Simple Summary
Cell–cell communication mechanisms are gathering growing scientific interest, particularly in the context of cancer cells and the tumor microenvironment. Extracellular vesicles are gaining increased interest due to their relevance in tumor molecular characterization, classification, diagnosis, prognosis evaluation, and response to treatment. Many advances have been made in the clinical and therapeutic fields, exploiting increasingly precise biomolecular engineering strategies. This review aims to focus on the role of extracellular vesicles (EVs) as diagnostic and therapeutic tools in lung cancer.
Abstract
Lung cancer represents the leading cause of cancer-related mortality worldwide, with around 1.8 million deaths in 2020. For this reason, there is an enormous interest in finding early diagnostic tools and novel therapeutic approaches, one of which is extracellular vesicles (EVs). EVs are nanoscale membranous particles that can carry proteins, lipids, and nucleic acids (DNA and RNA), mediating various biological processes, especially in cell–cell communication. As such, they represent an interesting biomarker for diagnostic analysis that can be performed easily by liquid biopsy. Moreover, their growing dataset shows promising results as drug delivery cargo. The aim of our work is to summarize the recent advances in and possible implications of EVs for early diagnosis and innovative therapies for lung cancer.
1. Introduction
Cancer is the leading cause of mortality globally [1], and a massive effort is being focused on finding novel therapeutic approaches and standardizing methods that can contribute to early neoplastic detection. Non-invasive techniques that do not involve radiation analysis represent a crucial goal. Among different tumors, the principal cause of death is lung cancer [1]. Lung cancer can be classified into two main histological subtypes: Small-Cell Lung Carcinoma (SCLC) and Non-Small Cell Lung Carcinoma (NSCLC), with a higher prevalence of NSCLC (about 80–85%) [2]. In the last decade, the high level of mortality due to lung cancer has prompted the onset of many multicenter studies seeking to improve early tumor detection by consolidated analysis (imaging by x-ray, PET, and PET/CT) and blood tests correlation. The 2004 COSMOS study (Continuous Observation of Smoking Subject) (ClinicalTrials.gov ID NCT01248806) enrolled more than 5000 asymptomatic smoker volunteers from the population because of their higher risk of developing lung cancer. Subjects were followed for 5 years with blood tests, spirometry, and annual low-dose spiral CT radiological examinations for nodules, alongside an evaluation of the correlation between COPD and lung cancer. Furthermore, many more studies comprising thousands of healthy patients have evaluated circulating biomarkers and radiomic analyses. For example, the CLEARLY study (Circulating and Imaging Biomarkers to Improve Lung Cancer EARLY Detection) (ClinicalTrials.gov ID NCT04323579), which started in 2018, is a multifactorial “bio-radiomic” protocol designed to detect early lung cancer in association with circulating biomarkers and radiomic data. Prognostic radiomic profiles for early detection have been correlated with molecular and cellular biomarkers such as microRNAs (miRNAs), proteins, circulating tumor cells (CTCs), and extracellular vesicles (EVs). EVs are involved in various processes, such as cell proliferation, differentiation, and the inflammatory response.
During the last ten years, circulating EVs have gained growing attention not only as biomarkers, but also for their ability to mediate cell–cell regulation and be manipulated for therapeutic purposes [3]. EV components have been implicated in many biological processes, and among them, a clear involvement in cancer invasion and metastasis has been observed [4]. Particularly noteworthy are the modulatory effects of EVs released from tumors and non-tumor cells such as mesenchymal stromal cells (MSCs) [5,6]. Many studies have been carried out to evaluate the effects and compositions of different EVs in tumor progression. The presence of regulatory messenger RNA (mRNA), which can modulate cancer cell proliferation, has been found within EVs [7]. Additionally, EV analysis has revealed the presence of controller proteins from neighboring cells [8], such as from the tumoral counterpart. Released EVs shuttle molecules involved in cell adhesion, migration, aggressiveness, and resistance to chemotherapeutic treatments [9]. The most remarkable molecules carried by EVs are miRNAs, which modulate multiple processes (growth, differentiation, apoptosis, migration, and drug/radioresistance) by their interaction with non-coding RNAs (ncRNAs), such as mRNAs, long non-coding RNAs (lncRNAs), and circular RNAs (circRNAs) [10]. Through these interactions, a single miRNA strand can control multiple genes, inhibiting their translation. This uniqueness gives relevance both to regulation processes and diagnosis and therapy. Engineering EVs with specific ncRNAs represents a promising outcome of the last few years, whereas the identification of an miRNA-specific signature from onset tumors still represents a challenging target. This review focuses on the role of EVs in diagnosis as components of liquid biopsy and in therapies for lung cancer, exploiting their use as theranostic agents. Despite many groups in the past describing the relationship between EVs and lung cancer, we hope that our work can help to suggest future diagnostic and therapeutic directions, improving their applications in fighting lung cancer [11,12,13].
2. Extracellular Vesicles
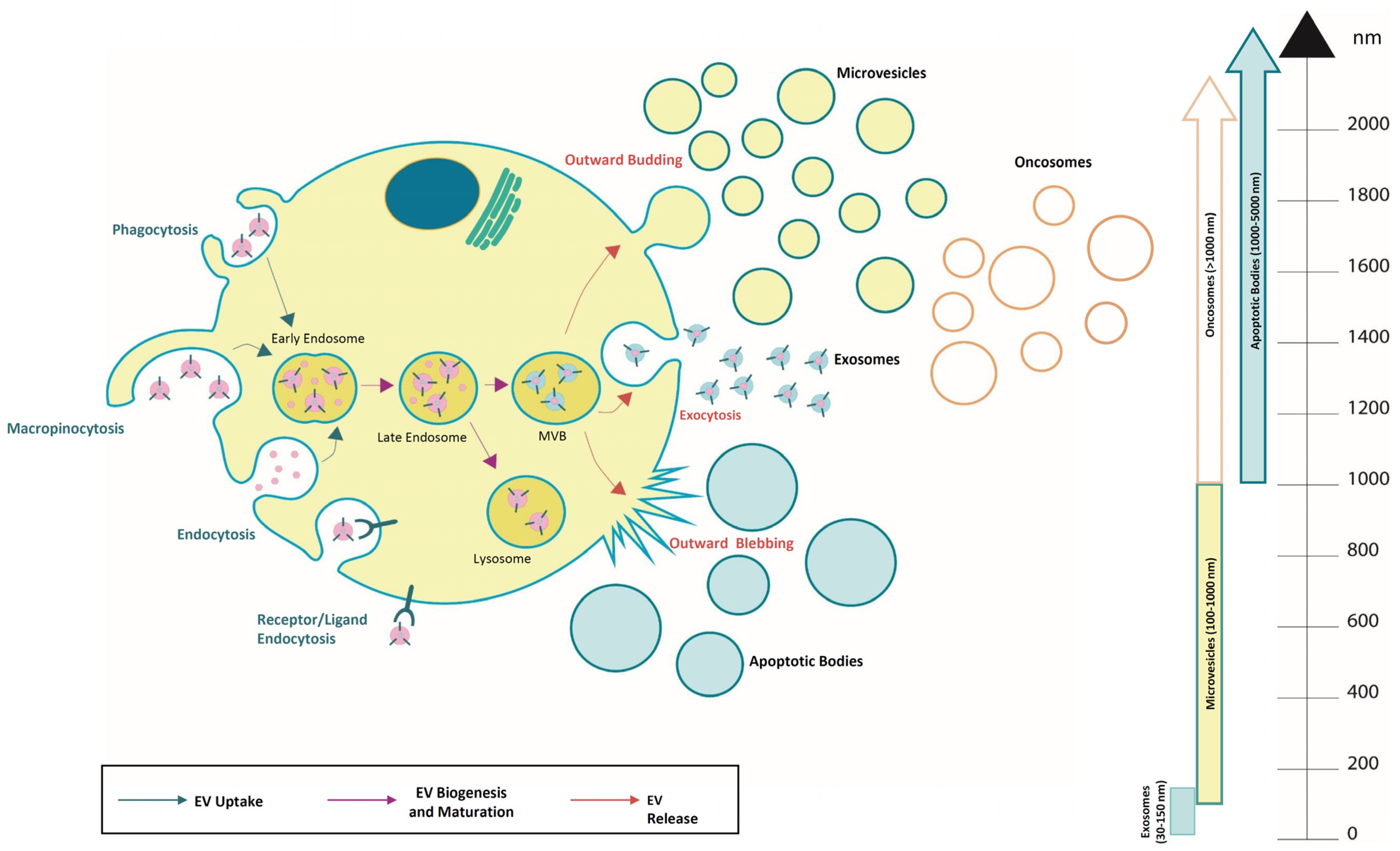
Extracellular vesicles (EVs) represent a crucial functional component of intercellular communication, acting as important mediators in both physiological and pathological processes in different organs and pathologies [14,15]. The classification of EVs reveals a complex landscape characterized by several factors. EVs were originally isolated from blood cells and showed significant variability in terms of their cellular origin, molecular content, size, and therapeutic efficacy [16,17]. Their classification based on size categorizes EVs into apoptotic bodies (1–5 µm), microvesicles (0.1–1 µm), and exosomes (30–150 nm) [18]. However, alternative classifications have been proposed, introducing considerations such as tissue of origin (e.g., prostasomes and oncosomes) and functional parameters [19]. EV proteins constitute a key aspect of their classification, reflecting both the cellular origin and the contents of the originating compartments. Exosomes (Exo) are generated by the endocytic pathway through the interaction between the endocytic vesicles and the endosomal sorting complex required for transport (ESCRT) system, and afterwards, they are released by the fusion of multivesicular bodies (MVBs) with the plasma membrane [20]. ESCRTs are involved in Exo production regulation also through the autophagy system. Autophagy-related genes (Atg) represent key factors for Exo release and their expression has been found to be deregulated in cancer cells, promoting proliferation and metastasis [21]. The complex network between autophagy and Exo trafficking includes many regulatory proteins and was recently revised by Zubkova and coworkers [22]. Conversely, microvesicles (MV) and apoptotic bodies arise directly from the plasma membrane [22]. In particular, MVs derive from membrane budding, whereas apoptotic bodies form from the blebbing of cells that undergo apoptosis. Cancer cells promote EV release to induce cancer development, proliferation, and metastasis. Among the EVs derived from cancer cells are oncosomes, which differ by size and composition from other EVs (Figure 1, Table 1).
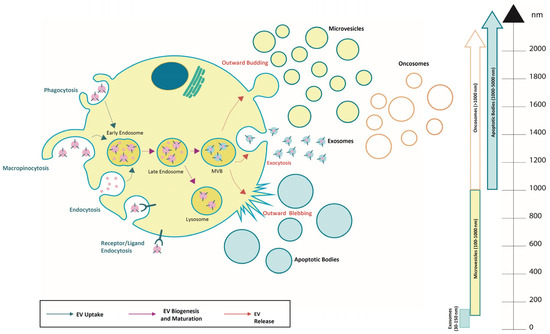
Figure 1.
Overview of extracellular vesicle subtypes and their uptake, biogenesis, and release. They are classified into different sub-classes and are generated through the endosomal pathway, released as exosomes (30–150 nm), microvesicles (0.1–1 μm), apoptotic bodies (1–5 µm), and oncosomes (>1 µm).

Table 1.
EV classification.
Integral membrane proteins, specifically tetraspanins like CD9, CD63, and CD81, stand out as important markers. Furthermore, EVs may contain membrane and cytoskeletal proteins, lysosomal enzymes, cytokines, chemokines, antigen presentation-related proteins (MHC class I and II complexes), and nucleic acids such as DNA, mRNAs, and miRNAs, all of which contribute significantly to EV classification [23,36]. The existence of DNA in EVs demonstrated in the past decade adds an intriguing dimension to their molecular composition. DNA in EVs, different in type (single- or double-stranded, mitochondrial) and form (fragment or chromatin-bound), may aid in discriminating EVs based on their cell of origin [37,38]. However, due to a lack of sufficient biomarkers and an overlap in size range, it is difficult to discriminate between the various types of vesicles.
EVs function as messengers and can be involved in key physiological conditions such as coagulation, pregnancy, metabolism, immunity, and apoptosis [39,40,41,42,43]. Under pathological or stress conditions induced by various stimuli, EVs show dynamic responses by altering both their quantity and molecular composition [44,45,46,47,48]. These altered vesicles hold promise as prospective biomarkers for various diseases, serving as reservoirs for potentially dangerous molecules. The pivotal role of EVs extends to their involvement in neurodegenerative diseases [47], blood disorders [49], metabolic processes [50], and cancer progression [51], where they act as intercellular communicators between cells and distant organs. EVs carry functional biomolecules, such as mRNA, proteins, miRNA, and metabolites, and can deliver them to cells across short and long distances, using the blood as a transport medium. The growing interest in EVs as disease biomarkers is reflected in their detectability across various body fluids.
The innate targeting capacity of EVs has shown considerable potential in cancer therapy [52,53,54], where, to mitigate challenges such as rapid clearance, low uptake rates, and off-target effects, researchers have explored EV engineering strategies that involve the modification of the EV surface and internal cargos [55]. Recent studies have demonstrated that EV surface cargos significantly influence their uptake, providing a basis for engineering strategies. The surface markers, including integrins, CD63, and tetraspanin 8 [56,57], contribute to EV tropism and are susceptible to engineering to improve their uptake efficiency [58]. EVs’ potential in cancer therapy extends to artificial targeting strategies, where specific surface molecules are designed to bind to molecules expressed on the surface of the desired recipient cells. This approach includes receptor–ligand interactions, enzymatic modifications, and antigen–antibody combinations [55]. In particular, engineered EVs with ankyrin repeat proteins expressed on the surface of the cell membrane exhibited specific binding to HER2-positive breast cancer cells, showing the potential of the receptor–ligand interaction strategy [59]. Antibody-mediated strategies involve engineering EV surfaces with anti-CD3 and anti-EGFR antibodies, leading to the T-cell-mediated elimination of EGFR-positive cancer cells [60]. Enzymatic strategies using hyaluronidase on the EV surface aim to increase EV uptake by degrading the tumor extracellular matrix, improving permeability for both tumor-specific CD8 T cells and drugs in the tumor microenvironment [61].
Upon uptake, the EV cargo modulates the activity of recipient cells [62,63], and, in this context, EVs secreted by MSCs (MSC-EVs) are a promising therapeutic component of the MSC secretome. Most preclinical studies involving MSC-EV therapy are based on vesicles produced by MSCs [3,64,65]. Moreover, to potentiate the functional activity of MSC-EVs, the strategy of priming/preconditioning their cells of origin was explored by using chemicals, cytokines, and growth factors, as well as specific culture conditions [3,64,66,67,68,69]. For instance, human MSC-EVs produced after stimulation with dimethyloxaloylglycine further stimulated angiogenesis through the Akt/mTOR pathway to enhance bone healing [70]. Tumor necrosis factor-alpha (TNF-α) was able to prime MSCs and improve the bone regenerative properties of MSC-derived EVs, as evidenced by the increased proliferation and osteogenic differentiation of osteoblastic cells in vitro [71]. Furthermore, several studies explored the effects of inflammatory priming on MSC-EVs, revealing distinct EV functions compared to other priming conditions. For instance, it was recently demonstrated that EVs derived from IFN-γ-primed MSCs have improved immunomodulatory properties compared to the 3D culture priming of MSC-EVs, which instead showed enhanced angiogenic properties [66]. In this scenario, the yield, size, and surface marker composition of MSC-derived EVs exhibited substantial variations with various priming treatments, and it is intriguing to understand how the EV content and their beneficial properties can be modulated. These studies will no doubt lay the foundation for potential advancements in MSC-EV therapeutics.
3. EVs in Diagnosis
While lung cancer represents, in most cases, a very inoperable disease with a low response to radiation therapy or chemotherapy and a low survival rate (with <17% for NSCLC and <7% for SCLC), the most important factor contributing to an increase in survival rate is early diagnosis and the selection of specific targeted therapeutic procedures. The identification of tumor characteristics based on molecular markers plays a key role in treatment effectiveness. Recently, a minimally invasive approach known as liquid biopsy was introduced, which involves sampling a small portion of body fluids to search for circulating tumor cells (CTCs), circulating proteins, and nucleic acids [72]. In this scenario, EVs, and particularly Exo, contain mediators influencing tumor progression as components of carcinogenesis that can help to identify and classify tumor onset and prevent its diffusion.
Several methods can be used to isolate EVs, such as differential ultracentrifugation, size exclusion chromatography, gradient centrifugation, the co-precipitation method, and microfluidic devices [73]. Yet, this represents a major challenge for EV application, since the development of high-throughput methodologies to allow for the rapid isolation of EVs from many samples would enhance their use in diagnosis [74].
EVs are known to participate in intercellular communication, immune responses, metabolism, and tumor progression, as they are capable of horizontally transmitting a wide range of biomolecules to target cells, making them important biomarkers for diagnosis, as well as promising molecular carriers for targeted therapies. The information they carry can influence the behavior of target cells in multiple ways. In particular, they can act as indicators, transferring membrane proteins and receptors to target cells, or even altering their phenotype through the horizontal transfer of genetic information. It has been demonstrated that EVs can deliver not only proteins or lipids, but also miRNAs, other ncRNAs, and mRNAs [75]. The analysis of EV miRNA levels in lung cancer patients showed a significant difference compared to control samples, suggesting that circulating EV miRNAs might represent a useful screening tool [76]. Compared to other circulating biomarkers such as cell-free DNA (cfDNA) and CTC, EVs have the advantage of being more abundant and more stable, given their lipid layer, which also protects the transported cargo. These characteristics are very important in order to establish sensitive and easily repeatable protocols for the early diagnosis of disease. Their role is central in certain pathological phenomena; for instance, it is now widely demonstrated that a tumor cell can release more than 20,000 of these vesicles in 48 h [77], with a role in conditioning the tumor microenvironment (TME). The TME includes several components such as the extracellular matrix (ECM), endothelial cells, cancer-associated fibroblasts (CAFs), and a strong immune component such as tumor-associated macrophages (TAMs), natural killer cells (NK), and T and B lymphocytes. Sanchez and coworkers examined the involvement of EVs and their miRNA cargo in the TME, demonstrating how they stimulate the formation of the neointima by activating macrophages within the TME, thus generating a niche for inflammation [78]. The analysis of EVs can represent a low-impact source for lung cancer characterization; notably, it has been demonstrated that EVs derived from bronchoalveolar lavage fluid (BALF) liquid biopsy can be used proficiently for epidermal growth factor receptor (EGFR) genotyping and the evaluation of EGFR mutations [79]. This method, together with the digital droplet PCR (ddPCR) and next-generation sequencing (NGS) techniques, can allow for the stratification of patients for TKI treatment without invasive methods such as tissue biopsy [79]. In this way, it is possible to quantify (copies/mL) and identify, if present, variants relating to the mutated EGFR, perhaps due to targetable resistance mechanisms involved in resistance to cancer therapy [80]. In this regard, a prospective phase 2 study was carried out to promote EGFR genotyping for subsequent therapeutic interventions through the analysis of EV-BALF liquid biopsy obtained from advanced NSCLC patients [81]. The study, for the first time, established that this platform represents a valid tool for immediate genotyping and allows for rapid results for therapeutic initiation in advanced NSCLC patients [81]. Moving forward, genotyping the miRNA content in EVs has been widely investigated. A recent study evaluated, with low-dose computed tomography (LDCT), the presence of indeterminate pulmonary nodules (IPNs) in association with circulating EV miRNAs [82]. The NGS analysis demonstrated a specific miRNA signature associated with the patient’s prognostic survival [82]. Similarly, another study described an miRNA signature (hsa-miR-106b-3p, hsa-miR-125a-5p, hsa-miR-3615, and hsa-miR-450b-5p) from plasma-circulating EVs with the identification of early-stage lung cancer [83]. An analogous result was obtained with the RT-PCR analysis of six miRNAs (miR-7, miR-21, miR-126, Let-7a, miR-17, and miR-19) in EV-BALF. Despite the limited number of patients, the study suggested a correlation between the expression of the analyzed miRNAs and early-stage lung cancer [84]. High-throughput transcriptomic analyses allowed for the identification of circular RNAs (circRNAs), resulting from the back-splicing of pre-mRNA, among numerous RNA strands. Although first described in the early 1970s, circRNAs were, until very recently, regarded as byproducts of splicing without any important biological function. The main function of circRNAs is the inhibition of miRNAs. They act as miRNA sponges, establishing a complex and precise system in the interaction with RNA-binding proteins and in the regulation of gene expression [85]. Recently, circRNAs were found to be enriched and stable in cancer EVs, suggesting their potential use as cancer biomarkers or therapeutic targets. It has been supposed that EVs could represent a mechanism for the release of circRNAs [86,87].
Cancer patients show circRNA expression levels in the ratio of 2:1 vs healthy controls [88]. A valid example of the role of EVs in prognosis is given by the Hongya et al. study on circVMP1, which was found to be correlated with the progression of NSCLC and resistance to cisplatin therapy [89].
Indeed, there is much evidence for circRNAs being involved in promoting tumor migration, NSCLC development, resistance to therapies, and tumorigenesis, with different pathways of molecular interaction. Through the miR377-5p/NOVA2 axis, circ_007288 promotes the development of NSCLC [90], while circ_0000376 stimulates tumorigenesis and promotes drug resistance by positively modulating the action of KPNA4 and sponging miR1298-5p [91].
Circ_0020123 is particularly interesting for the multiple interaction pathways in which it is involved in lung cancer and appears to be capable of promoting cell proliferation and migration on tumor growth in vivo, acting on the THBS2/miR590-5p axis [92] and favoring cisplatin resistance in NSCLC cells by targeting miR-14-3p [93].
In the study conducted by Wei et al., circ_0020123 acts as a competitive endogenous RNA (ceRNA) to interact with miR-1283, thus promoting the expression levels of PDZD8, a cytoskeletal regulatory protein involved in tumor migration and proliferation [94], also involved in the LARP1/miR-330-5p tumor axis mechanism with the homonymous CircRNA (circ_PDZD8) [95] or suppressing tumor growth either if not expressed [96] or through sponging miR-1299, regulating HMGB3 [97]. Many studies on circRNA in lung cancer have demonstrated a repressive role in the disease. The relevance of circRNAs and their RNA splice variants for tumor progression and therapy response has been demonstrated in preclinical models [98]. Given the plethora of pathways in which circRNAs are involved, the use of a specific database is fundamental to shed light on the many possible pathways, and this is one of the objectives with which CircInteractome was born [99].
Recent studies have explored the role of circRNAs derived from the lung and carried by EVs [100], and most of them are focused on their expression and role in lung cancer [101] (Table 2).
In a pioneering work in this field, Zhu and coworkers identified the presence of circHIPK3 in lung cancer released EVs [102]. This circRNA has been proposed as a novel EV-derived biomarker for lung cancer, whose action is connected with miR-637 reduction and acts as a tumor suppressor on cellular migration, invasion, and proliferation in NSLC [102].
Moreover, it was reported that the circRNAs contained in EVs act as novel genetic information molecules, mediating the interactions between cancer cells and other cells of the TME and regulating key steps in cancer progression [10,103,104]. Nowadays, the use of EV-circRNAs as biomarkers for cancer diagnosis and prognosis shows various limitations for sample sizes and a lack of standardized evaluation systems, so further analysis will support their specific application as early diagnostic markers.
On the other side, engineering strategies for EV-circRNAs could solve the limitations due to the size of circRNAs for efficient packaging and delivery systems, overcoming pharmacodynamics, pharmacokinetics, and safety considerations [105].

Table 2.
circRNA effects on lung cancer.
Table 2.
circRNA effects on lung cancer.
| CircRNA # | Function | Pathway | Reference |
|---|---|---|---|
| Circ_0012673 | Promote cell proliferation | Sponge miR-22; upregulate ErbB3 | [106] |
| Circ_0067934 | Promote cell proliferation, migration, and invasion | Modulate markers of epithelial-to-mesenchymal transition (EMT) | [107] |
| Circ_007288 | Promote cell proliferation | Sponge miR-377-5p/NOVA2 | [90] |
| Circ_0000376 | To induce resistance to cisplatin and promote tumorigenesis | Sponge miR-1298-5p/KPNA4 | [91] |
| Circ_PDZD8 | Promote cell proliferation | Sponge miR330-5p/LARP1 | [95] |
| Circ_0072309 | To promote tumor progression and invasion | Sponge miR607/FTO | [108] |
| Circ_ATAD1 | Enhance cancer progression | Sponge miR-191-5p | [109] |
| Circ_0092887 | Induce resistance to taxane | Sponge miR490-5p/UBE2T | [110] |
| Circ_0007385 | Promote cell proliferation, migration, tumourigenesis, and invasion | Sponge miR-181 | [111] |
| Circ_0013958 | Promote cell proliferation and invasion and prevent apoptosis | Sponge miR-134/cyclin D1 | [112] |
| Circ_0020123 | Inhibit proliferation and invasion | Sponge miR1299/HMGB3 | [97] |
| Circ_008305 | Inhibit tumor metastasis | Sponge miR-429/miR-200b-3p/PTK2 | [113] |
| Circ_CRIM1 | Inhibit tumor metastasis and invasion | Sponge miR-93 and miR-182; | [114] |
| Circ_RNF13 | Inhibit tumor proliferation and metastasis | Sponge miR-93-5p | [115] |
| CircSH3PXD2A | Inhibit tumor chemoresistance | miR-375-3p/YAP1 | [116] |
In addition to nucleic acid evaluation, recent progress in EV analysis has been implemented by looking at the protein content by proteomic profiling. Lung cancer EVs contain several tumor-associated proteins, such as EGFR, KRAS, inducer of extracellular matrix metalloproteinase, claudins, and RAB family proteins. In NSCLC, other proteins have been found such as exo markers like CD91, CD317, and EGFR. CD151, CD171, and tetraspanin 8 represent very reliable markers for lung cancer characterization and identification. Furthermore, METTL1 and the HIST family of proteins have been found to be overexpressed mostly in tumor samples [117]. Many studies are focusing on identifying the protein profiles of EVs from different stages and histologies of lung cancer, which is very important as a potential diagnostic tool [118,119]. A good example is given by Hoshino et al., who were able to characterize the complete proteomic profile of EVs from the plasma of 16 different cancer types and identified the proteins up- or down-regulated in cancer-associated EVs. Notably, the study revealed that cancer-derived proteins were not potential tumor tissue biomarkers and that approximately 50% of them arose from distant organs. Tumor-specific proteins were detected only in plasma, supporting the systemic nature of cancer and strengthening the potential use of EVs as liquid biopsy markers for early cancer diagnosis [117]. It has been reported that NSCLC-EVs shuttle specific proteins capable of inducing metastasis. Taverna et al. demonstrated that Amphiregulin, a ligand of EGFR contained in NSCLC-EVs, could induce metastasis, activating the EGFR pathway in pre-osteoclasts with an enhanced activity of proteolytic enzymes, leading to bone metastasis formation [120]. NSCLC EVs show an increased expression of FAM3C, a gene encoding for interleukin-like EMT inducer (ILEI). This results in an enhanced detection of FAM3C from lung tumor patients vs healthy subjects [121]. Furthermore, Du and coworkers identified that SCLC tumor-cell-derived EVs expressing PD-L1 play an important role in EVs and immune system crosstalk, suggesting a potential use of EV PD-L1 in the design of anticancer strategies [122]. From a prognostic point of view, the expression proteins of the A549 cell line (NSCLC) were analyzed before and after cisplatin treatment [123] by mass spectrometry (LC–MS/MS analysis). The results define a protein profile enriched for cholesterol metabolism pathway activation, indicating the role of EVs in lipogenesis activation and cell proliferation after chemotherapeutic treatment [123]. Nonetheless, a uniform consensus on protein markers from EVs is still missing for the restricted human sample datasets to drive interpretations of data analyses. To date, various resources have deposited the contents of EVs, especially regarding miRNAs, which can be consulted online: EVpedia [124,125] and Exocarta [126]. While the observation of new diagnostic information is strongly promoted, ctDNA represents an interesting target for liquid biopsy investigations in lung cancer detection [127]. However, the study of EVs and their protein cargo or CTCs has not yet entered clinical practice, and their application is limited to research studies (Table 3).

Table 3.
Diagnostic application of EVs from different body fluids in lung cancer.
4. EVs in Lung Cancer Therapy
Until a few years ago, the most common lung cancer treatment was chemotherapy. Recent progress in oncology has prompted the use of immune-checkpoint monoclonal antibody blockades in association with chemotherapeutic treatment [133] or as a single agent, depending on PD-1 IHC expression. On the other hand, next-generation sequencing technologies allow for the identification of the most recurrent mutations in lung cancers, providing a unique tool for evaluating oncogene addiction and the role of targeted therapy. Some of the identified mutations include epidermal growth factor receptor (EGFR), where mutations occur in 15% of NSCLC adenocarcinoma cases [134]. This allows for the targeting of these tumors by specific tyrosine kinase inhibitors (TKIs) and/or monoclonal antibodies, as recommended by current guidelines [135]. Different TKIs have been employed in several clinical trials, which have demonstrated a positive effect on progression-free survival (PFS) and fewer side effects compared to standard chemotherapy (platinum) [136]. Unfortunately, many patients have shown resistance to the specific EGFR inhibitor treatment. To overcome this problem, TKI treatment can be associated with anti-EGFR monoclonal antibodies (cetuximab, necitumumab, and panitumumab), as supported by numerous clinical trials reviewed by Ciardiello and colleagues [137]. Another therapeutic target identified in lung cancers is anaplastic lymphoma kinase (ALK), whose translocation with the EML4 gene affects 5% of NSCLC patients [138]. Specific TKI inhibitors have been identified: crizotinib, second-generation ceritinib and alectinib, and the new-generation lorlatinib, recently preferred for resistance mutations [139]. Interestingly, crizotinib has also been employed as a treatment for NSCLC patients positive for ROS-1 chromosomal rearrangements with clinical signs similar to ALK mutations [140,141]. Similar to NSCLC cancers, some mutations have been identified in mainly SCLC patients. In particular, these alterations concern the suppressor genes TP53 and RB1 [142]. Despite their identification, SCLC tumors do not show targetable mutations, and recently, researchers have been focusing their attention on RB1 as a potential therapy target, as demonstrated by in vivo studies [143,144]. Innovative therapeutic approaches have been studied in the last few years, revealing that EVs play a relevant role in physiological and pathological conditions, such as cancer and cardiovascular and neurodegenerative diseases. Over the last ten years, EV research has focused on their potential application as therapeutic agents. As already underlined, EVs can carry molecules, particularly non-coding RNAs, influencing cancer growth, progression, metastasis, or drug resistance [145]. Therefore, non-coding RNA has gained importance as a therapeutic tool and has been employed in several clinical studies (Table 4). Among the ncRNAs, a pivotal role is played by miRNA, which can be easily carried and delivered by EVs or other vectors. Specifically, miR34 has been widely studied in different tumors. Recently two different phase I multicenter trials were conducted to study by dose escalation the safety, pharmacokinetics, and pharmacodynamics of an miR-34 mimic (MRX34), administered by liposomal injection in patients with melanoma (NCT02862145) and other selected solid tumors: primary liver cancer SCLC, NSCLC, lymphoma, melanoma, multiple myeloma, and renal cell carcinoma (NCT01829971). The melanoma trial was withdrawn due to high toxicity, and the other study on solid tumors showed stable disease (SD) in 6 out of 47 patients [146]. This study represented the first miRNA-based clinical trial on cancer [147]. The capacity of miR-34 to inhibit tumor growth has been demonstrated by various studies, and the ability of EVs to carry this miRNA and inhibit tumor growth in a paracrine way has been assessed [148]. EVs can be considered a peculiar vector for anti-cancer delivery systems due to their natural and advantageous properties, such as their high biocompatibility and limited systemic toxicity. Specific nanocarrier-targeted action can be improved by engineering and functionalizing their surface, for example, by inducing the expression of specific proteins on the EV membrane or through the loading of miRNA, which can be inserted exogenously on isolated EVs (electroporation, sonication, and RNA cholesterol conjugation), or indirectly by genetic modification of the donor cells before EV isolation (RNA transfection, RNA encoding plasmid transfection, and virus transfection) [145]. For example, EVs isolated from mesenchymal stem cells have been demonstrated to transfer miRNA efficiently in different kinds of tumors. This observation has raised the possibility of engineering cells such as MSCs for miR-34 delivery to inhibit tumor growth by EV release [149]. Notable for their ability to migrate towards inflammation or tumoral regions, MSCs have the peculiar characteristic of being able to be genetically modified, and when employed for this purpose, they act as living delivery vectors [150,151]. It was observed recently that engineered bone marrow MSCs (BMSCs) can deliver miR-193a, reducing the cisplatin resistance of NSCLCs by targeting leucine-rich repeat-containing protein 1 (LRRC1) [152]. In the same way, BMSC-derived EVs carrying miR-126-3p suppressed the viability, migration, and invasion of NSCLC cells by targeting protein tyrosine phosphatase non-receptor type 9 (PTPN9) [153]. Similarly, another group showed that engineered BMSCs with miR-598 inhibited cell proliferation, migration, and invasion in NSCLC. They demonstrated that miR-598-loaded EVs acted in lung cells by down-regulating Derlin-1, the zinc finger E-box-binding homeobox 2 (ZEB2), and also Thrombospondin-2 (THBS2), in this way inhibiting growth and metastasis [154]. The same effect was obtained with exosomal miR-338-3p through the inhibition of MAPK signaling, reducing the cell adhesion molecule L1-like protein (CHL1) activity and the subsequent down-regulation of NSCLC proliferation and apoptosis [155]. Engineered exosomes loaded with miR-449a selectively inhibit the growth of homologous NSCLC [156]. Among them, Zhou and colleagues focused their attention on miR-449-a, which affects the migration and invasion of human NSCLC cells. They isolated exosomes from A549 cells and engineered them (miR-449a exo) to allow for the transfer of this miRNA, thereby demonstrating its anti-tumor activity both in in vitro and in vivo models [156]. Similarly, another group used MDA-MB-231 breast cancer cells as a source of engineered lung-targeted exosomes with miRNA-126, which reduced proliferation and migration through the PTEN/PI3K/AKT pathway in A549 cells and an in vivo lung metastasis mouse model [157].
Besides their application as miRNA carriers, EVs have been used for tumor RNA interference (RNAi) therapy through siRNA targeted against specific oncogenes. For example, KRAS, whose mutations account for 90% of pancreatic cancers and 20–25% of lung adenocarcinomas, represents an area of great interest for tumor-targeted gene therapy. Recently, lipid nanoparticles carrying KRAS siRNAs reduced its expression in several lung cancer cell lines, including human (A549 and H441) and mouse (CMT-167 and Lacun3) cells, and proliferation was observed through colony-forming assays [158].
During the last few years, various approaches have been studied and pursued to employ EVs as therapeutic applications or targets in lung cancer. It is well known that the EVs released by tumor cells can promote the spread and diffusion of the tumor and also counteract the immune response by inhibiting CD-positive T cells with anti-tumor functions [159] or favoring immune escape, attenuating cytotoxic CD8+ T cells through the expression of PD-L1, considered as a target for monoclonal therapy in NSCLC patients [160]. Because of these characteristics, EVs have been considered as target therapeutic strategies. Some pharmacological agents act on EV trafficking or lipid membrane metabolism and are extremely important for membrane fluidity and, as a consequence, for EV shedding/release. For example, GW4869 inhibits the membrane-neutral sphingomyelinase (nSMase) and exosome/EV biogenesis; it has been tested in PC9 lung adenocarcinoma cells, counteracting the antagonistic effects of gefitinib and cisplatin, which are widely used for NSCLC patient treatment [161].
Among the numerous molecular partners involved in membrane trafficking is Rab27A, a protein expressed in numerous cell types, including A549, which could regulate EV release. One research group demonstrated that specific shRNA against Rab27A carries a lower release of EVs and a reduction in tumor growth in an in vitro model of human lung adenocarcinoma cells [162].
Considering the impact of EVs on immune escape, over the years, clinical trials have been undertaken to apply them as a cancer vaccine [163,164,165,166,167] The EVs released by tumor cells proficiently trigger anti-tumor immunity; for example, in a study focused on EVs in vitro isolated from 3LL lung tumor cells, the activation of dendritic cells and T cells after being subjected to heat stress was induced through EV inflammatory chemokine contents [163]. Similarly, dendritic cells release vesicles (termed dexosomes) that have been demonstrated to prime T cells and present antigens to T CD8+ and CD4+ cells [168,169]. These cells and their secretome are of great scientific interest; indeed, dendritic cells were tested as autologous vaccinations in a clinical trial involving NSCLC patients, providing interesting immunologic responses [164]. A phase I clinical trial demonstrated the tolerance of engineering dexosomes with MAGE antigens in NSCLC patients’ MAGE+ [167]. These dexosomes were also used in a phase II trial on NSCLC patients, resulting in the stabilization of 32% of the recruited patients [166].
In a similar way to miRNA delivery, researchers are attempting to use EVs for drug/chemotherapy delivery. EVs loaded with paclitaxel were administered to a metastatic mouse model of NSCLC [170]. In particular, this research group demonstrated that exosomes efficiently vehicle the paclitaxel [171] and subsequently improved the formulation of these exosomes, demonstrating that this new delivery system exerts a higher ability to reach cancer cells with a better therapeutic effect [170]. Recently, exosomes isolated from M1 macrophages were evaluated as a drug vehicle for cisplatin, both in in vitro (Lewis lung cancer cells) and in vivo mouse models. The study demonstrated that the exosomes from M1 macrophages as chemotherapy carriers improved the anti-lung cancer effect of cisplatin and induced tumor cell death; specifically, in vitro experiments demonstrated the involvement of apoptosis through Bax and Caspase-3 [172]. In another in vitro study with two NSCLC cell lines (H1299 and A549), researchers used exosomes loaded with gold nanoparticles conjugated with doxorubicin, obtaining a greater particle uptake by target cells and drug release and more specific cytotoxicity with fewer side effects [173].

Table 4.
Therapeutic in vitro and in vivo application of EVs in lung cancers.
Table 4.
Therapeutic in vitro and in vivo application of EVs in lung cancers.
| Target/Study Models | Subject | Description | Reference |
|---|---|---|---|
| (Advanced) NSCLC | Vaccination trial with tumor antigen-loaded dendritic cell-derived exosomes | Maintenance immunotherapy in 47 patients with dexosomes to improve their PFS. | NCT01159288 |
| Solid tumors: primary liver cancer, SCLC, lymphoma, melanoma, multiple myeloma, renal cell carcinoma, NSCLC | Multicenter phase I study of MRX34, microRNA miR-RX34 liposomal injection | Phase I, open-label, multicenter, dose escalation study to investigate the safety, pharmacokinetics, and pharmacodynamics of the micro ribonucleic acid (microRNA) MRX34 in patients with unresectable primary liver cancer or advanced or metastatic cancer with or without liver involvement or hematologic malignancies. | NCT01829971 [147] |
| (Advanced) NSCLC | Phase I study of dexosome immunotherapy | Phase I study to evaluate safety and efficacy of autologous dexosomes loaded with tumor antigens (MAGE-A3, -A4, -A10, and MAGE-3DPO4), administered in 4 doses. Measurement of the immunologic responses and monitoring the clinical outcomes in 13 patients at different stages. | [167] |
| H1299 and A549 (NSCLC) | Nanosomes carrying doxorubicin anticancer activity against human lung cancer cells | In vitro analysis of gold nanoparticles (GNPs) loaded with doxorubicin to evaluate the release kinetics and the cytotoxic activity. | [173] |
| Mice injected with B16F10 cells to produce lung metastasis | EVs melanoma gold conjugated nanoparticle targeting lung tumors | The study provided an application system where exosomes isolated from cancer cells incorporated gold nanoparticles were tested in a mouse model to improve targeting system in metastatic foci. | [174] |
| In vitro: murine carcinoma cell line (3LL-M27); in vivo: mouse model with pulmonary metastases | Paclitaxel-loaded EVs against cancer cells | In vitro and in vivo study aims to introduce a new formulation for Paclitaxel distribution through exosomes (PTX-exo, fom RAW 264.7 cell line), providing high stability in tumor environment and a better effectiveness in vivo murine model. | [171] |
| In vitro: A549 and H1299 (NSCLC); In vivo: mouse model with lung cancer xenograft | Celastrol EVs formulation against lung cancer | Study focused on the effect of the natural compound celastrol loaded into exosomes, a new delivery system improved efficacy and reduced dose toxicity. | [175] |
| In vitro: A549 and H1299 (NSCLC); In vivo: nude mice with xenograft | Anthocyanidins EVs against multiple cancer types | The study aimed to obtain a nano-formulation of the natural derived compound, anthos, with exosomes. Exosomes enhanced the anti-proliferative and anti-inflammatory activity of anthos (vs the free compound) and the therapeutic affect toward lung cancer. | [176] |
| Nude mice with lung tumor xenografts | Milk-derived exosomes for oral delivery of paclitaxel | A study on chemotherapeutic paclitaxel delivery through exosomes in a formulation for oral administration, which exhibited greater therapeutic efficacy and lower systemic toxicity. | [177] |
5. Conclusions and Remarks
The potential applications of EVs in therapeutic and diagnostic approaches are far from being fully achieved. Over the last decade, the EV cancer field has experienced significant advancements that have fundamentally changed our understanding of intercellular communication and cancer biology.
However, a deeper knowledge of EV’s role in lung cancer is crucial in order to define biomarkers for prognosis and diagnosis, as well as to develop new therapeutic strategies for such deadly tumors [1]. So, to transfer this knowledge from bench to bedside, other studies need to be conducted to clarify and confirm the potential role of EVs in lung cancer and beyond. Tumor heterogeneity, in particular looking at EGFR mutations, is currently under investigation to further correlate cellular modifications with therapeutic response [81].
Their utility as delivery vehicles for various drugs, proteins, and nucleic acids has been evaluated by many laboratories. Their lipid composition contributes to their stability in body fluids and provides, at the same time, valid support for their cellular delivery by cell membrane fusion [178]. Moreover, the immunological properties of MSC offer a unique tool for EV secretion, combining their specific transfer ability aptitude (drugs, nucleic acids, and proteins) with immunomodulatory pharmacological effects [179] or new therapeutic approaches in numerous diseases, including lung cancer (Table 4). Despite MSCs’ natural tropism against tumors, which can represent a valid site-specific EV throughput tool, dendritic cell-derived exosomes can support the targeted tumor delivery of EVs and represent a promising example of vaccination due to their immunostimulatory capability (NCT01159288). On the other hand, from a diagnostic point of view and given the important role for cancer biology, the use of circulating EVs has gained a growing interest primarily for their availability. Conversely, one of the main challenges is represented from EVs’ origin, because their release is not exclusively related to the disease but can arise from any tissue. A wider analysis of EVs’ composition can support fast stratification and early detection. In this regard, a substantial analysis of EV circRNA signatures can identify lung-cancer-regulated miRNA [100,102]. Furthermore, a proteomic analysis of EV content offers the opportunity to acquire more information about EV biology and identify new biomarkers, contributing to early diagnosis and the design of valid treatments [180] (Figure 2). There are many difficulties and limitations, but the multi-omics approach has a very bright future and will undoubtedly provide much more information on these nano-sized biological entities. Despite numerous studies on experimental models and various pathologies, there are still many points that can be improved, for example, identifying cellular sources safe for immunogenicity and sources that can guarantee significant quantities, as well as trying to introduce standardized procedures to improve the workflow throughput. We hope that groundbreaking tests on the diagnostic and prognostic meaning of EV evaluation can draw new routine procedures for dissecting tumor heterogeneity and narrowing therapeutic intervention protocols.
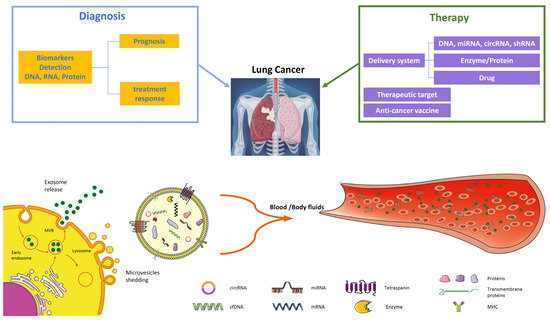
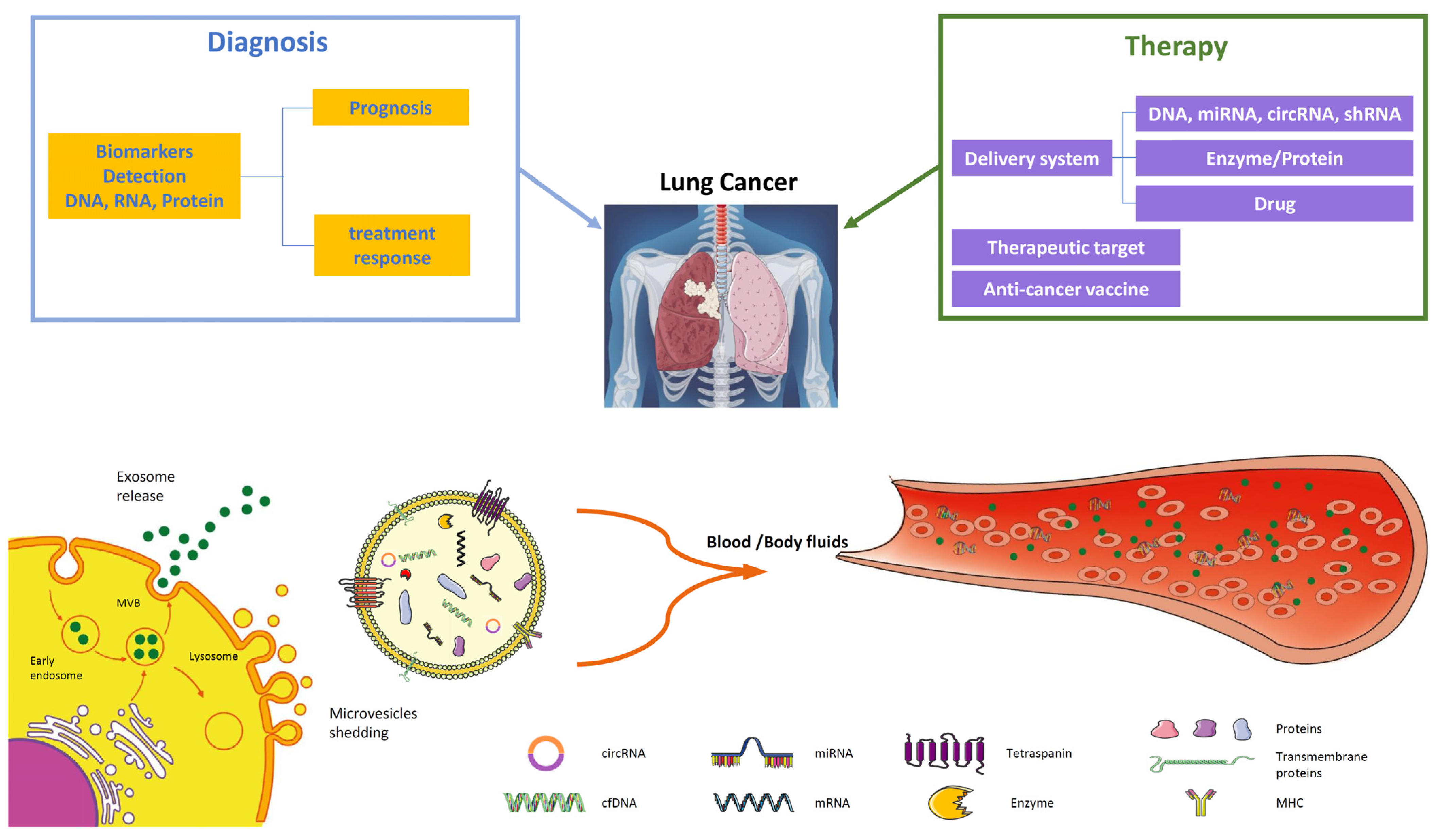
Figure 2.
EVs in lung cancer diagnosis and therapy. EVs are important players in intercellular communication, released through the endosomal pathway by the plasma membrane as exosomes (30–150 nm), microvesicles (0.1–1 μm), and apoptotic bodies (1–5 µm). Tumor-derived EVs are good candidates for liquid biopsy since they contain many components such as tumor-derived DNA, mRNA, miRNAs, and proteins. Their analysis from plasma or body fluids (BALF) offers significant information about tumor diagnosis through biomarkers crucial for early detection or prognosis and treatment response. The potential application of EV in therapy comprises their application in targeted therapy through the delivery of specific miRNAs, drug delivery of chemotherapy agents, or their employment as anti-cancer vaccines.
Last, but not least, scientists must investigate EVs’ structure deeply to maximize their engineering and applications as carrier systems (Figure 2). Another area to be further explored is related to their turnover. Studies have already focused on their release inhibition, and, considering the importance of the uptake step, it could be interesting to try to selectively reduce uptake mechanisms, although the pathways involved are numerous [181,182].
Author Contributions
A.P.C. and G.I. conceived and designed the review. Data collection and interpretation were carried out by A.P.C., R.T., V.M., A.G., V.G., L.I., M.P., S.T. and G.I. All authors approved the final draft of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
V.M. and G.I. work was supported by the Italian Ministry of Health, Ricerca Corrente.
Acknowledgments
The authors thank IRCCS ISMETT’s Language Services Department for editing the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Thai, A.A.; Solomon, B.J.; Sequist, L.V.; Gainor, J.F.; Heist, R.S. Lung cancer. Lancet 2021, 398, 535–554. [Google Scholar] [CrossRef]
- Miceli, V.; Zito, G.; Bulati, M.; Gallo, A.; Busa, R.; Iannolo, G.; Conaldi, P.G. Different priming strategies improve distinct therapeutic capabilities of mesenchymal stromal/stem cells: Potential implications for their clinical use. World J. Stem Cells 2023, 15, 400–420. [Google Scholar] [CrossRef]
- Grange, C.; Tapparo, M.; Collino, F.; Vitillo, L.; Damasco, C.; Deregibus, M.C.; Tetta, C.; Bussolati, B.; Camussi, G. Microvesicles released from human renal cancer stem cells stimulate angiogenesis and formation of lung premetastatic niche. Cancer Res. 2011, 71, 5346–5356. [Google Scholar] [CrossRef]
- Zhang, C.; Qin, C.; Dewanjee, S.; Bhattacharya, H.; Chakraborty, P.; Jha, N.K.; Gangopadhyay, M.; Jha, S.K.; Liu, Q. Tumor-derived small extracellular vesicles in cancer invasion and metastasis: Molecular mechanisms, and clinical significance. Mol. Cancer 2024, 23, 18. [Google Scholar] [CrossRef]
- Jothimani, G.; Pathak, S.; Dutta, S.; Duttaroy, A.K.; Banerjee, A. A Comprehensive Cancer-Associated MicroRNA Expression Profiling and Proteomic Analysis of Human Umbilical Cord Mesenchymal Stem Cell-Derived Exosomes. Tissue Eng. Regen. Med. 2022, 19, 1013–1031. [Google Scholar] [CrossRef]
- Xiao, H.; Lasser, C.; Shelke, G.V.; Wang, J.; Radinger, M.; Lunavat, T.R.; Malmhall, C.; Lin, L.H.; Li, J.; Li, L.; et al. Mast cell exosomes promote lung adenocarcinoma cell proliferation—Role of KIT-stem cell factor signaling. Cell Commun. Signal. CCS 2014, 12, 64. [Google Scholar] [CrossRef]
- Bhatta, B.; Luz, I.; Krueger, C.; Teo, F.X.; Lane, D.P.; Sabapathy, K.; Cooks, T. Cancer Cells Shuttle Extracellular Vesicles Containing Oncogenic Mutant p53 Proteins to the Tumor Microenvironment. Cancers 2021, 13, 2985. [Google Scholar] [CrossRef]
- Di Giuseppe, F.; Carluccio, M.; Zuccarini, M.; Giuliani, P.; Ricci-Vitiani, L.; Pallini, R.; De Sanctis, P.; Di Pietro, R.; Ciccarelli, R.; Angelucci, S. Proteomic Characterization of Two Extracellular Vesicle Subtypes Isolated from Human Glioblastoma Stem Cell Secretome by Sequential Centrifugal Ultrafiltration. Biomedicines 2021, 9, 146. [Google Scholar] [CrossRef]
- Cammarata, G.; de Miguel-Perez, D.; Russo, A.; Peleg, A.; Dolo, V.; Rolfo, C.; Taverna, S. Emerging noncoding RNAs contained in extracellular vesicles: Rising stars as biomarkers in lung cancer liquid biopsy. Ther. Adv. Med. Oncol. 2022, 14, 17588359221131229. [Google Scholar] [CrossRef]
- Kato, T.; Vykoukal, J.V.; Fahrmann, J.F.; Hanash, S. Extracellular Vesicles in Lung Cancer: Prospects for Diagnostic and Therapeutic Applications. Cancers 2021, 13, 4604. [Google Scholar] [CrossRef]
- Liu, Y.; Xia, Y.; Smollar, J.; Mao, W.; Wan, Y. The roles of small extracellular vesicles in lung cancer: Molecular pathology, mechanisms, diagnostics, and therapeutics. Biochim. Biophys. Acta Rev. Cancer 2021, 1876, 188539. [Google Scholar] [CrossRef]
- Tine, M.; Biondini, D.; Damin, M.; Semenzato, U.; Bazzan, E.; Turato, G. Extracellular Vesicles in Lung Cancer: Bystanders or Main Characters? Biology 2023, 12, 246. [Google Scholar] [CrossRef]
- Ginini, L.; Billan, S.; Fridman, E.; Gil, Z. Insight into Extracellular Vesicle-Cell Communication: From Cell Recognition to Intracellular Fate. Cells 2022, 11, 1375. [Google Scholar] [CrossRef]
- Alberti, G.; Russo, E.; Corrao, S.; Anzalone, R.; Kruzliak, P.; Miceli, V.; Conaldi, P.G.; Di Gaudio, F.; La Rocca, G. Current Perspectives on Adult Mesenchymal Stromal Cell-Derived Extracellular Vesicles: Biological Features and Clinical Indications. Biomedicines 2022, 10, 2822. [Google Scholar] [CrossRef]
- Russo, E.; Alberti, G.; Corrao, S.; Borlongan, C.V.; Miceli, V.; Conaldi, P.G.; Di Gaudio, F.; La Rocca, G. The Truth Is Out There: Biological Features and Clinical Indications of Extracellular Vesicles from Human Perinatal Stem Cells. Cells 2023, 12, 2347. [Google Scholar] [CrossRef]
- Johnstone, R.M.; Adam, M.; Hammond, J.R.; Orr, L.; Turbide, C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J. Biol. Chem. 1987, 262, 9412–9420. [Google Scholar] [CrossRef]
- Di Bella, M.A. Overview and Update on Extracellular Vesicles: Considerations on Exosomes and Their Application in Modern Medicine. Biology 2022, 11, 804. [Google Scholar] [CrossRef]
- Sailliet, N.; Ullah, M.; Dupuy, A.; Silva, A.K.A.; Gazeau, F.; Le Mai, H.; Brouard, S. Extracellular Vesicles in Transplantation. Front. Immunol. 2022, 13, 800018. [Google Scholar] [CrossRef]
- Gurung, S.; Perocheau, D.; Touramanidou, L.; Baruteau, J. The exosome journey: From biogenesis to uptake and intracellular signalling. Cell Commun. Signal. CCS 2021, 19, 47. [Google Scholar] [CrossRef]
- Guo, H.; Chitiprolu, M.; Roncevic, L.; Javalet, C.; Hemming, F.J.; Trung, M.T.; Meng, L.; Latreille, E.; Tanese de Souza, C.; McCulloch, D.; et al. Atg5 Disassociates the V(1)V(0)-ATPase to Promote Exosome Production and Tumor Metastasis Independent of Canonical Macroautophagy. Dev. Cell 2017, 43, 716–730.e717. [Google Scholar] [CrossRef]
- Zubkova, E.; Kalinin, A.; Bolotskaya, A.; Beloglazova, I.; Menshikov, M. Autophagy-Dependent Secretion: Crosstalk between Autophagy and Exosome Biogenesis. Curr. Issues Mol. Biol. 2024, 46, 2209–2235. [Google Scholar] [CrossRef]
- Thery, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Wei, H.; Chen, Q.; Lin, L.; Sha, C.; Li, T.; Liu, Y.; Yin, X.; Xu, Y.; Chen, L.; Gao, W.; et al. Regulation of exosome production and cargo sorting. Int. J. Biol. Sci. 2021, 17, 163–177. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, aau6977. [Google Scholar] [CrossRef]
- Raposo, G.; Stahl, P.D. Extracellular vesicles—On the cusp of a new language in the biological sciences. Extracell. Vesicles Circ. Nucl. Acids 2023, 4, 240–254. [Google Scholar] [CrossRef]
- Cable, J.; Witwer, K.W.; Coffey, R.J.; Milosavljevic, A.; von Lersner, A.K.; Jimenez, L.; Pucci, F.; Barr, M.M.; Dekker, N.; Barman, B.; et al. Exosomes, microvesicles, and other extracellular vesicles-a Keystone Symposia report. Ann. N. Y. Acad. Sci. 2023, 1523, 24–37. [Google Scholar] [CrossRef]
- Turchinovich, A.; Drapkina, O.; Tonevitsky, A. Transcriptome of Extracellular Vesicles: State-of-the-Art. Front. Immunol. 2019, 10, 202. [Google Scholar] [CrossRef]
- Clancy, J.W.; Sheehan, C.S.; Boomgarden, A.C.; D’Souza-Schorey, C. Recruitment of DNA to tumor-derived microvesicles. Cell Rep. 2022, 38, 110443. [Google Scholar] [CrossRef] [PubMed]
- Schmidtmann, M.; D’Souza-Schorey, C. Extracellular Vesicles: Biological Packages That Modulate Tumor Cell Invasion. Cancers 2023, 15, 5617. [Google Scholar] [CrossRef] [PubMed]
- Nicolini, A.; Ferrari, P.; Biava, P.M. Exosomes and Cell Communication: From Tumour-Derived Exosomes and Their Role in Tumour Progression to the Use of Exosomal Cargo for Cancer Treatment. Cancers 2021, 13, 822. [Google Scholar] [CrossRef]
- Minciacchi, V.R.; Freeman, M.R.; Di Vizio, D. Extracellular vesicles in cancer: Exosomes, microvesicles and the emerging role of large oncosomes. Semin. Cell Dev. Biol. 2015, 40, 41–51. [Google Scholar] [CrossRef]
- Crescitelli, R.; Lasser, C.; Szabo, T.G.; Kittel, A.; Eldh, M.; Dianzani, I.; Buzas, E.I.; Lotvall, J. Distinct RNA profiles in subpopulations of extracellular vesicles: Apoptotic bodies, microvesicles and exosomes. J. Extracell. Vesicles 2013, 2, 20677. [Google Scholar] [CrossRef]
- Liu, D.; Kou, X.; Chen, C.; Liu, S.; Liu, Y.; Yu, W.; Yu, T.; Yang, R.; Wang, R.; Zhou, Y.; et al. Circulating apoptotic bodies maintain mesenchymal stem cell homeostasis and ameliorate osteopenia via transferring multiple cellular factors. Cell Res. 2018, 28, 918–933. [Google Scholar] [CrossRef]
- Tang, H.; Luo, H.; Zhang, Z.; Yang, D. Mesenchymal Stem Cell-Derived Apoptotic Bodies: Biological Functions and Therapeutic Potential. Cells 2022, 11, 3879. [Google Scholar] [CrossRef]
- Li, A.; Zhang, T.; Zheng, M.; Liu, Y.; Chen, Z. Exosomal proteins as potential markers of tumor diagnosis. J. Hematol. Oncol. 2017, 10, 175. [Google Scholar] [CrossRef]
- Ghanam, J.; Chetty, V.K.; Barthel, L.; Reinhardt, D.; Hoyer, P.F.; Thakur, B.K. DNA in extracellular vesicles: From evolution to its current application in health and disease. Cell Biosci. 2022, 12, 37. [Google Scholar] [CrossRef]
- Thakur, B.K.; Zhang, H.; Becker, A.; Matei, I.; Huang, Y.; Costa-Silva, B.; Zheng, Y.; Hoshino, A.; Brazier, H.; Xiang, J.; et al. Double-stranded DNA in exosomes: A novel biomarker in cancer detection. Cell Res. 2014, 24, 766–769. [Google Scholar] [CrossRef]
- Huotari, J.; Helenius, A. Endosome maturation. EMBO J. 2011, 30, 3481–3500. [Google Scholar] [CrossRef]
- Soekmadji, C.; Li, B.; Huang, Y.; Wang, H.; An, T.; Liu, C.; Pan, W.; Chen, J.; Cheung, L.; Falcon-Perez, J.M.; et al. The future of Extracellular Vesicles as Theranostics—An ISEV meeting report. J. Extracell. Vesicles 2020, 9, 1809766. [Google Scholar] [CrossRef]
- Berckmans, R.J.; Nieuwland, R.; Boing, A.N.; Romijn, F.P.; Hack, C.E.; Sturk, A. Cell-derived microparticles circulate in healthy humans and support low grade thrombin generation. Thromb. Haemost. 2001, 85, 639–646. [Google Scholar]
- Zhang, J.; Li, H.; Fan, B.; Xu, W.; Zhang, X. Extracellular vesicles in normal pregnancy and pregnancy-related diseases. J. Cell. Mol. Med. 2020, 24, 4377–4388. [Google Scholar] [CrossRef]
- Akbar, N.; Azzimato, V.; Choudhury, R.P.; Aouadi, M. Extracellular vesicles in metabolic disease. Diabetologia 2019, 62, 2179–2187. [Google Scholar] [CrossRef]
- Bewicke-Copley, F.; Mulcahy, L.A.; Jacobs, L.A.; Samuel, P.; Akbar, N.; Pink, R.C.; Carter, D.R.F. Extracellular vesicles released following heat stress induce bystander effect in unstressed populations. J. Extracell. Vesicles 2017, 6, 1340746. [Google Scholar] [CrossRef]
- Chiaradia, E.; Tancini, B.; Emiliani, C.; Delo, F.; Pellegrino, R.M.; Tognoloni, A.; Urbanelli, L.; Buratta, S. Extracellular Vesicles under Oxidative Stress Conditions: Biological Properties and Physiological Roles. Cells 2021, 10, 1763. [Google Scholar] [CrossRef]
- Sproviero, D.; Gagliardi, S.; Zucca, S.; Arigoni, M.; Giannini, M.; Garofalo, M.; Fantini, V.; Pansarasa, O.; Avenali, M.; Ramusino, M.C.; et al. Extracellular Vesicles Derived from Plasma of Patients with Neurodegenerative Disease Have Common Transcriptomic Profiling. Front. Aging Neurosci. 2022, 14, 785741. [Google Scholar] [CrossRef]
- Thompson, A.G.; Gray, E.; Heman-Ackah, S.M.; Mager, I.; Talbot, K.; Andaloussi, S.E.; Wood, M.J.; Turner, M.R. Extracellular vesicles in neurodegenerative disease—Pathogenesis to biomarkers. Nat. Rev. Neurol. 2016, 12, 346–357. [Google Scholar] [CrossRef]
- Martucci, G.; Arcadipane, A.; Tuzzolino, F.; Occhipinti, G.; Panarello, G.; Carcione, C.; Bonicolini, E.; Vitiello, C.; Lorusso, R.; Conaldi, P.G.; et al. Identification of a Circulating miRNA Signature to Stratify Acute Respiratory Distress Syndrome Patients. J. Pers. Med. 2020, 11, 15. [Google Scholar] [CrossRef]
- Charla, E.; Mercer, J.; Maffia, P.; Nicklin, S.A. Extracellular vesicle signalling in atherosclerosis. Cell Signal. 2020, 75, 109751. [Google Scholar] [CrossRef] [PubMed]
- Royo, F.; Moreno, L.; Mleczko, J.; Palomo, L.; Gonzalez, E.; Cabrera, D.; Cogolludo, A.; Vizcaino, F.P.; van-Liempd, S.; Falcon-Perez, J.M. Hepatocyte-secreted extracellular vesicles modify blood metabolome and endothelial function by an arginase-dependent mechanism. Sci. Rep. 2017, 7, 42798. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Hou, J.; Yang, C.; Wang, H.; Wu, S.; Wu, Y.; Zhao, X.; Lu, C. Extracellular vesicles secreted by hypoxia pre-challenged mesenchymal stem cells promote non-small cell lung cancer cell growth and mobility as well as macrophage M2 polarization via miR-21-5p delivery. J. Exp. Clin. Cancer Res. CR 2019, 38, 62. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Chen, H.; He, K.; Wang, J. Human bone marrow mesenchymal stem cells-derived exosomes attenuated prostate cancer progression via the miR-99b-5p/IGF1R axis. Bioengineered 2022, 13, 2004–2016. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yan, G.; Yue, M.; Wang, L. Extracellular vesicles-derived microRNA-222 promotes immune escape via interacting with ATF3 to regulate AKT1 transcription in colorectal cancer. BMC Cancer 2021, 21, 349. [Google Scholar] [CrossRef] [PubMed]
- Ono, M.; Kosaka, N.; Tominaga, N.; Yoshioka, Y.; Takeshita, F.; Takahashi, R.U.; Yoshida, M.; Tsuda, H.; Tamura, K.; Ochiya, T. Exosomes from bone marrow mesenchymal stem cells contain a microRNA that promotes dormancy in metastatic breast cancer cells. Sci. Signal. 2014, 7, ra63. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, A.; Alini, M.; Baghaban Eslaminejad, M.; Hosseini, S. Engineering strategies for customizing extracellular vesicle uptake in a therapeutic context. Stem Cell Res. Ther. 2022, 13, 129. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.; Yue, S.; Stadel, D.; Zoller, M. Toward tailored exosomes: The exosomal tetraspanin web contributes to target cell selection. Int. J. Biochem. Cell Biol. 2012, 44, 1574–1584. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, A.; Costa-Silva, B.; Shen, T.L.; Rodrigues, G.; Hashimoto, A.; Tesic Mark, M.; Molina, H.; Kohsaka, S.; Di Giannatale, A.; Ceder, S.; et al. Tumour exosome integrins determine organotropic metastasis. Nature 2015, 527, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.; Pazos, R.; Royo, F.; Gonzalez, E.; Roura-Ferrer, M.; Martinez, A.; Gamiz, J.; Reichardt, N.C.; Falcon-Perez, J.M. Assessing the role of surface glycans of extracellular vesicles on cellular uptake. Sci. Rep. 2019, 9, 11920. [Google Scholar] [CrossRef] [PubMed]
- Limoni, S.K.; Moghadam, M.F.; Moazzeni, S.M.; Gomari, H.; Salimi, F. Engineered Exosomes for Targeted Transfer of siRNA to HER2 Positive Breast Cancer Cells. Appl. Biochem. Biotechnol. 2019, 187, 352–364. [Google Scholar] [CrossRef]
- Cheng, Q.; Shi, X.; Han, M.; Smbatyan, G.; Lenz, H.J.; Zhang, Y. Reprogramming Exosomes as Nanoscale Controllers of Cellular Immunity. J. Am. Chem. Soc. 2018, 140, 16413–16417. [Google Scholar] [CrossRef]
- Hong, Y.; Kim, Y.K.; Kim, G.B.; Nam, G.H.; Kim, S.A.; Park, Y.; Yang, Y.; Kim, I.S. Degradation of tumour stromal hyaluronan by small extracellular vesicle-PH20 stimulates CD103(+) dendritic cells and in combination with PD-L1 blockade boosts anti-tumour immunity. J. Extracell. Vesicles 2019, 8, 1670893. [Google Scholar] [CrossRef]
- Lee, T.H.; Chennakrishnaiah, S.; Audemard, E.; Montermini, L.; Meehan, B.; Rak, J. Oncogenic ras-driven cancer cell vesiculation leads to emission of double-stranded DNA capable of interacting with target cells. Biochem. Biophys. Res. Commun. 2014, 451, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Valadi, H.; Ekstrom, K.; Bossios, A.; Sjostrand, M.; Lee, J.J.; Lotvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef]
- Miceli, V.; Bulati, M.; Iannolo, G.; Zito, G.; Gallo, A.; Conaldi, P.G. Therapeutic Properties of Mesenchymal Stromal/Stem Cells: The Need of Cell Priming for Cell-Free Therapies in Regenerative Medicine. Int. J. Mol. Sci. 2021, 22, 763. [Google Scholar] [CrossRef] [PubMed]
- Miceli, V.; Bertani, A. Mesenchymal Stromal/Stem Cells and Their Products as a Therapeutic Tool to Advance Lung Transplantation. Cells 2022, 11, 826. [Google Scholar] [CrossRef]
- Bulati, M.; Gallo, A.; Zito, G.; Busa, R.; Iannolo, G.; Cuscino, N.; Castelbuono, S.; Carcione, C.; Centi, C.; Martucci, G.; et al. 3D Culture and Interferon-gamma Priming Modulates Characteristics of Mesenchymal Stromal/Stem Cells by Modifying the Expression of Both Intracellular and Exosomal microRNAs. Biology 2023, 12, 1063. [Google Scholar] [CrossRef]
- Bulati, M.; Miceli, V.; Gallo, A.; Amico, G.; Carcione, C.; Pampalone, M.; Conaldi, P.G. The Immunomodulatory Properties of the Human Amnion-Derived Mesenchymal Stromal/Stem Cells Are Induced by INF-gamma Produced by Activated Lymphomonocytes and Are Mediated by Cell-To-Cell Contact and Soluble Factors. Front. Immunol. 2020, 11, 54. [Google Scholar] [CrossRef] [PubMed]
- Miceli, V.; Pampalone, M.; Vella, S.; Carreca, A.P.; Amico, G.; Conaldi, P.G. Comparison of Immunosuppressive and Angiogenic Properties of Human Amnion-Derived Mesenchymal Stem Cells between 2D and 3D Culture Systems. Stem Cells Int. 2019, 2019, 7486279. [Google Scholar] [CrossRef]
- Miceli, V.; Chinnici, C.M.; Bulati, M.; Pampalone, M.; Amico, G.; Schmelzer, E.; Gerlach, J.C.; Conaldi, P.G. Comparative study of the production of soluble factors in human placenta-derived mesenchymal stromal/stem cells grown in adherent conditions or as aggregates in a catheter-like device. Biochem. Biophys. Res. Commun. 2020, 522, 171–176. [Google Scholar] [CrossRef]
- Liang, B.; Liang, J.M.; Ding, J.N.; Xu, J.; Xu, J.G.; Chai, Y.M. Dimethyloxaloylglycine-stimulated human bone marrow mesenchymal stem cell-derived exosomes enhance bone regeneration through angiogenesis by targeting the AKT/mTOR pathway. Stem Cell Res. Ther. 2019, 10, 335. [Google Scholar] [CrossRef]
- Lu, Z.; Chen, Y.; Dunstan, C.; Roohani-Esfahani, S.; Zreiqat, H. Priming Adipose Stem Cells with Tumor Necrosis Factor-Alpha Preconditioning Potentiates Their Exosome Efficacy for Bone Regeneration. Tissue Eng. Part A 2017, 23, 1212–1220. [Google Scholar] [CrossRef] [PubMed]
- Casagrande, G.M.S.; Silva, M.O.; Reis, R.M.; Leal, L.F. Liquid Biopsy for Lung Cancer: Up-to-Date and Perspectives for Screening Programs. Int. J. Mol. Sci. 2023, 24, 2505. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.; Im, H.; Castro, C.M.; Breakefield, X.; Weissleder, R.; Lee, H. New Technologies for Analysis of Extracellular Vesicles. Chem. Rev. 2018, 118, 1917–1950. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Hill, A.F. Therapeutically harnessing extracellular vesicles. Nat. Rev. Drug Discov. 2022, 21, 379–399. [Google Scholar] [CrossRef] [PubMed]
- Albanese, M.; Chen, Y.A.; Huls, C.; Gartner, K.; Tagawa, T.; Mejias-Perez, E.; Keppler, O.T.; Gobel, C.; Zeidler, R.; Shein, M.; et al. MicroRNAs are minor constituents of extracellular vesicles that are rarely delivered to target cells. PLoS Genet. 2021, 17, e1009951. [Google Scholar] [CrossRef] [PubMed]
- Rabinowits, G.; Gercel-Taylor, C.; Day, J.M.; Taylor, D.D.; Kloecker, G.H. Exosomal microRNA: A diagnostic marker for lung cancer. Clin. Lung Cancer 2009, 10, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Balaj, L.; Lessard, R.; Dai, L.; Cho, Y.J.; Pomeroy, S.L.; Breakefield, X.O.; Skog, J. Tumour microvesicles contain retrotransposon elements and amplified oncogene sequences. Nat. Commun. 2011, 2, 180. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, C.A.; Andahur, E.I.; Valenzuela, R.; Castellon, E.A.; Fulla, J.A.; Ramos, C.G.; Trivino, J.C. Exosomes from bulk and stem cells from human prostate cancer have a differential microRNA content that contributes cooperatively over local and pre-metastatic niche. Oncotarget 2016, 7, 3993–4008. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.A.; Hur, J.Y.; Kim, H.J.; Kim, W.S.; Lee, K.Y. Extracellular Vesicle-Based Bronchoalveolar Lavage Fluid Liquid Biopsy for EGFR Mutation Testing in Advanced Non-Squamous NSCLC. Cancers 2022, 14, 2744. [Google Scholar] [CrossRef]
- Girard, N. Optimizing outcomes in EGFR mutation-positive NSCLC: Which tyrosine kinase inhibitor and when? Future Oncol. 2018, 14, 1117–1132. [Google Scholar] [CrossRef]
- Kim, I.A.; Hur, J.Y.; Kim, H.J.; Kim, W.S.; Lee, K.Y. A prospective phase 2 study of expeditious EGFR genotyping and immediate therapeutic initiation through extracellular vesicles (EV)-based bronchoalveolar lavage fluid (BALF) liquid biopsy in advanced NSCLC patients. Transl. Lung Cancer Res. 2023, 12, 1425–1435. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Zhu, Y.; Zhang, J.; Zhang, W.; Wang, H.; Chen, H.; Wu, C.; Ni, J.; Xu, X.; Nian, B.; et al. Identification and evaluation of circulating small extracellular vesicle microRNAs as diagnostic biomarkers for patients with indeterminate pulmonary nodules. J. Nanobiotechnol. 2022, 20, 172. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Guo, W.; Liu, T.; Liang, N.; Ma, Q.; Gao, Y.; Tan, F.; Xue, Q.; He, J. Plasma extracellular vesicle microRNA profiling and the identification of a diagnostic signature for stage I lung adenocarcinoma. Cancer Sci. 2022, 113, 648–659. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Eom, J.S.; Kim, W.Y.; Jo, E.J.; Mok, J.; Lee, K.; Kim, K.U.; Park, H.K.; Lee, M.K.; Kim, M.H. Diagnostic value of microRNAs derived from exosomes in bronchoalveolar lavage fluid of early-stage lung adenocarcinoma: A pilot study. Thorac. Cancer 2018, 9, 911–915. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.L. The expanding regulatory mechanisms and cellular functions of circular RNAs. Nat. Rev. Mol. Cell Biol. 2020, 21, 475–490. [Google Scholar] [CrossRef] [PubMed]
- Lasda, E.; Parker, R. Circular RNAs Co-Precipitate with Extracellular Vesicles: A Possible Mechanism for circRNA Clearance. PLoS ONE 2016, 11, e0148407. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.M.; Abdelmohsen, K.; Mustapic, M.; Kapogiannis, D.; Gorospe, M. RNA in extracellular vesicles. Wiley Interdiscip. Rev. RNA 2017, 8, 1413. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zheng, Q.; Bao, C.; Li, S.; Guo, W.; Zhao, J.; Chen, D.; Gu, J.; He, X.; Huang, S. Circular RNA is enriched and stable in exosomes: A promising biomarker for cancer diagnosis. Cell Res. 2015, 25, 981–984. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Yao, J.; Wang, Y.; Ni, B. Exosome-transmitted circVMP1 facilitates the progression and cisplatin resistance of non-small cell lung cancer by targeting miR-524-5p-METTL3/SOX2 axis. Drug Deliv. 2022, 29, 1257–1271. [Google Scholar] [CrossRef]
- Tan, Z.; Cao, F.; Jia, B.; Xia, L. Circ_0072088 promotes the development of non-small cell lung cancer via the miR-377-5p/NOVA2 axis. Thorac. Cancer 2020, 11, 2224–2236. [Google Scholar] [CrossRef]
- Hu, S.; Zhang, Q.; Sun, J.; Xue, J.; Wang, C. Circular RNA circ_0000376 promotes paclitaxel resistance and tumorigenesis of non-small cell lung cancer via positively modulating KPNA4 by sponging miR-1298-5p. Thorac. Cancer 2023, 14, 2116–2126. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhao, L.; Wang, Y. Circular RNA circ_0020123 promotes non-small cell lung cancer progression by sponging miR-590-5p to regulate THBS2. Cancer Cell Int. 2020, 20, 387. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Zeng, J.; Rong, F.; Xu, Y.; Wei, R.; Zou, C. Circ_0020123 enhances the cisplatin resistance in non-small cell lung cancer cells partly by sponging miR-140-3p to regulate homeobox B5 (HOXB5). Bioengineered 2022, 13, 5126–5140. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Wang, C.; Wang, L.; Zhang, J. Circ_0020123 promotes cell proliferation and migration in lung adenocarcinoma via PDZD8. Open Med. 2022, 17, 536–549. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Du, T.; Chen, X.; Hu, P. Circ-PDZD8 promotes cell growth and glutamine metabolism in non-small cell lung cancer by enriching LARP1 via sequestering miR-330-5p. Thorac. Cancer 2023, 14, 2187–2197. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Huang, T.; Yuan, S.; Long, Y.; Tan, S.; Niu, G.; Zhang, P.; Yang, M. Circ_0020123 plays an oncogenic role in non-small cell lung cancer depending on the regulation of miR-512-3p/CORO1C. Thorac. Cancer 2022, 13, 1406–1418. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Yang, X.; Song, W.; Yu, N.; Lin, Q. Tanshinone IIA (TSIIA) represses the progression of non-small cell lung cancer by the circ_0020123/miR-1299/HMGB3 pathway. Mol. Cell. Biochem. 2023, 478, 1973–1986. [Google Scholar] [CrossRef] [PubMed]
- de Fraipont, F.; Gazzeri, S.; Cho, W.C.; Eymin, B. Circular RNAs and RNA Splice Variants as Biomarkers for Prognosis and Therapeutic Response in the Liquid Biopsies of Lung Cancer Patients. Front. Genet. 2019, 10, 390. [Google Scholar] [CrossRef] [PubMed]
- Dudekula, D.B.; Panda, A.C.; Grammatikakis, I.; De, S.; Abdelmohsen, K.; Gorospe, M. CircInteractome: A web tool for exploring circular RNAs and their interacting proteins and microRNAs. RNA Biol. 2016, 13, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Pedraz-Valdunciel, C.; Giannoukakos, S.; Gimenez-Capitan, A.; Fortunato, D.; Filipska, M.; Bertran-Alamillo, J.; Bracht, J.W.P.; Drozdowskyj, A.; Valarezo, J.; Zarovni, N.; et al. Multiplex Analysis of CircRNAs from Plasma Extracellular Vesicle-Enriched Samples for the Detection of Early-Stage Non-Small Cell Lung Cancer. Pharmaceutics 2022, 14, 2034. [Google Scholar] [CrossRef]
- Pedraz-Valdunciel, C.; Giannoukakos, S.; Potie, N.; Gimenez-Capitan, A.; Huang, C.Y.; Hackenberg, M.; Fernandez-Hilario, A.; Bracht, J.; Filipska, M.; Aldeguer, E.; et al. Digital multiplexed analysis of circular RNAs in FFPE and fresh non-small cell lung cancer specimens. Mol. Oncol. 2022, 16, 2367–2383. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Shen, L.; Xia, Q.; Tao, H.; Liu, Z.; Wang, M.; Zhang, X.; Zhang, J.; Lv, J. Extracellular vesicle-derived circHIPK3: Novel diagnostic biomarker for lung cancer. Adv. Med. Sci. 2023, 68, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Jiang, J.; Qian, H.; Yan, Y.; Xu, W. Exosomal circRNA: Emerging insights into cancer progression and clinical application potential. J. Hematol. Oncol. 2023, 16, 67. [Google Scholar] [CrossRef] [PubMed]
- Cammarata, G.; Barraco, N.; Giusti, I.; Gristina, V.; Dolo, V.; Taverna, S. Extracellular Vesicles-ceRNAs as Ovarian Cancer Biomarkers: Looking into circRNA-miRNA-mRNA Code. Cancers 2022, 14, 3404. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Wang, J.; Liu, L.; Zou, C.; Zhao, Y.; Xue, Z.; Sun, X.; Jiang, T.; Song, J. Presence and prospects of exosomal circRNAs in cancer (Review). Int. J. Oncol. 2023, 62, 5495. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhu, X.; Zhang, H.; Wei, S.; Chen, Y.; Chen, Y.; Wang, F.; Fan, X.; Han, S.; Wu, G. Increased circular RNA hsa_circ_0012673 acts as a sponge of miR-22 to promote lung adenocarcinoma proliferation. Biochem. Biophys. Res. Commun. 2018, 496, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, H. CircRNA circ_0067934 silencing inhibits the proliferation, migration and invasion of NSCLC cells and correlates with unfavorable prognosis in NSCLC. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 3053–3060. [Google Scholar] [CrossRef]
- Mo, W.L.; Deng, L.J.; Cheng, Y.; Yu, W.J.; Yang, Y.H.; Gu, W.D. Circular RNA hsa_circ_0072309 promotes tumorigenesis and invasion by regulating the miR-607/FTO axis in non-small cell lung carcinoma. Aging 2021, 13, 11629–11645. [Google Scholar] [CrossRef]
- Wan, Z.; Jia, S.; Lu, J.; Ge, X.; Chen, Q. circ-ATAD1 as Competing Endogenous RNA for miR-191-5p Forces Non-small Cell Lung Cancer Progression. Appl. Biochem. Biotechnol. 2023. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Z.; Tian, H. Hsa_circ_0092887 targeting miR-490-5p/UBE2T promotes paclitaxel resistance in non-small cell lung cancer. J. Clin. Lab. Anal. 2023, 37, e24781. [Google Scholar] [CrossRef]
- Jiang, M.M.; Mai, Z.T.; Wan, S.Z.; Chi, Y.M.; Zhang, X.; Sun, B.H.; Di, Q.G. Microarray profiles reveal that circular RNA hsa_circ_0007385 functions as an oncogene in non-small cell lung cancer tumorigenesis. J. Cancer Res. Clin. Oncol. 2018, 144, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Wang, X.; Wei, S.; Chen, Y.; Chen, Y.; Fan, X.; Han, S.; Wu, G. hsa_circ_0013958: A circular RNA and potential novel biomarker for lung adenocarcinoma. FEBS J. 2017, 284, 2170–2182. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Tong, X.; Zhou, Z.; Wang, S.; Lei, Z.; Zhang, T.; Liu, Z.; Zeng, Y.; Li, C.; Zhao, J.; et al. Circular RNA hsa_circ_0008305 (circPTK2) inhibits TGF-beta-induced epithelial-mesenchymal transition and metastasis by controlling TIF1gamma in non-small cell lung cancer. Mol. Cancer 2018, 17, 140. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liang, Y.; Mao, Q.; Xia, W.; Chen, B.; Shen, H.; Xu, L.; Jiang, F.; Dong, G. Circular RNA circCRIM1 inhibits invasion and metastasis in lung adenocarcinoma through the microRNA (miR)-182/miR-93-leukemia inhibitory factor receptor pathway. Cancer Sci. 2019, 110, 2960–2972. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, S.; Mao, Y.; Xu, J.; Yang, S.; Shen, H.; Xu, W.; Fan, W.; Wang, J. CircRNF13 regulates the invasion and metastasis in lung adenocarcinoma by targeting miR-93-5p. Gene 2018, 671, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Chao, F.; Zhang, Y.; Lv, L.; Wei, Y.; Dou, X.; Chang, N.; Yi, Q.; Li, M. Extracellular Vesicles Derived circSH3PXD2A Inhibits Chemoresistance of Small Cell Lung Cancer by miR-375-3p/YAP1. Int. J. Nanomed. 2023, 18, 2989–3006. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, A.; Kim, H.S.; Bojmar, L.; Gyan, K.E.; Cioffi, M.; Hernandez, J.; Zambirinis, C.P.; Rodrigues, G.; Molina, H.; Heissel, S.; et al. Extracellular Vesicle and Particle Biomarkers Define Multiple Human Cancers. Cell 2020, 182, 1044–1061.e1018. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, K.R.; Paulsen, B.S.; Baek, R.; Varming, K.; Sorensen, B.S.; Jorgensen, M.M. Exosomal proteins as potential diagnostic markers in advanced non-small cell lung carcinoma. J. Extracell. Vesicles 2015, 4, 26659. [Google Scholar] [CrossRef] [PubMed]
- Malla, R.R.; Pandrangi, S.; Kumari, S.; Gavara, M.M.; Badana, A.K. Exosomal tetraspanins as regulators of cancer progression and metastasis and novel diagnostic markers. Asia-Pac. J. Clin. Oncol. 2018, 14, 383–391. [Google Scholar] [CrossRef]
- Taverna, S.; Pucci, M.; Giallombardo, M.; Di Bella, M.A.; Santarpia, M.; Reclusa, P.; Gil-Bazo, I.; Rolfo, C.; Alessandro, R. Amphiregulin contained in NSCLC-exosomes induces osteoclast differentiation through the activation of EGFR pathway. Sci. Rep. 2017, 7, 3170. [Google Scholar] [CrossRef]
- Thuya, W.L.; Kong, L.R.; Syn, N.L.; Ding, L.W.; Cheow, E.S.H.; Wong, R.T.X.; Wang, T.; Goh, R.M.W.; Song, H.; Jayasinghe, M.K.; et al. FAM3C in circulating tumor-derived extracellular vesicles promotes non-small cell lung cancer growth in secondary sites. Theranostics 2023, 13, 621–638. [Google Scholar] [CrossRef]
- Dou, X.; Hua, Y.; Chen, Z.; Chao, F.; Li, M. Extracellular vesicles containing PD-L1 contribute to CD8+ T-cell immune suppression and predict poor outcomes in small cell lung cancer. Clin. Exp. Immunol. 2022, 207, 307–317. [Google Scholar] [CrossRef]
- Xu, J.; Wang, L.; Yin, N.; Chen, A.; Yi, J.; Tang, J.; Xiang, J. Proteomic profiling of extracellular vesicles and particles reveals the cellular response to cisplatin in NSCLC. Thorac. Cancer 2021, 12, 2601–2610. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.K.; Kang, B.; Kim, O.Y.; Choi, D.S.; Lee, J.; Kim, S.R.; Go, G.; Yoon, Y.J.; Kim, J.H.; Jang, S.C.; et al. EVpedia: An integrated database of high-throughput data for systemic analyses of extracellular vesicles. J. Extracell. Vesicles 2013, 2, 20384. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.; Li, Y.; Zhang, H.; Hu, J.; Liao, J.; Su, Y.; Li, Q.; Chen, B.; Li, C.; Wang, Z.; et al. exoRBase 2.0: An atlas of mRNA, lncRNA and circRNA in extracellular vesicles from human biofluids. Nucleic Acids Res. 2022, 50, D118–D128. [Google Scholar] [CrossRef]
- Keerthikumar, S.; Chisanga, D.; Ariyaratne, D.; Al Saffar, H.; Anand, S.; Zhao, K.; Samuel, M.; Pathan, M.; Jois, M.; Chilamkurti, N.; et al. ExoCarta: A Web-Based Compendium of Exosomal Cargo. J. Mol. Biol. 2016, 428, 688–692. [Google Scholar] [CrossRef] [PubMed]
- Rolfo, C.; Mack, P.C.; Scagliotti, G.V.; Baas, P.; Barlesi, F.; Bivona, T.G.; Herbst, R.S.; Mok, T.S.; Peled, N.; Pirker, R.; et al. Liquid Biopsy for Advanced Non-Small Cell Lung Cancer (NSCLC): A Statement Paper from the IASLC. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2018, 13, 1248–1268. [Google Scholar] [CrossRef]
- Carvalho, A.S.; Moraes, M.C.S.; Hyun Na, C.; Fierro-Monti, I.; Henriques, A.; Zahedi, S.; Bodo, C.; Tranfield, E.M.; Sousa, A.L.; Farinho, A.; et al. Is the Proteome of Bronchoalveolar Lavage Extracellular Vesicles a Marker of Advanced Lung Cancer? Cancers 2020, 12, 3450. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Park, H.Y.; Hur, J.Y.; Kim, H.J.; Kim, I.A.; Kim, W.S.; Lee, K.Y. Genomic profiling of extracellular vesicle-derived DNA from bronchoalveolar lavage fluid of patients with lung adenocarcinoma. Transl. Lung Cancer Res. 2021, 10, 104–116. [Google Scholar] [CrossRef]
- Hur, J.Y.; Kim, H.J.; Lee, J.S.; Choi, C.M.; Lee, J.C.; Jung, M.K.; Pack, C.G.; Lee, K.Y. Extracellular vesicle-derived DNA for performing EGFR genotyping of NSCLC patients. Mol. Cancer 2018, 17, 15. [Google Scholar] [CrossRef]
- Park, J.; Lee, C.; Eom, J.S.; Kim, M.H.; Cho, Y.K. Detection of EGFR Mutations Using Bronchial Washing-Derived Extracellular Vesicles in Patients with Non-Small-Cell Lung Carcinoma. Cancers 2020, 12, 2822. [Google Scholar] [CrossRef] [PubMed]
- Javadi, J.; Gorgens, A.; Vanky, H.; Gupta, D.; Hjerpe, A.; El-Andaloussi, S.; Hagey, D.; Dobra, K. Diagnostic and Prognostic Utility of the Extracellular Vesicles Subpopulations Present in Pleural Effusion. Biomolecules 2021, 11, 1606. [Google Scholar] [CrossRef] [PubMed]
- Duma, N.; Santana-Davila, R.; Molina, J.R. Non-Small Cell Lung Cancer: Epidemiology, Screening, Diagnosis, and Treatment. Mayo Clin. Proc. 2019, 94, 1623–1640. [Google Scholar] [CrossRef] [PubMed]
- Barta, J.A.; Powell, C.A.; Wisnivesky, J.P. Global Epidemiology of Lung Cancer. Ann. Glob. Health 2019, 85, 8. [Google Scholar] [CrossRef] [PubMed]
- Planchard, D.; Popat, S.; Kerr, K.; Novello, S.; Smit, E.F.; Faivre-Finn, C.; Mok, T.S.; Reck, M.; Van Schil, P.E.; Hellmann, M.D.; et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2018, 29, iv192–iv237. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.K.; Wu, Y.L.; Ding, P.N.; Lord, S.J.; Inoue, A.; Zhou, C.; Mitsudomi, T.; Rosell, R.; Pavlakis, N.; Links, M.; et al. Impact of Specific Epidermal Growth Factor Receptor (EGFR) Mutations and Clinical Characteristics on Outcomes After Treatment with EGFR Tyrosine Kinase Inhibitors Versus Chemotherapy in EGFR-Mutant Lung Cancer: A Meta-Analysis. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015, 33, 1958–1965. [Google Scholar] [CrossRef] [PubMed]
- Ciardiello, F.; Hirsch, F.R.; Pirker, R.; Felip, E.; Valencia, C.; Smit, E.F. The role of anti-EGFR therapies in EGFR-TKI-resistant advanced non-small cell lung cancer. Cancer Treat. Rev. 2024, 122, 102664. [Google Scholar] [CrossRef]
- Lei, Y.; Lei, Y.; Shi, X.; Wang, J. EML4-ALK fusion gene in non-small cell lung cancer. Oncol. Lett. 2022, 24, 277. [Google Scholar] [CrossRef]
- Baglivo, S.; Ricciuti, B.; Ludovini, V.; Metro, G.; Siggillino, A.; De Giglio, A.; Chiari, R. Dramatic Response to Lorlatinib in a Heavily Pretreated Lung Adenocarcinoma Patient Harboring G1202R Mutation and a Synchronous Novel R1192P ALK Point Mutation. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2018, 13, e145–e147. [Google Scholar] [CrossRef]
- Shaw, A.T.; Ou, S.H.; Bang, Y.J.; Camidge, D.R.; Solomon, B.J.; Salgia, R.; Riely, G.J.; Varella-Garcia, M.; Shapiro, G.I.; Costa, D.B.; et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N. Engl. J. Med. 2014, 371, 1963–1971. [Google Scholar] [CrossRef]
- Drilon, A.; Somwar, R.; Wagner, J.P.; Vellore, N.A.; Eide, C.A.; Zabriskie, M.S.; Arcila, M.E.; Hechtman, J.F.; Wang, L.; Smith, R.S.; et al. A Novel Crizotinib-Resistant Solvent-Front Mutation Responsive to Cabozantinib Therapy in a Patient with ROS1-Rearranged Lung Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2016, 22, 2351–2358. [Google Scholar] [CrossRef] [PubMed]
- Rudin, C.M.; Brambilla, E.; Faivre-Finn, C.; Sage, J. Small-cell lung cancer. Nat. Rev. Dis. Primers 2021, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Su, J.; Li, F.L.; Chen, T.; Mayner, J.; Engler, A.; Ma, S.; Li, Q.; Guan, K.L. YAP silencing by RB1 mutation is essential for small-cell lung cancer metastasis. Nat. Commun. 2023, 14, 5916. [Google Scholar] [CrossRef] [PubMed]
- Wildey, G.; Shay, A.M.; McColl, K.S.; Yoon, S.; Shatat, M.A.; Perwez, A.; Spainhower, K.B.; Kresak, A.M.; Lipka, M.; Yang, M.; et al. Retinoblastoma Expression and Targeting by CDK4/6 Inhibitors in Small Cell Lung Cancer. Mol. Cancer Ther. 2023, 22, 264–273. [Google Scholar] [CrossRef]
- Paramanantham, A.; Asfiya, R.; Das, S.; McCully, G.; Srivastava, A. Extracellular Vesicle (EVs) Associated Non-Coding RNAs in Lung Cancer and Therapeutics. Int. J. Mol. Sci. 2022, 23, 13637. [Google Scholar] [CrossRef] [PubMed]
- Beg, M.S.; Brenner, A.J.; Sachdev, J.; Borad, M.; Kang, Y.K.; Stoudemire, J.; Smith, S.; Bader, A.G.; Kim, S.; Hong, D.S. Phase I study of MRX34, a liposomal miR-34a mimic, administered twice weekly in patients with advanced solid tumors. Investig. New Drugs 2017, 35, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.S.; Kang, Y.K.; Borad, M.; Sachdev, J.; Ejadi, S.; Lim, H.Y.; Brenner, A.J.; Park, K.; Lee, J.L.; Kim, T.Y.; et al. Phase 1 study of MRX34, a liposomal miR-34a mimic, in patients with advanced solid tumours. Br. J. Cancer 2020, 122, 1630–1637. [Google Scholar] [CrossRef] [PubMed]
- Badami, E.; Carcione, C.; Chinnici, C.M.; Tinnirello, R.; Conaldi, P.G.; Iannolo, G. HCV Interplay with Mir34a: Implications in Hepatocellular Carcinoma. Front. Oncol. 2021, 11, 803278. [Google Scholar] [CrossRef]
- Vakhshiteh, F.; Rahmani, S.; Ostad, S.N.; Madjd, Z.; Dinarvand, R.; Atyabi, F. Exosomes derived from miR-34a-overexpressing mesenchymal stem cells inhibit in vitro tumor growth: A new approach for drug delivery. Life Sci. 2021, 266, 118871. [Google Scholar] [CrossRef]
- Shan, C.; Liang, Y.; Wang, K.; Li, P. Mesenchymal Stem Cell-Derived Extracellular Vesicles in Cancer Therapy Resistance: From Biology to Clinical Opportunity. Int. J. Biol. Sci. 2024, 20, 347–366. [Google Scholar] [CrossRef]
- Sohrabi, B.; Dayeri, B.; Zahedi, E.; Khoshbakht, S.; Nezamabadi Pour, N.; Ranjbar, H.; Davari Nejad, A.; Noureddini, M.; Alani, B. Mesenchymal stem cell (MSC)-derived exosomes as novel vehicles for delivery of miRNAs in cancer therapy. Cancer Gene Ther. 2022, 29, 1105–1116. [Google Scholar] [CrossRef]
- Wu, H.; Mu, X.; Liu, L.; Wu, H.; Hu, X.; Chen, L.; Liu, J.; Mu, Y.; Yuan, F.; Liu, W.; et al. Bone marrow mesenchymal stem cells-derived exosomal microRNA-193a reduces cisplatin resistance of non-small cell lung cancer cells via targeting LRRC1. Cell Death Dis. 2020, 11, 801. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ding, C.; Yang, X.; Zhao, J. BMSCs-Derived Exosomal MiR-126-3p Inhibits the Viability of NSCLC Cells by Targeting PTPN9. JBUON Off. J. Balk. Union Oncol. 2021, 26, 1832–1841. [Google Scholar]
- Li, X.; Wu, F. Mesenchymal stem cell-derived extracellular vesicles transfer miR-598 to inhibit the growth and metastasis of non-small-cell lung cancer by targeting THBS2. Cell Death Discov. 2023, 9, 3. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Yang, X.; Yang, H.; Lv, M.; Sun, X.; Zhou, B. Exosomal miR-338-3p suppresses non-small-cell lung cancer cells metastasis by inhibiting CHL1 through the MAPK signaling pathway. Cell Death Dis. 2021, 12, 1030. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Xu, M.; Wang, Z.; Yang, M. Engineered exosomes loaded with miR-449a selectively inhibit the growth of homologous non-small cell lung cancer. Cancer Cell Int. 2021, 21, 485. [Google Scholar] [CrossRef] [PubMed]
- Nie, H.; Xie, X.; Zhang, D.; Zhou, Y.; Li, B.; Li, F.; Li, F.; Cheng, Y.; Mei, H.; Meng, H.; et al. Use of lung-specific exosomes for miRNA-126 delivery in non-small cell lung cancer. Nanoscale 2020, 12, 877–887. [Google Scholar] [CrossRef] [PubMed]
- Anthiya, S.; Ozturk, S.C.; Yanik, H.; Tavukcuoglu, E.; Sahin, A.; Datta, D.; Charisse, K.; Alvarez, D.M.; Loza, M.I.; Calvo, A.; et al. Targeted siRNA lipid nanoparticles for the treatment of KRAS-mutant tumors. J. Control. Release Off. J. Control. Release Soc. 2023, 357, 67–83. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.H.; Li, Y.; Zhang, J.; Rong, J.; Ye, S. Epidermal growth factor receptor-containing exosomes induce tumor-specific regulatory T cells. Cancer Investig. 2013, 31, 330–335. [Google Scholar] [CrossRef] [PubMed]
- de Miguel-Perez, D.; Russo, A.; Arrieta, O.; Ak, M.; Barron, F.; Gunasekaran, M.; Mamindla, P.; Lara-Mejia, L.; Peterson, C.B.; Er, M.E.; et al. Extracellular vesicle PD-L1 dynamics predict durable response to immune-checkpoint inhibitors and survival in patients with non-small cell lung cancer. J. Exp. Clin. Cancer Res. CR 2022, 41, 186. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Q.; Liu, J.T.; Fan, L.L.; Liu, Y.; Cheng, L.; Wang, F.; Yu, H.Q.; Gao, J.; Wei, W.; Wang, H.; et al. Exosomes derived from gefitinib-treated EGFR-mutant lung cancer cells alter cisplatin sensitivity via up-regulating autophagy. Oncotarget 2016, 7, 24585–24595. [Google Scholar] [CrossRef]
- Li, W.; Hu, Y.; Jiang, T.; Han, Y.; Han, G.; Chen, J.; Li, X. Rab27A regulates exosome secretion from lung adenocarcinoma cells A549: Involvement of EPI64. APMIS Acta Pathol. Microbiol. Immunol. Scand. 2014, 122, 1080–1087. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Guo, J.; Yang, M.; Zhu, X.; Cao, X. Chemokine-containing exosomes are released from heat-stressed tumor cells via lipid raft-dependent pathway and act as efficient tumor vaccine. J. Immunol. 2011, 186, 2219–2228. [Google Scholar] [CrossRef] [PubMed]
- Hirschowitz, E.A.; Foody, T.; Kryscio, R.; Dickson, L.; Sturgill, J.; Yannelli, J. Autologous dendritic cell vaccines for non-small-cell lung cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2004, 22, 2808–2815. [Google Scholar] [CrossRef] [PubMed]
- Markov, O.; Oshchepkova, A.; Mironova, N. Immunotherapy Based on Dendritic Cell-Targeted/-Derived Extracellular Vesicles-A Novel Strategy for Enhancement of the Anti-tumor Immune Response. Front. Pharmacol. 2019, 10, 1152. [Google Scholar] [CrossRef] [PubMed]
- Pitt, J.M.; Andre, F.; Amigorena, S.; Soria, J.C.; Eggermont, A.; Kroemer, G.; Zitvogel, L. Dendritic cell-derived exosomes for cancer therapy. J. Clin. Investig. 2016, 126, 1224–1232. [Google Scholar] [CrossRef] [PubMed]
- Morse, M.A.; Garst, J.; Osada, T.; Khan, S.; Hobeika, A.; Clay, T.M.; Valente, N.; Shreeniwas, R.; Sutton, M.A.; Delcayre, A.; et al. A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer. J. Transl. Med. 2005, 3, 9. [Google Scholar] [CrossRef] [PubMed]
- Andre, F.; Escudier, B.; Angevin, E.; Tursz, T.; Zitvogel, L. Exosomes for cancer immunotherapy. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2004, 15 (Suppl. S4), iv141–iv144. [Google Scholar] [CrossRef]
- Zitvogel, L.; Regnault, A.; Lozier, A.; Wolfers, J.; Flament, C.; Tenza, D.; Ricciardi-Castagnoli, P.; Raposo, G.; Amigorena, S. Eradication of established murine tumors using a novel cell-free vaccine: Dendritic cell-derived exosomes. Nat. Med. 1998, 4, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Haney, M.J.; Zhao, Y.; Yuan, D.; Deygen, I.; Klyachko, N.L.; Kabanov, A.V.; Batrakova, E.V. Engineering macrophage-derived exosomes for targeted paclitaxel delivery to pulmonary metastases: In vitro and in vivo evaluations. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 195–204. [Google Scholar] [CrossRef]
- Kim, M.S.; Haney, M.J.; Zhao, Y.; Mahajan, V.; Deygen, I.; Klyachko, N.L.; Inskoe, E.; Piroyan, A.; Sokolsky, M.; Okolie, O.; et al. Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, N.; Wang, J. M1 macrophage-derived exosome-encapsulated cisplatin can enhance its anti-lung cancer effect. Minerva Medica 2023, 114, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Amreddy, N.; Babu, A.; Panneerselvam, J.; Mehta, M.; Muralidharan, R.; Chen, A.; Zhao, Y.D.; Razaq, M.; Riedinger, N.; et al. Nanosomes carrying doxorubicin exhibit potent anticancer activity against human lung cancer cells. Sci. Rep. 2016, 6, 38541. [Google Scholar] [CrossRef] [PubMed]
- Lara, P.; Palma-Florez, S.; Salas-Huenuleo, E.; Polakovicova, I.; Guerrero, S.; Lobos-Gonzalez, L.; Campos, A.; Munoz, L.; Jorquera-Cordero, C.; Varas-Godoy, M.; et al. Gold nanoparticle based double-labeling of melanoma extracellular vesicles to determine the specificity of uptake by cells and preferential accumulation in small metastatic lung tumors. J. Nanobiotechnol. 2020, 18, 20. [Google Scholar] [CrossRef]
- Aqil, F.; Kausar, H.; Agrawal, A.K.; Jeyabalan, J.; Kyakulaga, A.H.; Munagala, R.; Gupta, R. Exosomal formulation enhances therapeutic response of celastrol against lung cancer. Exp. Mol. Pathol. 2016, 101, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Munagala, R.; Aqil, F.; Jeyabalan, J.; Agrawal, A.K.; Mudd, A.M.; Kyakulaga, A.H.; Singh, I.P.; Vadhanam, M.V.; Gupta, R.C. Exosomal formulation of anthocyanidins against multiple cancer types. Cancer Lett. 2017, 393, 94–102. [Google Scholar] [CrossRef]
- Agrawal, A.K.; Aqil, F.; Jeyabalan, J.; Spencer, W.A.; Beck, J.; Gachuki, B.W.; Alhakeem, S.S.; Oben, K.; Munagala, R.; Bondada, S.; et al. Milk-derived exosomes for oral delivery of paclitaxel. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1627–1636. [Google Scholar] [CrossRef] [PubMed]
- Bari, E.; Ferrarotti, I.; Torre, M.L.; Corsico, A.G.; Perteghella, S. Mesenchymal stem/stromal cell secretome for lung regeneration: The long way through “pharmaceuticalization” for the best formulation. J. Control. Release Off. J. Control. Release Soc. 2019, 309, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Chinnici, C.M.; Russelli, G.; Bulati, M.; Miceli, V.; Gallo, A.; Busa, R.; Tinnirello, R.; Conaldi, P.G.; Iannolo, G. Mesenchymal stromal cell secretome in liver failure: Perspectives on COVID-19 infection treatment. World J. Gastroenterol. 2021, 27, 1905–1919. [Google Scholar] [CrossRef]
- Yao, X.; Liao, B.; Chen, F.; Liu, L.; Wu, K.; Hao, Y.; Li, Y.; Wang, Y.; Fan, R.; Yin, J.; et al. Comparison of proteomic landscape of extracellular vesicles in pleural effusions isolated by three strategies. Front. Bioeng. Biotechnol. 2023, 11, 1108952. [Google Scholar] [CrossRef]
- French, K.C.; Antonyak, M.A.; Cerione, R.A. Extracellular vesicle docking at the cellular port: Extracellular vesicle binding and uptake. Semin. Cell Dev. Biol. 2017, 67, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, M.; Martin-Jaular, L.; Lavieu, G.; Thery, C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 2019, 21, 9–17. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).