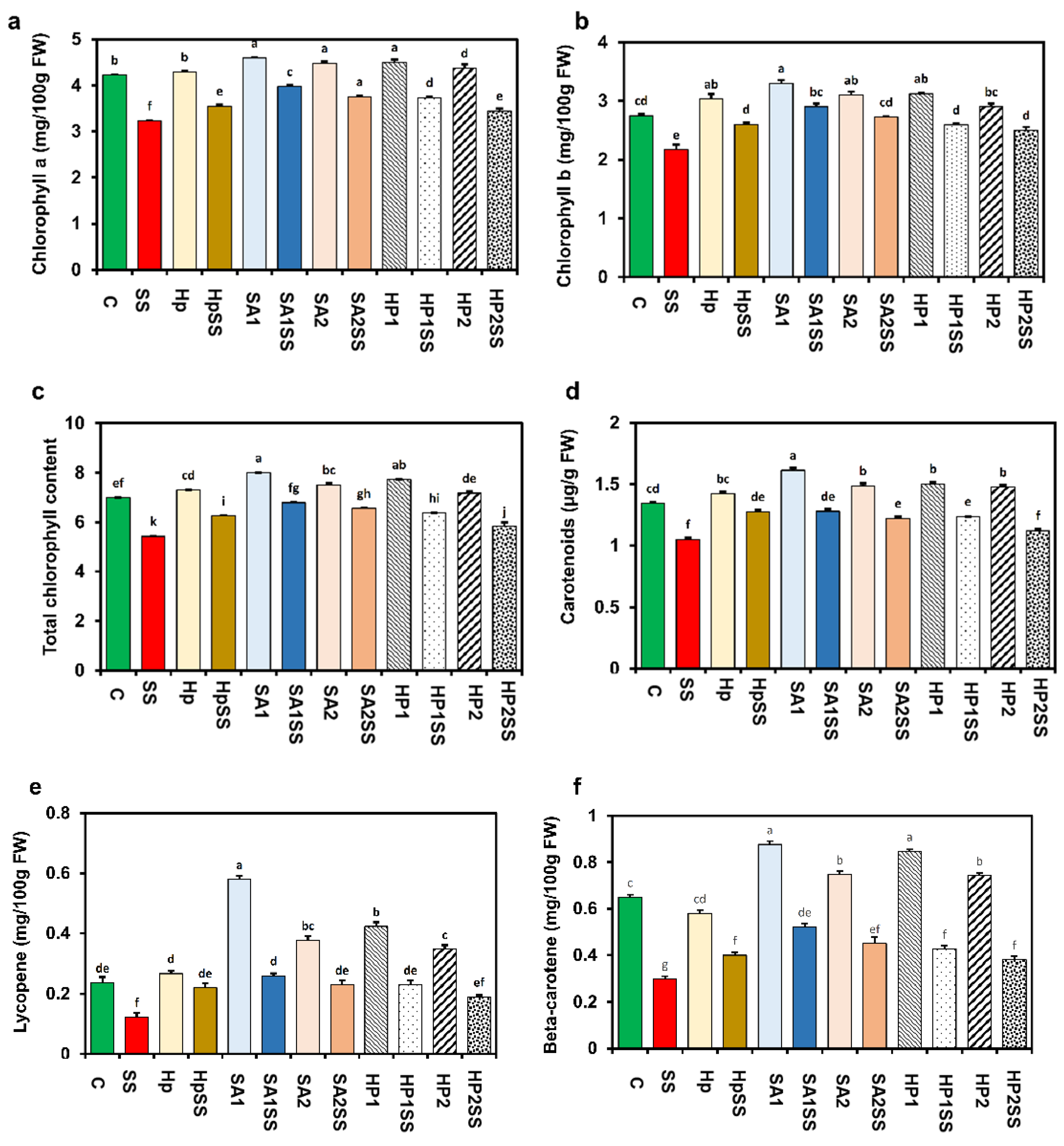
A significant fluctuation of Chl pigment contents was detected due to applied salt stress (Figure 3). A considerable decline in total Chl content (22.3%) including Chl a (23.5%) and Chl b (21%) in bean leaves due to salt stress compared to the control (Figure 3a–c). The supplementation of different concentrations of SA and H2O2 remarkably augmented Chl a, Chl b, and total Chl contents. Pigment analysis also revealed that carotenoids (21.8%), lycopene (48.1%), and beta-carotene (53.8%) were also reduced due to salt stress, and the supplementation of SA and H2O2 significantly increased the pigments (Figure 3c–f). The supplementation of SA1 under salt stress increased the maximum carotenoids (17.9%), lycopene (52.6%), and beta-carotene (42.6%) contents.
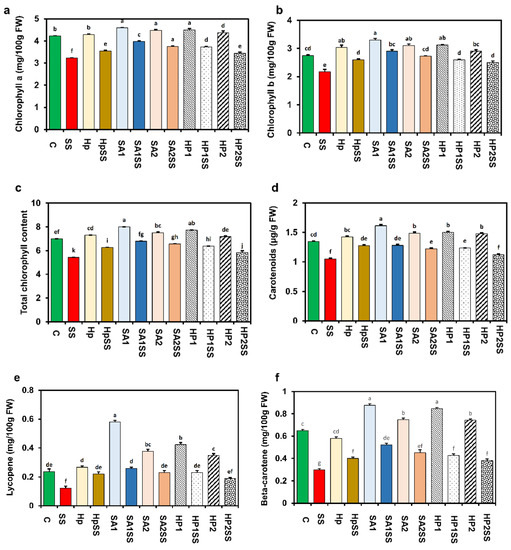
Figure 3.
Effects of SA-, H2O2-, and hydro-priming on the photosynthetic pigments of kidney bean under salt stress. (a) Chlorophyll a; (b) chlorophyll a; (c) total chlorophyll; (d) carotenoids; (e) lycopene; (f) beta-carotene. The error bar represents the standard error. Different letters among treatments were analyzed by Tukey’s test: p < 0.05.
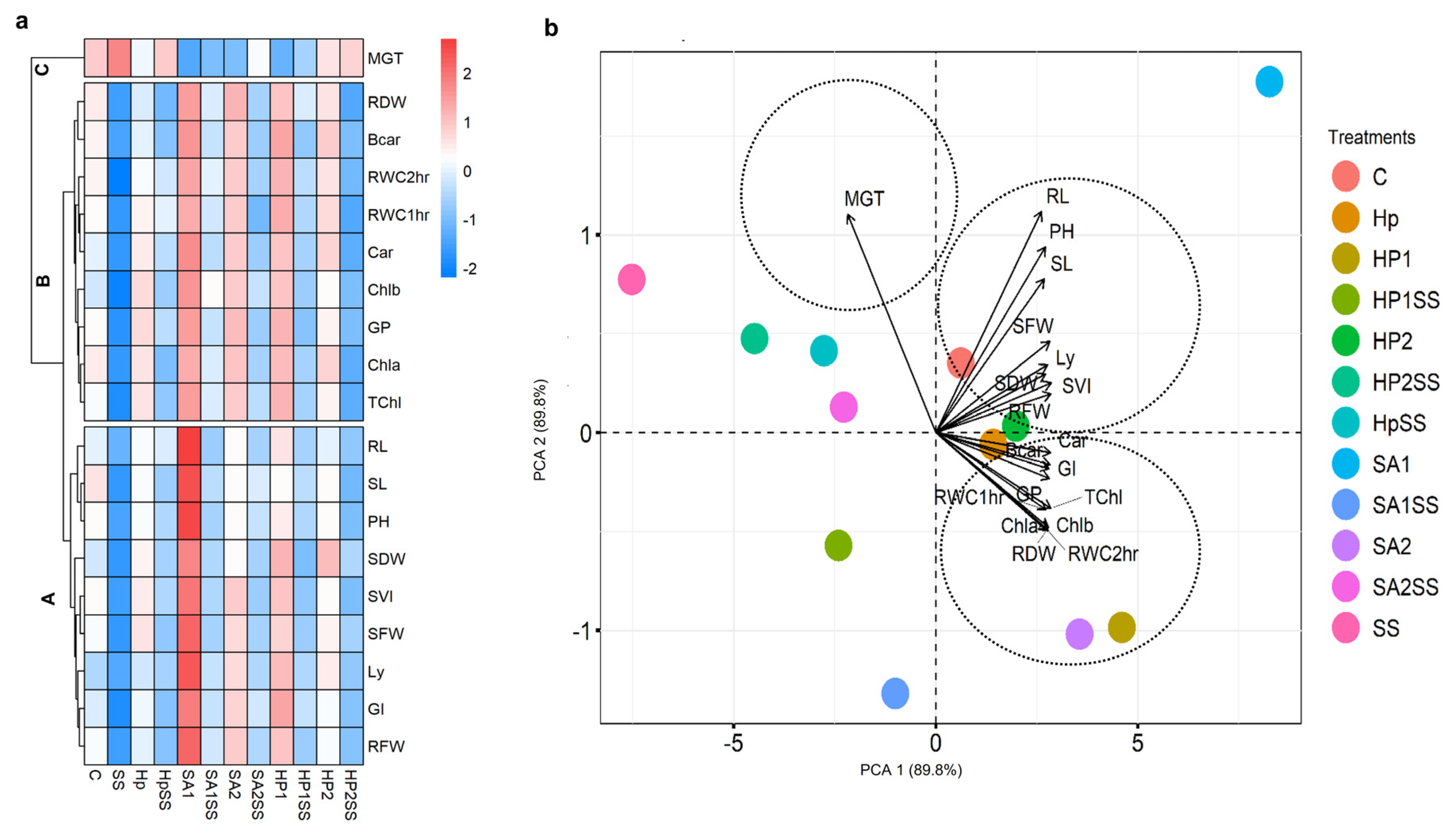
3.4. Estimation of Treatment-Variable Interactions through Heatmap and PCA
The mean values of germination indices, seedling traits, and physiological parameters were used to create a heatmap with hierarchical clustering and PCA (Figure 4). Three clusters (Cluster-A, -B, and -C) were found in the variable axis from the hierarchical clustering (Figure 4a). Cluster-A concerns RWC, GI, lycopene (Ly), SFW, SVI, SDW, plant height (PH), SL, and RL. Cluster-A parameters displayed decreasing trends in SS conditions and increasing trends in Hp, HpSS, SA1, SA1SS, SA2, SA2SS, HP1, HP1SS, HP2, and HP2SS conditions. Cluster-B includes the total Chl (TChl), Chl a, Chl b, GP, carotenoid (Car), RWC, beta-carotenoid (Bcar), and RDW. Similar to cluster-A, cluster-B parameters exhibited decreasing trends in SS conditions and increasing trends in other conditions. The variable MGT was clustered in the C group. The PCA components showed PCA 1 (89.8%) and PCA 2 (89.8%) data variability (Figure 4b). The PCA illustrated that cluster-A and B variables are closely associated with different treating conditions, whereas cluster-c was associated with the SS condition.
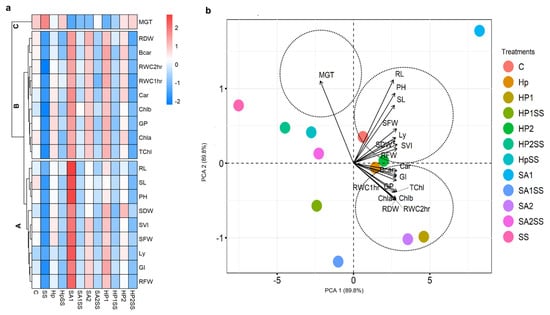
Figure 4.
Visualization of the interactions between treatments and all examined factors; a hierarchical clustered heatmap and principal component analysis (PCA) were used. (a) Heatmap with clustering method is used to display the scaled average values of all analyzed kidney bean parameters. (b) All data were analyzed using PCA. The investigated variables are germination percentage (GP), mean germination time (MGT), germination index (GI), seed vigor index (SVI), shoot length (SL), root length (RL), shoot fresh weight (SFW), shoot dry weight (SDW), root fresh weight (RFW), root dry weight (DRW), lycopene (Ly), plant height (PH), total chlorophyll (TChl), chlorophyll a (Chla), chlorophyll b (Chlb), carotenoid (Car), beta-carotene (Bcar), and relative water content (RWC).
4. Discussion
Successful seed germination is the most significant and fundamental step in the plant growth cycle since it is crucial for seedling development and subsequent productivity [34]. A number of studies described seed priming as a commonly used technique for promoting germination, improving morphological characteristics, and enhancing plant developmental progressions in both non-stress and stress situations [14,35]. Salt stress is a leading form of stress that causes severe reduction in germination and crop establishment. The literature shows that priming and the supplementation of diverse signaling molecules can impart salinity resistance to different crops such as wheat, faba bean, and rice [9,23,26]. Recently, we reported that the priming of wheat seeds with SA and H2O2 enhanced germination and seedling parameters under salt stress [26]. The current research was performed to uncover the role of SA and H2O2 in the priming of kidney bean seeds under salt stress. The results demonstrated that salt stress considerably decreased GP, GI, and SVI (Figure 1). Salt stress also reduced SL, RL, SFW, SDW, RFW, and RDW (Table 2). The presented results indicated that SA, H2O2, and hydro-priming increased GP, GI, SL, RL, and SVI of kidney beans under salt stress. The priming of seeds with these signaling molecules effectively relieved the adverse effects of salt stress on germinating seeds in our current experiment, as evidenced by significantly reduced MGT by SA, H2O2, and hydro-priming. These results are consistent with previous research that found that several priming agents significantly reduced the negative effects of salt stress on seed germination-related parameters in wheat [36], maize [37], and rice [38]. Furthermore, heatmap analysis showed that growth traits are increased by priming under salt stress (Figure 4a), and PCA also revealed that seedling traits are closely associated with different priming conditions (Figure 4b). Seed priming with various chemicals may weaken the cell membrane of seeds, resulting in enhanced nutrient utilization capability and faster germination and growth. Additionally, increasing the root length in the present experiment may have allowed for better soil moisture and nutrient uptake, as well as improved overall plant height or growth performance (Figure 2a) [39,40].
Under salt stress, maintaining an adequate water level in plants is a vital physiological step for maintaining normal growth progression [41]. RWC is a well-recognized water status indicator in plants since it is a water-related characteristic [42]. Salinity decreases the water potential in the soil, which has been linked to a decrease in RWC in leaves and a reduction in photosynthesis [43]. Exogenous chemical supplementation has been shown to improve the water status of various plant groups [44,45,46]. The findings obtained from the study showed RWC declined due to the salt stress (Figure 2b), and this is because the salt-induced damage disrupted the cell wall structure in the leaves, resulting in a decrease in water intake [47]. The results also demonstrated that salt-inhibited RWC is repressed by hydro-, SA-, and H2O2-priming and the supplementation of these agents (Figure 2b). This finding indicates that several priming and exogenous substances may be involved in the uptake of additional water from the soil in order to adjust the water level within plant organs.
A common symptom of salt stress is the deterioration of photosynthetic pigments. The present findings showed that salt stress decreased Chl a, Chl b, total Chl, carotenoids, lycopene, and beta carotene, and the priming and exogenous application of different agents improved the photosynthetic pigments under salt stress (Figure 3). This finding was consistent with earlier studies that found that priming rice and wheat seeds with distinct signaling molecules increased photosynthetic pigments [38,48]. The PCA and heatmap also clarified their interaction with treatment agents and stress conditions (Figure 4). These results indicate that different priming agents may aid the photosynthesis system by safeguarding chloroplast pigments from the toxicity of salt, most likely through the oxidative protection of chloroplasts and enhancing the enzyme’s activity by regulating chlorophyll biosynthesis [49,50].
Therefore, hydro-, SA-, and H2O2-priming enhance the germination and seedling traits of kidney beans under salt stress. Though all agents were determined to be viable options for improving kidney bean salinity tolerance, the lowest concentration of SA was found to be a more promising candidate for the improvement of salt-inhibited germination and seedling traits of kidney beans under salt stress.
5. Conclusions
The findings concluded that salt stress reduces germination indices, growth traits, leaf water status, and photosynthetic pigments of kidney beans. The priming and exogenous application of SA and H2O2 enhance the germination percentage, germination index, seed vigor index, shoot and root length, leaf water status, and photosynthetic pigments of kidney beans under salt stress. Our results also suggest that lower concentrations of SA and H2O2 can be used to achieve success in kidney bean cultivation. In spite of this, it is recommended that future research conducts a trial at the field level to validate our findings.
Author Contributions
Conceptualization, M.S.R.; methodology, M.S.R.; software, S.S.T.; validation, M.S.R., M.A.H., and Y.M.; formal analysis, S.S.T.; investigation, S.S.T., F.R., and M.M.R., M.H.K.; writing—original draft preparation, S.S.T. and F.R.; writing—review and editing, M.S.R. and Y.M.; visualization, M.S.R., M.A.H., and Y.M.; supervision, M.S.R. All authors have read and agreed to the published version of the manuscript.
Funding
The present investigation did not receive any external funds.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Finch-Savage, W.E.; Bassel, G.W. Seed vigour and crop establishment: Extending performance beyond adaptation. J. Exp. Bot. 2016, 67, 567–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rhaman, M.S.; Kibria, M.G.; Hoque, A. Climate Change and Its Adverse Impacts on Plant Growth in South Asia: Current Status and Upcoming Challenges. Phyton 2022, 91, 695. [Google Scholar]
- Arun, M.N.; Hebbar, S.S.; Senthivel, T.; Nair, A.K.; Padmavathi, G.; Pandey, P.; Singh, A. Seed Priming: The Way Forward to Mitigate Abiotic Stress in Crops. In Plant Stress Physiology-Perspectives in Agriculture. In Plant Stress Physiology-Perspectives in Agriculture; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Isayenkov, S.V.; Maathuis, F.J. Plant salinity stress: Many unanswered questions remain. Front. Plant Sci. 2019, 10, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gopalakrishnan, T.; Kumar, L. Modeling and mapping of soil salinity and its impact on Paddy Lands in Jaffna Peninsula, Sri Lanka. Sustainability 2020, 12, 8317. [Google Scholar] [CrossRef]
- Machado, R.M.A.; Serralheiro, R.P. Soil Salinity: Effect on Vegetable Crop Growth. Management Practices to Prevent and Mitigate Soil Salinization. Horticulturae 2017, 3, 30. [Google Scholar] [CrossRef]
- Rhaman, M.S.; Kibria, M.G.; Hossain, M.; Hoque, M.A. Effects of organic manure and bio-slurries with chemical fertilizers on growth and yield of rice (cv. BRRI dhan28). Int. J. Expt. Agric. 2016, 6, 36–42. [Google Scholar]
- Rhaman, M.S.; Rauf, F.; Tania, S.S.; Karim, M.M.; Sagar, A.; Robin, A.H.K.; Murata, Y. Seed priming and exogenous application of salicylic acid enhance growth and productivity of okra (Abelmoschus esculentus L.) by regulating photosynthetic attributes. J. Exp. Biol. Agric. Sci. 2021, 9, 759–769. [Google Scholar] [CrossRef]
- Abdel Latef, A.A.H.; Tahjib-Ul-Arif, M.; Rhaman, M.S. Exogenous auxin-mediated salt stress alleviation in faba bean (Vicia faba L.). Agronomy 2021, 11, 547. [Google Scholar] [CrossRef]
- Rasel, M.; Tahjib-Ul-Arif, M.; Hossain, M.A.; Hassan, L.; Farzana, S.; Brestic, M. Screening of salt-tolerant rice landraces by seedling stage phenotyping and dissecting biochemical determinants of tolerance mechanism. J. Plant Growth Regul. 2021, 40, 1853–1868. [Google Scholar] [CrossRef]
- Tania, S.S.; Rhaman, M.S.; Hossain, M.M. Hydro-priming and halo-priming improve seed germination, yield and yield contributing characters of okra (Abelmoschus esculentus L.). Trop. Plant Res. 2020, 7, 86–93. [Google Scholar] [CrossRef]
- Rhaman, M.S.; Rauf, F.; Tania, S.S.; Khatun, M. Seed priming methods: Application in field crops and future perspectives. Asian J. Res. Crop Sci. 2020, 5, 8–19. [Google Scholar] [CrossRef]
- Zhu, Z.H.; Sami, A.; Xu, Q.Q.; Wu, L.L.; Zheng, W.Y.; Chen, Z.P.; Jin, X.Z.; Zhang, H.; Li, Y.; Yu, Y.; et al. Effects of seed priming treatments on the germination and development of two rapeseed (Brassica napus L.) varieties under the co-influence of low temperature and drought. PLoS ONE 2021, 16, e0257236. [Google Scholar] [CrossRef] [PubMed]
- Rhaman, M.S.; Imran, S.; Rauf, F.; Khatun, M.; Baskin, C.C.; Murata, Y.; Hasanuzzaman, M. Seed priming with phytohormones: An effective approach for the mitigation of abiotic stress. Plants 2021, 10, 37. [Google Scholar] [CrossRef] [PubMed]
- Pastor, V.; Luna, E.; Mauch-Mani, B.; Ton, J.; Flors, V. Primed plants do not forget. Environ. Exp. Bot. 2013, 94, 46–56. [Google Scholar] [CrossRef]
- Borges, A.A.; Jiménez-Arias, D.; Expósito-Rodríguez, M.; Sandalio, L.M.; Pérez, J.A. Priming crops against biotic and abiotic stresses: MSB as a tool for studying mechanisms. Front. Plant. Sci. 2014, 5, 642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azooz, M.M. Salt stress mitigation by seed priming with salicylic acid in two faba bean genotypes differing in salt tolerance. Int. J. Agric. Biol. 2009, 11, 343–350. [Google Scholar]
- Roy, P.R.; Tahjib-Ul-Arif, M.; Polash, M.A.; Hossen, M.; Hossain, M.A. Physiological mechanisms of exogenous calcium on alleviating salinity-induced stress in rice (Oryza sativa L.). Physiol. Mol. Biol. Plants 2019, 25, 611–624. [Google Scholar] [CrossRef]
- Khan, W.; Prithiviraj, B.; Smith, D.L. Photosynthetic responses of corn and soybean to foliar application of salicylates. J. Plant Physiol. 2003, 160, 485–492. [Google Scholar] [CrossRef]
- Koo, Y.M.; Heo, A.Y.; Choi, H.W. Salicylic acid as a safe plant protector and growth regulator. Plant Pathol. J. 2020, 36, 1. [Google Scholar] [CrossRef]
- Uchida, A.; Jagendorf, A.T.; Hibino, T.; Takabe, T. Effects of hydrogen peroxide and nitric oxide on both salt and heat stress tolerance in rice. Plant Sci. 2002, 163, 515–523. [Google Scholar] [CrossRef]
- Silva, P.C.; AzevedoNeto, A.D.; Gheyi, H.R.; Ribas, R.F.; Silva, C.R.; Cova, A.M. Seed priming with H2O2 improves photosynthetic efficiency and biomass production in sunflower plants under salt stress. Arid. Land Res. Manag. 2021, 5, 1–5. [Google Scholar] [CrossRef]
- Roy, P.R.; Tahjib-Ul-Arif, M.; Akter, T.; Ray, S.R.; Sayed, M.A. Exogenous ascorbic acid and hydrogen peroxide alleviates salt-induced oxidative stress in rice (Oryza sativa L.) by enhancing antioxidant enzyme activities and proline content. Adv. Environ. Biol. 2016, 10, 148–155. [Google Scholar]
- Hemalatha, G.; Renugadevi, J.; Eevera, T. Studies on seed priming with hydrogen peroxide for mitigating salt stress in rice. Int. J. Curr. Microbiol. App. Sci. 2017, 6, 691–695. [Google Scholar] [CrossRef] [Green Version]
- Damalas, C.A.; Koutroubas, S.D.; Fotiadis, S. Hydro-priming effects on seed germination and field performance of faba bean in spring sowing. Agriculture 2019, 9, 201. [Google Scholar] [CrossRef] [Green Version]
- Tania, S.S.; Rahaman, M.; Rauf, F.; Afroj Suborna, M.; Humayun Kabir, M.; Hoque, A.; Rhaman, M.S. Seed priming with Salicylic Acid (SA) and Hydrogen Peroxide (H2O2) Improve Germination and Seedling Growth of Wheat (Triticum aestivum L.) under Salt Stress. Asian J. Res. Crop Sci. 2021, 6, 60–69. [Google Scholar] [CrossRef]
- Fatema, R.; Rahman, J.; Shozib, H.B.; Nazrul, M.I.; Fatima, K. Genetic diversity and nutritional components evaluation of Bangladeshi germplasms of kidney bean (Phaseolus vulgaris L.). J. Genet. Res. 2019, 5, 83–96. [Google Scholar]
- Mena, E.; Leiva-Mora, M.; Jayawardana, E.K.; García, L.; Veitía, N.; Bermúdez-Caraballoso, I.; Collado, R.; Ortíz, R.C. Effect of salt stress on seed germination and seedlings growth of Phaseolus vulgaris L. Cult. Trop. 2015, 36, 71–74. [Google Scholar]
- USDA (National Nutrient Database). Cut Green Beans. 29 August 2017. Available online: https://ndb.nal.usda.gov/ (accessed on 1 April 2022).
- Azooz, M.M.; Alzahrani, A.M.; Youssef, M.M. The potential role of seed priming with ascorbic acid and nicotinamide and their interactions to enhance salt tolerance in broad bean (‘Vicia faba’L.). Aust. J. Crop Sci. 2013, 7, 2091–2100. [Google Scholar]
- Nouairi, I.; Jalali, K.; Zribi, F.; Barhoumi, F.; Zribi, K.; Mhadhbi, H. Seed priming with calcium chloride improves the photosynthesis performance of faba bean plants subjected to cadmium stress. Photosynthetica 2019, 57, 438–445. [Google Scholar] [CrossRef] [Green Version]
- Mostofa, M.G.; Fujita, M. Salicylic acid alleviates copper toxicity in rice (Oryza sativa L.) seedlings by up-regulating antioxidative and glyoxalase systems. Ecotoxicology 2013, 22, 959–973. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic bio-membranes. Meth. Enzymol. 1987, 148, 350–382. [Google Scholar]
- Shiade, S.R.; Boelt, B. Seed germination and seedling growth parameters in nine tall fescue varieties under salinity stress. Acta Agric. Scand.-B Soil Plant Sci. 2020, 17, 485–494. [Google Scholar] [CrossRef]
- Muhei, S.H. Seed priming with phytohormones to improve germination under dormant and abiotic stress conditions. Adv. Crop Sci. Technol. 2018, 6, 403–409. [Google Scholar] [CrossRef]
- Farooq, M.; Basra, S.M.A.; Rehman, H.; Saleem, B.A. Seed priming enhances the performance of late sown wheat (Triticum aestivum L.) by improving chilling tolerance. J. Agron. Crop. Sci. 2008, 194, 55–60. [Google Scholar] [CrossRef]
- Ashraf, M.; Rauf, H. Inducing salt tolerance in maize (Zea mays L.) through seed priming with chloride salts: Growth and ion transport at early growth stages. Acta Physiol. Plant. 2001, 23, 407–414. [Google Scholar] [CrossRef]
- Tahjib-Ul-Arif, M.; Afrin, S.; Polash, M.A.S.; Akter, T.; Ray, S.R.; Hossain, M.; Hossain, M.A. Role of exogenous signaling molecules in alleviating salt-induced oxidative stress in rice (Oryza sativa L.): A comparative study. Acta Physiol. Plant. 2019, 41, 1–14. [Google Scholar] [CrossRef]
- Muhammad, I.; Kolla, M.; Volker, R.; Günter, N. Impact of nutrient seed priming on germination, seedling development, nutritional status and grain yield of maize. J. Plant Nutr. 2015, 38, 1803–1821. [Google Scholar] [CrossRef]
- Adhikari, B.; Dhital, P.R.; Ranabhat, S.; Poudel, H. Effect of seed hydro-priming durations on germination and seedling growth of bitter gourd (Momordica charantia). PLoS ONE. 2021, 16, e0255258. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Agarwal, R.M. Salinity stress induced alterations in antioxidant metabolism and nitrogen assimilation in wheat (Triticum aestivum L.) as influenced by potassium supplementation. Plant Physiol. Biochem. 2017, 115, 449–460. [Google Scholar] [CrossRef]
- Li, Y.; Li, H.; Li, Y.; Zhang, S. Improving water-use efficiency by decreasing stomatal conductance and transpiration rate to maintain higher ear photosynthetic rate in drought-resistant wheat. Crop J. 2017, 5, 231–239. [Google Scholar] [CrossRef]
- Pattanagul, W.; Thitisaksakul, M. Effect of salinity stress on growth and carbohydrate metabolism in three rice (Oryza sativa L.) cultivars differing in salinity tolerance. Indian J. Exp. Biol. 2008, 46, 736–742. [Google Scholar] [PubMed]
- Saboon, R.I.; Ahmad, N.; Ilyas, N.; Batool, N.; Gul, S. Salicylic acid enhances wheat plant growth under water stress conditions. Int. J. Biol. Biotech. 2015, 12, 329–336. [Google Scholar]
- Tahjib-Ul-Arif, M.; Siddiqui, M.N.; Sohag, A.A.; Sakil, M.A.; Rahman, M.M.; Polash, M.A.; Mostofa, M.G.; Tran, L.S. Salicylic acid-mediated enhancement of photosynthesis attributes and antioxidant capacity contributes to yield improvement of maize plants under salt stress. J. Plant Growth Regul. 2018, 37, 1318–1330. [Google Scholar] [CrossRef]
- Hernández, J.A.; Díaz-Vivancos, P.; Acosta-Motos, J.R.; Barba-Espín, G. Potassium nitrate treatment is associated with modulation of seed water uptake, Antioxidative Metabolism and Phytohormone Levels of Pea Seedlings. Seeds 2022, 1, 5–15. [Google Scholar] [CrossRef]
- Parvin, K.; Hasanuzzaman, M.; Bhuyan, M.H.; Nahar, K.; Mohsin, S.M.; Fujita, M. Comparative physiological and biochemical changes in tomato (Solanum lycopersicum L.) under salt stress and recovery: Role of antioxidant defense and glyoxalase systems. Antioxidants 2019, 8, 350. [Google Scholar] [CrossRef] [Green Version]
- Farzana, S.; Rasel, M.; Tahjib Ul Arif, M.; Hossain, M.A.; Azam, M.G.; Al Galib, M.A.; Mahamud, A.S.U.; Hossain, M.A. Salicylic acid and thiourea ameliorate the negative impact of salt stress in wheat (Triticum aestivum L.) seedlings by up-regulating photosynthetic pigments, leaf water status, and antioxidant defense system. J. Phytol. 2021, 13, 130–145. [Google Scholar] [CrossRef]
- Amin, A.A.; AEK, A.A.; Abouziena, H.F.; El-Awadi, M.; Gharib, F.A. Effects of benzoic acid and thiourea on growth and productivity of wheat (Triticum aestivum L.) plants. Int. Sci. Res. J. 2016, 72, 132–149. [Google Scholar]
- Ma, X.; Zheng, J.; Zhang, X.; Hu, Q.; Qian, R. Salicylic acid alleviates the adverse effects of salt stress on Dianthus superbus (Caryophyllaceae) by activating photosynthesis, protecting morphological structure, and enhancing the antioxidant system. Front. Plant Sci. 2017, 8, 600. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).